1. Introduction
Short bowel syndrome (SBS) is defined as the malabsorptive condition most often caused by massive resection of the small intestine [
1]. SBS is the most common cause of intestinal failure, which is the state when an individual’s gastrointestinal function is inadequate to maintain nutrient, growth and hydration status without intravenous or enteral supplementation [
1]. In children most cases of SBS originate in the newborn period and result from congenital anomalies or necrotizing enterocolitis [
2]. Gut failure can now be successfully managed, due to prolonged parenteral nutrition in hospital and/or at home [
3]. Loss of gut mucosa during resection does not only mean loss of absorption area but also deprives organism of many immunocompetent cells representing innate and adaptive response. Gut associated lymphoid tissue (GALT), which is regarded as the largest immune organ in human body contains a variety of immune cell types, particularly lymphocytes [
4]. GALT plays a critical role in the development of the systemic immune response. As a primary site of antigen exposure it primes naïve T- and B-lymphocytes, that develop into effector cells which migrate from the intestine to other sites of the body to protect against immune challenges, such as invading pathogens [
5].
Although nascent Peyer’s patches are evident in the newborn, the epithelium and lamina propria are devoid of mononuclear cells. T lymphocytes migrating from the thymus rapidly populate the thymus—dependent areas of Peyer’s patches and the epithelium but exposure to micro-organisms in the normal environment is necessary to develop the B cell population and their germinal follicles as shown by experimental studies [
6]. Children in whom large part of bowel was resected in neonatal period are obviously deprived of this large immune ,,training” area for their adaptive response. Therefore some investigators turned towards assessment of immunity in children with SBS. The publications however are sparse and focus on selected elements of immunity. To the best of our knowledge none of the studies investigated lymphocyte populations in children with SBS. Based on these notions we aimed to answer the question if immune deficiency expressed by peripheral lymphocytes counts and serum immunoglobulins can be long term consequence of massive bowel resection in newborn period.
2. Patients and methods
15 patients (aged 4 months–10 years) with short bowel disease, being under the care of a nutrition outpatient clinic at Department of Pediatrics, Pediatric Gastroenterology, Allergology and Nutrition of Medical University of Gdansk were enrolled to the study. All the patients underwent bowel resection in neonatal period, subsequently required total parenteral nutrition, which was continued at home.
Patients did not present with any signs of infection at evaluation. Clinical characteristics of the studied group are summarized in
Table 1.
12 healthy (2-5 years) children constituted control group.
Peripheral venous blood samples (5 ml) were collected during routine clinical control, while planned venipuncture. Serum immunoglobulins were determined in all the participants with the use of immunoturbidimetric method.
2.1. Ethics
The study was approved by the Independent Bioethical Committee of Medical University of Gdansk and was conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from legal guardians of all the participants included in the study.
2.2. Statistical analysis
The significance of differences between studied and control group was verified with Mann-Whitney U-test. The relationships between pairs of variables were analyzed on the basis of Spearman’s rank correlation coefficients. The results of all tests were considered significant at p<0.05. All analyses were performed with Statistica 10 software (Stat Soft. Inc., USA).
2.3. Antibodies, flowcytometry
Peripheral blood lymphocytes subsets were tested using “lyse no wash” method. Briefly, EDTA whole blood was stained with either CD45FITC/CD4RD-1/CD8ECD/CD3PC5 or CD45FITC/CD56RD-1/CD16PE/CD19ECD/CD3PC5 or CD3-FITC/HLA-DR-PE antibody cocktail (all from Beckman Coulter, USA) according to manufacturer procedure. Then lysis was performed with the use of Immunoprep Reagent System and TQ-Prep Workstation (Beckman Coulter, USA). Finally, Flow-Count Fluorospheres (Beckman Coulter, USA) was added for absolute lymphocytes counting and the sample readout was done with Navios flow cytometer (Beckman Coulter, USA). For each sample minimum 100.000 events were recorded, and CD45 gating was applied for T/B/NK lymphocytes enumeration, while activated T cells were identified as CD3/HLA-DR double positive cells.
3. Results
Median age was 6 years for SBS group and 4 years for control group.
Proportions and absolute counts of lymphocyte populations in the studied and control groups are shown in the
Table 2.
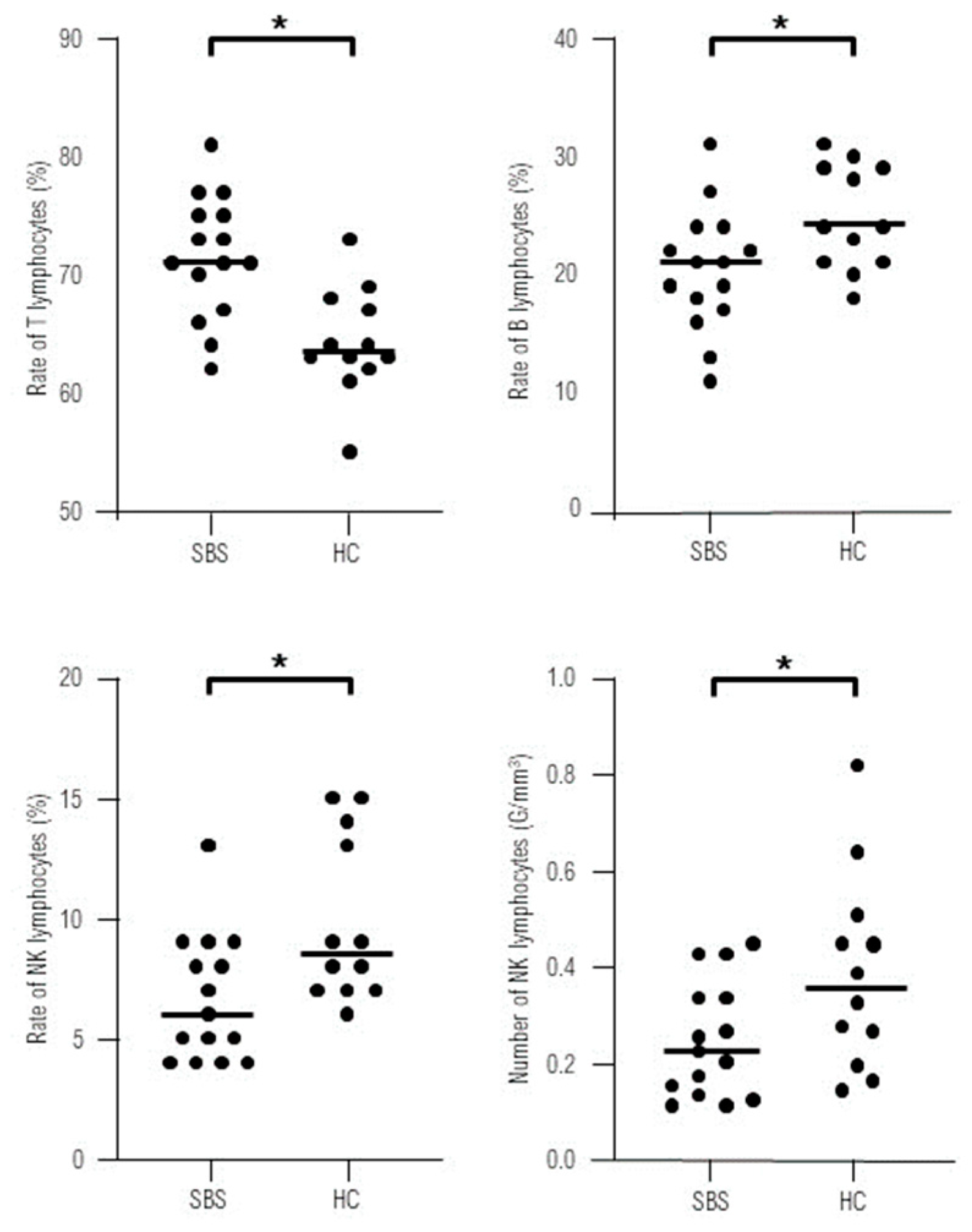
No statistically significant differences were found in the absolute numbers of lymphocytes T, B, CD4+, CD8+ and activated T cells in the study group comparing to control group. The percentage of B lymphocytes was reduced, while the rates of T lymphocytes were higher in SBS children. We documented significantly lower absolute count and proportion of NK cells in SBS group comparing to healthy controls (
Figure 1).
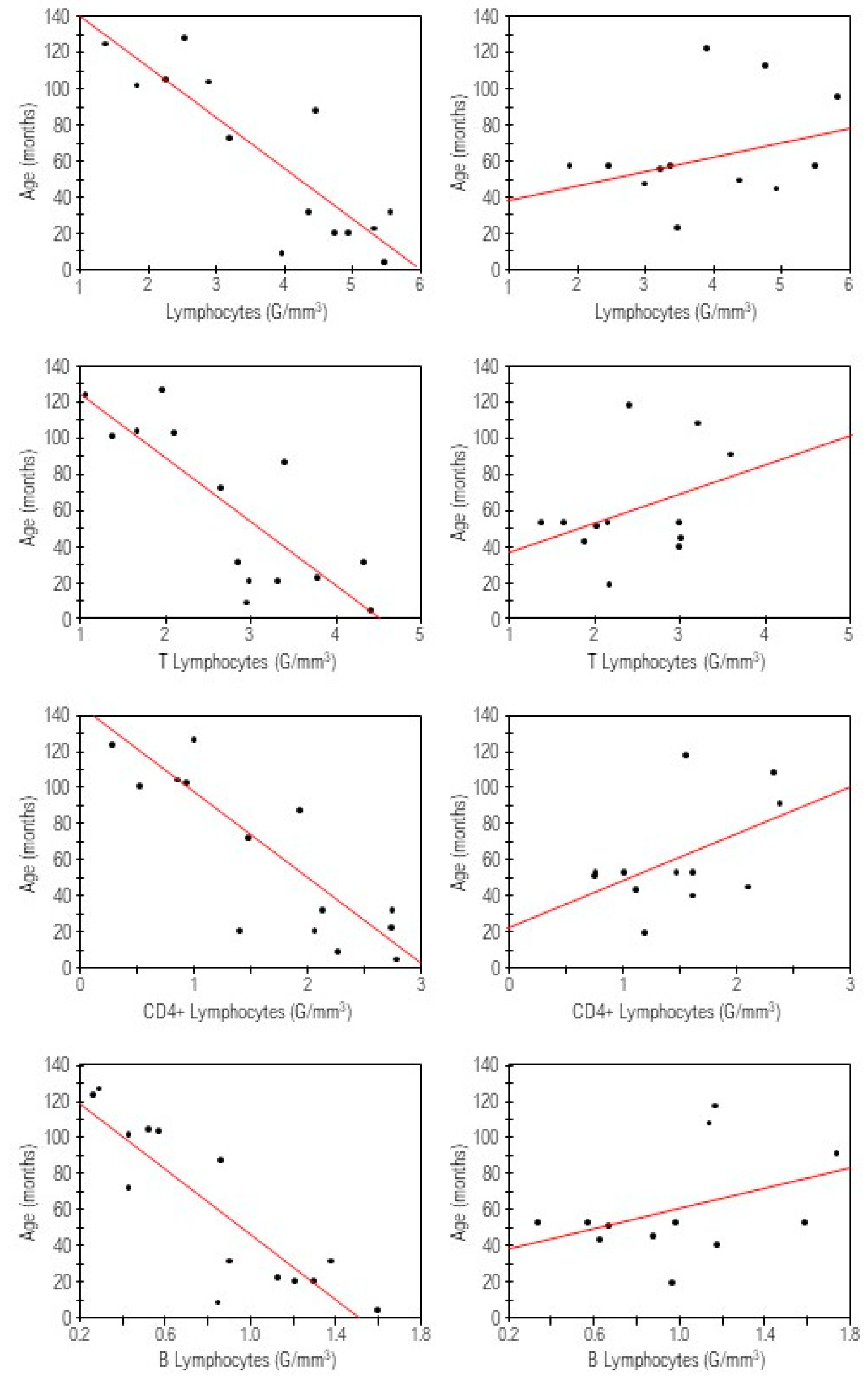
We found negative correlation between the rate of CD4+ cells and the length of resected bowel. No correlations between other investigated parameters and the length of resected bowel were noted.
Absolute counts of lymphocytes, lymphocytes B, T, CD4+ and percentages of lymphocytes CD4+, and activated T cells inversely correlated with the time after resection, while positive correlation was found for the percentage of CD8+ cells (
Figure 2).
No statistically significant differences were found between the levels of IgA, IgM and IgG in the studied and the control group. (p=0,62, p=0,60, p=0,24 respectively).
No patient demonstrated clinical signs of immunodeficiency.
4. Discussion
Studies concerning immunity in children with short bowel syndrome are sparse and refer only to selected immunological aspects. Realizing the crucial role of GALT for development of mucosal and systemic immunity, massive bowel resection could potentially result in serious immune dysfunction. Factors that may contribute to immune disturbances in SBS include: reduction of bowel surface for GALT development, lack of normal food antigen processing, bacterial overgrowth, increased permeability, bacterial translocation and influence of TPN on systemic immunity [
7].
Some publications dealing with immunological consequences of short bowel syndrome in children have been revised by Duran, but none of them investigated mechanism of systemic adaptive immune response [
7]. Moreover, all the analyzed publications concerned patients receiving parenteral nutrition and none of the studies related to late immune consequences of bowel resection. The only existing study describing lymphocyte subsets in short bowel syndrome refers to adults. It documents that 10 SBS patients presented with normal values of CD3+, CD4+, CD8+ lymphocytes, but reduced B lymphocytes in comparison to healthy individuals [
8]. One has to note, however, that bowel resection in adulthood is a completely distinct state, as the removal of intestine takes place in the individuals in whom immunity had previously undergone physiological development. In our cohort no differences in absolute counts of lymphocytes T, including CD4 +, CD8+cells and also B lymphocytes were found. Only the proportions of lymphocytes T and B in the studied group significantly differed from the control group, showing reduction in B lymphocytes frequency and increased rates of T lymphocytes.
We have also noted normal rate and number of activated T lymphocytes in SBS patients. This group of lymphocytes was particularly interesting, as T cell activation being the crucial process of development of adaptive immune response takes place mainly in mucosal associated lymphoid tissue including GALT. We have documented significantly lower absolute count and proportion of NK cells in SBS group comparing to healthy controls. This population of cells was not investigated by the authors of the quoted publication. The decrease in NK cells has been observed in some congenital and acquired conditions, mostly autoimmune diseases, but has never been reported in SBS [
9,
10]. We have to note that, although significantly lower, NK numbers were within the normal range in all our patients, thus cannot be considered as a sign of immunodeficiency. This preliminary observation needs to be confirmed in a larger cohort. Moreover, all the subjects displayed normal levels of immunoglobulins, what is an additional argument supporting our observation that immunodeficiency is not a late effect of bowel resection. Furthermore none of our patients demonstrated clinical signs of impaired immunity according to criteria developed by Jeffrey Model Foundation widely accepted by clinical immunologists [
11]. Although 4 patients had undergone catheter-related sepsis during TPN, no other severe infections were observed in the studied group. Likewise the quoted publication, our study was retrospective assessment of peripheral lymphocytes at different time after surgery, meaning that the studied group was not homogenous. The shortest time after resection in our cohort was 8 months, while the longest amounted to 10 years. It is worth noting that even the youngest subject in the studied group presented with the normal values of the studied subpopulations, what may indicate that although reduced, the remaining bowel surface is sufficient for the lymphocytes T and B to populate bowel and get activated. It has not however been clarified how long the reconstruction of GALT lasts. Probably some transient immunological imbalance appears after bowel resection, as it has been demonstrated in experimental studies. Barrena et al. reported systemic immune alterations, such as reduction in CD4+ lymphocytes and B lymphocytes, in SBS animal model 7 days after surgery [
12]. It may however reflect surgical stress rather than immunological imbalance caused by bowel removal, especially that analogical disturbances were also observed after other surgical interventions [
13]. Another study demonstrated changes in circulating lymphocytes numbers also 3 weeks after surgery [
14]. Short duration of the mentioned studies does not allow to conclude whether the presented abnormalities persist or rather they tend to evolve into full immune reconstruction in experimental animals. Therefore, future prospective studies are needed to clarify evolution of immune alterations following bowel resection in animals and in humans, especially children in whom intestinal loss takes place in newborn period.
We also examined relationships between lymphocytes subets and the length of resected bowel. Statistically significant inverse correlation was noted only for the rate of CD4+ cells. It could be explained by the reduction of the surface for antigen stimulation, which is necessary for maturation and proliferation of CD4+ cells [
15]. We do not consider this finding relevant, as it referred only to the rate, but not an absolute number of CD4 + and may result from small sample size. Analysis of the relations between the studied subses and the time after surgery revealed more findings. Surprisingly we found negative correlations for the absolute numbers of: lymphocytes, lymphocytes B, T, CD4+ and percentages of lymphocytes CD4+ and activated T cells. We would rather expect an increase of these amounts over time as a sign of immune reconstruction and maturation. It should be noted that in our subjects operated in the first days of life, the time since resection exactly corresponds to the child’s age. Therefore we also evaluated the correlation between the lymphocyte subpopulations and the age of children in control group, but we noted no statistically significant results. Thus, it can be speculated that, if this trend is sustained, the patients might develop immune deficits in future, which points out to the need for longer follow up in SBS patients.
In conclusion, our results suggest that children with short bowel present neither with clinical signs of immunodeficiency, nor with deficits in peripheral lymphocyte subsets and serum immunoglobulins. Decreased number of NK cells in SBS patients compared to healthy controls needs to be verified in larger cohort. The tendency of the lymphocyte numbers to decrease over time after resection indicates the necessity for longer follow- up, since it can be speculated that SBS patients might develop immune deficits in future. Further research concerning other parameters of adoptive immunity in children with short bowel syndrome is warranted.
Author Contributions
KS- conceptualization, methodology, investigation, writing- original draft preparation; AB- data curation, investigation; AZ- investigation, writing- original draft preparation; MM data curation, investigation; M Za investigation, visualization; MZi—methodology, writing- original draft preparation; PT—methodology, writing- review, supervision; MŁ- data curation, investigation,; ASS- conceptualization, writing- review, supervision.
Funding
The study was supported by internal university grant ST-60. and received no funding from any other organizations or institutions.
Institutional Review Board Statement
The study was approved by the Independent Bioethical Committee of Medical University of Gdansk—protocol code NKBBN/331/2019. The study was conducted according to the principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from legal guardians of all individual participants included in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
SBS- short bowel syndrome, GALT- gut associated lymphoid tissue, CD -cluster of differentiation, NK- natural killers.
References
- O’keefe, S.J.; Buchman, A.L.; Fishbein, T.M.; Jeejeebhoy, K.N.; Jeppesen, P.B.; Shaffer, J. Short Bowel Syndrome and Intestinal Failure: Consensus Definitions and Overview. Clin. Gastroenterol. Hepatol. 2006, 4, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Duro, D.; Kamin, D.; Duggan, C. Overview of Pediatric Short Bowel Syndrome. J. Pediatr. Gastroenterol. Nutr. 2008, 47, S33–S36. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Kesavan, A. Current treatment paradigms in pediatric short bowel syndrome. Clin. J. Gastroenterol. 2017, 11, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wershil, B.; Furuta, G. 4. Gastrointestinal mucosal immunity. J. Allergy Clin. Immunol. 2008, 121, S380–S383. [Google Scholar] [CrossRef] [PubMed]
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013, 4, 27–27. [Google Scholar] [CrossRef] [PubMed]
- Doe, W.F. The intestinal immune system. Gut 1989, 30, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Duran, B. The effects of long-term total parenteral nutrition on gut mucosal immunity in children with short bowel syndrome: a systematic review. BMC Nurs. 2005, 4, 2. [Google Scholar] [CrossRef]
- Turato, W.M.; Sales-Campos, H.; Braga, C.B.M.; Cunha, S.F.C.; Silvah, J.H.; da Silva, J.S.; Marchini, J.S.; Cardoso, C.R.d.B. The impact of intestinal resection on the immune function of short bowel syndrome patients. Hum. Immunol. 2016, 77, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef]
- Orange, J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013, 132, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Conley, M.E.; Notarangelo, L.D.; Etzioni, A. Diagnostic Criteria for Primary Immunodeficiencies. Clin. Immunol. 1999, 93, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Barrena, M.; Eizaguirre, I.; Aldazabal, P.; Cuadrado, E.; Bachiller, P.; Wang, W.; Tovar, J. Lymphocyte subpopulations after extensive small bowel resection in the rat. J. Pediatr. Surg. 1995, 30, 1447–1449. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Sato, N.; Sato, N.; Nakamura, T.; Masunaga, H. Changes in Lymphocyte Phenotypes and Cytokine Production by Surgical Stress in a Rat Small Intestinal Resection Model. J. Clin. Biochem. Nutr. 2007, 40, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chen, J.; Li, M.; Chou, M.; Lo, H. Oral Antibiotics Attenuate Bowel Segment Reversal–Induced Alterations in Subpopulation and Function of Peripheral Blood Leukocytes, Thymocytes, and Splenocytes in Massive Bowel-Resected Rats. J. Parenter. Enter. Nutr. 2008, 33, 90–101. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. J. Immunol. Res. 2012, 2012, 925135. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).