Submitted:
06 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. The Forbidden Apple
1.2. The Behavior of Living Beings
- -
- competition between species for the use of resources: the individuals of the species that reproduce most successfully in this historical period and in this environment are selected; the individuals that make up the populations of a species are constantly changing and in constant competition in the exploitation of the environment with individuals of many other species [10,11,12];
- -
- the cooperation of individuals in the use of resources: whatever the species, it is a complex system of individuals adapted to each other in order to grow, a kind of supra-organism contained in other larger systems, making the best possible use of resources and the available energy of an environment that is changing with it [3,13,14].
1.3. Toumaï and the Anthropocene
1.4. The Forests of the World
1.4.1. State of the Art
1.4.1. Can Humans Live far from Earth?
1.4.1. What If “Forest Ecosystem Services” Didn’t Exist?
1.4.1. Indisputable Facts
2. The Soil
2.1. In the Future of the Living Forest, There Is a Dead Forest
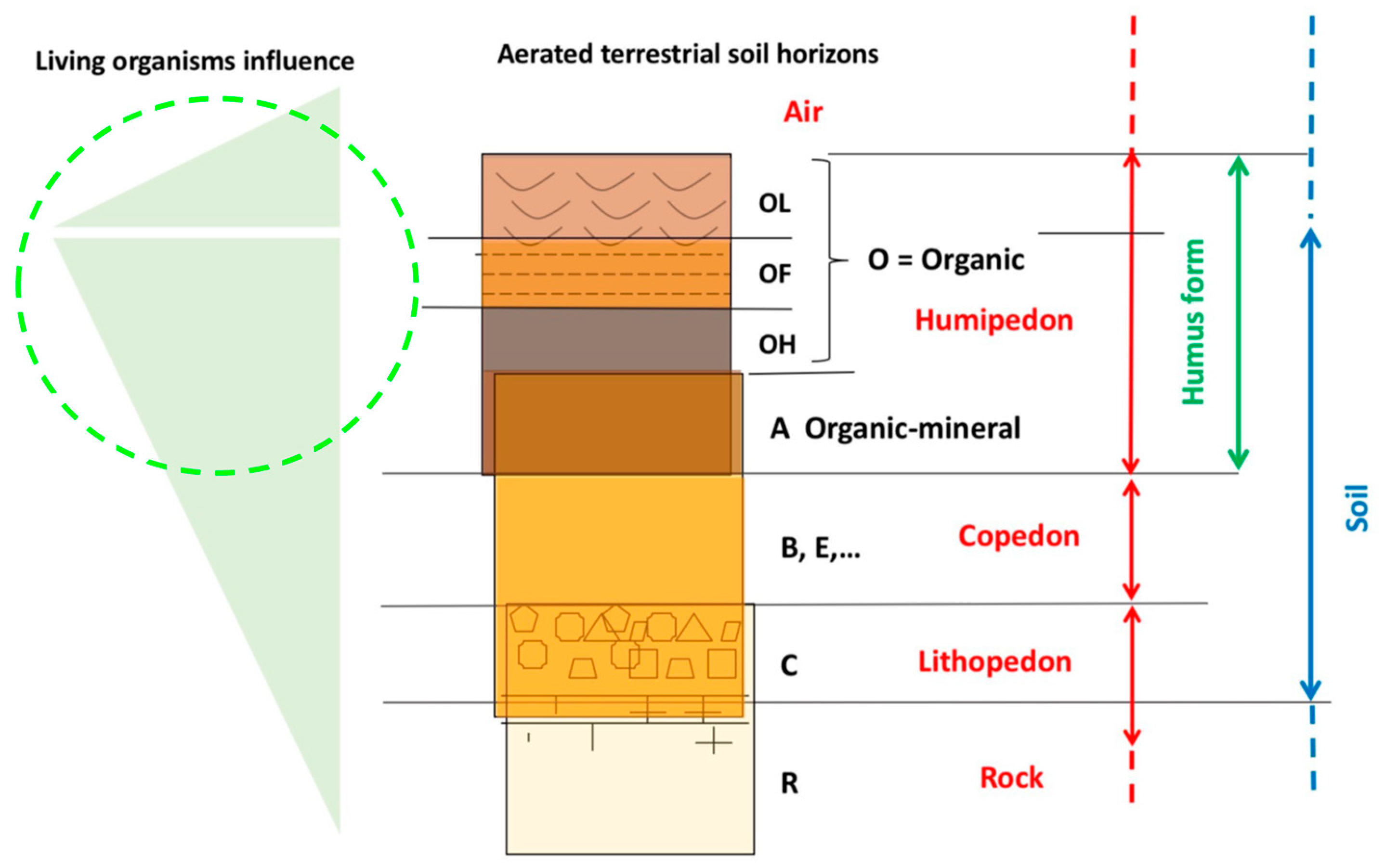
2.2. Either It Is Alive, in Which Case It Is Really “Soil”, or It Is “Rock”
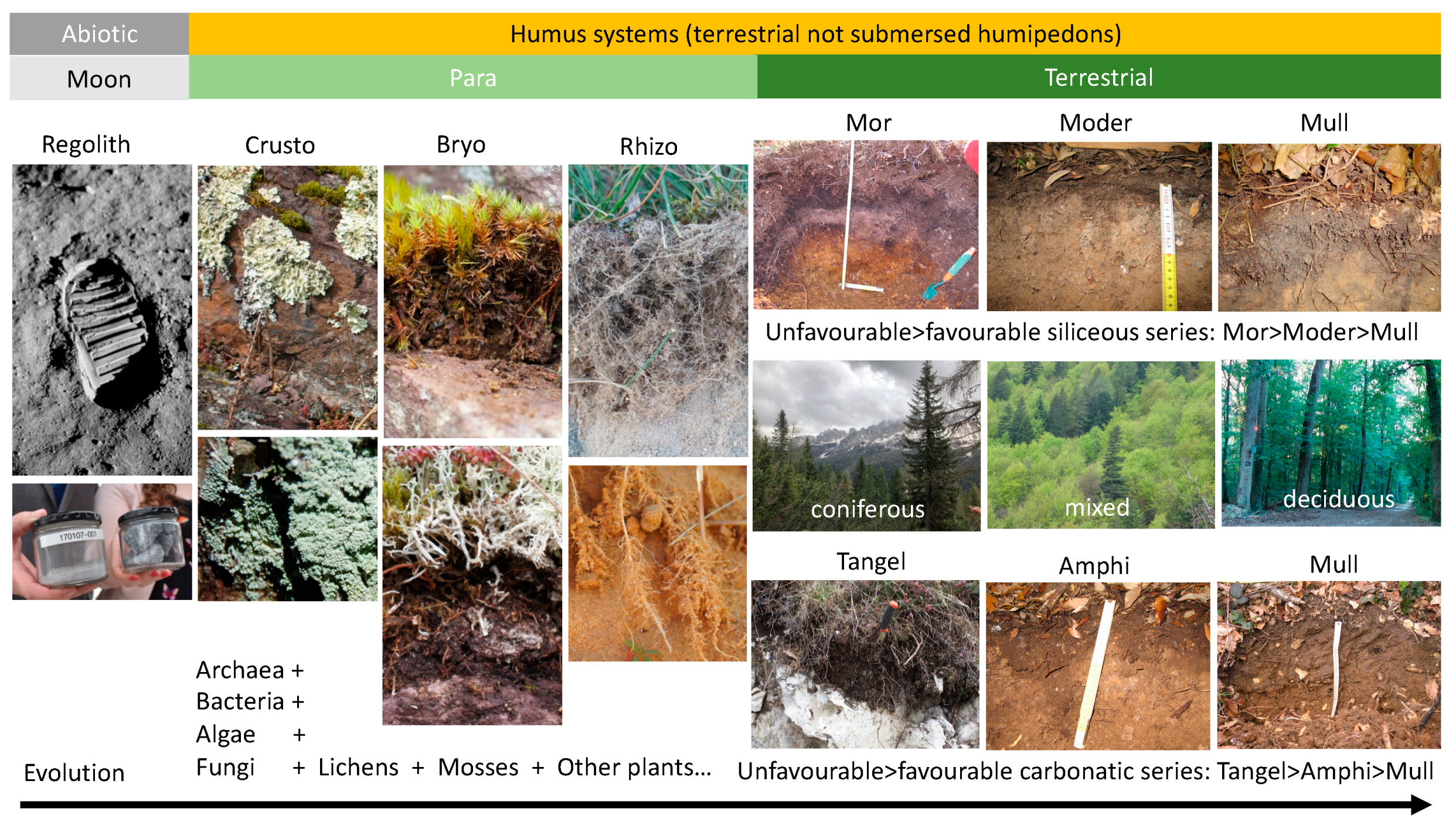
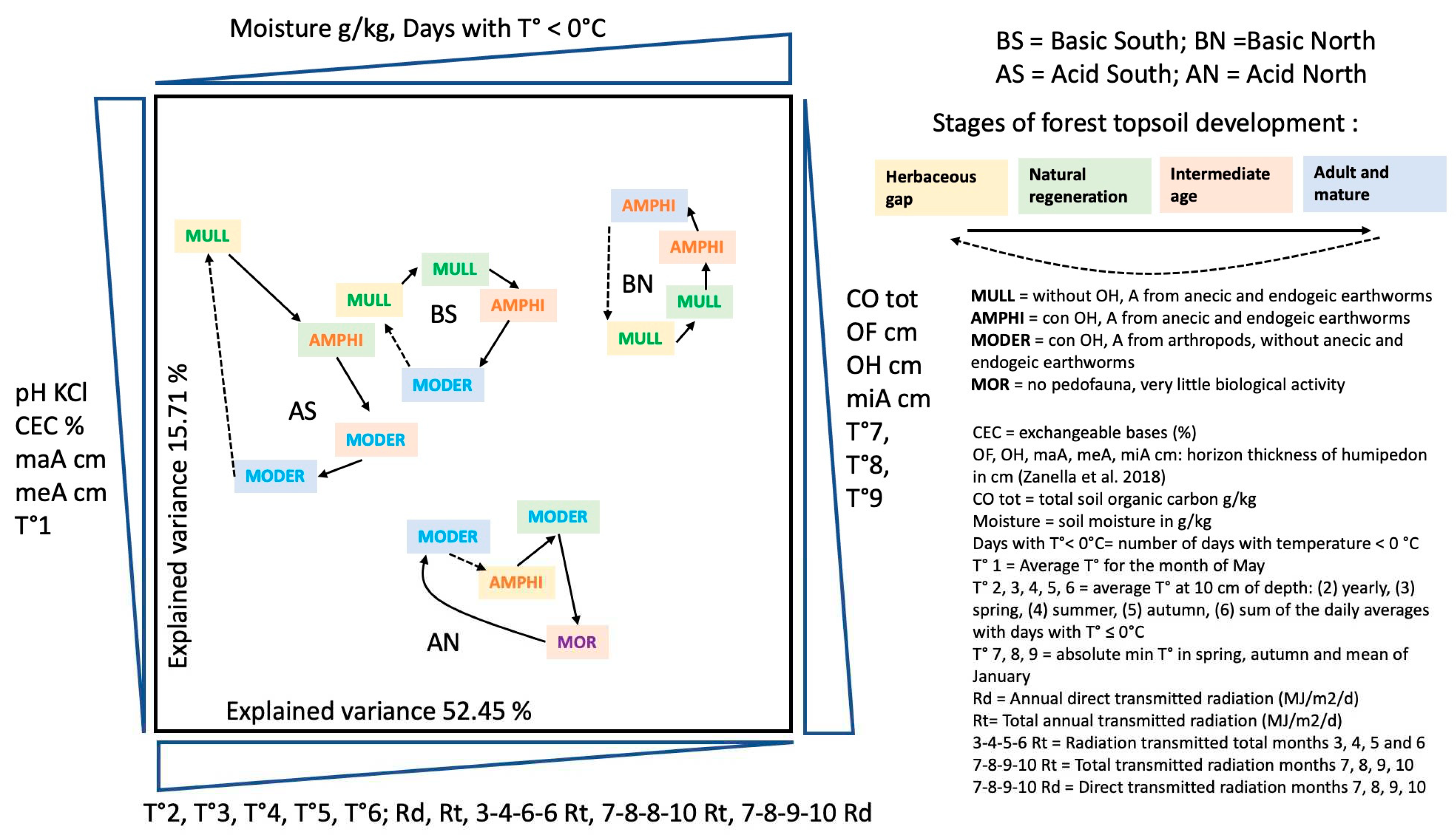
2.3. Humipedon and Forest Soil Functioning
- -
- the thicknesses of meA and maA (from earthworms) oppose the thicknesses of OF, OH and miA from arthropods;
- -
- the temperature at 10 cm depth opposes the humidity of the soil;
- -
- the total organic carbon of the soil increases under conditions of difficult bio-degradation of the litter.
3. A Soil-Conscious Forestry
3.1. Autopoiesis
3.2. Foundations of Practical Action Soil-Conscious Forestry
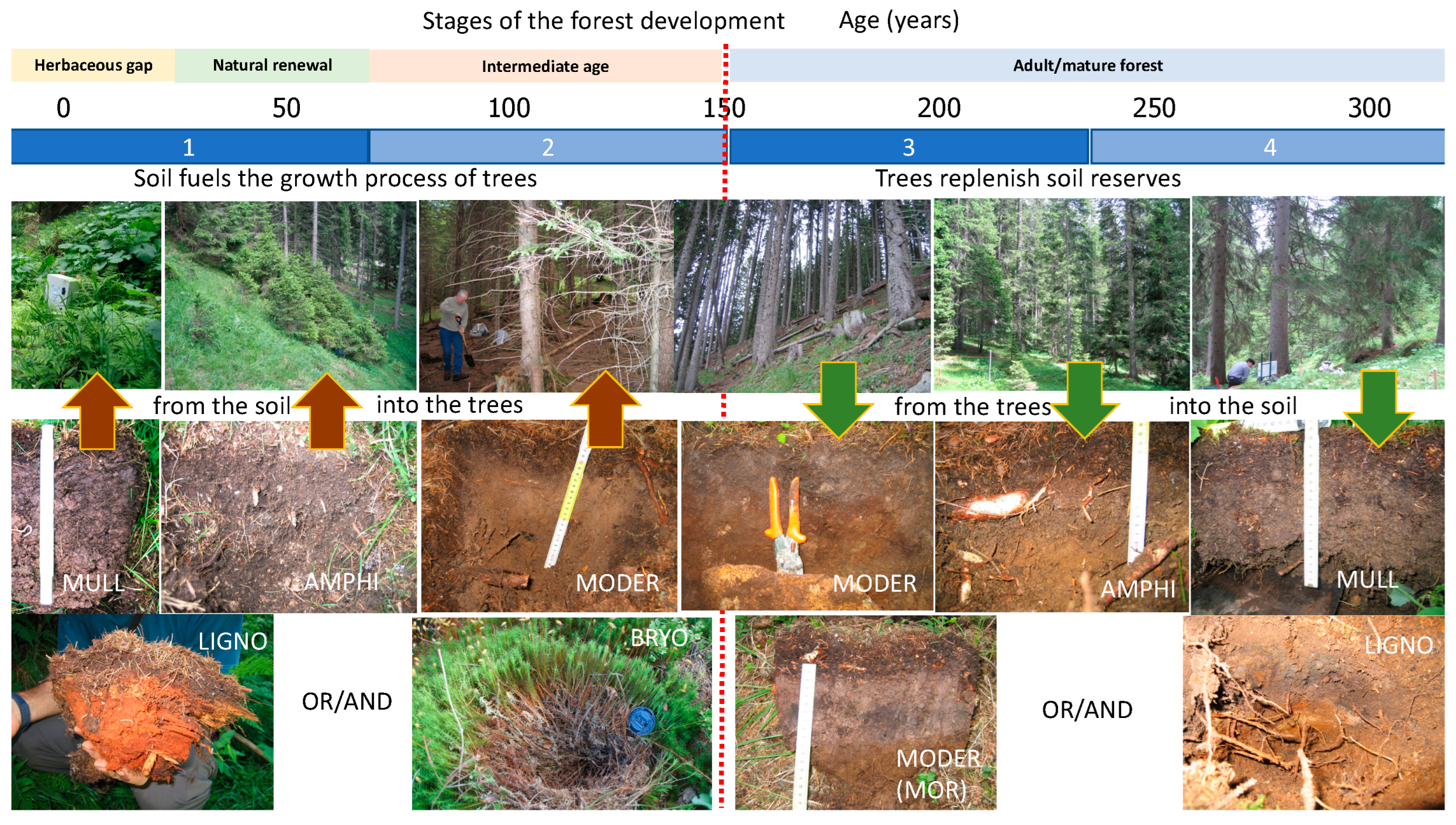
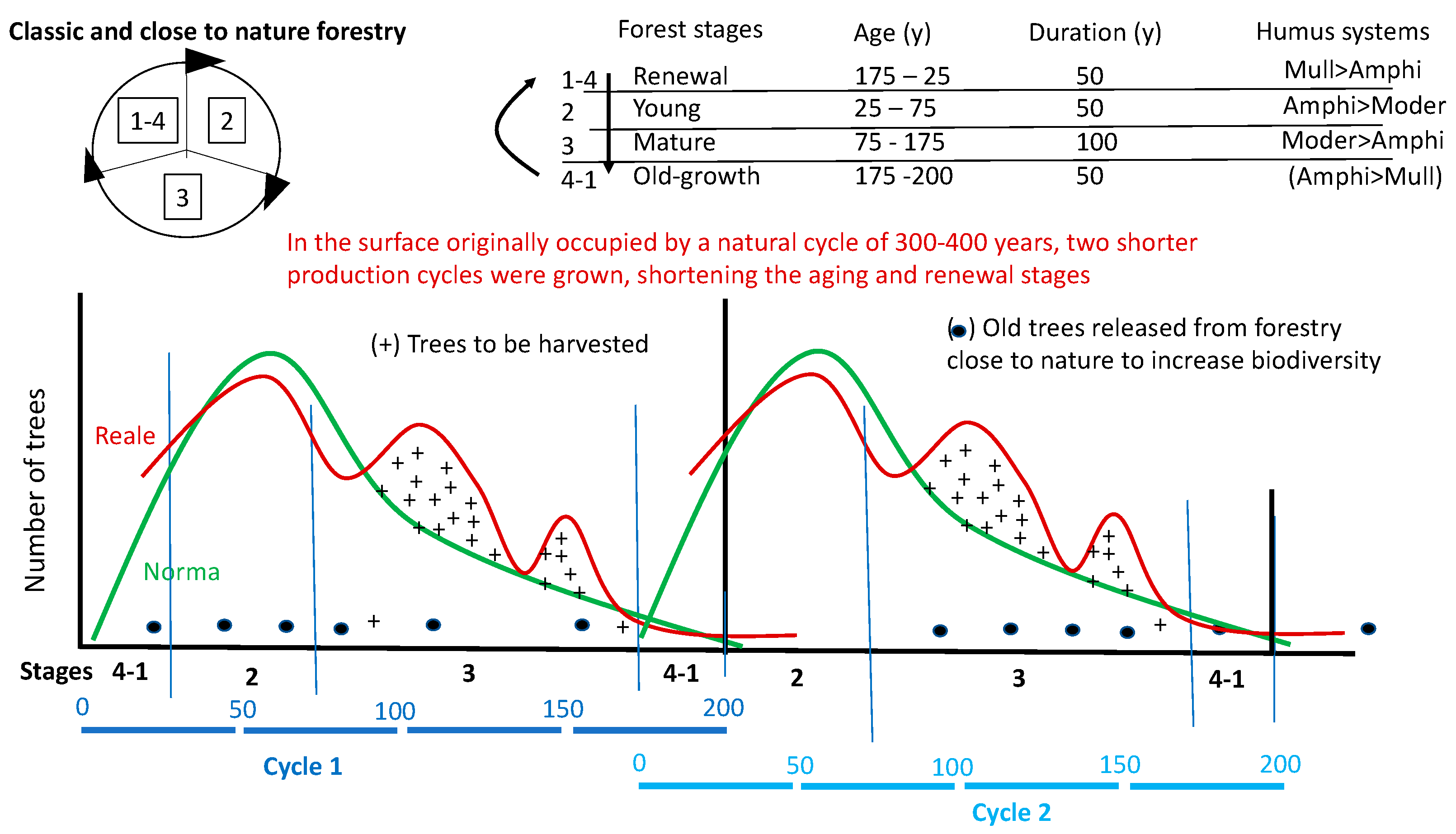
3.2.1. The Renewal of the Forest
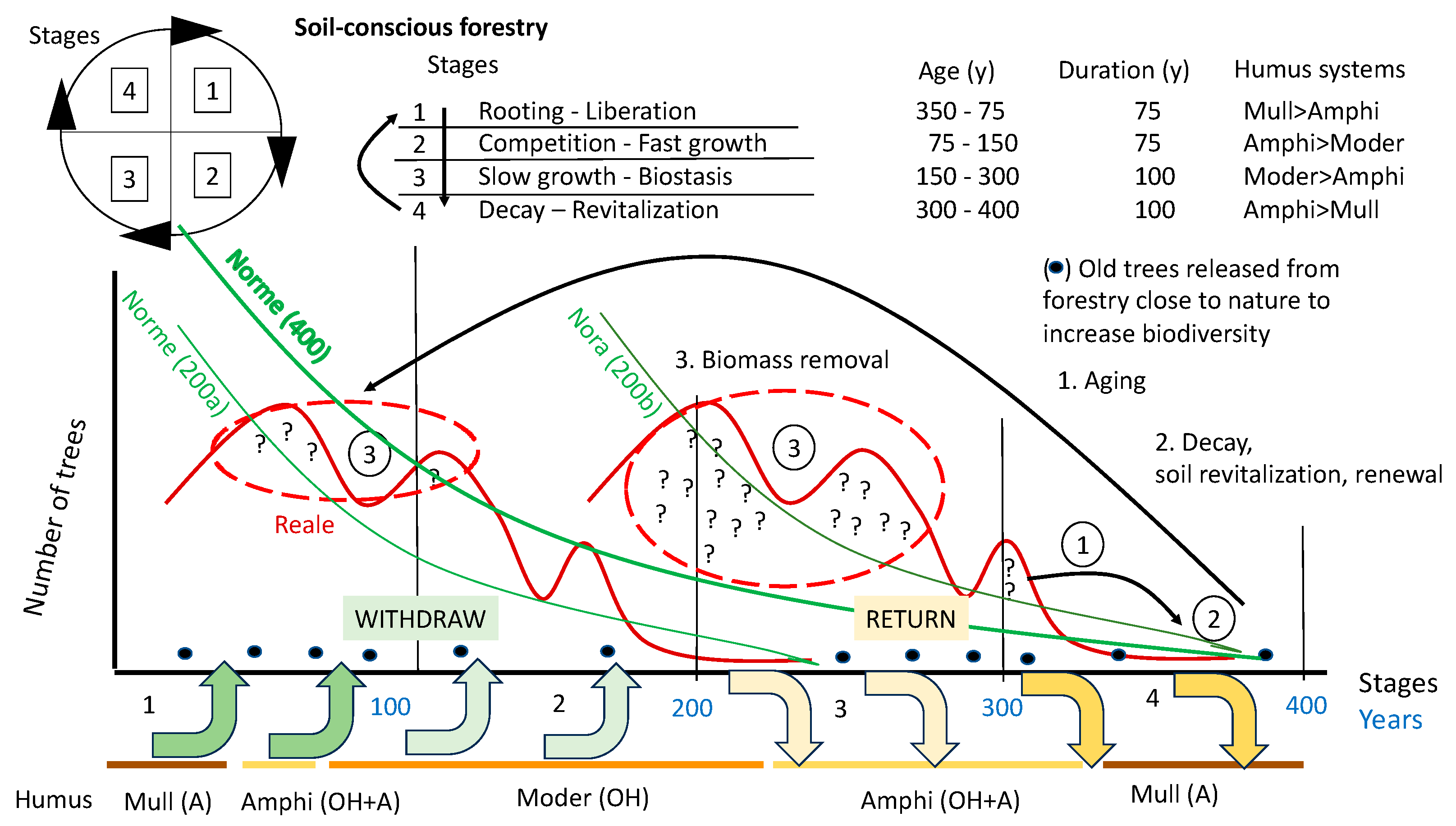
3.2.2. Supporting the Becoming of the Forest
3.2.3. Forest Biodiversity
3.2.4. Exotic Species
3.2.5. The Growth of the Forest
3.2.6. Human Economy and Harvesting
- -
- minimum harvesting: cutting and removing the minimum possible (instead of removing the maximum while respecting the vitality of the forest);
- -
- the maximum prolongation of the life of the trees: maintaining even the decaying stage of the forest (instead of interrupting the cycle of production in the moment of stasis of growth to obtain a high productivity – which, unfortunately, as we know is inevitably short-term, as happened with the depletion of agricultural land due to intensive agriculture);
- -
- respect for the structure that the forest has and will have in the future: careful thinning at all stages of the forest (instead of thinning to plan the growth and final cutting of a mature stand);
- -
- -
- replenishment tor energy reserves and mineral elements in the forest soil: it is recognized that the decline stage of the forest floor must be maintained because it ensures the reconstruction of the soil’s nutrient capital of the soil.
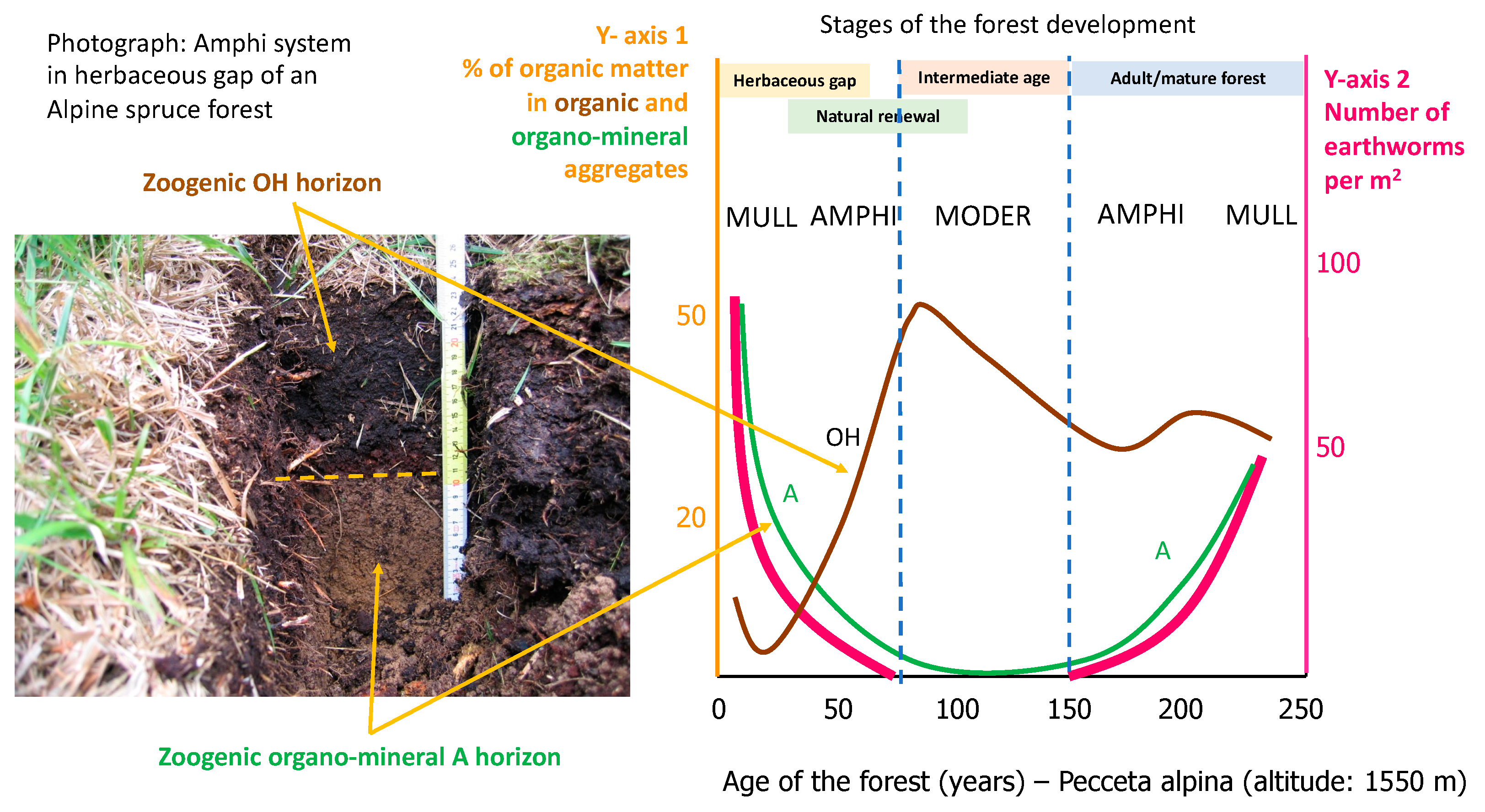
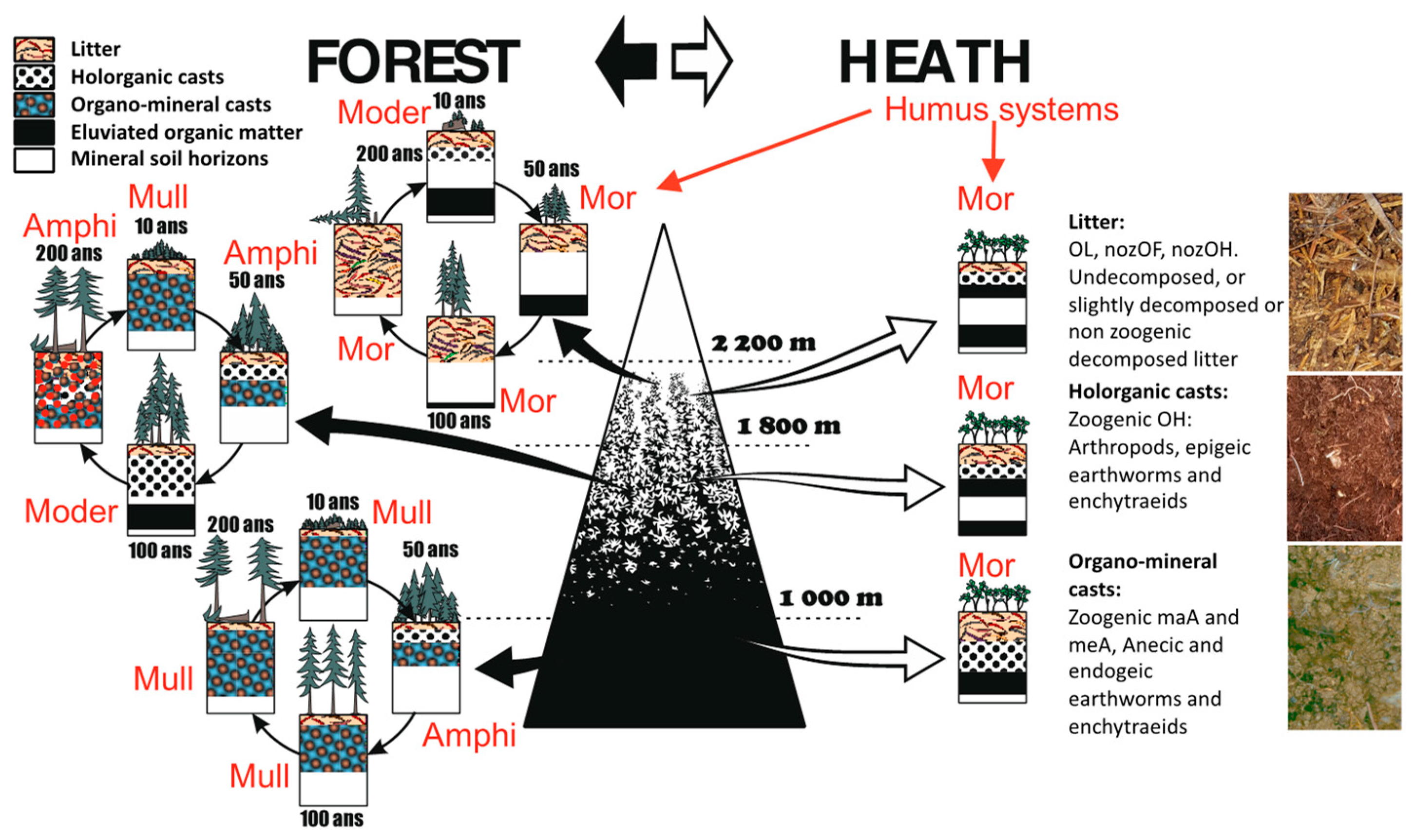
3.2.2. Examples in Alpine Environment
- -
- in the senescent stage and in the early juvenile stages, the A horizon of the Mull system (earthworms) and the ligOF and ligOH horizons of the Ligno system (fungi and wood fauna) develop;
- -
- in the intermediate stages the OH horizon increases in thickness at the expense of an A horizon which tends to disappear (from a thick A from earthworms in the Amphi of the first stages of development of the tall forest, to a thin A not from earthworms in the Moder of the dense perches and in the adult tall forest still growing strongly;
- -
- in the adult stages of relative stability, a redistribution of nutrients takes place outside and inside the soil, with a consequent decrease in the thickness of the OH horizon, storage of nutrient elements in the soil in the form of macroaggregates of the A horizon, or in any case of more organic substance easily recyclable in dead wood and partially biodegraded.
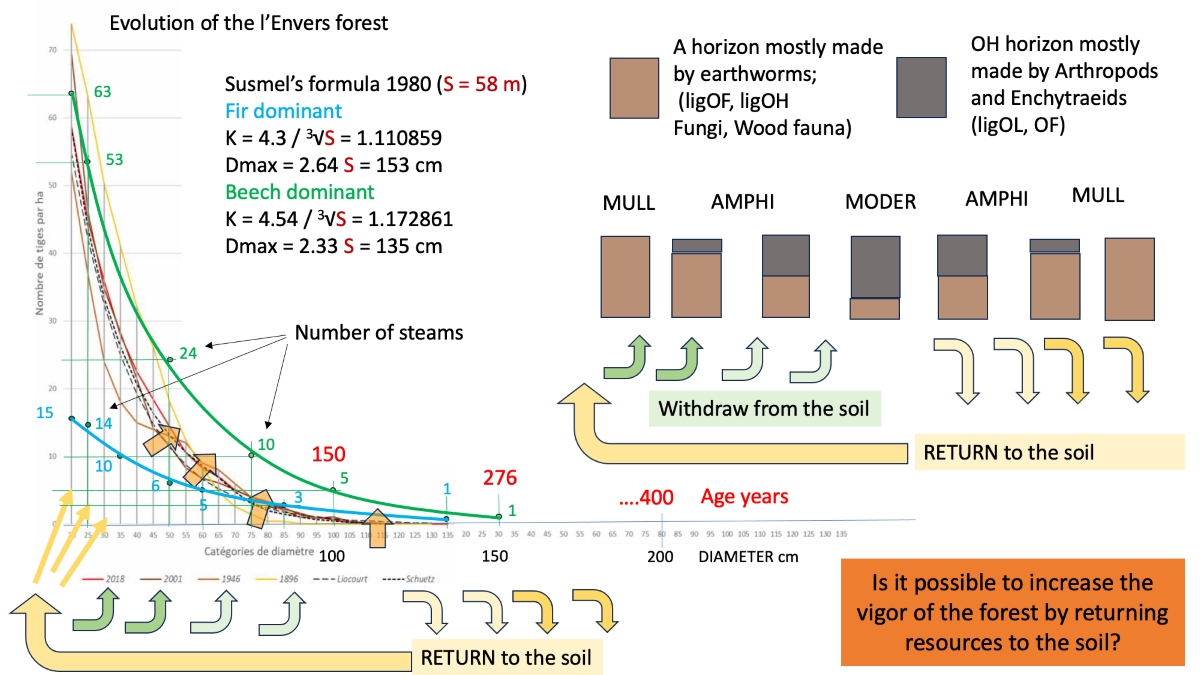
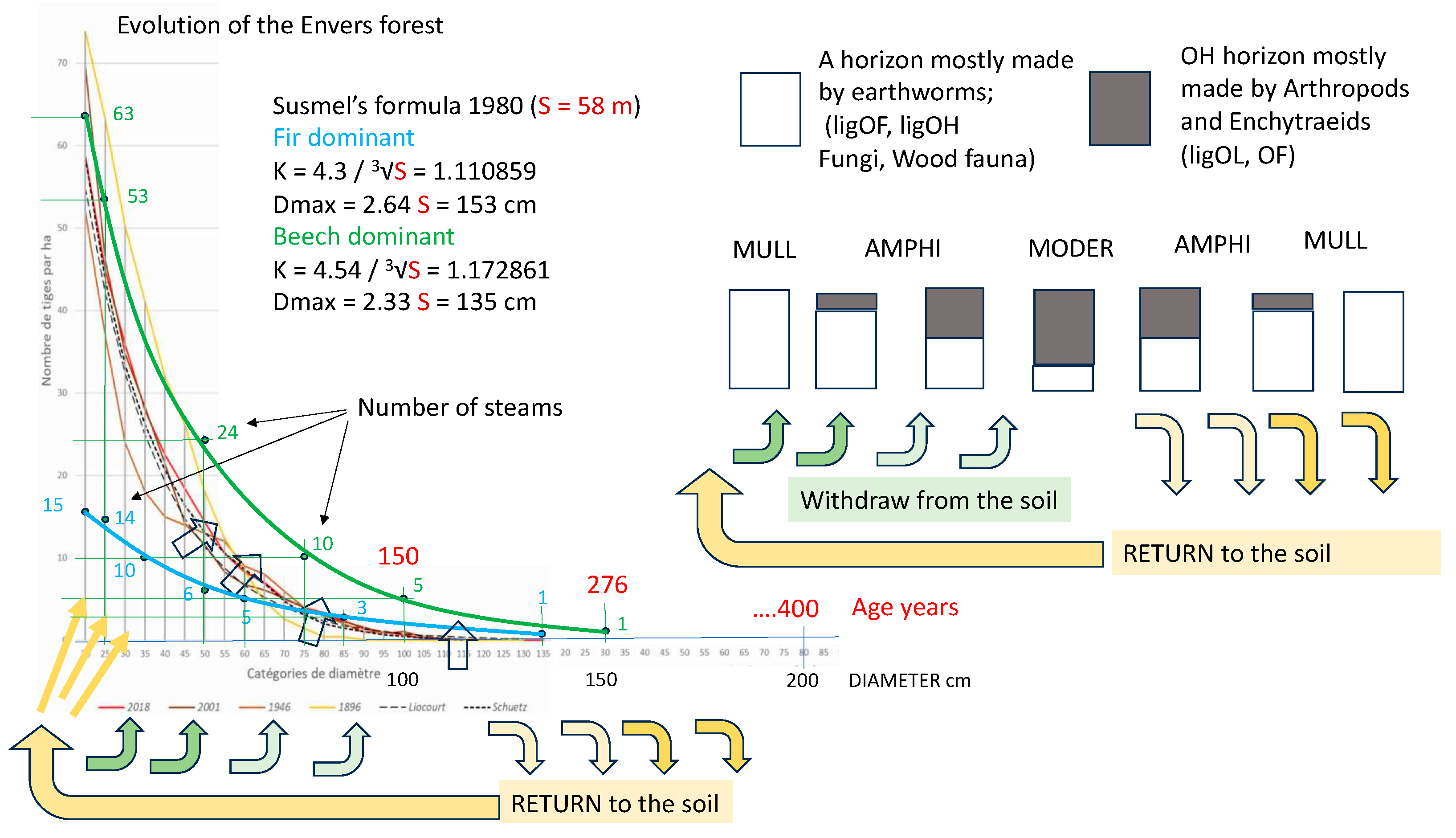
- with fir dominant: K = 4.3 / 3√S = 1.110859; Dmax = 2.64 S = 153 cm;
- with beech dominant: K = 4.54 / 3√S = 1.172861; Dmax = 2.33 S = 135 cm.
3.2.3. Planet Forest Management
3.2.4. Soil-Conscious Forestry and New Forestry Plantings in Climate Change
3.2.5. Coppice Forest, Wood Arboriculture, Urban Parks
3.2.6. Agroforestry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunet, M.; Beauvilain, A.; Coppens, Y.; Heintz, E.; Moutaye, A.H.E.; Pilbeam, D. The first australopithecine 2,500 kilometres west of the Rift Valley (Chad). Nature 1995, 378, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Dobzhansky, T. Nothing in Biology Makes Sense except in the Light of Evolution. Am. Biol. Teach. 1973, 35, 125–129. [Google Scholar] [CrossRef]
- Lovelock, J.E.; Margulis, L. Atmospheric homeostasis by and for the biosphere: the gaia hypothesis. Tellus 1974, 26, 2–10. [Google Scholar] [CrossRef]
- Dahl, T.W.; Arens, S.K. The impacts of land plant evolution on Earth’s climate and oxygenation state – An interdisciplinary review. Chem. Geol. 2020, 547, 119665. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Volterra, V. Fluctuations in the Abundance of a Species considered Mathematically1. Nature 1926, 118, 558–560. [Google Scholar] [CrossRef]
- http://10.0.4.14/118558a0.
- Lotka, A.J. Analytical Note on Certain Rhythmic Relations in Organic Systems. Proc. Natl. Acad. Sci. USA 1920, 6, 410–415. [Google Scholar] [CrossRef]
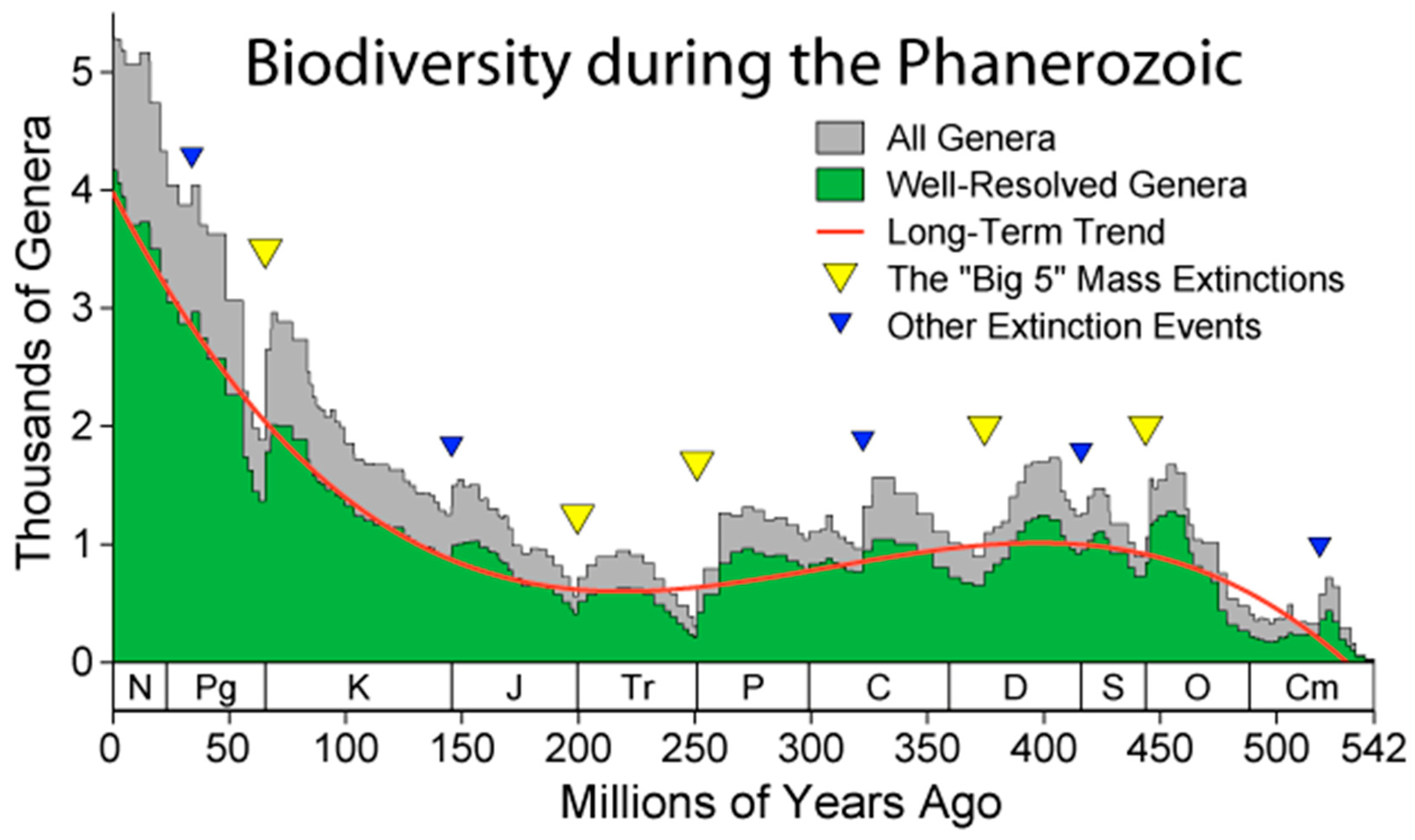
- Rohde, R.A.; Muller, R.A. Cycles in fossil diversity. Nature 2005, 434, 208–210. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef]
- Darwin, C.M.A. On the Origin of Species by Means of Natural Selection or the Preservation of favoured races in the struggle for life; John Murray: Albemarle Street, London, UK, 1859; p. 502. [Google Scholar]
- Safran, R.J.; Nosil, P. Speciation: The Origin of New Species. Nat. Educ. Knowl. 2012, 3. Available online: https://www.nature.com/scitable/knowledge/library/speciation-the-origin-of-newspecies-26230527/.
- Dawkins, R. The Selfish Gene, Oxford Uni ed.; Oxford, 1976. [Google Scholar]
- Margulis, L. Symbiotic Planet {A new Look at Evolution}, Science Ma ed.; Perseus Books Group: New York, NY, USA, 1998; pp. 147–147. [Google Scholar]
- Khakhina, L.A.N.C.; Nikolaevna, L.; Margulis, R.C.L. (Eds.) Concepts of symbiogenesis: A Historical and Critical Study of the Researchof the Russian Botanists (Bio-Origins Series); Yale University Press: New Haven, CT, USA, 1992; p. 177. [Google Scholar]
- Brunet, M.; Guy, F.; Pilbeam, D.; Mackaye, H.T.; Likius, A.; Ahounta, D.; Beauvilain, A.; Blondel, C.; Bocherens, H.; Boisserie, J.-R.; et al. A new hominid from the Upper Miocene of Chad, Central Africa. Nature 2002, 418, 145–151. [Google Scholar] [CrossRef]
- Coppens, Y. Lucy’s Knee: The Story of Man and the Story of His Story (Paperback). Protea Boekhuis; English ed. edition (March 15, 2012), 2012, p. 176.
- Diamond, J.J. Guns, germs and steel - A short history of Everybody for the last 13,000 years; Vintage, 1998. [Google Scholar]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef]
- Vela, I.C.; Vilches, T.B.; Berndes, G.; Johnsson, F.; Thunman, H. Co-recycling of natural and synthetic carbon materials for a sustainable circular economy. J. Clean. Prod. 2022, 365. [Google Scholar] [CrossRef]
- Smil, V. Energy in World History—Energy and Civilization: A History; The MIT Press: Cambridge, MA, USA, 2017; p. 552. [Google Scholar]
- I. W. G. I, IPCC Sixth Assessment Report, Climate Change 2021: The physical Science Basis. Technical Summary. 2022. Available online: https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_FrontMatter.pdf.
- Condamine, F.L.; Rolland, J.; Morlon, H. Assessing the causes of diversification slowdowns: temperature-dependent and diversity-dependent models receive equivalent support. Ecol. Lett. 2019, 22, 1900–1912. [Google Scholar] [CrossRef]
- Lynas, M. Our Final Warning - Six Degrees of Climate Emergency; Fourth Estate, 2020. [Google Scholar]
- Fao and Unep, The State of the World’s Forests 2020. Forest, Biodiversity and People; Food and Agriculture Organization: Rome, Italy, 2020. [Google Scholar]
- Potapov, P.; Hansen, M.C.; Pickens, A.; Hernandez-Serna, A.; Tyukavina, A.; Turubanova, S.; Zalles, V.; Li, X.; Khan, A.; Stolle, F.; et al. The Global 2000-2020 Land Cover and Land Use Change Dataset Derived From the Landsat Archive: First Results. Front. Remote. Sens. 2022, 3. [Google Scholar] [CrossRef]
- Fao, Global Forest Resources Assessment 2020: Main report. Rome, 2020.
- Zhouri, A. “Adverse Forces” in the Brazilian Amazon: Developmentalism Versus Environmentalism and Indigenous Rights. J. Environ. Dev. 2010, 19, 252–273. [Google Scholar] [CrossRef]
- Fao, Ifad, Unicef, Wfp, and Who. The State of Food Security and Nutrition in the World 2022. Repurposing food and agricultural policies to make healthy diets more affordable; FAO: Rome, Italy, 2022. [Google Scholar]
- Jon, C. The International Space Station is home to potentially dangerous bacteria. An in-depth analysis of an air filter and vacuum bags discovers a unique microbiome. Science 2015. [Google Scholar] [CrossRef]
- Tršan, M.; Seme, K.; Srčič, S. The environmental monitoring in hospital pharmacy cleanroom and microbiota catalogue preparation. Saudi Pharm. J. 2019, 27, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Weir, A. Tha Martian, Penguin Ra ed.; Del Rey, 2011; p. 369. [Google Scholar]
- Stinca, A.; Musarella, C.M.; Rosati, L.; Laface, V.L.A.; Licht, W.; Fanfarillo, E.; Wagensommer, R.P.; Galasso, G.; Fascetti, S.; Esposito, A.; et al. Italian Vascular Flora: New Findings, Updates and Exploration of Floristic Similarities between Regions. Diversity 2021, 13, 600. [Google Scholar] [CrossRef]
- Loy, A.; et al. Mammals of Italy: an annotated checklist. Hystrix 2019, 30, 87–106. [Google Scholar] [CrossRef]
- F. a. UNEP. The State of the World’s Forests 2020; ed: FAO and UNEP; 2020. [Google Scholar]
- Mannion, P.D.; Upchurch, P.; Benson, R.B.; Goswami, A. The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. 2014, 29, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.N.; Pimm, S.L.; Joppa, L.N. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, E2602–10. [Google Scholar] [CrossRef] [PubMed]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal Gradients of Biodiversity: Pattern, Process, Scale, and Synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Tierney, J.E.; Poulsen, C.J.; Montañez, I.P.; Bhattacharya, T.; Feng, R.; Ford, H.L.; Hönisch, B.; Inglis, G.N.; Petersen, S.V.; Sagoo, N.; et al. Past climates inform our future. Science 2020, 370, 680. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Zanella, A.; Bolzonella, C.; Squartini, A.; Xu, G.-L.; Banas, D.; Rosatti, M.; Longo, E.; Pindo, M.; Concheri, G.; et al. Land Use, Microorganisms, and Soil Organic Carbon: Putting the Pieces Together. Diversity 2022, 14, 638. [Google Scholar] [CrossRef]
- Soong, J.L.; Fuchslueger, L.; Marañon-Jimenez, S.; Torn, M.S.; Janssens, I.A.; Penuelas, J.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob. Chang. Biol. 2019, 26, 1953–1961. [Google Scholar] [CrossRef]
- Pennanen, T.; Fritze, H.; De Boer, W.; Baldrian, P. Editorial: special issue on the ecology of soil microorganisms. FEMS Microbiol. Ecol. 2019, 95. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Bologna, M.; Aquino, G. Deforestation and world population sustainability: a quantitative analysis. Sci. Rep. 2020, 10, 7631. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Barrios, E.; Valencia, V.; Jonsson, M.; Brauman, A.; Hairiah, K.; Mortimer, P.E.; Okubo, S. Contribution of trees to the conservation of biodiversity and ecosystem services in agricultural landscapes. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2017, 14, 1–16. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Guirado, E.; Reich, P.B.; Ochoa-Hueso, R.; Berdugo, M.; Sáez-Sandino, T.; Blanco-Pastor, J.L.; Tedersoo, L.; Plaza, C.; Ding, J.; et al. The global contribution of soil mosses to ecosystem services. Nat. Geosci. 2023, 16, 430–438. [Google Scholar] [CrossRef]
- Holland, R.; Eigenbrod, F.; Muggeridge, A.; Brown, G.; Clarke, D.; Taylor, G. A synthesis of the ecosystem services impact of second generation bioenergy crop production. Renew. Sustain. Energy Rev. 2015, 46, 30–40. [Google Scholar] [CrossRef]
- Sapp, J. Evolution by Association. A historyof Symbiosis; Oxford University Press, Inc.: New York, NY, USA, 1994; p. 255. [Google Scholar]
- Michaelian, K. Entropy Production and the Origin of Life. J. Mod. Phys. 2011, 02, 595–601. [Google Scholar] [CrossRef]
- Aszalós, R.; Thom, D.; Aakala, T.; Angelstam, P.; Brūmelis, G.; Gálhidy, L.; Gratzer, G.; Hlásny, T.; Katzensteiner, K.; Kovács, B.; et al. Natural disturbance regimes as a guide for sustainable forest management in Europe. Ecol. Appl. 2022, 32, e2596. [Google Scholar] [CrossRef] [PubMed]
- Cagliero, E.; et al. Exploring the long-term vegetation and fire-disturbance history of the Biogradska Gora old-growth forest (Montenegro); EGU: Vienna, Austria, 2020; Available online: https://meetingorganizer.copernicus.org/EGU2020/EGU2020-9910.html. [CrossRef]
- Motta, R. Why do we have to increase deadwood in our forests? How much deadwood does the forest need? For. Riv. Di Selvic. Ed Ecol. For. 2020, 17, 92–100. [Google Scholar] [CrossRef]
- Motta, R.; Ascoli, D.; Corona, P.; Marchetti, M.; Vacchiano, G. Silviculture and wind damages. The storm “Vaia”. For. Riv. Di Selvic. Ed Ecol. For. 2018, 15, 94–98. [Google Scholar] [CrossRef]
- Motta, R.; Larsen, J. A new paradigm for sustainable forest management: closeR to nature forest management. For. Riv. Di Selvic. Ed Ecol. For. 2022, 19, 52–62. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Keeton, W.S.; Lindner, M.; Svoboda, M.; Verkerk, P.J.; Bauhus, J.; Bruelheide, H.; Burrascano, S.; Debaive, N.; Duarte, I.; et al. Protection gaps and restoration opportunities for primary forests in Europe. Divers. Distrib. 2020, 26, 1646–1662. [Google Scholar] [CrossRef]
- Adamic, M.; Diaci, J.; Rozman, A.; Hladnik, D. Long-term use of uneven-aged silviculture in mixed mountain Dinaric forests: a comparison of old-growth and managed stands. For. Int. J. For. Res. 2016, 90, 279–291. [Google Scholar] [CrossRef]
- Hilbert, J.; Wiensczyk, A. Old-growth definitions and management: A literature review. J. Ecosyst. Manag. 2007, 8, 15–31. Available online: http://www.forrex.org/publications/jem/ISS39/vol8_no1_art2.pdf. [CrossRef]
- Leckie, S.; Vellend, M.; Bell, G.; Waterway, M.J.; Lechowicz, M.J. The seed bank in an old-growth, temperate deciduous forest. Can. J. Bot. 2000, 78, 181–192. [Google Scholar] [CrossRef]
- Potapov, P.; Hansen, M.C.; Laestadius, L.; Turubanova, S.; Yaroshenko, A.; Thies, C.; Smith, W.; Zhuravleva, I.; Komarova, A.; Minnemeyer, S.; et al. The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 2017, 3, e1600821. [Google Scholar] [CrossRef]
- Almond, R.E.A.; Grooten, M.; Bignoli, D.J.; Petersen, T. World Wildlife Fund (WWF) and Zoological Society of London (2022). Living Planet Report 2022—Building a nature-positive society. Available online: https://ourworldindata.org/biodiversity.
- Fao, Global forest sector outlook 2050: Assessing future demand and sources of timber for a sustainable economy – Background paper for The State of the World’s Forests 2022; FAO Forestry Working Paper, No. 31; FAO, 2022.
- Jernigan, A.; Kao-Kniffin, J.; Pethybridge, S.; Wickings, K. Soil microarthropod effects on plant growth and development. Plant Soil 2022, 483, 27–45. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: diversity, complexity and dynamics. FEMS Microbiol. Rev. 2016, 41, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Bernier, N.; Ponge, J. Humus form dynamics during the sylvogenetic cycle in a mountain spruce forest. Soil Biol. Biochem. 1994, 26, 183–220. [Google Scholar] [CrossRef]
- Bernier, N. Earthworm feeding activity and development of the humus profile. Biol. Fertil. Soils 1998, 26, 215–223. [Google Scholar] [CrossRef]
- Zampedri, R. Relazione tra clima, forme di humus e dinamica forestale in ambiente di pecceta altimontana. Tesi di Dottorato. Ph.D. Thesis, Territorio e Sistemi Agro-Foestali (TESAF), Padova, Italy, 2005. [Google Scholar]
- André, J. Activité et diversité des organismes hétérotrophes: les clés du bouclage des cycles biogéochimiques et sylvigénétiques; Vallauri, D., André, J., Dodelin, B., Eynard-Machet, R., Rambaud, D., Eds.; Lavoisier, 2005; p. 404. [Google Scholar]
- Bernier, N. Fonctionnement biologique des humus et dynamique des pessières alpines. Le cas de la forêt de Macot-la-Plagne (Savoie). Ecologie 1997, 28, 23–44. Available online: https://www.researchgate.net/publication/233758152_Fonctionnement_biologique_des_humus_et_dynamique_des_pessieres_alpines_Le_cas_de_la_foret_de_Macot-La-Plagne_SavoieHumus_biological_organization_and_alpine_spruce_forest_dynamics_The_example_of_Macot-#ful.
- Bernier, N. Fonctionnement biologique des humus et dynamique des pessières alpines. Le cas de la forêt de Macot-La-Plagne (Savoie). [Humus biological organization and alpine spruce forest dynamics. The example of Macot-La-Plagne forest (Savoie, France)]. Ecologie 1997, 28, 23–44. [Google Scholar]
- Zampedri, R.; Zanella, A.; Giannini, R. Soil, humipedon and forest management. For. - Riv. di Selvic. ed Ecol. For. 2023, 20, 13–19. [Google Scholar] [CrossRef]
- Hartmann, F.; Marcuzzi, G. Diagnosi degli humus forestali su basi biomorfologiche; CEDAM Padova: Padova, Italy, 1970; pp. 284–284. [Google Scholar]
- Zanella, A.; et al. Humus Forestali - Manuale di ecologia per il riconoscimento e l’interpretazione - Applicazione alle faggete, CEA ed.; San Michele all’Adige, Trento (Italia): 321 p.: Centro Ecologia Alpina, Fondazione Edmund Mach; 2001; p. 320. [Google Scholar]
- Graefe, U. Gibt es in Deutschland die Humusform Amphi? Mitteilungen der Deutschen Bodenkundlichen Gesellschaft 2007, 110, 459–460. Available online: https://www.researchgate.net/profile/Ulfert_Graefe/publication/261286541_Gibt_es_in_Deutschland_die_Humusform_Amphi/links/00463533c43b6e792b000000.pdf.
- Zampedri, R.; Bernier, N.; Zanella, A.; Giannini, R.; Menta, C.; Visentin, F.; Mairota, P.; Mei, G.; Zandegiacomo, G.; Carollo, S.; et al. Soil, Humipedon, Forest Life and Management. Int. J. Plant Biol. 2023, 14, 571–592. [Google Scholar] [CrossRef]
- Gensac, P. Régénération en altitude de l’épicéa (Picea abies (L) Karst) sur les souches dans les Alpes françaises. Ann. For. Sci. 1990, 47, 173–182. [Google Scholar] [CrossRef]
- Motta, R.; Brang, P.; Frehner, M.; Ott, E. Copertura muscinale e rinnovazione di abete rosso (Picea abies L.) nella pecceta subalpina di Sedrum (Grigioni, Svizzera). Monti e Boschi 1994, 3, 49–56. [Google Scholar]
- Zanella, A.; Ponge, J.-F.; Fritz, I.; Pietrasiak, N.; Matteodo, M.; Nadporozhskaya, M.; Juilleret, J.; Tatti, D.; Le Bayon, R.-C.; Rothschild, L.; et al. Humusica 2, article 13: Para humus systems and forms. Appl. Soil Ecol. 2018, 122, 181–199. [Google Scholar] [CrossRef]
- Tatti, D.; Fatton, V.; Sartori, L.; Gobat, J.-M.; Le Bayon, R.-C. What does ‘lignoform’ really mean? Appl. Soil Ecol. 2018, 123, 632–645. [Google Scholar] [CrossRef]
- Lugato, E.; Bampa, F.; Panagos, P.; Montanarella, L.; Jones, A. Potential carbon sequestration of European arable soils estimated by modelling a comprehensive set of management practices. Glob. Chang. Biol. 2014, 20, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2020, 100, 104950. [Google Scholar] [CrossRef]
- Maturana, H.R.; Varela, F.J. Autopoiesis and Cognition (Boston Studies in the Philosophy and History of Science); Springer: Dordrecht, The Netherlands, 1980; p. 146. [Google Scholar]
- Zanella, A. Tipologia dei boschi: uno strumento per l’interpretazione e la gestione su basi ecologiche dei sistemi forestali. Parchi 1999, 3, 2–15. [Google Scholar]
- Del Favero, R. I boschi delle regioni alpine italiane. Tipologia, funzionamento, selvicoltura; CLEUP: Padova, Italy, 2004; p. 600. [Google Scholar]
- Del Favero, R. I boschi delle regioni alpine italiane. Tipologia, funzionamento, selvicoltura. Con CD-ROM; Edizione 2, Scienze forestali ed.; CLEUP, 2013; p. 602. [Google Scholar]
- Del Favero, R. I boschi delle regioni dell’Italia centrale. Tipologia, funzionamento, selvicoltura. Con CD-ROM, Scienze agrarie ed.; CLEUP, 2010; p. 430. [Google Scholar]
- Del Favero, R. I boschi delle regioni meridionali e insulari d’Italia. Tipologia, funzionamento, selvicoltura, Scienze Agrarie ed.; CLEUP, 2018; p. 469. [Google Scholar]
- Berretti, R.; Lingua, E.; Motta, R.; Piussi, P. Classificazione strutturale dei popolamenti forestali nella Riserva forestale integrale della Valbona a Paneveggio (TN). L’ Italia Forestale e Montana 2004, 59, 98–118. [Google Scholar]
- Dellagiacoma, F.; Motta, R.; Piussi. Ricerche sull’ecologia della pecceta subalpina nella foresta di Paneveggio. Dendronatura 1996, 17, 77–86. [Google Scholar]
- Piussi, P; Sulli, A.; MMencuccini. 30 Years of seed production in a subalpine Norway Spruce forest. Patterns of temporal and spatial variation. For. Ecol.Manag. 1995, 76, 109–125. [Google Scholar]
- Motta, R.; Berretti, R.; Castagneri, D.; Lingua, E.; Nola, P.; Vacchiano, G. Stand and coarse woody debris dynamics in subalpine Norway spruce forests withdrawn from regular management. Ann. For. Sci. 2010, 67, 803–803. [Google Scholar] [CrossRef]
- Motta, R.; Nola, P.; Piussi, P. Structure and stand development in three subalpine Norway spruce (Picea abies (L.) Karst.) stands in Paneveggio (Trento, Italy). Glob. Ecol.Biodivers. 1999, 8, 455–473. [Google Scholar] [CrossRef]
- Motta, R.; Nola, P.; Piussi, P. Long-term investigations in a strict forest reserve in the eastern Italian Alps: spatio-temporal origin and development in two multi-layered subalpine stands. J. Ecol. 2002, 90, 495–507. [Google Scholar] [CrossRef]
- Pignatti, G. Forest vegetation in view of some scenarios of climate change in Italy. For. Riv. Di Selvic. Ed Ecol. For. 2011, 8, 1–12. [Google Scholar] [CrossRef]
- Susmel, L. Normalizzazione delle foreste Alpine: basi ecosistemiche, equilibrio, modelli colturali, produttività: con applicazione alle foreste del Trentino; Liviana: Padova, Italy, 1980; p. 437. [Google Scholar]
- Savilaakso, S.; Johansson, A.; Häkkilä, M.; Uusitalo, A.; Sandgren, T.; Mönkkönen, M.; Puttonen, P. What are the effects of even-aged and uneven-aged forest management on boreal forest biodiversity in Fennoscandia and European Russia? A systematic review. Environ. Évid. 2021, 10, 1–38. [Google Scholar] [CrossRef]
- Xu, C.; De Frenne, P.; Blondeel, H.; De Pauw, K.; Landuyt, D.; Lorer, E.; Sanczuk, P.; Verheyen, K.; De Lombaerde, E. Light more than warming impacts understory tree seedling growth in a temperate deciduous forest. For. Ecol. Manag. 2023, 549. [Google Scholar] [CrossRef]
- Brown, R.W.; Jones, D.L. Plasticity of microbial substrate carbon use efficiency in response to changes in plant carbon input and soil organic matter status. Soil Biol. Biochem. 2023. [Google Scholar] [CrossRef]
- Zanella, A.; Ponge, J.-F.; Jabiol, B.; Van Delft, B.; De Waal, R.; Katzensteiner, K.; Kolb, E.; Bernier, N.; Mei, G.; Blouin, M.; et al. A Standardized Morpho-Functional Classification of the Planet’s Humipedons. Soil Syst. 2022, 6, 59. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Rillig, M.C.; Shi, L.; Dippold, M.A.; Zeng, Z.; Kuzyakov, Y.; Zang, H.; Jones, D.L.; Blagodatskaya, E. Restricted power: Can microorganisms maintain soil organic matter stability under warming exceeding 2 degrees? Glob. Ecol. Biogeogr. 2023, 32, 919–930. [Google Scholar] [CrossRef]
- Ette, J.-S.; Sallmannshofer, M.; Geburek, T. Assessing Forest Biodiversity: A Novel Index to Consider Ecosystem, Species, and Genetic Diversity. Forests 2023, 14, 709. [Google Scholar] [CrossRef]
- Kooch, Y.; Zarei, F.D. Soil function indicators below shrublands with different species composition. CATENA 2023, 227, 107111. [Google Scholar] [CrossRef]
- Della Rocca, F.; Stefanelli, S.; Pasquaretta, C.; Campanaro, A.; Bogliani, G. Effect of deadwood management on saproxylic beetle richness in the floodplain forests of northern Italy: some measures for deadwood sustainable use. J. Insect Conserv. 2014, 18, 121–136. [Google Scholar] [CrossRef]
- Lachat, T.; et al. Bois mort en forêt. Formation, importance et conservation; Institut fédéral de recherches WSL CH-8903 Birmensdorf: Birmensdorf, CH, USA, 2014; pp. 1–12. [Google Scholar]
- Müller, J.; Bütler, R. A review of habitat thresholds for dead wood: a baseline for management recommendations in European forests. Eur. J. For. Res. 2010, 129, 981–992. [Google Scholar] [CrossRef]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Coomes, D.A.; Lines, E.R.; Morris, W.K.; Rüger, N.; et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 507, 90–93. [Google Scholar] [CrossRef]
- Zanella, A.; Berg, B.; Ponge, J.-F.; Kemmers, R.H. Humusica 1, article 2: Essential bases—Functional considerations. Appl. Soil Ecol. 2018, 122, 22–41. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.-D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef]
- Ciancio, O.; Nocentini, S. The forest and man: the evolution of forestry thought from modern humanism to the culture of complexity. Systemic silviculture and management on natural bases; Ciancio, O., Ed.; Accademia Italiana di Scienze Forestali, Firenze (Italia): Florence, Italy, 1997; p. 115. [Google Scholar]
- Nocentini, S.; Ciancio, O.; Portoghesi, L.; Corona, P. Historical roots and the evolving science of forest management under a systemic perspective. Can. J. For. Res. 2021, 51, 163–171. [Google Scholar] [CrossRef]
- Ciancio, O. La teoria della selvicoltura sistemica, le “sterili disquisizioni” e il sonnambulismo dell’intellighenzia forestale; Accademia Italiana di Scienze Forestali, 2010; ISBN 9788887553192. [Google Scholar]
- Ciancio, O. Storia del pensiero forestale. Selvicoltura, filosofia, etica; Rubettino, 2014; p. 546. [Google Scholar] [CrossRef]
- Ciancio, O.; Iovino, F.; Nocentini, S. The theory of the normal forest. La teoria del bosco normale. In collaborazione con O. Ciancio e F. Iovino. L’Italia For. Emont. 1994, 49, 446–462. [Google Scholar]
- F. Provincia Autonoma di Trento - Servizio Foreste e, Linee Tecniche per la Pianificazione Forestale Aziendale - Versione approvata con determinazione del Dirigente del Servizio Foreste e Fauna n. 56 di data 19 dicembre 2016 Art. 9 del D.P.G.P. n. 35 del 26 agosto 2008. Provincia Autonoma di Trento - Servizio Foreste e Fauna, 2016, pp. 135-135.
- Thomasius, H. Geschichte, Theorie und Praxis des Dauerwaldes: erweiterte Fassung eines Vortrages anläßlich der Jahrestagung 1996 des Landesforstvereins Sachsen-Anhalt am 14.05.1996 in Garitz bei Dessau. Salzland Druck GmbH, 1996.
- Cappelli, M. Selvicoltura Generale. Edagricole 1987, 300. [Google Scholar]
- Margulis, L.; Sagan, D.; Eldredge, N. What is Life? University of California Press: Berkeley and Los Angeles, CA, USA, 2000. [Google Scholar]
- A. Ripartizione per le Foreste Ufficio Pianificazione Forestale Provincia Autonoma di Bolzano Alto; Tipologie forestali dell’Alto Adige - VOLUME 1: tipi forestali, regioni forestali, chiave dei tipi forestali; Provincia Autonoma di Bolzano-Alto Adige Ripartizione per le foreste Ufficio Pianificazione forestale: Bolzano, Italy, 2010; p. 310.
- Szewczyk, J.; Szwagrzyk, J. Tree regeneration on rotten wood and on soil in old-growth stand. Plant Ecol. 1996, 122, 37–46. [Google Scholar] [CrossRef]
- Rodriguez, A.; Perestelo, F.; Carnicero, A.; Regalado, V.; Perez, R.; Fuente, G.; Falcon, M. Degradation of natural lignins and lignocellulosic substrates by soil-inhabiting fungi imperfecti. FEMS Microbiol. Ecol. 1996, 21, 213–219. [Google Scholar] [CrossRef]
- Hardersen, S.; Zapponi, L. Wood degradation and the role of saproxylic insects for lignoforms. Appl. Soil Ecol. 2018, 123, 334–338. [Google Scholar] [CrossRef]
- Hu, M.; Lehtonen, A.; Minunno, F.; Mäkelä, A. Age effect on tree structure and biomass allocation in Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies [L.] Karst.). Ann. For. Sci. 2020, 77, 1–15. [Google Scholar] [CrossRef]
- Suzuki, M.; Umeki, K.; Orman, O.; Shibata, M.; Tanaka, H.; Iida, S.; Nakashizuka, T.; Masaki, T. When and why do trees begin to decrease their resource allocation to apical growth? The importance of the reproductive onset. Oecologia 2019, 191, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Villarino, S.H.; Pinto, P.; Jackson, R.B.; Piñeiro, G. Plant rhizodeposition: A key factor for soil organic matter formation in stable fractions. Sci. Adv. 2021, 7, eabd3176. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Reverdy, A.; She, Q.; Sun, B.; Chai, Y. The role of rhizodeposits in shaping rhizomicrobiome. Environ. Microbiol. Rep. 2019, 12, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Lowenfels, J. Teaming with bacteria. The Organic Gardener’s Guide to Endophytic Bacteria and the Rhizophagy Cycle; Timber Press, Inc.: Portland, OR, USA, 2022. [Google Scholar]
- Radmanović, S.; Nikolić, N.; Đorđević, A. Humic Acids Optical Properties Of Rendzina Soils In Diverse Environmental Conditions Of Serbia. Arch. Tech. Sci. 2018, 1, 63–70. [Google Scholar] [CrossRef]
- Susmel, L. Leggi di variazione dei parametri della foresta disetanea normale. L’Italia For. Emont. 1956, 3, 105–116. [Google Scholar]
- Klepac, D. Corsi di cultura in ecologia 1976-1977. Pianifucazione e governo delle abetine disetanee e dei rovereti della Croazia; Padova, Italy, 1978. [Google Scholar]
- Susmel, L. Conservazione e miglioramento delle abetine delle Alpi Orientali. In Atti del Congresso Nazionale di Selvicoltura; Accademie Nazionale di Scienze Forestali: Firenze, Italy, 1954; pp. 331–372. [Google Scholar]
- Susmel, L. Riordinamento su basi bioecologiche delle abetine di S. Vito di Cadore; Pubbl. n. 9.; Stazione Sperimentale di Selvicoltura: Firenze, Italy, 1955. [Google Scholar]
- Pesendorfer, M.B.; Bogdziewicz, M.; Szymkowiak, J.; Borowski, Z.; Kantorowicz, W.; Espelta, J.M.; Fernández-Martínez, M. Investigating the relationship between climate, stand age, and temporal trends in masting behavior of European forest trees. Glob. Chang. Biol. 2020, 26, 1654–1667. [Google Scholar] [CrossRef]
- Mei, G.; Pesaresi, S.; Corti, G.; Cocco, S.; Colpi, C.; Taffetani, F. Changes in vascular plant species composition, top-soil and seed-bank along coppice rotation in an Ostrya carpinifolia forest. Plant Biosyst. - Int. J. Deal. all Asp. Plant Biol. 2019, 154, 259–268. [Google Scholar] [CrossRef]
- Altman, J.; Hédl, R.; Szabó, P.; Mazůrek, P.; Riedl, V.; Müllerová, J.; Kopecký, M.; Doležal, J. Tree-Rings Mirror Management Legacy: Dramatic Response of Standard Oaks to Past Coppicing in Central Europe. PLoS ONE 2013, 8, e55770. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Buckley, G.P. Ecology and Management of Coppice Woodlands; Chapman and Hall: London, UK, 1992; p. 366. [Google Scholar] [CrossRef]
- Piussi, P.; Alberti, G. General Forestry. Woods, society and management techniques, Collana Sc ed.; Compagnia delle Foreste S.r.l.: Arezzo, 2015; p. 432. [Google Scholar]
- Buckley, P. Coppice restoration and conservation: a European perspective. J. For. Res. 2020, 25, 125–133. [Google Scholar] [CrossRef]
- Mairota, P.; Manetti, M.; Amorini, E.; Pelleri, F.; Terradura, M.; Frattegiani, M.; Savini, P.; Grohmann, F.; Mori, P.; Terzuolo, P.; et al. Opportunities for coppice management at the landscape level: the Italian experience. iForest - Biogeosciences For. 2016, 9, 775–782. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




