1. Introduction
Although the etiology of idiopathic scoliosis (IS) is considered to be multifactorial, involving the influence of pathologies related to the musculoskeletal system, developmental and genetic factors, nutritional deficiencies, early exposure to toxins and hormonal dysregulation [
1]; neuropathological mechanisms occurring at the level of the brain and spinal cord may especially trigger or enhance the spine curvature progression and related neurological deficits [
2]. Development of pathological lateral curvature and axial rotation of the spine during ontogenesis in patients with IS affect the anatomical relationships of the spinal cord in the central canal, leading primarily to abnormalities in the activity of gray matter nerve centers and transmission of afferent and efferent impulses in the axons of white matter funiculi [
3,
4]. The incidence of spinal cord pathology in pediatric patients with scoliosis has been reported to be frequently detected even at 20%; with preoperative magnetic resonance imaging (MRI) demonstrating various intraspinal abnormalities [
5,
6]. Secondarily to IS origin, disorders in the conduction of neural impulses within the spinal roots, development of neuropathy in the peripheral nervous system, and neurogenic changes in the muscular system occur [
4]. Results of the clinical neurophysiology diagnostics have proven the high incidence of the mentioned abnormalities before treatment, the axonal-type injury symptoms in peroneal motor fibers, which postoperatively improved on the concave side of IS in parallel with the lumbar ventral roots neural motor conduction [
7]. Although these pathologies can be considered as subclinical, the surgical intervention restores the proper relations of the lumbar ventral roots in the spinal central canal, with symptoms resembling their decompression.
Kinesiotherapeutic treatment [
8,
9] and bracing [
10,
11,
12,
13] for improving the proper body posture, in the majority of IS patients provide only partial benefits for slowing down the progression of the pathological curvature. Negrini et al. [
14,
15] concluded on the necessity for surgical intervention in IS patients with a primary Cobb angle of 40–45 degrees when a fast worsening of the pathology is expected. This supports data from Diebo et al. [
16] and Addai et al. [
9] which indicated that 47% of patients were brace-eligible when the primary IS curvature ranged 20-45 degrees and 21% were surgical candidates for correction with Cobb angle more than 45 degrees. The curvature correction following selective thoracolumbar fusions of idiopathic scoliosis is successfully realized by posterior and anterior approaches. The purpose of surgical treatment is to achieve a three-dimensional symmetry of the affected spine while minimizing the number of fused levels [
17]. A fundamental aspect of surgical IS treatment is also the restoration of the sagittal balance; back pain, disc-root conflicts, and progressive degenerative disk disorder may be the consequences of non-treated disease [
18]. Surgical correction of scoliosis includes many procedures, during which the spinal cord, the nerve roots, and the key blood vessels are exposed at risk of frequent injury. The current data indicate that in 6.3% of patients through various mechanisms, including direct trauma to the spinal cord, ischemia, and stretch during IS deformity correction neurologic complications may occur [
19]. In pediatric surgeries, intraoperative neuromonitoring provides a safe and useful warning to minimize spinal cord injury incidence [
20].
Clinical assessment, including also classical X-ray and neuroimaging, in patients with scoliosis is a routine examination performed pre- and postoperatively [
21,
22], rarely in long-term follow-up [
23,
24], aimed at indicating the validity of the surgical procedure and at the observation of its effects in relation to biomechanical and aesthetic features of spine. In a few cases, it influences the decision to perform reoperation. In a review of the literature on this topic, if long-term clinical evaluation of patients with IS was performed, it usually involved the evaluation of radiographs; the results of available clinical tests assessing the function of the muscular and nervous systems were not reported.
The neurophysiological assessment has the most important intraoperative significance during neuromonitoring of the neural impulses conduction within the spinal cord pathways [
25], although preoperatively in patients with IS it supports the clinical assessment in making decisions about surgical treatment [
26]. Few studies in the field of clinical neurophysiology presenting postoperative recordings, especially of motor evoked potentials, proved an immediate functional improvement of efferent conduction of spinal pathways in IS patients [
7]. This does not imply that preoperative monitoring techniques are inherently more sensitive than intraoperative ones; the different assessments, pre- and intraoperative serve different purposes and may provide complementary insights into the neurological aspects of idiopathic scoliosis.
Neurophysiological evaluation of the patients after the surgical correction of idio-pathic scoliosis in long-term follow-up has not been presented in detail. Therefore, the aim of this paper is to present results of the comparative bilateral results of surface-recorded electromyography (EMG) and motor evoked potentials (MEP) recorded from tibialis anterior muscle, as well as the peroneal nerves electroneurography (ENG), not only before and after the scoliosis correction but also six months postoperatively. We will verify the main hypothesis of whether the effect of direct improvement of the neural impulses efferent conduction of spinal cord pathways following curvature correction in IS patients remains unchanged or continues to progress in the long-term follow-up. The null hypothesis in this study is that there is no difference in the parameters of electromyographic, electroneurographic, and transcranially evoked motor potentials recorded bilaterally from muscles of lower extremities in IS patients pre- and in long-lasting observation period postoperatively.
2. Materials and Methods
2.1. Participants and Study Protocol
Data from forty-five girls with IS treated between 2019 and 2023 years in Wiktor Dega Orthopedic and Rehabilitation Hospital in Poznań, Poland were selected for this study from the cohort of 372 patients.
The inclusion criteria were primary right thoracic and secondary left lumbar IS with Cobb angles included in similar ranges based on the measurements from anterior-posterior and lateral X-rays, Lenke 1-3 (mainly 2) type of IS [
27], matching the same technique of the patients’ surgery using Nova Spine corrective instrumentation with a similar number of the applied pedicle screws between 8 to 19 (13 on average), data acquisition from the same set of three diagnostic clinical neurophysiology tests performed preoperatively (T0), one week postoperatively (T1) and six months after surgery (T2) (
Table 1).
Three times performed neurophysiological evaluation of patients included bilateral tibialis anterior (TA) muscle electromyography during maximal contraction with surface electrodes (mcsEMG,) peroneal nerve electroneurography (ENG) recorded from extensor digitorum brevis (EXT) muscle following the electrical stimulation at the ankle, and motor evoked potential (MEP) recordings from tibialis anterior muscles following transcranial magnetic stimulation (TMS). Exclusion criteria covered the same contraindications as for diagnostic TMS and transcranial electrical stimulation utilized during the neuromonitoring procedures; including head trauma, epilepsy, cardiac disease, pacemakers or other implanted biomedical devices, and pregnancy [
28]. In the preoperative studies, the patients were fully aware and cooperative and there was no need to apply anesthetics, nor in the postoperative observations.
A control group of 80 healthy volunteers was examined to establish reference values for mcsEMG, ENG and MEP neurophysiological recordings. To ensure comparability, the control group’s demographics (gender, age, height, and weight) were adjusted to match those of the patient group. We have preliminarily chosen and matched the subjects of both groups taking into account the similar demographic and anthropometric properties; the significant differences in age, height, and weight between the patients and healthy volunteers in the control group were not observed (
Table 1).
Patients were surgically treated and clinically evaluated three times in T0-T2 periods of observation by the same team of four experienced spine surgeons; neurophysiological studies were performed by the same two neurophysiologists.
Ethical considerations were in agreement with the Helsinki Declaration. Approval was received from the Bioethical Committee of the University of Medical Sciences in Poznań, Poland (including studies on healthy people), decisions No. 942/21. Each subject (and her parent/legal guardian) was informed about the aim of the study and gave written consent for examinations and data publication.
2.2. Treatment
Before the surgical treatment, twenty-five out of the 45 patients were treated with the Cheneau brace but all of them had applied the physiotherapeutic exercises aimed at correcting the body posture. During the scoliotic spine surgery, the implantation of a Nova Spine corrective instrumentation system (Amiens, France) was utilized by a posterior approach in a prone position of patients (
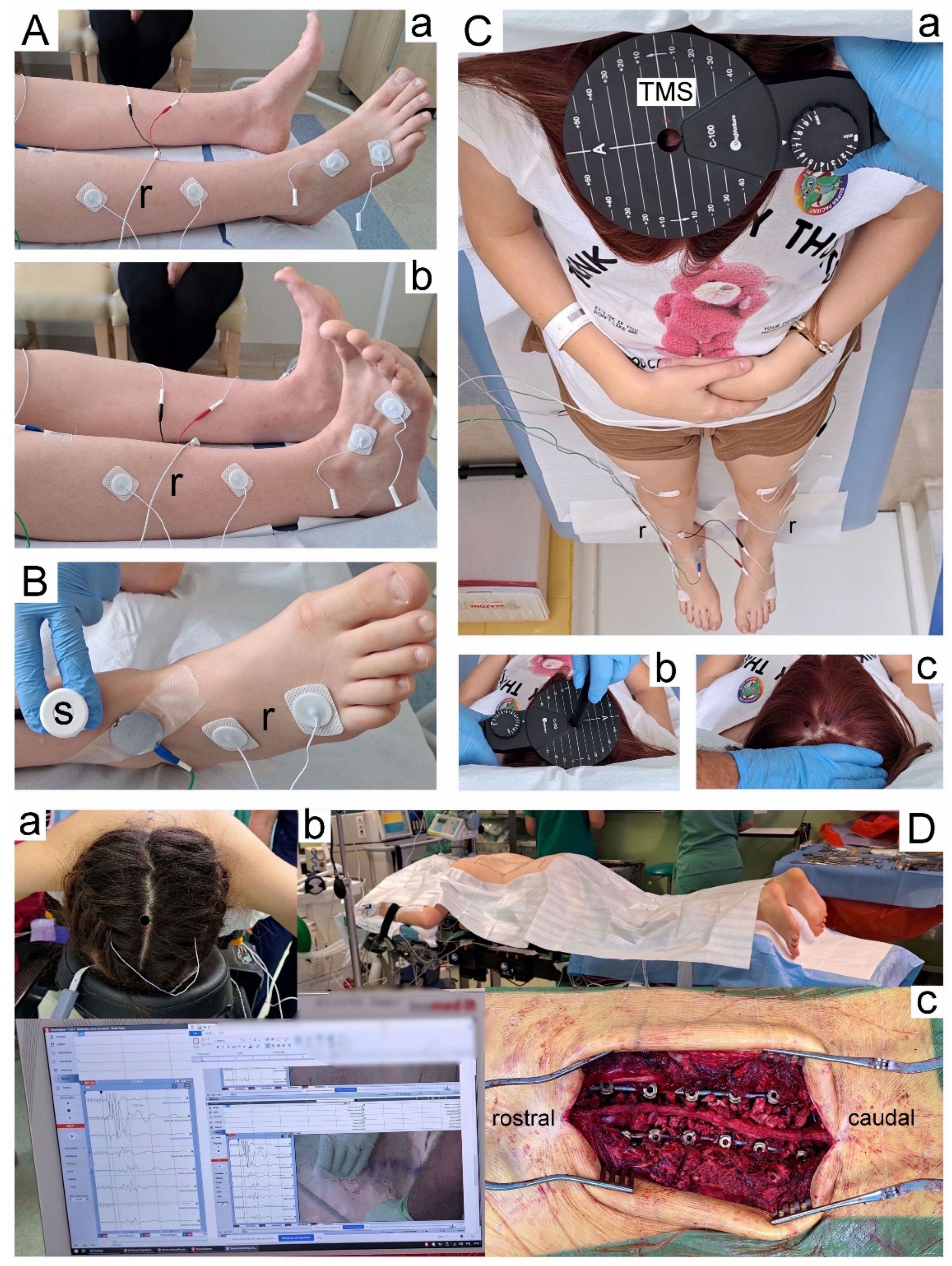
Figure 1Db). The deformity was corrected following the pedicle polyaxial and monoaxial screws implantation and implementation of two corrective rods (5.5 mm in diameter) made of titanium alloy (
Figure 1Dc). The manoeuvers of convex rod rotation, apical translation, segmental derotation, distraction on the concave side, and compression on the convex side were performed to obtain spine fusion. All mentioned surgical procedures were applied under the control of X-ray C-arm and neuromonitoring navigations (
Figure 1Da,c). Further details regarding the surgical procedures and intraoperative neurophysiological recordings are described elsewhere [
7].
2.3. Neurophysiological Recordings
Principles of the neurophysiological studies’ methodology performed at three periods of observation (preoperatively-T0, postoperatively one week after surgery-T1, and 6 months after the surgery-T2) are presented in
Figure 1. Tests were performed in the same diagnostic room with a controlled temperature of 22 °C; all patients were examined in a supine position. The KeyPoint Diagnostic System (Medtronic A/S, Skøvlunde, Denmark) was used for recordings of all neurophysiological tests.
Electromyographical recordings were performed using surface electrodes (mcsEMG), bilaterally applied to the tibialis anterior muscle (TA) (
Figure 1Aa), to assess the motor unit recruitment during the attempt of the maximal contraction lasting 5 s (
Figure 1Ab). Disposable Ag/AgCl surface recording electrodes (surface of 5 mm
2), with an active electrode placed on the muscle belly, a reference electrode on the distal tendon of the same muscle, and a ground electrode on the distal part of the examined muscle were used. The resistance between the electrode surface and the skin was decreased with electroconductive gel.
During the mcsEMG recordings acquisition and interpretation, we followed standards according to the Guidelines of the International Federation of Clinical Neurophysiology—European Chapter [
29,
30,
31]. Three attempts of the maximal muscle contraction for 5 s were performed by the patient each time; the neurophysiologist selected the best recording with the highest mean amplitude measured peak-to-peak with reference to the isoelectric line for analysis. The amplitude measured in μV and the frequency of muscle motor unit action potential recruitment measured in Hz were the output data from mcsEMG recordings. The frequency index (FI, 3–0) based on the calculations of motor units action potential recruitment during maximal contraction: 3 = 95–70 Hz—normal, 2 = 65–40 Hz—moderate abnormality, 1 = 35–10 Hz—severe abnormality; 0 = no contraction was analyzed. All mcsEMG recordings in both healthy volunteers and patients were performed at a base time of 80 ms/D and an amplification of 20–1000 μV/D. The upper 10 kHz and the lower 20 Hz filters were set in the recorder.
To assess the transmission of neural impulses in the motor peripheral fibers of the peroneal nerves, bilateral electroneurography (ENG) was performed (
Figure 1B). This method assessed if the abnormal muscle function or the efferent transmission were caused by the pathologies in the L5 ventral root fibers’ neural conduction and/or the consequences of peripheral neuropathies. Electrical stimulation with rectangular pulses of 0.2 ms duration at a frequency of 1 Hz and an intensity ranging from 0 to 80 mA using bipolar stimulating electrodes placed over the skin along the anatomical passages of the nerve at the ankle evoked the compound muscle action potentials (M-waves, CMAP) and F-waves which were recorded from the extensor digitorum brevis muscle (EXT). Recordings of these potentials were assumed to verify the transmission of neuronal impulses in the motor fibers peripherally and within L5 ventral spinal roots, respectively. The amplification of 100–5000 µV/D and a time base of 2–10 ms/D for ENG recordings were adjusted in a recorder. The parameters of amplitudes (in µV) and latencies (in ms) for M–waves, interlatencies of recorded M-F waves (in ms), and frequencies for F-waves (usually not less than 14 during evoking 20 positive, successive recordings of M—waves) were the outcome measures. The normative values recorded in healthy volunteer subjects were compared with the test results of the patients. The details on the methodology of acquisition and interpretation of ENG studies are described in other papers [
32,
33].
Motor evoked potentials (MEPs) were induced using a single, biphasic, 5 ms lasting magnetic stimulus delivered transcranially (TMS,
Figure 1Ca) with a magnetic circular coil (C-100, 12 cm in diameter) placed over the scalp in the area of the M1 motor cortex. The MagPro X100 magnetic stimulator (Medtronic A/S, Skøvlunde, Denmark) was used for the stimulus generation. The excitation was targeted with an angle for the corona radiate excitation, where the fibers of the corticospinal tract for innervation of lower extremities muscles take the origin from. MEPs were recorded with surface electrodes from TA muscles bilaterally. The magnetic field stream delivered from the coil at the strength 70–80% of the resting motor threshold (RMT; 0.84–0.96 T) excited all neural structures up to 3–5 cm deep. With such a condition, the cells of origin of the rubrospinal tract in the midbrain are expected to be also excited. The latency and amplitude parameters were analyzed as the outcome measures to assess the primary motor cortex output and evaluate the global efferent transmission of neural impulses to effectors via spinal cord descending tracts. The location of an optimal stimulation (a hot spot in the area where TMS elicited the largest recorded MEP amplitude;
Figure 1Bb and c) was searched following the consecutive tracking distanced 5 mm from each other. The reproducibility of the MEPs recordings at T0-T2 was supported by the accurate photographic documentation of hot spots marked at a similar location of the transcranial stimulation. The MEP amplitude was measured from peak to peak of the signal, the latency from the stimulus application marked by the artefact in the recording to the onset of the positive inflexion of potential. The stimulation was not reported by the subjects as painful. During MEPs acquisition, the low-pass filter of recorder was set to 20 Hz, the high-pass filter to 10 kHz, the time base at 10 ms/D, and the amplification of signals between 200 and 5000 µV. A bandwidth of 10 Hz to 1000 Hz and digitalization at 2000 samples per second and channel were used during recordings. Further details on the methodology of MEP recordings have been described in detail elsewhere [
34,
35].
Neuromonitoring sessions were performed in the theatre using the ISIS system (Inomed Medizintechnik, Emmendinger, Germany) (
Figure 1Dc). Motor evoked potentials were induced as a result of transcranial electrical stimulation (
Figure 1Da) in areas of the cortical motor fields for innervation of the thumb and selected muscles of the lower extremities following the application of a sequence of four stimuli (duration of a single pulse 500 µs) with an intensity of 105 mA on an average via bipolar subcutaneous electrodes. We used our experience in the application of the surface electrodes for MEP recording from TA and other upper and lower extremities muscles according to the previous descriptions [
7].
The choice in this study for the presentation of the results of MEPs and sEMG recordings from the tibialis anterior muscle as the key muscle was dictated the most often presented comparisons of the recorded parameters in scientific reports of other authors related to the treatment of IS patients when the neurophysiological tests were utilized for their evaluation.
2.4. Statistical Analysis
Statistica, version 13.1 (StatSoft, Kraków, Poland) was used for the data analysis. Descriptive statistics were reported as minimal and maximal values (range), with mean and standard deviation (SD). The normality distribution and homogeneity of variances were studied with the Shapiro–Wilk test and with Levene’s test. The frequency mcsEMG index and recorded F-wave frequencies were of the ordinal scale type, while amplitudes and latencies of other analysed neurophysiological tests were of the interval scale type. However, they did not represent a normal distribution; therefore, the non-parametric tests had to be used. None of the collected data represented a normal distribution or was of the ordinal scale type; therefore, the Wilcoxon’s signed-rank test was used to compare the differences between results obtained before and after treatment, as well as to compare results at the T0, T1 and T2 periods of observation. In the cases of independent variables, the non-parametric Mann–Whitney test was used. Any p-values of ≤0.05 were considered statistically significant. The cumulative data from parameters of mcsEMG, ENG and MEP recordings performed on both sides were used for comparison in T0, T1, and T2. The results from all neurophysiological tests performed on patients were also calculated from the group of healthy subjects (control group) to achieve the normative parameters used to compare the health status between the patients and the controls. Results did not reveal any significant difference in values of parameters recorded in neurophysiological tests on the left and right sides in controls. Attention was paid to matching patients and healthy controls’ demographic and anthropometric properties, including gender, age, height, weight, and BMI. Statistical software (StatSoft, Kraków, Poland) was used to determine the required sample size using the primary outcome variable of sEMG and MEPs amplitudes recorded from TA muscles before and after treatment with a power of 80% and a significance level of 0.05 (two-tailed). The mean and standard deviation (SD) were calculated using the data from the first 20 patients, and the sample size software estimated that more than 40 patients were needed for this study.
3. Results
In the vast majority of patients described in this paper, the Cheneau brace or the physiotherapeutic exercises, if applied, were not effective forms of treatment for diminishing the scoliosis progression.
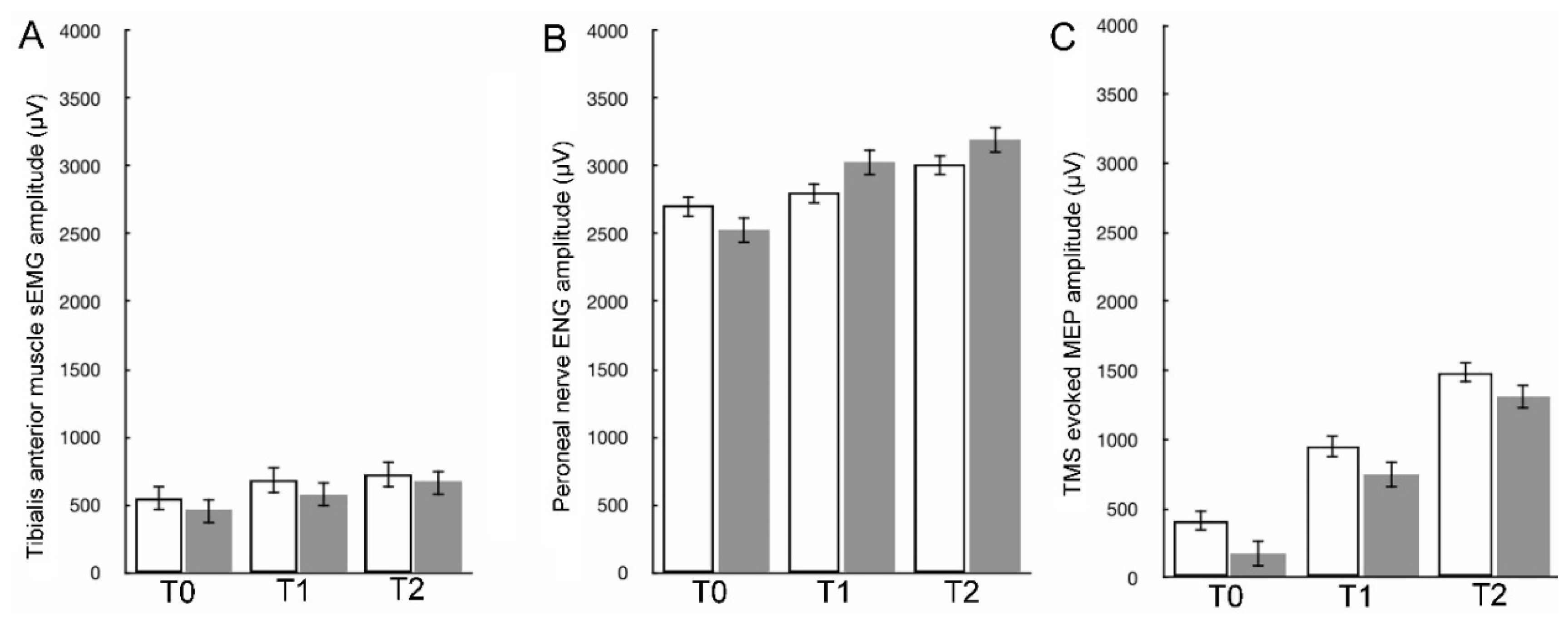
The mcsEMG amplitude recording parameters, ENG parameters and MEPs amplitude parameters recorded in the observation periods between T0 and T1 differed significantly at
p = 0.032-0.049, showing trends of the values increasing (see
Table 2, right side). This trend gradually increased for all parameters recorded in all tests performed in the observation periods between T0 and T2 when the differences were ascertained at
p = 0.019-0.045. This suggests that efferent neural conduction function both centrally and peripherally as well as TA muscle function improve immediately after surgical correction of scoliosis and further normalization appears after six months in long-term follow-up.
Comparison of more amplitudes than FI parameters from mcsEMG recordings indicated, that patients’ TA muscle motor units activity differed in T0 from the healthy controls at
p = 0.025-0.037, but this difference became less significant in T1 at
p = 0.026-0.042 and the least significant in T2 at
p = 0.043-0.046 (
Table 2, see also
Figure 2Aa-Ca and
Figure 3 A). It seems that the TA muscle motor unit recruitment function after half a year from surgical treatment in IS patients is comparable to the normal condition.
In ENG recordings performed preoperatively, early postoperatively, and in a long-term observation, the tendency to increase values of M-waves amplitudes (at
p = 0.007-0.011, see also
Figure 3B) and decreasing values of latencies (at
p = 0.034-0.075,
Table 2) indicated the gradually retreat the symptoms of peroneal motor fibers injury of mainly the axonal type. The parallel change of increase in the F-waves frequencies parameters in ENG recordings following the application of twenty electrical stimuli (
p = 0.042-0.055), provided evidence that surgeries improved also the lumbar ventral roots’ neural transmission (
Figure 2Ab-Cb), to the functional status considered normal. The M-F waves interlatency parameter analysis in T0-T2 revealed a similar improvement at
p = 0.031-0.062. In healthy volunteers, the strength of the electrical current to evoke the maximal M-wave amplitudes in ENG recordings ranged from 17 to 39 mA with a mean of 25.4 ± 2.1 mA, while in patients in T2 period of observation, it ranged from 30 to 45 mA (mean of 27.1 ± 1.9 mA), suggesting a lower, more physiological threshold of excitation for motor fibers of peroneal nerves.
The difference between parameters of MEPs amplitudes from recordings of healthy volunteers and patients before surgery was at
p = 0.009-0.01 (
Table 2, bottom; see also
Figure 2Ac and
Figure 3C). After the surgical scoliosis correction in T1 (
Figure 2Bc and
Figure 3C) it was at
p = 0.019−0.027, indicating a slight improvement in the efferent transmission of neural impulses within the fibers of the spinal tracts. In long-lasting T2 observation, this difference further diminished and the amplitudes differed only at
p = 0.041-0.046 bilaterally (
Figure 2Cc and
Figure 3C).
Preoperatively (T0), the results of all neurophysiological study parameters in IS patients were asymmetrical at
p = 0.036-0.05 and recorded as worse on the concave side, suggesting the lateralization of neurological motor deficits (
Table 2,
Figure 2Aa,c). One week postoperatively (T1), this asymmetry was recorded as gradually reduced (
Figure 2Ba,c), showing almost no difference between the right and left sides six months later (T2) (
Figure 2Ca,c).
Abbreviations: mcsEMG—muscle maximal contraction surface electromyography recordings; FI—frequency index (3-0)—frequency of motor units action potentials recruitment during maximal contraction (3—95–70Hz—normal; 2—65–40Hz—moderate abnormality; 1—35–10Hz—severe abnormality; 0—no contraction); ENG—electroneurography recordings; TMS—transcranial magnetic stimulation; MEP—muscle recorded motor evoked potential; NA—non-applicable; p ≤ 0.05 determines significant statistical differences marked in bold.
4. Discussion
The results of this study in patients treated surgically because of idiopathic scoliosis confirmed the previous findings [
7], that transmission of the efferent neural impulses in the spinal cord tracts and within the fibers of the peripheral nervous system slightly improves immediately after surgical correction of scoliosis. Moreover, we have ascertained further normalization of the motor function together with the improvement of TA muscle motor unit recruitment after half a year from surgical treatment in IS patients, in which mcsEMG parameters can be comparable to the normal condition.
Apart from revealing the improvement regarding biomechanical and aesthetic features of the spine following the applied surgeries for IS patients in clinical studies, the neurophysiological approach enables the investigation of the lateralization of the neurological motor deficits, which are gradually reduced postoperatively, showing almost no difference between the right and left sides in long-lasting observation. Although the test results referring to the efferent function evaluation presented in
Table 2 are consistent and complementary, one can raise an objection that they have been presented in patients with IS representing different types of scoliotic curvature according to the classification of Lenke et al. [
27], what might be one of this study limitations. The patients examined in this study mostly represented type 2, with the main right-sided curvature of the spine in the thoracic vertebrae, and to a lesser extent, the secondary one with an angle and opposite direction in the lumbar vertebrae, which is excessive in type 3 curvature. These cases are different from the type of scoliosis with an exclusively thoracic angle of curvature occurring in type 1. However, a greater decrease in the amplitude parameter and a slight increase of the latency parameter of MEPs recorded bilaterally from the TA muscle was observed similarly on the concave side of scoliosis, as well as changes in the conductivity of the roots of lumbar neuromeres with their consequences in the symptoms of neuropathy within the lower limb nerves on the same side. Nevertheless, the possible variations in the pathology pattern which are reflected in the abnormalities found in the functional studies can only be explained by structural MRI studies.
A possible explanation for the immediate improvement of the overall efferent conduction shown in the results of sEMG, ENG and MEP studies after IS spine surgery in T1 observation may be the restoration of normal anatomical and functional relationships of neural structures in the central canal of the deformed spine mainly through surgical procedures of distraction and derotation. This applies not only to axons in the lateral and ventral funiculi of the white matter but also in the spinal roots. This is clearly visible in the results of ENG tests showing symptoms of axonal damage in the motor fibers of the peroneal nerves (M-wave parameters abnormalities), recovering mainly on the concave side of scoliosis, as well as in tests of nerve impulse conduction in the ventral roots (decreased frequency of the recorded F-wave), which suggests that derotation and distraction may result in the restoration of normal relationships of the lumbar ventral roots in the central spinal canal, resembling their decompression. It can be assumed that another significant improvement in efferent conduction visible in the test results during the T2 observation period is the result of structural or more functional regeneration processes occurring in the spinal cord funiculi and/or the spinal ventral root structures.
The results of the presented study are unique and it is difficult to compare them with the results of similar studies presented by other authors, because they concern the functional assessment of patients after IS surgery in a long-term follow-up, and the results presented so far in this topic were postoperative X-ray evaluations. The comparison of the MEPs recording parameters in this study when recorded from the TA muscle with the reports of other authors provides different data [
36,
37]. The only consistent comparison refers to the latency parameter presented by Lo et al. [
38] and earlier by Edmonds et al. [
39], but the mean parameter of amplitude at about 500 µV is far beyond the calculated in our MEPs recordings. Moreover, the data cited above come mainly from intraoperative neuromonitoring observations accompanying scoliosis correction, rather than diagnostic data recorded postoperatively.
One might object to the use of the tibialis anterior muscle of the lower extremities for neurophysiological assessment instead of the paraspinal muscles, which better meet the criteria for functional assessment of the motor function in patients with IS because these muscles preoperatively show asymmetric activity of motor units [
4]. However, it should be remembered that these muscles are surgically incised and retracted in the midline to expose the surgical field during the curvature correction, and comparison of their preoperative and postoperative function, especially in short-term follow-up, would be fraught with inevitable iatrogenic structural damage. Hence, MEP and sEMG recordings using the surface electrodes from the tibialis anterior muscle bilaterally are more and more widely used not only in pre- and postoperative diagnostic purposes but also their activity has been proven to be precise enough for intraoperative monitoring [
40,
41].
5. Conclusions
The presented algorithm of the diagnostic proceeding pre-, early post-, and long-lasting postoperatively using mcsEMG, MEP, and ENG neurophysiological examinations can be significant not only in making the final decision regarding surgical treatment and its personalization but also helps in precise ascertaining its effects as well as in predicting the final result of IS treatment.
A comprehensive preoperative radiological and neurological assessment supplemented with precise functional examinations, such as clinical neurophysiology tests, convinces the surgeon of the need to undertake the surgical treatment, and postoperatively, about the effectiveness of the treatment when the neurological symptoms retreat and the radiological assessment is satisfactory.
Author Contributions
Conceptualisation, P.D., J.H. and T.K.; methodology, P.D., J.H., K.K. and T.K.; software, J.H.; validation, P.D., J.H., K.K. and T.K.; formal analysis, P.D., J.H., K.K. and T.K.; investigation, P.D., J.H., K.K., P.J., P.G., M.T., Ł.K., M.D. and T.K.; resources, P.D. and J.H.; data curation, P.D., J.H., K.K. and T.K.; writing—original draft preparation, P.D. and J.H.; writing—review and editing, P.D., J.H., P.J., P.G., M.T., Ł.K., M.D. and T.K.; visualisation, J.H.; supervision, P.D., J.H. and T.K.; project administration, P.D. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of Poznan University of Medical Science, decision no 942/21 dated on 13 January 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All the data generated or analysed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Burwell, R.G.; Dangerfield, P.H.; Lowe, T.G.; Margulies, J.Y. (Eds.) Etiology of Adolescent Idiopathic Scoliosis: Current Trends and Relevance to New Treatment Approaches; State of the Art Reviews, Spine; Hanley & Belfus Inc.: Philadelphia, PA, USA, 2000. [Google Scholar]
- Lowe, T.G.; Edgar, M.; Margulies, J.Y.; Miller, N.H.; Raso, V.J.; Reinker, K.A.; Rivard, C.H. Etiology of idiopathic scoliosis: Current trends in research. J. Bone Joint Surg. 2000, 82, 1157–1168. [Google Scholar] [CrossRef]
- Hawes, M.C.; O’Brien, J.P. The transformation of spinal curvature into spinal deformity: Pathological processes and implications for treatment. Scoliosis 2000, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Rogala, P. Etiopathogenesis of the adolescent idiopathic scoliosis basing on neuroimaging and neurophysiological examinations with the special emphazing of motor evoked potentials (MEP). Stud. Health Technol. Inform. 2012, 176, 446. [Google Scholar]
- Gupta, P.; Lenke, L.G.; Bridwell, K.H. Incidence of neural axis abnormalities in infantile and juvenile patients with spinal deformity. Is a magnetic resonance image screening necessary? Spine 1998, 23, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.B.; Lonstein, J.E.; Heithoff, K.B.; Kirkham, J.A. Magnetic resonance imaging evaluation of the adolescent patient with idiopathic scoliosis before spinal instrumentation and fusion. A prospective, double-blinded study of 140 patients. Spine 1997, 22, 855–858. [Google Scholar] [CrossRef]
- Daroszewski, P.; Huber, J.; Kaczmarek, K.; Janusz, P.; Główka, P.; Tomaszewski, M.; Domagalska, M.; Kotwicki, T. Comparison of Motor Evoked Potentials Neuromonitoring Following Pre- and Postoperative Transcranial Magnetic Stimulation and Intraoperative Electrical Stimulation in Patients Undergoing Surgical Correction of Idiopathic Scoliosis. J. Clin. Med. 2023, 12, 6312. [Google Scholar] [CrossRef]
- Negrini, S.; Antonini, G.; Carabalona, R.; Minozzi, S. Physical exercises as a treatment for adolescent idiopathic scoliosis. A systematic review. Pediatr. Rehabil. 2003, 6, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Addai, D.; Zarkos, J.; Bowey, A.J. Current concepts in the diagnosis and management of adolescent idiopathic scoliosis. Child’s Nerv. Syst. 2020, 36, 1111–1119. [Google Scholar] [CrossRef]
- Negrini, S.; Minozzi, S.; Bettany-Saltikov, J.; Zaina, F.; Chockalingam, N.; Grivas, T.B.; Kotwicki, T.; Maruyama, T.; Romano, M.; Vasiliadis, E.S. Braces for idiopathic scoliosis in adolescents. Spine 2010, 35, 1285–1293. [Google Scholar] [CrossRef]
- Weinstein, S.L.; Dolan, L.A.; Wright, J.G.; Dobbs, M.B. Effects of bracing in adolescents with idiopathic scoliosis. N. Engl. J. Med. 2013, 369, 1512–1521. [Google Scholar] [CrossRef]
- Kelly, J.; Shah, N.; Freetly, T.; Dekis, J.; Hariri, O.; Walker, S.; Borrelli, J.; Post, N.H.; Diebo, B.G.; Urban, W.P.; et al. Treatment of adolescent idiopathic scoliosis and evaluation of the adolescent patient. Curr. Orthop. Pract. 2018, 29, 424–429. [Google Scholar] [CrossRef]
- Pepke, W.; Morani, W.; Schiltenwolf, M.; Bruckner, T.; Renkawitz, T.; Hemmer, S.; Akbar, M. Outcome of Conservative Therapy of Adolescent Idiopathic Scoliosis (AIS) with Chêneau-Brace. J. Clin. Med. 2023, 12, 2507. [Google Scholar] [CrossRef]
- Negrini, S.; Aulisa, L.; Ferraro, C.; Fraschini, P.; Masiero, S.; Simonazzi, P.; Tedeschi, C.; Venturin, A. Italian guidelines on rehabilitation treatment of adolescents with scoliosis or other spinal deformities. Eur. Med. 2005, 41, 183–201. [Google Scholar]
- Negrini, S.; Donzelli, S.; Aulisa, A.G.; Czaprowski, D.; Schreiber, S.; de Mauroy, J.C.; Diers, H.; Grivas, T.B.; Knott, P.; Kotwicki, T.; et al. SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018, 13, 3. [Google Scholar] [CrossRef]
- Diebo, B.G.; Segreto, F.A.; Solow, M.; Messina, J.C.; Paltoo, K.; Burekhovich, S.A.; Bloom, L.R.; Cautela, F.S.; Shah, N.V.; et al. Spine 2017; 17 (Supplement): S213.
- Patel, P.N.; Upasani, V.V.; Bastrom, T.P.; Marks, M.C.; Pawelek, J.B.; Betz, R.R.; Lenke, L.G.; Newton, P.O. Spontaneous lumbar curve correction in selective thoracic fusions of idiopathic scoliosis: A comparison of anterior and posterior approaches. Spine 2008, 33, 1068–1073. [Google Scholar] [CrossRef]
- Luk, K.D.K.; Vidyadhara, S.; Lu, D.S.; Wong, Y.W.; Cheung, W.Y.; Cheung, K.M.C. Coupling between sagittal and frontal plane deformity correction in idiopathic thoracic scoliosis and its relationship with postoperative sagittal alignment. Spine 2010, 35, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.K.; Loh, K.W.; Chung, W.H.; Hasan, M.S.; Chan, C.Y.W. Perioperative outcome and complications following single-staged posterior spinal fusion using pedicle screw instrumentation in adolescent idiopathic scoliosis(AIS): A review of 1057 cases from a single centre. BMC Musculoskelet. Disord. 2021, 22, 413. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Hwang, S.W.; Tataryn, Z.; Samdani, A.F. Neuromonitoring changes in pediatric spinal deformity surgery: A single-institution experience. J. Neurosurg. Pediatr. 2014, 13, 247–254. [Google Scholar] [CrossRef]
- Ozturk, C.; Karadereler, S.; Ornek, I.; Enercan, M.; Ganiyusufoglu, K.; Hamzaoglu, A. The role of routine magnetic resonance imaging in the preoperative evaluation of adolescent idiopathic scoliosis. Internat. Orthopaed. 2010, 34, 543–546. [Google Scholar] [CrossRef]
- Scaramuzzo, L.; Giudici, F.; Archetti, M.; Minoia, L.; Zagra, A.; Bongetta, D. Clinical relevance of preoperative MRI in adolescent idiopathic scoliosis: Is hydromyelia a predictive factor of intraoperative electrophysiological monitoring alterations? Clin Spine Surg. 2019, 32, E183–E187. [Google Scholar] [CrossRef]
- Chen, K.; Chen, Y.; Shao, J.; Zhoutian, J.; Wang, F.; Chen, Z.; Li, M. Long-Term Follow-up of Posterior Selective Thoracolumbar/Lumbar Fusion in Patients With Lenke 5C Adolescent Idiopathic Scoliosis: An Analysis of 10-Year Outcomes. Glob. Spine J. 2022, 12, 840–850. [Google Scholar] [CrossRef]
- Ghandhari, H.; Ameri, E.; Nikouei, F.; Mahdavi, S.M.; Chehrassan, M.; Motalebi, M. Selective Thoracolumbar/Lumbar Fusion in Adolescent Idiopathic Scoliosis: A Comprehensive Review of the Literature. Archiv. Bone Joint Surg. 2023, 11, 313–320. [Google Scholar] [CrossRef]
- Pastorelli, F.; Di Silvestre, M.; Plasmati, R.; Michelucci, R.; Greggi, T.; Morigi, A.; Bacchin, M.R.; Bonarelli, S.; Cioni, A.; Vommaro, F.; et al. The prevention of neural complications in the surgical treatment of scoliosis: The role of the neurophysiological intraoperative monitoring. Eur. Spine J. 2011, 20, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Daroszewski, P.; Garasz, A.; Huber, J.; Kaczmarek, K.; Janusz, P.; Główka, P.; Tomaszewski, M.; Kotwicki, T. Update on neuromonitoring procedures applied during surgery of the spine—Observational study. Reumatologia 2023, 61, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lenke, L.G.; Betz, R.R.; Harms, J.; Bridwell, K.H.; Clements, D.H.; Lowe, T.G.; Blanke, K. Adolescent idiopathic scoliosis: A new classification to determine extent of spinal arthrodesis. J. Bone Jt. Surg. 2001, 83, 1169–1181. [Google Scholar] [CrossRef]
- MacDonald, D.B. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J. Clin. Neurophysiol. 2002, 19, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.M.; Huber, J.; Leszczyńska, K.; Wietrzak, P.; Kaczmarek, K. Relationships between the Clinical Test Results and Neurophysiological Findings in Patients with Thoracic Outlet Syndrome. Bioengineering 2022, 9, 598. [Google Scholar] [CrossRef]
- Wesołek, A.; Daroszewski, P.; Huber, J. Neurophysiological Evaluation of the Functional State of Muscular and Nervous Systems in High-Maneuvering Jet Fighters. Appl. Sci. 2023, 13, 1120. [Google Scholar] [CrossRef]
- Leszczyńska, K.; Huber, J. Unveiling the Correlations between Clinical Assessment of Spasticity and Muscle Strength and Neurophysiological Testing of Muscle Activity in Incomplete Spinal Cord Injury Patients: The Importance of a Comprehensive Evaluation. Appl. Sci. 2023, 13, 7609. [Google Scholar] [CrossRef]
- Huber, J.; Leszczyńska, K.; Wincek, A.; Szymankiewicz-Szukała, A.; Fortuna, W.; Okurowski, S.; Tabakow, P. The Role of Peripheral Nerve Electrotherapy in Functional Recovery of Muscle Motor Units in Patients after Incomplete Spinal Cord Injury. Appl. Sci. 2021, 11, 9764. [Google Scholar] [CrossRef]
- Wiertel-Krawczuk, A.; Huber, J.; Szymankiewicz-Szukała, A.; Wincek, A. Neurophysiological Evaluation of Neural Transmission in Brachial Plexus Motor Fibers with the Use of Magnetic versus Electrical Stimuli. Sensors 2023, 23, 4175. [Google Scholar] [CrossRef] [PubMed]
- Wincek, A.; Huber, J.; Leszczyńska, K.; Fortuna, W.; Okurowski, S.; Chmielak, K.; Tabakow, P. The Long-Term Effect of Treatment Using the Transcranial Magnetic Stimulation rTMS in Patients after Incomplete Cervical or Thoracic Spinal Cord Injury. J. Clin. Med. 2021, 10, 2975. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, K.; Huber, J. Comparing Parameters of Motor Potentials Recordings Evoked Transcranially with Neuroimaging Results in Patients with Incomplete Spinal Cord Injury: Assessment and Diagnostic Capabilities. Biomedicines 2023, 11, 2602. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Park, Y.G.; Kim, D.H.; Yoon, S.Y. Monitoring of Motor and Somatosensory Evoked Potentials During Spine Surgery: Intraoperative Changes and Postoperative Outcomes. Ann Rehabil Med. 2016, 40, 470–480. [Google Scholar] [CrossRef]
- Kimiskidis, V.K.; Potoupnis, M.; Papagiannopoulos, S.K.; Dimopoulos, G.; Kazis, D.A.; Markou, K.; Zara, F.; Kapetanos, G.; Kazis, A.D. Idiopathic scoliosis: A transcranial magnetic stimulation study. J. Musculoskelet. Neuronal Interact. 2007, 7, 155–160. [Google Scholar] [PubMed]
- Lo, Y.L.; Dan, Y.F.; Tan, Y.E.; Tan, C.T.; Raman, S. Intra-operative monitoring in scoliosis surgery with multi-pulse cortical stimuli and desflurane anesthesia. Spinal Cord 2004, 42, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, H.L., Jr.; Paloheimo, M.P.; Backman, M.H.; Johnson, J.R.; Holt, R.T.; Shields, C.B. Transcranial magnetic motor evoked potentials (tcMMEP) for functional monitoring of motor pathways during scoliosis surgery. Spine 1989, 14, 683–686. [Google Scholar] [CrossRef]
- Gadella, M.C.; Dulfer, S.E.; Absalom, A.R.; Lange, F.; Scholtens-Henzen, C.H.; Groen, R.J.; Wapstra, F.H.; Faber, C.; Tamási, K.; Sahinovic, M.M.; et al. Comparing Motor-Evoked Potential Characteristics of Needle versus Surface Recording Electrodes during Spinal Cord Monitoring-The NERFACE Study Part I. J. Clin. Med. 2023, 12, 1404. [Google Scholar] [CrossRef]
- Dulfer, S.E.; Gadella, M.C.; Tamási, K.; Absalom, A.R.; Lange, F.; Scholtens-Henzen, C.H.; Faber, C.; Wapstra, F.H.; Groen, R.J.; Sahinovic, M.M.; et al. Use of Needle Versus Surface Recording Electrodes for Detection of Intraoperative Motor Warnings: A Non-Inferiority Trial. The NERFACE Study Part II. J. Clin. Med. 2023, 12, 1753. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).