Submitted:
06 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
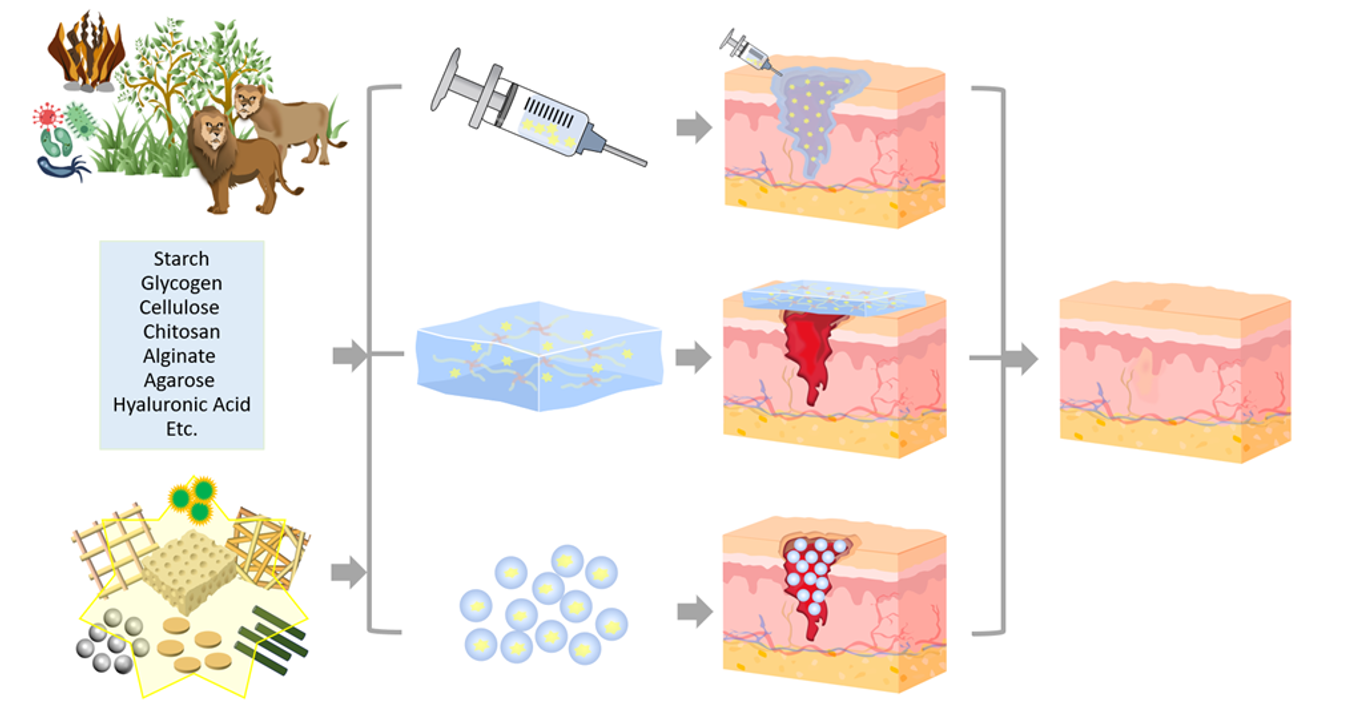
Keywords:
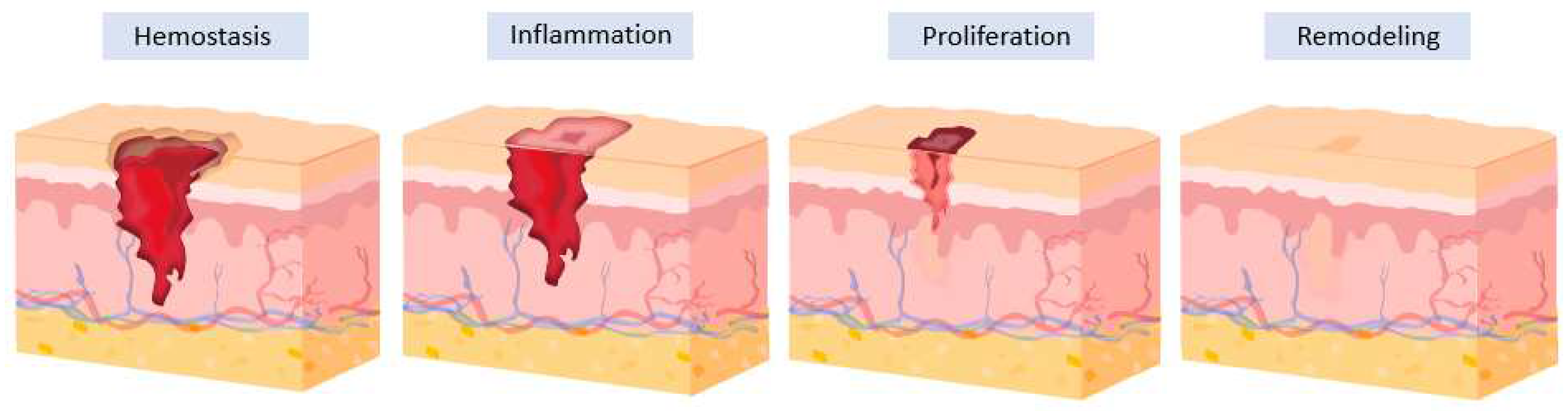
1. Introduction
2. Natural Polysaccharide-Based Composite Hydrogel Dressings (CHDs)
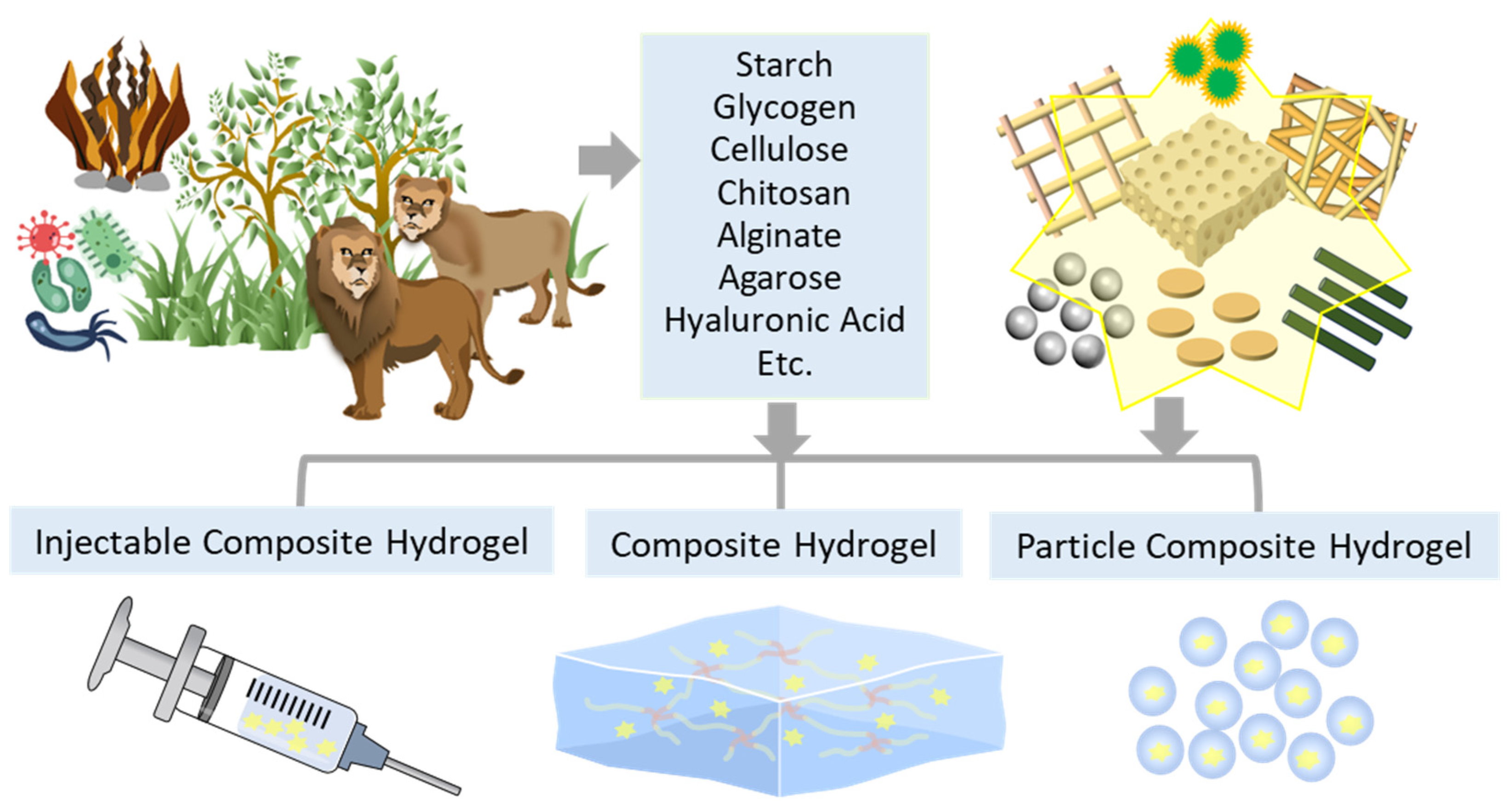
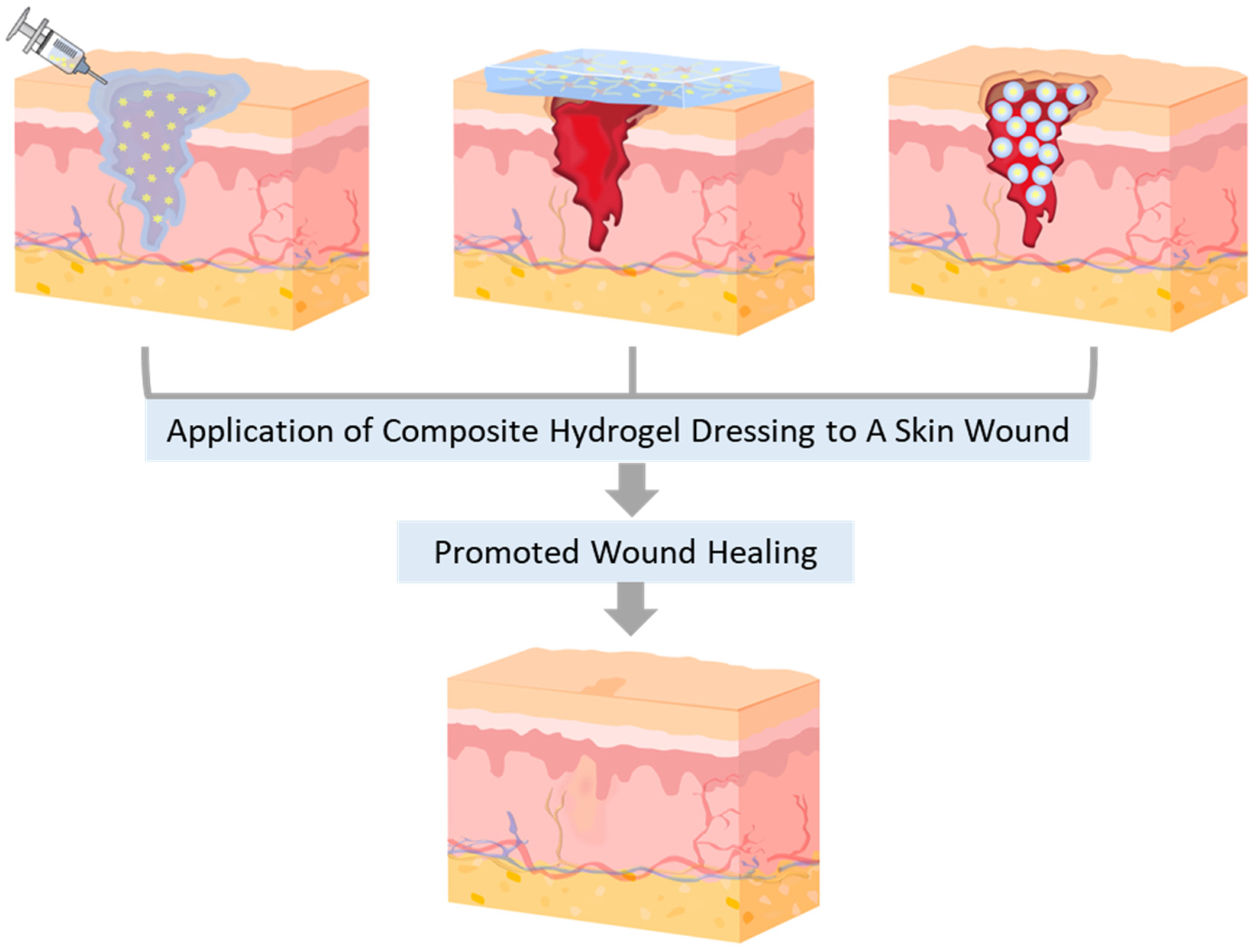
2.1. Starch (St)-based CHDs
2.2. Glycogen (Gly)-based CHDs
2.3. Cellulose (Cel)-based CHDs
2.4. Chitosan (CS)-based CHDs
2.5. Sodium Alginate (SA)-based CHDs
2.6. Agarose (AG)-based CHDs
2.7. Hyaluronic Acid (HA)-based CHDs
3. Conclusions and Challenges
| Name | Components | Features | Applications | Ref. |
|---|---|---|---|---|
| Starch (St) | ||||
| CoSt | Aldehyde-St, DP-conjugated Col(here, Co), CaCO3 | Injectability, self-healing ability, shape adaptability, hemostatic efficiency, strong wet tissue adhesiveness (62 ± 4.8 Kpa), high sealing performance (153.2 ± 35.1 mmHg)., wound healing efficacy. | Emergency wounds, Non-pressing, hemostasis |
[30] |
| Fe3O4@St-IANCH |
IA-modified St, Fe3O4 MNPs (ThA: GFN) |
pH-sensitive and magnetic response, cytocompatibility, controlled GFN release, wound healing efficacy. | General wounds | [40] |
| CMS@CuO |
Sodium CMS, CuO NPs | Solution casting for gel synthesis, biocompatible, antioxidant, and antimicrobial properties, wound healing efficacy. | General wounds | [41] |
| Glycogen (Gly) | ||||
| CG@ZnONP | Gly(here, G), CS(here, C), ZnO NPs, Cotton pads | Nanocomposite, antibacterial properties, high thermal stability and mechanical properties, excellent epithelialization and tissue generation, lower inflammation, flawless wound healing. | General wounds | [46] |
| C/G/H |
Gly(here, G), Col(here, C), HAP NPs (here, H) |
Gly as a crosslinking agent, gelation by Schiff base and electrostatic interactions, desirable mechanical properties for bMSCs to differentiate (Young’s modulus: 10-70 kPa and compressive modulus: 30-432 kPa), great cell adhesiveness. | Bone repair | [52] |
| BC-HCP/ siRNA (or BC-HCP/ siMMP-9) |
BC, four HCP (Gly-DMAPA, Gly-D4, Amyp-DMAPA, Amyp-D4), (ThA: siMMP-9) |
BC-HCPs as gene carriers, antibacterial properties, biocompatibility, wound healing enhanced though the inhibition of MMP-9 by the controlled release of siMMP-9. | Diabetic wounds | [53] |
| Cellulose (Cel) | ||||
| rBC/MXene | rBC, MXene, ECH |
Dual crosslinking (hydrogen bonding/van der Waals interaction and ECH crosslinking), EF-regulated wound healing, high surface roughness, wound healing efficacy. | Skin wounds | [63] |
| MC/TA/Fe |
MC, TA, Fe3+ (ThA: TA) |
Fast gelation, dual crosslinking (coordination/hydrogen bonds in TA/Fe and hydrophobic interactions in MC), pH and temperature sensitive, antibacterial, and antioxidant properties, photothermal and UV-blocking behavior, wound healing efficacy. | General wounds, beauty devices | [64] |
| CMC/HACC | CMCBA, HACC, CuS@C (ThA: Curcumin) |
Injectable, self-healing, EF-responsive, photocatalytic properties, excellent light-induced antibacterial activity, wound healing efficacy. | General wounds | [65] |
| Chitosan (CS) | ||||
| C-CTS/SA-Ag/dECM | CTS(C-CS/SF/TA), SA, Ag NPs, L-DOPA (ThA: dECM) |
Robust wet-tissue adhesiveness (151.40 ± 1.50 kPa), fast multimodal self-healing ability, excellent antibacterial property, higher swelling, hemostatic efficiency, wound healing efficacy. | Massive hemostasis, organ incision, deep wounds | [78] |
| CS/β-GP | CS, H₂O₂-loaded PLA MPs, β-GP (ThA: AM, H2O2) |
Injectability, oxygen-generating performance, hemocompatibility (hemolysis rate: <5%), thermosensitive and antibacterial properties, wound healing efficacy. | General wounds | [79] |
| CEC/PF/ CNT | CEC, PF127, CNT (ThA: Mox) |
Conductive, self-healing, hemostatic, and antibacterial properties, would healing using photothermal therapy. |
Infected wounds, hemostasis | [68] |
| OCEN |
CMC, OCS, EPL-PR, CS@SeNPs, (ThA: ICPs) | Injectable, self-healing, and pH-sensitive properties, shape-adaptivity, excellent adhesiveness, antibacterial activities, biocompatibility, free radical scavenging properties, large absorbance of wound exudate. | Diabetic wounds, hemostasis | [80] |
| Sodium Alginate (SA) | ||||
| BP-SA | SA, BP NSs |
Light-responsive and antibacterial properties, Proper modulus (G’: ~15 kPa), wound healing efficacy. | General wounds | [86] |
| SA-nHA-SiQDs |
SA-SiQDs, nHA NPs, Ca2+ (ThA: ADSCs) |
UME-responsive 3D-printing, laser-activated ROS production, enhanced scaffold stiffness (G’: ~100 kPa), controlled degradation, wound healing efficacy. | Scarless memory repair of urethra | [87] |
| SD-PFD |
SA-DP (SD), PFD NPs (ThA: DOX) |
Injectable and self-healing behaviors, pH sensitiveness, temperature sensitiveness, excellent photothermal and antibacterial properties, adhesiveness, wound healing efficacy. | Melanoma care | [85] |
| SA-COS-ZnO | Oxidized SA, COS, ZnO | Good MVTR, excellent blood compatibility, antibacterial and mechanical properties, wound healing efficacy. | Scald wounds | [84] |
| Agarose (AG) | ||||
| CMA-Ag | CMA (modified AG), Ag+ ions | Crosslinks by ionic interaction, pH and temperature responsiveness, antibacterial properties, biocompatibility, hemocompatibility, wound healing efficacy. | Infected wounds | [93] |
| ATF | AG, TA-Fe NPs | Good tensile strength (ATF-5: 58.5 kPa), superior photothermal sterilization effect, good biocompatibility, antibacterial activity, wound healing efficacy. | Infected wounds | [92] |
| Lignin–AG/SF/ ZnCr2O4 |
Lignin, AG, SF ZnCr2O4 NPs | Self-healing, high swelling (815 ± 14%), enhanced mechanical properties (elastic modulus: 29.51 ± 0.05 MPa and tensile strength: 176.2 ± 1.4 MPa), biocompatibility, antimicrobial, anti-infective, and antioxidant properties, hemocompatibility, fast wound healing time (5 days). | General wounds, tissue engineering | [92] |
| Hyaluronic acid (HA) | ||||
| US@GOx@VEGF (UGV) |
HA, GOx, MnO2 PLGA, PFH, (ThA: VEGF, GOx-MnO2), |
Injectable, self-healing, and sound-responsive properties, real-time monitoring of blood sugar levels, wound healing promoted by controlled VEGF release. | Diabetic wounds | [102] |
| HA-NB/HA-CDH | HA, PLGA-NB (ThA: TGFβ) |
Injectable and adhesive properties, nanobubbles (D: ~220 μm), scarless wound healing. | Diabetic wounds, | [103] |
| FHHA-S/Fe | HHA, Fe3+ | Crosslinking of electrospun HA nanofibers with F3+ ions (D: ~ 60 nm), at higher Fe3+ ions, higher mechanical stability and G’, antibacterial property, wound healing efficacy. | Chronic diabetic wounds | [97] |
| ARTiCAR/ (NanoM1-BMP2) |
SA, HA, PCL (ThA: MSCs) |
A combined wound dressing (PCL electrospun nanofibers and SA/HA hydrogels with MSCs), promoted subchondral bone and cartilage regeneration. | Bone wounds, osteochondral and tendon regeneration | [105] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walters, K. A.; Roberts, M. S. , The structure and function of skin. In Dermatological and transdermal formulations, CRC press: 2002; pp 19-58.
- Farahani, M.; Shafiee, A. , Wound Healing: From Passive to Smart Dressings. Advanced Healthcare Materials 2021, 10, 2100477. [Google Scholar] [CrossRef] [PubMed]
- Tomic-Canic, M.; Burgess, J. L.; O’Neill, K. E.; Strbo, N.; Pastar, I. , Skin Microbiota and its Interplay with Wound Healing. American Journal of Clinical Dermatology 2020, 21, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Min, T.; Bian, X.; Dong, Y.; Zhang, P.; Wen, Y. , Rational Design of Intelligent and Multifunctional Dressing to Promote Acute/Chronic Wound Healing. ACS Applied Bio Materials 2022, 5, 4055–4085. [Google Scholar] [CrossRef]
- Kirsner, R. S.; Eaglstein, W. H. , The Wound Healing Process. Dermatologic Clinics 1993, 11, 629–640. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D. J.; Hager, S.; Hauser, J.; Mirastschijski, U. , Skin Wound Healing: An Update on the Current Knowledge and Concepts. European Surgical Research 2016, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zheng, Y.; Cui, D.; Haick, H. , Multifunctional Dressing for Wound Diagnosis and Rehabilitation. Advanced Healthcare Materials 2021, 10, 2101292. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. , Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Powers, J. G.; Higham, C.; Broussard, K.; Phillips, T. J. , Wound healing and treating wounds: Chronic wound care and management. Journal of the American Academy of Dermatology 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J. R.; Giacopelli, J. A. , A review of wound healing and wound dressing products. The Journal of Foot and Ankle Surgery 1997, 36, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. , Wound dressings: Current advances and future directions. Journal of Applied Polymer Science 2019, 136, 47738. [Google Scholar] [CrossRef]
- Lee, J. H.; Bucknall, D. G. , Swelling behavior and network structure of hydrogels synthesized using controlled UV-initiated free radical polymerization. Journal of Polymer Science Part B: Polymer Physics 2008, 46, 1450–1462. [Google Scholar] [CrossRef]
- Lee, J. H.; Lee, D. S.; Jung, Y. C.; Oh, J.-W.; Na, Y. H. , Development of a Tough, Self-Healing Polyampholyte Terpolymer Hydrogel Patch with Enhanced Skin Adhesion via Tuning the Density and Strength of Ion-Pair Associations. ACS Applied Materials & Interfaces 2021, 13, 8889–8900. [Google Scholar]
- Richbourg, N. R.; Peppas, N. A. , The swollen polymer network hypothesis: Quantitative models of hydrogel swelling, stiffness, and solute transport. Progress in Polymer Science 2020, 105, 101243. [Google Scholar] [CrossRef]
- Barman, S. R.; Chan, S.-W.; Kao, F.-C.; Ho, H.-Y.; Khan, I.; Pal, A.; Huang, C.-C.; Lin, Z.-H. , A self-powered multifunctional dressing for active infection prevention and accelerated wound healing. Science Advances 2023, 9, eadc8758. [Google Scholar] [CrossRef] [PubMed]
- Han, W. J.; Lee, J. H.; Lee, J.-K.; Choi, H. J. , Remote-controllable, tough, ultrastretchable, and magneto-sensitive nanocomposite hydrogels with homogeneous nanoparticle dispersion as biomedical actuators, and their tuned structure, properties, and performances. Composites Part B: Engineering 2022, 236, 109802. [Google Scholar] [CrossRef]
- Lu, G.; Ling, K.; Zhao, P.; Xu, Z.; Deng, C.; Zheng, H.; Huang, J.; Chen, J. , A novel in situ-formed hydrogel wound dressing by the photocross-linking of a chitosan derivative. Wound Repair and Regeneration 2010, 18, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Fan, D. , A Combination Therapy Using Electrical Stimulation and Adaptive, Conductive Hydrogels Loaded with Self-Assembled Nanogels Incorporating Short Interfering RNA Promotes the Repair of Diabetic Chronic Wounds. Advanced Science 2022, 9, 2201425. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, E. E.; Kang, N.; Hamad, M. A.; Wooley, K. L.; Elsabahy, M. , Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nature Communications 2019, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pan, Z.; Zhao, Z.-Y.; Wang, M.-H.; Dong, L.; Gao, H.-L.; Liu, C.-Y.; Zhou, P.; Chen, L.; Shi, C.-J.; Zhang, Z.-Y.; Yang, C.; Yu, S.-H.; Zou, D.-H. , Anti-Swelling, Robust, and Adhesive Extracellular Matrix-Mimicking Hydrogel Used as Intraoral Dressing. Advanced Materials 2022, 34, 2200115. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. , Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. Journal of Colloid and Interface Science 2019, 556, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kim, J. S.; Kim, D. W.; Park, E. S.; Youn, Y. S.; Din, F. u.; Kim, J. O.; Ku, S. K.; Jin, S. G.; Choi, H.-G. , Novel composite double-layered dressing with improved mechanical properties and wound recovery for thermosensitive drug, Lactobacillus brevis. Composites Part B: Engineering 2021, 225, 109276. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Ickowicz, D. E.; Domb, A. J.; Khan, W. , Polysaccharide Biomaterials. Israel Journal of Chemistry 2013, 53, 787–794. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z. , Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]
- Guo, M. Q.; Hu, X.; Wang, C.; Ai, L. , Polysaccharides: structure and solubility. Solubility of polysaccharides 2017, 2, 8–21. [Google Scholar] [CrossRef]
- Delgado, L. L.; Masuelli, M. , Polysaccharides: concepts and classification. Evolution in Polymer Technology Journal 2019, 2. [Google Scholar]
- Mohammed, A. S. A.; Naveed, M.; Jost, N. , Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). Journal of Polymers and the Environment 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C. K. , Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Alipour, R.; Khorshidi, A.; Shojaei, A. F.; Mashayekhi, F.; Moghaddam, M. J. M. , Skin wound healing acceleration by Ag nanoparticles embedded in PVA/PVP/Pectin/Mafenide acetate composite nanofibers. Polymer Testing 2019, 79, 106022. [Google Scholar] [CrossRef]
- Yang, W.; Kang, X.; Gao, X.; Zhuang, Y.; Fan, C.; Shen, H.; Chen, Y.; Dai, J. , Biomimetic Natural Biopolymer-Based Wet-Tissue Adhesive for Tough Adhesion, Seamless Sealed, Emergency/Nonpressing Hemostasis, and Promoted Wound Healing. Advanced Functional Materials 2023, 33, 2211340. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Zhang, Y.; Chen, Y. , The application of natural polymer–based hydrogels in tissue engineering. In Hydrogels based on natural polymers, Elsevier: 2020; pp 273-307.
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. , Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Advanced Healthcare Materials 2019, 8, 1900670. [Google Scholar] [CrossRef] [PubMed]
- Mayet, N.; Choonara, Y. E.; Kumar, P.; Tomar, L. K.; Tyagi, C.; Du Toit, L. C.; Pillay, V. , A Comprehensive Review of Advanced Biopolymeric Wound Healing Systems. Journal of Pharmaceutical Sciences 2014, 103, 2211–2230. [Google Scholar] [CrossRef]
- Ai, Y.; Jane, J.-l. , Chapter 3 - Understanding Starch Structure and Functionality. In Starch in Food (Second Edition), Sjöö, M.; Nilsson, L., Eds. Woodhead Publishing: 2018; pp 151-178.
- Cui, C.; Jia, Y.; Sun, Q.; Yu, M.; Ji, N.; Dai, L.; Wang, Y.; Qin, Y.; Xiong, L.; Sun, Q. , Recent advances in the preparation, characterization, and food application of starch-based hydrogels. Carbohydrate Polymers 2022, 291, 119624. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M. P.; Fernández-Torres, A.; Peponi, L. , Humidity-activated shape memory effect on plasticized starch-based biomaterials. Carbohydrate Polymers 2018, 179, 93–99. [Google Scholar] [CrossRef]
- Garcia, M. A. V. T.; Garcia, C. F.; Faraco, A. A. G. , Pharmaceutical and Biomedical Applications of Native and Modified Starch: A Review. Starch - Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Hamaker, B. R. , Current and future challenges in starch research. Current Opinion in Food Science 2021, 40, 46–50. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M. I. H. , An overview on starch-based sustainable hydrogels: Potential applications and aspects. Journal of Polymers and the Environment 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Nezami, S.; Sadeghi, M.; Mohajerani, H. , A novel pH-sensitive and magnetic starch-based nanocomposite hydrogel as a controlled drug delivery system for wound healing. Polymer Degradation and Stability 2020, 179, 109255. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Zare, E. N.; Salimi, F.; Goudarzi, I.; Tay, F. R.; Makvandi, P. , Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. International Journal of Molecular Sciences 2021, 22, 2531. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M. M.; González-Lucán, M.; Donapetry-García, C.; Fernández-Fernández, C.; Ameneiros-Rodríguez, E. , Glycogen metabolism in humans. BBA clinical 2016, 5, 85–100. [Google Scholar] [CrossRef]
- Wang, H.-D.; Zhong, Z.-H.; Weng, Y.-J.; Wei, Z.-Z.; Zhang, Y.-Q. , Degraded Sericin Significantly Regulates Blood Glucose Levels and Improves Impaired Liver Function in T2D Rats by Reducing Oxidative Stress. Biomolecules 2021, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Vigh-Larsen, J. F.; Ørtenblad, N.; Spriet, L. L.; Overgaard, K.; Mohr, M. , Muscle Glycogen Metabolism and High-Intensity Exercise Performance: A Narrative Review. Sports Medicine 2021, 51, 1855–1874. [Google Scholar] [CrossRef]
- Melendez, R.; Melendez-Hevia, E.; Canela, E. I. , The fractal structure of glycogen: a clever solution to optimize cell metabolism. Biophysical journal 1999, 77, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Sayed, S. M.; Liu, S.; Oderinde, O.; Yao, F.; Fu, G. , Glycogen-based self-healing hydrogels with ultra-stretchable, flexible, and enhanced mechanical properties via sacrificial bond interactions. International Journal of Biological Macromolecules 2018, 117, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Daghlas, S. A.; Mohiuddin, S. S. , Biochemistry, Glycogen. 2019.
- Rich, L. R.; Harris, W.; Brown, A. M. , The role of brain glycogen in supporting physiological function. Frontiers in neuroscience 2019, 13, 1176. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Giri, T. K. , Chapter 2 - Glycogen-based hydrogels. In Polysaccharide Hydrogels for Drug Delivery and Regenerative Medicine, Giri, T. K.; Ghosh, B.; Badwaik, H., Eds. Elsevier: 2024; pp 21-34.
- Tanna, S.; Taylor, M.; Adams, G. , Insulin delivery governed by covalently modified lectin-glycogen gels sensitive to glucose. Journal of pharmacy and pharmacology 1999, 51, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Swielam, E. M.; Atwa, N. A.; Agwa, M. M. , Novel design of bandages using cotton pads, doped with chitosan, glycogen and ZnO nanoparticles, having enhanced antimicrobial and wounds healing effects. International Journal of Biological Macromolecules 2022, 197, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, J.; Ying, H.; Zhou, Y.; Lai, J.; Chen, J. , Glycogen as a crosslinking agent of collagen and nanohydroxyapatite to form hydrogels for bmsc differentiation. ACS Sustainable Chemistry & Engineering 2020, 8, 2106–2114. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Pan, C.; Saw, P. E.; Ren, M.; Lan, B.; Wu, J.; Wang, X.; Zeng, T.; Zhou, L. , Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta biomaterialia 2020, 102, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. , Multifunctional cellulose-based hydrogels for biomedical applications. Journal of Materials Chemistry B 2019, 7, 1541–1562. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, Q.; Jing, S.; Li, C.; Chen, Q.; Chen, B.; Pang, Z.; Jiang, B.; Gan, W.; Chen, G. , Strong, hydrostable, and degradable straws based on cellulose-lignin reinforced composites. Small 2021, 17, 2008011. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. , Mechanical and water-resistant properties of eco-friendly chitosan membrane reinforced with cellulose nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Zhao, L.; Yazdkhasti, A.; Mao, Y.; Siciliano, A. P.; Dai, J.; Jing, S.; Xie, H.; Li, Z. , A scalable high-porosity wood for sound absorption and thermal insulation. Nature Sustainability 2023, 6, 306–315. [Google Scholar] [CrossRef]
- Ribeiro, A. M.; Magalhães, M.; Veiga, F.; Figueiras, A. , Cellulose-Based Hydrogels in Topical Drug Delivery: A Challenge in Medical Devices. In Cellulose-Based Superabsorbent Hydrogels, Mondal, M. I. H., Ed. Springer International Publishing: Cham, 2018; pp 1-29.
- Chen, C.; Xi, Y.; Weng, Y. , Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Leal, C. R.; Almeida, P. L.; Sobral, R. G. , Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microbial biotechnology 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Farshi, P.; Salarian, R.; Rabiee, M.; Alizadeh, S.; Gholipourmalekabadi, M.; Ahmadi, S.; Rabiee, N. , Design, preparation, and characterization of silk fibroin/carboxymethyl cellulose wound dressing for skin tissue regeneration applications. Polymer Engineering & Science 2022, 62, 2741–2749. [Google Scholar] [CrossRef]
- Sharif, F.; Muhammad, N.; Zafar, T. , Cellulose based biomaterials: Benefits and challenges. Biofibers and Biopolymers for Biocomposites: Synthesis, Characterization and Properties 2020, 229-246.
- Mao, L.; Hu, S.; Gao, Y.; Wang, L.; Zhao, W.; Fu, L.; Cheng, H.; Xia, L.; Xie, S.; Ye, W.; Shi, Z.; Yang, G. , Biodegradable and Electroactive Regenerated Bacterial Cellulose/MXene (Ti3C2Tx) Composite Hydrogel as Wound Dressing for Accelerating Skin Wound Healing under Electrical Stimulation. Advanced Healthcare Materials 2020, 9, 2000872. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y. H.; Chathuranga, K.; Lee, J. S.; Koo, J.; Park, W. H. , Multifunctional and thermoresponsive methylcellulose composite hydrogels with photothermal effect. Carbohydrate Polymers 2022, 277, 118834. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, H.; Chen, W.; Han, P.; Yao, X.; Tang, B.; Duan, W.; Li, P.; Wei, X.; Chu, P. K.; Zhang, X. , An injectable, self-healing composite hydrogel with enhanced near-infrared photo-antibacterial therapeutic effects for accelerated wound healing. Chemical Engineering Journal 2023, 452, 139474. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. , Chitosan-based biomaterials for tissue engineering. European Polymer Journal 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P. X.; Guo, B. , Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chemical Engineering Journal 2019, 362, 548–560. [Google Scholar] [CrossRef]
- He, J.; Shi, M.; Liang, Y.; Guo, B. , Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chemical Engineering Journal 2020, 394, 124888. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P. X.; Guo, B. , Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. , A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydrate Polymers 2018, 180, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Y.; Zhao, L.; Feng, Z.; Peng, K.; Wei, A.; Wang, Y.; Tong, Z.; Cheng, B. , Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Composites Part B: Engineering 2020, 197, 108139. [Google Scholar] [CrossRef]
- Yang, E.; Hou, W.; Liu, K.; Yang, H.; Wei, W.; Kang, H.; Dai, H. , A multifunctional chitosan hydrogel dressing for liver hemostasis and infected wound healing. Carbohydrate Polymers 2022, 291, 119631. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dong, Y.; Wang, M.; Lu, X.; Li, X.; Yu, J.; Ding, B. , Hydrogel/nanofibrous membrane composites with enhanced water retention, stretchability and self-healing capability for wound healing. Composites Part B: Engineering 2023, 257, 110672. [Google Scholar] [CrossRef]
- Ibrahim, H.; El-Zairy, E. , Chitosan as a biomaterial—structure, properties, and electrospun nanofibers. Concepts, compounds and the alternatives of antibacterials 2015, 1, 81–101. [Google Scholar] [CrossRef]
- Teng, L.; Shao, Z.; Bai, Q.; Zhang, X.; He, Y.-S.; Lu, J.; Zou, D.; Feng, C.; Dong, C.-M. , Biomimetic Glycopolypeptide Hydrogels with Tunable Adhesion and Microporous Structure for Fast Hemostasis and Highly Efficient Wound Healing. Advanced Functional Materials 2021, 31, 2105628. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Cao, Y.; Guo, Z.; Li, F.; Wang, L. , Construction of Chitosan-Based Hydrogel Incorporated with Antimonene Nanosheets for Rapid Capture and Elimination of Bacteria. Advanced Functional Materials 2020, 30, 2003196. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Li, X.; Lin, Z.; Chen, L.; Chen, H.; Jin, Y.; Zhang, T.; Xia, H.; Lu, Y.; Zhang, Y. , Ultrafast in-situ forming halloysite nanotube-doped chitosan/oxidized dextran hydrogels for hemostasis and wound repair. Carbohydrate Polymers 2021, 267, 118155. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Li, F.; He, S.; Li, W.; Wu, Q.; He, J.; Ruan, R.; Xiao, Z.; Zhang, J.; Yang, H. , Mussel- and Barnacle Cement Proteins-Inspired Dual-Bionic Bioadhesive with Repeatable Wet-Tissue Adhesion, Multimodal Self-Healing, and Antibacterial Capability for Nonpressing Hemostasis and Promoted Wound Healing. Advanced Functional Materials 2022, 32, 2200908. [Google Scholar] [CrossRef]
- Dadkhah Tehrani, F.; Shabani, I.; Shabani, A. , A hybrid oxygen-generating wound dressing based on chitosan thermosensitive hydrogel and decellularized amniotic membrane. Carbohydrate Polymers 2022, 281, 119020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yuan, W. , Self-healing and shape-adaptive nanocomposite hydrogels with anti-inflammatory, antioxidant, antibacterial activities and hemostasis for real-time visual regeneration of diabetic wounds. Composites Part B: Engineering 2023, 262, 110819. [Google Scholar] [CrossRef]
- Keykhaee, M.; Rahimifard, M.; Najafi, A.; Baeeri, M.; Abdollahi, M.; Mottaghitalab, F.; Farokhi, M.; Khoobi, M. , Alginate/gum arabic-based biomimetic hydrogel enriched with immobilized nerve growth factor and carnosine improves diabetic wound regeneration. Carbohydrate Polymers 2023, 321, 121179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, D.; Zhao, W.; Zhang, R.; Yu, B.; Ma, G.; Li, Y.; Hao, D.; Xu, F.-J. , Engineering Platelet-Rich Plasma Based Dual-Network Hydrogel as a Bioactive Wound Dressing with Potential Clinical Translational Value. Advanced Functional Materials 2021, 31, 2009258. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. , Ions-induced gelation of alginate: Mechanisms and applications. International Journal of Biological Macromolecules 2021, 177, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. , Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydrate Polymers 2021, 266, 118100. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. , NIR- and pH-responsive injectable nanocomposite alginate-graft-dopamine hydrogel for melanoma suppression and wound repair. Carbohydrate Polymers 2023, 314, 120899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xie, J.; Xing, J.; Li, C.; Wong, T. M.; Yu, H.; Li, Y.; Yang, F.; Tian, Y.; Zhang, H.; Li, W.; Ning, C.; Wang, X.; Yu, P. , Light-Programmable Nanocomposite Hydrogel for State-Switchable Wound Healing Promotion and Bacterial Infection Elimination. Advanced Healthcare Materials 2023, 12, 2201565. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Y.; Fang, C.; Song, L.; Wang, Y.; Lu, L.; Yang, R.; Bu, Z.; Liang, X.; Zhang, K.; Fu, Q. , Urine-Microenvironment-Initiated Composite Hydrogel Patch Reconfiguration Propels Scarless Memory Repair and Reinvigoration of the Urethra. Advanced Materials 2022, 34, 2109522. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. , Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Comprehensive Reviews in Food Science and Food Safety 2019, 18, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Indovina, P.; Tettamanti, E.; Micciancio-Giammarinaro, M.; Palma, M. , Thermal hysteresis and reversibility of gel–sol transition in agarose–water systemsa. The Journal of Chemical Physics 1979, 70, 2841–2847. [Google Scholar] [CrossRef]
- Fernández, E.; López, D.; Mijangos, C.; Duskova-Smrckova, M.; Ilavsky, M.; Dusek, K. , Rheological and thermal properties of agarose aqueous solutions and hydrogels. Journal of Polymer Science Part B: Polymer Physics 2008, 46, 322–328. [Google Scholar] [CrossRef]
- Kim, J. H.; Yun, E. J.; Seo, N.; Yu, S.; Kim, D. H.; Cho, K. M.; An, H. J.; Kim, J.-H.; Choi, I.-G.; Kim, K. H. , Enzymatic liquefaction of agarose above the sol–gel transition temperature using a thermostable endo-type β-agarase, Aga16B. Applied microbiology and biotechnology 2017, 101, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yu, Z.; Chen, S.; Fei, L.; Sha, Q.; Zhou, N.; Chen, Z.; Xu, C. , Facile and eco-friendly fabrication of polysaccharides-based nanocomposite hydrogel for photothermal treatment of wound infection. Carbohydrate Polymers 2020, 230, 115565. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Ying, R.; Wang, W.; Guo, Y.; He, Y.; Mo, X.; Xue, C.; Mao, X. , A Macroporous Hydrogel Dressing with Enhanced Antibacterial and Anti-Inflammatory Capabilities for Accelerated Wound Healing. Advanced Functional Materials 2020, 30, 2000644. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Aliabadi, H. A. M.; Radinekiyan, F.; Sobhani, M.; Maleki, A.; Madanchi, H.; Mahdavi, M.; Shalan, A. E. , Investigation of the biological activity, mechanical properties and wound healing application of a novel scaffold based on lignin–agarose hydrogel and silk fibroin embedded zinc chromite nanoparticles. RSC advances 2021, 11, 17914–17923. [Google Scholar] [CrossRef]
- Meyer, K. , Biochemistry and biology of mucopolysaccharides. The American Journal of Medicine 1969, 47, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. , Hyaluronic acid (hyaluronan): a review. Veterinarni medicina 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Yu, J.; Shao, N.; Lu, H.; Guo, J.; Qiu, X.; Zhou, D.; Huang, Y. , Absorbable Thioether Grafted Hyaluronic Acid Nanofibrous Hydrogel for Synergistic Modulation of Inflammation Microenvironment to Accelerate Chronic Diabetic Wound Healing. Advanced Healthcare Materials 2020, 9, 2000198. [Google Scholar] [CrossRef] [PubMed]
- Collins, M. N. , Hyaluronic acid for biomedical and pharmaceutical applications. Smithers Rapra: 2014.
- Bukhari, S. N. A.; Roswandi, N. L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N. A.; Thu, H. E.; Hussain, Z. , Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. International Journal of Biological Macromolecules 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Graça, M. F. P.; Miguel, S. P.; Cabral, C. S. D.; Correia, I. J. , Hyaluronic acid—Based wound dressings: A review. Carbohydrate Polymers 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, F.; Longobardo, G.; Fabozzi, A.; di Gennaro, M.; Borzacchiello, A. , Hyaluronic Acid-Based Wound Dressing with Antimicrobial Properties for Wound Healing Application. Applied Sciences 2022, 12, 3091. [Google Scholar] [CrossRef]
- Han, X.; Chen, S.; Cai, Z.; Zhu, Y.; Yi, W.; Guan, M.; Liao, B.; Zhang, Y.; Shen, J.; Cui, W.; Bai, D. , A Diagnostic and Therapeutic Hydrogel to Promote Vascularization via Blood Sugar Reduction for Wound Healing. Advanced Functional Materials 2023, 33, 2213008. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Lee, J.; Hua, J.; Li, S.; Panchamukhi, A.; Yue, J.; Gou, X.; Xia, Z.; Zhu, L.; Wu, X. , A pulsatile release platform based on photo-induced imine-crosslinking hydrogel promotes scarless wound healing. Nature Communications 2021, 12, 1670. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R. J.; Hata, A. , Targeting the TGFβ signalling pathway in disease. Nature Reviews Drug Discovery 2012, 11, 790–811. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Pijnenburg, L.; Idoux-Gillet, Y.; Bornert, F.; Benameur, L.; Tabrizian, M.; Auvray, P.; Rosset, P.; María Gonzalo-Daganzo, R.; Gómez Barrena, E.; Gentile, L.; Benkirane-Jessel, N. , Preclinical safety study of a combined therapeutic bone wound dressing for osteoarticular regeneration. Nature Communications 2019, 10, 2156. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





