1. Introduction
Free radicals are highly reactive molecular species with several detrimental effects on the living being. Reactive oxygen species (ROS) such as hydroxyl radicals (
•OH), superoxide anions (O
2•-), singlet oxygen (
1O
2), and hydrogen peroxide (H
2O
2) are the most common subtypes of free radicals that are consistently generated in the living organisms via various metabolic processes and countered by endogenous enzymatic and non-enzymatic antioxidant defense system to maintain cellular homeostasis [
1]. However, several internal and external factors such as age, disease, pollution, smoking, alcohol, radiation, and consumption of certain drugs (like cyclosporine, bleomycin, and gentamycin) are the causative agents for the excessive cellular production of reactive oxygen species (ROS) that is beyond the threshold limit of the endogenous antioxidant’s leads to oxidative stress [
1]. ROS-induced oxidative stress has destructive ramifications on all classes of biomolecules, mainly lipids, proteins, and DNA [
2], and has been accredited as a direct or indirect reason behind more than 100 types of human ailments [
3,
4]. The intimate involvement of oxidative stress to stimulate different kinases incurring pathways and transcription factors like nuclear factor kappa B (NFκB) and activator protein 1 (AP-1) suggests the deep impact of oxidative stress on the induction of inflammation and inflammation-related disorders [
5,
6].
Several clinical and preclinical studies implemented oxidative stress and its induced effects as a major culprit for the onset of several neurological, kidney, and respiratory disorders [
1]. A notable effect of oxidative stress is well-established for hypertension, ischemia, atherosclerosis, cardiomyopathy, cardiac hypertrophy, and other cardiovascular diseases. Oxidative stress is also established to impose significant impact on circulating low-density lipoproteins (LDL), leading to LDL oxidation (oxLDL) [
1]. The oxLDL are easily recognized/probed by scavenger receptors on macrophages spurs foam cell generation leads to lipid accumulation and atherosclerotic plaque formation [
7]. Interestingly, high-density lipoproteins (HDL) inhibit LDL oxidation and have a great impact on atherosclerosis. HDL, owing to its antioxidant activity attributed by enzymes such as paraoxonase 1 (PON-1), lecithin-cholesterol acyltransferase (LACT), and platelet-activating factor acetylhydrolase (PAF-AH) inhibit and prevent the LDL oxidation [
8] and the consequent impact on atherosclerosis. Nevertheless, the lipid and protein constituents within HDL is also susceptible to oxidative alterations [
9], impairing HDL functionality and thereby increasing the risk of atherosclerosis and various other diseases.
Knowing the numerous detrimental effects of oxidative stress, its management is imperative, which can be accomplished by the supplementation of external antioxidants that work indigenously or with a conjunction of endogenous antioxidant system for the effective elimination of surplus ROS. Several synthetic and natural compounds of plant and animal origin have been documented for their potential antioxidant activities [
10,
11] using different
in vitro and
in vivo assays. However, a limited number of them have undergone human testing to demonstrate their effectiveness as antioxidants for human consumption.
Beeswax alcohol (BWA) is a blend of six linear aliphatic alcohols (of carbon chain C24 to C34) extracted from beeswax [
12]. BWA is well recognized for its diverse bio-functionality, including antioxidant, anti-platelet, and anti-inflammatory activity, by inhibiting cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) [
12,
13]. However, the most noteworthy role of BWA was established as a gastroprotective agent. Several pharmacological and clinical studies confirmed the impact of BWA on the quantity and quality of gastric mucous [
14,
15]. Recently, the Korean Food and Drug Administration (KFDA) approved BWA as a functional food ingredient for improving joint and gastrointestinal health [
12]. Despite the several health-beneficial effects, BWA has not been tested with respect to its impact on paraoxonase -1 (PON-1) activity and preventive agent against oxidative damage of LDL and HDL.
Keeping these into consideration, the present study aimed to evaluate the effect of BWA on HDL-associated PON-1 activity and the prevention of LDL and HDL oxidative damage. Additionally, the protective effect of BWA was tested in zebrafish embryos against carboxymethyllysine (CML) induced stress. Finally, BWA (100 mg/day) was processed for the preliminary clinical trials for assessing the effect of BWA on oxidative variables, anthropometric profile, liver and kidney function biomarkers, and lipid profile of middle-aged and older human subjects of both genders.
2. Materials and Methods
2.1. Materials
Beeswax alcohol (BWA) was complementary provided by Raydel Austrailia, Pty, Ltd. (Thornleigh, NSW, Australia). The BWA was specified with six high molecular weight alcohols [i.e., triacontanol (C
30H
62O) (25-35%), dotriacontanol (C
32H
66O) (18-25%), octacosanol (C
28H
58O) (12-20%), hexacosanol (C
26H
54O) (1-20%), tetracosanol (C
24H
50O) (6-15%) and tetratriacontanol (C
34H
70O) (≤7.5%)] extracted from beeswax (
Apis mellifera L.) [
12]. All other chemicals else otherwise stated were of the highest purity and used as supplied. DPPH (Cat. No. 9286), paraoxon-ethyl (Cat. No. 9286), and phosphotungstic acid (Cat. No. 9286),
N-ε-carboxymethyllysine (Cat No. 14580-5g), coenzyme (Q
10 CoQ
10) (Cat. No. C9538-100mg), dihydroethidium (Cat. No. 37291) and acridine orange (Cat. No.A9231) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. DPPH Free Radical Scavenging Activity
The antioxidant activity of BWA and CoQ
10 was determined by DPPH free radical assay as an earlier adopted method [
16]. In brief, 950 μL DPPH solution (24 μg/mL in methanol) was mixed with 50 μL of BWA or CoQ
10 to obtain the final concentration of 0.125, 0.25, 0.5, 2.5, and 5 μM. Simultaneously, DPPH solution devoid of BWA and CoQ
10 was prepared that served as control. DPPH radical scavenging activity was measured after 120 min incubation at 25
oC by taking absorbance at 517 nm using a spectrophotometer (UV-2600i-Shimadzu, Kyoto, Japan).
2.3. Isolation of LDL, and HDL
LDL, and HDL from human blood were isolated using density gradient centrifugation as the previously described method [
17,
18]. For the isolation of LDL (1.019<d<1.063), and HDL (1.063<d<1.225) density of the gradient was adjusted by NaCl and NaBr using the standard method [
19], and the content was centrifuged at 100,000×
g. After 24 hr centrifugation, LDL, and HDL were recovered from the respective density fractions and dialyzed overnight against Tris buffered saline (pH 8.0) to remove the trace of NaBr. The dialyzed LDL, and HDL were preserved in the refrigerator for further use.
2.4. Paraoxonase Assay
Paraoxonase-1 (PON-1) activity was determined using HDL following the previously described method [
20,
21]. In brief, HDL (2 mg/mL, 20 μL) was mixed individually with varying concentrations (0.01-2.5 μM) of BWA or CoQ
10. Subsequently, the substrate paraoxon-ethyl (550 mM) was added to the reaction mixture, and the content was incubated at 25
oC. After 215 min incubation, an absorbance of 415 nm was recorded to quantify
p-nitrophenol production, a hydrolysis product of paraoxon-ethyl catalyzed by PON-1. The PON-1 activity is expressed as μU/L/min and μU is defined as the production of 1 μmol/L
p-nitrophenol by unit volume of the enzyme at the unit time considering 17×10
3 M
-1cm
-1 molar absorption coefficient for
p-nitrophenol.
2.5. Oxidation of LDL, Electrophoresis, and Quantification of LDL Oxidized Products
The CuSO
4-induced oxidation of LDL and the effect of BWA and CoQ
10 on LDL oxidation was determined by the previously described method [
22,
23]. In brief, LDL (final 1 mg/mL, 75 μL) was mixed with CuSO
4 (final 0.5 μM, 7.5 μL) with the subsequent addition of BWA or CoQ
10 to achieve a final concentration of 10, 20 and 30 μM. After 30 min incubation at 37
oC, the content was electrophoresed on 0.5% agarose gel (non-denaturing conditions) using Tris-EDTA running buffer (pH 8.0) at a constant electric supply of 50 V. After about an hour, electrophoresis was terminated, and the separated proteins LDL/apo-B were visualized by Coomassie blue staining (final 1.25%). The degree of oxidation in LDL was quantified by thiobarbituric acid reactive substance (TBARS) assay using malondialdehyde (MDA) as the standard following the earlier described method [
17,
24].
2.6. Electron Microscopic Examination of LDL
Transmission electron microscopic (TEM) examination of LDL and oxidized LDL (oxLDL) treated with BWA (10-30 μM) was performed as the previously described method [
17] with slight modification. In brief, an equal amount (5 μL) of LDL suspension, which was stained with 2% phosphotungstate acid (pH 7.4), was placed over carbon-coated 200 mesh copper grids. After 2 min, the excess content was blotted, and the LDL-attached copper grid was incubated for 2 hr at 50
oC. Finally, LDL particle morphology was observed under TEM (Hitachi H-7800; Ibaraki, Japan) at 80 kV acceleration voltage.
2.7. Exposure of HDL with Ferrous Ions
The effect of BWA to prevent the ferrous ion (Fe
2+) mediate oxidative damage of HDL was examined by SDS-polyacrylamide gel electrophoresis (PAGE) as the previously described method [
25]. In brief, HDL (2 mg/mL) was mixed with ferrous sulfate (FeSO
4)(60 μM final), and subsequently, different amounts of BWA (0, 10, 20, and 30 μM) were added. The content was incubated at 37
oC for 72 hr in the presence of 5% CO
2 following 15% PAGE. The separated bands were stained with commissive brilliant blue (final 1.25%) to document the stability of apoA-I in HDL. The ferrous ion-mediated damage was examined by quantifying the band intensity using Image J software (
http://rsb.info.nih.gov/ij, 1.53r version, accessed on Jan 16, 2023). Further the morphological analysis of HDL was examined using TEM analysis following the methodology outlined in section 2.6.
2.8. Microinjection of Zebrafish Embryos
Zebrafish embryos were produced as the previously described method [
26]. In brief, zebrafish (of both sexes) were segregated from each other for 16 h, followed by coupling of male and female (2:1) in the breeding tank to produce embryos. The produced embryos (1.5 hr post fertilization) were randomly divided into four groups (each group n=150). In Group I and II, Embryos were microinjected with 10 nL PBS (vehicle) and 10 nL PBS containing 500 ng CML. The embryos in Group III and IV were microinjected with 500 ng CML together with 10 nL of CoQ
10 (final 15 ng) or BWA (final 15 ng), respectively. Embryos were constantly monitored under the microscope (Motic SMZ 168; Hong Kong) to examine the developmental deformities and survivability, and the images were captured at 5 hr and 24 hr post treatment.
2.9. Reactive Oxygen Species (ROS) and Apoptosis in Zebrafish Embryos
Reactive oxygen species (ROS) in embryos were evaluated by DHE fluorescent staining as a previously described method [
26]. In brief, zebrafish embryos (n=20) at 5 hr post-treatment were stained with DHE (30 μM). After 30 min incubation in the dark, embryos were rinsed twice with 1×PBS and visualized under a fluorescent microscope (Nikon Eclipse TE2000, Tokyo, Japan) at the excitation and emission wavelength of 588 nm and 605 nm, respectively. The apoptosis in the embryos was examined by AO fluorescent staining [
26]. At 5 hr post-treatment, zebrafish embryos (n=20) from different groups were suspended in 500 μL of AO (5 μg/mL). After 1 h staining, embryos were rinsed two times with 1×PBS and visualized under a fluorescent microscope (Nikon Eclipse TE2000, Tokyo, Japan) at the excitation and emission wavelength of 502 nm and 525 nm, respectively. Image J software was employed for quantifying the fluorescently stained area (
http://rsb.info.nih.gov/ij, 1.53r version, accessed on Jan 16, 2023).
2.10. Clinical Study
2.10.1. Participants
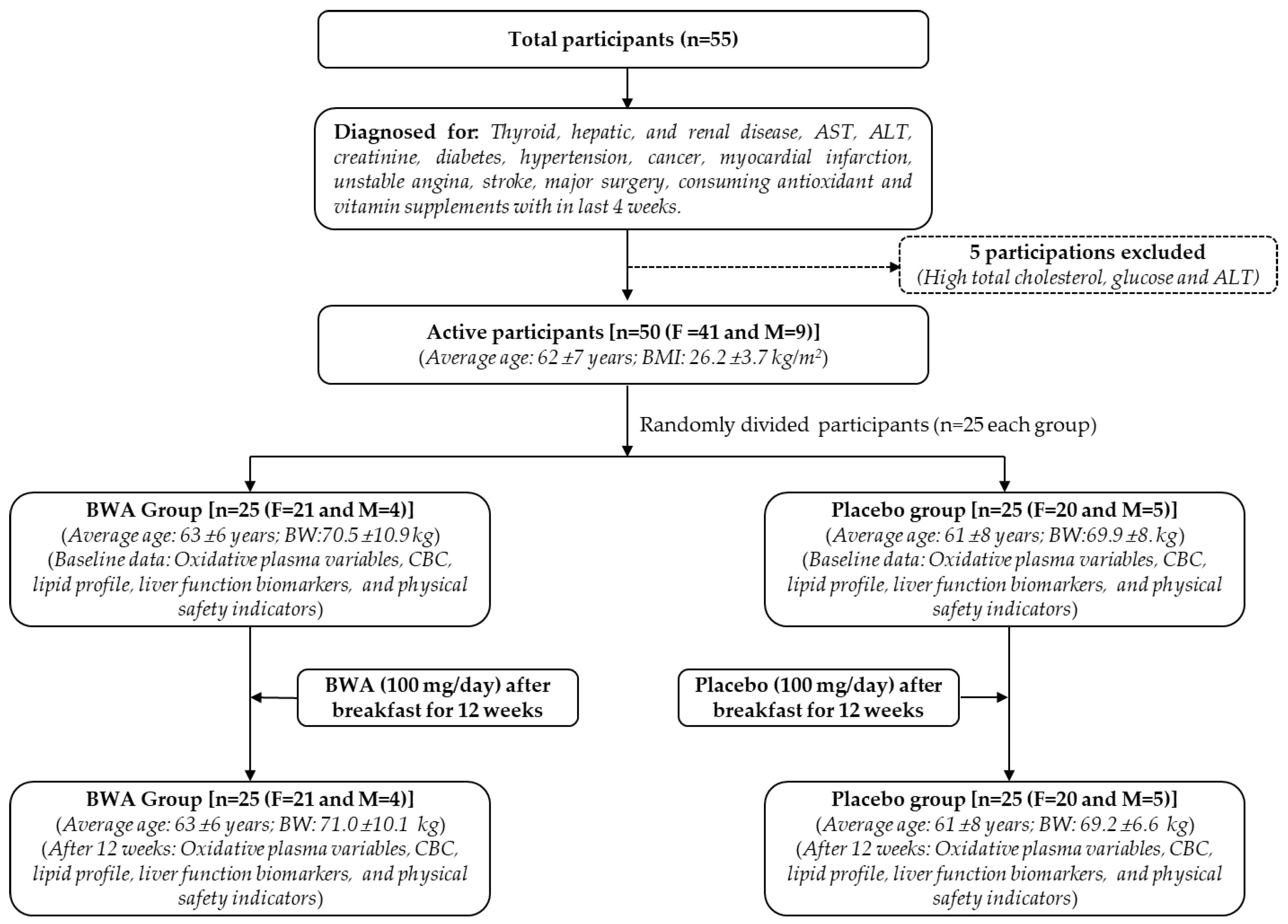
The enrolled human subjects were of both sexes, aged between 40 to 75 years, and recruited voluntarily after signing the informed content. All the participants were in healthy state per their medical history, anthropometric examination, and laboratory tests. Subjects diagnosed with thyroid, hepatic, and renal diseases, diabetes (fasting glucose >7 mmol/L), hypertension (diastolic pressure ≥ 100 mmHg), cancer, and following values for the blood indicators: alanine aminotransferase (ALT) or aspartate aminotransferase (ALT) >55 UI, creatinine >130 μmol/L, or if they had suffered myocardial infarction, unstable angina, stroke, ischemic transient attacks, major surgery or disease-related hospitalization within the six months prior to the study were excluded. Subjects that were consuming antioxidant or vitamin supplements within one month prior to the study were also excluded.
2.10.2. Study Layout
The study was conducted in a randomized, double-blind, placebo-controlled manner in accordance with the declaration of Helsinki, as well as the recommendation of the World Health Organization and the Cuban regulations on good clinical practice. The study protocol was approved by the independent Clinical Research Ethics Committee of the Surgical Medical Research Center (IRB approval number. 04-19), Havana, Cuba, and registered in the Public Registry of Clinical Trials of Cuba (RPCEC00000401). In total, 55 participants were registered, with 50 included in the active treatment phase. Five subjects were not included due to high baseline values for cholesterol (1 subject), glucose >7 mmol/L (3 subjects), and ALT >55 UI (1 subject). The 50 participants (both male and female) were randomly divided into two groups (n=25 in each group) and started to consume two 50 mg tablets of BWA (Raydel
®)/day (i.e., 100 mg) or placebo up to 12 weeks as documented in
Figure 1.
2.10.3. Anthropometric and Blood Analysis
The participant's body weight, body mass index (BMI), and pulse rate in both groups were analyzed at the beginning (baseline) and after 12 weeks of BWA or placebo consumption. Similarly, the blood was collected from the participants of both groups after overnight fasting in the EDTA rinsed tubes. Blood samples were centrifuged at 3000×g for 15 min at 4oC to obtain plasma. Hematological indicators (hemoglobin, haematocit, red blood count, white blood cell count, platelet count) and blood biochemistry indicators (ALT, AST, glucose, creatinine, total cholesterol, and triglycerides) were assessed with enzymatic routine methods using reagent kits (Roche, Switzerland) in the Hitachi 719 autoanalyzer (Tokyo, Japan) of Surgical Medical Research Center (Havana, Cuba).
2.10.4. Oxidative Variables
In both the groups (BWA or placebo) at baseline and after 12 weeks, lipid peroxides were assessed through the quantification of plasma MDA using the colorimetric method [
27]. Plasma (0.5 mL) was mixed with sodium dodecyl sulfate (SDS) (final 8.1%, 0.2 mL), 3.5 M sodium acetate buffer (pH 4.0, 1.5 mL), and an aqueous solution of thiobarbituric acid (TBA) (final 0.8%, 1.5 mL). Finally, the volume was adjusted to 4 mL by distilled water followed by 45 min heating at 95
oC. After cooling content was centrifuged at 1500×
g for 10 min, and an absorbance of 532 nm was recorded. The concentration of thiobarbituric acid reactive substance (TBARS) was expressed as MDA equivalent/mL using a standard curve of MDA.
Total plasma hydroperoxides level (TOH) was determined by ferrous oxidation in xylenol orange (FOX) assay as earlier described method [
28]. In brief, 0.1 mL plasma was mixed with 0.9 mL of FOX reagent and incubated at 37
oC for 30 min. The absorbance of 560 nm was measured, and TOH was quantified using a standard curve of cumene hydroperoxide. The total antioxidant capacity of plasma was evaluated by a colorimetric kit (Randox, NX22332, Crumlin, UK) that incubated 2,2´-Azido-di-(3-ethylbenzthiazoline sulphonate) (ABTS) with a peroxidase (metmyoglobin) and hydrogen peroxide (H
2O
2) which produces a blue-green radical cation (ABTS
+) detected at 600 nm.
2.11. Statistical Analysis
The outcomes are illustrated as mean ± SD for all the experiments conducted in triplicates. The pairwise statistical differences between the groups were determined through One-way Analysis of Variance (ANOVA) followed by Tukey's multiple range test, utilizing SPSS software (version 29.0; SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
3.1. Antioxidant Activity of BWA
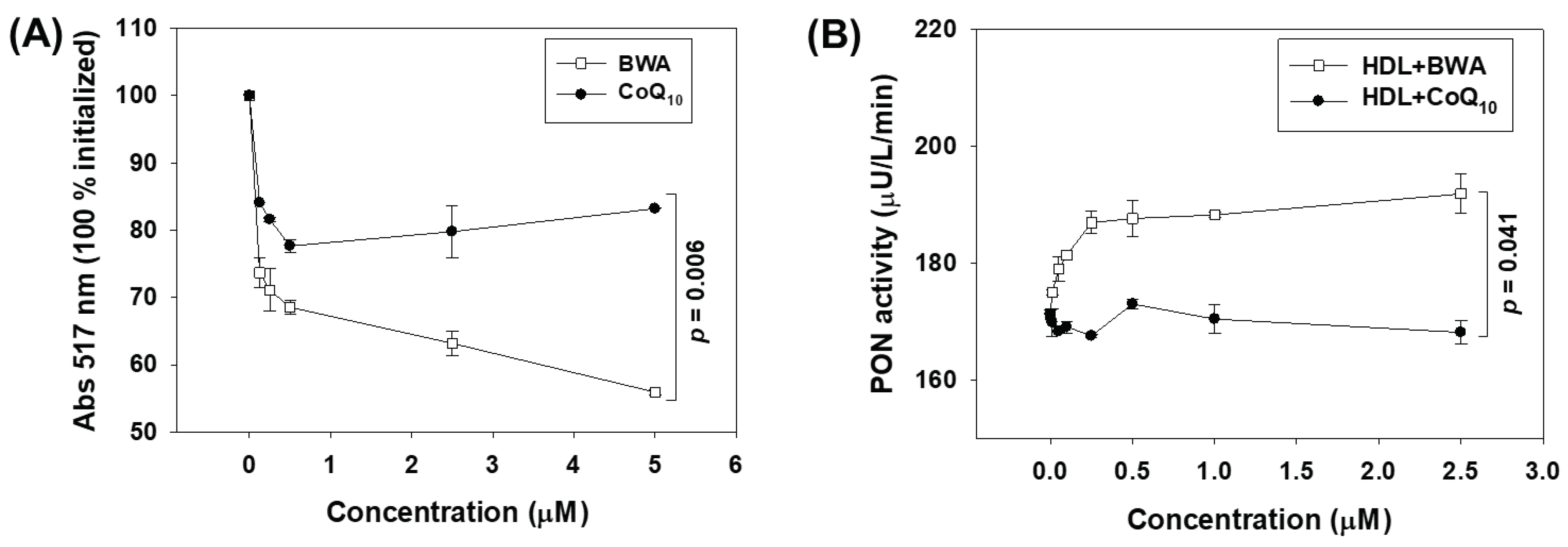
The antioxidant activity of BWA was first assessed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay. As depicted in
Figure 2A, a 28.8%, 31.4%, 36.8%, and 44.2% reduction of DPPH free radicals was observed at 0.25, 0.5, 2.5, and 5 μM BWA concentrations, respectively, which is significantly better than the activity of CoQ
10 at the respective concentrations. Precisely at 5 μM concentration, BWA displayed a significantly 2.7-fold higher (
p=0.006) reduction of DPPH free radicals than that of the CoQ
10 at the same concentration
. These results indicate that BWA can scavenge the free radicals and have an antioxidant activity. The results are consistent with the earlier study [
29] documenting the DPPH radical scavenging activity of octacosanol, an essential BWA component. Unlike [
29], we observed a slightly better activity that can be justified by the presence of different aliphatic alcohols in BWA that synergistically work compared to the octacosanol. Additionally [
30], a positive correlation of policosanol content in milk thistle was established for DPPH and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity, corroborating the present findings. Also, the ability of BWA to scavenge endogenous hydroxyl radicals in gastric mucosa supports the BWA antioxidant nature [
31].
Further, the effect of BWA was evaluated on the activity of PON-1, which is an important antioxidant enzyme associated with HDL [
32,
33]. Therefore, any substance that can enhance PON-1 activity has a direct impact on the functionality of HDL. Knowing this, BWA was tested for its impact on PON-1 activity, and the outcomes were compared with the influence of CoQ
10 (an endogenous antioxidant of humans and other animals) on the PON-1 activity. The current results demonstrate a noteworthy enhancement of PON-1 activity in the presence of BWA (
Figure 2A). A 163.2 μU/L/min PON-1 activity was noticed in the control (without BWA or CoQ
10) that enhanced in the presence of BWA (0.01-2.5 μM) and attained a maximum of 194.2 μU/L/min at 2.5 μM BWA concentration, representing 19% activity enhancement. The PON-1 activity enhanced linearly by increasing BWA concentration from 0 to 0.5 μM and, after that, remained almost constant with the further enhancement of the BWA concentration. While compared to CoQ
10, BWA displayed a significantly 20% higher (
p=0.041) PON-1 activity at 2.5 μM concentration. Interestingly, the PON-1 activity in response to CoQ
10 was found to be nearly similar to the activity observed in control (without BWA or CoQ
10), signifying no effect of CoQ
10 at the tested concentrations (0.01-2.5 μM) on the activity enhancement of PON-1. To the best of our knowledge, so far, no study has documented the effect of BWA on the activity enhancement of the antioxidant enzyme PON-1. However, some previous studies document the impact of BWA on the activity enhancement of antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase [
14,
34]. The outcome of the present study adds the value that BWA can enhance PON-1 activity and strengthen the use of BWA as a suitable nutraceutical precisely in relation to the bio-functionality of HDL.
3.2. Inhibition of Cupric ions (Cu2+) Mediated LDL Oxidation by BWA
LDL is vulnerable towards oxidation, which leads to the accumulation of oxidative LDL (oxLDL) that is readily uptake by the macrophages via CD36 and Sr-A scavenger receptors, rendering macrophages into foam cells and causing atherosclerotic lesions [
35,
36,
37]. Knowing the several detrimental consequences of oxLDL, in the present study, the effect of BWA was evaluated to inhibit the oxidation of LDL induced by CuSO
4, which is considered an excellent external stressor owing to the ability of Cu
2+ ions to the oxidative damage of LDL protein (apo-B) and lipid portion [
22]. At the onset, the effect of BWA was evaluated to inhibit Cu
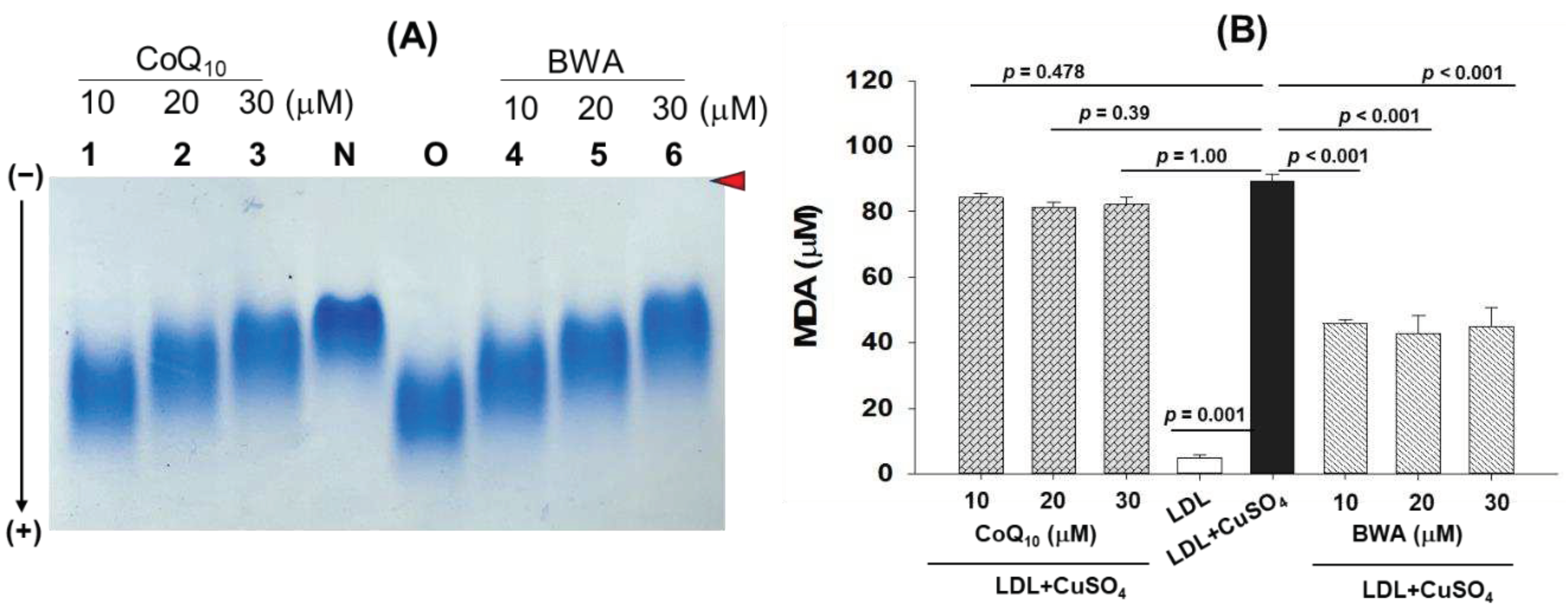
2+ ions mediated oxidation of LDL (apo-B) using native gel electrophoresis. As depicted in
Figure 3A, the native LDL (lane N) showed the retardation of electrophoretic mobility, while the oxLDL (LDL+ CuSO
4) (lane O) showed higher electrophoretic mobility. The treatment of BWA efficiently prevents CuSO
4-mediated oxidative damage of LDL in a dose-dependent manner (lanes 4-6). As documented in
Figure 3A, the electrophoretic mobility of LDL progressively retarded in response to BWA (10-30 μM) treatment. Noticeably, at 30 μM concentration of BWA, the LDL electrophoretic mobility front (lane 6) is as same as the electrophoretic mobility front of native LDL (lane N), signifying the impact of BWA to prevent the oxidative damage of LDL. On the other side CoQ
10 (10-30 μM) also displayed a dose-dependent effect to prevent LDL oxidation as evidenced by retardation of electrophoretic mobility (lane 1-3). Notability compared to CoQ
10, BWA showed slightly better prevention of LDL oxidation at the tested concentrations (10-30 μM), as evident by the higher band intensity with more retarded electrophoretic mobility at the respective concentrations.
Furthermore, the extent of lipid oxidation in LDL, which is exposed to CuSO
4 and subsequently treated with BWA or CoQ10, was evaluated. Results demonstrate maximum lipid oxidation (evident by the higher MDA quantification) in LDL treated with CuSO
4 (
Figure 3B). A maximum of 91.2 μM MDA was quantified in LDL treated with CuSO
4, which is significantly 21-fold higher (
p=0.001) than the MDA level quantified in native LDL. The treatment of BWA effectively inhibits CuSO
4-mediated lipid oxidation in a dose-dependent manner. At 10, 20, and 30 μM BWA concentrations, a 1.9-fold (
p<0.001), 2.1-fold (
p<0.001), and 2-fold (
p<0.001) low LDL oxidation was observed as compared to the LDL treated with CuSO
4, signifying the role of BWA to prevent lipid oxidation of LDL. The preventive effect of BWA was further confirmed by performing CuSO
4-mediated LDL oxidation in the higher amount of BWA (0-100 μM). The results suggest a dose-dependent effect of BWA to prevent Cu2+ ions-mediated oxidation of LDL (apo-B) and confirm the effective role of BWA in preventing LDL oxidation (
Supplementary Figure S1). Surprisingly, the CoQ10 displayed a nonsignificant effect on LDL lipid peroxidation altered by CuSO
4. The most probable reason for BWA to prevent LDL oxidation relates to its antioxidant activity, which scavenges harmful free radicals and ions, in turn creating a healing effect against oxidative stress. A prior study [
29] highlighted the metal-chelating activity of octacosanol, thereby corroborating the current results, given that octacosanol is an essential component of BWA. Additionally, the results follow the previous reports suggesting the impact of BWA on the prevention of lipid peroxidation and oxidative damage of protein in the gastric mucosa of rats [
13,
14,
38]. However, to the best of our knowledge, no study so far documented the effect of BWA on preventing the LDL protein (apo-B) and lipid peroxidation.
3.3. BWA Restored Cupric ions (Cu2+) Mediated Morphological Changes of LDL
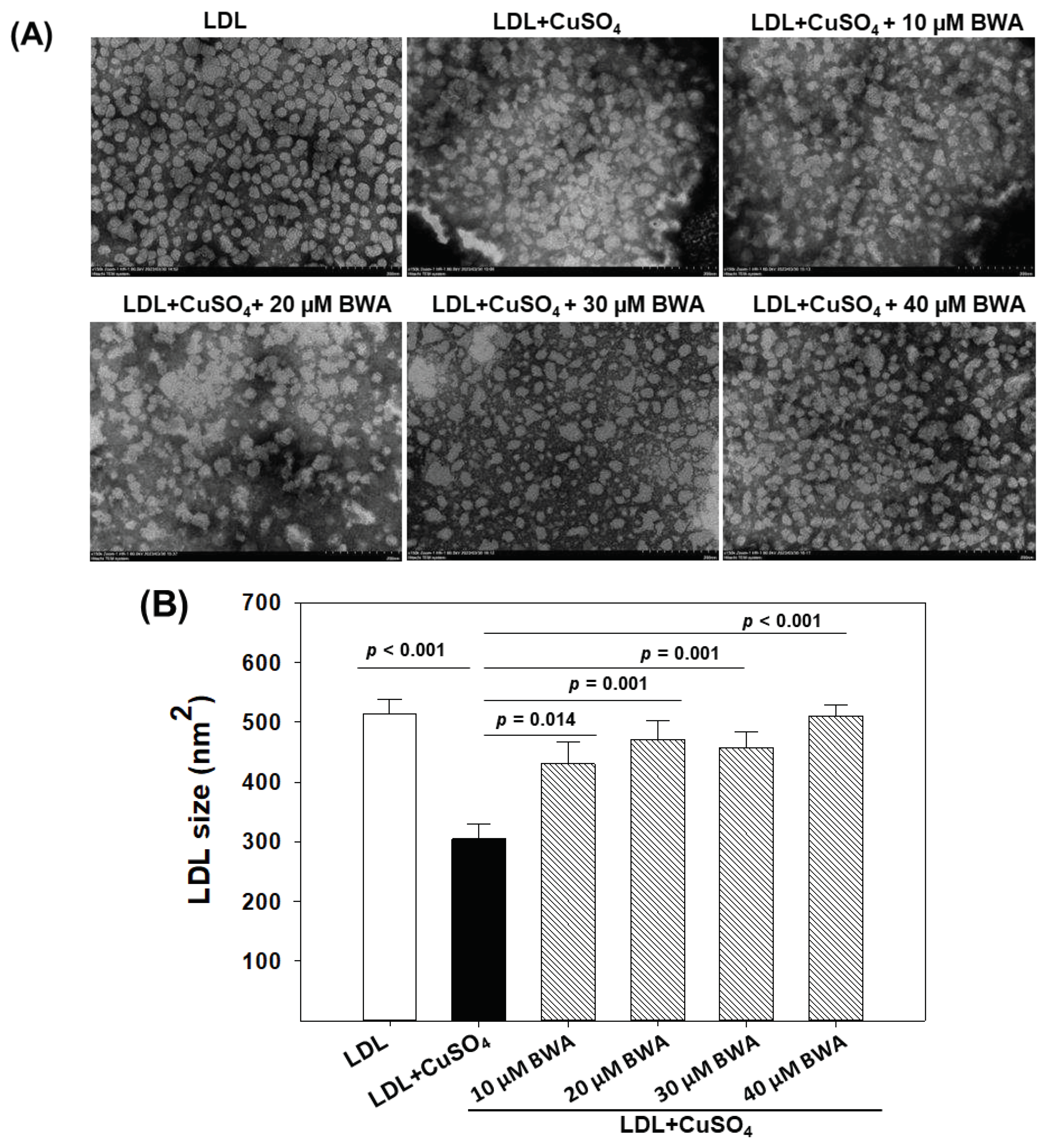
Transmission electron microscopy (TEM) is an useful technique to provide crucial insight into the structural characteristics of LDL particles [
17]. As depicted in
Figure 4, the native LDL appeared with a circular shape with even surface morphology without any particle aggregation with the highest average particle size 514.2±23.8 nm
2. The typical LDL particle characteristic is adversely impacted by the exposure of CuSO
4. As depicted in
Figure 4 an irregular size, distorted surface morphology with notable particle aggregation, and reduced LDL particle size was observed in LDL treated with CuSO
4. The average particle size of native LDL (514.2±23.8 nm
2) decreased significantly by 40.8% (
p<0.001) in response to the exposure of CuSO
4 suggesting the adversity of CuSO
4 towards morphological alteration of LDL. Conversely when LDL was exposed to BWA at the concentration of 10-40 μM a noteworthy reversal in the CuSO
4-mediated structural alteration was observed. At 10, 20, 30, and 40 μM BWA concentration the LDL particle size significantly enhanced by 18.4% (
p=0.014), 29.45% (
p=0.001), 25.9% (
p=0.001) and 40.2% (
p<0.001), respectively as compared to the particle size appeared in CuSO
4 treated LDL. Moreover, the LDL particle morphology altered by CuSO
4 improved in response to BWA, precisely at 40 μM concentration LDL tends towards the normal morphology as appeared for the native LDL. The findings collectively suggest that BWA possesses a counteractive effect against the oxidative challenge posed by CuSO
4, thereby affording the protection of LDL particles from the detrimental effect of oxidative stress. Previously, several studies documented the antioxidant effect of BWA [
12,
39]; however, no study described the effect of BWA to prevent LDL morphological changes induced by oxidative stress.
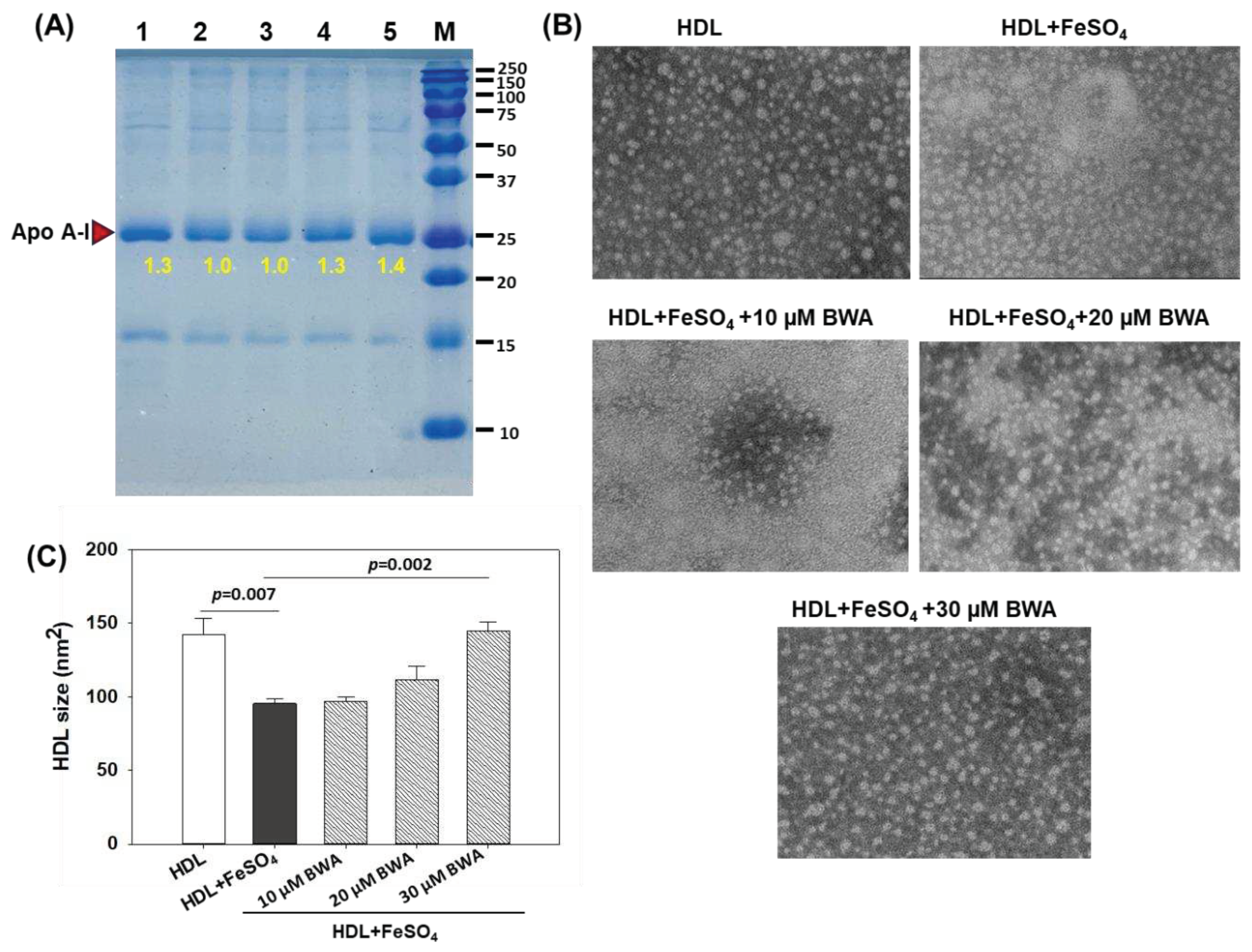
3.4. BWA Prevents HDL Degradation by Ferrous Ions (Fe2+)
HDL is the most important lipoprotein with multifaceted functions, including cardiovascular health [
33,
40]. Notably, the important role of HDL in preventing LDL oxidation is a key feature to deter atherosclerotic lesions. Perceiving the imperative role of HDL in cardiovascular and other diseases impact of BWA was evaluated to prevent the degradation of HDL stimulated by FeSO
4.
Figure 5 depicts a compelling visual representation of the effect of various treatments on HDL (apoA-I) integrity at 72 hr of treatment. The native HDL (lane 1) exhibited the baseline conditions with a sharp, intensified band. However, HLD exposed to Fe
2+ ions (final 60 μM) suffered severe degradation (lane 2), evident by a 1.3-fold lower band intensity compared to the band intensity of native HDL (lane 1). The co-treatment of HDL with BWA, specifically with BWA at 20 and 30 μM, effectively prevents the degradation of HDL provoked by Fe
2+ ions evident by 1.3 fold and 1.4 fold higher band intensities (lanes 4 and 5), respectively, compared to HDL-treated with Fe
2+ ions (lane 2).
The morphology of HDL in response to different treatments was evaluated by TEM, which is an efficient tool to access the structural deformities of HDL [
20]. It has been well established that external stress has a significant effect on HDL morphology and quality that eventually affects the functionality of HDL [
20]. The results depicted in Figures 5A and B revealed an even surface morphology with a bigger particle size (142.3±11.2 nm
2) of the native HDL that significantly reduced by 33.1% (
p=0.007) when HDL was exposed to Fe
2+ ions. Treatment of BWA explicitly 30 μM efficiently reverted Fe
2+ ions impaired structural changes. A 144.5±6.2 nm
2 HDL particle size was observed in 30 μM treated HDL, i.e., significantly 34.4% higher (
p=0.002) than the particle size observed in HDL treated with Fe
2+ ions, signifying the protective effect of BWA to counter the adversity posed by the exposure of Fe
2+ ions.
These results serve as compelling evidence to support the notion that BWA plays a crucial preventive role in mitigating the oxidative damage inflicted upon HDL by Fe
2+ ions. The protective function of BWA underscores its significance in maintaining the structural integrity and functional prowess of HDL impaired by oxidative challenge. So far, no study has elucidated the effect of BWA on preventing HDL degradation; however, few studies have documented the effects of some biological materials to prevent HDL degradation against the challenge posed by Fe
2+ ions primarily by their antioxidant role [
41] and thereby strengthening the present notion that BWA owing to its antioxidant property preventing the HDL degradation.
3.5. BWA Enhances the CML Impaired Embryo Survivability
Due to high genomic similarity with humans, zebrafish are considered an excellent animal model for preclinical studies [
42,
43]. Herein, the effect of BWA was evaluated to protect the zebrafish embryos against carboxymethyllysine (CML) induced toxicity. CML is an important advanced end glycation (AGE) product that provokes oxidative stress and inflammation and consequently has several detrimental effects [
26,
41,
44]. As depicted in
Figure 6A, the highest embryo survivability was observed in the PBS alone group, while the embryo injected with CML showed a gradual decline in embryo survivability, reaching 47.3% at 5 hr post-treatment. Conversely, the injection of BWA (15 ng) effectively enhances the CML-impaired embryo survivability. At 5 hr post-treatment, 66.6% of embryo survivability was observed in the BWA+CML injected group, which is significantly 1.4-fold higher (
p=0.041) than the embryo survivability in only the CML injected group. Surprisingly, no impact of CoQ
10 (15 ng) was observed on the CML-impaired embryo survivability; even more, much lower embryo survivability at 5 hr post-treatment was observed in CoQ
10+CML injected group compared to only the CML injected group.
Morphological changes of the developing embryos were examined at 5 and 24 hr post-treatment (
Figure 6B). A severe embryo death (indicated by red arrow) was observed in the CML inject group contrast to this, no embryo death was observed in the PBS injected group. A delayed embryo development with several developmental deformities concerning tail fin curvature (indicated by the black arrow), yolk sac edema (indicated by the blue arrow), and pericardial edema (indicated by the green arrow) was observed in embryos injected with CML. The embryo co-injected with BWA documented the prevention of developmental impairment induced by CML and aligned well with the survivability results present in
Figure 6A. In contrast, the non-plausible effect of CoQ
10 was observed in embryo development altered by the CML. Notably, a curvature of the tail (indicated by black arrow) and pericardial edema (indicated by green arrow) were observed in the embryos injected with CoQ
10+CML. The findings demonstrated the protective effect of BWA against CML-induced developmental abnormalities and mortality in zebrafish embryos.
The fluorescent images of DHE and AO staining document the extent of ROS production and apoptosis induced by CML (
Figure 6 C & D). The results of DHE staining unveiled a massive ROS production in response to CML, which is significantly 9.5-fold higher (
p=0.024) than the ROS level in the PBS-injected embryos. The treatment of BWA efficiently thwarts CML-induced ROS production. A significantly 6-fold lower (
p=0.031) DHE fluorescent intensity was quantified in BWA+CML injected embryos compared to only CML injected embryos. The AO staining revealed the highest fluorescent intensity in the CML injected group, which was significantly 5.1-fold higher (
p=0.009) than the fluorescent intensity observed in PBS injected group, implying a high extent of apoptosis. BWA significantly prevents CML-induced apoptosis, as evidenced by significantly 3.4-fold lower (
p=0.014) AO fluorescent intensity compared to the CML-injected embryos. In contrast, no preventing effect of CoQ
10 was observed against CML-induced apoptosis.
The findings of DHE and AO staining collectively endorse the efficacy of BWA in preventing CML-induced ROS production and apoptosis. The antioxidant nature of BWA is a major cause that efficiently counters the CML-induced ROS and consequently improves embryo survivability. Results are in good agreement with previous reports suggesting the antioxidants can revert the CML-induced ROS production and apoptosis, consequently rescuing the zebrafish embryos from CML-induced toxicity [
26].
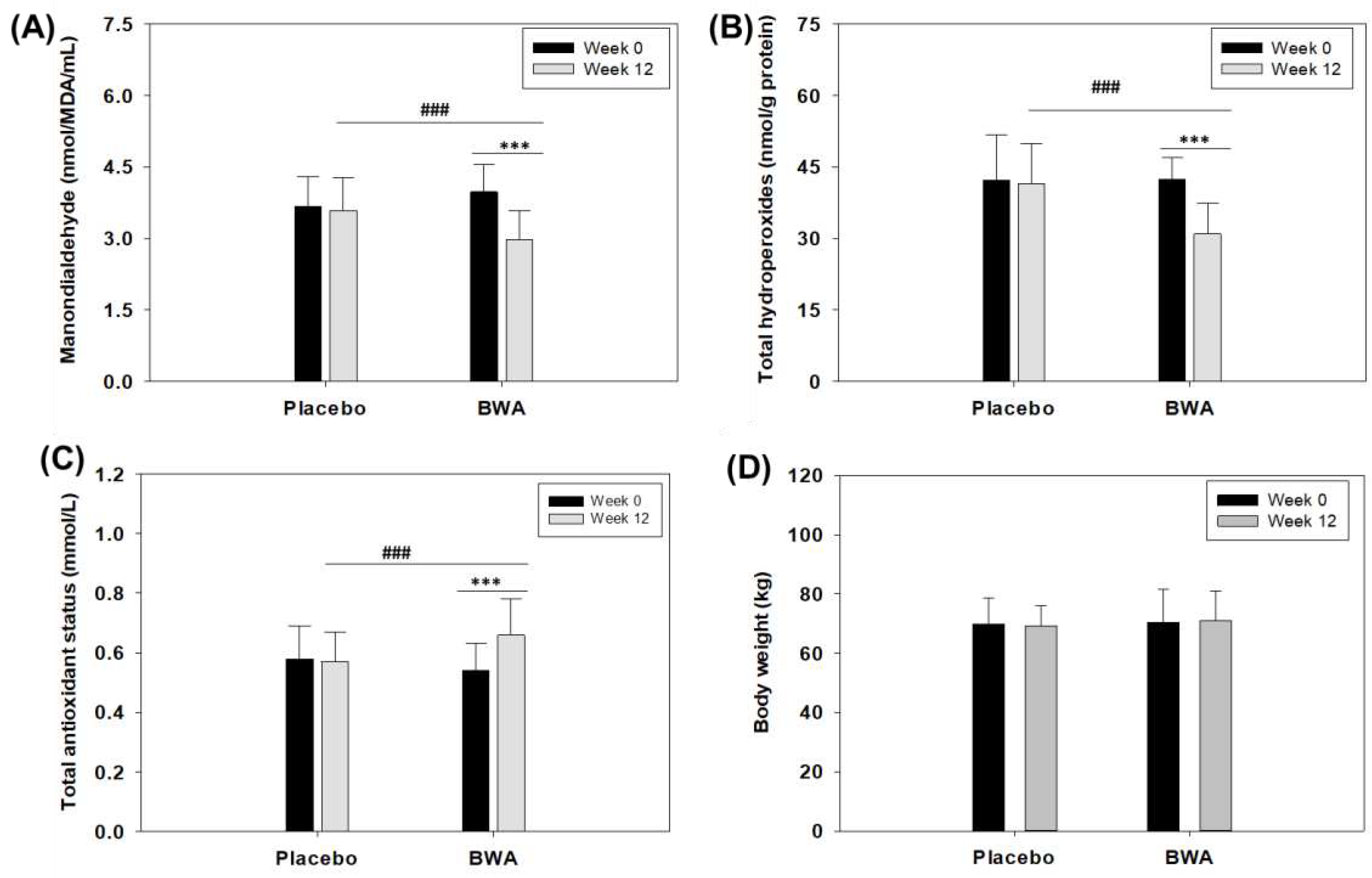
3.6. Supplementation of BWA Improved Oxidative Plasma Variables in Human Subjects
The
in vitro and preclinical studies performed in the zebrafish suggested the antioxidant properties of BWA. To extend the
in vitro findings, a preliminary clinical study was conducted to evaluate the impact of BWA (100 mg/day) consumption for 12 weeks on middle-aged and older human oxidative plasma variables. The choice of participants encompassed middle-aged and older individuals due to the well-established correlation between aging and reduction in the inherent antioxidant defense system, resulting in an elevated level of oxidative stress associated with various age-related diseases [
45,
46]. Initially, the MDA level was evaluated, which is considered an important marker of lipid peroxidation. The results demonstrated a significant 25% reduction (
p<0.001) in MDA level after 12 weeks of consumption of BWA compared to the baseline values (
Figure 7A). In contrast, no significant changes in MDA level were noticed after 12-week consumption in the placebo group when compared to their baseline values.
Further, total hydroperoxide level was evaluated in both BWA and placebo groups. Hydroperoxides are the important ROS [
47] formed by the peroxidation of unsaturated fatty acids and cholesterol [
28] and serve as a good indicator of oxidative stress [
48]. The results document a significant 27% reduction (
p<0.001) of plasma total hydroperoxides in the BWA-consuming group compared to their baseline value. In contrast, non-significant changes in serum total hydroperoxides levels were observed in placebo groups, signifying the effect of BWA on the reduction of total hydroperoxides (
Figure 7B). Simultaneously, a significant elevation of 22.2% in plasma total antioxidant status was noticed after 12 weeks of BWA consumption compared to the baseline value. Contrary to this, the placebo group's total antioxidant status remains unchanged (
Figure 7C).
The collective outcomes from the plasma oxidative variables demonstrate BWA's effectiveness in reducing oxidative stress among middle-aged and elderly individuals, and results are in good agreement with the earlier reports documenting the impact of BWA on lipid peroxidation [
49] and oxidative markers [
50]. Likewise, another report deciphered the impact of BWA on the reduction of hydroxyl radical generation, lipid peroxidation and activity enhancement of antioxidant enzymes in the gastric mucosa of rats [
51,
52,
53]. Similarly, there have been reports highlighting BWA’s influence on the production of hydroxyl radicals, protein oxidation, and the augmentation of antioxidant enzyme activities like catalase, superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) [
14,
38], as well as plasma total antioxidant status [
39,
49,
54], reinforcing the current finding.
3.7. BWA have no Adverse Effect on Anthropometric Profile, Liver and Kidney Biomarkers and Lipid Profile
To assess the suitability of BWA for human consumption, it is imperative to evaluate its safety assessment. No discernible impact of BWA was observed on the anthropometric profile with respect to body weight (
Figure 7D), pulse rate (beat/min) and blood pressure during 12 weeks of consumption (
Table 1). As depicted in
Table 1, the anthropometric profile remains unchanged in both the placebo and BWA-supplemented group during 12 weeks compared to baseline values. Furthermore, the blood analysis revealed no adverse effect of BWA consumption on glucose, hemoglobin, haematocratin,
red blood cell count, white blood cell, and total platelets count, along with
aspartate amine transferase (AST), alanine amine transferase (ALT), and creatinine level (
Table 1). Also, BWA consumption did not have any effect on total cholesterol and triglyceride levels throughout the study, and the value remained consistently unchanged with respect to the baseline values. Notably, a minor adverse effect was observed in 5 subjects. Among them, one belongs to the BWA group, reporting mild diarrhea, while the remaining four were from the placebo group, comprising two incidences of mild diarrhea and two incidences of mild urinary problems. The findings collectively affirm the safety profile of BWA and its favorable tolerability in the context of the study.
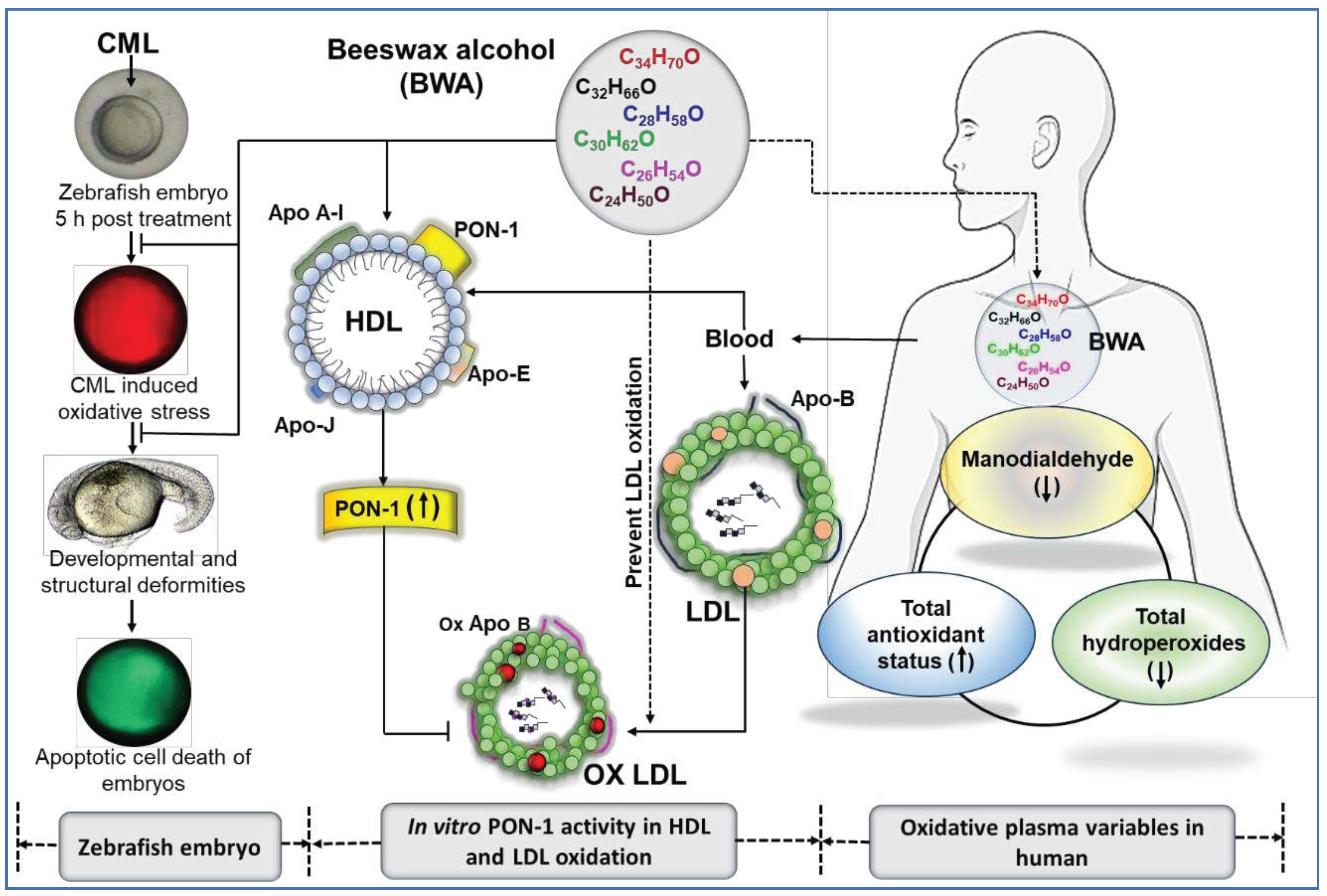
4. Conclusions
BWA demonstrated substantial antioxidant properties and enhanced PON-1 activity within HDL. BWA antioxidative effect efficiently thwarted LDL oxidation and significantly preserved the integrity and structural stability of HDL/ApoA-I. Also, BWA was marked to prevent the CML-induced oxidative stress and apoptosis, consequently rescuing zebrafish embryos from developmental deformities and mortality (
Figure 8). A preliminary clinical investigation involving middle-aged and older individuals confirmed the safety of BWA and its potency in reducing oxidative stress variables and elevating total antioxidant status. Nonetheless, the dual functionality of BWA in activating PON-1 within HDL and inhibiting LDL oxidation indicates the effectiveness of BWA against cardiovascular disorders that require a comprehensive investigation in the future. Collectively, the outcome of the present study (preclinical and clinical) endorses BWA as a safe supplement to prevent oxidative stress and related disorders.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Figure S1.
Author Contributions
Conceptualization, K.-H.C.; methodology, J.C.F.-T., L.E.L.-G., J.I.-F., V.M.-C., A.O.-Y., Y.P.-G., M.M.-A., S.M.-C., M.G.-P., S.J.-D., and A.B; data curation, writing—original draft preparation, K.-H.C., and J.C.F.-T.; Funding—review and editing, K.-H.C., supervision, K-H.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by Committee of Animal Care and Use of Raydel Research Institute (approval code RRI-20-004).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Human study
The clinical study was approved by the Clinical Research Ethics Committee of the Surgical Medical Research Center (IRB approval number. 04-19), Havana, Cuba, and registered in the Public Registry of Clinical Trials of Cuba (RPCEC00000401).
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Saleh, E.A.M.; Al-Dolaimy, F.; Baymakov, S.; Ullah, M.I.; Khlewee, I.H.; Bisht, Y.S.; Alsaalamy, A.H. Oxidative stress affects the beginning of the growth of cancer cells through a variety of routes. Pathol. Res. Prac. 2023, 154664. [Google Scholar]
- Gutteridge, J.M. Free radicals in diseases processes: a compilation of cause and consequence. Free Radic. Res. Commun. 1993, 19, 141–158. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31–38. [Google Scholar] [CrossRef]
- Hoshino, Y.; Mishima, M. Redox-based therapeutics for lung diseases. Antioxid. Redox Signal. 2008, 10, 701–704. [Google Scholar] [CrossRef]
- MacNee, W. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 2001, 429, 195–207. [Google Scholar] [CrossRef]
- Tabet, F.; Rye, K.A. High-density lipoproteins, inflammation and oxidative stress. Clin. Sci. 2009, 116, 87–98. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Ito, F.; Ito, T. High-density lipoprotein (HDL) triglyceride and oxidized HDL: New lipid biomarkers of lipoprotein-related atherosclerotic cardiovascular disease. Antioxidants 2020, 9, 362. [Google Scholar] [CrossRef]
- Lourenço, S. C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Huang, X.; Ahn, D.U. Plant- and animal-based antioxidants' structure, efficacy, mechanisms, and applications: A review. Antioxidants 2022, 11, 1025. [Google Scholar] [CrossRef]
- Han, Y.; Zee, S.; Cho, K.-H. Beeswax alcohol and fermented black rice bran synergistically ameliorated hepatic injury and dyslipidemia to exert antioxidant and anti-inflammatory activity in ethanol-supplemented Zebrafish. Biomolecules 2023, 13, 136. [Google Scholar] [CrossRef]
- Molina, V.; Mas, R.; Carbajal, D. D-002 (beeswax alcohols): Concurrent joint health benefits and gastroprotection. Indian J. Pharm. Sci. 2015, 77, 127–134. [Google Scholar] [CrossRef]
- Pérez, Y.; Oyárzabal, A.; Mas, R.; Molina, V.; Jiménez, S. Protective effect of D-002, a mixture of beeswax alcohols, against indomethacin-induced gastric ulcers and mechanism of action. J. Nat. Med. 2013, 67, 182–189. [Google Scholar] [CrossRef]
- Puig, M.N.; Castaño, S.M.; Ferreiro, R.M.; Clara, M.V.; Hernansez, N.M. Effects of oral administration of D-002 (Beeswax alcohols) on histological and functional outcomes in a Rat model of antigen-induced arthritis: Preliminary Study. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 5, 60–68. [Google Scholar]
- Cho, K.-H.; Kang, D.-J.; Nam, H.-S.; Kim, J.-H.; Kim, S.-Y.; Lee, J.-O.; Kim, B.-J. Ozonated sunflower oil exerted protective effect for embryo and cell survival via potent reduction power and antioxidant activity in HDL with strong antimicrobial activity. Antioxidants 2021, 10, 1651. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Baek, S.-H.; Kang, D.-J.; Na, H.; Komatsu, T.; Uehara, Y. Beneficial effect of Cuban policosanol on blood pressure and serum lipoproteins accompanied with lowered glycated hemoglobin and enhanced high-density lipoprotein functionalities in a randomized, placebo-controlled, and double-blinded trial with healthy Japanese. Int. J. Mol. Sci. 2023, 24, 5185. [Google Scholar] [CrossRef]
- Park, K.H.; Shin, D.G.; Kim, J.R.; Cho, K.H. Senescence-related truncation and multimerization of apolipoprotein A-I in high density lipoprotein with an elevated level of advanced glycated end products and cholesteryl ester transfer activity. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 600–610. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-R.; Lee, I.-C.; Kwon, H.-J. Native high-density lipoproteins (HDL) with higher paraoxonase exert a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Kang, D.-J.; Zee, S.; Park, M.-H. Enhancement of high-density lipoprotein (HDL) quantity and quality by regular and habitual exercise in middle-aged women with improvements in lipid and apolipoprotein profiles: Larger particle Ssze and higher antioxidant ability of HDL. Int. J. Mol. Sci. 2023, 24, 1151. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Kang, D.-J.; Park, M.-H.; Kim, J.-H. Long-Term Alcohol Consumption Caused a Significant Decrease in Serum High-Density Lipoprotein (HDL)-Cholesterol and Apolipoprotein A-I with the Atherogenic Changes of HDL in Middle-Aged Korean Women. Int. J. Mol. Sci. 2022, 23, 8623. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, S.-M.; Kim, S.-J.; Lee, E.-Y.; Kim, J.-R.; Cho, K.-H. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int. J. Mol. Med. 2017, 39, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yadav, D.; Kim, S.-J.; Kim, J.-R.; Cho, K. High Consumption of Iron Exacerbates Hyperlipidemia, Atherosclerosis, and Female Sterility in Zebrafish via Acceleration of Glycation and Degradation of Serum Lipoproteins. Nutrients 2017, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Bahuguna, A.; Kang, D.-J. Long-term supplementation of ozonated Sunflower oil improves dyslipidemia and hepatic inflammation in hyperlipidemic Zebrafish: suppression of oxidative stress and inflammation against carboxymethyllysine Toxicity. Antioxidants 2023, 12, 1240. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar]
- Nourooz-Zadeh, J. Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol. 1999, 300, 58–62. [Google Scholar] [PubMed]
- Sengupta, S.; Nandi, I.; Bhattacharyya, D.K.; Ghosh, M. Anti-oxidant and anti-bacterial properties of 1-octacosanol isolated from rice bran wax. J. Plant Biochem. Physiol. 2018, 6, 1. [Google Scholar]
- Harrabi, S.; Ferchichi, A.; Bacheli, A.; Fellah, H. Policosanol composition, antioxidant and anti-arthritic activities of milk thistle (Silybium marianum L.) oil at different seed maturity stages. Lipids Health Dis. 2018, 16, 82. [Google Scholar] [CrossRef]
- Guerra, Y.P.; Yera, Á.O.; Despaigne, S.J.; Cuevas, C.V.M.; Ferreiro, R.M. Scavenger effect of D-002 on hydroxyl radicals in the gastric mucosa. Rev. Cuba. Farm. 2012, 46, 87–96. [Google Scholar]
- Shokri, Y.; Variji, A.; Nosrati, M.; Khonakdar-Tarsi, A.; Kianmehr, A.; Kashi, Z.; Bahar, A.; Bagheri, A.; Mahrooz, A. Importance of paraoxonase 1 (PON1) as an antioxidant and antiatherogenic enzyme in the cardiovascular complications of type 2 diabetes: Genotypic and phenotypic evaluation. Diabetes Res. Clin. Pract. 2020, 161, 108067. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H. The Current status of research on high-density lipoproteins (HDL): A paradigm shift from HDL quantity to HDL quality and HDL functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef] [PubMed]
- Zamora, Z.; Molina, V.; Mas, R.; Ravelo, Y.; Perez, Y.; Oyarzabal, A.; Jiménez, S. D-002 treatment attenuates esophagitis in a model of chronic gastroesophageal reflux in rats. IOSR J. Pharm. 2015, 5, 36–40. [Google Scholar]
- Pentikainen, M.O.; Oorni, K.; Ala-Korpela, M.; Kovanen, P.T. Modified LDL-trigger of atherosclerosis and inflammation in the arterial intima. J. Intern. Med. 2000, 247, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic. Res. 2000, 33, 85–97. [Google Scholar]
- Aviram, M.; Rosenblat, M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic. Biol. Med. 2004, 37, 1304–1316. [Google Scholar] [CrossRef]
- Molina, V.; Valdés, S.; Carbajal, D.; Arruzazabala, L.; Menéndez, R.; Mas, R. Antioxidant effects of D-002 on gastric mucosa of rats with injury induced experimentally. J. Med. Food. 2001, 4, 79–84. [Google Scholar] [CrossRef]
- Menéndez, R.; Más, R.; Amor, A.M.; Pérez, Y.; González, R.M.; Fernández, J.; Molina, V.; Jiménez, S. Antioxidant effects of D002 on the in vitro susceptibility of whole plasma in healthy volunteers. Arch. Med. Res. 2001, 32, 436–441. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs context. 2018, 7, 212525. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Kang, D.-J.; Na, H.-J. Anti-inflammatory activity of CIGB-258 against acute toxicity of carboxymethyllysine in paralyzed Zebrafish via enhancement of high-density lipoproteins stability and functionality. Int. J. Mol. Sci. 2022, 23, 10130. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Guerra-Librero, A.; Rodríguez-Santana, C.; Escames, G.; Acuña-Castroviejo, D. The Zebrafish, an outstanding model for biomedical research in the field of melatonin and human diseases. Int. J. Mol. Sci. 2022, 23, 7438. [Google Scholar] [CrossRef] [PubMed]
- Ruchika; Sharma, A.; Saneja, A. Zebrafish as a powerful alternative model organism for preclinical investigation of nanomedicines. Drug Discov. Today 2022, 27, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Hsu, Y.W.; Pan, T.M. Suppression of dimerumic acid on hepatic fibrosis caused from carboxymethyl-lysine (CML) by attenuating oxidative stress depends on Nrf2 activation in hepatic stellate cells (HSCs). Food Chem. Toxicol. 2013, 62, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, G.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Madhusoodanan, U.K.; Sharanabasappa, M.; Ghosh, S.; Jacob, J. Measurement of plasma hydroperoxide concentration by FOX-1 assay in conjunction with triphenylphosphine. Clin. Chim. Acta 2003, 337, 147–152. [Google Scholar] [CrossRef]
- Santas, J.; Guardiola, F.; Rafecas, M.; Bou, R. Determination of total plasma hydroperoxides using a diphenyl-1-pyrenylphosphine fluorescent probe. Anal. Biochem. 2013, 434, 172–177. [Google Scholar] [CrossRef]
- López, E.; Illnai, J.; Molina, V.; Oyarzábal, A.; Fernández, L.; Pérez, Y.; Mas, R.; Mesa, M.; Fernandez, J.; Mendoza, S. Effects of D-002 (beeswax alcohols) on lipid peroxidation in middle-aged and older subjects. Lat. Am. J. Pharm. 2008, 27, 695–703. [Google Scholar]
- Illnait, J.; Rodriguez, I.; Molina, V.; Mendoza, S.; Mas, R.; Fernandez, L.; Oyarzabal, A.; Perez, Y.; Mesa, M.; Fernández, J. Effects of D-002 (beeswax alcohols) on gastrointestinal symptoms and oxidative markers in middle-aged and older subjects. Lat. Am. J. Pharm. 2013, 32, 166–174. [Google Scholar]
- Carbajal, D.; Molina, V.; Noa, M.; Valdes, S.; Arruzazabala, M.L.; Aguiar, M.; Mas, R. Effects of D-002 on gastric mucus composition in ethanol induced ulcer. Pharmacol. Res. 2000, 42, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, D.; Molina, V.; Valdés, S.; Arruzazabala, M.L.; Mas, R. Antiulcer activity of higher primary alcohols of beeswax. J. Pharm. Phamacol. 1995, 47, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, D.; Molina, V.; Valdés, S.; Arruzazabala, M.L.; Rodeiro, I.; Mas, R.; Magraner, J. Possible cytoprotective mechanism in rats of D-002 an anti-ulcerogenic product isolated from beeswax. J. Pharm. Pharmacol. 1996, 48, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.; Illnait, J.; Molina, V.; Oyarzabal, A.; Fernandez, L.; Mesa, M.; Más, R.; Mendoza, S.; Gámez, R.; Jiménez, S. Comparison of the antioxidant effects of D-002 (beeswax alcohols) and grape seed extract (GSE) on plasma oxidative variables in healthy subjects. Lat. Am. J. Pharm. 2010, 29, 255–262. [Google Scholar]
Figure 1.
Study layout for the clinical trials of beeswax alcohol (BWA). Subjects with ≥100 mmHg (diastolic pressure), >55 UI for ALT and AST, >130 μmol/L creatinine, and >7 mmol/L glucose (fasting) were excluded from the study. AST, ALT, BMI, BW, and CBC are abbreviated for aspartate aminotransferase, alanine transaminase, body mass index, body weight and complete blood count, respectively.
Figure 1.
Study layout for the clinical trials of beeswax alcohol (BWA). Subjects with ≥100 mmHg (diastolic pressure), >55 UI for ALT and AST, >130 μmol/L creatinine, and >7 mmol/L glucose (fasting) were excluded from the study. AST, ALT, BMI, BW, and CBC are abbreviated for aspartate aminotransferase, alanine transaminase, body mass index, body weight and complete blood count, respectively.
Figure 2.
Figure 2. A comparative effect of beeswax alcohol (BWA) and coenzyme Q10 (CoQ10) on free radical scavenging and paraoxonase 1 (PON-1) activity. (A) DPPH free radical scavenging activity. (B) Paraoxonase 1 (PON-1) activity in HDL. The PON-1 activity is expressed as the formation of 1 μmol p-nitrophenol from the substrate paraoxon-ethyl at a unit volume of the enzyme and time. P value represents the statistical difference between the groups employing one-way ANOVA following Tukey's Post Hoc analysis.
Figure 2.
Figure 2. A comparative effect of beeswax alcohol (BWA) and coenzyme Q10 (CoQ10) on free radical scavenging and paraoxonase 1 (PON-1) activity. (A) DPPH free radical scavenging activity. (B) Paraoxonase 1 (PON-1) activity in HDL. The PON-1 activity is expressed as the formation of 1 μmol p-nitrophenol from the substrate paraoxon-ethyl at a unit volume of the enzyme and time. P value represents the statistical difference between the groups employing one-way ANOVA following Tukey's Post Hoc analysis.
Figure 3.
Effect of beeswax alcohol (BWA) and coenzyme Q10 (CoQ10) on CuSO4 induced oxidation of LDL. (A) Electrophoretic mobility of apo-B fraction of LDL. Lanes 1, 2, and 3 represent LDL+CuSO4 treated with 10, 20, and 30 μM CoQ10, respectively. Lanes N and O reflect a native non-oxidized LDL and CuSO4 oxidized LDL, respectively. Lanes 4, 5, and 6 symbolize LDL+CuSO4 treated with 10, 20, and 30 μM BWA. Electrophoresis was performed on 0.5% agarose gel using Tris-EDTA buffer (pH 8.0) at a constant voltage (50 V). (B) Quantification of CuSO4-induced LDL oxidation in the presence and absence of BWA and CoQ10. The LDL oxidation was quantified by thiobarbituric acid reactive substance (TBARS) assay using malondialdehyde (MDA) as reference. P value represents the statistical difference between the groups with respect to oxidized LDL employing one-way ANOVA following Tukey's Post Hoc analysis.
Figure 3.
Effect of beeswax alcohol (BWA) and coenzyme Q10 (CoQ10) on CuSO4 induced oxidation of LDL. (A) Electrophoretic mobility of apo-B fraction of LDL. Lanes 1, 2, and 3 represent LDL+CuSO4 treated with 10, 20, and 30 μM CoQ10, respectively. Lanes N and O reflect a native non-oxidized LDL and CuSO4 oxidized LDL, respectively. Lanes 4, 5, and 6 symbolize LDL+CuSO4 treated with 10, 20, and 30 μM BWA. Electrophoresis was performed on 0.5% agarose gel using Tris-EDTA buffer (pH 8.0) at a constant voltage (50 V). (B) Quantification of CuSO4-induced LDL oxidation in the presence and absence of BWA and CoQ10. The LDL oxidation was quantified by thiobarbituric acid reactive substance (TBARS) assay using malondialdehyde (MDA) as reference. P value represents the statistical difference between the groups with respect to oxidized LDL employing one-way ANOVA following Tukey's Post Hoc analysis.
Figure 4.
Effect of beeswax alcohol (BWA) and coenzyme Q10 (CoQ10) on size and structural alteration of LDL probed by transmission electron microscope (TEM). (A) TEM images of LDL in the presence and absence of CuSO4, CuSO4+BWA (10-30 μM), and CuSO4+CoQ10 (10-30 μM). Images were captured at 150K magnification after negative stained with phosphotungstic acid [graphic scale=200 nm]. (B) Quantification of LDL size in response to different treatments. P value represents the statistical difference between the groups with respect to oxidized LDL employing one-way ANOVA following Tukey's Post Hoc analysis.
Figure 4.
Effect of beeswax alcohol (BWA) and coenzyme Q10 (CoQ10) on size and structural alteration of LDL probed by transmission electron microscope (TEM). (A) TEM images of LDL in the presence and absence of CuSO4, CuSO4+BWA (10-30 μM), and CuSO4+CoQ10 (10-30 μM). Images were captured at 150K magnification after negative stained with phosphotungstic acid [graphic scale=200 nm]. (B) Quantification of LDL size in response to different treatments. P value represents the statistical difference between the groups with respect to oxidized LDL employing one-way ANOVA following Tukey's Post Hoc analysis.
Figure 5.
Effect of beeswax alcohol (BWA) on ferrous ion (Fe2+) mediated proteolytic degradation and structural alteration in HDL. (A) Electrophoretic mobility of apoA-I fraction of LDL. Samples were run in 15% PAGE after 72 hr incubation and subsequently stained with Coomassie brilliant blue (0.125%). Lanes 0 and 1 represent HDL alone at 0 hr and 72 hr incubation. Lane 2 represents HDL+FeSO4 (60 μM), while lanes 3, 4, and 5 contain HDL+FeSO4 (60 μM) in the presence of 10, 20, and 30 μM BWA, respectively. Lane M molecular weight marker (10-250 kDa). Samples in lanes 1-5 were incubated at 72 hr. (B) TEM images of HLD in the presence and absence of FeSO4, FeSO4+BWA (10-30 μM). (C) Quantification of HDL size in response to different treatments. P value represents the statistical difference between the groups with respect to oxidized LDL employing one-way ANOVA following Tukey's Post Hoc analysis.
Figure 5.
Effect of beeswax alcohol (BWA) on ferrous ion (Fe2+) mediated proteolytic degradation and structural alteration in HDL. (A) Electrophoretic mobility of apoA-I fraction of LDL. Samples were run in 15% PAGE after 72 hr incubation and subsequently stained with Coomassie brilliant blue (0.125%). Lanes 0 and 1 represent HDL alone at 0 hr and 72 hr incubation. Lane 2 represents HDL+FeSO4 (60 μM), while lanes 3, 4, and 5 contain HDL+FeSO4 (60 μM) in the presence of 10, 20, and 30 μM BWA, respectively. Lane M molecular weight marker (10-250 kDa). Samples in lanes 1-5 were incubated at 72 hr. (B) TEM images of HLD in the presence and absence of FeSO4, FeSO4+BWA (10-30 μM). (C) Quantification of HDL size in response to different treatments. P value represents the statistical difference between the groups with respect to oxidized LDL employing one-way ANOVA following Tukey's Post Hoc analysis.
Figure 6.
A comparative effect of beeswax alcohol (BWA) and coenzyme Q
10 (CoQ
10) to prevent carboxymethyllysisne (CML) induced toxicity in zebrafish embryos.
(A) Survivability rate of zebrafish embryos during 5 hr post-treatment.
(B) Images representing the developmental deformities at 5 hr and 24 hr post-treatment. The black and red arrows represent the embryo death and tail curvature, while the green and blue arrows represent pericardial and yolk sac cdema, respectively.
(C) Fluorescent images of dihydroethidium (DHE) and acridine orange (AO) representing the reactive oxygen species (ROS) production and apoptosis in the embryos of zebrafish.
(D) Quantification of the fluorescent intensity. Image J software was utilized to compute the fluorescent intensity of DHE and AO staining (
http://rsb.info.nih.gov/ij/ version 1.53, accessed on Jan 23, 2023). PBS and CML groups representing the embryos microinjected with 10 nL of PBS and 500 ng CML/10 nL PBS, respectively, while embryos in CML+BWA or CoQ
10 groups were microinjected with 500 ng CML suspended in 10 nL of BWA or CoQ
10 (final 15 μM). ** represents
p<0.01, * represents
p<0.05 compared with the AO fluorescent intensity of only CML injected group while
## represents
p<0.01, and
#represents
p<0.05 compared with the DHE fluorescent intensity of only CML injected group; “ns” representing a non significant difference between the groups.
Figure 6.
A comparative effect of beeswax alcohol (BWA) and coenzyme Q
10 (CoQ
10) to prevent carboxymethyllysisne (CML) induced toxicity in zebrafish embryos.
(A) Survivability rate of zebrafish embryos during 5 hr post-treatment.
(B) Images representing the developmental deformities at 5 hr and 24 hr post-treatment. The black and red arrows represent the embryo death and tail curvature, while the green and blue arrows represent pericardial and yolk sac cdema, respectively.
(C) Fluorescent images of dihydroethidium (DHE) and acridine orange (AO) representing the reactive oxygen species (ROS) production and apoptosis in the embryos of zebrafish.
(D) Quantification of the fluorescent intensity. Image J software was utilized to compute the fluorescent intensity of DHE and AO staining (
http://rsb.info.nih.gov/ij/ version 1.53, accessed on Jan 23, 2023). PBS and CML groups representing the embryos microinjected with 10 nL of PBS and 500 ng CML/10 nL PBS, respectively, while embryos in CML+BWA or CoQ
10 groups were microinjected with 500 ng CML suspended in 10 nL of BWA or CoQ
10 (final 15 μM). ** represents
p<0.01, * represents
p<0.05 compared with the AO fluorescent intensity of only CML injected group while
## represents
p<0.01, and
#represents
p<0.05 compared with the DHE fluorescent intensity of only CML injected group; “ns” representing a non significant difference between the groups.
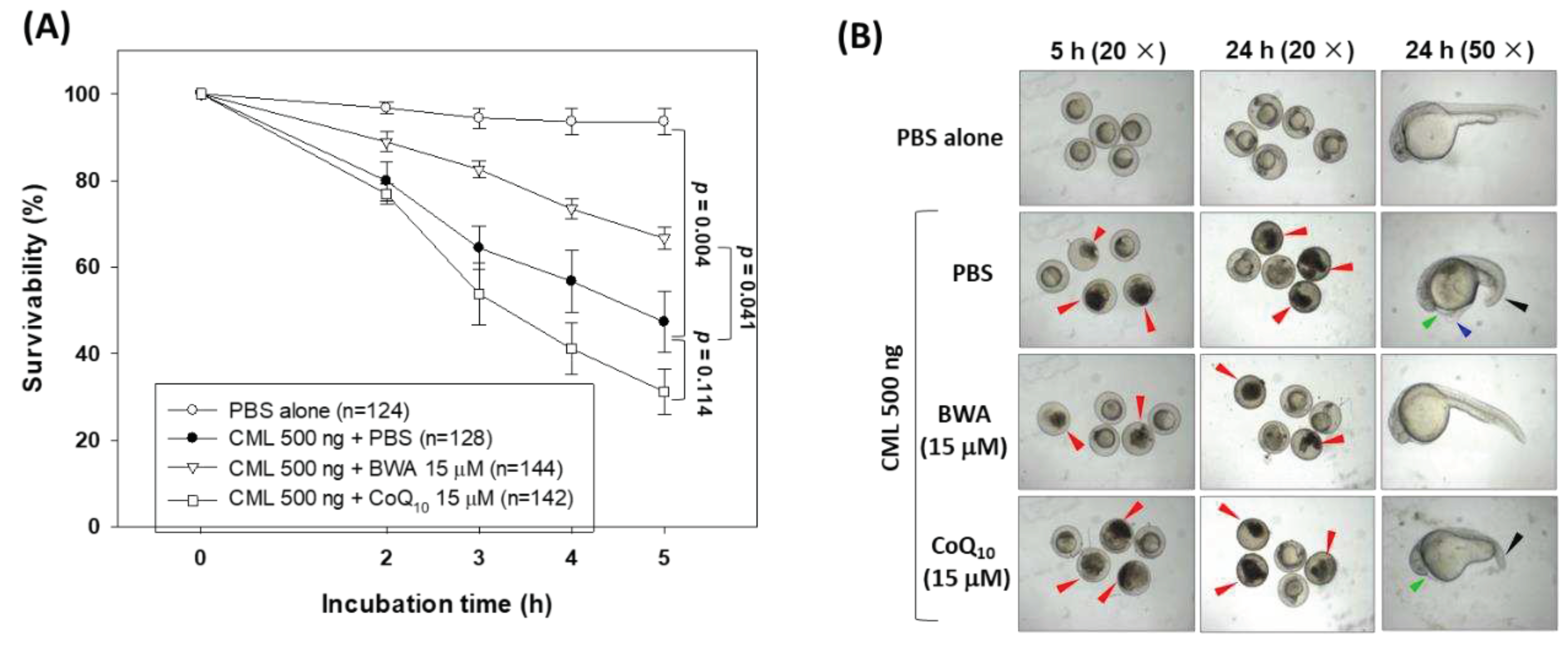
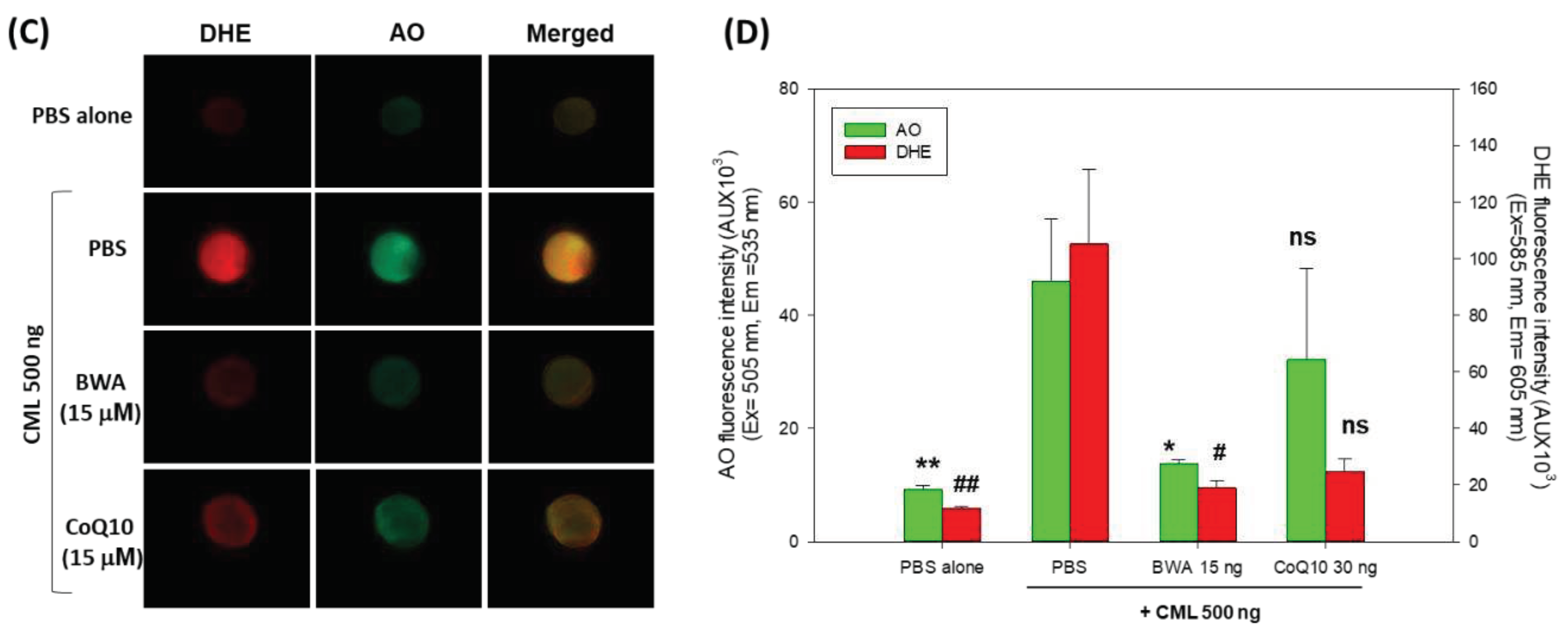
Figure 7.
Oxidative plasma variables and body weight of humans (n=50) before and after 12 weeks of intake of beeswax alcohol (BWA) (100 mg/day) and placebo. (A) Malondialdehyde (MDA). (B) Total hydroperoxides. (C) Total antioxidant status. (D) Body weight. *** represents the comparison between the 12 weeks intake of BWA with its baseline values at p<0.001, while ### represents a comparison between the group's 12 weeks intake of BWA and placebo at p<0.001.
Figure 7.
Oxidative plasma variables and body weight of humans (n=50) before and after 12 weeks of intake of beeswax alcohol (BWA) (100 mg/day) and placebo. (A) Malondialdehyde (MDA). (B) Total hydroperoxides. (C) Total antioxidant status. (D) Body weight. *** represents the comparison between the 12 weeks intake of BWA with its baseline values at p<0.001, while ### represents a comparison between the group's 12 weeks intake of BWA and placebo at p<0.001.
Figure 8.
Summary of antioxidant effect of beeswax alcohol (BWA) to prevent oxidative damage of zebrafish embryos, low-density lipoproteins (LDL), enhancement of paraoxonase 1 (PON-1) activity of HDL, and improvement of human oxidative plasma variable.
Figure 8.
Summary of antioxidant effect of beeswax alcohol (BWA) to prevent oxidative damage of zebrafish embryos, low-density lipoproteins (LDL), enhancement of paraoxonase 1 (PON-1) activity of HDL, and improvement of human oxidative plasma variable.
Table 1.
Effect of 12 weeks intake of beeswax alcohol (BWA) (100 mg/day) and placebo on anthropometric profile, blood analysis, liver and kidney function biomarkers and lipid profile.
Table 1.
Effect of 12 weeks intake of beeswax alcohol (BWA) (100 mg/day) and placebo on anthropometric profile, blood analysis, liver and kidney function biomarkers and lipid profile.
| Treatment |
Baseline (week 0) |
Week 12 |
| |
Pulse (beats/min) |
| Placebo |
70.08 ± 3.34 |
70.25 ± 1.07 |
| BWA |
71.36 ± 3.09 |
70.56 ± 1.47 |
| |
Diastolic blood pressure (mmHg) |
| Placebo |
79.20 ± 4.93 |
78.33 ± 3.61 |
| BWA |
78.80 ± 4.40 |
78.40 ± 3.74 |
| |
Systolic blood pressure (mmHg) |
| Placebo |
123.40 ± 11.06 |
125.20 ± 5.41 |
| BWA |
125.60 ± 11.21 |
125.00 ± 6.12 |
| |
Haemoglobin (g/L) |
| Placebo |
12.91 ± 1.31 |
12.98 ± 1.15 |
| BWA |
12.77 ± 0.83 |
12.80 ± 0.85 |
| |
Haematocrit (%) |
| Placebo |
39.31 ± 2.64 |
39.46 ± 2.06 |
| BWA |
38.98 ± 2.06 |
39.50 ± 1.81 |
| |
Red blood cell count (cell × 103) |
| Placebo |
4.23 ± 0.38 |
4.17 ± 0.20 |
| BWA |
4.22 ± 0.33 |
4.26 ± 0.28 |
| |
White blood cell count (cell × 103) |
| Placebo |
6.00 ± 1.43 |
6.09 ± 1.15 |
| BWA |
6.38 ± 1.34 |
6.15 ± 0.98 |
| |
Platelet count (cell × 103) |
| Placebo |
225.70 ± 39.33 |
227.41 ± 35.56 |
| BWA |
213.84 ± 42.42 |
215.36 ± 35.78 |
| |
Aspartate aminotransferase (U/L) |
| Placebo |
24.80 ± 6.26 |
24.75 ± 3.82 |
| BWA |
26.28 ± 5.42 |
25.60 ± 4.23 |
| |
Alanine aminotransferase (U/L) |
| Placebo |
16.88 ± 5.59 |
17.58 ± 4.57 |
| BWA |
17.88 ± 7.13 |
17.32 ± 4.23 |
| |
Glucose (mmol/L) |
| Placebo |
4.36 ± 0.90 |
4.39 ± 0.72 |
| BWA |
4.92 ± 1.05 |
4.61 ± 0.93 |
| |
Creatinine (μmol/L)
|
| Placebo |
76.08 ± 16.91 |
77.28 ± 12.10 |
| BWA |
76.12 ± 20.29 |
77.67 ± 14.33 |
| |
Total cholesterol (mmol/L) |
| Placebo |
6.31 ± 1.37 |
6.11 ± 1.10 |
| BWA |
6.34 ± 1.12 |
6.08 ± 0.78 |
| |
Triglycerides (mmol/L) |
| Placebo |
1.95 ± 0.98 |
1.71 ± 0.53 |
| BWA |
1.78 ± 0.74 |
1.68 ± 0.49 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).