1. Introduction
Hospital admission rates for congestive heart failure were the highest in 2015 in Germany with a rate of 3.7 per 1000 inhabitants compared to the European average rate of 2.4 per 1000 inhabitants despite high-quality primary care causing tremendous costs for the health care system [
1]. One solution might be cardiac resynchronization therapy (CRT) for a selected group of heart failure patients. It proved to reduce both hospitalization rates [
2,
3,
4,
5,
6,
8] and mortality [
7] in multiple trials. Therefore, current guidelines recommend CRT implantation for heart failure (HF) patients with reduced LVEF as a class IA indication in case of left bundle branch block (LBBB) of ≥ 150 ms and in case of complete heart block in AF or sinus rhythm to avoid right ventricular pacing as well as class IIA indication for less QRS width (130–149 ms) in sinus rhythm [
9,
10]. Digitalis is currently recommended as a class IB [
11] indication to establish rate control in patients with atrial fibrillation and HF with LVEF ≤ 40% and as second line treatment in patients with suboptimal heart rate control or worsening symptoms under beta-blocker and/or non-dihydropyridine calcium-antagonist treatment [
11]. To treat HF, the ESC HF guidelines recommend digoxin as a class II B indication to treat symptomatic patients with reduced LVEF in sinus rhythm despite guideline directed medical therapy to reduce the risk of hospitalization (both all-cause and HF-hospitalizations) [
10]. Only the latter indication has been investigated in the randomized controlled DIG trial. This trial, published in the nineties, failed to show a reduction in mortality but showed lower hospitalization rates in patients randomized to digitalis therapy instead [
12]. Within the last decade, medical heart failure therapy has been broadened to wide-spread use of angiotensin-converting-enzyme inhibitors (ACEI), aldosterone antagonists, angiotensin-neprilysin-inhibitors (ARNI) and lately SGLT-2 inhibitors, which all proved to reduce both mortality and heart failure related events [
13,
14,
25]. In two previous meta-analyses comprising over 1,000,000 patients, we could demonstrate that digoxin is associated with increased mortality in both patients with AF and/or HF [
15,
16]. When aiming for targeted therapeutic serum digoxin levels by regular blood controls, digoxin use might not lead to higher mortality rates compared to digoxin non-users in a HF patient collective [
17]. In is also noteworthy, that even with an extremely close monitoring strategy, it is only possible to maintain serum digoxin levels in the target range in less than 50% of patients [
17]. ICD patients also showed a higher mortality rate when allocated to digitalis therapy compared to patients not on digitalis [
18]. Furthermore, digitalis therapy might induce fatal ventricular arrhythmias, as CRT and ICD patients experienced more high-rate VT/VF episodes in a subgroup analysis of the MADIT-CRT trial [
19].
Whether digitalis therapy has an impact on mortality and CRT response in this context was investigated in this large non-randomized multicenter CRT-D study.
2. Methods
Patient population. This non-randomized tri-center observational study is based on the analysis of data collected in consecutive patients who received a CRT-Defibrillator at the J. W. Goethe University (Frankfurt, Germany), at the Medical Centre of the Hungarian Defense Forces (Budapest, Hungary) and at the Bethel-Clinic (Bielefeld, Germany) between 2005 and 2019. All patients analyzed were followed at the institution where the implantation was performed. Devices from various manufacturers were used (Medtronic, USA; St. Jude Medical/Ventritex, USA; Guidant/Boston Scientific, USA; ELA/Sorin, Italy; Biotronik, Germany). The study was approved by the IRB of the J.W. Goethe University and conforms to the ethical guidelines of the Declaration of Helsinki.
Data collection. Data were prospectively and in two centers partly retrospectively collected from the index hospitalization at the time of initial CRT-D implantation and at each follow-up visit which took place every six months or at the time of unscheduled visits in the out- or in-patient clinic. Data collection included patient characteristics such as age, indication for CRT, left LVEF and LVEDD, and relevant comorbid conditions. LVEF was evaluated by experienced cardiologists independently (not aware of the purpose of the study) as well as by automatic measurements (GE Healthcare, AutoEF). ECG parameters such as atrioventricular (AV) conduction or QRS width before and after CRT-implantation and biventricular pacing properties were additionally assessed. NYHA-classification was calculated at implantation and every follow-up visit. Pertinent medication use (beta-blockers, ACE inhibitors or angiotensin receptor blockers, diuretics, aldosterone antagonists, digitalis glycosides, antiarrhythmic drugs) was documented. Digitalis was used to treat heart failure and/or to control heart rate in atrial fibrillation, according to current guideline recommendations [
10,
11]. Data were also collected from blood tests. Serum digitalis levels were checked at the discretion of the treating physician. All relevant information was entered into a customized database. For missing data, particularly in case of missed follow-up visits, family members, treating physicians, or other hospitals were contacted to retrieve the missing information.
Outcomes. The primary outcome measure was time to all-cause mortality. Cause-specific mortality was defined according to the Hinkle and Thaler classification [
20]. Secondary endpoints were CRT-response defined as an improvement of ≥1 NYHA class and improvement of LVEF by at least 5% within the first year after CRT-D implantation. Moreover, QRS shortening by at least 10ms during follow-up was assessed. The incidence of appropriate and inappropriate ICD shocks were also collected and compared between the two groups.
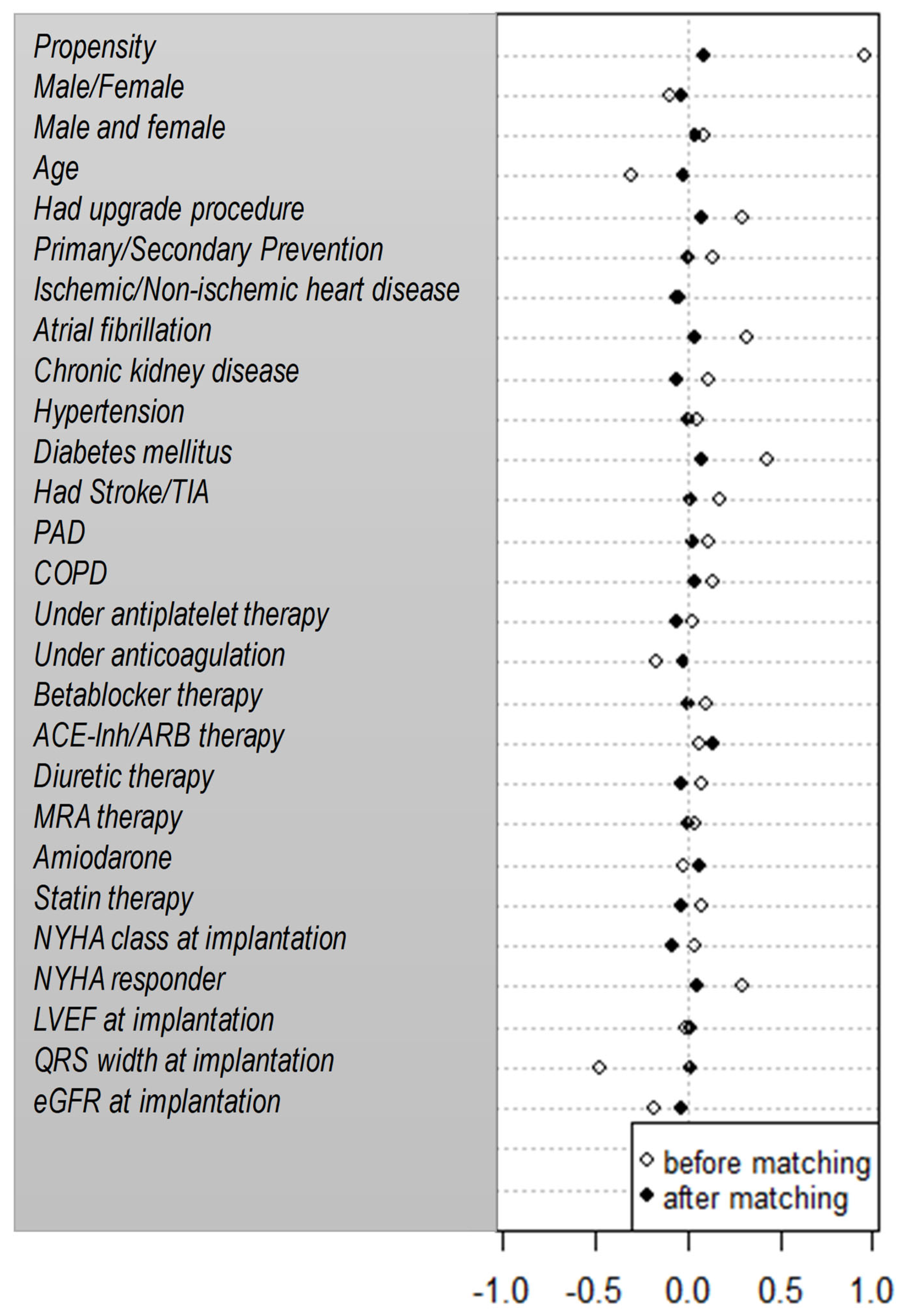
Statistics. Statistical analysis was performed using SPSS version 22 program (IBM, USA) and R core (GNU GPL, USA). Baseline characteristics were compared by the Wilcoxon Mann Whitney U-test (continuous variables) and the Chi²-test or Fisher exact test (categorical variables). Survival analysis was performed using Kaplan-Meier-analysis. Survival curves were compared using the log-rank test and Wald test for the Cox proportional hazard model. Crude and adjusted hazard ratios (HR) with 95% confidence intervals for digitalis use were calculated for potential confounding factors including age, gender, primary/secondary prevention indication, ischemic/non-ischemic heart disease, NYHA class, LVEF, upgrade/de novo implantation, hypertension, QRS width, documented atrial fibrillation, diabetes mellitus, chronic renal disease and amiodarone use. Additionally, propensity score matching was performed using the nearest neighborhood method with a 1:1 matching ratio and a caliper of 0.2. Within this matched cohort a Kaplan-Meier survival analysis was repeated and groups were compared using log-rank test. Only two-sided tests were used and p-values p<0.05 were considered statistically significant.
3. Results
Patient population. A total of 575 heart failure patients received a CRT-D device for primary or secondary prevention from sudden cardiac death in 2005 to 2015 in Frankfurt, Bielefeld and Budapest. Of those, 552 patients were regularly followed for a median of 28 months (0.1-126.8) in the in- and outpatient-clinics and form the basis of this report.
Our patient collective consists of mainly men (77%) with a mean age of 67±11 years and symptomatic heart failure due either to ischemic (n=298; 54%) or non-ischemic heart disease (n=254; 46%). Left ventricular function was severely reduced with a mean LVEF of 25±7% and broad QRS was present in all patients indicating intraventricular conduction disturbances (QRS width mean=160±28ms) with 76% having left bundle branch block, 10% having right bundle branch block and 14% having a non-specific intraventricular conduction delay or wide paced QRS based on the definition of the European Society of Cardiology [
29]. The most frequent comorbidities were atrial fibrillation (n=200; 36%), hypertension (n=381; 69%), diabetes mellitus (n=194; 35%) or chronic kidney disease (n=284; 51%) (
Table 1). 219 patients received digitalis in addition to heart failure medication (40%;
Table 2) and 333 patients did not (60%). Patients on digitalis were younger (mean age 64±11 vs 67±11 years; p<0.001) and had better renal function (median eGFR 63 vs 54 ml/min, p=0.004), but suffered from more advanced heart failure with a lower LVEF (mean 23±7 vs 27±7%, p<0.001) and more frequently NYHA III symptoms (71 vs 57%, p=0.002) compared to patients not on digitalis. Additionally, significantly more patients on digitalis had episodes of atrial fibrillation documented before CRT-D implantation (47 vs 29%; p<0.001).
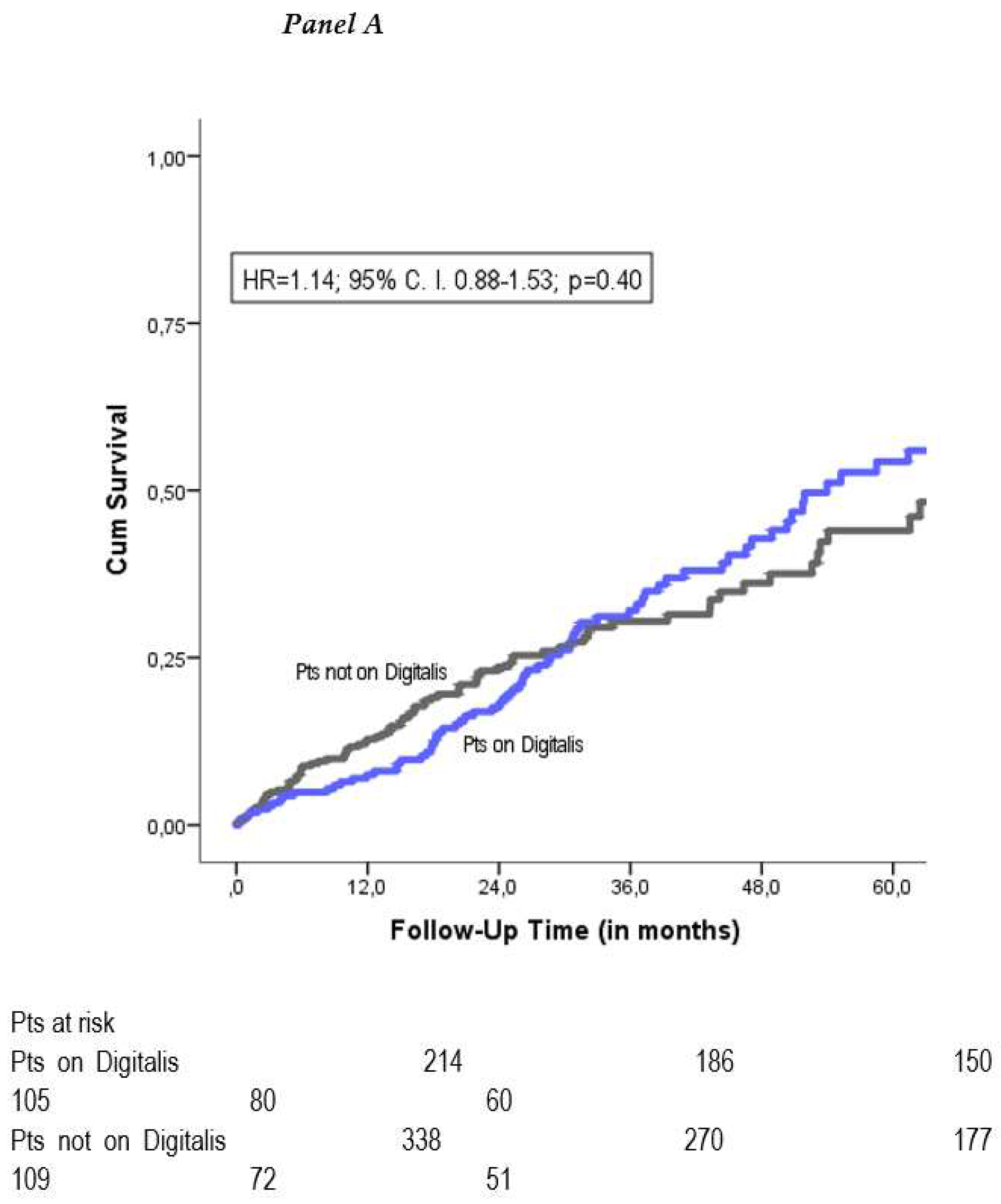
All-cause mortality. During a follow-up period of 37±28 months, 176 patients died (32%). Crude Kaplan-Meier analysis showed no significant difference in mortality between the two groups (HR=1.14; 95% CI 0.88-1.53; p=0.40) (
Figure 1A). To correct for potential confounders, we performed a multivariate Cox-Regression analysis for all baseline variables. This yielded the following independent predictors of mortality: age, male gender, chronic kidney disease, higher NYHA class and a CRT implanted as an upgrade of a preimplanted pacemaker or defibrillator (
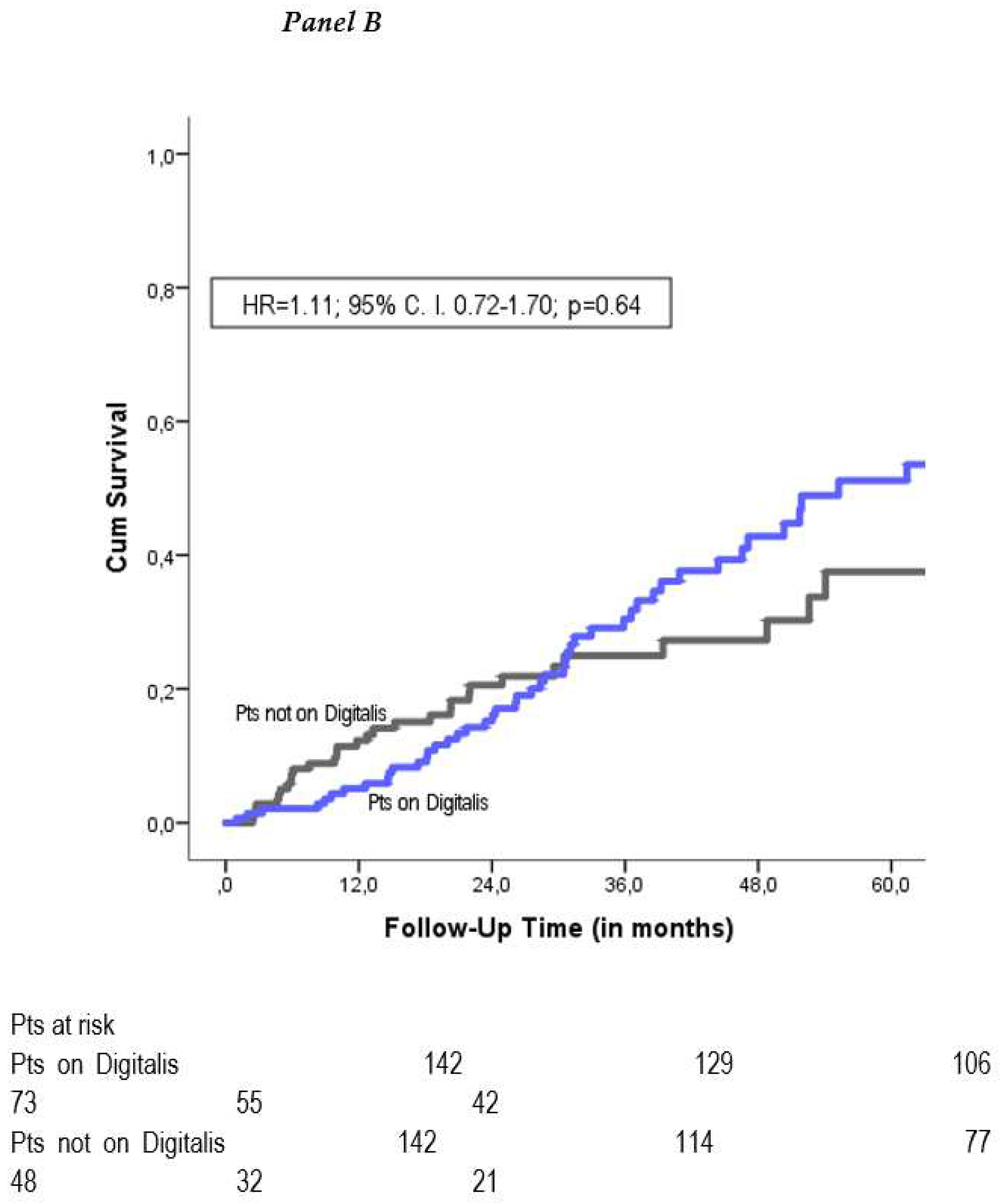
Table 3). After adjustment, the risk for mortality continued to be similar among patients on digitalis and patients not on digitalis (adjusted HR=1.04; 95% CI 0.75-1.45; p=0.82). The propensity-score matched cohort consisted of 286 patients with 143 patients on digitalis and 143 patients not on digitalis and had equally balanced baseline characteristics (
Figure 2). Kaplan-Meier mortality analysis was performed and demonstrated no significant mortality difference between the two groups (propensity adjusted HR=1.11; 95% CI 0.72-1.70; p=0.64) (
Figure 1B).
Cause-specific mortality. In 23 of 114 death events, cause-specific mortality could not be assessed as the circumstances surrounding death remained unknown (21%). Cardiac non-arrhythmic death due to progressive heart failure was the predominant cause of death in both, patients receiving digitalis and patients not receiving digitalis (both 34%; p=0.57), followed by non-cardiovascular deaths (21 vs 23%), cardiac arrhythmic deaths (19 vs 18%) and other cardiovascular deaths (3 vs 9%) (p=n. s. for all comparisons) (
Table 4).
Arrhythmia burden and digitalis therapy. We assessed episodes of ventricular arrhythmias in a subgroup analysis of the Frankfurt cohort. Tachycardia information was available in 58% of the patients (n=212). Ventricular arrhythmia (non-sustained/sustained VT and VF) occurred in 72 patients (32%), with a trend towards a higher incidence in patients on digitalis (n=50; 38% vs n=22; 27%; p=0.07). However, patients on digitalis developed significantly more frequently VT/VF episodes requiring ICD shock therapy (23% vs 10%; p=0.01). The incidence of inappropriate shock therapy was low and not different between the patient groups (8% vs 5%; p=0.50).
CRT-response and digitalis therapy. 336 out of 575 patients (58%) were CRT-responders during the 37 ± 28 month follow-up period. Of these, 125 patients were on digitalis (37%) and 211 patients were not (63%). Digitalis non-users showed significantly improved HF symptoms measured in improvement in NYHA-class by at least one class (HR=0.57; 95% CI 0.47-0.70; p<0.01) six months after CRT implantation. Pairwise echocardiographic measurements (baseline and 6 months follow-up) were available in 358 patients for LVEF (65%). Consecutively, digitalis intake decreased CRT response rates measured in improvement in LVEF by at least 5% at 6 months (HR=0.60; 95% CI 0.45-0.78; p<0.01). Furthermore, QRS shortening (≥10ms) was more often present in digitalis non-users (HR=0.62; 95% C. I. 0.46-0.83; p=0.001). Accordingly, biventricular pacing properties were significantly lower in patients on digitalis (92.5% ± 15.1% versus 95.5% ± 10.8%; p=0.02).
Serum digitalis levels throughout the study. From the total of 219 patients on any digitalis substance 86 patients (39%) had at least one serum digitalis level value available, and 29 patients (13%) had five or more digitalis level values. The mean serum digoxin level (in 50 patients) was 0.77±0.61 ng/ml (min-max 0.0-6.8). The mean digitoxin level (in 36 patients) was 20.97±9.1 ng/ml (min-max <0.15-40.0). Notably, three patients from these changed from digoxin to digitoxin during follow-up.
4. Discussion
Main findings. In our multicenter observational study comprising a large cohort of over 500 CRT-D recipients, digitalis therapy had no impact on mortality, but it negatively altered the likelihood to respond to CRT and was associated with an increased risk of ventricular tachyarrhythmias requiring ICD shock.
Impact of digitalis on mortality. Digitalis glycosides are either used to improve rate control in patients with atrial fibrillation or to lessen symptoms of advanced stage heart failure or for both indications according to current guideline recommendations [
10,
11]. These guideline recommendations are based on only one randomized controlled trial conduced over twenty years ago with patients receiving solely beta-blocker for heart failure therapy. Under these circumstances, digitalis therapy was associated with a 23% risk reduction for heart failure hospitalization [
12]. In the meantime, substances like ACE-inhibitors, aldosterone antagonists, ARNI and most recently SGLT-2 inhibitors have revolutionized medical heart failure therapy as they demonstrated to be able to reduce both heart failure related events and mortality [
13,
14,
25]. McMurray and colleagues demonstrated in the PARADIGM-HF trial a 16% relative risk reduction in mortality in 8442 patients and a 21% risk reduction in heart failure related events (both p ≤ 0.001) due to sacrubitril/valsartan compared to enalapril intake. This patient population suffered from severe heart failure with a mean LVEF of 29%, similar to our collective. Most interestingly, 30% of the patients in each treatment arm were on digitalis medication as well [
14]. Notably, ARNI or SGLT-2 inhibitors were still not prescribed in a regular fashion to the patients of the current study. For CRT, the COMPANION trial showed a 41% relative risk reduction for heart failure related hospitalizations [
3] and a 36% relative risk reduction for death in patients implanted with a CRT defibrillator [
4]. Of note, 78-79% of the patients received digitalis in the randomized groups [
3,
4]. Multiple observational studies demonstrated an increased risk of death in heart failure patients with or without atrial fibrillation due to digitalis administration [
17,
18,
19,
20,
21,
22,
23]. Relative risk for death ranged from 14% to 72% [
17,
19,
20]. Especially patients with severely impaired left ventricular function did not benefit from digoxin intake in further studies [
21,
23].
In view of such study reports it is somehow unexpected that digitalis therapy had no impact on mortality in the current multicenter observational study of CRT-D recipients [
26]. Notably, also a subgroup analysis form the MADIT-CRT trial demonstrated that digitalis therapy does not alter the risk for death (HR=0.93; 95% C. I. 0.67-1.32; p=0.71).
Another possible explanation for the observation that mortality was not increased by digitalis in our study with CRT patients might be based on the fact that proarrhythmic effects of digitalis were less life threatening because all patients were fitted with a defibrillator. This hypothesis is supported by the finding that VT/VF episodes requiring ICD shock therapy were more frequent in patients with digitalis.
Further explanation for a better-than-expected survival could be the better controlled serum digitalis concentration in this cohort. We could provide at least one serum digitalis level value in 39% of the included patients. The majority of these patients were treated with digoxin, in whom the mean digoxin concentration proved to be 0.77 ng/ml. Based on a post hoc analysis of the DIG trial [
27] and some later observational studies [
17] digitalis plasma concentrations under 0.9 ng/ml might be less harmful or have even neutral effect on all-cause mortality.
Cause-specific mortality and arrhythmia. In our CRT-D population, progressive heart failure was the predominating cause of death in 34% of the patients, followed by non-cardiovascular and cardiac arrhythmic deaths (22% and 18%). Our results are in line with the findings of a subgroup analysis of the COMPANION trial, where 44% of the patients died due to pump failure [
23]. Digitalis therapy was not associated with either one of these death modes classified according to Hinkle and Thaler [
20]. It is of common knowledge that digitalis therapy increases the risk for cardiac arrhythmias and causes atrial arrhythmias, conduction disturbances and multiple forms of ventricular tachycardia [
24]. Patients on digitalis experienced numerically more ventricular arrhythmias in our collective. The number of ventricular tachyarrhythmias leading to appropriate shock was significantly higher among patients on digitalis (p=0.01). Digitalis therapy was associated with an increased risk for ventricular arrhythmias, especially with heart rates ≥ 200 beats per minute in the MADIT-CRT subgroup analysis (HR=1.41; 95% CI 1.14-1.75; p=0.002; HR=1.65; 95% CI 1.27-2.15; p≤0.001) [
19]. This finding is also supported by a previous analysis of a large ICD collective which demonstrated a significant 30% higher risk of ICD shock therapy in patients on digitalis [
18].
CRT-response and digitalis therapy. Approximately 60-70% of CRT-recipients respond to CRT-therapy by improvement of at least one NYHA class, improvement in 6-minute-walk-test, or by increasing LVEF, respectively decreasing LVEDD [
2,
3,
4,
5,
6,
7]. To the best of our knowledge, this is the first study that demonstrates that CRT-response was negatively affected by digitalis therapy concerning both improvement in NYHA functional class and improvement in LVEF. Consecutively, biventricular pacing percentage was significantly lower in patients on digitalis. Although we observed normal digoxin level in a subset of patients in our study, the reduced CRT-response might be explained, at least in part, by results of a preclinical study showing that toxic effects of digoxin can even be detected with subtherapeutic level negatively affecting intracellular calcium homeostasis in the myocardium [
28]. In contrast, a recently published retrospective study on 297 HF patients in sinus rhythm compared on cardiovascular endpoints (HF hospitalization, heart transplantation and all-cause mortality) based on receiving treatment with digitalis versus receiving no treatment with digitalis [
26] showed no difference in CRT responder rates. Furthermore, it is debatable whether the increased morbidity in our study population and higher patient number might have contributed to a significantly different response rate to CRT based on digitalis treatment. It should also be noted, that patients on digitalis had less often QRS narrowing in our analysis, which can also explain the decreased echocardiographic and clinical response in this patient group [
30].
Limitations
Our study is retrospective in nature, hence all potential limitations of such a design apply to this analysis. We aimed to minimize potential confounding by carefully adjusting data to important patient characteristics found on univariate and multivariate analyses and therefore performed a propensity-score matched analysis. Despite this, residual confounding cannot be entirely excluded. Digitalis use was assessed at CRT-D implantation but not during follow-up or at time of death. Serum digitalis levels were checked at the discretion of the treating physician resulting in incomplete data. Also, CRT-response was mainly assessed clinically by improvement of NYHA functional class, which is a rather subjective parameter. Echocardiographic data was not available for the whole study collective. Moreover, data and ICD shock discharge were only available for the Frankfurt CRT-cohort. Strengths of our study consist of the large patient cohort, the long follow-up duration and the heterogenous, multicenter collective.
5. Conclusions
Digitalis therapy had no discernible effect on mortality in our large multicenter cohort of CRT-D recipients. In contrast, digitalis administration significantly decreased CRT response rates and increased the incidence for fast ventricular arrhythmias leading to appropriate ICD shock therapy.
Funding
There was no funding of this study.
Acknowledgments
The statistical expertise of Professor Eva Herrmann, Head of Department of Biostatistics, J.W. Goethe University, and the assistance in data requisition of Antje Steidl are greatly appreciated by the authors.
Conflicts of Interest
Julia W. Erath reports receiving consultant fees, travel support and lecture fees from ZOLL Medical, travel grants from Bayer Vital, St. Jude Medical/Abbott, Novartis and lecture fees from Servier, Pfizer and Bayer and was a fellow of the Boston Scientific heart rhythm fellowship program. Mate Vamos reports consulting fees and/or nonfinancial support from Biotronik, Medtronic, and Pfizer, outside the submitted work. David Pilecky has nothing to disclose. Nikolett Vigh has nothing to disclose. Carsten W. Israel reports consulting fees from Medtronic, St Jude Medical, lecture fees from Biotronik, Boston Scientific, Medtronic, MicroPort/Livanova and St. Jude Medical and sponsored research from Boston Scientific, Medtronic, and MicroPort/Livanova, outside the submitted work. Gabor Z. Duray reports speakers’ bureau from Medtronic, Biotronik, St. Jude Medical and Johnson&Johnson, outside the submitted work.
Abbreviations
| CRT |
Cardiac resynchronization therapy |
| NYHA |
New York Heart Association Classification for symptomatic heart failure |
| LVEF |
Left ventricular ejection fraction |
| ICD |
Implantable cardioverter-defibrillator |
| HR |
Hazard ratio |
| CI |
Confidence interval |
| HF |
Heart failure |
| AF |
Atrial fibrillation |
| SGLT-2 |
Sodium-glucose-uptake inhibitors |
| VT/VF |
Ventricular tachycardia/ventricular fibrillation |
| ACE |
Angiotensin-converting-enzyme |
References
- OECD Health policy studies. Cardiovascular Disease and Diabetes: Policies for Better Health and Quality of Care. June 2015.
- Abraham WT, Fisher WG, Smith AL et al. Cardiac resynchronization therapy in chronic heart failure. N Engl J Med 2002, 346, 1845–53. [CrossRef]
- Anand IS, Carson P, Galle E et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation 2009, 119, 969-77. [CrossRef]
- Bristow MR, Saxon LA, Boehmer J et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004, 2140-50. [CrossRef]
- Linde C, Abraham WT, Gold MR, Sutton MJ, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. JACC 2008, 52, 1834–43. [CrossRef]
- Moss AJ, Hall WJ, Cannom DS et al. Cardiac resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2010, 363, 2385–95. [CrossRef]
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005, 352, 1539–49. [CrossRef]
- Healey JS, Hohnloser SH, Exner DV, Birnie DH, Parkash R, Connolly SJ, Krahn AD, Simpson CS, Thibault B, Basta M, Philippon F, Dorian P, Nair GM, Sivakumaran S, Yetisir E, Wells GA, Tang AS, RAFT investigators. Cardiac resynchronization therapy in patients with permanent atrial fibrillation: results from the Resynchronization for Ambulatory Heart Failure Trial (RAFT). Circ Heart Fail 2012, 5, 566–70. [CrossRef]
- Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM; ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021 Sep 14;42(35):3427-3520. [CrossRef]
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-3726.
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021, 42, 373–498. [CrossRef]
- The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 1997, 8, 525–533. [CrossRef]
- The RALES investigators. Effectiveness of Spironolactone Added to an Angiotensin-Converting Enzyme Inhibitor and a Loop Diuretic for Severe Chronic Congestive Heart Failure (The Randomized Aldactone Evaluation Study). Am J Cardiol 1996, 78, 902–7. [CrossRef]
- McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Paradigm-HF Investigators. Angiotensin-Neprilysin Inhibition versus Enalpril in Heart Failure. N Engl J Med 2014, 371, 993–1004. [CrossRef]
- Vamos M, Erath JW, Hohnloser SH. Digoxin-associated mortality: A systematic review and meta-analysis of the literature. Eur Heart J 2015. [CrossRef]
- Vamos M, Erath JW, Benz AP, Lopes RD, Hohnloser SH. Meta-Analysis of Effects of Digoxin on Survival in Patients with Atrial Fibrillation or Heart Failure: An Update. Am J Cardiol. 2019, 123, 69–74. [CrossRef]
- Muk B, Vámos M, Bógyi P, Szabó B, Dékány M, Vágány D, Majoros Z, Borsányi T, Duray GZ, Kiss RG, Nyolczas N. The impact of serum concentration-guided digoxin therapy on mortality of heart failure patients: A long-term follow-up, propensity-matched cohort study. Clin Cardiol. 2020, 43, 1641–48. [CrossRef]
- Erath JW, Vamos M, Hohnloser SH. Effects of Digitalis on Mortality in a Large Cohort of ICD-recipients: Results of a long-term Follow-Up-Study in 1020 Patients. Eur Heart J Cardiovasc Pharmcother 2016. [CrossRef]
- Lee YA, Kutyifa V, Ruwald MH, McNitt S, Polonsky B, Zareba W. Digoxin therapy and associated clinical outcomes in the MADIT-CRT trial. Heart Rhythm 2015, 16, 2010–17. [CrossRef]
- Hinkle LE Jr, Thaler HAT. Clinical classification of cardiac deaths. Circulation 1982, 65, 457–64. [CrossRef]
- Kass DA, Chen CH, Curry C, Talbot M, Berger R, Fetics B, Nevo E. Improved left ventricular mechanisms from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 1999, 99, 1567–73. [CrossRef]
- Breithardt OA, Sinha AM, Schwammenthal E, Bidaoui N, Markus KU, Franke A, Stellbrink C. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. JACC 2003, 41, 765–70. [CrossRef]
- Carson P, Anand I, O´Connor C, Jaski B, Steinberg J, Lwin A, Lindenfeld J, Ghali J, Barnet JH, Feldman AM, Bristow MR. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. JACC 2005, 46, 2329–34. [CrossRef]
- Eckardt L, Breithardt G. Drug-induced ventricular tachycardia. In: Zipes D, Jalife J (eds) Cardiac electrophysiology. From cell to bedside. Elsevier Saunders 6th edition Philadelphia 2014, pp 1001-8. [CrossRef]
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020, 383, 1413–1424. [CrossRef]
- Fernandes C, António N, Marinho V, Milner J, Madeira M, Sousa PA, Ventura M, Cristóvão J, Elvas L, Gonçalves L. Digoxin in Patients With Advanced Heart Failure and Sinus Rhythm Submitted to Cardiac Resynchronization Therapy—Is There Any Benefit? Journal of Cardiovascular Pharmacology 2022, 79. [CrossRef]
- Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003, 289, 871–878. [CrossRef]
- Farghaly HSM, Ashry IEM, Hareedy MS. High doses of digoxin increase the myocardial nuclear factor-kB and CaV1.2 channels in healthy mice. A possible mechanism of digitalis toxicity. Biomed Pharmacother. 2018, 105, 533–539. [CrossRef]
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE; ESC Committee for Practice Guidelines (CPG), Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bänsch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013, 34, 2281–329. [CrossRef]
- Pilecky D, Duray GZ, Elsner D, Israel CW, Erath-Honold JW, Vamos M. Association between electrical and mechanical remodeling after cardiac resynchronization therapy: systematic review and meta-analysis of observational studies. Heart Fail Rev. 2022, 27, 2165–2176. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).