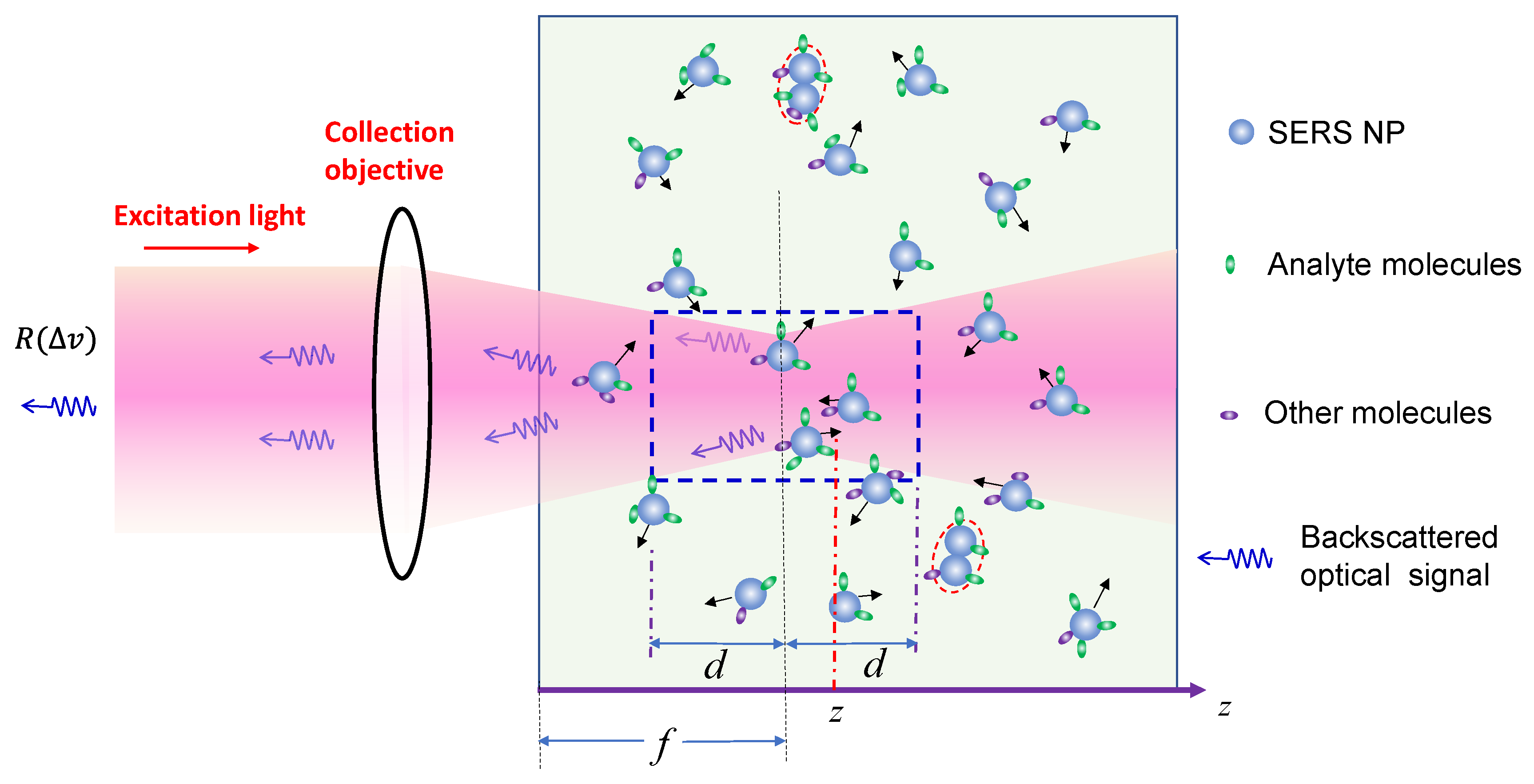
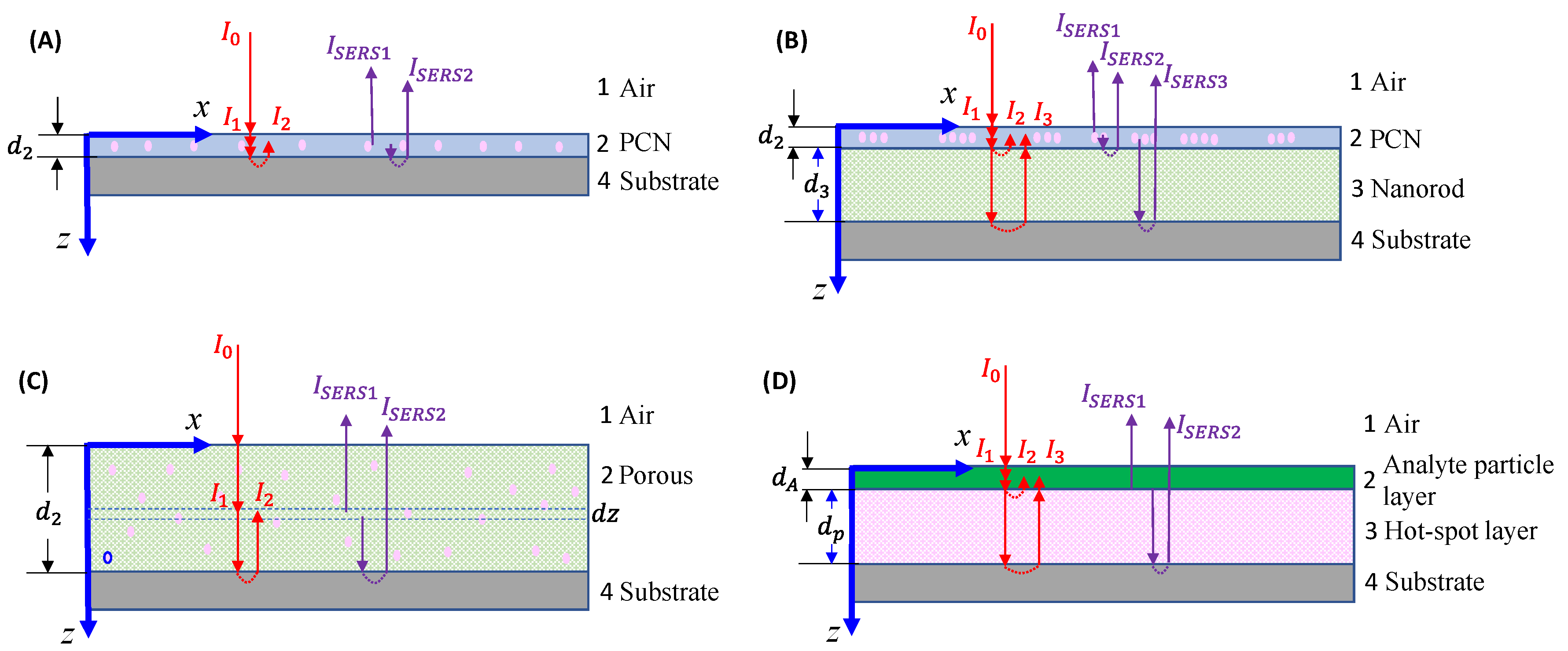
3.1.1. The Analytes Are Much Smaller than the Size of the Hot-Spots
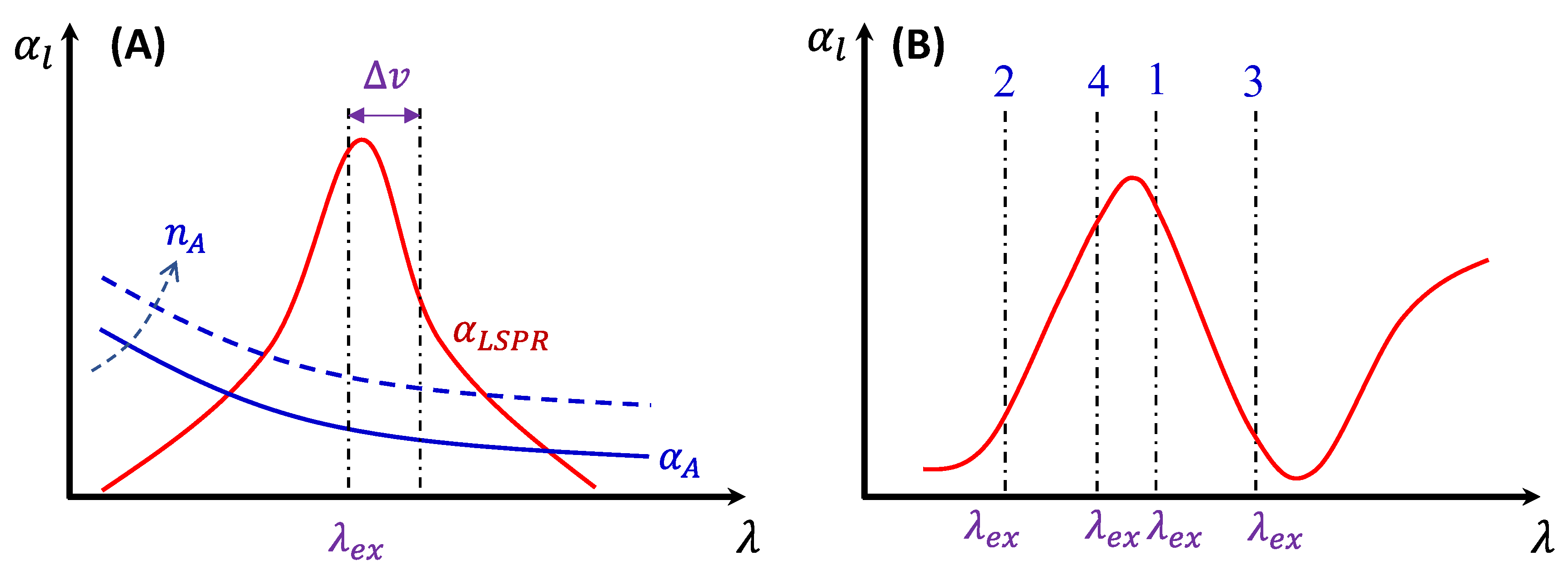
In the plasmonic research community, it is well-established that the hot-spot size of a PCN is typically in the range of 5-10 nm near its surface. When the size of the analytes is much smaller than the hot-spot size, these analytes can adsorb onto hot-spot locations, generating substantial SERS signals. Given that effective EF is in the range from 106 to 108, even a small fraction of analytes adsorbed inside the hot-spots can dominate the collected Raman signal. Consequently, understanding the factors influencing during the SERS measurement is crucial.
According to Le Ru et al.,[
6] various factors can impact
, including:
1) Excitation wavelength .
2) Polarization of the excitation laser.
3) PCN morphology.
4) Variation in PCN size and shape.
5) Orientation of the adsorbed analytes.
6) Fraction of analytes in hot-spot locations.
Let’s first discuss the average EF for a single PCN. It is important to note that the discussions presented here focus on scenarios involving sub-monolayer or single monolayer coverage of analytes on a PCN.
Spherical PCNs: In solution-based detection, the behavior of dispersed PCNs largely influences
, determined by the shape, size, and aggregates of these PCNs. Consider a scenario where PCNs are individual Au or Ag nanoparticles with a specific
. When
is very close to
, the SERS signal is maximized.[
7] Let's assume all PCNs are spherical in shape (
Figure 2A). The estimation of the average
depends on the following factors: the polarization of the excitation laser, the orientation of the adsorbed analytes, analyte coverage, and PCN Brownian motion.
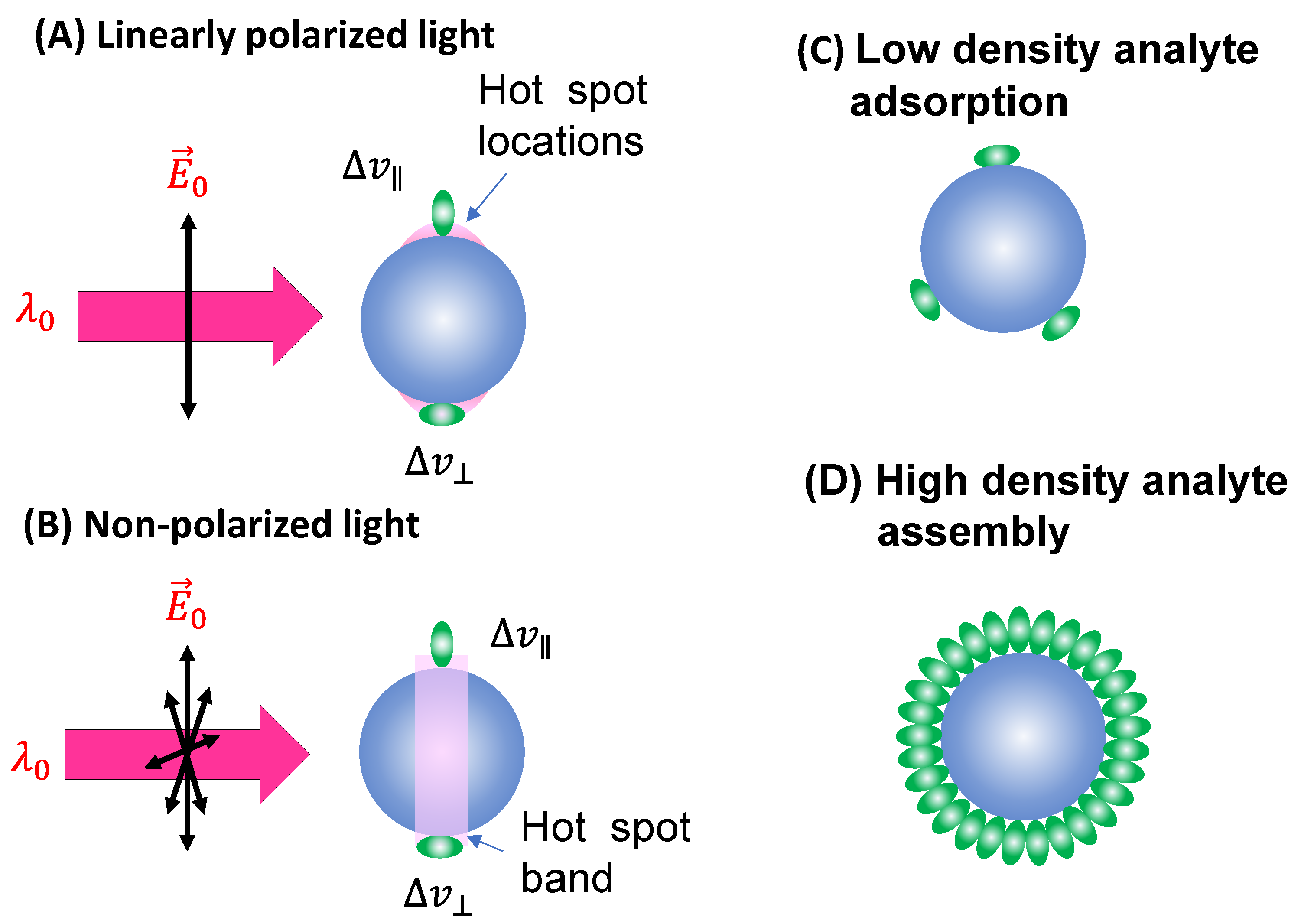
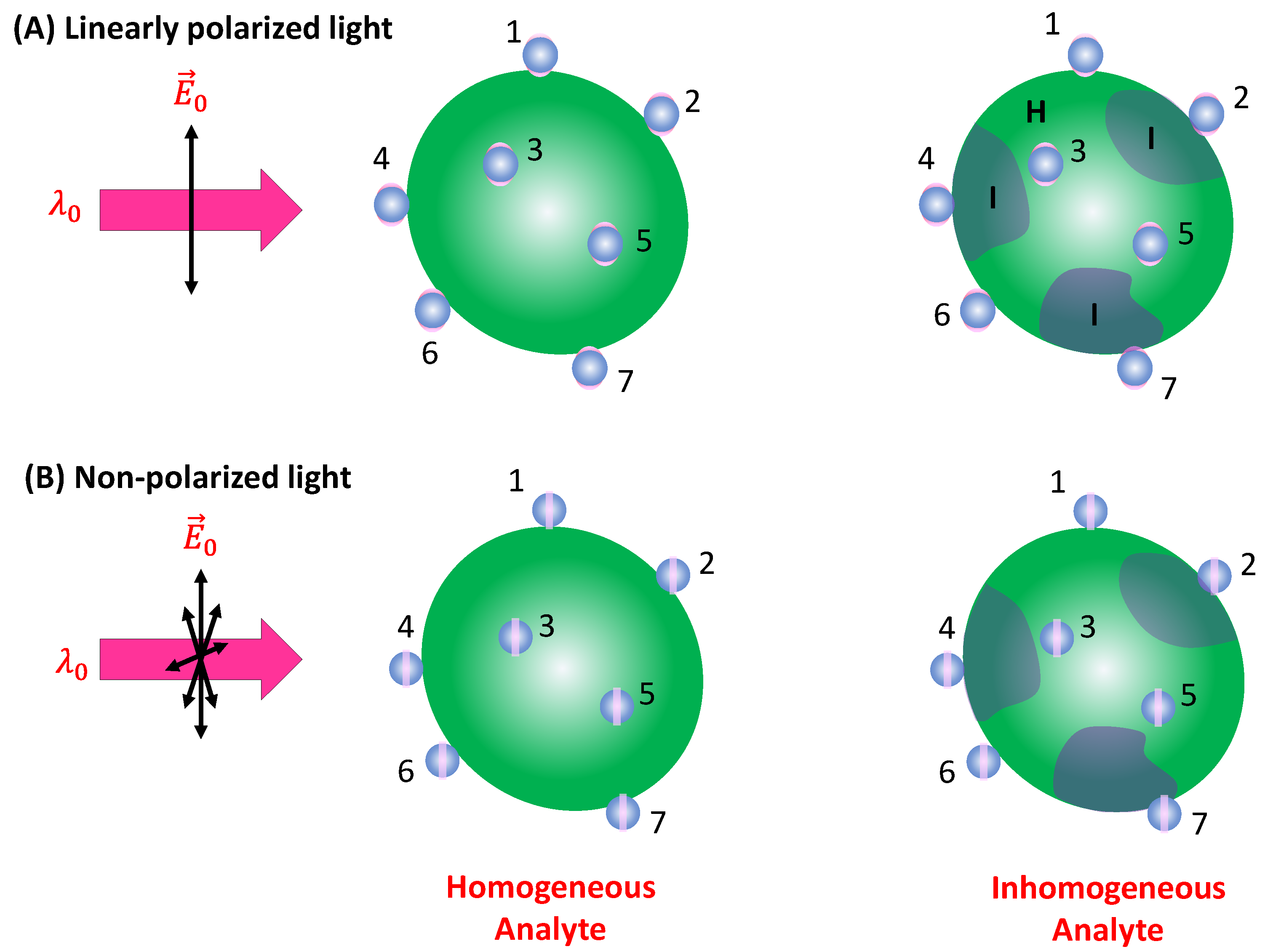
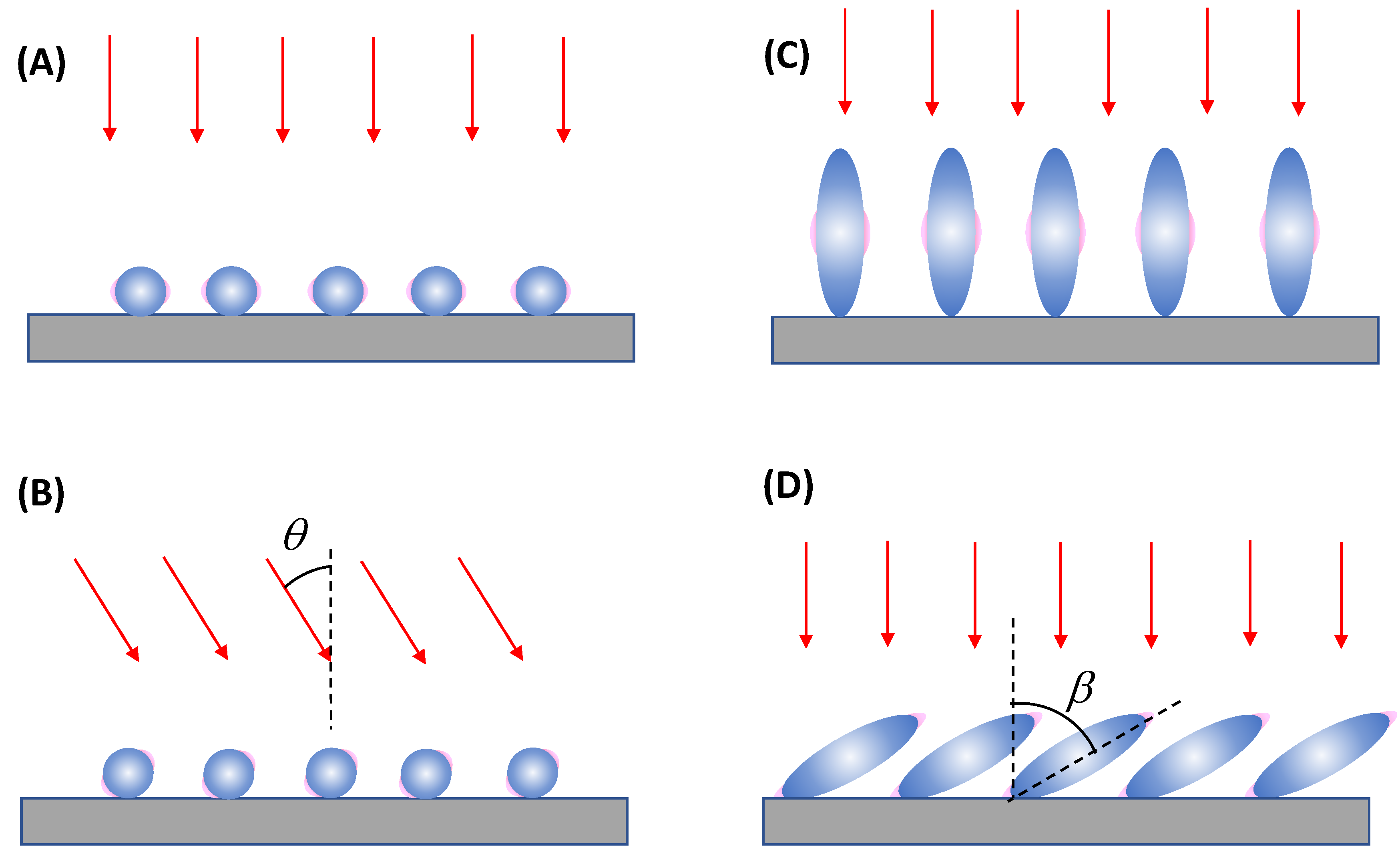
In the case of a vertically linear polarized excitation laser, hot spots on a spherical PCN are typically located at the top and bottom poles of the PCN, aligned with the polarization direction (
Figure 2A). If an analyte adheres to the top surface of the PCN with its long axis perpendicular to the surface, the Raman active mode (
mode) with vibrational components along the analyte's axis will be enhanced. However, if the analyte's orientation on the PCN surface rotates by 90 degrees, as depicted on the bottom surface in
Figure 2A, the
mode won't be enhanced. Instead, the Raman active mode with a vibrational component perpendicular to the molecule's axis (
mode) will be enhanced. This non-uniform enhancement of vibrational modes can alter the shape of the SERS spectrum.
Representing the SERS scattering cross-sections of the analyte with its axis parallel (
) and perpendicular (
) to the polarization direction as
and
respectively, and considering the orientation distribution of analyte molecules as
(refer to
Figure 2C) with respect to the polarization direction, the SERS EF
, accounting for the orientation effect, can be expressed as follows,
, (13)
where is the ideal EF of a spherical PCN when an analyte adsorbs on the hot-spot, , and is the average SERS scattering cross-section at ,
. (14)
Consider the comparison between a scenario where analyte molecules are randomly adsorbed (
Figure 2C) and a case where analyte molecules are well-oriented due to self-assembly (
Figure 2D). In
Figure 2D, the SERS spectrum will be primarily governed by
, whereas in
Figure 2C, both
and
contribute to the final SERS spectrum. It's evident that if the analyte possesses a complex structure with varying symmetry, Eq. 13 would become more intricate. Consequently, due to potential changes in analyte orientation, not only can the shape of the SERS spectrum be altered, but the effective EF might also differ at various
values.
In solution-based SERS measurements, PCNs undergo Brownian motion, both translationally and rotationally. Therefore, the
represents the average of
when the sites of adsorbed analytes become hot-spot location. Assuming a very low analyte density (as depicted in
Figure 2C), where only a few analytes (
MA) are adsorbed on the PCN surface, let's consider that the hot-spot has a solid angle of
in the spherical PCN, denoted as
, where
h is the projected radius of the hot-spot on the spherical PCN, and
r is the PCN radius. The probability of an analyte in a hot-spot location is
, then the average
for a single PCN becomes,
. (15)
If a PCN is entirely coated with a layer of analytes, these analytes may tend to align around the PCNs in a specific orientation, as illustrated in
Figure 2D. In this case, irrespective of the PCN's orientation, there will always be analyte molecules present in the hot-spot locations. Let's assume that each analyte occupies a small solid angle
on the surface of a PCN. Given that there are always
analytes situated in a hot-spot, the average
for a single PCN becomes,
. (16)
Here
, i.e., the equation
holds, making Eq. 16 equivalent to Eq. 15. However, Eq. 16 remains constant over time, whereas Eq. 15 represents a time-averaged result, depending strongly on random motion. This dependence could offer a method to measure PCN size, similar to principles used in dynamic light scattering.[
8]
When unpolarized light is used for excitation, hot-spots will form around the equatorial band of the PCN, as depicted in
Figure 2B. This is due to the electric fields being equally distributed in all directions perpendicular to the light's incident direction. Although this change in polarization does not significantly impact the distribution of analyte orientations in the final SERS spectrum (i.e., the discussion of
and
for Eq. 13 remains valid), the projected intensity of the excitation laser in a specific direction reduces to
. As shown in
Figure 3, taking into account the probability of analytes being adsorbed in the hot-spot area
, we get
. (17)
when , , making Eqs. 15 and 16 to become , which is smaller than the obtained in Eq. 17. Therefore, in the context of spherical PCN suspension in solutions, using unpolarized excitation light can yield a higher .
In addition, experimentally there is always a distribution of the size s and shape of the PCNs, or even aggregation of PCNs. In this case, is not a constant, and neither is . Thus, the effective can be expressed as
, (18)
where is the probability density function of s and Σ, with .
Certainly, if two or more PCNs aggregate, as outlined by the red dashed ovals in
Figure 1, the
can undergo a significant red-shift due to plasmonic coupling/hybridization.[
9,
10] Therefore the contribution from aggregate particles to the final SERS intensity can often be neglected. However, if
is tuned to
of the aggregated PCNs, the primary contribution to SERS will stem from the aggregated PCNs, not the monodispersed ones.
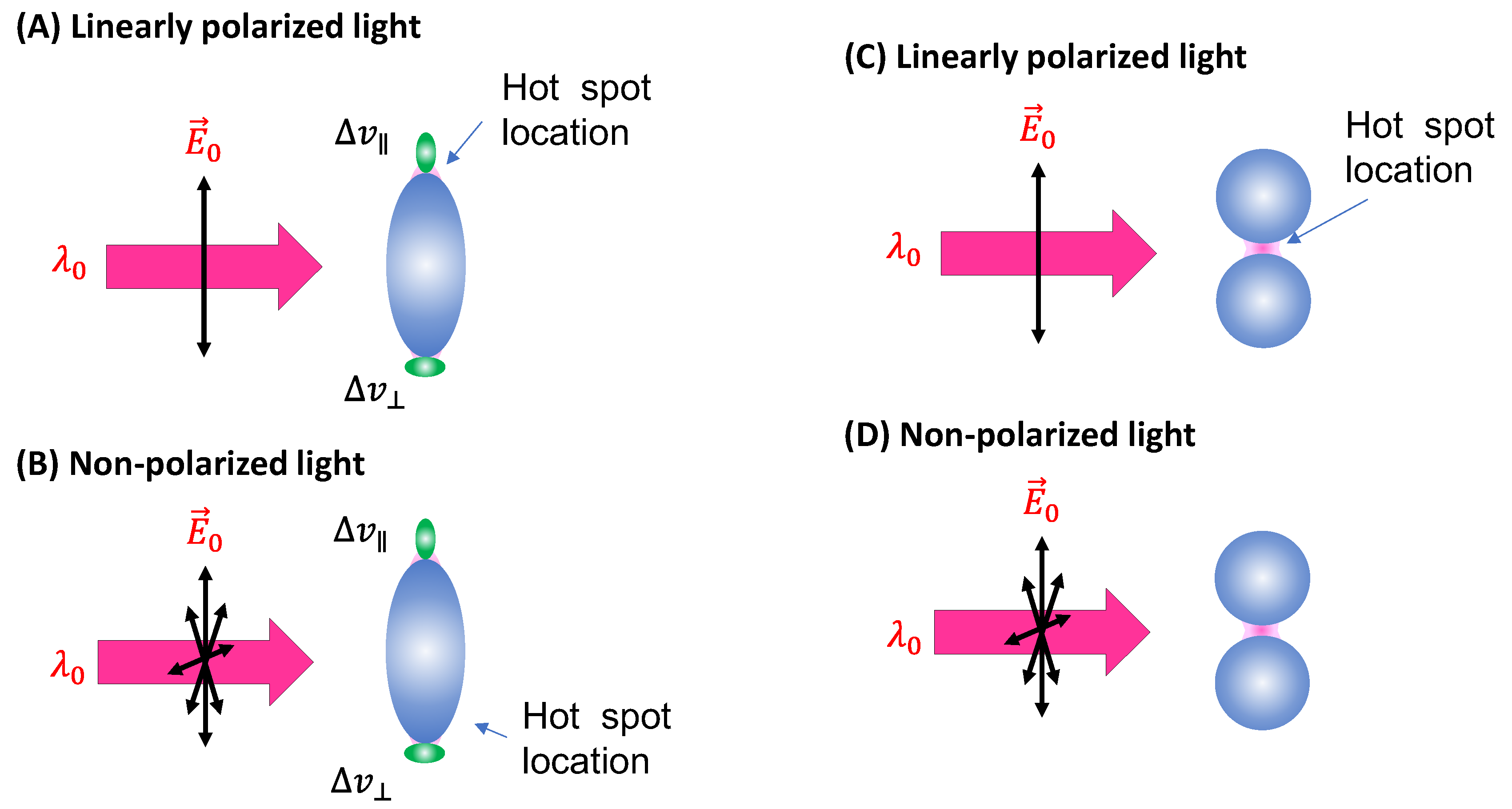
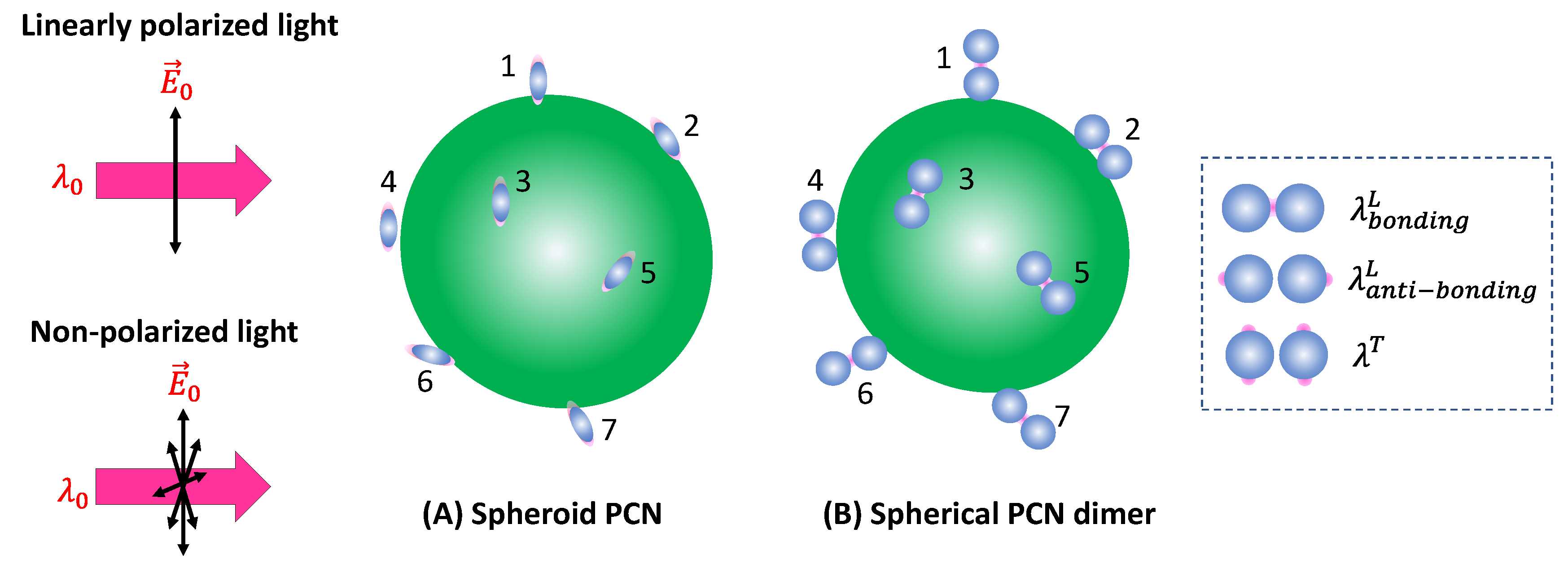
Spheroid PCNs: If the PCNs are anisotropic, like the spheroid particles shown in
Figure 3A, the estimation of
will be very different. Monodispersed PCN spheroids possess two LSPR wavelengths (specifically considering prolate PCNs): a longitudinal mode (
) excited along the axis of the spheroid, and a transverse mode (
) with resonance direction perpendicular to the spheroid’s axis.[
11] Depending on the aspect ratio of the spheroid, the values of
and
could be very close (in the case of small aspect ratio) or significantly apart from each other (in the case of high aspect ratio). When linearly polarized light with
excites the PCN spheroid along its axis, a very high local electric field (
) appears at the two poles along the axis. On the other hand, when linearly polarized light with
is used perpendicular to its axis, the local electric fields (
) at the two poles (the hot-spot locations) perpendicular to the axis has a much smaller magnitude than
. Typically, researchers opt to use
to generate SERS signals from PCN spheroid. In this case, unlike the situation with spherical PCNs, the hot-spots are site-specific. Specifically, SERS signals are produced only when analyte molecules are adsorbed on the two poles of the spheroid, given that the spheroid's long axis partially aligns with the polarization direction. Thus, the average EF
for a single spheroid is influenced by the orientation of analytes in the hot-spots, the likelihood of analytes being inside the hot-spots, and the orientation of the spheroid with respect to the polarization direction.
To explore the effect of analyte molecule adsorption orientation, Eq. 13 is still valid. To estimate the probability of analytes inside the hot-spot, we can consider two scenarios: analytes having an equal likelihood of adsorbing on any surface location of the PCN, and the adsorption probability depending on the curvature of the location. [
12,
13]
Let’s consider the first scenario. We can maintain the assumption that the hot-spot on the tip of the spheroid projects a circular area with a radius h on the spheroid. Assuming that the long axis radius of the spheroid is c and short axis radius is a, the probability of finding one of the total adsorbed analytes located at the two hot-spots is given by
, (19)
where the numerator in Eq. 19 is the area of the hot-spot on a pole of the spheroid, and the denominator is the total area of a PCN, , , . Thus, the average for a single prolate PCN can be written as,
. (20)
However, since only the external electric field parallel to the axis of spheroid can generate the SERS signal, if these two vectors make an angle , then the contribution of this particularly orientated spheroid to the SERS signal can be written as,
. (21)
Considering that the orientation of the spheroid particle can be uniformly distributed at any orientation due to Brownian motion, we eventually obtain,
. (22)
For the second scenario, if the analyte’s adsorption probability depends on the local curvature of a PCN, then Eq. 19 can be rewritten as
, (23)
where is the curvature () dependent adsorption probability density of analytes on a small surface dA. The integration is conducted over the entire hot-spot area. Except for the calculation of , the other expressions for the EF shall remain the same. Eq. 22 represents the result of orientational averaging of the PCN spheroids. Clearly, if all the spheroid particles could be aligned along the polarization direction, the maximum SERS signal could be obtained from the spheroid PCNs.
For non-polarized excitation, only the light polarized along the long axis of the spheroid can excite the SERS signal, accounting for only . Assuming that analyte adsorption is independent of the curvature, then
, (24)
i.e.,
. (25)
Thus, compared to Eq. 22, the for the non-polarized excitation (Eq. 25) is larger than that of linear polarization.
Spherical PCN Dimers: Another typical PCN configuration is a spherical colloid dimer with extremely small gaps, ranging from 1 to 5 nm, as depicted in
Figure 3C.[
14] Clearly such a dimer particle is also anisotropic, meaning the formation of hot-spots depends on the polarization of the incident light. Moreover, to obtain a high SERS intensity, the analytes must be located within the gaps; if the analytes are outside the gaps, the SERS signal will be significantly reduced.
The calculation of for PCN dimers is similar to that for PCN spheroids, as the hot-spot is location-specific, and its excitation is highly dependent on the relative orientation of the dimer's long axis and the polarization direction. Therefore, all the discussions applicable to spheroid PCNs are also valid for PCN dimers. As the dimer consists of two spheres, if there are no other effects and the analytes have an equal probability of adsorbing on any surface location of the PCNs (considering only surface adsorption), then,
, (26)
And for a single PCN dimer is,
. (27)
Considering the orientation distribution of the dimers in the solution, according to Eqs. 21 and 22, one has
. (28)
For non-polarized light, the discussions for spheroid PCN can be applied, and Eq. 25 is valid.
Practically, both PCN spheroids and dimers exhibit size and shape distributions. Therefore, the derived Eqs. 22, 25, and 28 must undergo shape and size averaging, similar to the process outlined in Eq. 18, to determine the ultimate .
3.1.2. The Analyte Particles Are Much Larger than the Size of the PCNs
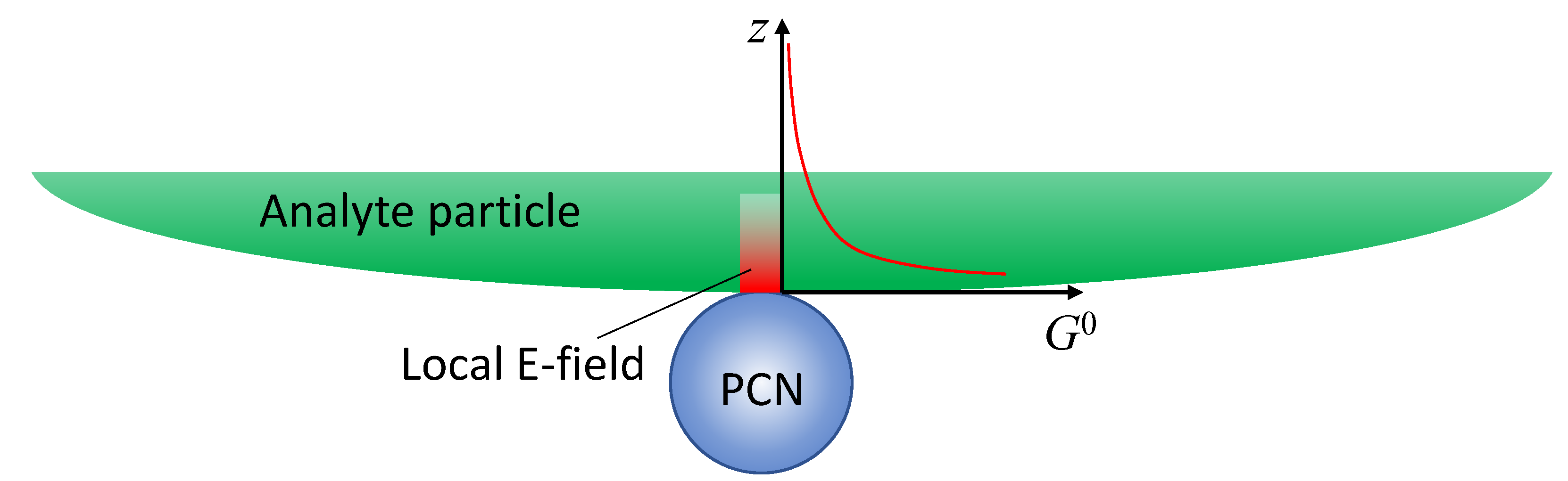
If the analyte is not a small molecule but a biological organism like a virus, bacteria, or tissues, the expression of SERS intensity in solution-based measurements diverges significantly from those in
Section 3.1.1 because the adsorption configuration of PCNs and analyte particles is changed. The PCN can only adsorb onto a very small fraction of the surface of the analyte particles as shown in
Figure 4, and the local electric field from the hot-spot would penetrate into the analyte’s surface following either an exponential or power-law decay relationship. In other words, molecules from various depths within the analyte surface would contribute to the overall SERS spectrum. Let
(
),[
15,
16] and consider an ideal scenario with a spherical PCN as shown in
Figure 4, the layer density of molecules on the analyte surface
varies with depth, leading to distinct SERS scattering cross-sections (
). Then the effective SERS intensity
from a single hot-spot can be expressed as,
. (29)
In this case, defining a SERS EF becomes impractical for several reasons. Firstly, the depth-dependent nature of the analyte particle may not be uniform; different layers at various depths could contain diverse molecules, such as viruses or bacteria, each contributing distinct SERS spectral features. Secondly, accurately estimating the number of molecules contributing to the final SERS spectrum is exceedingly challenging. Finally, determining the contribution of molecules from the limited layer of the analyte particle to the normal Raman intensity presents a formidable task. Due to the high complexity and inhomogeneity of analyte particles, the Raman spectrum is influenced not only by the surface components of the particle but also by contents inside the particles. As a result, the SERS spectrum and the Raman spectrum may exhibit significant differences. Moreover, determining the number of specific molecules responsible for the Raman spectrum is exceptionally difficult. Nevertheless, if we assume that the analyte particle is uniform and possesses a constant surface density (such as a polystyrene colloidal bead), denoted by , , then
, (30)
where the number of the analyte molecules contributing to SERS is .
Spherical PCNs: When a linearly polarized excitation is applied, and the spherical PCNs are significantly smaller than the size of the analyte particle, they can randomly adsorb onto the analyte surface with equal probability. In this scenario, only PCNs adsorbed in locations with a local surface normal component aligned with the polarization direction can generate the SERS signal. This condition applies to PCN particles numbered 1, 2, 6, 7 in
Figure 5A. Let
represent the probability of a spherical PCN adsorbed on the analyte surface. Consequently, both
and
become functions of
and
. If
denotes the average number of spherical PCNs adsorbed on an analyte particle,
represents the density of the analyte particles in the solution, and
is the volume of the detection (the blue dashed box in
Figure 2), then
. (31)
where . Note that should be a function of and , where is the density of spherical PCNs. If the orientation of the surface molecules on the analyte particle surface in the hot-spot regions has a distribution, then in Eq. 31 shall be replaced by , which is determined by Eq. 13.
In the case of non-polarized excitation (
Figure 5B), the hot-spot region forms a band on the PCN, allowing more surface molecules on the analyte particle to contribute to the SERS signal. Due to the symmetry, both
and
become functions of
only. Eq. 31 remains valid with the modification
, thus,
. (32)
In comparison to the expression for linear polarized excitation, Eq. 31, it is anticipated that non-polarized excitation can significantly enhance the SERS intensity.
If the analyte particle possesses an inhomogeneous surface, as shown in
Figure 5, featuring two distinct regions (as seen in bacterial membranes), denoted as I and H, respectively, with different surface molecules characterized by corresponding scattering cross-sections
and
, the situation becomes more complex. Assuming
fraction of PCNs adsorbs on Region I, and
fraction on Region H (where
), then
, (33)
where and are theoretical EFs of corresponding molecules. Eq. 33 shows that in principle the overall SERS spectra is a linear combination of and (both could be depth dependent as shown in Eq. 29). However, the coefficients in this linear combination do not just rely on and , but also on their corresponding SERS EFs ( and ). If the PCNs are not specifically designed to preferentially bind to any region, , where and represent the surface areas of regions I and H on the analyte particle. If the PCNs are selectively modified by certain chemical functionalization groups, will be highly specific. If the surface of the analyte particle comprises more than two inhomogeneous regions, Eq. 33 will be an accumulation of SERS spectra from different surface regions, i.e., Eq. 33 can be extended to situation involving three or more surface components.
Spheroid PCNs: If spheroid PCNs are employed as a SERS substrate to detect analyte particles significantly larger than the PCNs (as illustrated in
Figure 6A) under linearly polarized light, with
, only PCNs with one of their poles adsorbed on the analyte particle and oriented in alignment with the polarization direction can contribute to the SERS signal. This includes particles numbered 1, 6, and 7 in
Figure 6A. The probability of the spheroid poles adsorbed on the analyte particle is given by Eq. 32. If there are a total number of
particles on the analyte particle surface, the number of PCN particles that could potentially produce SERS is
. Only those PCNs adsorbed at locations with a local surface normal component aligned with the polarization direction can generate the SERS signal. Based on Eq. 31, one has,
, (34)
with . For the non-polarized excitation, the argument for Eq. 32 remains valid, and the total SERS intensity will increase by .
Similar argument for inhomogeneous analyte particle is also valid.
Spherical PCN Dimers: If PCN dimer particles are used as shown in
Figure 6B under linearly polarized excitation, it becomes evident that none of the surface molecules of the analyte particle can be located inside the hot-spot positions (gaps). Consequently, using
to excite the hot-spot gap for generating the SERS signal is not advantageous. In the scenario where two PCN spherical particles form a dimer, plasmon hybridization results in two longitudinal modes and one transverse mode. [
17] The hot-spot gap emerges due to the bonding longitudinal mode with a resonant wavelength
, while the anti-bonding mode
and the transverse mode
. For
, the hot-spots are at the two ends of the dimer along the long-axis direction, whereas for
the hot-spots are at the four tops of the two spheres perpendicular to the dimer's long axis, as indicated by the dashed rectangle in
Figure 6B. Therefore, to generate a sufficient SERS signal, one must choose
or
. These two cases align precisely with the spheroid PCN situations discussed earlier. It is anticipated that the produced SERS intensities will be determined by Eq. 34.
This configuration demonstrates that the hot-spot arrangement in a SERS substrate may not necessarily be consistent for different analytes. While the hot-spot gap configuration in PCN dimers is useful for explaining SERS signals when analyte molecules are much smaller than the gap size, as the size of analyte molecules becomes comparable or even larger than the gap, the hot-spot might shift to different locations on the two spherical PCNs. Consequently, adjustments in are necessary to obtain the maximum SERS intensity.