Submitted:
07 November 2023
Posted:
08 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI data acquisition
- MT-weighted: TR = 20 ms, echo time (TE) = 4.76 ms, flip angle (FA) = 8°, scan time 5 min 40 s;
- T1-weighted: TR =16 ms, TE = 4.76 ms, FA =18°, scan time 4 min 32 s;
- Proton-density-weighted: TR= 16 ms, TE = 4.76 ms, FA= 3°, scan time 4 min 32 s.
- 3D FLAIR-SPACE-FSE: TR = 5000 ms, TE = 390 ms, TI = 1800 ms and
- 3D T2-SPACE-FSE: TR=3000ms, TE=335ms.
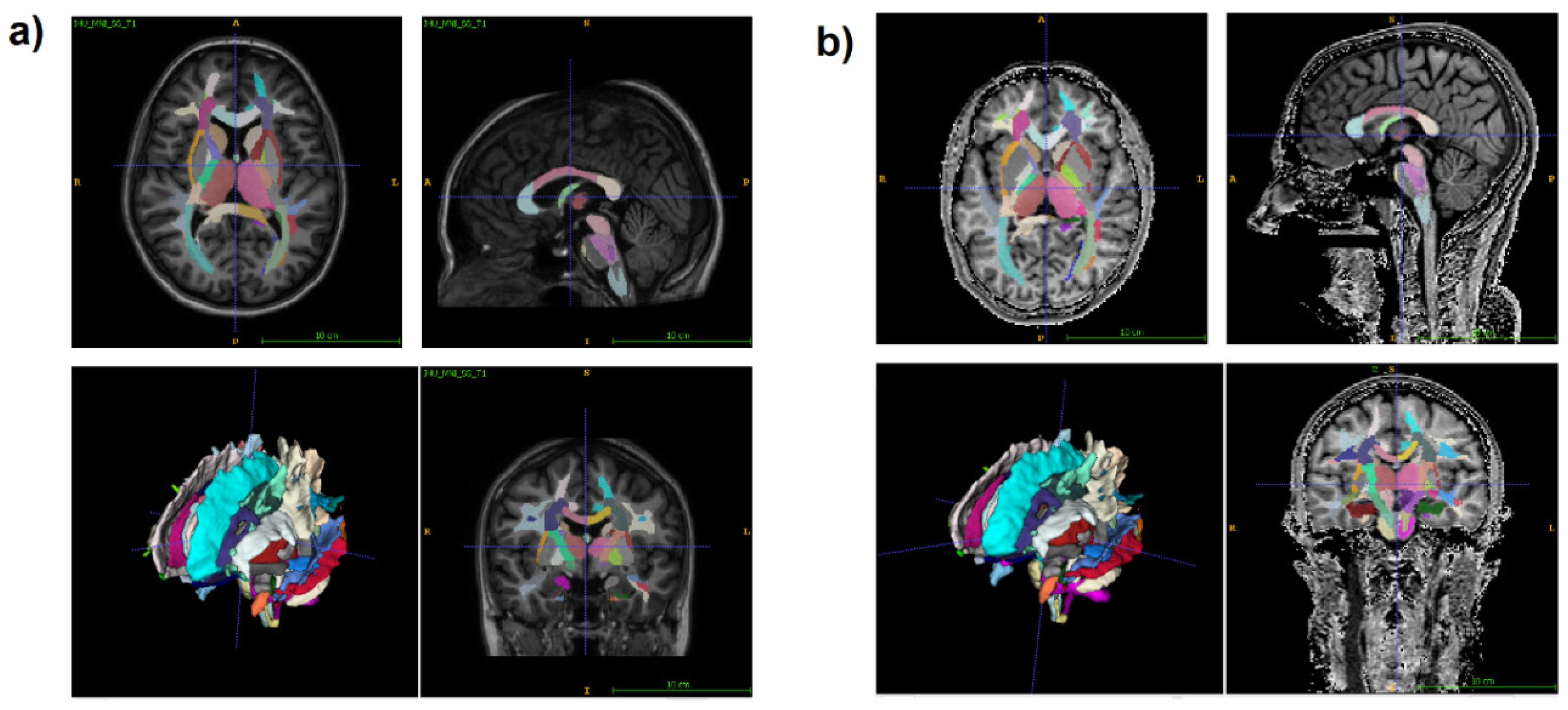
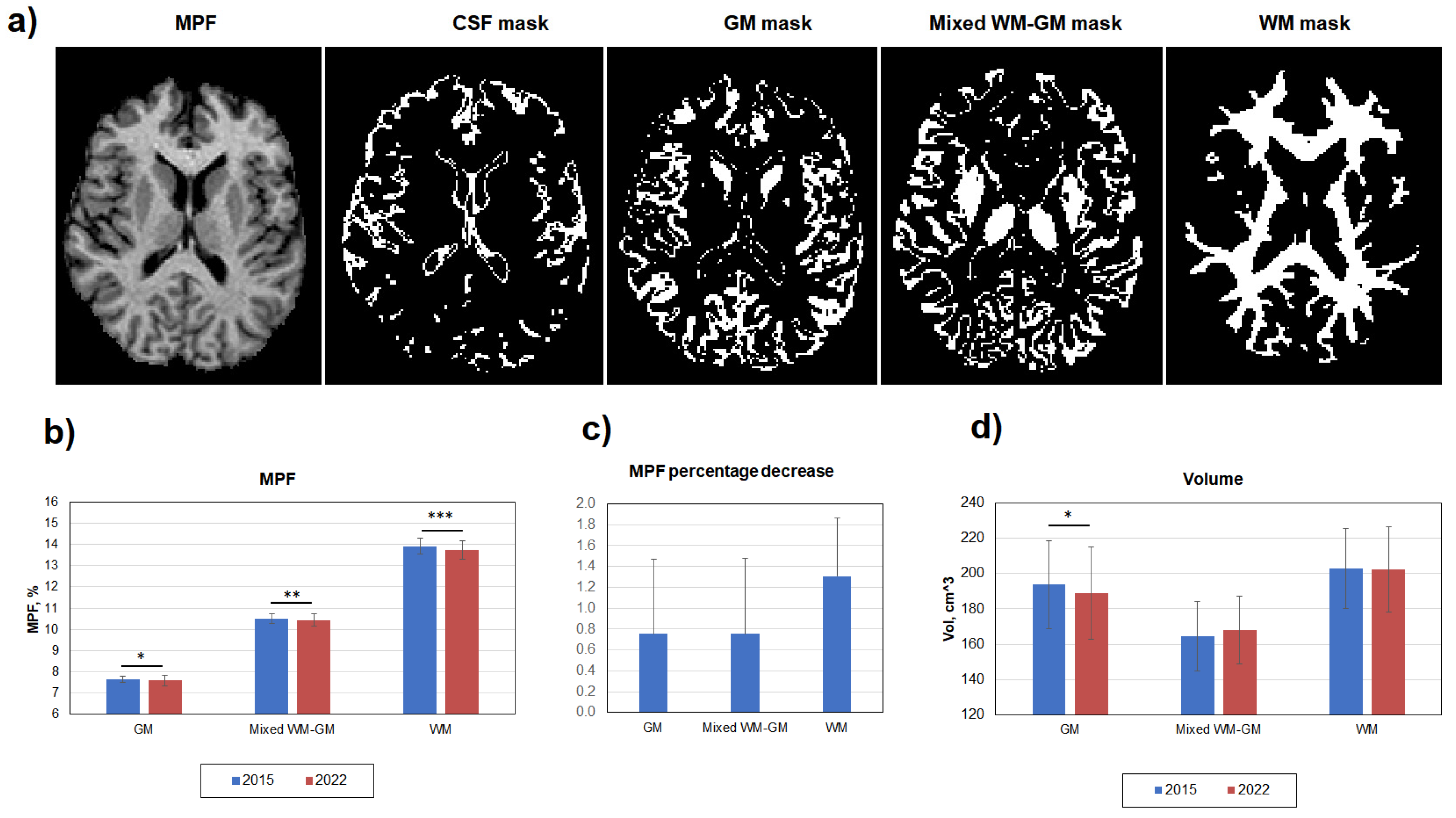
2.3. Image processing
2.4. ELISA
2.5. Statistical analysis
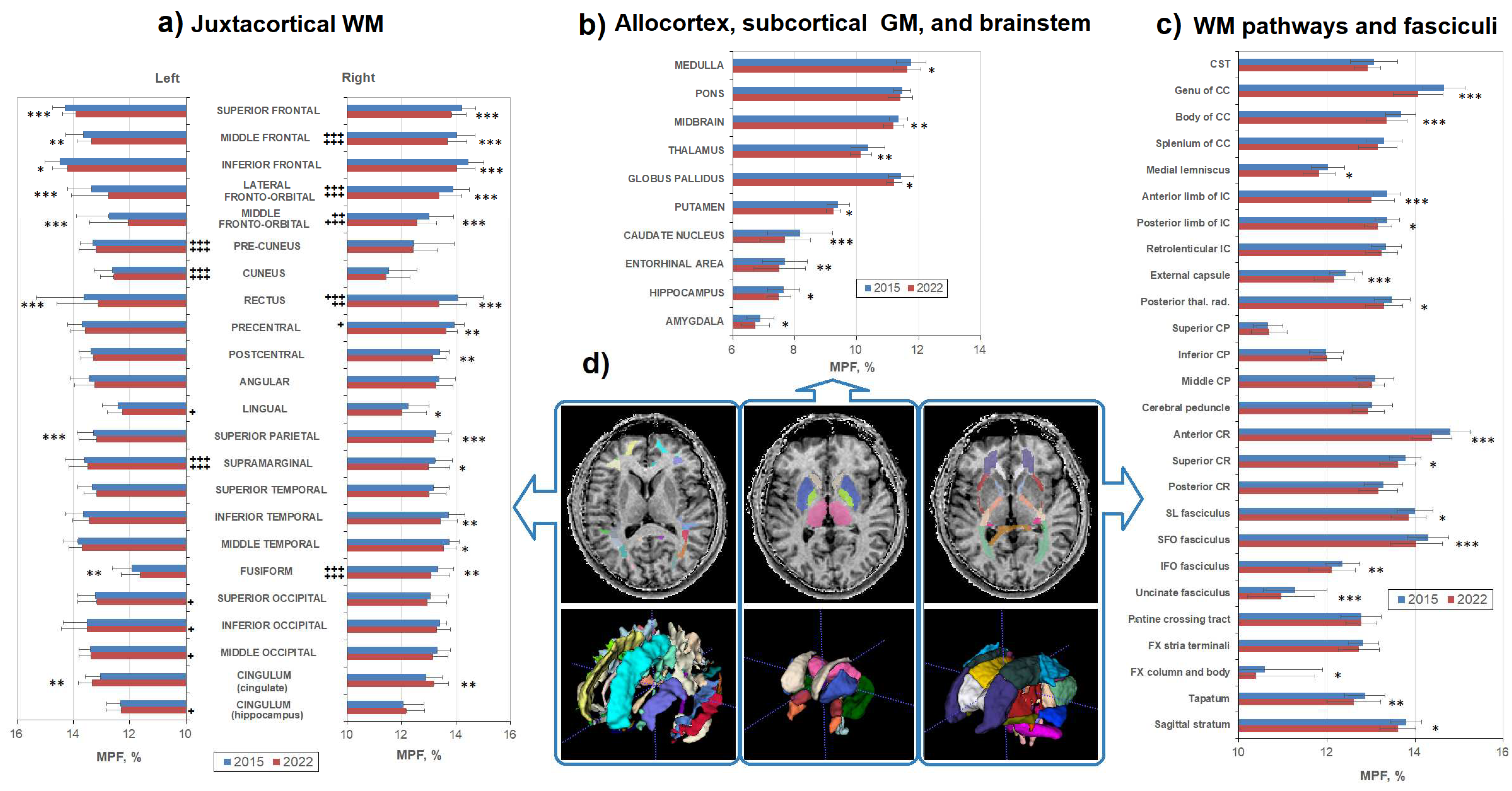
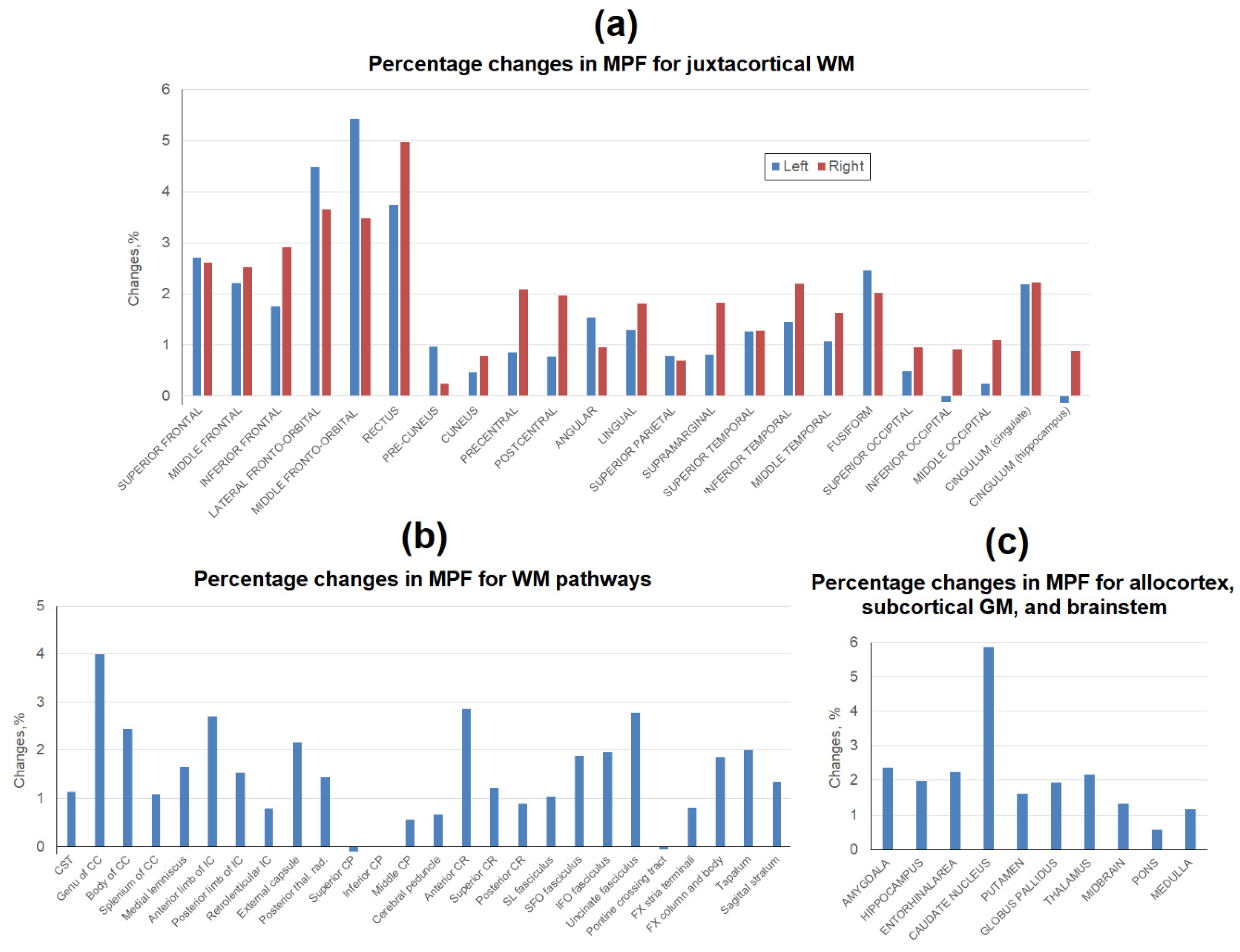
3. Results
3.1. Age-related global changes in the brain myelination
3.2. Age-related changes in separate WM and GM structures
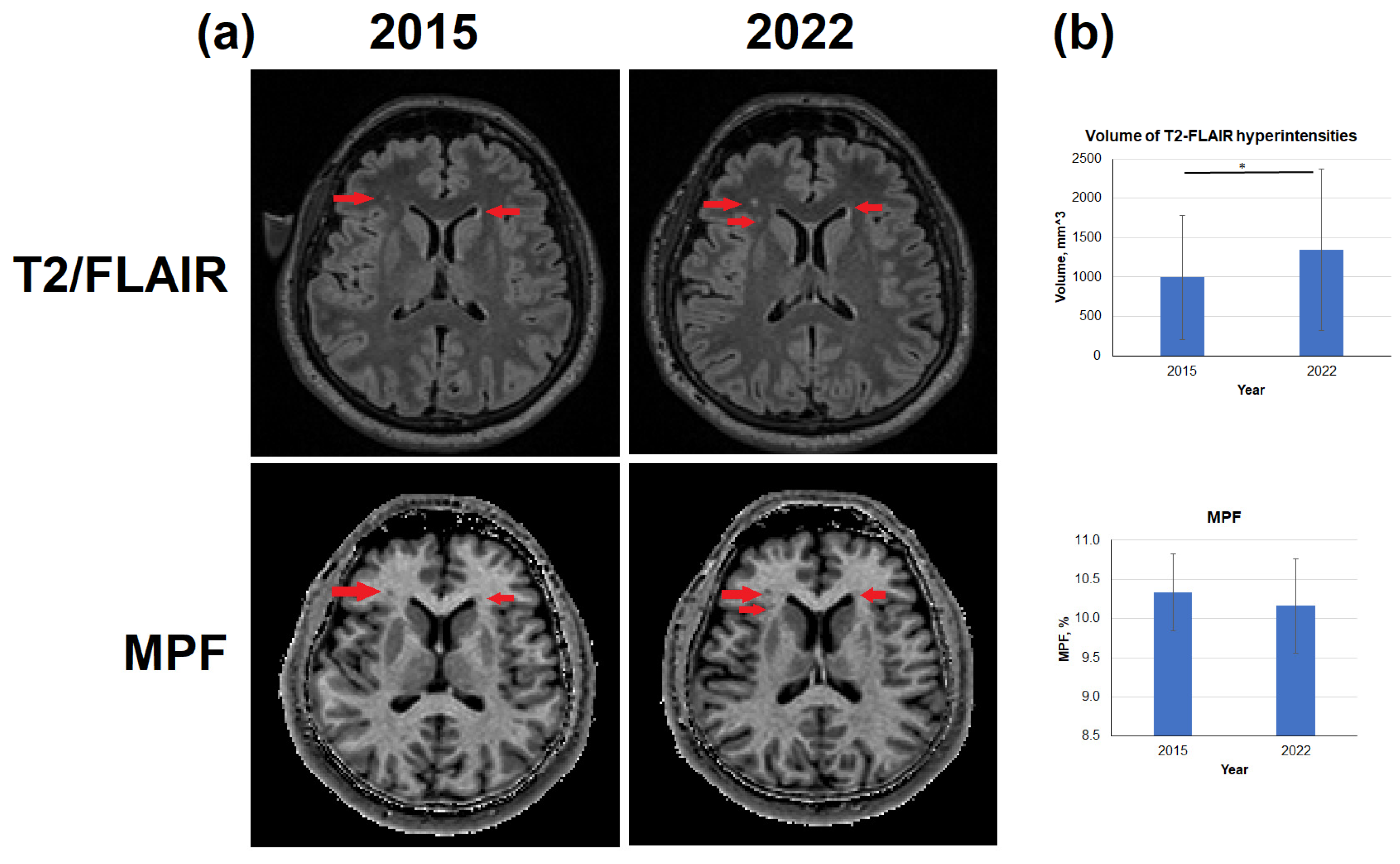
3.3. Age-related changes in the volume of T2-FLAIR hyperintensities
3.4. Age-related changes in myelin-related autoantibodies
4. Discussion
5. Conclusions
6. Study limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, J.H.; Hof, P.R. Life and Death of Neurons in the Aging Brain. Science 1997, 278, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Morrison, J.H.; Rosene, D.L.; Hyman, B.T. Are Neurons Lost from the Primate Cerebral Cortex during Normal Aging ? Cerebral Cortex 1998, 8, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Liang, P.; Fu, H.A.N.; Zhang, J.I.U.C.; Chen, J.U.N. Effects of Normal Aging on Myelin Sheath Ultrastructures in the Somatic Sensorimotor System of Rats. Molecular Medicine Reports 2014, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Peters, A. The Effects of Normal Aging on Myelinated Nerve Fibers in Monkey Central Nervous System. Frontiers in Neuroanatomy 2009, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Peters, A. The Effects of Normal Aging on Nerve Fibers and Neuroglia in the Central Nervous System. Journal of Neurocytology 2002, 31, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Li, A.M.; Grutzendler, J.; Haven, N.; Haven, N. Lifelong Cortical Myelin Plasticity and Age-Related Degeneration in the Live Mammalian Brain. Nat Neurosci. 2018, 21, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Kemper, T.L. Neuroanatomical and Neuropathological Changes during Aging. In Clinical neurology of aging; Albert, M.L., Knoefel, J.E., Eds.; Oxford University Press, 1994; pp. 3–67. [Google Scholar]
- Lintl, P.; Braak, H. Loss of Intracortical Myelinated Fibers: A Distinctive Age-Related Alteration in the Human Striate Area. Acta Neuropathologica 1983, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Nyengaard, J.R.; Pakkenberg, B.; Gundersen, H.J.G. Age-Induced White Matter Changes in the Human Brain: A Stereological Investigation. Neurobiology of Aging 1997, 18, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Franco Piredda, G.; Hilbert, T.; Thiran, J.-P.P.; Kober, T.; Piredda, G.F.; Hilbert, T.; Thiran, J.-P.P.; Kober, T. Probing Myelin Content of the Human Brain with MRI: A Review. Magnetic Resonance in Medicine 2021, 85, 627–652. [Google Scholar] [CrossRef] [PubMed]
- Salat, D.H.; Tuch, D.S.; Greve, D.N.; Kouwe, A.J.W. Van Der; Hevelone, N.D.; Zaleta, A.K.; Rosen, B.R.; Fischl, B.; Corkin, S.; Rosas, H.D.; et al. Age-Related Alterations in White Matter Microstructure Measured by Diffusion Tensor Imaging. Neurobiology of Aging 2005, 26, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Jones, D.K.; Summers, P.E.; Morris, R.G.; Williams, S.C.R.; Markus, H.S. Evidence for Cortical “Disconnection” as a Mechanism of Age-Related Cognitive Decline. Neurology 2001, 57, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Stanley, J.A.; Raz, N. Adult Age Differences in Subcortical Myelin Content Are Consistent with Protracted Myelination and Unrelated to Diffusion Tensor Imaging Indices. NeuroImage 2016, 143, 26–39. [Google Scholar] [CrossRef]
- Faizy, T.D.; Thaler, C.; Broocks, G.; Flottmann, F.; Leischner, H.; Kniep, H.; Nawabi, J.; Schön, G.; Stellmann, J.-P.; Kemmling, A.; et al. The Myelin Water Fraction Serves as a Marker for Age-Related Myelin Alterations in the Cerebral White Matter – A Multiparametric MRI Aging Study. Frontiers in Neuroscience 2020, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Underhill, H.R.; Yuan, C.; Yarnykh, V.L. Direct Quantitative Comparison between Cross-Relaxation Imaging and Diffusion Tensor Imaging of the Human Brain at 3.0 T. NeuroImage 2009, 47, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Whittall, K.P.; MacKay, A.L.; Graeb, D.A.; Nugent, R.A.; Li, D.K.B.; Paty, D.W. In Vivo Measurement of T2 Distributions and Water Contents in Normal Human Brain. Magnetic Resonance in Medicine 1997, 37, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.L.L.; Rutt, B.K.; Arun, T.; Pierpaoli, C.; Jones, D.K. Gleaning Multicomponent T 1 and T 2 Information from Steady-State Imaging Data. Magnetic Resonance in Medicine 2008, 60, 1372–1387. [Google Scholar] [CrossRef] [PubMed]
- Faizy, T.D.; Kumar, D.; Broocks, G.; Thaler, C.; Flottmann, F.; Leischner, H.; Kutzner, D.; Hewera, S.; Dotzauer, D.; Reddy, R.; et al. Age-Related Measurements of the Myelin Water Fraction Derived from 3D Multi-Echo GRASE Reflect Myelin Content of the Cerebral White Matter. Scientific Reports 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.J.M.; Yip, E.; Mackay, A.L.; Thornton, A.E.; Vila-rodriguez, F.; Macewan, G.W.; Kopala, L.C.; Smith, G.N.; Laule, C.; Macrae, C.B.; et al. 48 Echo T2 Myelin Imaging of White Matter in Fi Rst-Episode Schizophrenia: Evidence for Aberrant Myelination. NeuroImage: Clinical 2014, 6, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Grydeland, H.; Walhovd, K.B.; Tamnes, C.K.; Westlye, L.T.; Fjell, A.M. Intracortical Myelin Links with Performance Variability across the Human Lifespan: Results from T1- and T2- Weighted MRI Myelin Mapping and Diffusion Tensor Imaging. The Journal of Neuroscience 2013, 33, 18618–18630. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, J.D.; Wandell, B.A.; Mezer, A.A. Lifespan Maturation and Degeneration of Human Brain White Matter. Nature Communications 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Birkl, C.; Birkl-toeglhofer, A.M.; Endmayr, V.; Romana, H.; Rauscher, A.; Kasprian, G.; Krebs, C.; Haybaeck, J. The in Fluence of Brain Iron on Myelin Water Imaging. NeuroImage 2019, 199, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Seiler, A.; Schöngrundner, S.; Stock, B.; Nöth, U.; Hattingen, E.; Steinmetz, H.; Klein, J.C.; Baudrexel, S.; Wagner, M.; Gracien, R. Cortical Aging – New Insights with Multiparametric Quantitative MRI. Aging 2020, 12, 10–12. [Google Scholar] [CrossRef]
- Seiler, S.; Ropele, S.; Schmidt, R. Magnetization Transfer Imaging for in Vivo Detection of Microstructural Tissue Changes in Aging and Dementia: A Short Literature Review. Journal of Alzheimer’s Disease 2014, 42, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kisel, A.A.; Naumova, A.V.; Yarnykh, V.L. Macromolecular Proton Fraction as a Myelin Biomarker: Principles, Validation, and Applications. Frontiers in Neuroscience 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Underhill, H.R.; Rostomily, R.C.; Mikheev, A.M.; Yuan, C.; Yarnykh, V.L. Fast Bound Pool Fraction Imaging of the in Vivo Rat Brain: Association with Myelin Content and Validation in the C6 Glioma Model. NeuroImage 2011, 54, 2052–2065. [Google Scholar] [CrossRef]
- Yarnykh, V.L.L.; Bowen, J.D.D.; Samsonov, A.; Repovic, P.; Mayadev, A.; Qian, P.; Gangadharan, B.; Keogh, B.P.P.; Maravilla, K.R.R.; Henson, L.K.J.; et al. Fast Whole-Brain Three-Dimensional Macromolecular Proton Fraction Mapping in Multiple Sclerosis. Radiology 2014, 274, 210–220. [Google Scholar] [CrossRef]
- Draganski, B.; Ashburner, J.; Hutton, C.; Kherif, F.; Frackowiak, R.S.J.; Helms, G.; Weiskopf, N. NeuroImage Regional Speci Fi City of MRI Contrast Parameter Changes in Normal Ageing Revealed by Voxel-Based Quanti Fi Cation ( VBQ ). NeuroImage 2011, 55, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.F.; Freund, P.; Draganski, B.; Anderson, E.; Cappelletti, M.; Chowdhury, R.; Diedrichsen, J.; Fitzgerald, T.H.B.; Smittenaar, P.; Helms, G.; et al. Neurobiology of Aging Widespread Age-Related Differences in the Human Brain Microstructure Revealed by Quantitative Magnetic Resonance Imaging q. Neurobiology of Aging 2014, 35, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Karolis, V.R.; Callaghan, M.F.; Tseng, J.C.; Hope, T.; Weiskopf, N.; Rees, G.; Cappelletti, M. Spatial Gradients of Healthy Ageing: A Study of Myelin-Sensitive Maps. Neurobiology of Aging 2019, 79, 83–92. [Google Scholar] [CrossRef]
- Biel, D.; Steiger, T.K.; Bunzeck, N. Age-Related Iron Accumulation and Demyelination in the Basal Ganglia Are Closely Related to Verbal Memory and Executive Functioning. Scientific Reports 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mole, J.P.; Fasano, F.; Evans, J.; Sims, R.; Hamilton, D.A.; Kidd, E.; Metzler-baddeley, C. Genetic Risk of Dementia Modifies Obesity Effects on White Matter Myelin in Cognitively Healthy Adults. Neurobiology of Aging 2020, 94, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Yarnykh, V.L. Time-Efficient, High-Resolution, Whole Brain Three-Dimensional Macromolecular Proton Fraction Mapping. Magnetic Resonance in Medicine 2016, 75, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Yarnykh, V.L. Fast Macromolecular Proton Fraction Mapping from a Single Off-Resonance Magnetization Transfer Measurement. Magnetic Resonance in Medicine 2012, 68, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Naumova, A.V.; Akulov, A.E.; Khodanovich, M.Y.; Yarnykh, V.L. High-Resolution Three-Dimensional Macromolecular Proton Fraction Mapping for Quantitative Neuroanatomical Imaging of the Rodent Brain in Ultra-High Magnetic Fields. NeuroImage 2017, 147, 985–993. [Google Scholar] [CrossRef]
- Yarnykh, V.L.; Krutenkova, E.P.; Aitmagambetova, G.; Henson, L.K.J.; Piedmont, H.; Repovic, P.; Mayadev, A.; Qian, P.; Gangadharan, B. Iron-Insensitive Quantitative Assessment of Subcortical Gray Matter Demyelination in Multiple Sclerosis Using Macromolecular Proton Fraction. American Journal of Neuroradiology 2018, 39, 618–625. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.Y.; Sorokina, I.V.V.; Glazacheva, V.Y.Y.; Akulov, A.E.E.; Nemirovich-Danchenko, N.M.M.; Romashchenko, A.V.V.; Tolstikova, T.G.G.; Mustafina, L.R.R.; Yarnykh, V.L.L. Histological Validation of Fast Macromolecular Proton Fraction Mapping as a Quantitative Myelin Imaging Method in the Cuprizone Demyelination Model. Scientific Reports 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yarnykh, V.L.; Kisel, A.A.; Khodanovich, M.Y. Scan–Rescan Repeatability and Impact of B 0 and B 1 Field Nonuniformity Corrections in Single-Point Whole-Brain Macromolecular Proton Fraction Mapping. Journal of Magnetic Resonance Imaging 2020, 51, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, L.P.; Yarnykh, V.L.; Parshukova, D.A.; Kornetova, E.G.; Semke, A.V.; Usova, A.V.; Pishchelko, A.O.; Khodanovich, M.Y.; Ivanova, S.A. Global Hypomyelination of the Brain White and Gray Matter in Schizophrenia: Quantitative Imaging Using Macromolecular Proton Fraction. Translational psychiatry 2021, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Khodanovich, M.Y.; Pishchelko, A.O.; Glazacheva, V.Y.; Pan, E.S.; Akulov, A.E.; Svetlik, M.V.; Tyumentseva, Y.A.; Anan’ina, T.V. Yarnykh Vasily Leonidovich Quantitative Imaging of White and Gray Matter Remyelination in the Cuprizone Demyelination Model Using the Macromolecular Proton Fraction. Cells 2019, 8, 1204. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Kisel, A.A.; Akulov, A.E.; Atochin, D.N.; Kudabaeva, M.S.; Glazacheva, V.Y.; Svetlik, M.V.; Medvednikova, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Quantitative Assessment of Demyelination in Ischemic Stroke in Vivo Using Macromolecular Proton Fraction Mapping. Journal of Cerebral Blood Flow and Metabolism 2018, 38, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Khodanovich, M.Y.; Gubskiy, I.L.; Kudabaeva, M.S.; Namestnikova, D.D.; Kisel, A.A.; Anan’ina, T.V.; Tumentceva, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Long-Term Monitoring of Chronic Demyelination and Remyelination in a Rat Ischemic Stroke Model Using Macromolecular Proton Fraction Mapping. Journal of Cerebral Blood Flow and Metabolism 2021, 41, 2856–2869. [Google Scholar] [CrossRef] [PubMed]
- Drobyshevsky, A.; Synowiec, S.; Goussakov, I.; Lu, J.; Gascoigne, D.; Aksenov, D.P.; Yarnykh, V. NeuroImage Temporal Trajectories of Normal Myelination and Axonal Development Assessed by Quantitative Macromolecular and Diffusion MRI: Ultrastructural and Immunochemical Validation in a Rabbit Model. NeuroImage 2023, 270, 119974. [Google Scholar] [CrossRef] [PubMed]
- Petrie, E.C.; Cross, D.J.; Yarnykh, V.L.; Richards, T.; Martin, N.M.; Pagulayan, K.; Hoff, D.; Hart, K.; Mayer, C.; Tarabochia, M.; et al. Neuroimaging, Behavioral, and Psychological Sequelae of Repetitive Combined Blast/Impact Mild Traumatic Brain Injury in Iraq and Afghanistan War Veterans. Journal of Neurotrauma 2014, 31, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Yarnykh, V.L.L.; Prihod’ko, I.Y.Y.; Savelov, A.A.A.; Korostyshevskaya, A.M.M. Quantitative Assessment of Normal Fetal Brain Myelination Using Fast Macromolecular Proton Fraction Mapping. American Journal of Neuroradiology 2018, 39, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Korostyshevskaya, A.M.M.; Prihod’ko, I.Y.Y.; Savelov, A.A.A.; Yarnykh, V.L.L. Direct Comparison between Apparent Diffusion Coefficient and Macromolecular Proton Fraction as Quantitative Biomarkers of the Human Fetal Brain Maturation. Journal of Magnetic Resonance Imaging 2019, 50, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; Corrigan, N.M.; Yarnykh, V.L.; Ramírez, N.F.; Kuhl, P.K. Language Experience during Infancy Predicts White Matter Myelination at Age 2 Years. The Journal of Neuroscience 2023, 43, 1590–1599. [Google Scholar] [CrossRef]
- Corrigan, N.M.; Yarnykh, V.L.; Huber, E.; Zhao, T.C.; Kuhl, P.K. Brain Myelination at 7 Months of Age Predicts Later Language Development. NeuroImage 2023, 263, 119641. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.M.; Yarnykh, V.L.; Hippe, D.S.; Owen, J.P.; Huber, E.; Zhao, T.C.; Kuhl, P.K. Myelin Development in Cerebral Gray and White Matter during Adolescence and Late Childhood. NeuroImage 2021, 227, 117678. [Google Scholar] [CrossRef]
- Yarnykh, V.L.; Tartaglione, E.V.; Ioannou, G.N. Fast Macromolecular Proton Fraction Mapping of the Human Liver in Vivo for Quantitative Assessment of Hepatic Fibrosis. NMR in Biomedicine 2015, 28, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behavioural Neurology 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carrera, I.; Frise, E.; Verena, K.; Mark, L.; Tobias, P.; Stephan, P.; Curtis, R.; Stephan, S.; Benjamin, S.; et al. Fiji - an Open Platform for Biological Image Analysis. Nature Methods 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, C.; Smith, G.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Avants, B.B.; Tustison, N.J.; Song, G.; Cook, P.A.; Klein, A.; Gee, J.C. NeuroImage A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. NeuroImage 2011, 54, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Avants, B.B.; Yushkevich, P.; Pluta, J.; Minkoff, D.; Korczykowski, M.; Detre, J.; Gee, J.C. NeuroImage The Optimal Template Effect in Hippocampus Studies of Diseased Populations. NeuroImage 2010, 49, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Faria, A.; Jiang, H.; Li, X.; Akhter, K.; Zhang, J.; Hsu, J.T.; Miller, M.I.; Zijl, P.C.M. Van; Albert, M.; et al. NeuroImage Atlas-Based Whole Brain White Matter Analysis Using Large Deformation Diffeomorphic Metric Mapping: Application to Normal Elderly and Alzheimer ’ s Disease Participants. NeuroImage 2009, 46, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G.; Lu, P.H.; Tingus, K.; Mendez, M.F.; Richard, A.; Peters, D.G.; Oluwadara, B.; Barrall, K.A.; Finn, J.P.; Villablanca, P.; et al. Lifespan Trajectory of Myelin Integrity and Maximum Motor Speed. Neurobiology of Aging 2010, 31, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.L.; Traipe, E.; Hunter, J.V; John, C.; Ledakis, G.E.; Tallent, E.M.; Buchem, M.A. Van; Armstrong, C.L.; Traipe, E.; Hunter, J.V; et al. Medial-Lateral Differences in Myelin Integrity in Vivo in the Normal Adult Brain Age-Related, Regional, Hemispheric, and Medial-Lateral Differences in Myelin Integrity in Vivo in the Normal Adult Brain. American Journal of Neuroradiology 2004, 25, 977–984. [Google Scholar] [PubMed]
- Akhonda, M.A.B.S.; Faulkner, M.E.; Gong, Z.; Church, S.; Agostino, J.D.; Bergeron, J.; Bergeron, C.M.; Ferrucci, L.; Bouhrara, M. The Effect of the Human Brainstem Myelination on Gait Speed in Normative Aging. The journals of gerontology. Series A, Biological sciences and medical sciences, 1–8. [CrossRef]
- Westlye, L.T.; Walhovd, K.B.; Dale, A.M.; Bjørnerud, A.; Due-tønnessen, P.; Engvig, A.; Tamnes, C.K.; Østby, Y. Life-Span Changes of the Human Brain White Matter: Diffusion Tensor Imaging ( DTI ) and Volumetry. Cerebral Cortex 2010, 20, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, E.; Cooper, E. Antibodies to Myelin Basic Protein, Myelin Oligodendrocytes Peptides, a - b -Crystallin, Lymphocyte Activation and Cytokine Production in Patients with Multiple Sclerosis. Journal of Internal Medicine 2003, 254, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Rubner, P.; Schautzer, F.; Egg, R.; Ulmer, H.; Mayringer, I.; Dilitz, E.; Deisenhammer, F.; Reindl, M. Antimyelin Antibodies as a Predictor of Clinically Definite Multiple Sclerosis after a First Demyelinating Event. New England Journal of Medicine 2003, 349, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.M.; Trifilieff, E.; Pender, M.P. Correlation Between Anti-Myelin Proteolipid Protein ( PLP ) Antibodies and Disease Severity in Multiple Sclerosis Patients With PLP Response-Permissive HLA Types. Frontiers in Immunology 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, C.J.; Chen, N.; Sellebjerg, F.; Sørensen, P.S.; Leslie, R.G.Q.; Bendtzen, K.; Nielsen, C.H. Autoantibodies to Myelin Basic Protein (MBP) in Healthy Individuals and in Patients with Multiple Sclerosis: A Role in Regulating Cytokine Responses to MBP. Immunology 2009, 128. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Kövari, E.; Herrmann, F.R.; Cuvinciuc, V.; Tomm, A.M.; Zulian, G.B.; Lovblad, K.O.; Giannakopoulos, P.; Bouras, C. Do Brain T2/FLAIR White Matter Hyperintensities Correspond to Myelin Loss in Normal Aging? A Radiologic-Neuropathologic Correlation Study. Acta Neuropathologica Communications 2014, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ylikoski, A.; Erkinjuntti, T.; Raininko, R.; Sarna, R.; Sulkava, R.; Tilvis, R. White Matter Hyperintensities on Mri in the Neurologically Nondiseased Elderly: Analysis of Cohorts of Consecutive Subjects Aged 55 to 85 Years Living at Home. Stroke 1995, 26, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Markus, H.S. The Clinical Importance of White Matter Hyperintensities on Brain Magnetic Resonance Imaging: Systematic Review and Meta-Analysis. BMJ (Online) 2010, 341, 288. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Total | Male | Female |
|---|---|---|---|
| Sample size (%) | 11(100) | 6(55) | 5(45) |
| Age, 2nd study, years (SD) | 52.2 (8.6) | 54.7 (9.1) | 49.2 (7.7) |
| Age, 1st study, median (min-max) | 44 (33-60) | 48.5(36-60) | 41(33-54) |
| Age, 2nd study, median (min-max) | 51 (40-67) | 55.5(43-67) | 48(40-61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





