1. Introduction
Plant pathogens secrete large amount of effectors to surmount host immunity and hijack host metabolism. Plants develop two layers of immunity to prevent the infection of pathogens, the pathogen-associated molecular patterns (PAMPs)-triggered immunity (PTI), and the effector-triggered immunity (ETI) [
1,
2]. Correspondingly, the successful pathogens evolve mechanisms to overcome the plant immunity, mainly by secreting massive effectors of diverse functions [
3]. Although most of the effectors identified so far are small secreted proteins without domains of characterized function and existing only in some lineages of a species, there are also ‘core effectors’ that are broadly distributed among different pathogens [
4,
5,
6,
7].
The core effectors play crucial roles at different cellular compartments during the interaction between pathogens and their hosts. The lysine motif (LysM) containing proteins are the effectors broadly distributed in many organisms and are extensively studied. At the apoplast, the LysM effectors compete with the plant PAMPs recognition receptor CEBiP for the binding of chitin oligosaccharides, and therefore prevent the chitin oligosaccharides triggered immunity in the plant cells [
8,
9,
10]. The necrosis-inducing secreted protein 1 (NIS1) is a small secreted effector without conserved domain but is broadly distributed in filamentous fungi. The NIS1 homologs could bind to receptor-like cytoplasmic kinase (RLCK) BIK1 to inhibit the PAMPs-induced ROS burst [
4,
11,
12]. The fungal pathogens also secrete the Common in Fungal Extracellular Membrane (CFEM) domain-containing effectors, which may target different cellular compartments to facilitate infection [
13,
14]. In
Magnaporthe oryzae, MoCDIP2 is a CFEM-containing protein that functions at the late infection stages and could induce cell death when transiently expressed in tobacco leaves [
15]. In
Fusarium graminearum, the CFEM effectors target two maize extracellular proteins associated with the wall-associated protein kinase ZmWAK17 to suppress ZmWAK17-triggered cell death [
16]. In
Colletotrichum fructicola, the CFEM-containing effector CfEC12 competes with NPR1 for the binding of MdNIMIN2 to compromise the interaction between NPR1 and its regulator MdNIMIN2, and consequently suppresses plant immunity [
17]. There are also some species-specific ‘core effectors’ had been reported, such as the Pep1 in smut fungus [
18], the PID4-containing protein in several plant associated fungi and bacteria [
19]. Understanding the function of core effectors in different fungal pathogens may put insights into the mechanism underlying the interaction between pathogens and their hosts [
5,
20].
The CAP (cysteine-rich secretory protein (CRISP)/antigen 5/pathogenesis related-1) superfamily proteins are broadly distributed from prokaryotes to eukaryotes [
21]. These proteins play important roles in different cellular processes, including the development and immune response in animals and plants [
22,
23], and the virulence in pathogens [
24]. In
Candida albicans, deletion of
RBE1 or
RBT4, caused significant attenuation in pathogenicity [
25]. In
F. oxysporum and
F. graminearum, the PR-1-like proteins are required for the virulence on mammalian or plant hosts [
26,
27]. In
F. verticillioides, FvSCP1 is required for virulence on maize [
28]. In the wheat stripe rust fungus
Puccinia striiformis f. sp.
tritici, three PsCAP proteins were involved in the pathogenicity [
24]. In
Cytospora chrysosperma, CcCAP1 is required for the suppression of immunity in poplar plants [
29]. So far, only a few studies demonstrated that the CAP proteins are required for lipid export and sterol binding in fungus [
30], and a recent study in
Ustilago maydis uncovered that it could hijack maize cathepsin B-like 3 (CatB3) to cleave the PR1-like protein UmPR-1La, leading to the release of functional CAPE-like peptides for the interruption of plant CAPE mediated immunity [
31]. However, whether these CAP proteins function could target other ligands or proteins during infection remain largely unknown. Notably, recent study in potato PR1 proteins showed that they target subunits of the AMPK kinase complex in
Phytophthora infestans to suppress the pathogenicity [
32], indicating that the CAP proteins may function by interacting with different proteins.
In
M. oryzae, the causal agent of the destructive blast disease of rice, only several core effectors have been functionally characterized, including Slp1 [
8], NIS1 [
4], MC69 [
33], BAS2 [
34], as well as effectors containing conserved domains, such as MoCDIP2, a CFEM domain containing protein [
15]. In this study, we identified 145 putative core effectors (PCEs), functionally characterized 14 PCEs and found that MoPce1 (MGG_05100) was required for full virulence of
M. oryzae, likely through the inhibition of plant immunity by interacting with the rice target OsDi19-5.
2. Results
2.1. Identification of putative core effectors involved in pathogenicity
We identified putative core effectors (PCEs) according to the criteria: (1) with signal peptide but without transmembrane domain; (2) presented in all of the six
Pyricularia/
Magnaporthe isolates (70-15, BR32, DS0505, EI9604, SV9610 and P1609), and at least three of the other 6 species selected, including
Botrytis cinerea,
Colletotrichum gloeosporioides,
Fusarium graminearum,
Sclerotinia sclerotiorum,
Trichoderma reesei and
Ustilago maydis. A total of 145 PCEs was identified (
Table S1). Among them, five were also identified by Saitoh et al (2012). Functional analysis on 14 of the PCEs showed that two of the candidates
MoPCE1 (
MGG_05100) and
MoPEC2 (
MGG_13578) are likely involved in the pathogenicity of
M. oryzae (
Figure S1 and
Table S2).
MoPCE1 encodes a CAP (cysteine-rich secretory protein (CRISP) / antigen 5 / pathogenesis related-1) domain-containing protein [
29].
MoPCE2 encodes a sulphydryl oxidase. We chose
MoPCE1 for further study.
Multiple sequence alignment showed that MoPce1 contains all the conserved motifs of CAP proteins, including the four α- helices (α1-4) and three β-sheet (β1-3), albeit some of the residuals in these motifs differ. However, the three cysteine residuals in β2 and β3 were replaced with other amino acids (
Figure S2A). MoPce1 was phylogenetically most closely related to the homologs from
M. oryzae and other species, such as CcCap2 from
C. chrysosperma, FGSG_03109 from
F. graminearum, and FOXG_14109 from
F. oxysporum, followed by the PR1 homologs from
Arabidopsis thaliana and
Nicotiana tabacum, and then the CAP proteins from other species, such as the yeast PRY1-3 (
Figure S2B).
2.2. MoPce1 is required for the pathogenicity but not asexual development
To further investigate the role of MoPce1 in asexual development and pathogenicity, we generated the complemented strain and performed phenotypic analysis. The Δ
Mopce1 strain was compatible to the wild type and complemented strain in fungal growth, conidiogenesis, and appressorial development (
Figure S3). In the spray inoculation assays, the Δ
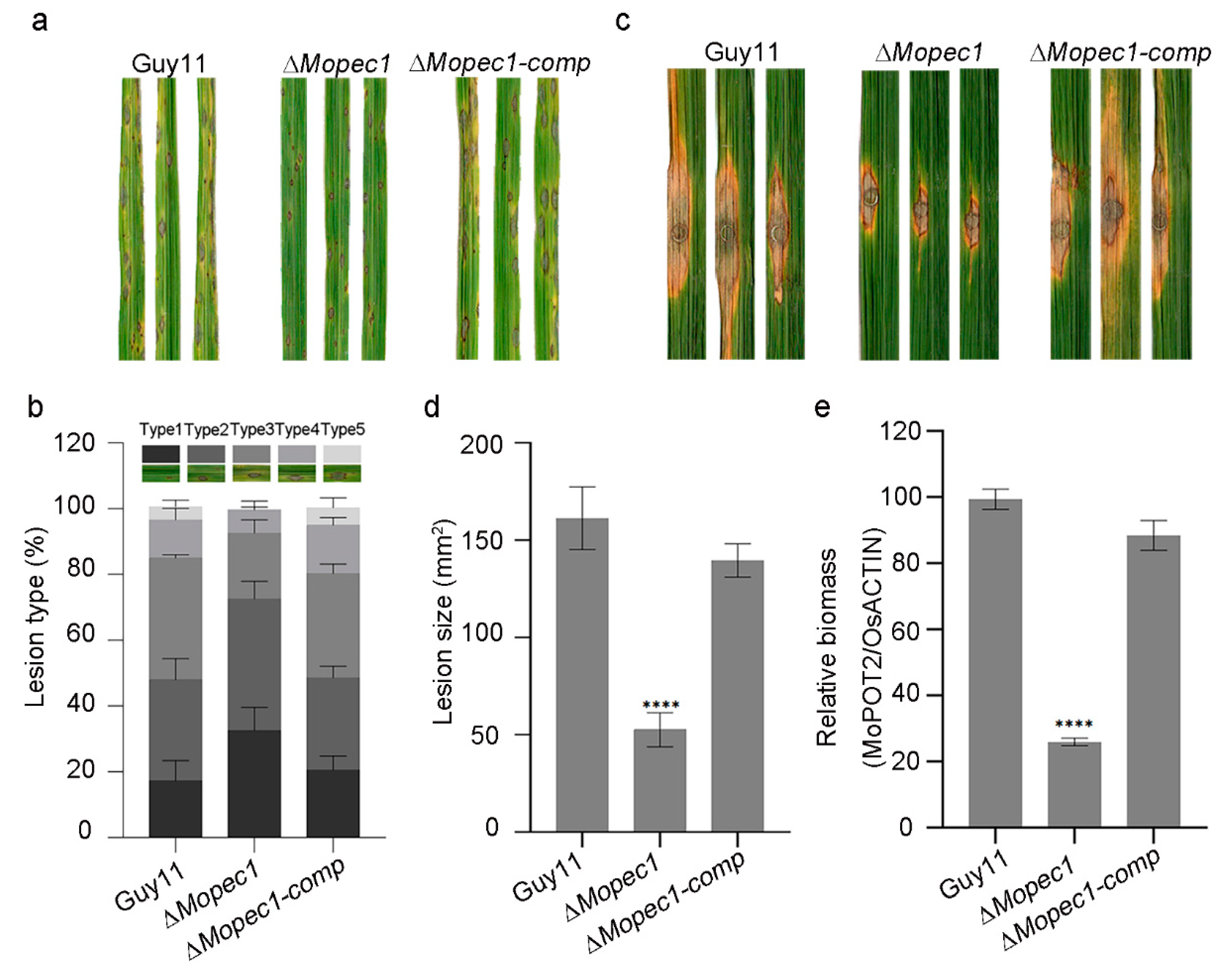
Mopce1 strain caused fewer susceptible lesions than the wild type and complemented strains (
Figure 1a,b). Similarly, in the punch inoculation assays, both the size of and relative biomass of lesions generated by the Δ
Mopce1 strain were significantly reduced compared to that of the wild type and complemented strains (
Figure 2c-e). These results suggested that MoPce1 was required for the pathogenicity but not the vegetative growth, conidiation, appressorial development of the rice blast fungus.
2.3. MoPce1 is a secreted protein localized in the nuclei of plant cells
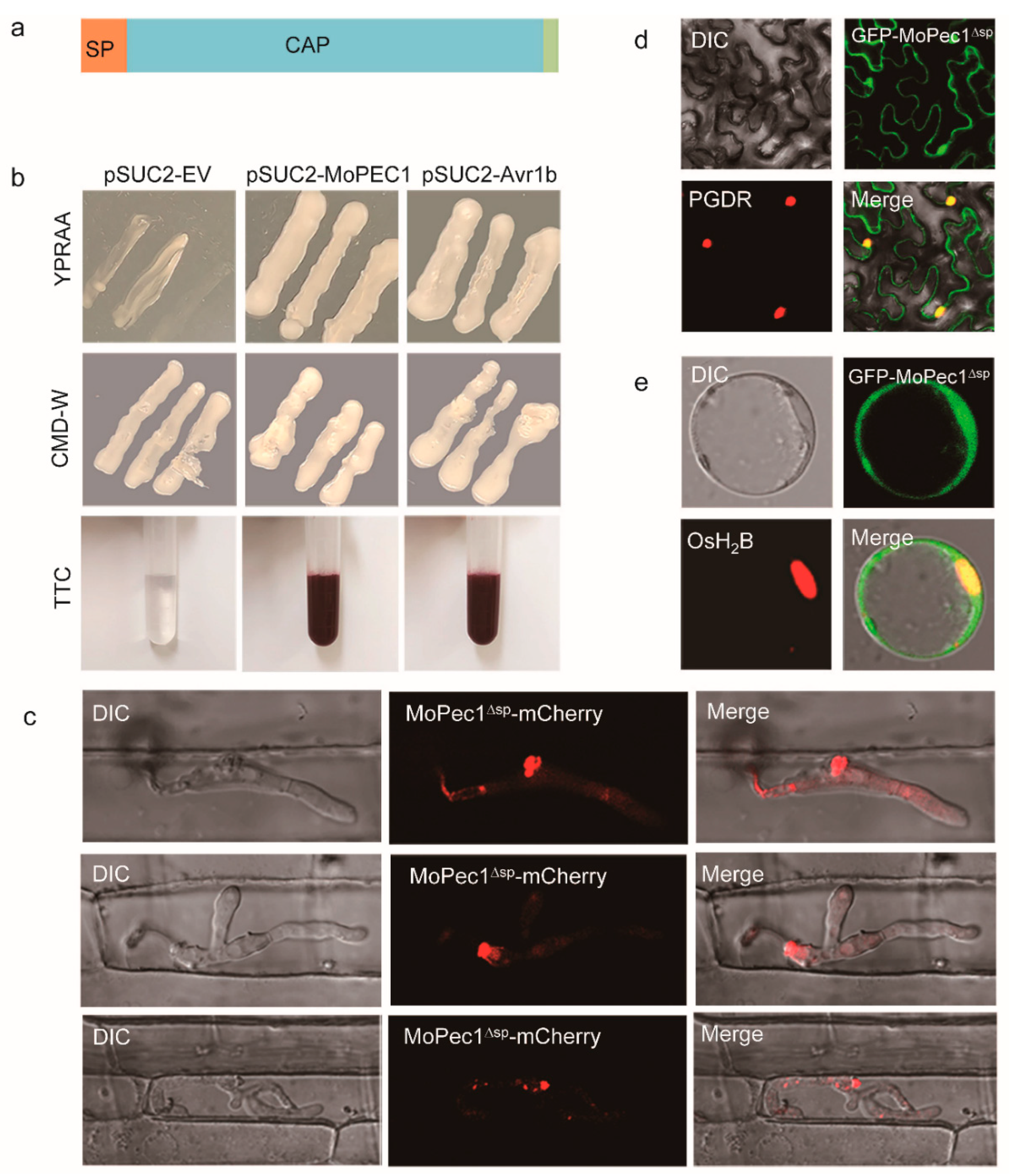
To validate whether MoPce1 is a bona fide secreted protein, we first performed functional evaluation of the predicted secreted peptide (SP) using the yeast signal peptide screen method. Our results showed that fusion of MoPce1_SP enables the growth of
Saccharomyces cerevisiae YTK12 strain on CMD-W and YPRAA media, as well as the induction of red color reaction (
Figure 2a,b). These results indicated that the SP of MoPce1 is functional. We them investigated the subcellular localization of MoPce1. To this aim, we first investigated the function of MoPce1-mCherry and GFP-MoPce1, and found that both fusions could rescue the defects in pathogenicity of
Mopce1 strain (
Figure S4). After the failure in detecting the fluorescence signal by using the
PmPCE1 native promoter, the
PWL2 promoter was used to drive the expression of
MoPCE1-
mCherry fusion. The live-cell imaging assays showed that the fluorescence was accumulated in the biotrophic interfacial complex (BIC) during infection (
Figure 2c), which suggested that MoPce1 is a cytoplasmic effector. Since no overt signal was detected in the rice cell compartments, we also investigated the subcellular localization of MoPce1 in plant cells by ectopically expressing the MoPCE1-GFP fusion in tobacco leaves or the rice protoplasts. And both results showed an accumulation of fluorescence in the nuclei (
Figure 2d,e), indicative of a nuclear localization of this protein.
2.4. Ectopic expression of MoPce1 in rice compromises the plant immunity
To further investigate the function of MoPce1 during the interaction between
M. oryzae and rice, we generated transgenic rice lines ectopically expressing the
MoPCE1ΔSP-
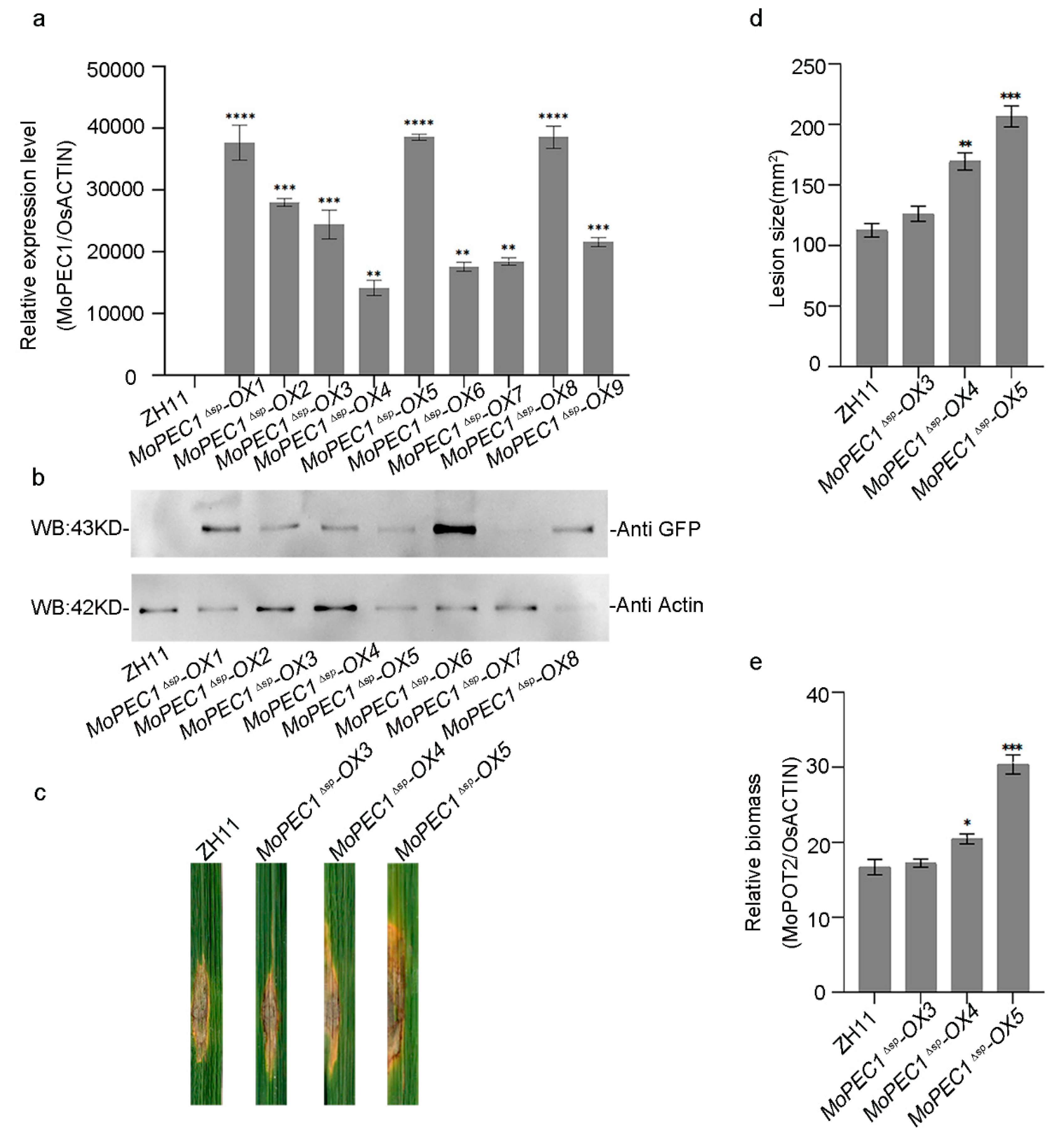
GFP fusion. Night individual lines expressing the
MoPCE1ΔSP-
GFP fusion was identified using both qRT-PCR and Western blot assays (
Figure 3a,b). Live-cell imaging results showed that the MoPce1
ΔSP-GFP fusion may localized in the nuclei of rice cells (
Figure 3c). We then chose three lines (#3, #4 and #5) representing different expressing level for further study. When challenged with the
M. oryzae Guy11 spore solution in the punch inoculation assays, the size and relative biomass of lesions generated on
MoPCE1ΔSP-
OX lines are larger than that of wild type rice cultivar ZH11 (
Figure 3d,e). These results suggested that transgenic rice plants ectopically expressing
MoPCE1ΔSP are more susceptible to rice blast fungus.
We them investigated whether the increased susceptibility in
MoPCE1ΔSP-
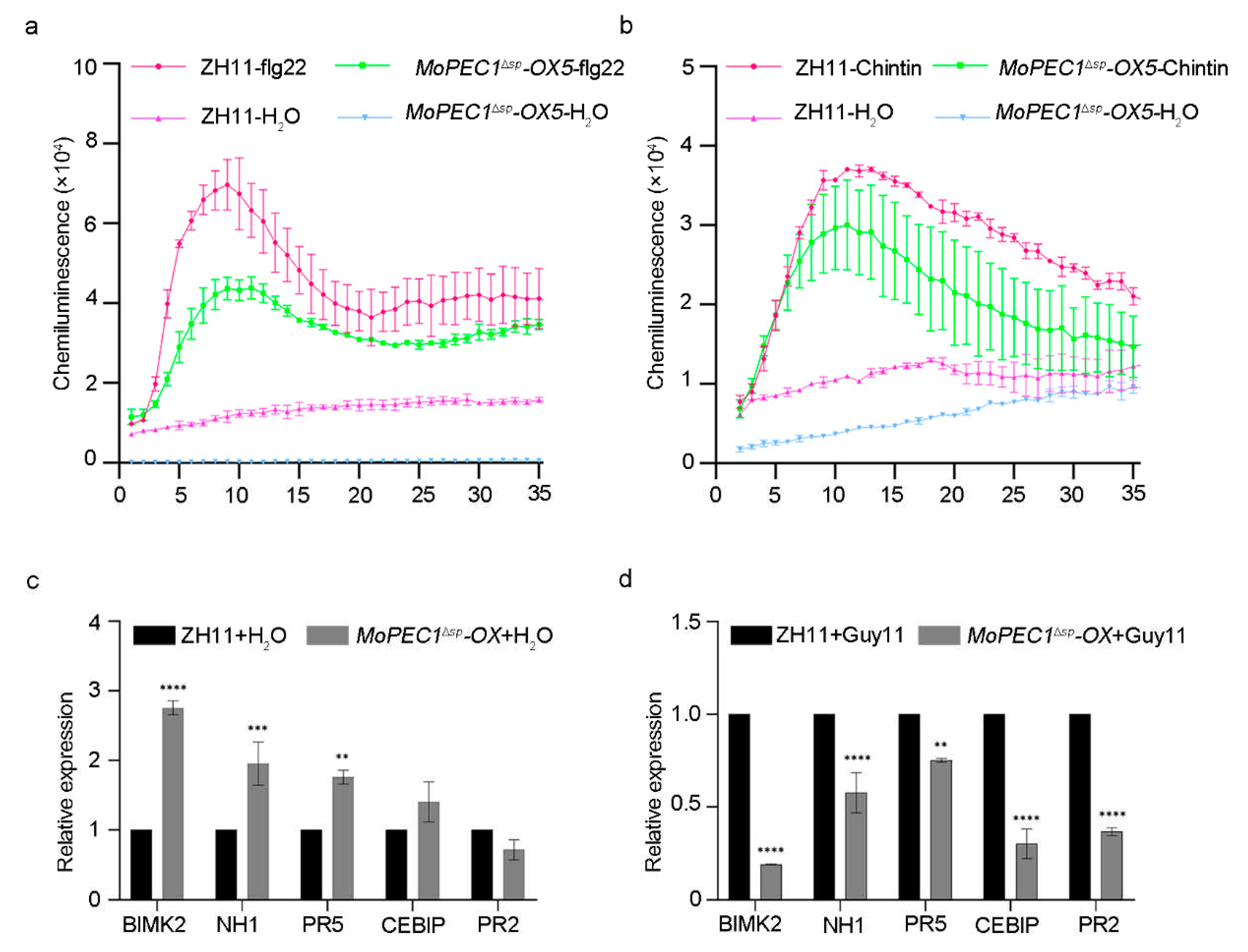
OX lines was caused by the compromised immune response. Upon the treatment of either flg22 or chitin, the
MoPCE1ΔSP-
OX plants displayed a weaker oxidative burst compared with the wild type cultivar ZH11, (
Figure 4a,b). We also found that, upon the treatment of Guy11 spore solution, the related expression level of the pathogenesis-related genes (
PRs) in the
MoPCE1ΔSP-
OX plants were decreased compared with ZH11 wild type (
Figure 4c,d).
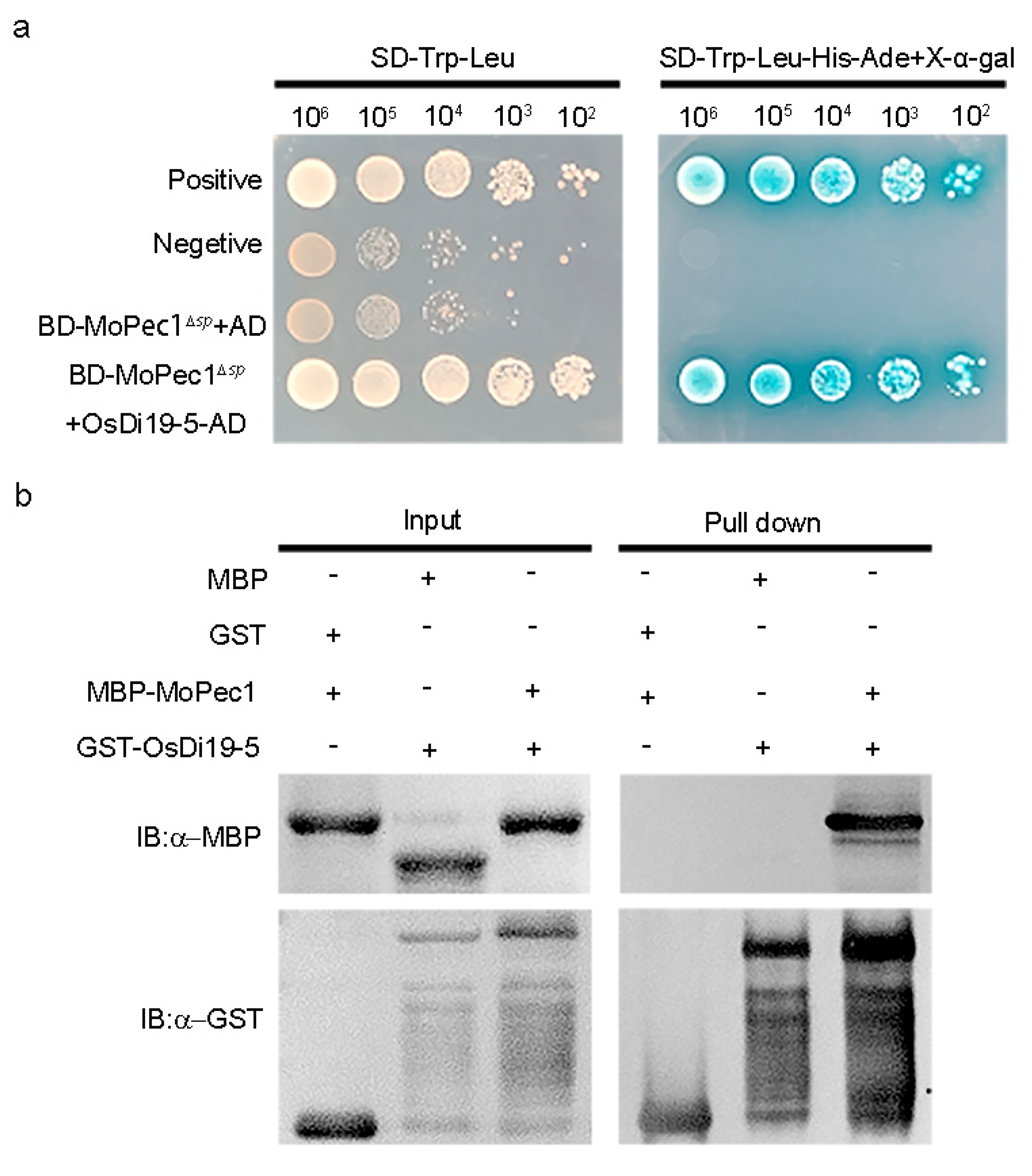
2.5. MoPce1 interacts with OsDi19-5
To explore the mechanisms on how the MoPce1 functions, we performed yeast-two-hybrid to screen the rice cDNA library for MoPce1 interacting proteins (PIPs). Our results showed that OsDi19-5, a transcriptional repressor acting negatively in salt stress response [
35], could be a candidate of PIP. To validate the interaction between Mopce1 and OsDi19-5, both yeast-two-hybrid and pull-down assays were conducted. In the yeast-two-hybrid assays, BD-MoPce1 was able to interact with OsDi19-5-AD (
Figure 5a). In the pull-down assays, MBP-MoPce1 interacts with the GST-OsDi19-5 but not the GST-tag (
Figure 5b). Taken together, our results suggested that MoPce1 could target OsDi19-5.
3. Discussion
In this study, we characterized the function of ‘core effectors’ in rice blast fungus, and revealed that MoPCE1, a CAP domain containing protein is required for the full virulence. In addition, we also found that MoPce1 targets OsDi19-5, which may contribute to the compromised immune response in transgenic rice overexpressing MoPCE1.
The CAP domain containing proteins are broadly distributed in diverse species across all kingdoms. They are characterized by two motifs responsible for sterol binding and fatty acid binding, respectively, and contain several conserved cysteine residues [
36]. In this study, we showed that MoPce1 contains all the conserved motifs but lost all the three conserved cysteine residues of CAP domain. Intriguingly, MoPce1 is phylogenetically closed to PR1 from Arabidopsis compared to the orthologs from many fungal species, such as the PRY1 from yeast. This result is in agree with the hypothesis that these CAP proteins could be expended and evolved independently in
M. oryzae after the horizontally transfer from plants [
36].
The CAP proteins have diversified function in different fungal species. In nonpathogenic fungus yeast, PRY1 and PRY2 are required for the detoxification of compounds like the plant oil eugenol [
37], and the cell wall protein Pry3 plays a negative role in mating [
38]. In the fungal pathogens, increasing evidence demonstrated the roles of CAP proteins in their interaction with the hosts. For example, the RBT4 from
Candida albicans [
25,
39], MpPR-1 from
Moniliophthora perniciosa [
40], Fpr1 from
F. oxysporum [
26], FgPR-1L-4 from
F. graminearum [
27], CAP proteins from the wheat stripe rust Fungus
P. striiformis f. sp.
tritici [
24], CcCAP1 from
C. chrysosperma [
29], and UmPR-1La from
U. maydis [
38]. In this study, we found that deletion of MoPCE1 compromised the pathogenicity of rice blast fungus, and ectopic expression of MoPCE1 in rice reduced immune response in the plants, resulting in a much more susceptibility. However, the
Mopce1 strain still caused susceptible lesions. It’s possible that the other CAP proteins may act redundantly during infection.
The CAP proteins may facilitate the infection of fungal pathogens through different mechanisms. The involvement of CAP proteins in sterol binding and lipid export is well documented and may be required for their role in pathogenicity [
25,
26,
27,
40,
41]. Although direct evidence is still lacking for the role of sterol binding of CAP domain during infection of fungal pathogens, study in tobacco PR-1 revealed that plants could inhibit pathogen growth through the sequestration of sterol from pathogens by PR-1 protein [
42]. In
U. maydis, UmPR-1La not only participates in sensing phenolics and eliciting hyphal-like formation, but also releases CAPE-like peptides to subvert plant immunity [
31]. Besides, pathogens evolved specific clade of CAP protein lacking the conserved cysteine residues required for intramolecular disulfide bridges, and were proposed to bind novel ligands [
36]. The targeting of subunits of AMPK complex in
P. infestans by potato PR1 [
32], and the binding of Rcr3
pim by the venom allergen-like effector protein Gr-VAP1 of
Globodera rostochiensis, also demonstrated that the CAP family proteins may interact with diverse proteins. In this study, the absence of the three cysteine residuals suggested that MoPce1 may act in other pathway rather than sterol export. We showed that MoPce1 was localized at BIC during infection, indicative of a cytoplasmic effector, which was in consistent with the nuclear localization of MoPce1 when ectopically expressed in tobacco leaves and rice protoplasts. In addition, we found that MoPce1 interacts with OsDi19-5, a transcription factor in rice. Since the colleague in our lab had revealed a role of OsDi19-5 in response to
M. oryzae infection (personal communications), it’s very likely that MoPce1 may inhibit plant immunity by targeting OsDi19-5.
4. Materials and Methods
4.1. Identification of the PCE proteins
To identify the putative core effectors (PCEs), we search for the homologs of the secreted proteins identified in the
Pyricularia penniseti isolate P1609 [
43] using orthofinder [
44], from six
Pyricularia/
Magnaporthe isolates 70-15, BR32, DS0505, EI9604 and SV9610 [
45], and the other six species, including
B. cinerea,
C. gloeosporioides,
F. graminearum,
S. sclerotiorum,
T. reesei and
U. maydis. A total of 146 proteins were identified as the PCE candidates (
Table S1). After the initial screening through a pathogenicity analysis using the knock-out mutant of 14 genes (able S2),
MGG_05100 encoding a protein with CAP domain was chose for further investigation. NCBI database and UniProt [
46] was used to predict homologous proteins that have been studied and CAP superfamily members from selected species. SignalP 4.1 was used to predict signal peptides (
https://services.healthtech. dtu.dk/services/SignalP-4.1/). Additionally, we used Mega X and Clustalx to construct a phylogenetic tree of CAP protein homologs. Multiple sequence alignment was performed using ClustalW (
https://www.genome.jp/ tools-bin/clustalw) and ESPript (
https://espript.ibcp.fr/ESPript/ESPript/ index.php).
4.2. Culture conditions of fungal strains
Manipulation of fungal strains was performed as described previously [
47]. The indicated strains were cultured at 28°C in complete medium (CM: 6 g/L yeast extract, 6 g/L acid-hydrolyzed casein, 10 g/L sucrose, 20 g/L agarose). Fungal mycelia were harvested by culturing in liquid CM medium at 28°C in the dark. For the induction of conidiation, fungal blocks were transferred from CM plates to rice bran medium (RBM: 40 g/L rice bran, adjusted to pH 6.0-6.5 with NaOH, 20 g/L agar). The cultures were incubated in the dark at 28°C for 5-7 days, followed by 3 days of light exposure to obtain conidia.
4.3. Generation of deletion strains and its complemented strains
To construct
MoPEC1 deletion mutants and complemented strains, we used the knockout vector pCX62 and the marker fusion method with PEG-mediated transformation. The full-length open reading frame (ORF) sequence of
MoPEC1 was deleted. First, the upstream and downstream flanking sequences of
MoPEC1 (973 bp and 1022 bp, respectively) were amplified using the primers MoPEC1-AF/AR and MoPEC1-BF/BR (
Table S3). Then, the PCR products were inserted into the pCX62 vector containing the hygromycin phosphotransferase (HPH) cassette. Finally, the fusion fragment (AH and HB) was amplified and transformed into the protoplasts of Guy11. The transformed colonies were screened using the primer pairs MG5100tF/tR and UAF/H853 (
Table S3). Southern blot analysis was performed to confirm the deletion mutants.
To construct complemented strains of
MoPEC1, we amplified a 3.2 kb sequence containing the upstream- and downstream region of
MoPEC1 using the primers 5100com_KpnIF/5100com_HindIIIR (
Table S3), which was inserted into the pKNT vector. The resultant plansmid was then introduced into the
Mopce1 protoplasts.
4.4. Pathogenicity and live-cell imaging assays
Spore solution of each M. oryzae strain (5×104 spores/mL in 0.02% Tween 20) was used to inoculate leaves of approximately 3-week-old rice plants. Disease symptoms caused by M. oryzae were recorded and calculated at 5-7 days after inoculation (Stella Cesari, et al., 2022).
To further analyze the effect of
MoPEC1 deletion on the pathogenicity of
M. oryzae, we performed Punch inoculation. Spore solution of strains indicated was adjusted to 5×10
5 spores/mL using sterilized water containing 0.02% vol/vol Tween 20. A 10 μL spore suspension was inoculated into wounded rice leaves, and the inoculated rice plants were placed in a greenhouse. Disease symptoms were investigated and recorded 10 days after inoculation. DNA extraction was performed on the punched rice leaves using the CTAB method, followed by quantitative fluorescence analysis using the primer pairs MoPot2-qRT-F/R and OsUG-qRT-F/R [
48].
For live-cell imaging assays, spore solution (5×105 spores/mL in sterilized water) of WT, ΔMopec1, and the complement strains were inoculated into sheaths of approximately 4-week-old rice plants, which were then kept in darkness and high humidity. The hyphal invasion efficiency was investigated 24 hours after inoculation using Nikon A1R laser scanning confocal microscope system (Nikon, Japan).
4.5. Functional analysis of signal peptide
To verify the function of
MoPEC1 signal peptide, we integrated the signal peptide sequence into the pSUC2 vector (which can synthesize tryptophan and carry a sucrose utilization gene fragment lacking signal peptide). The constructed vector was transformed into the yeast strain YTK12 (a nutritional-deficient strain lacking sucrose utilization gene and unable to grow in the absence of tryptophan) using lithium acetate method. The transformed yeast was plated on CMD-W (tryptophan-deficient plate) and incubated in darkness at 30°C for 3 days. Then, single colonies grown on CMD-W plate were transferred to YPRAA plate (10 g/L yeast extract, 20 g/L peptone, 20 g/L sucrose, supplemented with 20 μg/ml agar) as a carbon source. When the signal peptide with secretion function was connected to the pSUC2 vector, it allowed YTK12 strain to grow on YPRAA plate, and the activity of sucrose utilization enzyme was determined by reducing 2,3,5-triphenyltetrazolium chloride (TTC) to insoluble red 1,3,5-triphenylformazan (TPF) [
49,
50].
4.6. Transient expression in Nicotiana benthamiana leaves
The coding sequence of
MoPCE1 lacking the signal peptide (
MoPCE1Δsp) was amplified using Pcxsn-MoPec1-F/R and inserted into pCXSN vector, and the constructed vector was introduced into
Agrobacterium tumefaciens GV3101 competent cells. After the screening on LB agar plates supplemented with 200 μg/mL kanamycin and 100 μg/mL rifampicin, the colonies were validated through PCR-based genotyping using the primers PCXSN-seq-F/R (Additional file 2:
Table S3).
The preparation of inoculum was performed as described previously [
51]. In brief, transformed strains were inoculated into liquid LB medium supplemented with 200 μg/mL kanamycin and 100 μg/mL rifampicin for 24-36 hours. Bacteria was collected and suspended in the Agrobacterium infiltration buffer (10 mM MES, 10 mM magnesium chloride, 150 μM acetosyringone; OD
600=0.4). Incubate the bacterial suspension in a culture chamber at 28°C for 4 hours, then infiltrated into 4-week-old tobacco leaves. The inoculated seedlings were cultured in the dark for 48 hours. Microscopic examination was performed using a Nikon A1R laser scanning confocal microscope system (Nikon, Japan).
4.7. Transformation of rice protoplasts
The transformation of rice protoplasts was performed as described previously [
52]. Rice seeds were germinated and grown in the dark for about 10 days. The yellowing shoots were then cut into 0.5 mm sections and placed in a sterile conical flask containing pre-cooled 0.6 M mannitol. The cut tissues were subjected to vacuum infiltration in the dark for 20 minutes, and then the supernatant was discarded. Next, 20 mL of enzyme solution (0.6 M mannitol, 10 mM MES, 1% cellulase RS, 0.5% pectolyase R10, 0.1% bovine serum albumin, 1 mM calcium chloride) was added to the conical flask, and the tissues were subjected to vacuum infiltration in the dark for 15 minutes. The flask was then incubated at 28 °C with shaking at 70 rpm for 4 hours to allow protoplast release. The enzyme solution was then discarded, and the rice tissues were resuspended in 20 mL of W5 medium (154 mM sodium chloride, 125 mM calcium chloride, 5 mM potassium chloride, 5 mM glucose, 2 mM MES, pH adjusted to 5.7 with potassium hydroxide) to release the protoplasts. The rice tissues were filtered through a miracloth, and the collected rice protoplasts were transferred to a 50 mL EP tube. The plant tissue was washed once with W5 medium, and then the tube was centrifuged at 4 °C, 790 g to collect the pellet. The pellet was washed once with W5 medium, and the supernatant was discarded. The protoplasts were resuspended in W5 medium to a concentration of 2.5 × 10
6 cells/mL. The protoplasts were then divided into 2.0 mL centrifuge tubes, with 150 μL per tube, and incubated on ice for 30 minutes before transformation.
10 ng of plasmid (pHF223-GFP-MoPCE1) was added to 150 μL of protoplasts and gently mixed. An equal volume of 40% PEG was then added, and the mixture was gently inverted. The rice protoplasts were incubated in a 25°C incubator in the dark for 10 minutes, and then the mixture was diluted with double volume of W5 medium. After centrifugation at 790 g for 3 minutes, the supernatant was discarded, and 500 μL of W5 medium was added. The protoplasts were incubated at 25°C in the dark for 16 hours, and the localization was observed under a Nikon A1R laser scanning confocal microscope system (Nikon, Japan).
4.8. Construction and validation of transgenic rice plants
The transgenic rice plants ectopically expressing MoPCE1Δsp-GFP fusion in the ZH11 background (pCXUN-MoPCE1Δsp-GFP) was constructed by Wuhan Boyuan Company. Plant DNA was extracted from its leaves using the CTAB method. A small amount of plant leaves was placed in a 2 ml EP tube with steel beads and frozen in liquid nitrogen. The sample was then ground using a tissue grinder (TISSUEL YSER-2L, Shanghai Jingxin Industrial Development Co., Ltd., China) at 45 Hz for 90 s, with a repeated grinding step. Next, 500 μl of preheated CTAB extraction buffer (2% CTAB, 1.42 M NaCl, 20 mM EDTA, 100 mM Tris HCl) and 6.5 μl of RNase were added to each tube, followed by 1 min of shaking and 10~15 min of incubation at 65 ℃. After that, 0.7 times the volume of chloroform was added, and the mixture was vigorously shaken for 1 min. The sample was then centrifuged at 12,000rpm for 10 min, and 350 μl of the supernatant was transferred to a new 1.5 ml EP tube. An equal volume of isopropanol was added, and the mixture was incubated at -20℃ for 2 hours. After centrifugation at 12,000 rpm for 10 min, the supernatant was discarded, and the precipitate was washed with 70% ethanol. Finally, the precipitate was dissolved in 50 μl of ddH2O using the MoPEC1-tF/tR primers for preliminary verification (Chen, 2004).
For protein extraction, an appropriate amount of leaves was frozen in liquid nitrogen and stored at -80℃. The leaves were ground into powder in liquid nitrogen, and the powder was transferred to a 15 ml EP tube. An appropriate amount of protein lysis buffer (50 mL, lysis buffer: 500ul PMSF + 500 μl protease inhibitor) was added, mixed thoroughly, and placed on ice for 30 min. The sample was inverted every 10 min to promote lysis. Then, the sample was centrifuged at 4℃, 12,000rpm for 10 min, and the supernatant was filtered into a new EP tube. 120 μl of the supernatant was taken as the input sample. 20 μl of GFP beads (Tiandi Renhe, Smart-Lifesciences, China) was added to a 1.5 ml EP tube, and each tube of beads was washed with 500 μl of washing buffer for three times. The washed beads were transferred to the protein solution and incubated at 4℃ with rotation for 3 hours. The beads were then captured using a magnetic rack, and the supernatant was removed. The beads were washed with 500 μl of washing buffer for three times. After that, 80 μl of washing buffer and the corresponding 5×loading buffer were added, and the mixture was boiled at 100℃ for 10 min. Finally, Western blot analysis was performed using anti-GFP antibody (M20004; Abmart, China), Anti-plant actin antibody (ABL1055; Abbkine Scientific, China), and Goat Anti-Mouse IgG HRP (M21001; Abmart, China. Western Bright ECL HRP substrate (Advansta, Menlo Park, USA) was used for the detection of chemiluminescent signals.
4.9. Measurement of ROS in rice leaves
Rice leaf samples from approximately six-week-old transgenic rice plants were punched with a hole puncher to obtain 2 mm diameter rice discs (care should be taken to avoid secondary damage to the rice leaves during punching, and ensure that the size of each leaf is consistent). The prepared rice discs were placed in sterile water and incubated in the dark for 10 hours. The rice discs were then placed in a 96-well plate, with 2 rice discs per well, and 90 μL of a working solution containing 100 μM luminol, 5 μg/mL of horseradish peroxidase, and 8 nM hexa-N-acetyl-chito-hexaose. The samples were incubated in the dark for 30 minutes, and then 10 μL of 500 nM flg22 or 80 nM chitin was added to each well, with water as a control. The production of ROS in the rice tissues was immediately measured using a luminometer (Thermo scientific, USA) [
53].
4.10. Yeast two-hybrid and Pull down assays
To screen for targets of MoPec1 in host cells, we employed the yeast two-hybrid technique using the lithium acetate method to select rice proteins that interact with MoPec1 from a rice cDNA library. Subsequently, we identified OsDi19-5 as a target of MoPec1. To validate this interaction, we constructed p
BD-MoPec1 and p
AD-OsDi19-5 plasmids and co-transformed them into the yeast strain AH109. The candidate clones were grown on SD/-His/-Leu/-Trp medium and supplemented with X-α-Gal for β-galactosidase analysis. The results indicated that the two proteins could interact. To further confirm the interaction between these two proteins, we constructed p
GEX-4T-OsDi19-5 and p
Mal-c2X-MoPCE1 vectors and transformed them into Escherichia coli strain BL21. Single colonies were inoculated in 5 mL LB liquid medium and incubated overnight at 37°C with shaking at 180 rpm. Then, 500 μL of the overnight culture was transferred to 50 mL of fresh LB medium and incubated at 37°C until the optical density at 600 nm (OD
600) reached 0.4-0.6. Isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to induce protein expression at a final concentration of 1 mM, followed by incubation at 16°C with shaking at 150 rpm for 12 hours. The bacterial culture was then transferred to 50 mL centrifuge tubes and centrifuged at 4°C and 7,000 rpm to collect the bacterial cells. The supernatant was discarded, and the cell pellet was resuspended in GST lysis buffer containing 20 mM KH
2PO
4-K
2HPO
4 and 300 mM sodium chloride. Before use, PMSF, DTT, and Triton X-100 were added to achieve final concentrations of 1 mM PMSF, 1 mM DTT, and 1% Triton X-100. The cell suspension was centrifuged at 4°C and 7,000 rpm to collect the bacterial cells. Then, 8 mL of GST lysis buffer (containing 1 mM PMSF, 1 mM DTT, and 1% Triton X-100) was added to the bacterial cells without generating bubbles. The cells were then disrupted using an ultrasonic cell disruptor (working for 4 s and resting for 6 s, with a total processing time of 12 min per sample). After cell lysis, the mixture was centrifuged at 4°C and 12,000 rpm for 30 min to collect the supernatant. Meanwhile, the GST magnetic beads (Smart-Lifesciences, China) were equilibrated by washing with GST buffer six times. The supernatant containing GST fusion protein was mixed with the equilibrated GST magnetic beads (avoiding bubble formation) and incubated at 4°C for 3 hours. The supernatant was removed using a magnetic stand, and the GST magnetic beads were washed six times with GST buffer. Then, the GST magnetic beads were transferred into the supernatant of MBP (maltose-binding protein) and mixed gently to remove bubbles. A 100 μL aliquot of the supernatant was collected as the input sample, and the remaining mixture was incubated at 4°C for 3 hours. After incubation, the supernatant was removed, and the GST magnetic beads were washed six times. The maltose-binding protein (MBP) and GST-tagged proteins were purified, followed by analysis using Western blotting [
48]. The Western blotting was performed using anti-GST antibody (M20007; Abmart, China), anti-MBP antibody (M20051; Abmart, China), and Goat Anti-Mouse IgG HRP (M21001; Abmart, China). Western Bright ECL HRP substrate (Advansta, Menlo Park, USA) was used for the detection of chemiluminescent signals.
4.11. Statistical analysis
The statistical analysis was performed using the
t-test in GraphPad Prism 7.0 Software with the setting for 2-samples assuming unequal variances (GraphPad Software, San Diego, California, USA,
www.graphpad.com).
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1,
Figure S1: Deletion of
MoPCE1 or
MoPCE2 compromised the pathogenicity of rice blast fungus. (
a) strategy for the generation of MoPCE1. (
b) Evaluation of Mopce1 candidates through Southern blot. c,d Deletion of MoPCE1 (
c) or MoPCE2 (d) resulted in reduce pathogenicity in the mutant strains in the punch inoculation assays.
Figure S2: MoPce1 is a conserved CAP protein. (a) Multiple sequence alignment of MoPce1 and CAP proteins from F. graminearum (FGSG_03109), C. chrysosperma (CcCap2), S. cerevisiae (PRY1), A. thaliana (PR-1), and U. maydis (UMAG_01204). The four α-helices (α1-4) and three β-sheet (β1-3) were marked with blue lines; red arrows indicate the conserved cysteine residuals. (b) Phylogenetic tree was constructed using the CAP homologs from C. chrysosperma (CcCAP1-3), Valsa malicola (VMCG_03776), F. oxysporum (FOZG_13166, FOXG_09795, FOXG_06245, FOXG_10300, FOXG_12292 and FOXG_14109), C. albicans (Rbt4, CAALFM_C110810WA, CAALFM_C107580CA, CAALFM_C114120CA and CAALFM_C107040CA), S. cerevisiae (PRY1-3), Solanum lycopersicum (P14a), A. thaliana (PR-1), N. tabacum (PR1a, PR1b and PR1c), Oryza sativa (Os01g0971100), Neurospora crassa (NCU_02470, NCU_05618), Aspergillus fumigatus (Afu1g02040, and Afu1g12350), Aspergillus nidulans (AN1058 and AN10057), Botrytis cinerea (BCIG_08280 and BCIG_09594), F. graminearum (FGSG_02744, FGSG_09548, FGSG_03109 and FGSG_03312), M. oryzae (MGG_07807, MGG_03085, MGG_13936 and MGG_03755), U. maydis (UMAG_01204 and UMAG_04343), and Coccidioides immitis (CIMG_09974 and CIMG_06897).
Figure S3: MoCap4 is dispensable for asexual development of M. oryzae. a,b Colony morphology (a) and diameter (b) of Guy11 wild type, Mopce1, and complemented strains grown on CM plates. c Number of conidia collected from a 7-cm rice bran plate at 3 days after induction of conidiation.
Figure S4: The GFP fusions of MoPce1 were functional. (a-c) The morphology (a), size (b) and relative biomass (c) of lesions caused by Guy11 wild type, Δ
Mopce1, and Δ
Mopce1 strains ectopically expressing the
MoPCE1-GFP (Δ
Mopce1/ MoPce1-GFP) or
GFP-MoPCE1 (Δ
Mopce1/ GFP-MoPce1).
Author Contributions
Conceptualization, X.X. and Y.Y.; methodology, X.X.; software, X.X.; validation, X.X., Y.Y. and Z.Z.; formal analysis, X.X.; investigation, X.X.; resources, X.X.; data curation, X.X.; writing—original draft preparation, X.X.; writing—review and editing, X.X.; visualization, X.X.; supervision, X.X.; project administration, X.X.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the National Natural Science Foundation of China (32172365 and 32272513), Central Guidance on Local Science and Technology Development Fund of Fujian Province (2022L3088), and the Innovative Research Funding of Fujian Agriculture and Forestry University, China (CXZX2020153D).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI crosstalk: an integrative view of plant immunity. Current opinion in plant biology 2021, 62, 102030. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Giraldo, M.C.; Valent, B. Filamentous plant pathogen effectors in action. Nature reviews. Microbiology 2013, 11, 800–814. [Google Scholar] [CrossRef]
- Irieda, H.; Inoue, Y.; Mori, M.; Yamada, K.; Oshikawa, Y.; Saitoh, H.; Uemura, A.; Terauchi, R.; Kitakura, S.; Kosaka, A.; et al. Conserved fungal effector suppresses PAMP-triggered immunity by targeting plant immune kinases. Proceedings of the National Academy of Sciences of the United States of America 2019, 116, 496–505. [Google Scholar] [CrossRef]
- Chen, H.; King, R.; Smith, D.; Bayon, C.; Ashfield, T.; Torriani, S.; Kanyuka, K.; Hammond-Kosack, K.; Bieri, S.; Rudd, J. Combined pangenomics and transcriptomics reveals core and redundant virulence processes in a rapidly evolving fungal plant pathogen. BMC biology 2023, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Hoang, C.V.; Bhaskar, C.K.; Ma, L.S. A novel core effector Vp1 promotes fungal colonization and virulence of Ustilago maydis. Journal of fungi (Basel, Switzerland) 2021, 7. [Google Scholar] [CrossRef]
- Morgan, W.; Kamoun, S. RXLR effectors of plant pathogenic oomycetes. Current opinion in microbiology 2007, 10, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Mentlak, T.A.; Kombrink, A.; Shinya, T.; Ryder, L.S.; Otomo, I.; Saitoh, H.; Terauchi, R.; Nishizawa, Y.; Shibuya, N.; Thomma, B.P.; et al. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. The Plant cell 2012, 24, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Cen, K.; Li, B.; Lu, Y.; Zhang, S.; Wang, C. Divergent LysM effectors contribute to the virulence of Beauveria bassiana by evasion of insect immune defenses. PLoS pathogens 2017, 13, e1006604. [Google Scholar] [CrossRef]
- Tian, H.; Fiorin, G.L.; Kombrink, A.; Mesters, J.R.; Thomma, B. Fungal dual-domain LysM effectors undergo chitin-induced intermolecular, and not intramolecular, dimerization. Plant physiology 2022, 190, 2033–2044. [Google Scholar] [CrossRef]
- Yoshino, K.; Irieda, H.; Sugimoto, F.; Yoshioka, H.; Okuno, T.; Takano, Y. Cell death of Nicotiana benthamiana is induced by secreted protein NIS1 of Colletotrichum orbiculare and is suppressed by a homologue of CgDN3. Molecular plant-microbe interactions : MPMI 2012, 25, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhou, W.; Lin, Y.; Liu, Z.; Yin, Z.; Huang, L. Two NIS1-like proteins from apple canker pathogen (Valsa mali) play distinct roles in plant recognition and pathogen virulence. Stress biology 2022, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.-d.; Jing, Z.-y.; Zhang, K.; Tan, Q.; Wang, G.L.; Liu, W. Bioinformatic analysis and functional characterization of the CFEM proteins in maize anthracnose fungus Colletotrichum graminicola. Journal of Integrative Agriculture 2021, 20, 2438–2449. [Google Scholar] [CrossRef]
- Qian, Y.; Zheng, X.; Wang, X.; Yang, J.; Zheng, X.; Zeng, Q.; Li, J.; Zhuge, Q.; Xiong, Q. Systematic identification and functional characterization of the CFEM proteins in poplar fungus Marssonina brunnea. Frontiers in cellular and infection microbiology 2022, 12, 1045615. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Songkumarn, P.; Venu, R.C.; Gowda, M.; Bellizzi, M.; Hu, J.; Liu, W.; Ebbole, D.; Meyers, B.; Mitchell, T.; et al. Identification and characterization of in planta-expressed secreted effector proteins from Magnaporthe oryzae that induce cell death in rice. Molecular plant-microbe interactions : MPMI 2013, 26, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zuo, N.; Bai, W.Z.; Wei, W.Q.; Yuan, T.L.; Zhang, D.; Wang, Y.Z.; Tang, W.H. Fungal CFEM effectors negatively regulate a maize wall-associated kinase by interacting with its alternatively spliced variant to dampen resistance. Cell reports 2022, 41, 111877. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Liu, G.; Zhang, S.; Liang, X.; Zhang, R.; Sun, G. A fungal CFEM-containing effector targets NPR1 regulator NIMIN2 to suppress plant immunity. Plant biotechnology journal 2023. [Google Scholar] [CrossRef]
- Hemetsberger, C.; Mueller, A.N.; Matei, A.; Herrberger, C.; Hensel, G.; Kumlehn, J.; Mishra, B.; Sharma, R.; Thines, M.; Hückelhoven, R.; et al. The fungal core effector Pep1 is conserved across smuts of dicots and monocots. The New phytologist 2015, 206, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Misas Villamil, J.C.; Mueller, A.N.; Demir, F.; Meyer, U.; Ökmen, B.; Schulze Hüynck, J.; Breuer, M.; Dauben, H.; Win, J.; Huesgen, P.F.; et al. A fungal substrate mimicking molecule suppresses plant immunity via an inter-kingdom conserved motif. Nature communications 2019, 10, 1576. [Google Scholar] [CrossRef]
- Khairi, M.H.F.; Nor Muhammad, N.A.; Bunawan, H.; Abdul Murad, A.M.; Ramzi, A.B. Unveiling the core effector proteins of oil palm pathogen Ganoderma boninense via pan-secretome analysis. Journal of fungi (Basel, Switzerland) 2022, 8. [Google Scholar] [CrossRef]
- Abraham, A.; Chandler, D.E. Tracing the evolutionary history of the CAP superfamily of proteins using amino acid sequence homology and conservation of splice sites. Journal of molecular evolution 2017, 85, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xiong, D.; Schneiter, R.; Tian, C. The function of plant PR1 and other members of the CAP protein superfamily in plant-pathogen interactions. Molecular plant pathology 2023, 24, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, G.M.; Roelants, K.; O'Bryan, M.K. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocrine reviews 2008, 29, 865–897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, Y.; Guo, H.; Gan, P.; Cai, M.; Kang, Z.; Cheng, Y. Identification and functional analysis of CAP genes from the wheat stripe rust fungus Puccinia striiformis f. sp. tritici. Journal of fungi (Basel, Switzerland) 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Röhm, M.; Lindemann, E.; Hiller, E.; Ermert, D.; Lemuth, K.; Trkulja, D.; Sogukpinar, O.; Brunner, H.; Rupp, S.; Urban, C.F.; et al. A family of secreted pathogenesis-related proteins in Candida albicans. Molecular microbiology 2013, 87, 132–151. [Google Scholar] [CrossRef] [PubMed]
- Prados-Rosales, R.C.; Roldán-Rodríguez, R.; Serena, C.; López-Berges, M.S.; Guarro, J.; Martínez-del-Pozo, Á.; Di Pietro, A. A PR-1-like protein of Fusarium oxysporum functions in virulence on mammalian hosts. The Journal of biological chemistry 2012, 287, 21970–21979. [Google Scholar] [CrossRef]
- Lu, S.; Edwards, M.C. Molecular characterization and functional analysis of PR-1-like proteins identified from the wheat head blight fungus Fusarium graminearum. Phytopathology 2018, 108, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mukherjee, M.; Kim, J.E.; Yu, W.; Shim, W.B. Fsr1, a striatin homologue, forms an endomembrane-associated complex that regulates virulence in the maize pathogen Fusarium verticillioides. Molecular plant pathology 2018, 19, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xiong, D.; Xu, Z.; Liu, T.; Tian, C. The Cytospora chrysosperma virulence effector CcCAP1 mainly localizes to the plant nucleus to suppress plant immune responses. mSphere 2021, 6. [Google Scholar] [CrossRef]
- Choudhary, V.; Schneiter, R. Pathogen-Related Yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 16882–16887. [Google Scholar] [CrossRef]
- Lin, Y.H.; Xu, M.Y.; Hsu, C.C.; Damei, F.A.; Lee, H.C.; Tsai, W.L.; Hoang, C.V.; Chiang, Y.R.; Ma, L.S. Ustilago maydis PR-1-like protein has evolved two distinct domains for dual virulence activities. Nature communications 2023, 14, 5755. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tian, T.; Feng, L.; Yang, X.; Li, L.; Tan, X.; Wu, W.; Li, Z.; Treves, H.; Serneels, F.; et al. Pathogenesis-related protein 1 suppresses oomycete pathogen by targeting against AMPK kinase complex. Journal of advanced research 2023, 43, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Fujisawa, S.; Mitsuoka, C.; Ito, A.; Hirabuchi, A.; Ikeda, K.; Irieda, H.; Yoshino, K.; Yoshida, K.; Matsumura, H.; et al. Large-scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens. PLoS pathogens 2012, 8, e1002711. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, G.; Giraldo, M.C.; Khang, C.H.; Coughlan, S.; Valent, B. Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as Biotrophy-associated secreted proteins in rice blast disease. The Plant cell 2009, 21, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Kong, D.; Ji, L.; Kong, L.; Wang, Y.; Peng, L.; Xie, G. OsClo5 functions as a transcriptional co-repressor by interacting with OsDi19-5 to negatively affect salt stress tolerance in rice seedlings. The Plant journal : for cell and molecular biology 2021, 105, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Schneiter, R.; Di Pietro, A. The CAP protein superfamily: function in sterol export and fungal virulence. Biomolecular concepts 2013, 4, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils--a review. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Cottier, S.; Darwiche, R.; Meyenhofer, F.; Debelyy, M.O.; Schneiter, R. The yeast cell wall protein Pry3 inhibits mating through highly conserved residues within the CAP domain. Biology open 2020, 9. [Google Scholar] [CrossRef]
- Braun, B.R.; Head, W.S.; Wang, M.X.; Johnson, A.D. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 2000, 156, 31–44. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Thomazella, D.P.; Vidal, R.O.; do Prado, P.F.; Reis, O.; Baroni, R.M.; Franco, S.F.; Mieczkowski, P.; Pereira, G.A.; Mondego, J.M. The fungal pathogen Moniliophthora perniciosa has genes similar to plant PR-1 that are highly expressed during its interaction with cacao. PloS one 2012, 7, e45929. [Google Scholar] [CrossRef]
- Darwiche, R.; El Atab, O.; Baroni, R.M.; Teixeira, P.; Mondego, J.M.C.; Pereira, G.A.G.; Schneiter, R. Plant pathogenesis-related proteins of the cacao fungal pathogen Moniliophthora perniciosa differ in their lipid-binding specificities. The Journal of biological chemistry 2017, 292, 20558–20569. [Google Scholar] [CrossRef] [PubMed]
- Gamir, J.; Darwiche, R.; Van't Hof, P.; Choudhary, V.; Stumpe, M.; Schneiter, R.; Mauch, F. The sterol-binding activity of PATHOGENESIS-RELATED PROTEIN 1 reveals the mode of action of an antimicrobial protein. The Plant journal : for cell and molecular biology 2017, 89, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhong, Z.; Shi, M.; Zhang, L.; Lin, L.; Hong, Y.; Fang, T.; Zhu, Y.; Guo, J.; Zhang, L.; et al. Comparative genomic analysis revealed rapid differentiation in the pathogenicity-related gene repertoires between Pyricularia oryzae and Pyricularia penniseti isolated from a Pennisetum grass. BMC genomics 2018, 19, 927. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome biology 2015, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Norvienyeku, J.; Chen, M.; Bao, J.; Lin, L.; Chen, L.; Lin, Y.; Wu, X.; Cai, Z.; Zhang, Q.; et al. Directional selection from host plants is a major force driving host specificity in Magnaporthe Species. Scientific reports 2016, 6, 25591. [Google Scholar] [CrossRef] [PubMed]
- Consortium, U. UniProt: a hub for protein information. Nucleic acids research 2015, 43, D204–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Chen, S.T.; Qi, M.; Cao, X.Q.; Liang, N.; Li, Q.; Tang, W.; Lu, G.D.; Zhou, J.; Yu, W.Y.; et al. The putative elongator complex protein Elp3 is involved in asexual development and pathogenicity by regulating autophagy in the rice blast fungus. Journal of Integrative Agriculture 2021, 20, 2944–2956. [Google Scholar] [CrossRef]
- Han, Y.; Song, L.; Peng, C.; Liu, X.; Liu, L.; Zhang, Y.; Wang, W.; Zhou, J.; Wang, S.; Ebbole, D.; et al. A Magnaporthe chitinase interacts with a rice jacalin-related lectin to promote host colonization. Plant physiology 2019, 179, 1416–1430. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Chen, T.; Lin, Y.; Luo, C. Functional evaluation of the signal peptides of secreted proteins. Bio-protocol 2018, 8, e2839. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, C.; Wang, X.; Sun, S.; Zhao, J.; Kang, Z.; Wang, X. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nature communications 2019, 10, 5571. [Google Scholar] [CrossRef]
- Chen, X.; Pan, S.; Bai, H.; Fan, J.; Batool, W.; Shabbir, A.; Han, Y.; Zheng, H.; Lu, G.; Lin, L.; et al. A nonclassically secreted effector of Magnaporthe oryzae targets host nuclei and plays important roles in fungal growth and plant infection. Molecular plant pathology 2023, 24, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Chen, S.; Ning, Y.; Wang, G.L. Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Current protocols in plant biology 2016, 1, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chen, S.; Shirsekar, G.; Zhou, B.; Khang, C.H.; Songkumarn, P.; Afzal, A.J.; Ning, Y.; Wang, R.; Bellizzi, M.; et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. The Plant cell 2012, 24, 4748–4762. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).