1. Introduction
The battle against pest and pathogens is paramount for ensuring global food security, especially for staple crops such as soybean
(Glycine max). Root lesion nematodes, particularly Pratylenchus brachyurus (Godfrey) Filip Schurr-Steekh, pose formidable challenges in this struggle, known for their devastating impact as documented by [
1]
and [
2]
. These phytonematodes pose a substantial threat to soybean cultivation, causing relevant yield losses due to their wide distribution, extensive host range, and the severe, often irreversible damage [
3,
4,
5]
.
The presence of
P. brachyurus is characterized by the emergence of dark, reddish-brown to black lesions on plant roots. Over time, these lesions coalesce, forming extensive necrotic areas resulting from the oxidation of root tissues. Additionally, as these nematodes penetrate and feed on plant roots, they can establish peculiar interactions with other phytopathogenic microorganisms, ultimately compromising plant health [
6,
7,
8]. These nematodes thrive in tropical climates, taking advantage of favorable conditions and the abundance of host crops [
9]. Their effectiveness is further amplified by their array of enzymes, which enable them to move through root tissues and subdue the plant's defenses [
10,
11,
12].
While a myriad of management strategies has been deployed against
P. brachyurus, many have fallen short in terms of long-term sustainability and efficacy [
13,
14,
15]. With traditional methods waning, the scientific community is turning to innovative solutions. Among these, silver nanoparticles (AgNPs), particularly those green-synthesized from plant extracts, have emerged as potential game-changers. The prowess of AgNPs derived from various plant extracts in mitigating nematode threats is well-documented, pointing to their promise as potent nematicides.
AgNPs produced through green synthesis methods, particularly using plant leaf extracts, have attracted significant attention due to their effective management of plant-parasitic nematodes [
16]. A plethora of recent research has underscored the efficacy of such AgNPs in controlling
Meloidogyne species, through processes such as inhibition of egg hatching, obstructing entry of J2 juveniles into roots, and disrupting juvenile development [
17,
18,
19]. Moreover, a study by [
20] demonstrated the decline of
Heterodera sacchari population and the concurrent enhancement of vegetative growth and yield in rice plants, resulting from the application of green-synthesized AgNPs.
The choice of reducing agents in the synthesis of nanoparticles is crucial in determining the physicochemical characteristics and toxic effects of AgNPs. Leaf extracts of plants, which contain compounds like amino acids, flavonoids, phenolic compounds, sugars, and terpenoids, not only reduce silver ions and stabilize the AgNPs but also regulate their growth, aggregation, and can enhance their nematotoxic activity [
21,
22,
23,
24]. For example, AgNPs synthesized using medicinal plant leaf extracts such as
Conyza dioscoridis,
Melia azedarach, and
Moringa oleifera have demonstrated high efficacy against egg hatching and the J2 stage of
M. incognita [
25]. However, secondary metabolites in
C. dioscoridis leaf notably enhanced the nematicidal effect of AgNPs compared with other treatments. Leaf extracts from a variety of plants, such as
Euphorbia tirucalli,
Azadirachta indica,
Curcuma longa,
Acalypha wilkesiana,
Senna siamea, and
Ficus sycomorus, have been successfully utilized in the biosynthesis of AgNP, demonstrating effectiveness in reducing phytonematode populations [
26,
27,
28,
29,
30]. Interestingly, soybean, the very crop threatened by the nematodes, might hold the key to an effective countermeasure. Extracts from soybean leaves, an often-overlooked by-product, have shown promise for synthesizing potent AgNPs [
31,
32]. Nevertheless, the potential of these nanoparticles in combating plant parasitic nematodes has yet to be fully explored in research.
Our study delves into this uncharted territory, focusing on P. brachyurus, a nematode that does not form specialized feeding cells, thus representing a unique challenge. We venture to harness the potential of green-synthesized AgNPs from soybean derivatives, envisaging a sustainable and cyclical agricultural management approach. Our overarching goal is to amplify the value of soybean by-products in pest management, offering an innovative, sustainable, and efficient solution.
Given the unexplored potential of AgNPs synthesized from soybean components in controlling phytonematodes, our study sets out to undertake a comprehensive analysis, aiming to: (i) assess the effectiveness of AgNPs synthesized through green methods using various concentrations of soybean leaf extract; (ii) perform physical and chemical characterization of the synthesized nanoparticles; (iii) evaluate the in vitro and in vivo nematicidal activity of these AgNPs; (iv) investigate the biomolecules responsible for the reduction of Ag+ to Ag0 and the stabilization of the synthesized AgNPs; and (iv) study the mode of action of AgNPs on P. brachyurus specimens.
2. Materials and Methods
2.1. Soybean leaf extract
Healthy soybean plants (cv. Willians) were cultivated in a greenhouse at 25 ± 5°C at the University of Brasília, Brasília, Federal District, Brazil, for 45 days. The leaves were carefully harvested, washed thoroughly with a 1000× diluted neutral detergent solution, and rinsed with distilled water to remove any residue. The plant material was then air-dried at room temperature and stored in a sealed plastic bag at -20°C.
To prepare the plant extract, 2 g of the cold leaves were cut and mixed with 20 mL of boiling distilled water for 2 min. The extract was then meticulously filtered through nº 7 filter paper (14 µm) (Qualy Comercial Eireli, Passos, MG, Brazil) using a funnel to remove any solid residues. The resulting filtered aqueous extract had a distinct light green coloration (data not shown).
2.2. Synthesis and characterization of nanoparticles
Silver nanoparticles (AgNPs) were synthesized using soybean leaf extract as the reducing and stabilizing agent. Silver nitrate (AgNO3) (Sigma-Aldrich, St Louis, MO, USA) was selected as silver salt precursor and dissolved in type I water to a final concentration of 1 mmol L-1. The AgNPs reaction syntheses were conducted using varying concentrations of plant extract: 0.78, 1.56, 3.12, 6.25, 12.5, and 25 mg of leaf mL-1. The final suspensions were denoted as 1.56AgNPs, 3.12AgNP, 6.25AgNP, 12.5AgNP, and 25AgNP, respectively. The stock aqueous extract was diluted to these final concentrations, mixed with AgNO3 solution, and then incubated at 65°C for 2.5 h. The resulting AgNPs were preserved in a polypropylene tube at 4°C in the dark for further physical-chemical characterization and testing of biological activity.
To characterize the nanoparticles, an absorbance curve was generated by collecting measurements from a UV-Vis spectrophotometer (Shimadzu UV-1203, Kyoto, Japan) across a wavelength range of 350 to 550 nm for each reaction synthesis. The peak with the highest intensity indicated the presence of the surface plasmon resonance (SPR) effect. The physical characterization of the AgNPs was then performed using dynamic light scattering (DLS) and electrophoretic mobility techniques on a ZetaSizer Nano ZS (Malvern Instruments, Worcestershire, United Kingdom), equipped with a He-Ne laser operating at 633 nm. This methodology facilitated the determination of several parameters, including the hydrodynamic diameter (HD) of the particles, the size distribution of the particle subpopulations evaluated by the polydispersity index (PdI), and the electrophoretic mobility expressed as Zeta potential. The assessments of HD, PdI, and Zeta potential were performed in triplicates, with the measurements automated and the scattering angle set at 173° at 25°C. Moreover, the reaction syntheses and the measurements of HD, PdI, and Zeta potential of AgNPs were repeated three times to confirm reproducibility. The data generated were processed using ZetaSizer software developed by Malvern Instruments.
The identification of functional groups in the soybean leaf extract that might contribute to the reduction, capping, and stabilization process of AgNPs was performed using Fourier-transform infrared spectroscopy (FTIR). FTIR spectra were obtained in potassium bromide (KBr) tablet and in the total attenuated total reflectance (ATR) mode using a Vertex 70 spectrometer (Bruker Corporation, Billerica, Massachusetts, United State), outfitted with an ATR configuration specifically tailored for the samples. The samples were deposited onto a diamond crystal and analyzed within a spectral range of 4000 to 350 cm−1, at a resolution of 4 cm−1 with a total of 32 scans. The data collected were then acquired and analyzed using OPUS software v7.2 (Bruker Corporation).
2.3. P. brachyurus culture
The culture of the root lesion nematode, P. brachyurus, originating from the Pratylenchus culture collection of the University of Brasília, was conducted in the Department of Phytopathology, Brasília, Federal District, Brazil. The population was collected from naturally infected soybean plants harvested in a commercial area in the municipality of Luziânia, Goiás, in the Central region of Brazil. The nematode population was propagated in soybean plants growing within a controlled greenhouse environment at 25°C ± 3°C.
To confirm the species of the population, nematodes were extracted from the roots of soybean plants cultivated in the greenhouse. The extraction of nematodes was carried out using the methodology described by [
33], followed by molecular, morphological, and morphometric characterization of specimens. For the molecular characterization, DNA was extracted from the
Pratylenchus specimens using the DNeasy Blood and Tissue Kit (Qiagen). A pair of species-specific primers (18S-F and ACM7-R) designed to detect
P.
brachyurus was used, and the PCR process was accomplished as described by [
34].
For a detailed morphological and morphometric assessment, semi-permanent microscopy slides containing ten adult females were meticulously prepared. Various anatomical structures were studied, including body length (L), stylet length (ST), stylet bulb diameter (ØSTB), stylet bulb length (STB), tail length (T), esophagus length (ESO), distance from vulva to anus (VA), largest body diameter (ØL), body diameter at the anus (ØLA), body diameter at the vulva (ØLV), percentage distance of the vulva from the anterior end (V), and the overlap length of the esophageal glands (EG). Additionally, the De Man indices were also calculated: a (ratio of body length to largest body diameter), b (ratio of body length to esophagus length), c (ratio of body length to tail length), and c’ (ratio of tail length to tail diameter at anus height). The data were statistically analyzed using descriptive statistics, such as mean and standard deviation. The nematodes were classified according to the identification keys of [
3] using a Leica DM2500 optical microscope (Leica, Wetzlar, Germany).
For all bioassay experiments, the inoculum density was calibrated to ~1000 nematodes mL-1 using a Peters' counting chamber with the same optical microscope set to a 40× objective.
2.4. Direct exposure of P. brachyurus to 3.12AgNP and 6.25AgNP
This experiment assessed the inhibitory impact of AgNPs, synthesized using different leaf extract concentrations on the mobility of the root lesion nematode, P. brachyrus, in an in vitro setting. Two distinct formulations of AgNPs were prepared using 3.12 mg and 6.25 mg of leaves per mL and were termed 3.12AgNP and 6.25AgNP, respectively. These formulations were chosen based on their physicochemical attributes, which aligned with the requisite criteria for nanomaterials.
The experimental procedure involved exposing 1,000 nematodes to six varying concentrations (1, 10, 25, 50, 125, and 250 µmol L
-1) of both 3.12AgNP and 6.25AgNP formulations independently. The nematode-nanoparticle mixtures were incubated at room temperature for 48 h, after which they were transferred to Baermann funnels for an additional 48 h following the protocol outlined by [
35]. Subsequently, live nematodes were harvested from the terminal container after a total incubation period of 96 h and quantified to evaluate the inhibitory effect under each treatment condition. Sterile water served as the control solution. The experiment was conducted with five experimental units, each represented by one funnel, and was replicated twice at varying time intervals. This resulted in two distinct experimental batches, with a total of ten funnels evaluated under each treatment condition.
The minimum inhibitory concentration (IC50 and IC90 values, which represent the concentrations required to inhibit 50% and 90% of nematicidal activity, respectively) was ascertained through the analysis of two replicates, followed by logistic regression to fit curves to the data. An analysis of variance (ANOVA) was performed on these values, with treatment means compared using Fisher's Least Significant Difference test at a significance level of α = 0.05.
2.5. AgNP effects on soybean grown and nematode population in soil system
Only the AgNP and concentrations that exhibited the highest nematicidal efficacy in preliminary in vitro mobility studies were selected for in vivo testing. Initially, soybean seeds were sowed in bags filled with a sterilized mixture of soil, sand, and Bioplant® commercial substrate, mixed in a 1:1:1 ratio. Upon reaching the V2 stage, the rhizosphere of each soybean plant was inoculated with a 1 mL suspension containing 1000 individuals of P. brachyurus. Three days after nematode inoculation, a 1 mL dose of the green-synthesized 6.25AgNP was applied to the soil surrounding the rhizosphere. The in vivo nematicidal efficacy of AgNPs was then assessed through two separate experiments, using application timings and concentrations derived from prior in vitro trials.
In the first experiment, a single dose of green-synthesized 6.25AgNP was administered at concentrations of 125 and 250 µmol L-1. The second experiment involved a single dose of 6.25AgNP at 500 µmol L-1 and two subsequent doses at 250 µmol L-1, spaced a month apart. Control treatments consisted of water applications. Throughout the experiment, plants were housed in a greenhouse maintained at a temperature of 25 ± 3°C for 80 days.
Following 80 days of nanoparticle application, various plant metrics were evaluated, including plant height, shoot weight, leaf count, root weight, nematodes per gram of root, and the nematode reproduction factor (RF) for each treatment. The RF was calculated based on the contrast between the final population (Pf) and initial population (Pi), following the methodology proposed by [
36]. Nematode data per gram of root underwent an analysis of variance (ANOVA), with Fisher's least significant difference being calculated to a significance level of P=0.05 for treatment comparisons. Each treatment was composed of eight replicates with a single soybean plant in a 1 L pot. All infection sets were conducted twice at varying intervals.
2.6. Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
To determine the average dry size of the most effective AgNPs and investigate their effects on nematode cuticles, a two-pronged microscopy approach was employed: Transmission Electron Microscopy (TEM) for particle analysis and Scanning Electron Microscopy (SEM).
In the first stage, the AgNPs were diluted in water and dispersed onto copper grids for TEM analysis using a JEOL 1011 microscope. The particle diameters were precisely measured using the ImageJ software, and the resultant size distribution curve was plotted using R software. For the SEM analysis, nematodes were submerged in AgNPs suspension for 24 h, rinsed with water, and then fixed in Karnovsky's solution (2% glutaraldehyde and 2% paraformaldehyde) buffered with 0.05 M sodium cacodylate at pH 7.2) for 12 h [
37]. The nematodes were washed three times with 0.05 M sodium cacodylate and post-fixed with osmium tetroxide for 1 h. Subsequently, they were rinsed with distilled water and dehydrated through a sequential acetone series (50%, 70%, 90%, and twice at 100% concentrations). After dehydration, the samples were subjected to critical point drying using a Balzers CPD 030 with carbon dioxide as the transition fluid and mounted on supports adorned with carbon adhesive tape. The final step involved gold sputter coating using a Leica EM SCD 500, after which the samples were ready for SEM analysis on a JEOL JSM-7001F microscope. During each solution disposal stage, the material in 1.5 mL tubes was centrifuged at 2500 g for 3 min to ensure thorough separation.
4. Discussion
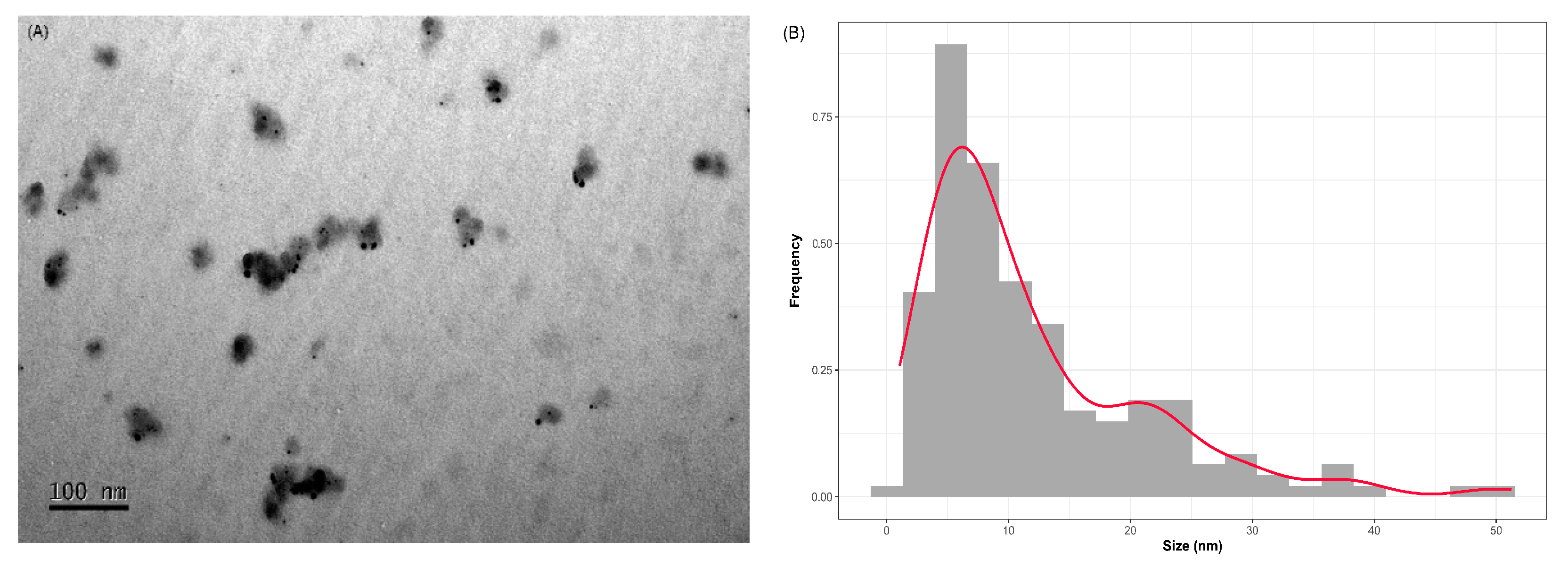
Modern agricultural research aims to promote sustainable practices that reduce reliance on external inputs and foster biodiversity. This involves employing non-polluting techniques and repurposing all by-products. Our study delves into this concept, demonstrating the use of lesser-utilized soybean by-products as reliable agents to reduce silver ions, generating AgNPs. These nanoparticles provide an eco-friendly solution to control the destructive phytonematode P. brachyurus, an important threat to tropical soybean crops. Using soybean leaf extract, we efficiently converted silver ions into spherical nanoparticles characterized by uniform distribution, low polydispersity, and hydrodynamic diameters ranging from 73 to 104 nm.
While previous research has explored the green synthesis of these nanoparticles through soybean leaf extract [
31,
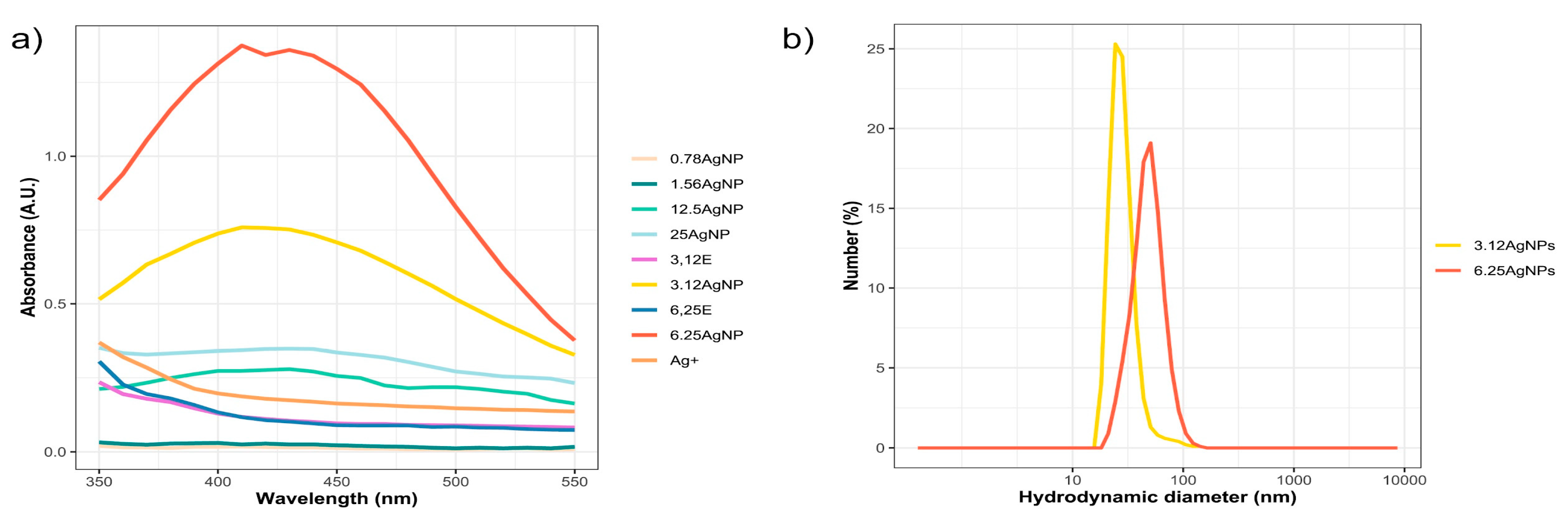
32], our study investigated the reproducibility of the reaction synthesis at different intervals. We also evaluated how fluctuating extract concentrations affect the physical and biological attributes of the AgNPs. We confirmed AgNPs formation using leaf concentrations of 3.12 and 6.25 mg mL, evidenced by color changes and UV-Vis absorption peaks (410-430 nm), demonstrating the SPR effect. Additionally, we observed an increase in absorbance as the extract concentration in the reactions increased, as shown in
Figure 2a. This enhanced absorbance, a manifestation of the SPR effect, could indicate alterations in the AgNPs’ size, shape, composition, reduction yield, surface characteristics, or potential shifts in biological activity [
39].
Our findings suggest that doubling the concentration of the leaf extract led to a decrease in AgNPs size while enhancing its effectiveness against
P. brachyurus. However, concentrations lower than 3.12 mg and higher than 6.25 mg of extract per mL inhibited the reduction of silver ions, thereby preventing AgNPs formation. This was evidenced by either minimal absorbance peaks below 400 nm or the absence of formation of nanometric particles sized less than 100 nm. The presence and/or concentration of specific constituents within the soybean extract could either inhibit or promote AgNPs formation, as well affect their dimensions and form [
40,
41]. Achieving reproducible physicochemical properties of AgNPs presents a significant challenge, given that minor variations in plant growth conditions can alter the properties of secondary metabolites responsible for the reduction of silver ions [
42,
43,
44,
45]. A noteworthy point from our study is the observed absence of reproductive anomalies, which might be attributed to the uniform handling of the soybean leaves. These leaves were collected simultaneously and stored securely until utilized for reaction syntheses, ensuring consistency in the material used.
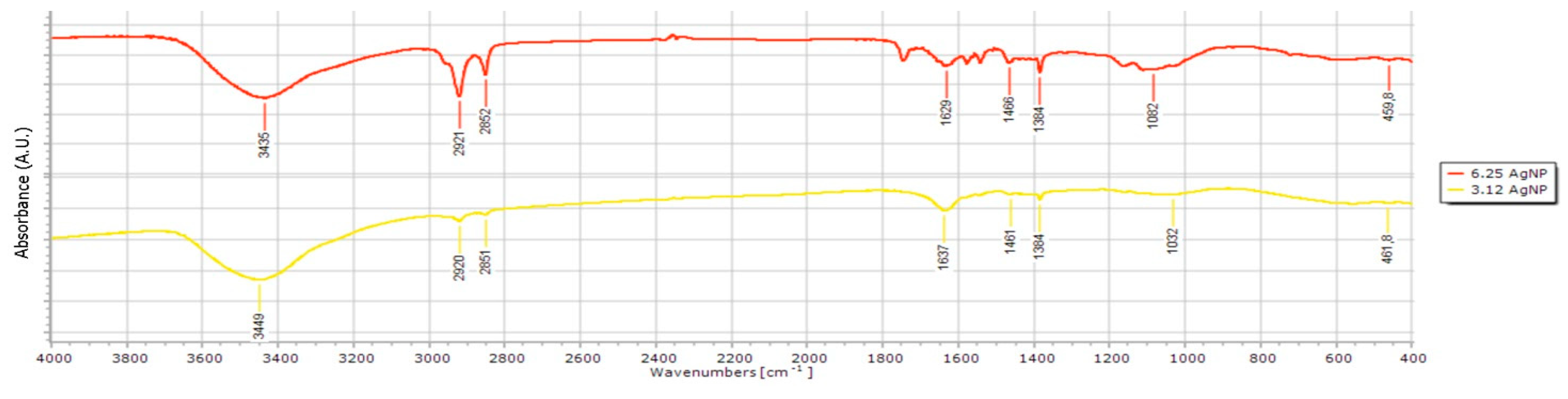
Various studies have highlighted a range of biomolecules pivotal in the formation and properties of nanoparticles, including polysaccharides, polyphenols, and alcohols [
46,
47,
48]. Building on this knowledge, we further investigated the specific functional groups in soybean leaves that facilitate the reduction of Ag⁺ ions to Ag⁰. We employed FTIR analysis to provide useful insights into the mechanisms underlying AgNPs synthesis mediated by soybean leaf extracts. Our results showcased the complex and multifunctional nature of compounds derived from soybean aqueous extracts, demonstrating a variety of biomolecules functional groups that can facilitate the reduction of metal ions. The presence of signals corresponding to alcohols and phenols, along with amide absorption bands, indicates the likely involvement of phenolic acids, and polysaccharides in the reduction process. This is consistent with earlier research that emphasized the expressive silver ion binding capacities of amino acids and polysaccharides present in soybean leaves [
49]. Other studies have proposed that agents such as flavonoids and isoflavones [
50,
51], as well as soluble soybean polysaccharides [
52,
53,
54], could also act as potential reducing agents in this process.
Although our synthesized AgNPs demonstrated varied toxicity towards
P. brachyurus, they did not cause any negative effects on soybean plants. In a broader context, different studies suggest that exposure for up to 3 days at 100-800 µmol L
-1 is needed to achieve noticeable reductions in phytonematode mobility or mortality [
55,
56,
57]. In our experiments, we observed that a 48 h exposure with AgNPs was required to neutralize over 50% of the nematode population's mobility (IC50) at concentrations ranging from 3.66 to 25.18 µmol L
-1. Our findings align with [
58], who reported similar effective concentrations of marine algae-derived AgNPs against tomato-infecting nematodes. Notably, even at identical concentrations, there were discernible differences in the effectiveness between the 3.12AgNPs and 6.25AgNPs treatments. The latter AgNPs demonstrated superior results. This can be attributed to the consensus that smaller nanoparticles, due to their increased surface area, display enhanced reactivity and efficacy [
59,
60]. Moreover,
in vitro experiments revealed a greater reduction in nematode mobility when exposed to AgNP concentrations of 125 and 250 µmol L
-1, especially to 6.25AgNP.
These promising
in vitro results were not replicated in
in vivo trials with
P. brachyurus. In the soybean rhizospheres, nematode proliferation at concentration of 125 and 250 µmol L
-1 reduced the number of nematodes in the root comparable to the control group. However, surprisingly, both concentrations resulted in a reproduction factor (RF) greater than 1, indicating nematode reproduction even after the application of AgNPs. The contrast between
in vitro and
in vivo findings highlights the complex interplay of AgNPs behavior within the soil environment. This complexity can be attributed to various factors, including AgNPs absorption by plant roots and their attachment to soil particles, resulting in dynamic nanoparticle distributions in the vicinity of the roots [
61], and their interactions with the multifaceted rhizosphere microbial community [
62]. Such behavior, highlighted in recent studies like that of [
63], points to the intricate challenge of predicting nanoparticle effects in natural settings and underscores the need for extensive research to unravel the underlying mechanisms at play.
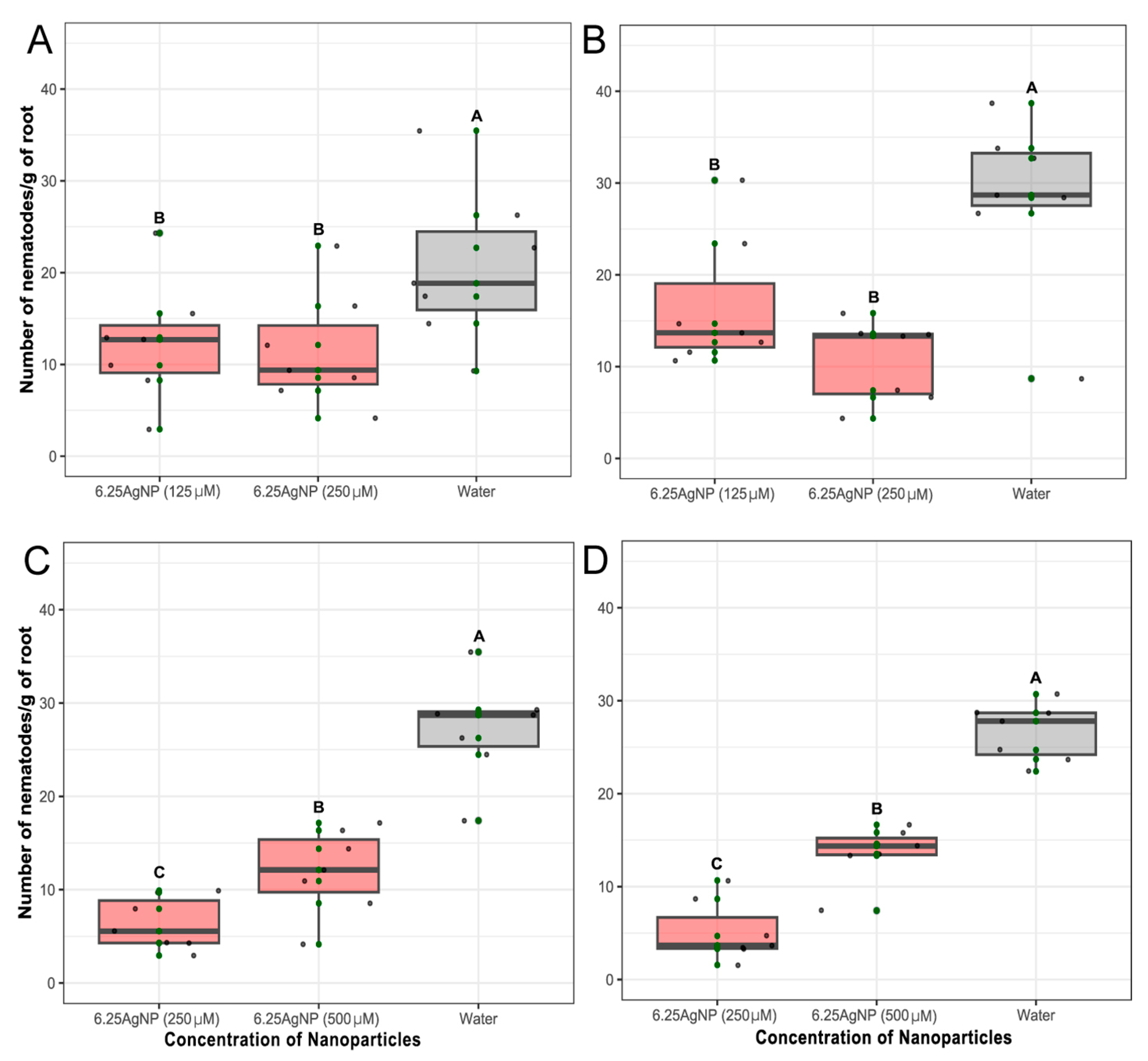
Another contributing factor could be that AgNPs show diminished effectiveness, possibly because of the nematodes' ability to evade, especially in mobile species like Pratylenchus species, or the lack of correlation between the dose to inhibit mobility and mortality. Importantly, the discrepancy in outcomes was lessened when either a single high-dosage application of 500 µmol L-1 was used, and mainly when 250 µmol L-1 was administered repeatedly in a second assay. The repeated exposure to the AgNPs may cause a cumulative or enhanced effect on the nematode population, disrupting their development or survival over time. This paves the way for further exploration into optimizing dosage strategies to harness the nematicidal potential of AgNPs.
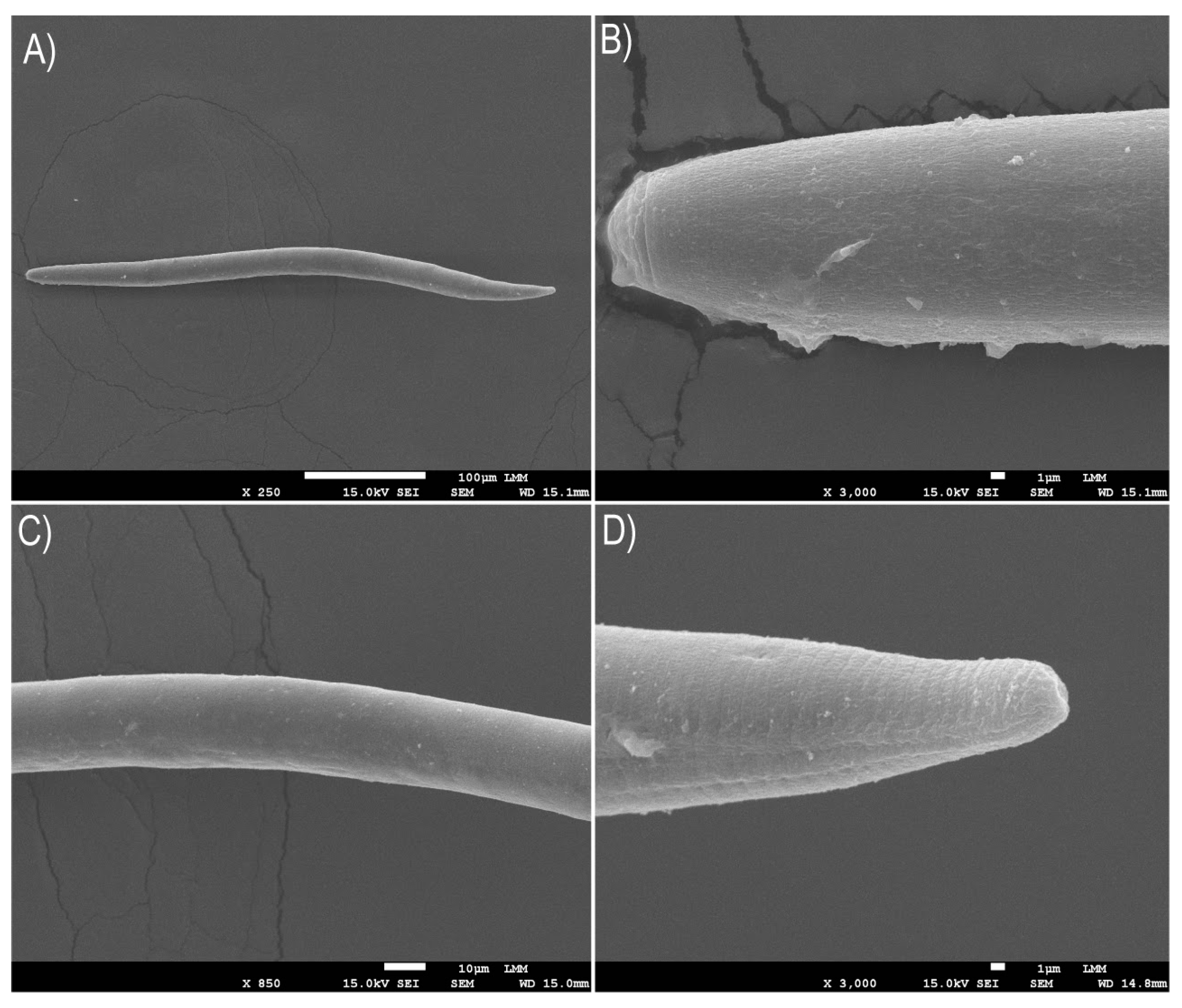
An in-depth analysis using SEM depicted AgNPs accumulation on the nematode cuticle, albeit without apparent immediate alterations to the cuticle, highlighting the need for further research to understand the prolonged influence of AgNPs on nematode physiology and behavior. Previous studies have suggested that AgNPs affect phytonematodes through complex and diverse cellular mechanisms [
64]. AgNPs have the potential to induce adverse effects, including germ cell death, reproductive problems, reduced life expectancy, and genetic damage across generations, as nematodes internalize these particles [
65,
66,
67,
68].
In accordance with environmental regulations, it's worth noting that regulatory agencies, including the Brazilian Ministry of Health has established a permissible level for elemental silver in drinking water at 50 µg mL
-1 [
69]. In our study, the applied silver concentrations consistently remained below this threshold, measuring at 0.084 µg mL
-1. Our findings underscore the relevance of sustainable nematode management in soybean cultivation. Green-synthesized AgNPs exhibit remarkable nematicidal action against
P. brachyurus, positioning them as a viable alternative to traditional chemical-based solutions. Their eco-friendliness further accentuates their appeal, alleviating environmental pollution concerns tied to conventional methods.
The potential of AgNPs as a nematode control strategy necessitates further exploration. Elucidating the long-term effects on nematode physiology and their reproductive, developmental, and behavioral aspects is vital. Understanding AgNPs' nematicidal action and soil AgNPs absorbance can refine their field application and dosage. Assessing their impact on non-target organisms and soil health will ensure their safe and sustainable application in tropical soybean cultivation regions.
In essence, our study unveils the promise of green-synthesized AgNPs against P. brachyurus in soybean agriculture. They emerge as an environmentally conscious and sustainable tool against nematode infestations. This research contributes substantially to the broader understanding of AgNPs' biological capabilities and practical applications, marking a pivotal move towards greener nematode control solutions in the agricultural realm.
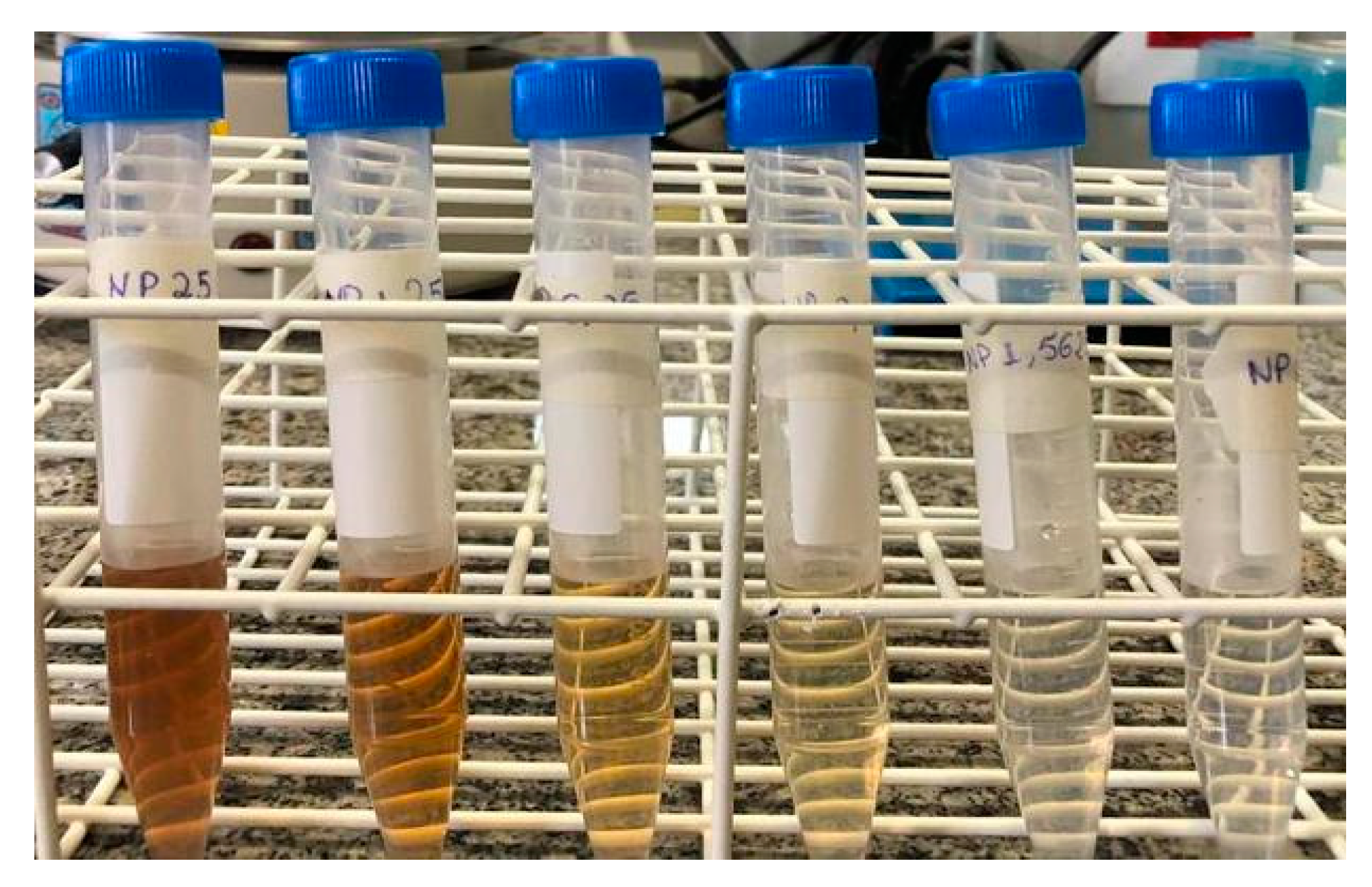
Figure 1.
Reaction tubes containing suspensions of AgNPs, synthesized using varying concentrations of soybean leaf aqueous extract: 25, 12.5, 6.25, 3.12, 1.56, and 0.78 mg mL-1, respectively.
Figure 1.
Reaction tubes containing suspensions of AgNPs, synthesized using varying concentrations of soybean leaf aqueous extract: 25, 12.5, 6.25, 3.12, 1.56, and 0.78 mg mL-1, respectively.
Figure 2.
(a) Visible absorbance curves of AgNPs and extracts (E) obtained by green synthesis with soy leaf aqueous extract obtained at 65°C for 2.5 h. (b) Hydrodynamic diameter dispersion of AgNPs synthesized using 3.12 (3.12AgNPs) and 6.25 mg mL-1 of soybean leaf aqueous extract (6.25AgNPs).
Figure 2.
(a) Visible absorbance curves of AgNPs and extracts (E) obtained by green synthesis with soy leaf aqueous extract obtained at 65°C for 2.5 h. (b) Hydrodynamic diameter dispersion of AgNPs synthesized using 3.12 (3.12AgNPs) and 6.25 mg mL-1 of soybean leaf aqueous extract (6.25AgNPs).
Figure 3.
Fourier-transform infrared spectroscopy (FTIR) spectra of AgNPs synthesized using 3.12 (3.12AgNPs; yellow line) and 6.25 mg mL-1 soybean leaf aqueous extract (6.25AgNPs; red line).
Figure 3.
Fourier-transform infrared spectroscopy (FTIR) spectra of AgNPs synthesized using 3.12 (3.12AgNPs; yellow line) and 6.25 mg mL-1 soybean leaf aqueous extract (6.25AgNPs; red line).
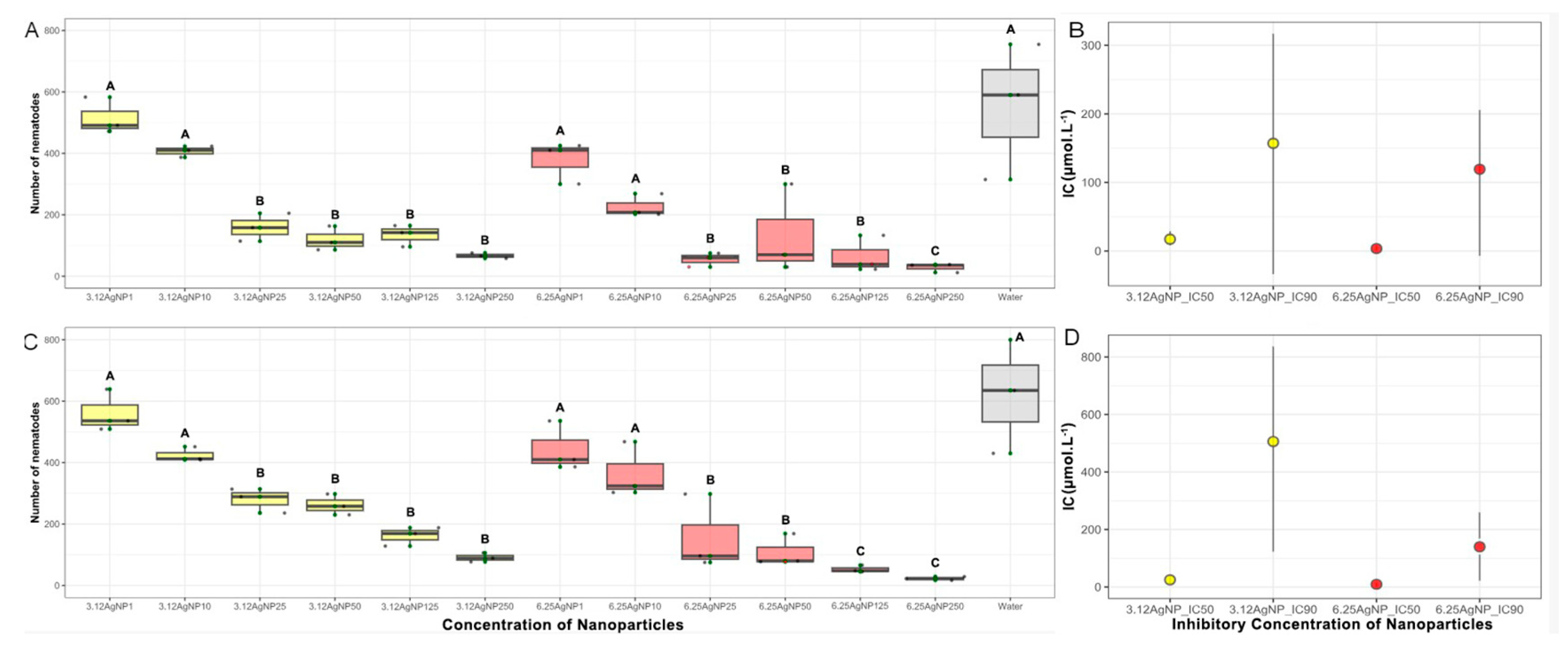
Figure 4.
Six different concentrations (1, 10, 25, 50, 125, and 250 µmol mL-1) of the AgNPs synthesized with 3.12 (3.12AgNP) and 6.25 mg mL-1 of aqueous soy leaf extract (6.25AgNP) in contact with Pratylenchus brachyurus specimens at room temperature for 48 h in first (a) and second (c) in vitro experiments. IC50 and IC90 values and confidence interval (bar) of AgNPs of treatment against P. brachyurus in first (b) and second (d) in vitro experiments. 3.12AgNP and 6.25AgNPs are represented by yellow and red color bars, respectively. The application of water was used as control represented by a gray color bar.
Figure 4.
Six different concentrations (1, 10, 25, 50, 125, and 250 µmol mL-1) of the AgNPs synthesized with 3.12 (3.12AgNP) and 6.25 mg mL-1 of aqueous soy leaf extract (6.25AgNP) in contact with Pratylenchus brachyurus specimens at room temperature for 48 h in first (a) and second (c) in vitro experiments. IC50 and IC90 values and confidence interval (bar) of AgNPs of treatment against P. brachyurus in first (b) and second (d) in vitro experiments. 3.12AgNP and 6.25AgNPs are represented by yellow and red color bars, respectively. The application of water was used as control represented by a gray color bar.
Figure 5.
(a-b) Number of Pratylenchus brachyurus specimens/gram of soybean root treated with one application of 6.25 mg mL-1 of soy leaf aqueous extract (6.25AgNP) at a concentration of 125 µmol L-1 and 250 µmol L-1. (c-d) Number of nematode/gram of soybean root treated with one application of 6.25 mg mL-1 of soybean leaf aqueous extract (6.25AgNP) at a concentration of 500 µmol L-1 and two application of 250 µmol L-1 spaced one month apart. The application of water was used as control. The number of nematodes was scored after 80 days of nematode inoculation. Mean of number of nematodes/gram of soybean root was calculated using data from eight repetitions. Error bars represent the SE calculated from eight repetitions. Treatments are indicated in the X-axis.
Figure 5.
(a-b) Number of Pratylenchus brachyurus specimens/gram of soybean root treated with one application of 6.25 mg mL-1 of soy leaf aqueous extract (6.25AgNP) at a concentration of 125 µmol L-1 and 250 µmol L-1. (c-d) Number of nematode/gram of soybean root treated with one application of 6.25 mg mL-1 of soybean leaf aqueous extract (6.25AgNP) at a concentration of 500 µmol L-1 and two application of 250 µmol L-1 spaced one month apart. The application of water was used as control. The number of nematodes was scored after 80 days of nematode inoculation. Mean of number of nematodes/gram of soybean root was calculated using data from eight repetitions. Error bars represent the SE calculated from eight repetitions. Treatments are indicated in the X-axis.
Figure 6.
(a) Images obtained by transmission electron microscopy (TEM) of AgNPs synthesized using 6.25 mg mL-1 of soybean leaf aqueous extract (6.25AgNPs) of soybean leaf aqueous extract and (b) particles size distribution for 6.25AgNPs in dry condition.
Figure 6.
(a) Images obtained by transmission electron microscopy (TEM) of AgNPs synthesized using 6.25 mg mL-1 of soybean leaf aqueous extract (6.25AgNPs) of soybean leaf aqueous extract and (b) particles size distribution for 6.25AgNPs in dry condition.
Figure 7.
Scanning electron microscopy (SEM) images were taken after exposing the Pratylenchus brachyurus specimens in a 250 μmol L-1 solution of AgNPs (synthesized using 6.25 mg mL-1 of soy leaf aqueous extract, termed 6.25AgNPs) for a period of 48 h. The images depict various parts of P. brachyurus adult: (a) the body, (b) labial region, (c) median region, and (d) tail terminus.
Figure 7.
Scanning electron microscopy (SEM) images were taken after exposing the Pratylenchus brachyurus specimens in a 250 μmol L-1 solution of AgNPs (synthesized using 6.25 mg mL-1 of soy leaf aqueous extract, termed 6.25AgNPs) for a period of 48 h. The images depict various parts of P. brachyurus adult: (a) the body, (b) labial region, (c) median region, and (d) tail terminus.
Table 1.
Physicochemical characteristic of the AgNPs synthesized with 3.12 (3.12AgNP) and 6.25 (6.25AgNP) mg mL-1 of soy leaf aqueous extract obtained by dynamic light scattering for obtaining the hydrodynamic diameter, polydispersity index (PdI), and Zeta potential synthesized three times.
Table 1.
Physicochemical characteristic of the AgNPs synthesized with 3.12 (3.12AgNP) and 6.25 (6.25AgNP) mg mL-1 of soy leaf aqueous extract obtained by dynamic light scattering for obtaining the hydrodynamic diameter, polydispersity index (PdI), and Zeta potential synthesized three times.
| |
First synthesis |
Second synthesis |
Third synthesis |
| Nanoparticles |
Size (d.nm) |
PdI |
Zeta Potential (mV) |
Size (d.nm) |
PdI |
Zeta Potential (mV) |
Size (d.nm) |
PdI |
Zeta Potential (mV) |
| Ag+ |
64.7±4.3 |
0.57±0.04 |
-19.1±1.3 |
- |
- |
- |
- |
- |
- |
| 3.12AgNP |
104.3±2.4 |
0.23±0.01 |
-25.1±0.5 |
101.1±3.4 |
0.19±0.01 |
-28.1±0.5 |
113.3±1.7 |
0.22±0.02 |
-20.2±1.4 |
| 6.25AgNP |
73.5±1.5 |
0.22±0.02 |
-23.3±0.2 |
70.2±2.2 |
0.20±0.03 |
-23.3±0.2 |
79.1±1.8 |
0.19±0.05 |
-19.7±2.1 |
Table 2.
IC50 and IC90 values and confidence interval (parentheses) of AgNPs of treatment against Pratylenchus brachyurus in first and second in vitro experiments.
Table 2.
IC50 and IC90 values and confidence interval (parentheses) of AgNPs of treatment against Pratylenchus brachyurus in first and second in vitro experiments.
| Nanoparticles |
Experiment_1 |
Experiment_2 |
| 3.12AgNP_IC50 |
17.21 (8.65-28.77) |
25.18 (13.65-36.71) |
| 6.25AgNP_IC50 |
3.66 (0.85-8.19) |
9.6 (0.68-12.9) |
| 3.12AgNP_IC90 |
157.02 (33.66-316.7) |
506.45 (123.33-836.26) |
| 6.25AgNP_IC90 |
119.29 (6.93-205.52) |
140.09 (22.35-212.53) |