1. Introduction
Chicory (
Cichorium intybus var.
sativum Bisch.) is industrially cultivated in Chile for the extraction of inulin and oligofructose from the roots [
1]. Inulin is a fructan polymer, which is used as a functional ingredient in the food industry because of its prebiotic properties that improve human health and reduce the risks of developing several diseases [
2]. Chicory was introduced to Chile in 2006, being cultivated mainly in the Biobío Region (36°46’22”S, 73°03’47”W), with exports of 13,000 tons per year for approximately USD 50 million FOB, which has ranked Chile as the third largest exporter of inulin worldwide [
3]. However, this industrial crop is affected by the ascomycete fungus
Boeremia exigua var.
exigua (Desm.) Aveskamp, Gruyter & Verkley, which is one of the pathogens associated with the complex of fungi and pseudo-fungi causing ‘root rot’ [
4]. In fact, this disease is an important yield-limiting factor in chicory production in the southern area of Chile, resulting in severe decreases in annual crop yields ranging from 3 to 80%.
Boeremia exigua var.
exigua can parasitize many plant species, but it can also grow on dead plant tissue [
5,
6]. The fungus acts as an opportunistic parasite that can cause pre- and post-harvest root rot, necrosis, and lesions of leaves and stems in various host plant species [
5,
7,
8]. In industrial chicory,
B. exigua var.
exigua causes dark brown to black necrotic lesions with a dry and firm consistency, mainly located in the upper part of the roots [
4]. Currently, there is no chemical or varietal control of this pathogen in industrial chicory [
9], while there is also a lack of technical knowledge to manage the disease. However, risks of root rot in industrial chicory can be minimized by using management practices, such as proper soil selection and adequate irrigation water supply, and by preventing excess moisture in the soil during the summer, particularly if temperatures exceed 20 ºC [
9] since these conditions favor pathogen attack and disease development in the crop. In fact, the optimum growth temperature for
B. exigua var.
exigua is between 20 and 25 °C, which implies that strategies for root rot control in industrial chicory need to be developed and implemented.
Interest in the biological control of crop diseases has increased in recent years due to increasing public concern about the impact of chemical pesticides on the environment, human health, and food safety [
10,
11]. In this sense, the use of microorganisms capable of controlling soil pathogens constitutes an environmentally friendly alternative for the control of industrial chicory root rot.
Bacteria of the
Pseudomonas fluorescens group have been intensively studied for disease control in agriculture [
10,
12,
13]. These rhizobacteria are mostly isolated from plant roots, contributing to the suppression or reduction of root diseases caused by different bacteria, fungi, pseudo-fungi and phytopathogenic nematodes in different crops [
10,
12,
14]. The protective mechanisms of these bacteria include induced systemic resistance, competition, and antibiosis associated with the production of antimicrobial secondary metabolites, such as 2,4-diacetylphloroglucinol (2,4-DAPG), pioluterine, pyrrolnitrin, phenazines, or hydrogen cyanide [
12,
13]. Ref. [
12] have indicated that bacteria capable of producing 2,4 DAPG (
phlD+) and pioluterin (
plt+) are molecularly and phenotypically different from other strains of
P. fluorescens and thus considered as a new species called
Pseudomonas protegens. The presence of 2,4-DAPG is associated with toxicity to a broad spectrum of organisms, such as bacteria, fungi, and nematodes [
15], while pioluterin is mainly known for its suppressive capacity against the oomycete
Pythium ultimum [
16,
17] as well as its antibacterial activity [
18]. The antifungal activity of 2,4-DAPG is characterized by the dissipation of the proton gradient across the mitochondrial membrane, affecting cellular respiration in pathogenic fungi [
19] and causing cell lysis in bacteria [
15].
To the best of the authors’ knowledge, there is no evidence of the ability of P. protegens to reduce the attack of B. exigua var. exigua in industrial chicory. Therefore, the objective of this research was to determine the effect of Pseudomonas protegens strains on the pathogenic fungus Boeremia exigua var. exigua under in vitro, in vivo, and field conditions. The root colonization capacity of these bacteria was also evaluated.
3. Discussion
2,4-DAPG-producing
Pseudomonas spp. have been isolated from numerous crops such as beans, alfalfa, lentils, barley, oats, and wheat [
14]. In the south of Chile, 2,4-DAPG-producing bacteria have been found in wheat crops and found responsible of the suppression of take-all disease caused by the fungus
Gaumannomyces tritici, a phenomenon known worldwide as take-all decline, induced by monoculture of wheat [
30,
31,
32]. However, there are no previous reports on the presence and biocontrol activity of these bacteria in industrial chicory.
The results obtained in the preselection of bacteria for the control of
B. exigua var.
exigua carried out in vitro and under controlled conditions in chicory roots, suggest that the inhibitory effect induced by the bacterial strains would be due to the diffusion of the antimycotic compounds that have been described for
P. protegens bacteria in the culture medium present in the Petri dish, such as 2,4-DAPG and pioluteorina. This occurs because this bacterial species is characterized by the presence of the
phlD gene (determined in the bacteria used) and
plt genes, which are associated with the production of the aforementioned compounds and contribute to the antagonism of
P. protegens against different kinds of bacteria, fungi and oomycetes [
18]. To our knowledge, the current study is the first report of the inhibitory effect exerted by
P. protegens bacteria on the fungus
B. exigua var.
exigua. The compounds influence the colonization of fungal tissues [
33], but sensitivity varies depending on the microorganism that is affected, e.g., pyoluteorin is twenty times more toxic than 2,4-DAPG for the growth of the bacterium
Erwinia amylovora [
34]. In addition, these two compounds also have different ecological roles. For example, 2,4-DAPG is toxic to the amoeba
Acanthamoeba castellanii, but not to pyoluteorin [
35]. This implies that, depending on the conditions faced by the bacterium, the production of one compound could be more advantageous in comparison to another [
34]. For this reason, we did not determine which of the two antibiotics, or if both, are toxic to the pathogen
B. exigua var.
exigua. However, the antifungal activity of 2,4-DAPG, which is given by its ability to dissipate the proton gradient that occurs in the mitochondrial membrane, affecting fungal cellular respiration [
19], suggests that this metabolite would be the main responsible for controlling
Boeremia. On the other hand, variability in root rot control in chicory could be explained by the fact that biosynthesis of antibiotics can be a metabolic burden for the bacterium. Although the biochemical and genetic determinants are not related to their biosynthesis, the production of 2,4-DAPG and pyoluteorin by
P. protegens prevails one over the other in the face of competitors and predators or depending on the habitats of these bacterial populations [
34].
Bacteria with antagonistic activity against soil-borne pathogens must establish and maintain population density in the rhizosphere environment [
36]. Plants are the main determining factor of the microbial community structure because they provide carbon and energy sources to the soil microbiota through root exudates, forming a unique environment for bacterial colonization [
37]. Our results indicate that the bacterial strains under study are capable of surviving in the chicory rhizosphere during crop development, which would indicate that root exudates released by industrial chicory plants favor the survival of
P. protegens. Under field conditions, the evaluation of the presence or absence of
P. protegens strains in chicory root indicates that the strains inoculated in the seed can increase in the chicory rhizosphere compared to the non-inoculated control. Determining the degree of bacterial colonization by seed inoculation was important given the capacity that the bacterial strains showed to control
B. exigua var.
exigua under controlled conditions, which may also account for the higher yields observed with their use in the field. As the bacteria used were originally isolated from wheat, it was important to determine their ability to survive and colonize the rhizosphere of industrial chicory, particularly considering that chicory plants have pivoting roots that can measure more than one meter in length and weigh up to 1,000 grams; whereas wheat root system is fasciculate, and thus is characterized by numerous thin roots.
In order for a microorganism to be beneficial and applicable to the crop, it is important that: (i) it establishes or colonizes; (ii) it has the capacity to protect the plant, and (iii) it manages to compete with the native microbiota [
38,
39]. In the field experiments, there was a decrease in the percentage of positive samples for the
phID gene in the evaluation at 90 DAS, while this was not observed in the pot trial. This difference could be explained by the fact that root growth rate increases up to 10 times at 90 DAS, tending to produce cracks in the tissue that release compounds rich in sugars; this could increase microbial populations and thus competition between them in the rhizosphere. In the case of the experiment with chicory plants under controlled conditions, the increase in root growth was not marked, possibly due to lower light and natural narrowness generated by the walls of the pots. In this sense, it is important to indicate that, according to our observations in the field, rot symptoms started in this period. This agrees with [
23], who reported that roots without wounds and inoculated with the pathogens
Phytophthora cryptogea and
B. exigua var.
exigua did not show rot symptoms, indicating that the infections caused by these pathogens on the roots are dependent on the presence of wounds or lesions.
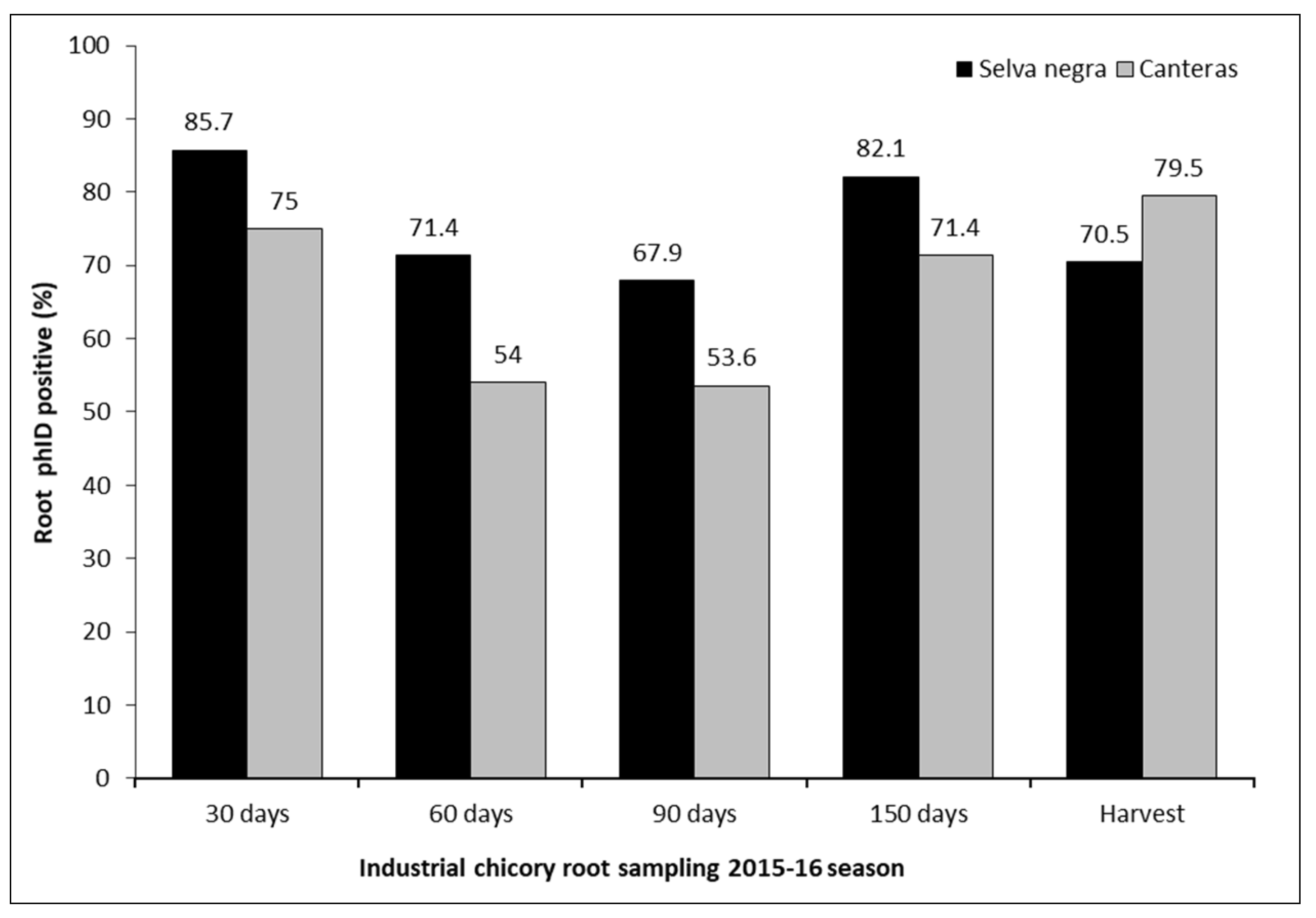
Higher percentages of samples positive for the
phID gene were observed in Selva Negra compared to Canteras. This could be explained by the differences in soil type and soil properties between the experimental stations. According to [
24], the soil in Selva Negra corresponds a type of Andisol (“trumao”) soil, with high organic matter content ranging from 8 to 12%, and excellent biological activity; while the soil in Canteras is sandy, with a low organic matter content, moderate biological activity, and excessive drainage. High drainage results in a rapid loss of water and nutrients applied to the crop, which may explain the lower yields observed in Canteras (
Table 2). Nevertheless, colonization by bacteria of the
P. fluorescens group depends on other factors or characteristics such as pH of the rhizosphere, temperature, water flow in the soil profile (irrigation and rainfall), plant species and even plant genotype [
40]. In this study, not all of these variables were determined. However, differences between the experimental sites could have influenced the fact that Selva Negra recorded higher percentages of samples positive for the
phID gene associated with populations of
P. protegens in the roots. Likewise, the existence of populations of native bacteria of
P. protegens naturally associated with the roots of industrial chicory were detected in both experimental sites, being higher in Selva Negra probably due to the higher content of organic matter in the soil.
There are few reports on the use of biological control agents of diseases that affect chicory cultivation in Chile [
41]. Globally, there is also very little information on the use of these agents in this plant. Diseases such as root rot reduce production, which results in lower income for farmers who have contracts with Beneo Orafti S.A. to grow industrial chicory since the company establishes acceptable quality levels for harvested chicory roots that show rot. In addition, the existence of roots damaged by root rot reduces efficiency of inulin extraction and affects safety of this product. Therefore, as a relevant finding of the present study, we would emphasize the increased yield of healthy roots and reduced root rot by the use of
P. protegens, which was evidenced in the weight of diseased roots and incidence and severity of the disease (%) under field conditions. This improvement in yield variables and plant health occurred both in the presence or absence of the fungal strain Pho669 of
B. exigua var.
exigua. It should be noted that the treatments inoculated with the mixture of the two Chilean strains of
P. protegens obtained a higher yield in both experimental sites. Studies that have evaluated species of the
P. fluorescens group isolated from suppressive soils and used as plant or soil inoculants [
42], have demonstrated the efficiency of these bacteria to colonize roots, protect plants from different diseases and increase plant productivity [
43,
44,
45,
46,
47]; also evidenced in our experience for industrial chicory.
The pathogen occurred naturally in the soil in which the study was conducted, since the treatment without inoculation of
B. exigua var.
exigua presented roots with typical root rot symptoms. This was expected because the experimental sites were not fumigated prior to seeding. In addition, a study conducted in Chile (Biobio Region) on seven crops of industrial chicory grown between San Carlos (36°25’29”S, 71°57’29” O) and Quilleco (37°28’00”S, 71°58’00”W), showed a high frequency of root rot symptoms of
B. exigua var.
exigua in all the sampled fields (unpublished data). Additionally, it is important to note that
B. exigua var.
exigua is characterized by being a facultative and opportunistic soil parasite that survives as a saprophyte in diseased or dead plant material through the formation of mycelium and/or pycnidia [
48]. In fact, these adaptive conditions allow this pathogen to survive in the soil for long periods of time. For this reason, inoculation with the fungus in the experimental sites allowed standardizing the level of inoculum among the experimental plots.
The greater weight of healthy roots (ton ha
-1) obtained with the seed treatments inoculated with the mixture of strains Ca10A and ChB7 in Canteras and Selva Negra, compared to the non-inoculated treatment and that inoculated with the fungus, may be associated with a growth-promoting effect when using both bacterial strains in the rhizosphere of chicory. Ref. [
49] evidenced the presence of growth-promoting metabolic mechanisms in strains Ca10A and ChB7, associated with phosphorous solubilizing activity and IAA (indole acetic acid) production. The author determined that strains Ca10A and ChB7 can solubilize 70% more phosphorus (mg of P
2O
4 L
-1) than others of the genus
Pseudomonas as reported by [
38], and produce higher levels of IAA, which influences the hormonal balance of plants, affecting growth [
50]. The ability to promote plant growth on industrial chicory and reduce incidence and severity of root rot of Chilean strains of
P. protegens is of commercial interest to the growers, since industrial chicory is marketed for its pivoting root, and thus an increase in root growth and weight of healthy roots results in increased yield.
4. Materials and Methods
4.1. Isolates of B. exigua var. exigua
The highly pathogenic fungus B. exigua var. exigua strain Pho669, which was obtained from diseased roots of industrial chicory, was used in all the experiments.
4.2. In Vitro Preselection of the Antagonistic Effect of Bacterial Isolates on the Pathogenic Fungus B. exigua var. exigua
A preselection of bacterial isolates with greater antibiosis against
B. exigua var.
exigua was conducted. Eighteen bacterial isolates, which were obtained from wheat plants [
20], were evaluated. They corresponded to
Pseudomonas fluorescens strains Ca1A, Ca9B, Ca11A, C21BD1, C12BC1, C21BC3, C9BA1, and C5BE4; and isolates of the bacterium
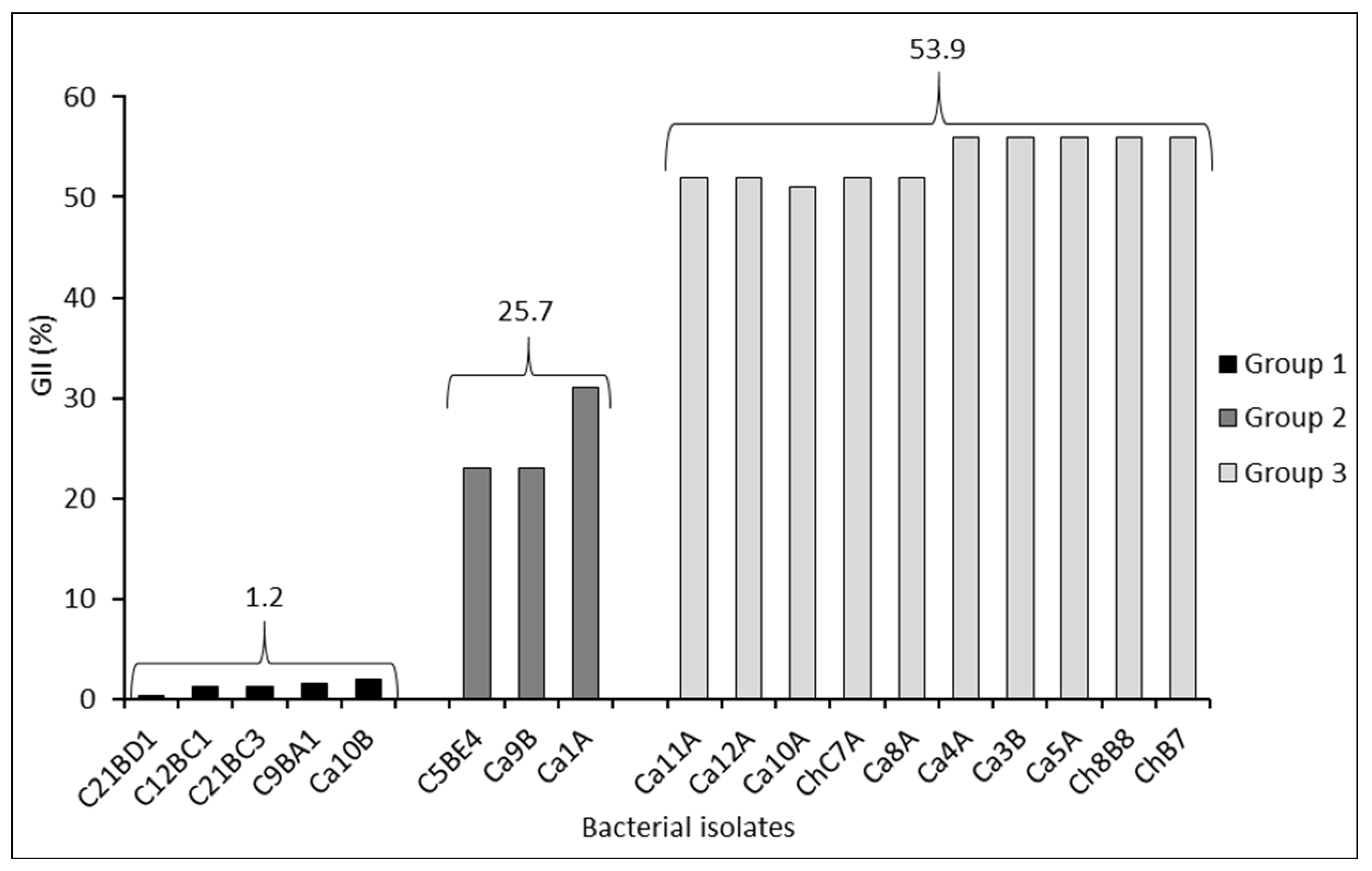
P. protegens Ca3B, Ca4A, Ca5A, Ca8A, Ca10A, Ca10B, Ca12A, Ch8B8, ChC7A and ChB7. All the isolates were obtained from the Phytopathology Laboratory of the Faculty of Agronomy, University of Concepción, Chile. Groups of 6 isolates were formed and their inhibitory capacity against
B. exigua var.
exigua was compared.
The bacterial isolates were previously incubated in King B (KB) broth (peptone protease, 20 g; anhydrous K
2HPO
4, 1.965 g; MgSO
4 7H2O, 1.5 g; 10 mL glycerol and 1000 mL water) at 25 ±1°C for 48 h with constant agitation at 150 rpm. An aliquot of 5 uL was placed equidistantly on a Petri dish with APD (20%) + KB medium, while a piece of isolated mycelium of the fungal strain Pho669 (5 mm in diameter), which was previously grown in APD medium at 24ºC for 10 days, was placed in the center of the dish. Petri dishes containing the fungus without bacteria were used as control. Prior to the experiments, the presence of the phlD gene, which is associated with the production of 2,4-DAPG, was determined in all the antagonistic strains under study. For this, the polymerase chain reaction (PCR) technique was used according to the protocol described by [
21].
Measurements of the mycelial growth of
B. exigua var.
exigua were made at 4, 7, 10 and 14 days of incubation by determining the distance between the antagonistic bacteria and the fungal hyphal edge. The growth inhibition index (GII) of the pathogen was determined and calculated once growth in the control (without bacteria) reached the edge of the Petri dish [
22], using the following Formula (1):
where: MGC = mycelial growth in the control (mm); MGT = mycelial growth under treatment (mm). The experiment included 4 repetitions and was replicated twice.
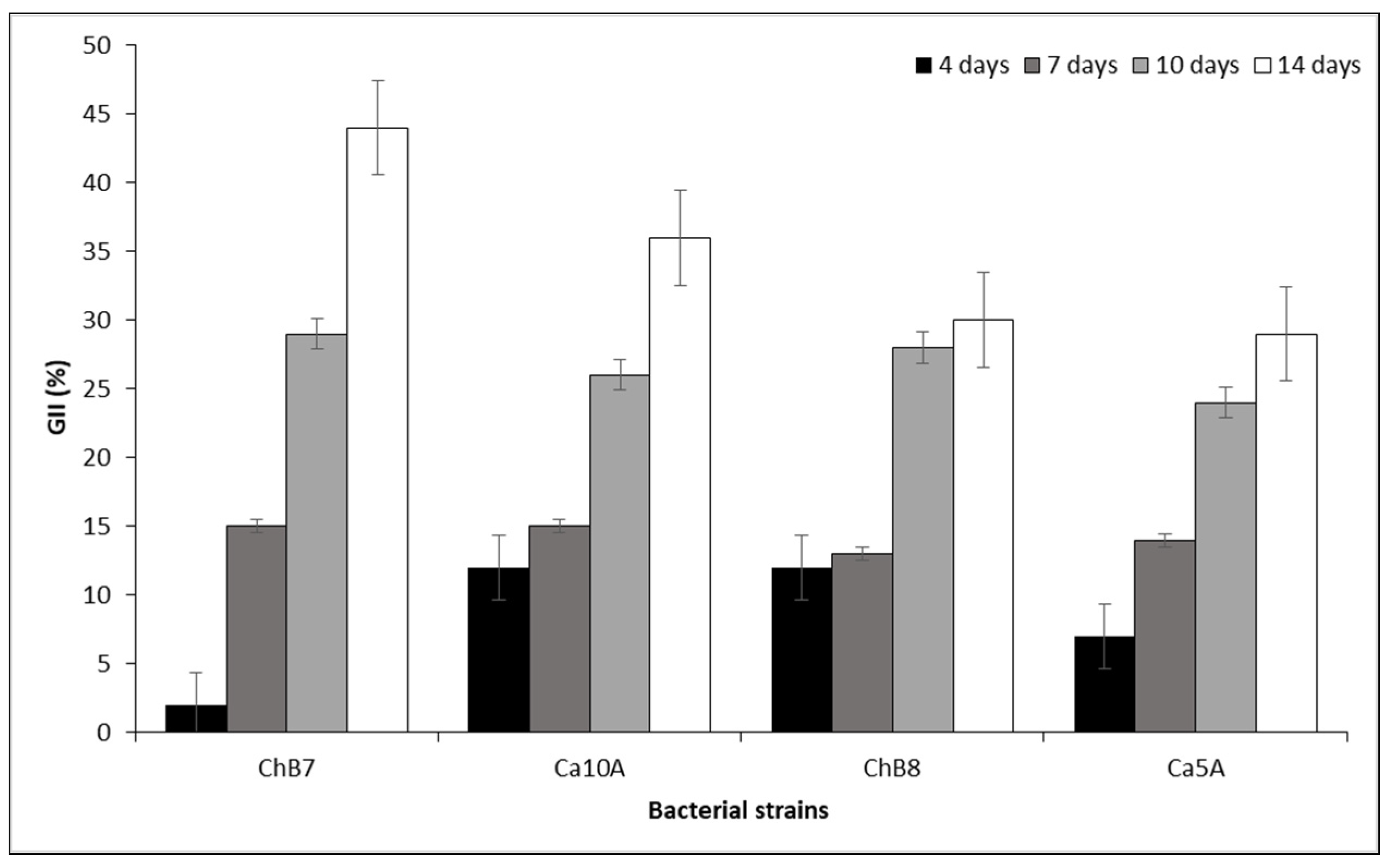
Dual cultures were used to evaluate antagonism against
B. exigua var.
exigua. For this, the three bacterial strains exhibiting the highest inhibition rates of pathogen growth were selected, while the strain CA10A was also used because it exhibited high antagonism against another pathogen that causes root rot in chicory, the pseudo-fungus
Phytophthora cryptogea (unpublished data) [
1].
To determine GII, measurements of the mycelial growth of the pathogens were made at 4, 7, 10 and 14 days of incubation by determining the distance between the antagonistic bacteria and the fungal hyphal edge. The experiment included three repetitions and was replicated twice. The two strains that showed the greatest inhibition of B. exigua var. exigua in dual cultures, were selected for subsequent evaluations.
4.3. Evaluation of the Control Effectiveness of P. protegens on B. exigua var. exigua in Roots of Industrial Chicory Grown under Controlled Conditions
To determine the effectiveness of P. protegens on the control of B. exigua var. exigua in industrial chicory, roots were collected from plants grown in two experimental stations: Selva Negra (36°85’44”S, 72°09’70”W), San Ignacio, Ñuble Region; and Canteras (37°50’45”S, 72°31’66”W), Quilleco, Biobío Region, Chile. The samples were placed in clean containers and transported the same day to the Phytopathology Laboratory of the Faculty of Agronomy, University of Concepción, Chile. Upon arrival to the laboratory, the roots were carefully cleaned and washed with distilled water to remove soil residues and impurities and then stored in a chamber at 4°C until use.
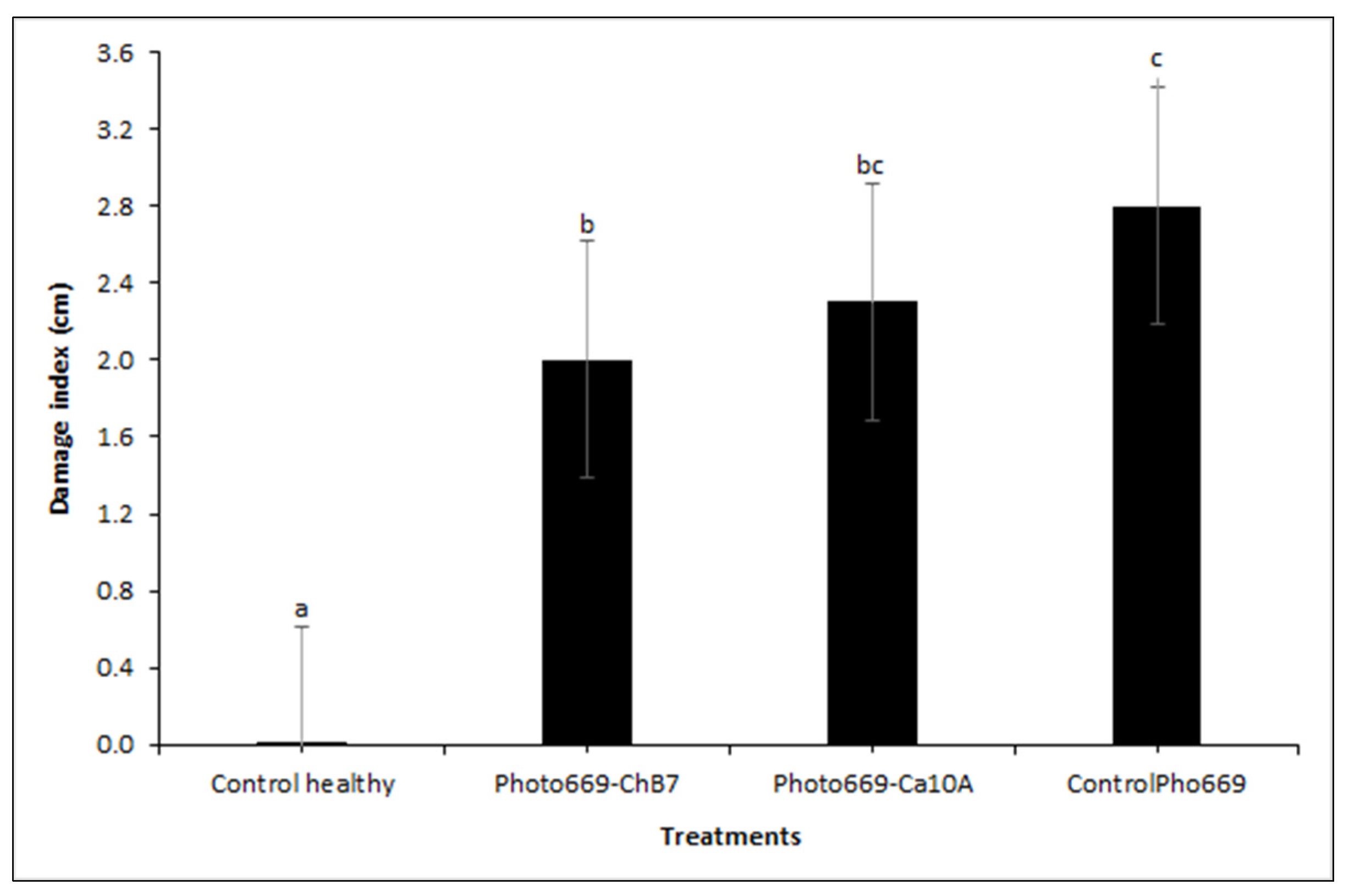
To conduct the experiment, the chicory roots were disinfected with a 10% sodium hypochlorite solution for 10 min (v/v), then rinsed twice with sterile distilled water and subsequently dried in a laminar flow chamber [
8,
23]. Subsequently, a piece of tissue (10 mm in diameter) from the upper part of the root was extracted using a cork borer and then inoculated in that area with 300 µL of bacterial culture of strains ChB7 and Ca10A at a concentration of 1x10
6 UFC mL
-1. After 48 h, a disc of actively growing mycelium (10 mm in diameter) of
B. exigua var.
exigua strain Pho669, previously grown in PDA medium at 25 ºC for 7 days. Subsequently, the wounds were sealed with plastic wrap to prevent dehydration and the roots were incubated in humid chambers at room temperature (±20°C) for 21 days. The following treatments were evaluated: non-inoculated control; control inoculated with fungal strain Pho669; inoculation with bacterial strain Ca10A + fungal strain Pho669; and inoculation with bacterial strain ChB7 + fungal strain Pho669. On day 21, the roots were cut transversally in equal parts and the disease damage index was determined by measuring the necrotic area (rot) on the root surface in a transversal and longitudinal way (mm) (Disease damage index = (transversal + longitudinal damage) /2). The initial bacterial concentration in each trial was determined by a bacterial count. For this, cultures grown in King B at 25 ± 1°C for 48 h were serially diluted seven times in sterile distilled water at a ratio of 1:10. Subsequently, a volume of 10 µL of each dilution was deposited on a Petri dish with King B (KB) agar. Once the microdrops were dry, the plates were incubated at 24 ºC for 24 h and a count of units forming colonies (CFU mL
-1) was performed.
The experiment was established using a completely randomized block design. Each block was represented by a humid chamber containing all the treatments under evaluation. The experiment was replicated twice and included four repetitions per treatment.
4.4. Evaluation of the Colonization Capacity of P. protegens in Industrial Chicory Roots Grown in Pots under Controlled Conditions
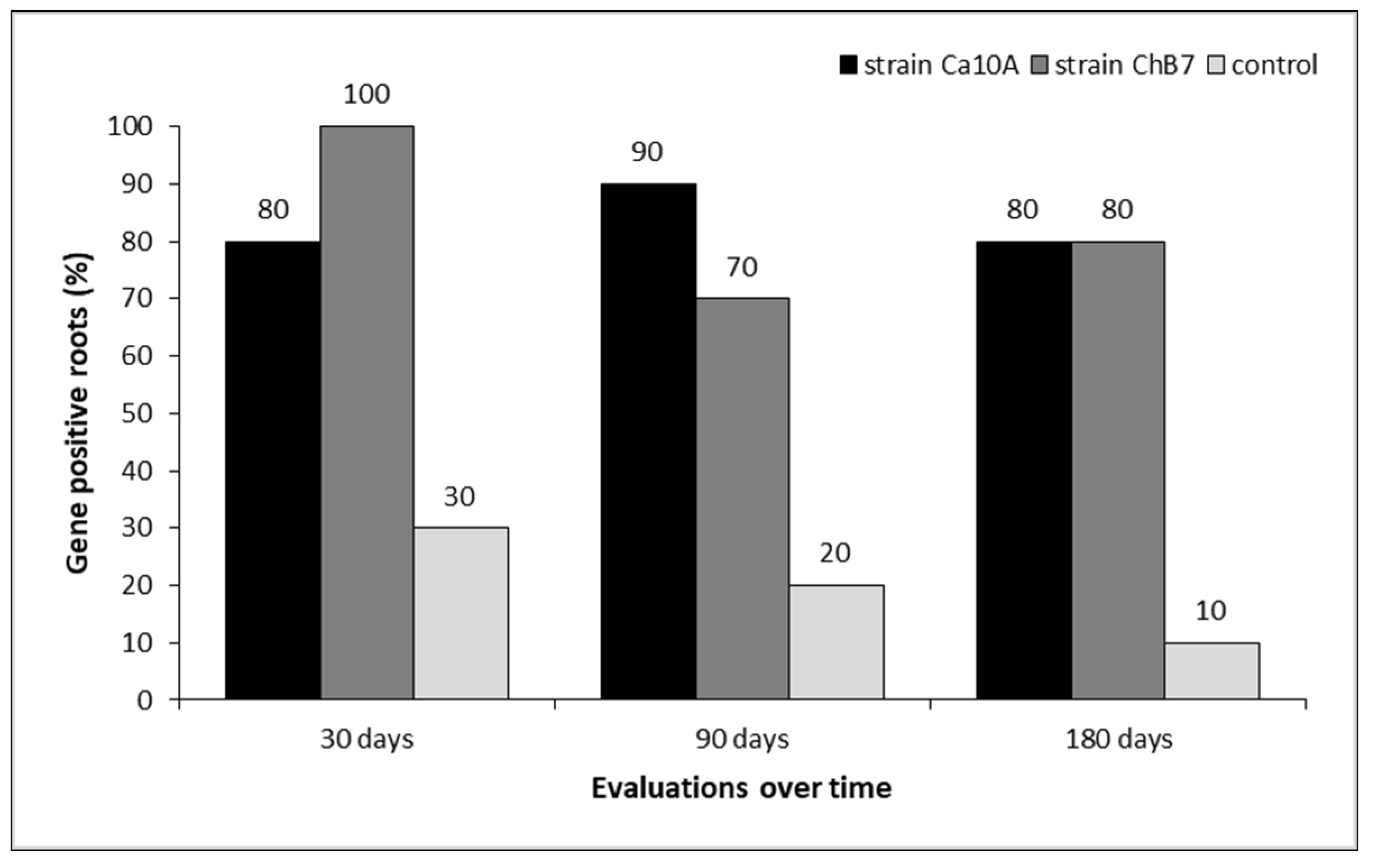
Ninety pre-germinated seeds of industrial chicory cv. Diesis were used. The seeds were placed in nursery trays of 50 alveoli (54.4 x 28.2 cm) with peat and perlite substrate (2:1) previously autoclaved (120°C for 15 min), and kept in a growth room under controlled conditions (24 ºC ± 1ºC)) and a photoperiod of 14 h light/10 h darkness (day/night), delivered by far-red (630-660 nm), yellow (615 nm), blue (460-490 nm) and white light, with a spectrum of 68 to 88.3 µmol m-2 s-1.
Seven days after seeding (DAS), 30 chicory seedlings were inoculated with the bacterial strain Ca10A using a micropipette; 30 chicory seedlings were inoculated with the strain ChB7; and 30 chicory seedlings were used as controls (non-inoculated with the antagonistic bacteria). The initial concentration of the bacteria was adjusted to 1x10
6 CFU mL
-1, which was confirmed by bacterial count. Thirty days after bacterial inoculation, seedlings were transplanted to individual pots of 12 cm in diameter and 40 cm high, containing a substrate made up of a mixture of peat and perlite (2:1), which was previously autoclaved at 120 °C for 15 min. The pots were kept in a growth room under the controlled conditions described above. The presence of bacteria in industrial chicory roots was evaluated at 30, 90 and 180 days after bacterial inoculation, using the method described by [
21]. Briefly, 1 g from the root surface was placed in a test tube with sterile distilled water for 24 h. Then, the liquid content of each tube was diluted four times (10
-1, 10
-2, 10
-3 and 10
-4) in ELISA microplates containing 150 µL of sterile water. Then, 50 µL of the dilutions 10
-2, 10
-3 and 10
-4 were added to 150 µL of KB broth (1/3) with antibiotics: ampicillin (40 µg mL
-1), chloramphenicol (13 µg mL
-1) and cyclohexamide (100 µg mL
-1) in microplates for a subsequent incubation at 25 °C for 48 h with constant agitation at 150 rpm. At the end of incubation period, presence of the
phlD gene in
P. protegens strains was determined using the polymerase chain reaction (PCR) technique. The
phlD+ samples were associated with the presence of the inoculated strains at the beginning of the trial. Measurements of fresh and dry weight of roots and leaves (g) were made at 180 days after bacterial inoculation.
The experiments were conducted using a completely randomized design and each pot represented an experimental unit.
4.5. Evaluation of the Root Colonization Capacity and Control Effectiveness of Antagonistic Bacteria on Root Rot Caused by the Pathogen B. exigua var. exigua in Industrial Chicory Grown under Field Conditions
Two experiments were carried out in Selva Negra (36°85’44”S, 72°09’70”W) and Canteras (37°50’45”S, 72°31’66”W) experimental stations, located in Ñuble and Biobío Regions, Chile, respectively. Both experimental stations are managed by the company Beneo Orafti S.A. In Selva Negra, the soil corresponds to an Andisol derived from volcanic ash, Pueblo Seco Series (associated with Arrayán Series); it is deep, well-drained, with a loam-to-silt loam texture, flat topography with slopes from 0 to 1%, and organic matter content ranging from 8 to 12% in the A horizon, thus presenting high biological activity [
24,
25]. In Canteras, the soil belongs to an Arenales series, whose parent material is recent volcanic, andesitic-basaltic sand; it is characterized by a sandy-loamy to sandy texture with or without gravel, with depth of 30 and 80 cm and low content of organic matter, thus presenting moderate biological activity [
24,
25].
The climatic conditions observed in both experimental stations at the time of the experiment were obtained from the National Agroclimatic Network (AGROMET) and are described in
Table 4 and
Table 5. In Selva Negra, rainfall reached 390 mm, being mainly concentrated in the months of September, October and April; average air temperature was warm, while average soil temperature was quite stable, with minimum and maximum temperature ranging from 13 to 17.6°C during the season. Similar climatic conditions were observed in Canteras, with an accumulated rainfall of 294 mm in the growing season; average air temperature was 2°C higher than in Selva Negra, while soil temperatures were less stable with minimum and maximum temperature ranging from 2.1 to 31°C.
Table 4.
Agrometeorological data during the 2015-16 industrial chicory season, obtained from the Santa Rosa Agrometeorological Station, Chillán, Ñuble Region, Chile. Data obtained from the National Agroclimatic Network (Agromet), online.
Table 4.
Agrometeorological data during the 2015-16 industrial chicory season, obtained from the Santa Rosa Agrometeorological Station, Chillán, Ñuble Region, Chile. Data obtained from the National Agroclimatic Network (Agromet), online.
| Month |
Rain accumulation
(mm) |
Evapotranspiration
(mm) |
Air average temperature
(°C) |
Soil average temperature
(°C) |
| September |
103.8 |
56.4 |
10.4 |
10.4 |
| October |
101.5 |
90.2 |
13.0 |
12.9 |
| November |
8.7 |
120.3 |
16.0 |
15.6 |
| December |
0.0 |
158.4 |
19.0 |
18.1 |
| January |
8.6 |
150.2 |
21.4 |
19.6 |
| February |
0.0 |
141.6 |
20.1 |
18.8 |
| March |
6.6 |
103.9 |
17.4 |
16.4 |
| April |
104.8 |
47.6 |
12.9 |
13.6 |
| May |
55.6 |
27.9 |
11.9 |
12.8 |
Table 5.
Agrometeorological data during the 2015-16 industrial chicory season, obtained from the Human Agrometeorological Station, Quilleco, Biobío Region, Chile. Data obtained from the National Agroclimatic Network (Agromet), online.
Table 5.
Agrometeorological data during the 2015-16 industrial chicory season, obtained from the Human Agrometeorological Station, Quilleco, Biobío Region, Chile. Data obtained from the National Agroclimatic Network (Agromet), online.
| Month |
Rain accumulation
(mm) |
Evapotranspiration
(mm) |
Air average temperature
(°C) |
Soil average temperature
(°C) |
| October |
93.2 |
95.4 |
13.1 |
13.1 |
| November |
22.4 |
120.7 |
13.9 |
16.1 |
| December |
6.4 |
160.1 |
19.1 |
18.7 |
| January |
0.9 |
160.4 |
21.4 |
22.5 |
| February |
0.2 |
145.8 |
21.1 |
21.5 |
| March |
20.1 |
117.8 |
17.9 |
18.7 |
| April |
109.8 |
51.7 |
12.2 |
12.6 |
| May |
40.7 |
29.1 |
10.7 |
11.7 |
Before seeding, seeds of industrial chicory cv. Diesis were inoculated with the beneficial bacteria using the following methodology: 4 Falcon tubes containing a volume of 40 mL of bacterial culture in KB broth were centrifuged at 20 C° for 8 min at 4000 rpm, and the supernatant was removed. The bacterial pellet was washed and resuspended in 25 mL of saline solution (0.9% NaCl) and centrifuged at 20 °C for 5 min at 4000 rpm. The supernatant was discarded, and 100 µL of sterile King B broth and 3 mL of carboxymethylcellulose (0.05%) were added to the pellet. The mixture was slightly vortexed, then added to the seed in order to obtain an inoculation dose of 7.5 mL of each concentrated microorganism per 100 g of chicory seeds, and finally left to dry at room temperature (~23°C) for 18 h.
The seeds were sown on September 17 and October 8, 2015 in Selva Negra and Canteras, respectively. In both experimental sites, a six-row precision planter (MS high-precision model, Monosem®, Paris, France) was used. The experimental units consisted of plots of 3 m x 7 m with six rows. Planting spacing was 10.2 cm between plants and 45 cm between rows. The treatments were established in a randomized complete block design with four repetitions. The evaluated treatments are described in
Table 6.
Table 6.
Treatments used to determine the capacity of Pseudomonas protegens bacteria to colonize industrial chicory roots and control root rot caused by the pathogen Boeremia exigua var. exigua strain Pho669. Experiment conducted in Selva Negra and Canteras experimental stations (Beneo-Orafti S.A), Ñuble and Biobío Regions, Chile.
Table 6.
Treatments used to determine the capacity of Pseudomonas protegens bacteria to colonize industrial chicory roots and control root rot caused by the pathogen Boeremia exigua var. exigua strain Pho669. Experiment conducted in Selva Negra and Canteras experimental stations (Beneo-Orafti S.A), Ñuble and Biobío Regions, Chile.
| Treatment |
Description |
| T1 |
Non-inoculated control |
| T2 |
Control inoculated with Pho669 |
| T3 |
Seed inoculated with strain ChB7 |
| T4 |
Seed inoculated with strains ChB7 and Pho669 |
| T5 |
Seed inoculated with strain Ca10A |
| T6 |
Seed inoculated with strains Ca10A and Pho669 |
| T7 |
Seed inoculated with strains Ca10A and ChB7 |
| T8 |
Seed inoculated with strains Ca10A and ChB7, and Pho669 |
Agronomic management of the experiments (weed control, irrigation, and fertilization) was conducted according to the standard management recommended by Beneo Orafti S.A. Fertilization was applied at seeding and consisted of a mixture of 30 units of N; 100 units of P2O5; 250 units of K2O; 2 units of B; 1.8 U Zn; 24 units of Mg; and 32 units of S ha-1. Accumulated irrigation of 380 mm was applied by fixed spray sprinklers. Leaf pest control was carried out using an insecticide based on Thiamethoxam (141 g L-1) and Lambda-cyhalothrin (106 g L-1) (Engeo® 247 ZC, Syngenta) applied at a dose of 150 mL ha-1 when plants reached the growth stage of 3-4 true leaves (45 DAS). For leaf disease control, two fungicide applications based on Fenpropidin (375 g L-1) and Difeconazole (100 g L-1) (Score Beta® 475 EC, Syngenta] were used in the growth stage of 20-25 true leaves (120 and 150 DAS). Weed control was carried out using standard herbicides; at pre-seeding, Trifluralin (48 g L-1) (Treflan® EC, Agrotechnology) at 1.5-2.0 L ha-1; in post-emergence (cotyledon stage), Propyzamide (50% w/w. 500 g kg-1) (Kerb® 50W, Dow AgroSciences LLC) at a dose of 0.5 kg ha-1 and Flumetsulam (80%) (Preside® 80WG at a dose of 10-20 g ha-1, Dow AgroSciences LLC.); at the growth stage of 2-2.5 true leaves, Imazamox (700 g kg-1) (Sweeper® 700 DG at a dose of 20 g ha-1, Basf Chile S.A.); at the stage of 3-4 true leaves, S-metallochlor (960 g L-1) (Dual® Gold 960 EC at a dose of 200 ml ha-1, Syngenta] and Dimethenamide-P (720 g L-1) (Frontier®-P EC at 200 ml ha-1, Basf Chile S.A.); and at post-emergence, an application of Lenocil (800 g kg-1) (Venzar® WP at a dose of 500 g ha-1, DuPont Chile S.A.).
Inoculation of
B. exigua var
exigua was carried out in the four central rows of the experimental plots. 10 g of inoculum was distributed manually per linear meter in a furrow of 8-10 cm deep at the side of the row. This was conducted at the growth stage of 9-10 true leaves between December 8 and 10, 2015 (approximately 80 DAS). For the assays under field conditions, preparation of the artificial inoculum of the strain Pho669 consisted of multiplying the pathogen for 30 days in 500 mL Erlenmeyer flasks with previously sterilized millet grains. For this, each flask was filled with 200 g of millet, 100 mL of distilled water and 30 discs (7 mm in diameter) of active growth of the fungus [
4,
26,
27].
The trials were harvested manually between May 16 and 17, 2016 (about 8 months after seeding). Weight of healthy and diseased roots (ton ha
-1) as well as leaf weight (ton ha
-1) were measured using a digital platform scale (model DY61, Maigas Comercial S.A., Chile). Additionally, colonization by
P. protegens bacteria was determined during different growth stages of chicory. For this, approximately 10 plants were obtained from each experimental plot at 30, 60, 90, 150 DAS and harvest, including the treatments without bacterial inoculation. The presence or absence of the antagonistic bacterial strains in the rhizosphere of the chicory plants was determined through the amplification of the phlD gene by means of PCR following the protocols described previously [
21]. In chicory, root growth rate increases after 100 DAS, and thus roots thicken considerably with accumulating food reserves for flowering. The presence of bacteria in the upper and lower parts of the root was evaluated at 150 DAS and harvest; a sample was considered positive for the
phlD gene when the upper and/or lower parts of the root were positive for the presence of this gene.
Sugar beet roots were rated for rot using a modified 7-level scale (Hanson, 2010) used by Beneo Orafti S.A., where 0= No rot symptoms; 1 = With surface rot spots; 2 = With rot band of < 5mm penetration; 3.1 = With less than 5 mm penetration into the rot band and < 30% root rot; 3.2 = With more than 5 mm of penetration in the rot band and > 30% rot; 4.1= Root tip rot and advance < 5 cm from apex, 4.2= Advanced tip rot > 5 cm advance from apex and 5= 100% completely rotten root. The evaluations were carried out in the field, once the four central rows of each plot were harvested. The data obtained were used to determine the incidence of the disease, which corresponded to the total number of roots with rot symptoms of the total number of roots harvested. Disease severity was evaluated through the following modified formula (2) of [
28]:
where: E: level on the root rot scale; N: total number of roots and G: maximum severity in the rot scale.
4.6. Statistical Analysis
The preselection was evaluated by Ward’s hierarchical clustering method, while average linkage was used for clustering. Data from each conglomerate or clusters were analyzed for normality using the Shapiro-Wilk test. The data obtained from the experiment in humid chambers were evaluated using the non-parametric Kruskal-Wallis test, while pairwise comparisons were used to determine differences between the treatments. Data on the biomass obtained from the bacterial colonization test in the growth chamber and on yield obtained in the field tests were analyzed for normality using the Shapiro-Wilk test. An analysis of variance (ANOVA) was used to evaluate the experiments in the growth chamber and in the field according to the experimental design, while significant differences between the treatments (P ≤ 0.05) were analyzed by Fisher’s LSD test (α = 0.05). The results (as percentages) were transformed using the Bliss Angular Transformation prior to the statistical analysis. All statistical analyzes were performed using SAS (Statistical Analysis System) software [
29].
Author Contributions
T.Q-D.: Conceptualization, data curation, formal analysis, investigation, methodology, writing-original draft, writing-review and editing, J.SM.: field investigation, B.R.: molecular investigation, writing-original draft, P.O.: laboratory investigation, M.V.: Conceptualization, methodology, supervision, writing-original draft, S.F.: Conceptualization, methodology, supervision, writing-original draft, P.C.: writing-review and editing, P.A.: field investigation, project administration, and E.M-E.: Conceptualization, funding acquisition, formal analysis, investigation, methodology, project administration, resources supervision, writing -review and editing. All authors have read and agreed to the published version of the manuscript.