Submitted:
07 November 2023
Posted:
08 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
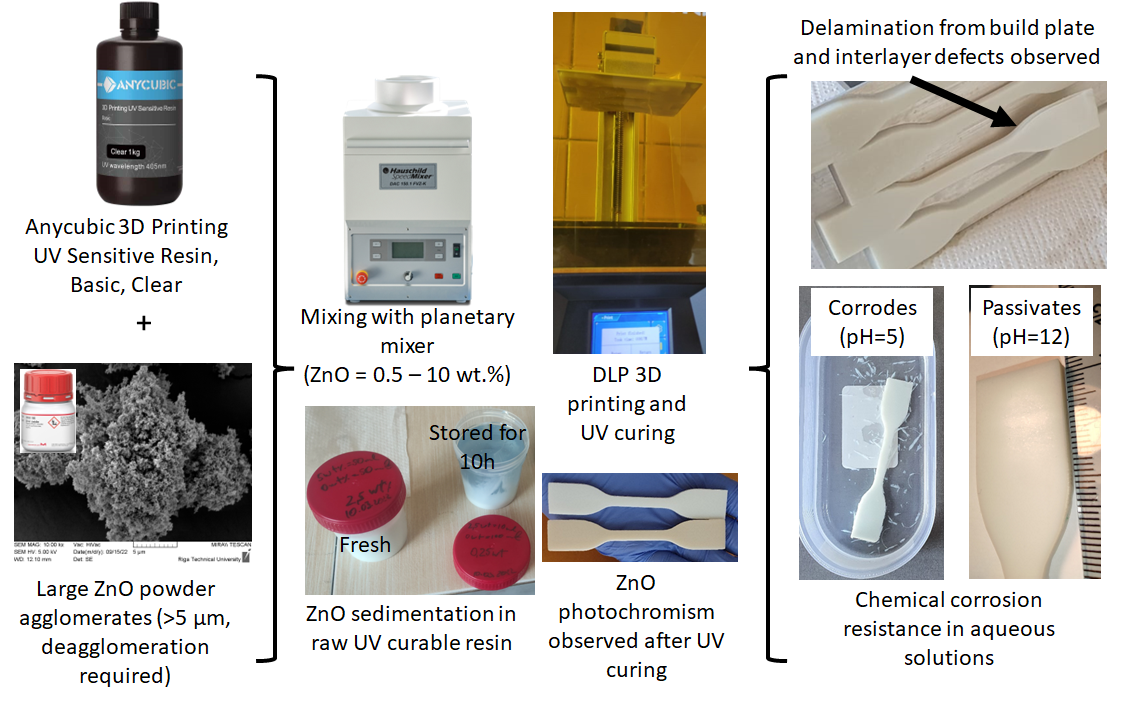
2.1. Applied Materials
2.2. Methods for Samples Manufacturing
2.3. Methods for Visual and Mechanical Characterization of Materials and Samples
2.4. Chemical Corrosion Tests
3. Results
3.1. Visual and Mechanical Properties of Materials and Samples
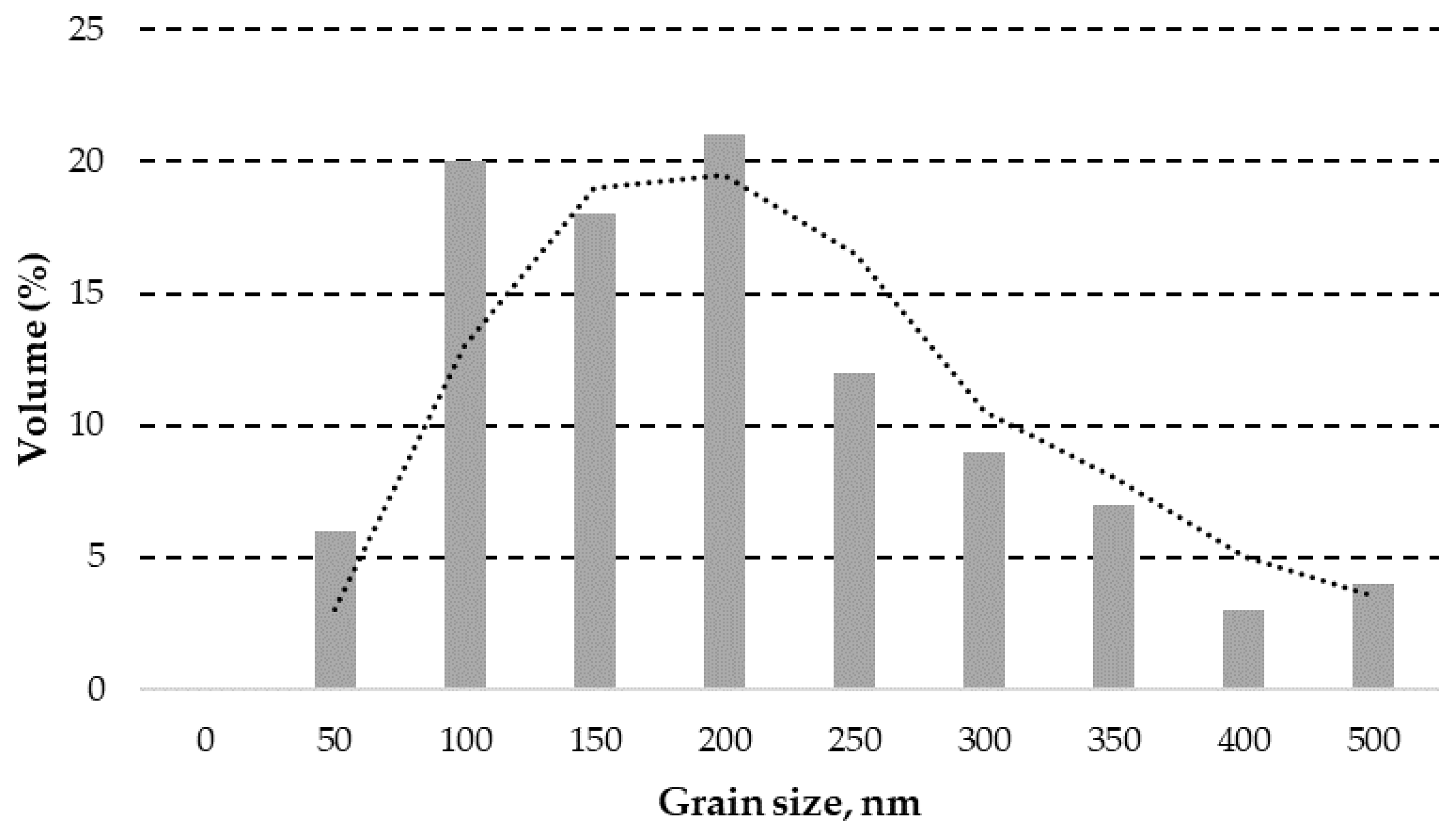
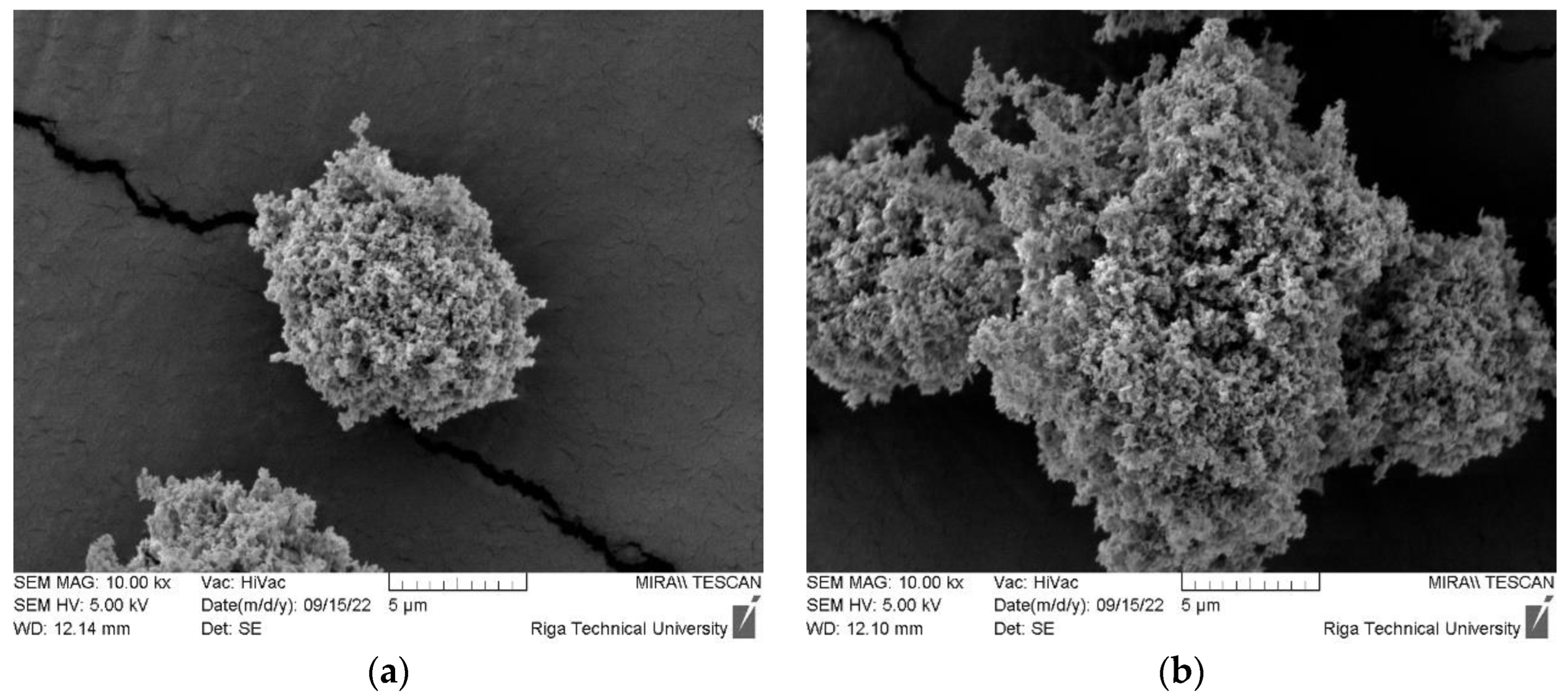
3.1.1. ZnO Powder Granulometric Analysis Result
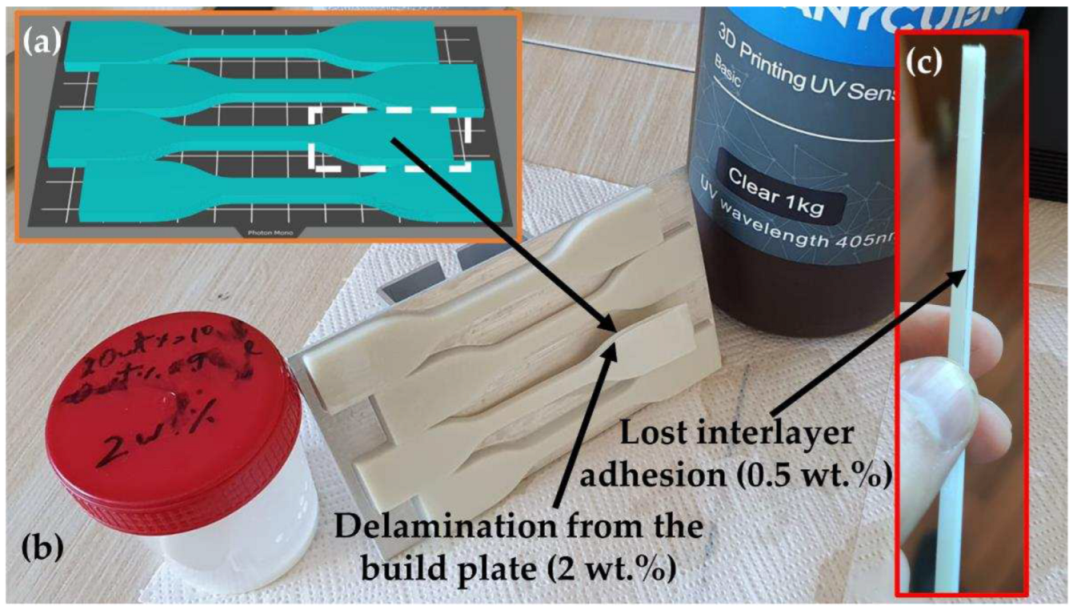
3.1.2. Visual Characteristics and Tensile Strength of Manufactured Samples
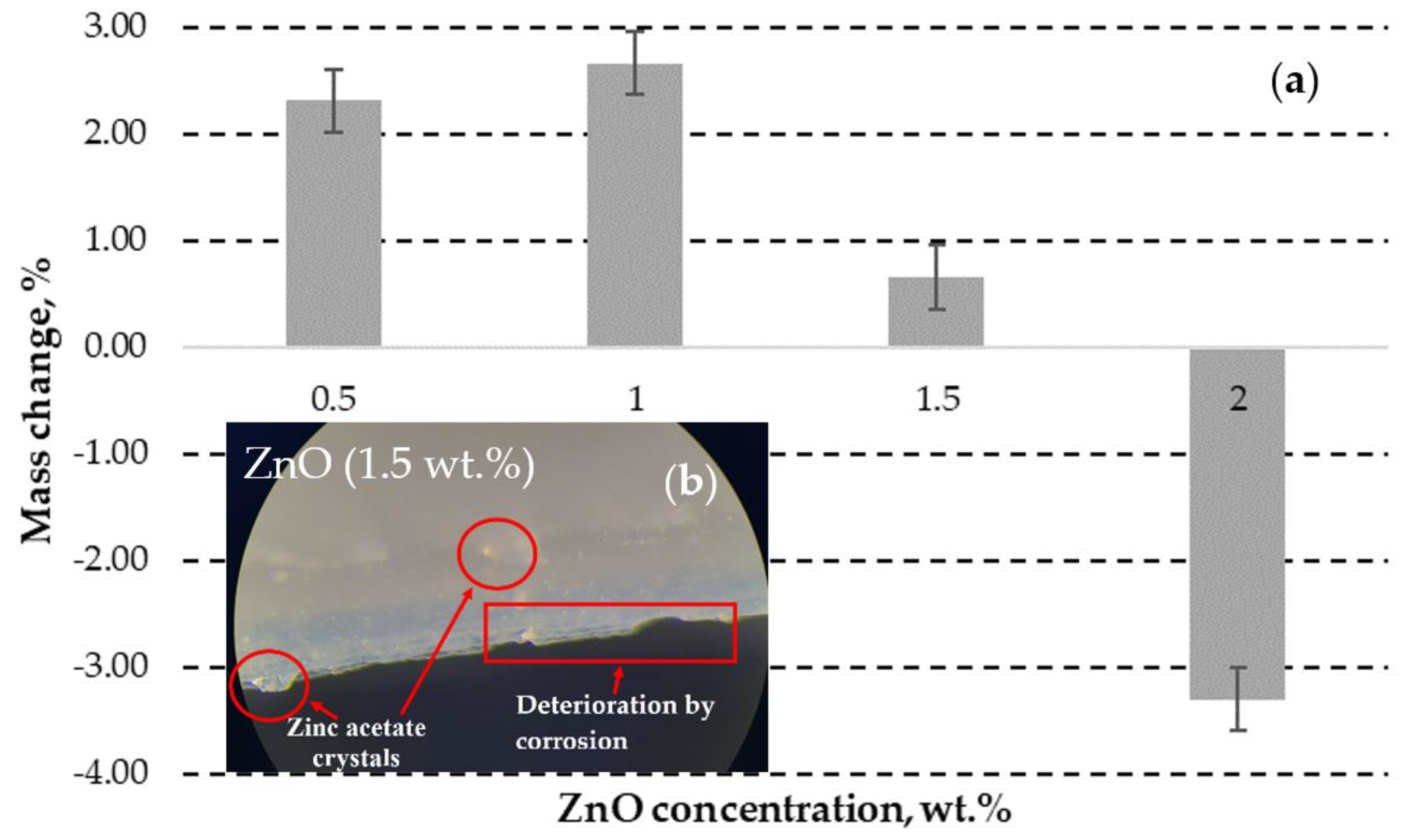
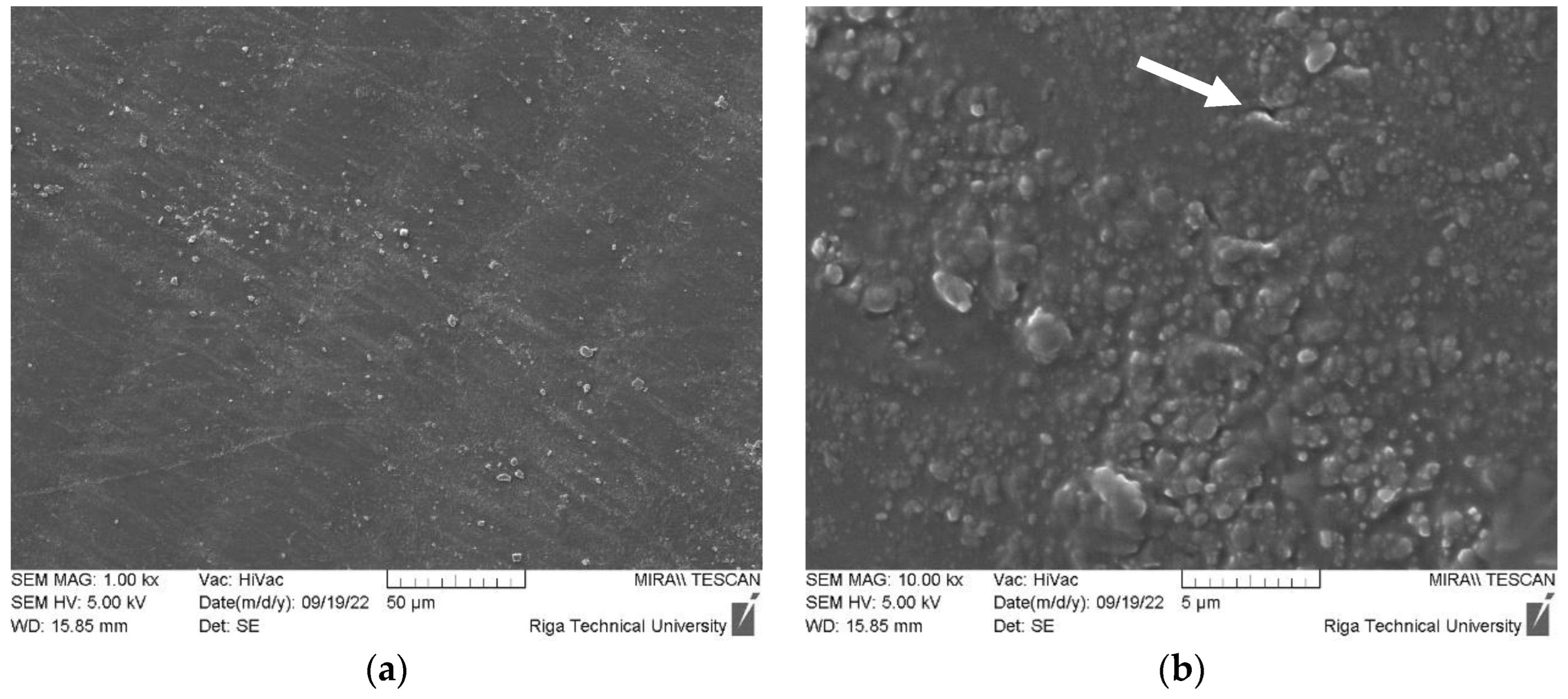
3.1.3. DLP ZnO Composites Corrosion Resistance in Acetic Acid Solution (pH=5)
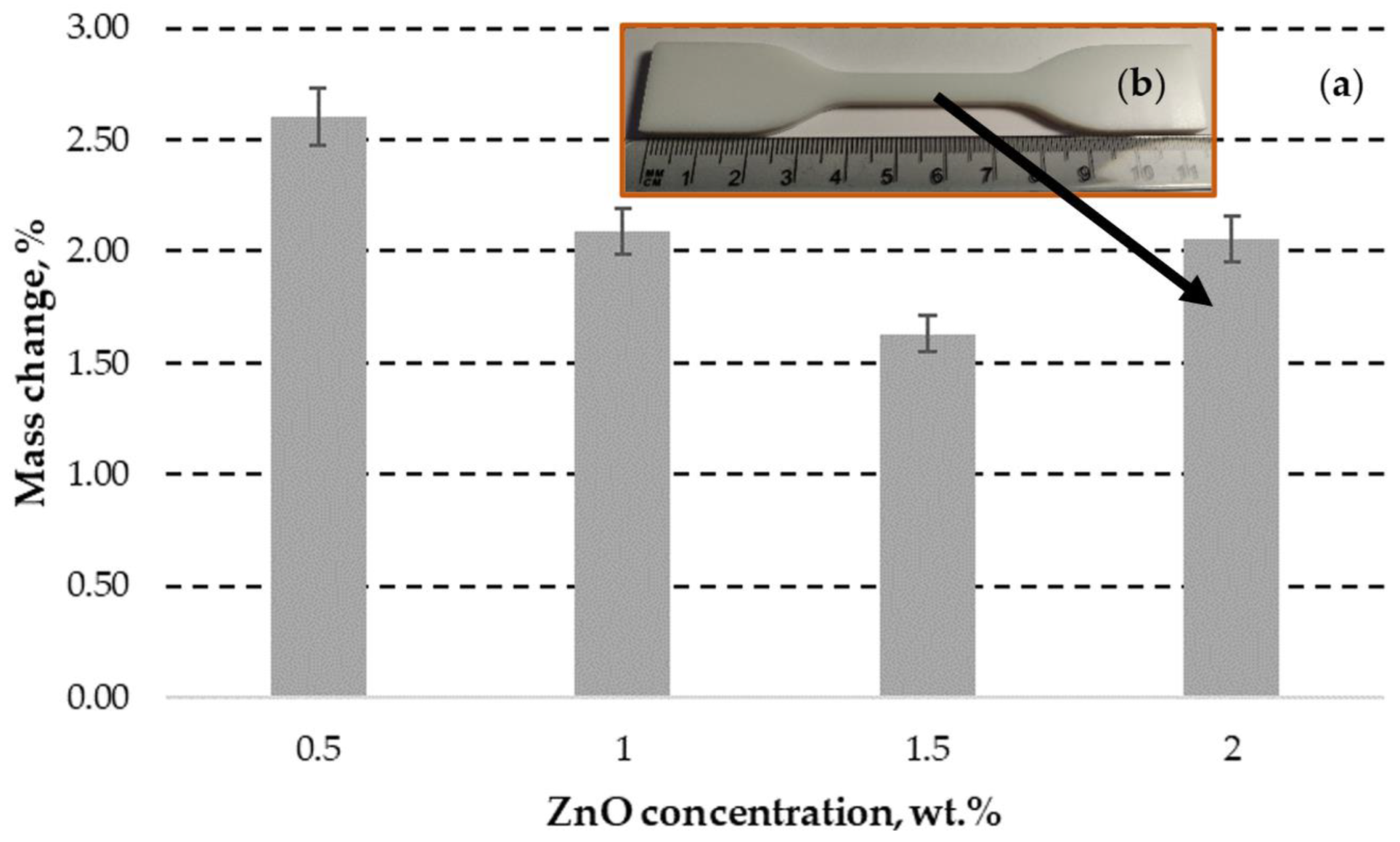
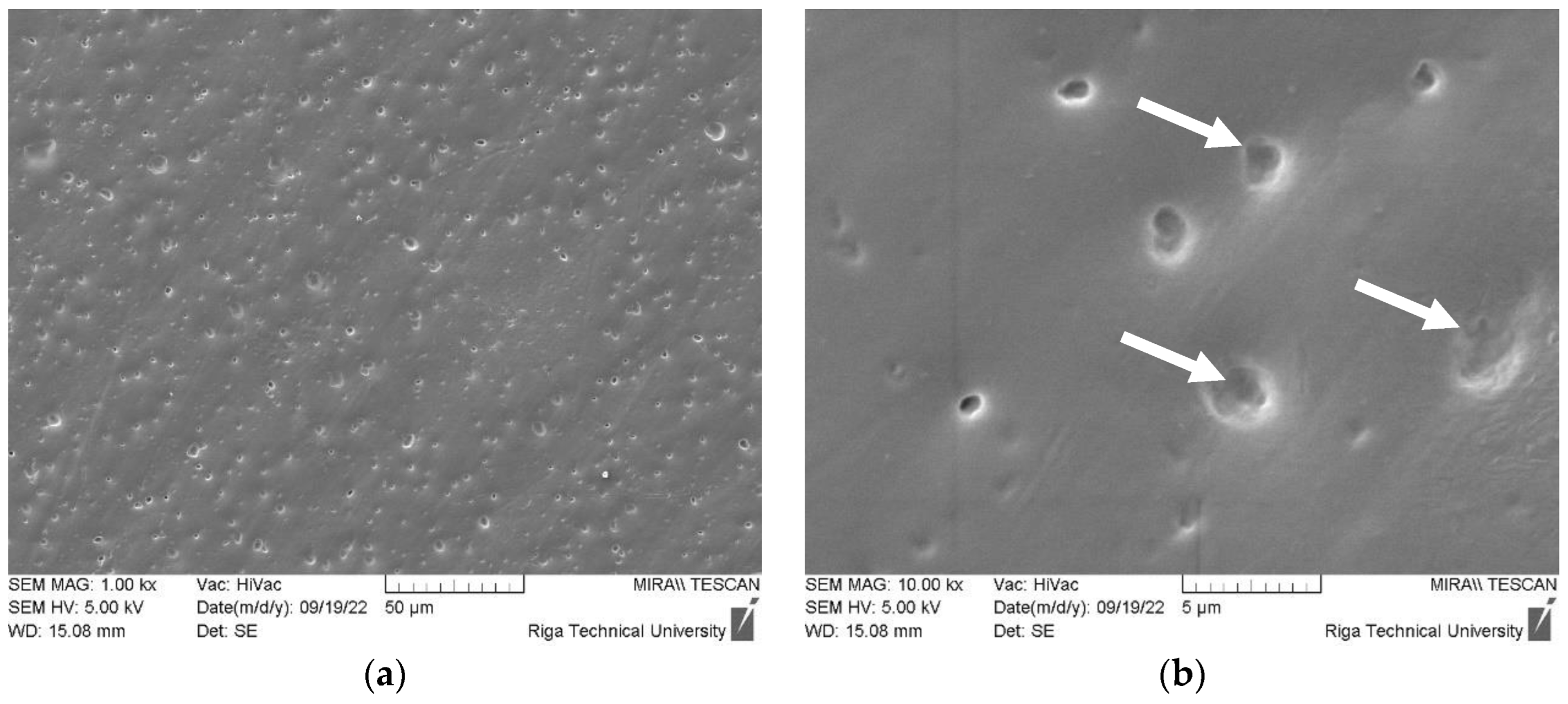
3.1.4. DLP ZnO Composites Corrosion Resistance in Sodium Hydroxide Solution (pH=12)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, O.; Simon, G.P. Epoxy Nanocomposites Based on Layered Silicates and Other Nanostructured Fillers. In Polymer Nanocomposites; Elsevier, 2006; pp. 29–56.
- Fuchi, Y.; Yoshida, K.; Kozako, M.; Hikita, M.; Kamei, N. Comparison of Electrical Insulation Properties of Hydrocarbon-Based Thermosetting Resin and Epoxy Resin. In Proceedings of the 2016 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), IEEE, October 2016; pp. 133–136. [Google Scholar]
- Tanaka, T. Dielectric Nanocomposites with Insulating Properties. IEEE Transactions on Dielectrics and Electrical Insulation 2005, 12, 914–928. [Google Scholar] [CrossRef]
- García, J.M.; García, F.C.; Serna, F.; de la Peña, J.L. High-Performance Aromatic Polyamides. Prog Polym Sci 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(Hydroxy Urethane)s. Chem Rev 2015, 115, 12407–12439. [Google Scholar] [CrossRef]
- Popov, V. V.; Lobanov, M.L.; Stepanov, S.I.; Qi, Y.; Muller-Kamskii, G.; Popova, E.N.; Katz-Demyanetz, A.; Popov, A.A. Texturing and Phase Evolution in Ti-6Al-4V: Effect of Electron Beam Melting Process, Powder Re-Using, and HIP Treatment. Materials 2021, 14, 4473. [Google Scholar] [CrossRef] [PubMed]
- Ron, T.; Leon, A.; Popov, V.; Strokin, E.; Eliezer, D.; Shirizly, A.; Aghion, E. Synthesis of Refractory High-Entropy Alloy WTaMoNbV by Powder Bed Fusion Process Using Mixed Elemental Alloying Powder. Materials 2022, 15, 4043. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.; Fleisher, A.; Muller-Kamskii, G.; Avraham, S.; Shishkin, A.; Katz-Demyanetz, A.; Travitzky, N.; Yacobi, Y.; Goel, S. Novel Hybrid Method to Additively Manufacture Denser Graphite Structures Using Binder Jetting. Sci Rep 2021, 11, 2438. [Google Scholar] [CrossRef]
- Varghese, G.; Moral, M.; Castro-García, M.; López-López, J.J.; Marín-Rueda, J.R.; Yagüe-Alcaraz, V.; Hernández-Afonso, L.; Ruiz-Morales, J.C.; Canales-Vázquez, J. Fabrication and Characterisation of Ceramics via Low-Cost DLP 3D Printing. Boletín de la Sociedad Española de Cerámica y Vidrio 2018, 57, 9–18. [Google Scholar] [CrossRef]
- Popov, V.; Koptyug, A.; Radulov, I.; Maccari, F.; Muller, G. Prospects of Additive Manufacturing of Rare-Earth and Non-Rare-Earth Permanent Magnets. Procedia Manuf 2018, 21, 100–108. [Google Scholar] [CrossRef]
- Fidan, I.; Huseynov, O.; Ali, M.A.; Alkunte, S.; Rajeshirke, M.; Gupta, A.; Hasanov, S.; Tantawi, K.; Yasa, E.; Yilmaz, O.; et al. Recent Inventions in Additive Manufacturing: Holistic Review. Inventions 2023, 8, 103. [Google Scholar] [CrossRef]
- Tubío, C.R.; Guitián, F.; Gil, A. Fabrication of ZnO Periodic Structures by 3D Printing. J Eur Ceram Soc 2016, 36. [Google Scholar] [CrossRef]
- Lu, J.; Dong, P.; Zhao, Y.; Zhao, Y.; Zeng, Y. 3D Printing of TPMS Structural ZnO Ceramics with Good Mechanical Properties. Ceram Int 2021, 47. [Google Scholar] [CrossRef]
- Waheed, S.; Rodas, M.; Kaur, H.; Kilah, N.L.; Paull, B.; Maya, F. In-Situ Growth of Metal-Organic Frameworks in a Reactive 3D Printable Material. Appl Mater Today 2021, 22. [Google Scholar] [CrossRef]
- Tubío, C.R.; Nóvoa, J.A.; Martín, J.; Guitián, F.; Salgueiro, J.R.; Gil, A. Broadband Terahertz ZnO Photonic Crystals Fabricated by 3D Printing. Ceram Int 2019, 45. [Google Scholar] [CrossRef]
- Lee, D.K.; Sin, K.S.; Shin, C.; Kim, J.H.; Hwang, K.T.; Kim, U.S.; Nahm, S.; Han, K.S. Fabrication of 3D Structure with Heterogeneous Compositions Using Inkjet Printing Process. Mater Today Commun 2023, 35. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Tzounis, L.; Mountakis, N.; Korlos, A.; Fischer-Griffiths, P.E.; Grammatikos, S. On the Mechanical Response of Silicon Dioxide Nanofiller Concentration on Fused Filament Fabrication 3d Printed Isotactic Polypropylene Nanocomposites. Polymers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, G.; Yang, Z.; Cao, X.; Jia, D.; Zhou, Y. Direct Ink Writing of Continuous SiO2 Fiber Reinforced Wave-Transparent Ceramics. Journal of Advanced Ceramics 2020, 9, 403–412. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Ning, L.; Sun, J.; Liu, L.; Zhao, K. Preparation and Properties of Nano-TiO2-Modified Photosensitive Materials for 3D Printing. E-Polymers 2022, 22, 686–695. [Google Scholar] [CrossRef]
- Veselý, P.; Froš, D.; Hudec, T.; Sedláček, J.; Ctibor, P.; Dušek, K. Dielectric Spectroscopy of PETG/TiO2 Composite Intended for 3D Printing. Virtual Phys Prototyp 2023, 18. [Google Scholar] [CrossRef]
- Xu, J.; Pang, W.; Shi, W. Synthesis of UV-Curable Organic–Inorganic Hybrid Urethane Acrylates and Properties of Cured Films. Thin Solid Films 2006, 514, 69–75. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Wang, D.; Sun, L.; Webster, T.J. Nanoscale 3D Bioprinting for Osseous Tissue Manufacturing. Int J Nanomedicine 2020, Volume 15, 215–226. [Google Scholar] [CrossRef]
- Flowers, J.; Rose, M.A. Stereolithography Grows Experimenters. Technology and Engineering Teacher 2020, 79, 15–18. [Google Scholar]
- Fu, J.; Yin, H.; Yu, X.; Xie, C.; Jiang, H.; Jin, Y.; Sheng, F. Combination of 3D Printing Technologies and Compressed Tablets for Preparation of Riboflavin Floating Tablet-in-Device (TiD) Systems. Int J Pharm 2018, 549, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Seo, J.; Khan, S.B.; Jang, E.S.; Han, H. Effect of Acrylic Acid on the Physical Properties of UV-Cured Poly(Urethane Acrylate-Co-Acrylic Acid) Films for Metal Coating. Prog Org Coat 2011, 71, 110–116. [Google Scholar] [CrossRef]
- Lü, N.; Lü, X.; Jin, X.; Lü, C. Preparation and Characterization of UV-Curable ZnO/Polymer Nanocomposite Films. Polym Int 2007, 56, 138–143. [Google Scholar] [CrossRef]
- Gaidukovs, S.; Medvids, A.; Onufrijevs, P.; Grase, L.; Brunavs, J. Development of UV-Cured Epoxy Resin Based on Marineline. Formulation for Applications as Efficient Repair System of Boat Tanks’ Protective Coating. In In Proceedings of the Baltic Polymer Symposium 2018; Institute of Physics Publishing (IOP): Jurmala, 2018; pp. 12–14. [Google Scholar]
- Wang, F.; Hu, J.Q.; Tu, W.P. Study on Microstructure of UV-Curable Polyurethane Acrylate Films. Prog Org Coat 2008, 62, 245–250. [Google Scholar] [CrossRef]
- Bao, F.; Shi, W. Synthesis and Properties of Hyperbranched Polyurethane Acrylate Used for UV Curing Coatings. Prog Org Coat 2010, 68, 334–339. [Google Scholar] [CrossRef]
- Clauser, J.; Gester, K.; Steinseifer, U.; Sonntag, S.J. Regulating Blood Cell Adhesion via Surface Modification of Polyurethanes. Advances in Polyurethane Biomaterials 2016, 287–318. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, G.; Zhang, J.; Huang, J.; Yu, X.; Shang, Q.; An, R.; Liu, C.; Hu, L.; Zhou, Y. Rubber Seed Oil-Based UV-Curable Polyurethane Acrylate Resins for Digital Light Processing (DLP) 3D Printing. Molecules 2021, 26, 5455. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural Engineering of Polyurethane Coatings for High Performance Applications. Prog Polym Sci 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Synthesis of Novel UV-Curable Difunctional Thiourethane Methacrylate and Studies on Organic–Inorganic Nanocomposite Hard Coatings for High Refractive Index Plastic Lenses. Prog Org Coat 2008, 62, 274–284. [Google Scholar] [CrossRef]
- Mishra, R.S.; Mishra, A.K.; Raju, K.V.S.N. Synthesis and Property Study of UV-Curable Hyperbranched Polyurethane Acrylate/ZnO Hybrid Coatings. Eur Polym J 2009, 45, 960–966. [Google Scholar] [CrossRef]
- Yoshimura, H.N.; Molisani, A.L.; Narita, N.E.; Manholetti, J.L.A.; Cavenaghi, J.M. Mechanical Properties and Microstructure of Zinc Oxide Varistor Ceramics. Materials Science Forum 2006, 530–531, 408–413. [Google Scholar] [CrossRef]
- Choi, H.M.; Kwon, S.; Jung, Y.-G.; Cho, Y.T. Comparison of Durability for PUA Type Resin Using Wear and Nano-Indentation Test. Journal of the Korean Society of Manufacturing Process Engineers 2018, 17, 8–15. [Google Scholar] [CrossRef]
- Nik Pauzi, N.N.P. ; A. Majid, R.; Dzulkifli, M.H.; Yahya, M.Y. Development of Rigid Bio-Based Polyurethane Foam Reinforced with Nanoclay. Compos B Eng 2014, 67, 521–526. [Google Scholar] [CrossRef]
- Madhan Kumar, A.; Mizanur Rahman, M.; Gasem, Z.M. A Promising Nanocomposite from CNTs and Nano-Ceria: Nanostructured Fillers in Polyurethane Coatings for Surface Protection. RSC Adv 2015, 5, 63537–63544. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Sun, Q.; Shi, B. Enhanced Mechanical Property, Chemical Resistance and Abrasion Durability of Waterborne Polyurethane Based Coating by Incorporating Highly Dispersed Polyacrylic Acid Modified Graphene Oxide. Prog Org Coat 2022, 170, 106949. [Google Scholar] [CrossRef]
- Rahman, M.M. Polyurethane/Zinc Oxide (PU/ZnO) Composite—Synthesis, Protective Property and Application. Polymers (Basel) 2020, 12, 1535. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, L.; Yu, H.; Haroon, M.; Haq, F.; Shi, W.; Wu, B.; Wang, L. Research Progress of UV-Curable Polyurethane Acrylate-Based Hardening Coatings. Prog Org Coat 2019, 131, 82–99. [Google Scholar] [CrossRef]
- Antonov, M.; Kers, J.; Liibert, L.; Shuliak, V.; Smirnov, A.; Bartolomé, J.F. Effect of Basalt Reinforcement Type and Content on the Abrasive Wear Behaviour of Polymer Composites. Key Eng Mater 2016, 674, 181–188. [Google Scholar] [CrossRef]
- Kumar, R.; Malaval, B.; Antonov, M.; Zhao, G. Performance of Polyimide and PTFE Based Composites under Sliding, Erosive and High Stress Abrasive Conditions. Tribol Int 2020, 147, 106282. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Experimental Investigation of Time-Dependent Mechanical Properties of PC-ABS Prototypes Processed by FDM Additive Manufacturing Process. Mater Lett 2017, 193, 58–62. [Google Scholar] [CrossRef]
- Eckel, Z.C.; Zhou, C.; Martin, J.H.; Jacobsen, A.J.; Carter, W.B.; Schaedler, T.A. Additive Manufacturing of Polymer-Derived Ceramics. Science (1979) 2016, 351, 58–62. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. de Nanocomposites for Food Packaging Applications. Food Research International 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Duncan, T. v. Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J Colloid Interface Sci 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Tang, E.; Liu, H.; Sun, L.; Zheng, E.; Cheng, G. Fabrication of Zinc Oxide/Poly(Styrene) Grafted Nanocomposite Latex and Its Dispersion. Eur Polym J 2007, 43, 4210–4218. [Google Scholar] [CrossRef]
- Yan, M.F. Zinc Oxide. Concise Encyclopedia of Advanced Ceramic Materials 1991, 523–525. [Google Scholar] [CrossRef]
- Song, Z.; Kelf, T.A.; Sanchez, W.H.; Roberts, M.S.; Rička, J.; Frenz, M.; Zvyagin, A. V. Characterization of Optical Properties of ZnO Nanoparticles for Quantitative Imaging of Transdermal Transport. Biomed Opt Express 2011, 2, 3321. [Google Scholar] [CrossRef]
- Khabazipour, M.; Anbia, M. Removal of Hydrogen Sulfide from Gas Streams Using Porous Materials: A Review. Ind Eng Chem Res 2019, 58, 22133–22164. [Google Scholar] [CrossRef]
- Ates, T.; Tatar, C.; Yakuphanoglu, F. Preparation of Semiconductor ZnO Powders by Sol–Gel Method: Humidity Sensors. Sens Actuators A Phys 2013, 190, 153–160. [Google Scholar] [CrossRef]
- Li, K.; de Rancourt de Mimérand, Y.; Jin, X.; Yi, J.; Guo, J. Metal Oxide (ZnO and TiO2) and Fe-Based Metal–Organic-Framework Nanoparticles on 3D-Printed Fractal Polymer Surfaces for Photocatalytic Degradation of Organic Pollutants. ACS Appl Nano Mater 2020, 3, 2830–2845. [Google Scholar] [CrossRef]
- Bykkam, S.; Narsingam, S.; Ahmadipour, M.; Dayakar, T.; Venkateswara Rao, K.; Shilpa Chakra, Ch.; Kalakotla, S. Few Layered Graphene Sheet Decorated by ZnO Nanoparticles for Anti-Bacterial Application. Superlattices Microstruct 2015, 83, 776–784. [Google Scholar] [CrossRef]
- Kim, D.; Jang, M.; Seo, J.; Nam, K.H.; Han, H.; Khan, S.B. UV-Cured Poly(Urethane Acrylate) Composite Films Containing Surface-Modified Tetrapod ZnO Whiskers. Compos Sci Technol 2013, 75, 84–92. [Google Scholar] [CrossRef]
- Siyanbola, T.O.; Sasidhar, · K; Rao, · B V S K; Narayan, R. ; Olaofe, · O; Akintayo, · E T; Raju, · K V S N Development of Functional Polyurethane-ZnO Hybrid Nanocomposite Coatings from Thevetia Peruviana Seed Oil. J Am Oil Chem Soc 2015, 92, 267–275. [Google Scholar] [CrossRef]
- Mhd Haniffa, M.A.C.; Ching, Y.C.; Chuah, C.H.; Ching, K.Y.; Liou, N.S. Synergistic Effect of (3-Aminopropyl)Trimethoxysilane Treated ZnO and Corundum Nanoparticles under UV-Irradiation on UV-Cutoff and IR-Absorption Spectra of Acrylic Polyurethane Based Nanocomposite Coating. Polym Degrad Stab 2019, 159, 205–216. [Google Scholar] [CrossRef]
- Soares, R.R.; Carone, C.; Einloft, S.; Ligabue, R.; Monteiro, W.F. Synthesis and Characterization of Waterborne Polyurethane/ZnO Composites. Polymer Bulletin 2014, 71, 829–838. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Hu, C.; Huang, W.; Su, L. Anti-Corrosive Properties of Waterborne Polyurethane/Poly(o-Toluidine)-ZnO Coatings in NaCl Solution. https://doi.org/10.1080/01694243.2018.1529881, 2019; 33, 1047–1065. [Google Scholar] [CrossRef]
- Siyanbola, T.O.; Sasidhar, K.; Rao, B.V.S.K.; Narayan, R.; Olaofe, O.; Akintayo, E.T.; Raju, K.V.S.N. Development of Functional Polyurethane–ZnO Hybrid Nanocomposite Coatings from Thevetia Peruviana Seed Oil. J Am Oil Chem Soc 2015, 92, 267–275. [Google Scholar] [CrossRef]
- Salazar-Bravo, P.; del Angel-López, D.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Brachetti-Sibaja, S.B.; Ferrel-Álvarez, A.C. Investigation of ZnO/Waterborne Polyurethane Hybrid Coatings for Corrosion Protection of AISI 1018 Carbon Steel Substrates. Metallurgical and Materials Transactions A 2019, 50, 4798–4813. [Google Scholar] [CrossRef]
- Posthumus, W.; Magusin, P.C.M.M.; Brokken-Zijp, J.C.M.; Tinnemans, A.H.A.; van der Linde, R. Surface Modification of Oxidic Nanoparticles Using 3-Methacryloxypropyltrimethoxysilane. J Colloid Interface Sci 2004, 269, 109–116. [Google Scholar] [CrossRef]
- Mai, N.T.; Anh, B.T.M.; Vuong, N.T. Acid and Alkali Resistance of Acrylic Polyurethane/R-SiO 2 Nanocomposite Coating. Vietnam Journal of Chemistry 2020, 58, 67–73. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; He, Z.; Hong, J.; Bao, J. Urethane Acrylate-based Photosensitive Resin for Three-dimensional Printing of Stereolithographic Elastomer. J Appl Polym Sci 2020, 137, 49294. [Google Scholar] [CrossRef]
- Anycubic Translucent UV Resin. Available online: https://www.anycubic.com/products/clear-uv-resin (accessed on 24 July 2022).
- MERCK Zinc Oxide. Available online: https://www.sigmaaldrich.com/LV/en/product/sigald/205532 (accessed on 24 July 2022).
- ASTM International ASTM D638 − 14. Standard Test Method for Tensile Properties of Plastics. In Standards and publications; West Conshohocken, 2014; p. 17.
- Anycubic ANYCUBIC Photon Mono. Available online: https://www.anycubic.com/products/photon-mono-resin-3d-printer (accessed on 25 July 2022).
- Ito, H.; Yoshioka, D.; Hamada, M.; Okamoto, T.; Kobori, Y.; Kobayashi, Y. Photochromism of Colloidal ZnO Nanocrystal Powders under Ambient Conditions. Photochemical & Photobiological Sciences. [CrossRef]
- Meng, F.; King, M.D.; Hassan, Y.A.; Ugaz, V.M. Localized Fluorescent Complexation Enables Rapid Monitoring of Airborne Nanoparticles. Environ Sci Nano 2014, 1, 358. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; He, Z.B.; Bai, H.W. Preparation and Mechanical Properties of T-ZnOw/PS Composites. Chinese Journal of Polymer Science (English Edition) 2009, 27, 173–181. [Google Scholar] [CrossRef]
- Zhou, J.P.; Qiu, K.Q.; Fu, W.L. The Surface Modification of ZnOw and Its Effect on the Mechanical Properties of Filled Polypropylene Composites. 2005, 39, 1931–1941. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Mangadlao, J.D.; Tiu, B.D.B.; Tritchler, G.C.; Advincula, R.C. High-Strength Stereolithographic 3D Printed Nanocomposites: Graphene Oxide Metastability. ACS Appl Mater Interfaces 2017, 9, 10085–10093. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.S.; Nancharaih, T.; Rao, V.V.S. Additive Manufacturing Techniques in Manufacturing -An Overview. Mater Today Proc 2018, 5, 3873–3882. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos B Eng 2018, 143, 172–196. [Google Scholar] [CrossRef]
- van Niekerk, J.N.; Schoening, F.R.L.; Talbot, J.H. The Crystal Structure of Zinc Acetate Dihydrate, Zn(CH3COO)2.2H2O. Acta Crystallogr 1953, 6, 720–723. [Google Scholar] [CrossRef]
- Zinc Acetate Dihydrate. In ACS Reagent Chemicals; American Chemical Society: Washington, DC, 2017.
- Vuong, N.T.; Hiep, N.A. The Alkaline Hydrolysis Degradation of a Water-Borne Styrene Acrylic Coating. Vietnam Journal of Chemistry 2016, 54, 249. [Google Scholar]
- Chartrain, N.A.; Williams, C.B.; Whittington, A.R. A Review on Fabricating Tissue Scaffolds Using Vat Photopolymerization. Acta Biomater 2018, 74, 90–111. [Google Scholar] [CrossRef]
- Bae, J.H.; Won, J.C.; Lim, W. bin; Min, J.G.; Lee, J.H.; Kwon, C.R.; Lee, G.H.; Huh, P. Synthesis and Characteristics of Eco-Friendly 3D Printing Material Based on Waterborne Polyurethane. Polymers 2020, 13, 44. [Google Scholar] [CrossRef]
- Bae, J.H.; Won, J.C.; Lim, W. bin; Lee, J.H.; Min, J.G.; Kim, S.W.; Kim, J.H.; Huh, P. Highly Flexible and Photo-Activating Acryl-Polyurethane for 3D Steric Architectures. Polymers 2021, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Do, T.V.; Ha, M.H.; Le, H.K.; Le, T.T.; Linh Nguyen, T.N.; Dam, X.T.; Lu, L.T.; Tran, D.L.; Vu, Q.T.; et al. Crosslinking Process, Mechanical and Antibacterial Properties of UV-Curable Acrylate/Fe3O4-Ag Nanocomposite Coating. Prog Org Coat 2020, 139, 105325. [Google Scholar] [CrossRef]
- Vuong, N.T.; Linh, N.T. The Accelerated Weathering Aging of a Water-Borne Styrene Acrylic Coating. Vietnam Journal of Chemistry 2016, 54, 139. [Google Scholar]
- Voicu, G.; Tiuca, G.A.; Badanoiu, A.I.; Holban, A.M. Nano and Mesoscopic SiO2 and ZnO Powders to Modulate Hydration, Hardening and Antibacterial Properties of Portland Cements. Journal of Building Engineering 2022, 57, 104862. [Google Scholar] [CrossRef]
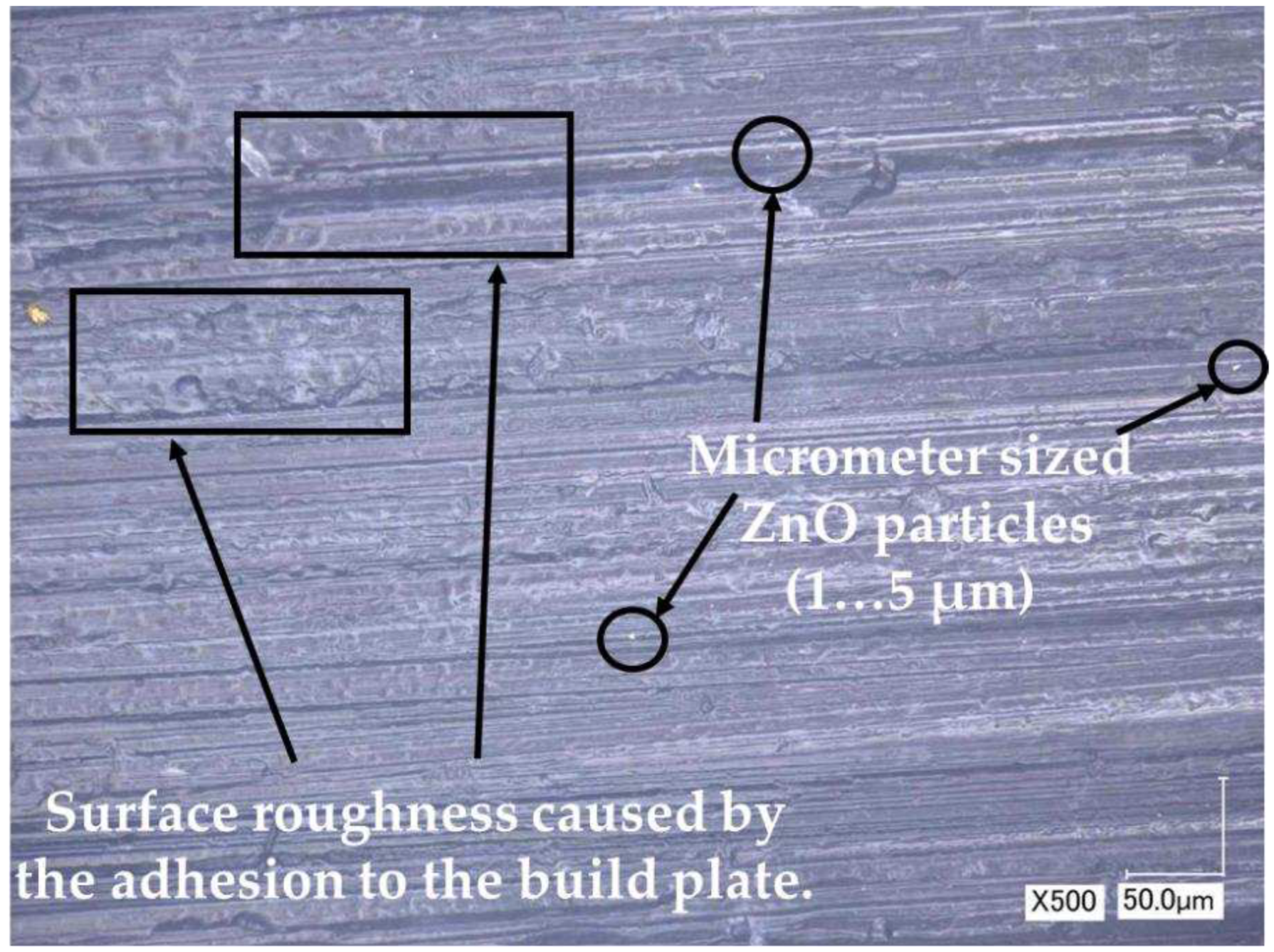
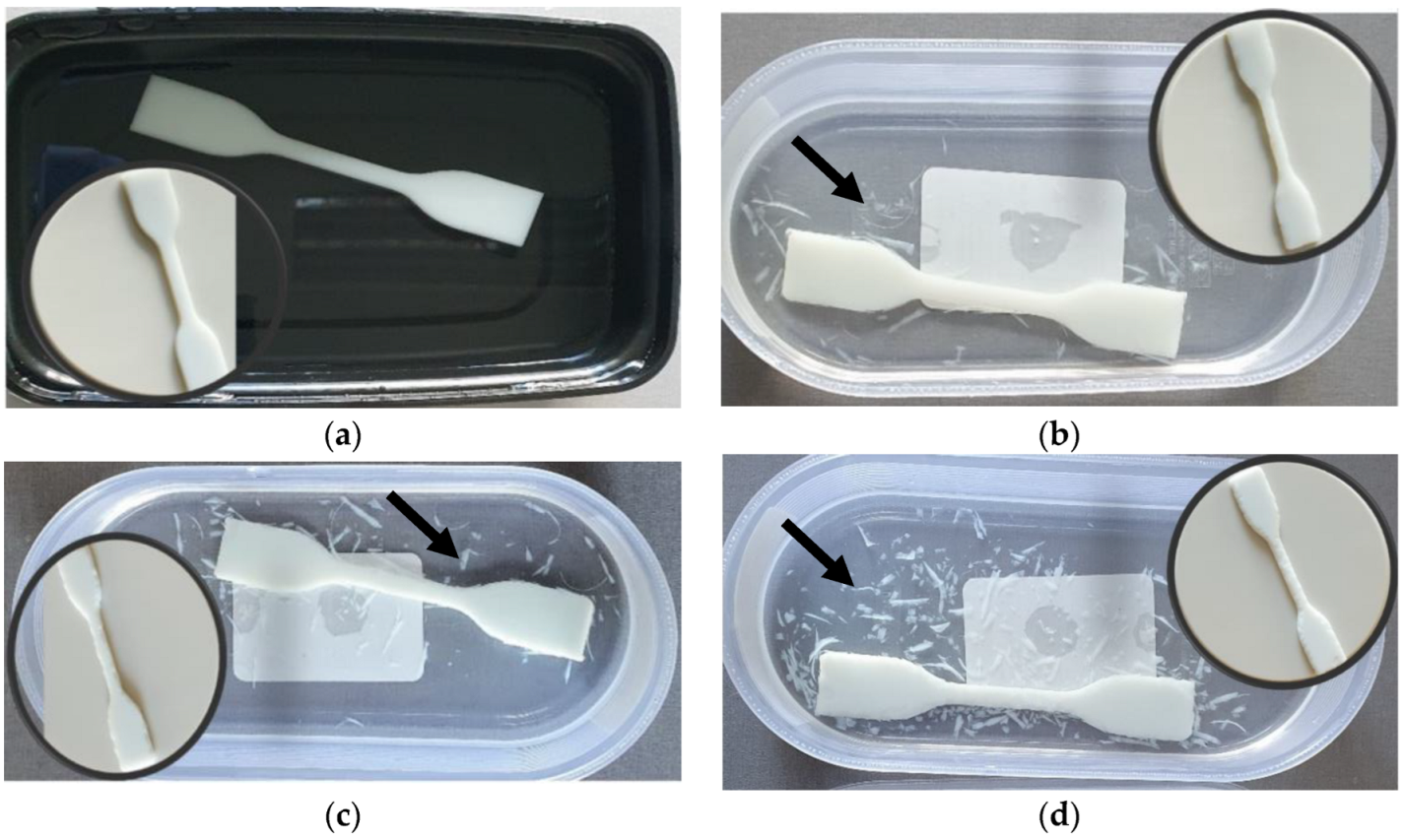

| No. | Filling substance | Particle size | Loading | Additive manufacturing method | Properties/applications | Ref. |
|---|---|---|---|---|---|---|
| 1 | ZnO | 0.7 µm | 44-52 vol% | IPL | The compressive strength ranges from 5.08 to 11.09 MPa at temperatures of 900 to 1500 °C. | [12] |
| 2 | ZnO | 100 nm | 38 wt.% | DLP | The compressive strength ranges from 1.26 to 6.82 MPa for materials with a Gyroid structure and Schwartz P structure. | [13] |
| 3 | ZnO | <130 nm | 10 wt.% | FDM | New devices are continuously emerging for pertinent applications in fields like environmental science, energy, and catalysis. | [14] |
| 4 | ZnO | Highly concentrated ZnO ink | 50 vol% | Robotic deposition equipment | ZnO optoelectronic devices operate at THz frequencies and can be seamlessly integrated with various optical components like waveguides and resonators. | [15] |
| 5 | SiO2 | 100 nm | 2 wt.% | IPL | The applicability of inkjet 3D printing in the electronics industry is promising with ink characteristics like a density of 1.05 g·ml-1 and a viscosity of 9.53 mPa·s, enabling precise and controlled deposition of conductive materials for circuit fabrication. | [16] |
| 6 | SiO2 | 5-15 nm | 0.5-4 wt.% | FFF | Tensile strength ranges from 31 to 35 MPa, with a corresponding tensile modulus of elasticity of 138-148 MPa. Additionally, it has a flexural strength of 40-47 MPa and a flexural modulus of elasticity spanning 786-927 MPa. The impact resistance falls within the range of 3.72-4.01 kJ·m-2, and the microhardness measures between 12.44 and 13.34 HV. | [17] |
| 7 | SiO2 | The diameter of the fiber is 6.5 μm.20 nm powder | 10 vol% (fiber)3.68-11.76 wt.% powder | Direct ink writing | The composite material exhibits a dielectric constant of 1.2 and a dielectric loss tangent of 1.5 x 10-2. Its bending strength ranges from 11.2±1.1 to 14.15±1.3 MPa, while the apparent porosity falls within the range of 24.36% to 24.48%. | [18] |
| 8 | TiO2 | 10 nm | 0-2.5% | SLA | The material demonstrates a tensile strength between 17 and 25 MPa, an impact resistance of 17.5 to 25 kJ·m-2, a hardness of 80 HV, and an elongation at break of 8 to 8.5%. | [19] |
| 9 | TiO2 | 50–300 µm | 10-20% | FDM | The grain size distribution plays a crucial role in the frequency-dependent variations of the dielectric constant and loss factor in this ceramic composite. These characteristics are essential for its performance in dielectric applications, including its use in capacitors for A/D converters, filtration capacitors, and dielectric resonant antennas. | [20] |
| Slice setting parameter | Value | Unit of measure |
|---|---|---|
| Layer thickness | 50 | µm |
| Normal exposure time | 2 | s |
| Off time | 0.5 | s |
| Bottom exposure time | 40 | s |
| Bottom layers | 6 | layers |
| Z axis lift distance (after printing of each layer) | 6 | mm |
| Z axis lift speed | 5 | mm·s-1 |
| Z axis retract speed | 6 | mm·s-1 |
| ZnO concentration | |||||
|---|---|---|---|---|---|
| 0 wt.% | 0.5 wt.% | 1 wt.% | 1.5 wt.% | 2 wt.% | |
| Average thickness, mm (deviation, mm) |
4.01 (±0.07) |
3.84 (±0.03) |
3.99 (±0.16) |
3.72 (±0.34) |
3.83 (±0.12) |
| Average deviation from the target thickness, mm | (~0.000) | −0.160 | −0.003 | −0.280 | −0.170 |
| Average width at the center, mm (deviation, mm) |
5.93 (±0.03) |
6.33 (±0.02) |
6.49 (±0.03) |
6.42 (±0.07) |
6.47 (±0.11) |
| Average deviation from the target width at the center, mm | -0.070 | +0.330 | +0.490 | +0.420 | +0.470 |
| Sample | |||||
|---|---|---|---|---|---|
| 0 wt.% | 0.5 wt.% | 1 wt.% | 1.5 wt.% | 2 wt.% | |
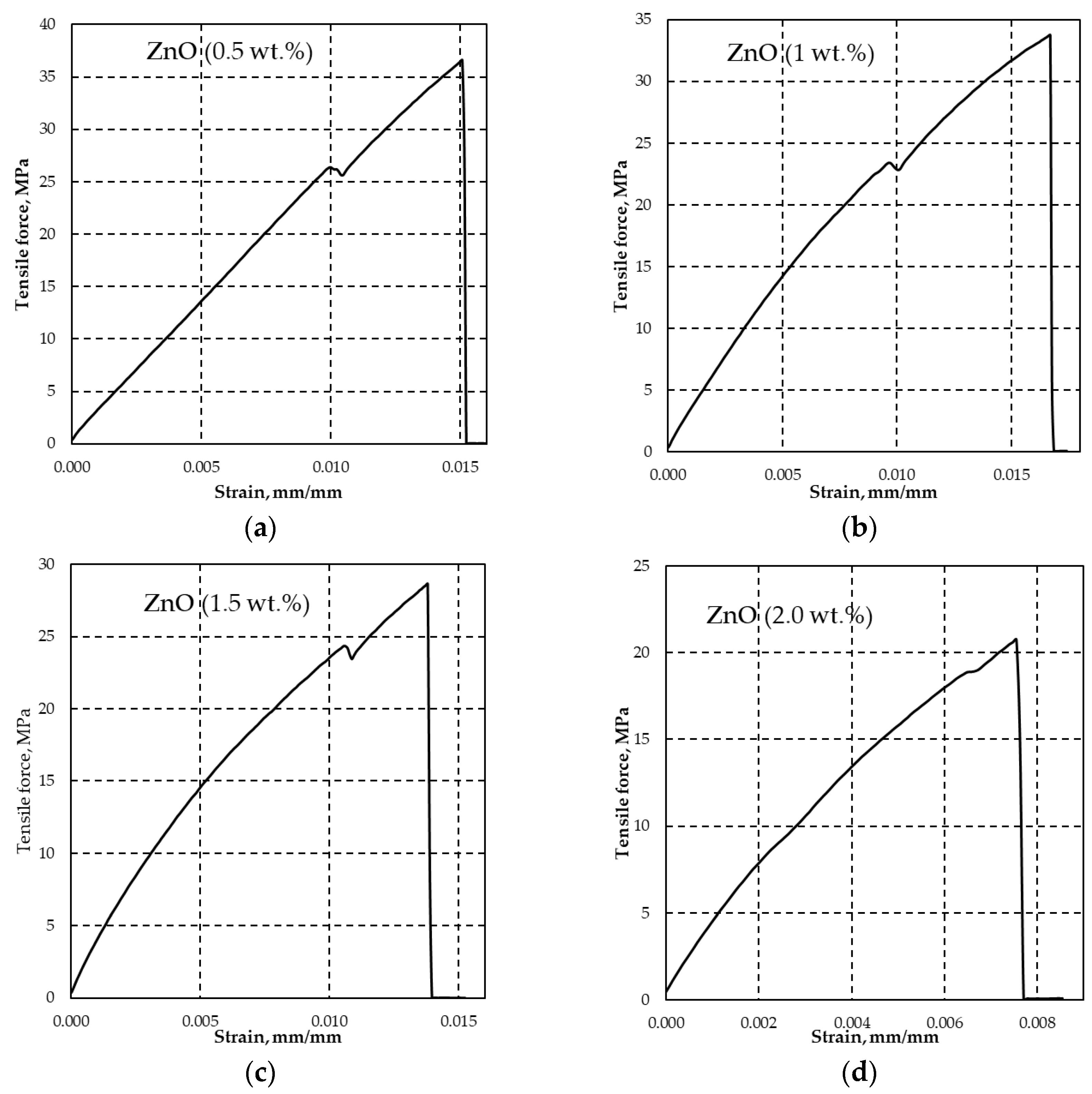
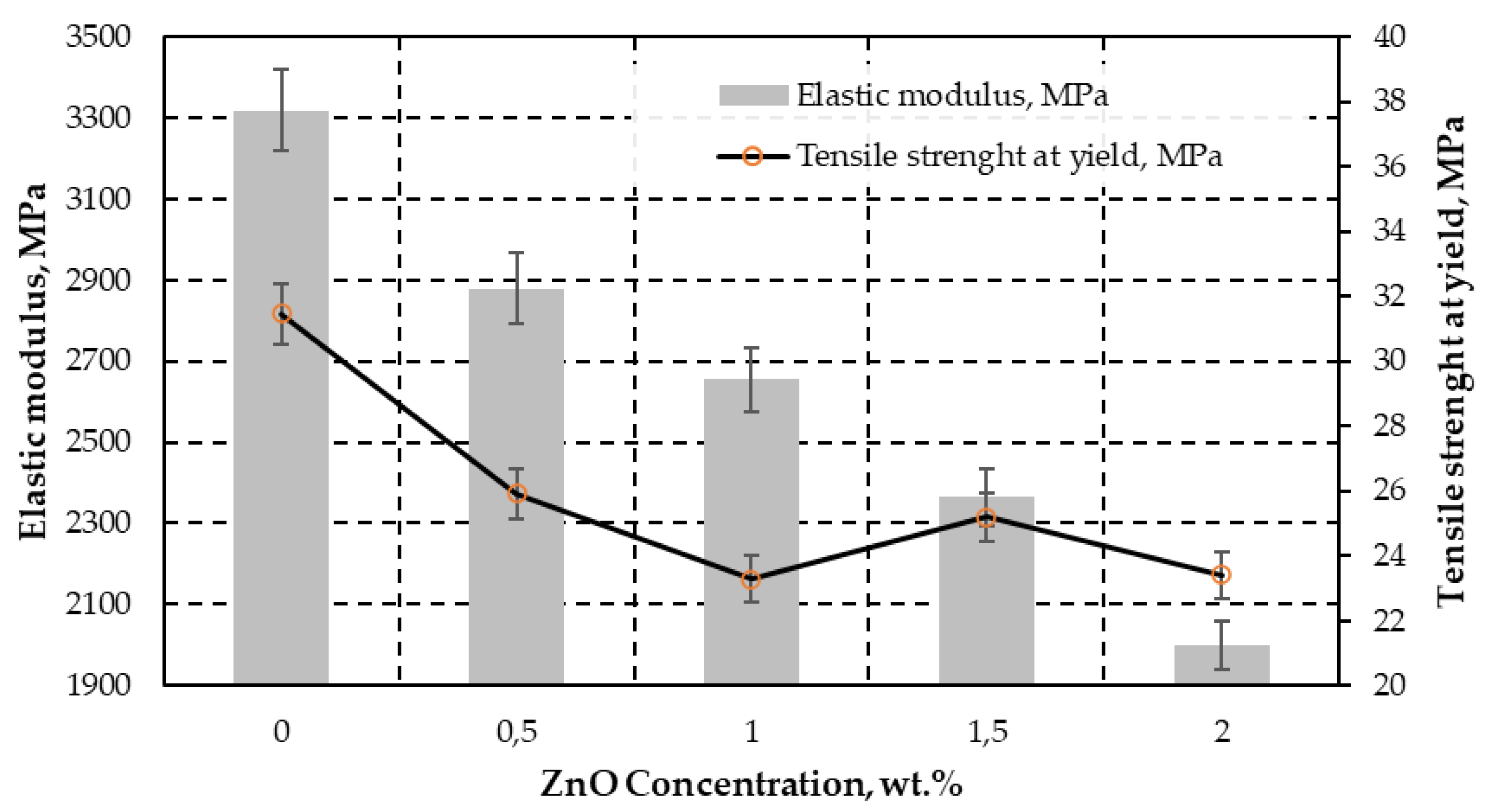
| Tensile strength at yield, σy (MPa) | - | 25.93 (±0.44) | 23.29 (±0.18) | 25.19 (±1.25) | 23.41 (±3.21) |
| Elongation / deformation at yield, ε (mm·mm-1) | - | 0.0091 (±0.0014) |
0.0089 (±0.0012) |
0.0110 (±0.0001) |
0.0091 (±0.0036) |
| Tensile strength at fracture, σUTS (MPa) | 43.1 (±4.06) | 38.76 (±0.03) | 35.40 (±0.03) | 29.93 (±0.03) | 24.04 (±0.03) |
| Elongation / deformation at fracture, εUTS (mm·mm-1) | - | 0.0156 (±0.0001) |
0.0172 (±0.0007) |
0.0146 (±0.0011) |
0.0112 (±0.0051) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





