1. Introduction
Asian seabass (
Lates calcarifer) is a euryhaline fish indigenous to the Indo-Pacific region and is extensively farmed in Southeast Asia, notably in Thailand. Its capacity to survive in fresh and saltwater environments makes it an essential aquacultural commodity in Thailand, with significant economic value. The growth of the Asian seabass farming industry in the region has been propelled by this high demand, leading to a substantial expansion of its cultivation. The cultivation of Asian seabass has not only enhanced its domestic popularity in Thailand but has also resulted in exportation to countries such as China, Taiwan, Vietnam, Singapore, and Malaysia [
1,
2,
3]. However, the Asian seabass farming industry is facing problems due to the outbreak of diseases such as bacterial and viral infections, which are causing significant damage to fish farms. Among those harmful diseases, streptococcosis and columnaris infections, which are caused by
Streptococcus iniae and
Flavobacterium covae, respectively, have been identified as major causative agents that significantly hamper Asian seabass aquaculture [
4,
5,
6].
To overcome disease problems in fish aquaculture, vaccines are considered one of the most effective methods for disease prevention, acting as an alternative to chemical and drug treatment methods, which always have many adverse side effects both in the environment and for consumers [
7]. Unlike in higher vertebrates, vaccination methods in fish can be conducted through injection, immersion, and oral administration. Of these, injection routes are classified as the most effective for immune responses and protection. However, these methods are time- and cost-consuming and always induce stress and mortality after vaccination [
8]. Additionally, this method is too difficult to booster vaccinate in the nursery or juvenile to adults during culture in the grow-out stages. Therefore, immersion and oral administration methods are intensively considered because these routes are easy to perform in small fish, and a high number of fish can be simultaneously vaccinated in hatcheries or nurseries, and booster vaccination can also be more practical [
9,
10].
Compared with the injection route, immersion and oral vaccination have relatively low immune responses and protection efficacy [
11]. To increase and meet the better efficacy of oral and immersion vaccines, nanotechnology is a novel technique developed to enhance vaccine efficacy in various fish species [
11,
12,
13,
14]. This technology involves reducing the size of vaccine particles, enabling them to adhere more efficiently to various mucosa-associated lymphoid tissues (MALTs), which effectively immune-function at the skin, nasal, gill, and gastrointestinal tract of fish, thus promoting better and longer-lasting immune responses and protection [
14,
15]. However, since various diseases occur at different times throughout the production cycle, administering bi- or polyvalent vaccines over the culture period can be targeted to effectively increase the quantity and quality of fish production and reduce production costs, which are preferable for fish farmers. Therefore, bivalent or multivalent vaccines would be a more attractive alternative.
Based on the current information, various bivalent nanovaccines have been developed and have shown excellent immune responses and protection in different fish species, including bivalent vaccines against columnaris and francisellosis in Nile tilapia [
12].
At present, however, there are no reports on using nanovaccines in the aquaculture industry to prevent diseases, especially streptococcosis and columnaris, which are severe diseases that can cause significant damage to the Asian seabass aquaculture industry. Therefore, this study aims to investigate the efficacy of nanovaccines derived from the inactivated bacteria S. iniae and F. covae in enhancing the immune response, gene expression related to immunity, and disease resistance against these two target pathogens in Asian seabass at nursery farm scales. The information obtained from this study is expected to provide valuable information that can be applied to improve disease prevention in the Asian seabass aquaculture industry.
2. Materials and Methods
2.1. Fish and experimental designs
Healthy Asian seabass (
Lates calcarifer) larvae [approximately 15 days after hatching (DAH)] with weights and lengths of 0.07 ± 0.02 g and 1.8 ± 0.17 cm, respectively, were raised at a nursery farm located in the Songklong subdistrict, Bangpakong district, Chachoengsao Province, Thailand. Fish larvae were raised and reared in 12 3.2 × 3.2 m
3 cement ponds containing approximately 5,000 L (50 cm in depth) of 5 ppt estuarine water with 50,000 fish/pond (10 fish/L stocking density). Four groups (3 ponds/group) in 4 zones (1-4) for further vaccination experiments were constructed in nursery farm conditions. The water quality of the nursery pond was closely monitored and controlled daily to ensure its optimal levels for fish, with a temperature range of 28-30°C, pH 7-8, salinity 3-5 ppt, and alkalinity 90-100 mg/L as CaCO
3. The feeding schedule consisted of commercial pelleted feed twice daily, 10% body weight, and the water was routinely changed every morning and evening, approximately 80%. Furthermore, routine fish sampling was conducted to test for potential diseases, including bacteria, viruses, and parasites, using standard microbiology methods [
16].
All described experiments in the current study were conducted according to the Ethical Principles and Guidelines for the Use of Animals National Research Council of Thailand and approved by the Animal Ethics Committee, KU, Thailand (ACKU63-FIS-003).
2.2. Bacterial culture and formalin-killed vaccine preparations
Streptococcus iniae (AQAHMSi2) and
Flavobacterium covae (AQAHMFc) were isolated from moribund Asian seabass [
17]. The bacterium was cultured in tryptic soy broth (TSB) (Difco™, USA) for
S. iniae and Shieh’s broth for
F. covae. Incubate these bacteria at 30°C for 18–24 hours (h). After incubation, the bacterial cultures were harvested via centrifugation at 5,000 rpm for 10 minutes (min), and the resulting supernatant was discarded. The obtained pellets were washed twice with 0.85% NaCl. The bacterial cells were fixed with 1.0% formaldehyde solution for 24 h and washed twice with 0.85% NaCl. The bacterial cell density was measured using a spectrophotometer (Thermo Fisher Scientific, MA, USA) by monitoring the optical density at 600 nm. The purity of the cultured bacterium was confirmed through microscopic examination and Gram staining for further nanovaccine preparation.
2.3. Formulation of bivalent nanovaccines
The dry form of the nanovaccine of each
S. iniae and
F. covae was prepared by complexing antigens with cationic biopolymers to form nanoparticles following the protocol of Kitiyodom et al. (2019) [
18] with some modifications. Both
S. iniae and
F. covae were separately prepared and combined when used as bivalent vaccinations. To formulate inactivated nanovaccine, both formalin-killed bacteria were adjusted based on an optical density until equivalent to 1 × 10
9 CFU/mL. The bacterial cells were sonicated at 40% amplitude for 10 minutes in an ice bath to breakdown them into nanosized particles. Chitosan solution (cationic biopolymer) (50–200 kDa, Sigma) at 0.25% w/v was prepared in 1% acetic acid.
To prepare the biopolymeric nanovaccine, sonicated bacterial cells were gently mixed with chitosan solution at a ratio of 1:0.5 (v/v). Chitosan was simultaneously complexed with antigen and formed cationic nanoparticles as mucoadhesive nanovaccines. The mixture was constantly stirred for 90 minutes at room temperature. Furthermore, the polymeric nanovaccine was transformed into dry form, treated with 2.5% sucrose, and then dried by a freeze-drying process. The vaccines were stored at -80°C. The physicochemical properties of the nanovaccine were measured by average diameter and zeta potential using a Malvern Instruments Zetasizer Nano ZX, employing the dynamic light scattering (DLS) technique described by Kitiyodom et al. (2019) [
18].
Before use, a dry form of bivalent nanovaccine of each S. iniae and F. covae nanovaccine was diluted with 0.85% NaCl to reach 5 × 108 CFU/mL. Diluted nanovaccines of each bacterium were mixed at a ratio of 1:1, providing a 1 × 109 CFU/mL mixture in a 2 L sterile bottle and further used immediately for immersion vaccination after preparation. A preliminary analysis based on a mixture of S. iniae and F. covae nanovaccines showed that this ratio yielded higher ratios of specific IgM to S. iniae concentrations and specific IgM to F. covae concentrations compared with ratios of 1:2, 1:3, 2:1 and 3:1. This information was used for both oral and immersion vaccination.
For the oral vaccine, a nanovaccine of each S. iniae and F. covae nanovaccine with a concentration of approximately 8.5 × 106 CFU was thoroughly mixed to provide a bivalent vaccine of the two bacteria of 1.7 × 107 CFU. This concentration was used to scramble to fish feed at 1.7 × 107 CFU/fish in feed vaccination trials below.
2.4. Immersion vaccination
Three ponds in zone 1 were used as a control, and three ponds in zone areas 2-4, which were maintained by different workers, were used for vaccine treatments. When the fish reached 30 DAH, the water in each nursing pond in zones 1-4 was reduced and remained 10 cm deep (1.0 m3 in volume). Then, 2 L of the above-prepared vaccines was premixed with 2 L of culture water and further thoroughly splashed in the nursing pond to reach the final concentrations of both bacteria at 2 × 106 CFU/mL; fish larvae were exposed to vaccines for a full 20 min. Subsequently, similar spared water was added to a 50 cm depth to maintain a 4 × 105 CFU/mL vaccine concentration. Fish larvae were exposed to the last concentration for 24 h. Two liters of 0.85% NaCl were added to the three control ponds, which served as the control group. Afterward, the water was replaced under the same conditions for 10 days. After this, the second immersion vaccination was conducted. Fish larvae at 40 DAH were booster-vaccinated with the same conditions in the first immersion vaccination. During this period, aeration was fully supplied throughout the experiments.
2.5. Experimental feed preparation and oral vaccination
When the experimental fish reached 50 DAH, the total amount of a bivalent nanovaccine prepared in section 2.3 was calculated based on the target concentration of 1.7 × 106 CFU/fish and then mixed with 70 mL 0.85% NaCl/kg feed. The vaccine solution was thoroughly mixed with commercial feed (Thai Union Feedmill, Thailand) at a 5% feeding rate and air-dried until completely dry. Afterward, experimental fish groups in zones 2-4 were fed 2 times with the prepared feed to reach a total vaccine concentration of 3.4 × 107 CFU/fish/day. Fish were further fed experimental feed for 3 consecutive days to receive a net oral vaccine dose of approximately 1 × 108 CFU/fish. For the control group, fish were fed with feed mixed with 0.85% NaCl under the same conditions as vaccinated fish.
2.6. Effects of bivalent nanovaccine on IgM and immune responses of larvae to fingerling stages of Asian seabass
2.6.1. Fish sampling
During the vaccination experiments, the whole body of Asian seabass was collected 10 days after each vaccination (10, 20, and 30 DAV or 40, 50, and 60 DAH, respectively). At each DAV, 24 fish were randomly selected. The first 8 fish were used for total IgM detection and IgM specific to S. iniae and F. covae by ELISA, lysozyme, and bactericidal activity. The other 8 fish were used for immune-related gene expression. The last 8 fish were prepared for histopathological changes in mucosal-associated lymphoid tissues (MALTs) in the experimental fish’s skin, gills, and gut.
2.6.2. Extraction of whole-body protein
At 10, 20, and 30 DAV, the whole body of Asian seabass was collected using sterilized scissors to finely cut fish into 1.5 mL Eppendorf tubes. Subsequently, sample tissues were preserved in a protein extraction buffer containing a protease inhibitor cocktail (HiMedia, Mumbai, India) at a ratio of 100 mg of tissue per 1 mL of protein extraction buffer. The Asian seabass samples were homogenized using pellet pestles in a stable temperature condition at 4°C. After centrifugation at 2,000-2,500 rpm for 5 min at 4°C, the supernatant was transferred to a fresh 1.5 mL Eppendorf tube and stored at -80°C for ELISA and innate immune parameter analyses below.
2.6.3. Total RNA extraction and preparation of first strand cDNA
The whole fish samples were preserved in 1.0 mL of TriZOLTM reagent (Thermo Fisher Scientific, MA, USA) according to the manufacturer’s protocol. Total RNA was subsequently isolated from the whole body, and the concentration was determined using a NanoDropTM spectrophotometer (Thermo Fisher Scientific, MA, USA). To synthesize first-strand cDNA, one microliter of 1,000 ng/μL total RNA was used as a template with the ReverTra Ace® qPCR RT Master Mix with gDNA Remover Kit (TOYOBO, Japan) according to the manufacturer’s protocol. The product of the first-strand cDNA synthesis was stored at -80°C for further experiments.
2.6.4. Histology analysis of mucosa-associated lymphoid tissues (MALTs)
Whole-body fish collected from all experimental groups after vaccination at each DAV were preserved and kept in Davidson’s fixative. The whole-body fish was then embedded in paraffin blocks after being dehydrated and processed by Fischer et al. (2008) [
19]. Hematoxylin and eosin (H&E) were used to stain the target MALTs, including the skin, gills and intestine. Histopathological changes were observed under a light microscope (Olympus, MA, USA).
2.6.5. Total serum IgM
Total serum IgM protein from the whole body of experimental Asian seabass at 10, 20, and 30 DAV was used in the ELISA. The first step was to coat a flat-bottomed 96-well plate with bicarbonate/carbonate coating buffer, pH 9.6, with a volume of 50 mL, and incubate it at room temperature (RT) for 2 h and discard the solution. The microplate wells were then incubated overnight at 4°C with 50 mL of fish serum protein. After incubation, the wells were washed three times with wash buffer containing phosphate-buffered saline (PBS) and 0.05% Tween-20 (PBST; pH 7.4). Then, 50 mL of VisualProtein-BlockPRO™ Blocking Buffer (Energenesis Biomedical Co., Ltd., Taipei, Taiwan) was added to each well and incubated for 2 h at RT. After incubation, the wells were washed thrice with wash buffer and incubated for 5 mins at RT. After incubation, rabbit anti-Asian seabass IgM antibody (GenScript Biotech, USA) diluted at a ratio of 1:2,000 was added to each well at a volume of 50 mL and incubated for 2 h at RT. After incubation, the wells were washed thrice with wash buffer using an ImmunoWash machine and set for 5 mins for the final wash. Then, goat anti-rabbit IgM (Sigma, USA) diluted at a ratio of 1:5,000 was added to a final volume of 50 mL and incubated at RT for 2 hours. Afterward, wells were washed with wash buffer using the ImmunoWash machine 6 times and further incubated at RT for 5 mins for the 6th wash step. The TMB substrate One Component HRP Microwell Substrate (Surmodics IVD, Inc., USA) was added to a volume of 50 mL and incubated for approximately 1 min at RT, and then TMB Stop Solution (Surmodics IVD, Inc., USA) was added to stop the ELISA reaction. Finally, the IgM levels were measured in the absorbance of each reaction using the iMark™ Microplate Absorbance Reader at 450 nanometers (Bio-Rad Laboratories Ltd., USA).
2.6.6. Assessment of serum IgM specific to S. iniae and F. covae antigens
Detection of specific IgM against S. iniae and F. covae was performed using ELISA. Formalin killed S. iniae and F. covae bacterial solutions at a 1×108 CFU/mL concentration, which were prepared with the above protocol. Bacterial cells were sonicated with a VCX130 ultrasonic sonicator (Sonics & Materials, Inc., CT, USA). The first step was to coat a flat-bottomed 96-well plate with bicarbonate/carbonate coating buffer, pH 9.6, with a volume of 50 mL, and incubate it at RT for 2 h before removing the solution. Next, the bacterial solution prepared earlier was added to each well with a volume of 50 mL and incubated overnight at 4°C. Further steps for serum IgM specific to S. iniae and F. covae were conducted with the same conditions described in section 2.6.5.
2.7. Cellular and humoral immune response assays
2.7.1. Lysozyme activity
The activity of serum lysozyme was evaluated on the lysis of
Micrococcus lysodeikticus (Sigma, MO, USA), as previously described [
20]. Lysozyme activity levels were determined using the following formula: units/mL enzyme = [(A540 sample - A540 blank) dilution factor]/(0.001) × (0.1)
2.7.2. Bactericidal activity
The bactericidal activity (BA) of the whole-body protein samples prepared as described in section 2.6.2 was tested using S. iniae as the representative pathogenic bacterium in the bivalent nanovaccine formulation. The bacterium was cultured and prepared as described above. The BA of the experimental serum was determined by incubating 40 mL of protein serum with 10 μL of 1×105 CFU/mL bacterial suspension of S. iniae bacterium in a 1.5 mL tube (total final pathogen cells in the reaction being 1×103 CFU). The mixture was incubated for 2 h at RT. The surviving bacteria were counted on media specific to S. iniae [trypticase soy broth (Difco™ & BBL™, USA)]. Samples without serum and samples without bacteria were used as negative and positive controls (100% survival or 0% BA), respectively. The BA was calculated as the percentage of surviving bacteria after exposure to serum protein and present after plating on trypticase soy agar (Merck KGaA, Darmstadt, Germany) using the following formula: BA (%) = [(T0 – T24)/T0] × 100, where T0 is the total initial bacteria and T24 is the number of bacteria present after 24 h of exposure.
2.8. Expression of immune-related genes of larvae to fingerling stages of Asian seabass using quantitative real-time PCR (qRT‒PCR)
The immune-related genes
IgM,
IgT,
IgD,
CD4,
MHCIIα, and
TCRα were selected as target in the expression analysis.
IgM and
IgT primers were specifically designed based on their secreted Ig forms. All the primers used in the study were validated in Asian seabass by RT‒PCR amplification and nucleotide sequencing and are listed in
Table 1.
The qRT‒PCR assays were performed using Brilliant III Ultra-Fast SYBR
® Green (Agilent, CA, USA) in an Mx3005P QPCR Systems instrument (Agilent, CA, USA). The qPCRs were optimized in 15 μL reactions, including 10 μL of 2×SYBR Green QPCR Master Mix, 1 μL of 0.5 mM forward and reverse primers, 1 μL of cDNA template, and distilled water to adjust the reaction to a final volume of 15 μL. The qPCR cycling conditions consisted of an initial condition of one cycle of 95°C for 5 min, 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 90 s, and a final extension at 72°C for 10 min. Triplicate qRT-PCRs were conducted for each sample. The housekeeping gene
β-actin was used to standardize the results by eliminating variation in mRNA and cDNA quantity and quality. The relative expression of immune-related genes in the whole body of Asian seabass at different time points was calculated using 2
-ΔΔCT analysis following the protocol of Livak and Schmittgen (2001) [
21].
2.9. Growth performance
The effects of bivalent nanovaccines on growth performance were assessed during a 30-day vaccination trial. The growth performances of all treatment groups were based on their body weight data. Thirty fish from each treatment group (10 fish/pond) were randomly monitored for growth performance parameters by weighing their bodies at 10, 20, and 30 DAV. Growth performances were reported as 1) absolute growth rate (AGR), including weight gain (WG) and average daily growth rate (ADG), and 2) specific growth rate (SGR). Moreover, the feed conversion ratio (FCR) was also measured in all experimental trials. All growth calculations were performed using the methods described by Bunnoy et al. (2019) [
22].
where W
t is the final weight/length, W
i is the initial weight/length, and t is the trial duration (30 days).
2.10. Challenge with S. iniae and F. covae
At the end of the 30 DAV, one hundred eighty fish from each pond of zones 1-4 were randomly selected and transferred into 6 250-L fiberglass tanks (30 fish/tank) for challenge tests, three tanks for
S. iniae and three tanks for
F. covae. The preliminary pathogenicity of virulent
S. iniae and
F. covae on Asian seabass was determined to validate the optimum dose for the experimental challenge trials. The fourteen-day median lethal concentration (LC
50) was assessed by direct immersion with the obtained final concentrations of 1 × 10
5 CFU/mL for
F. covae and 1× 10
7 CFU/mL for
S. iniae for 30 min, which was subsequently applied for the challenge test in this trial. The mortality and survival of the fish were recorded every 12 h for up to 14 days postchallenge. Reisolation of
S. iniae and
F. covae from moribund fish was performed to verify the cause of death in fish using a standard diagnostic protocol [
16]. Cumulative survival analysis of Asian seabass challenged with
S. iniae and
F. covae was calculated using the Kaplan‒Meier method [
15].
2.13. Statistical analysis
Protein serum IgM levels, innate immune response parameters, immune-related gene expression, and cumulative survival were obtained from three replicates, and the results are expressed as the mean ± standard deviation (SD). Data were statistically analyzed by one-way analysis of variance (ANOVA) and Duncan’s new multiple range test (DMRT) to determine differences among groups. All statistical analyses were performed using Statistical Package for Social Science (SPSS for Windows version 24.0, Chicago, IL, USA). The level of statistical significance between the control and experimental groups in the challenge test was indicated as * (p < 0.05), ** (p < 0.01), and ***(p < 0.001) using Student’s t test.
4. Discussion
Intensive Asian seabass cultures are constantly faced with an increase in the risk of disease outbreaks, leading to a reduction in fish production and mass mortalities [
23]. Streptococcosis and columnaris disease are common causes of disease outbreaks in many freshwater fish species worldwide, mainly in the Asian seabass culture in Thailand [
5,
24]. The efficacy of antibiotics and chemicals in managing these diseases is variable. Vaccination offers a pragmatic and trustworthy means of averting diseases in aquaculture, diminishing occurrences of mass mortality, and lessening the reliance on harmful antibiotics and chemicals [
25,
26]. These strategies have been commercially applied to many economic fish species [
27]. In comparison to the vaccination routes, injection administration is the most effective regarding immune responses and protection levels [
8]. However, this method is relatively unacceptable for commercial scales with high labor, high stress, mortality induction, booster vaccination difficulty during fish growth in grow-out ponds, cost requirements, etc. Therefore, immersion and oral vaccination have been developed to increase their efficacy in the fish aquaculture industry. These methods were previously found to exhibit low efficacy in protection but have been lately developed to meet an acceptable level important for industry. With the progress and development of nanotechnology, nanovaccines with mono- and bivalent characteristics have been effectively developed [
12,
28].
Therefore, the development of bivalent nanovaccines against S. iniae and F. covae was conducted in the present study to generate high efficacy in resistance to these two harmful pathogenic bacteria in Asian seabass larvae.
Based on the physical characteristics of
S. iniae and
F. covae nanovaccines, it was demonstrated that the average particle size and zeta potential were in line with the suitable and influential characteristics of nanovaccines that are smaller than 500 nm and have positive charges. The cationic biopolymer generated positively charged nanovaccines, enhancing adhesion on the mucosal surface and improving protection against diseases. These properties have been suggested as the optimal characteristics for effective and favorable vaccines to be efficiently absorbed by fish immune-associated organs or tissues [
29].
Recently, mono- and bivalent nanovaccines have been developed to provide better immune responses and protection in various fish species, especially tilapia, from several fish pathogens, such as
Aeromonas veronii [
30],
Flavobacterium oreochromis [
12],
Francisella orientalis [
14],
Streptococcus agalactiae [
31,
32,
33] and tilapia lake virus [
34,
35,
36]. These nanovaccines were formulated from various materials, including chitosan or its derivatives, alginate, nanoclay, halloysite nanotubes, and poly[(methyl methacrylate)-co-(methyl acrylate)-co-(methacrylic acid)]-poly(d,l-lactide-co-glycolide) (PMMMA-PLGA). With oral, immersion, and intramuscular injections, these nanovaccines effectively elevated protection from the pathogens mentioned above [
37]. Of these, chitosan and its derivatives are low-cost and effective adjuvants, enhancing better immune responses such as immunostimulants [
38,
39].
To date, various monovalent nanovaccines have been reported in some fish species; in the present study, chitosan-based bivalent nanovaccines created from
S.
iniae and
F.
covae bacteria were further applied to fish larvae in the nursery farms of farmers via the first immersion and the other two immersions during routine water exchanges of larval stages and oral booster vaccination during fingerling periods, respectively. These combination methods have been reported to prevent stress conditions that may severely rise during or after excessive handling or overcrowding conditions in vaccination periods [
40,
41]. Our research intends to explore cost-effective, safe, practical, and high-efficacy vaccines for dual-preventing harmful pathogenic bacteria causing streptococcosis and columnaris diseases at Asian seabass farm scales.
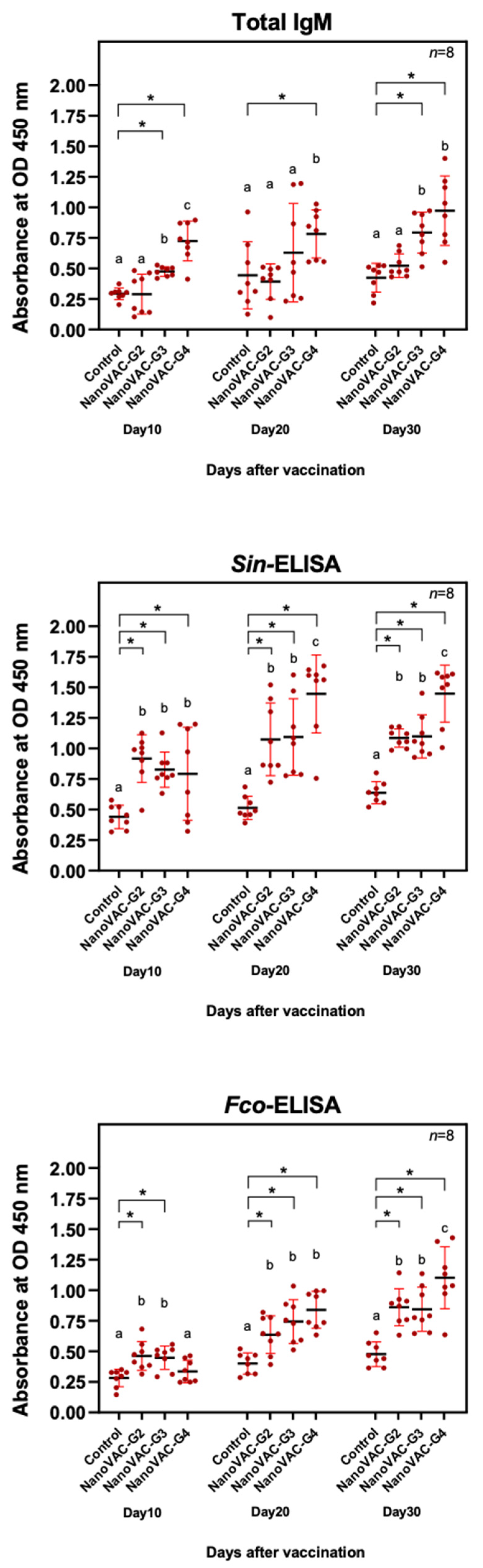
Principally, total serum IgM is expected to be the first indicator used to demonstrate immune responses after vaccination, and nanovaccines were effectively surpassed to increase this target parameter [
13]. The application of the current nanovaccines significantly increased serum IgM levels in the 3rd and 4th vaccinated groups at 10 and 30 DAVs, similar to bivalent mucoadhesive nanovaccines of
Flavobacterium oreochromis (For) and
Francisella orientalis (Fo) and monovalent nanovaccines of For and Fo, which showed significant IgM levels compared with the control group. However, when specific IgM to
S.
iniae or
F.
covae bacteria was measured, all nanovaccine-immunized groups showed significantly higher specific IgM than the unvaccinated control group at every DAV. In contrast to the previous study, total IgM levels of Nile tilapia in bivalent mucoadhesive nanovaccine
For- and
Fo-vaccinated groups were significantly suppressed compared with the
For or
Fo monovalent vaccines [
12], suggesting that the bivalent
S.
iniae or
F.
covae nanovaccine is strong enough to enhance specific IgM levels against both
S.
iniae or
F.
covae.
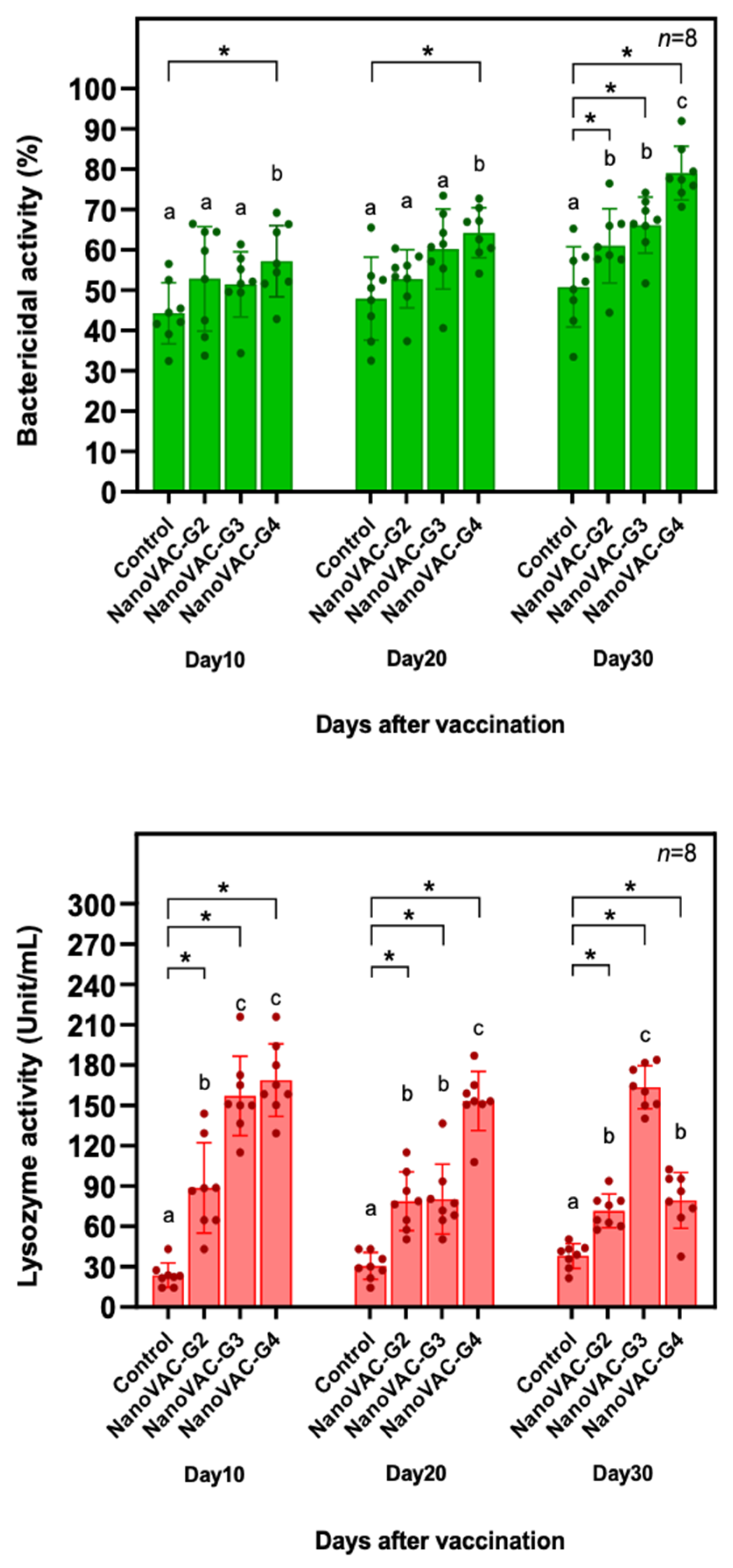
Additionally, our findings showed that fish immunized with bivalent nanovaccines showed an increase in innate immune parameters, including lysozyme activity and bactericidal activity. This information agreed with the results from previous studies conducted by Bunnoy et al. [
12], which showed that the application of bivalent mucoadhesive nanovaccines to prevent francisellosis and columnaris diseases in Nile tilapia also significantly elevated these innate immune-parameters against
Francisella orientalis and
Flavobacterium oreochromis, suggesting that the innate immune responses of fish are simultaneously induced by bivalent nanovaccine application. Furthermore, this vaccine effectively upregulates several immune-related genes, such as
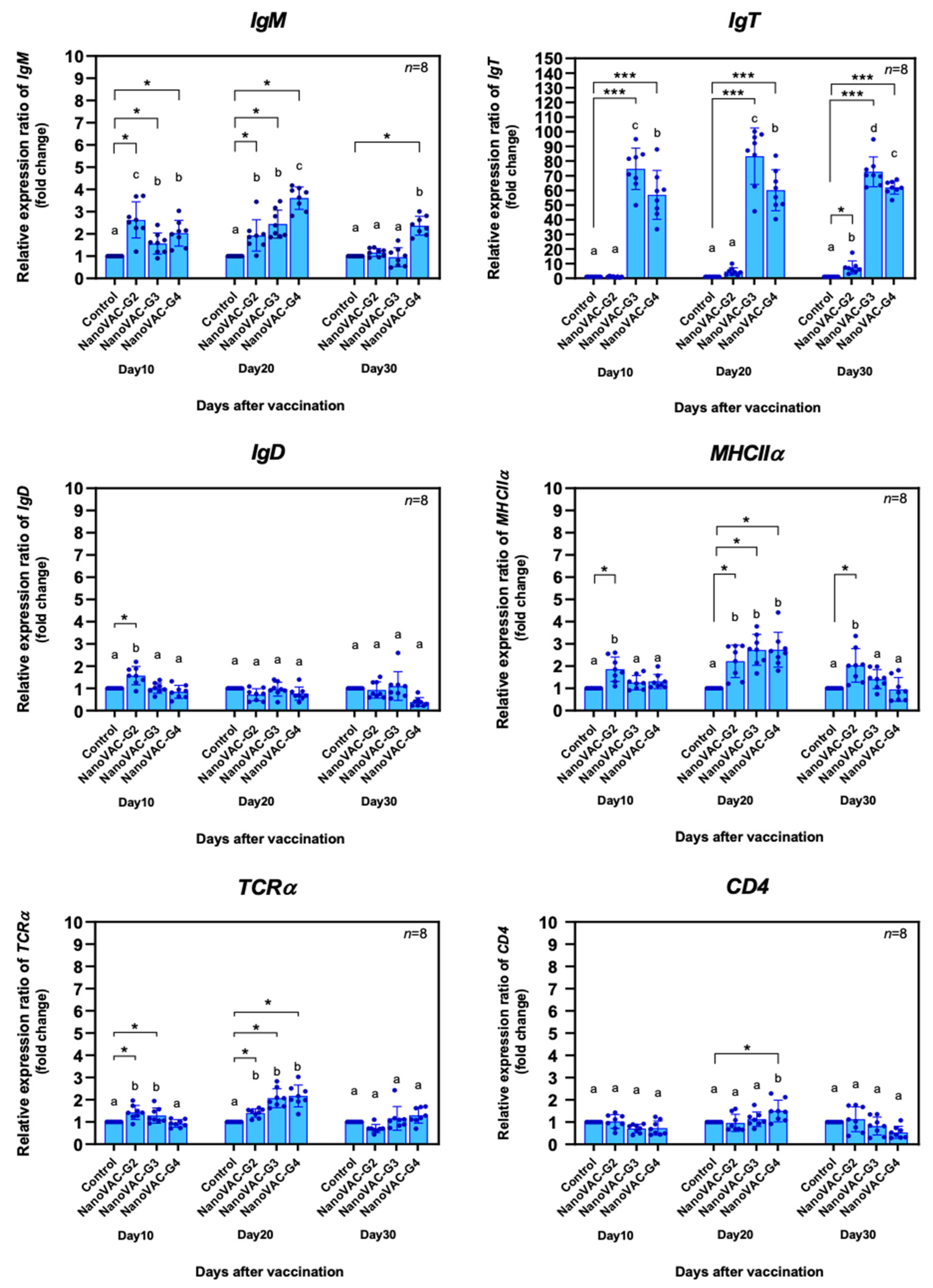
IgM,
IgD,
IgT,
MHCIIα,
TCRα, and
CD4, which are key components of specific immune systems. This information was firmly in line with previous studies [
12,
14,
28,
42,
43,
44], suggesting that the bivalent nanovaccines in the current study are potent immunogens that vigorously drive the two arms of both innate and adaptive immune systems [
12]. Particularly for
IgM and
IgT, almost all vaccinated treatments showed very high upregulation, indicating that two immersion and single booster vaccinations of the bivalent nanovaccines effectively enhance both systemic and local-specific immune responses. Similar responses were also observed in several monovalent nanovaccine experiments [
14,
18,
30] and a bivalent nanovaccine [
12].
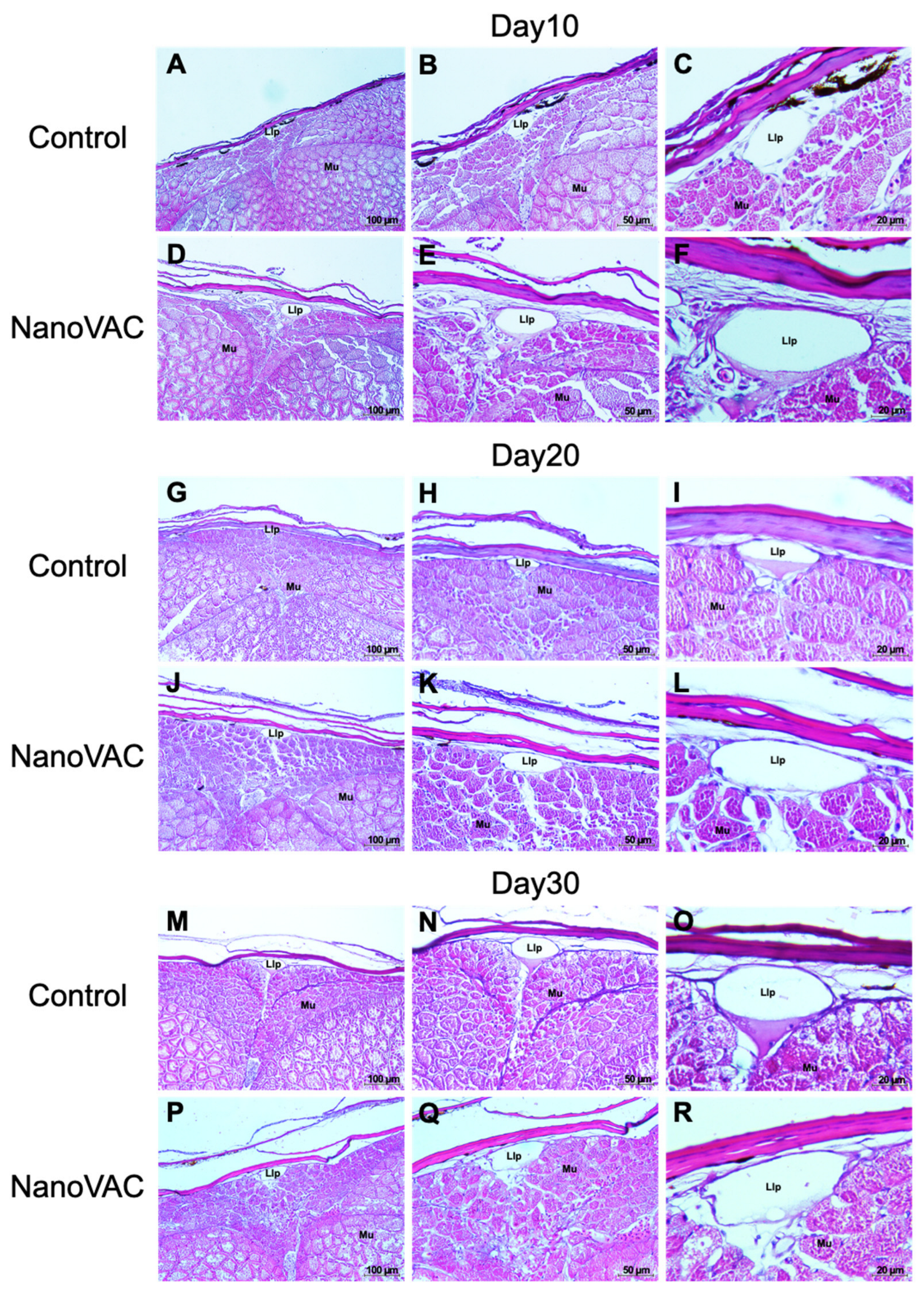
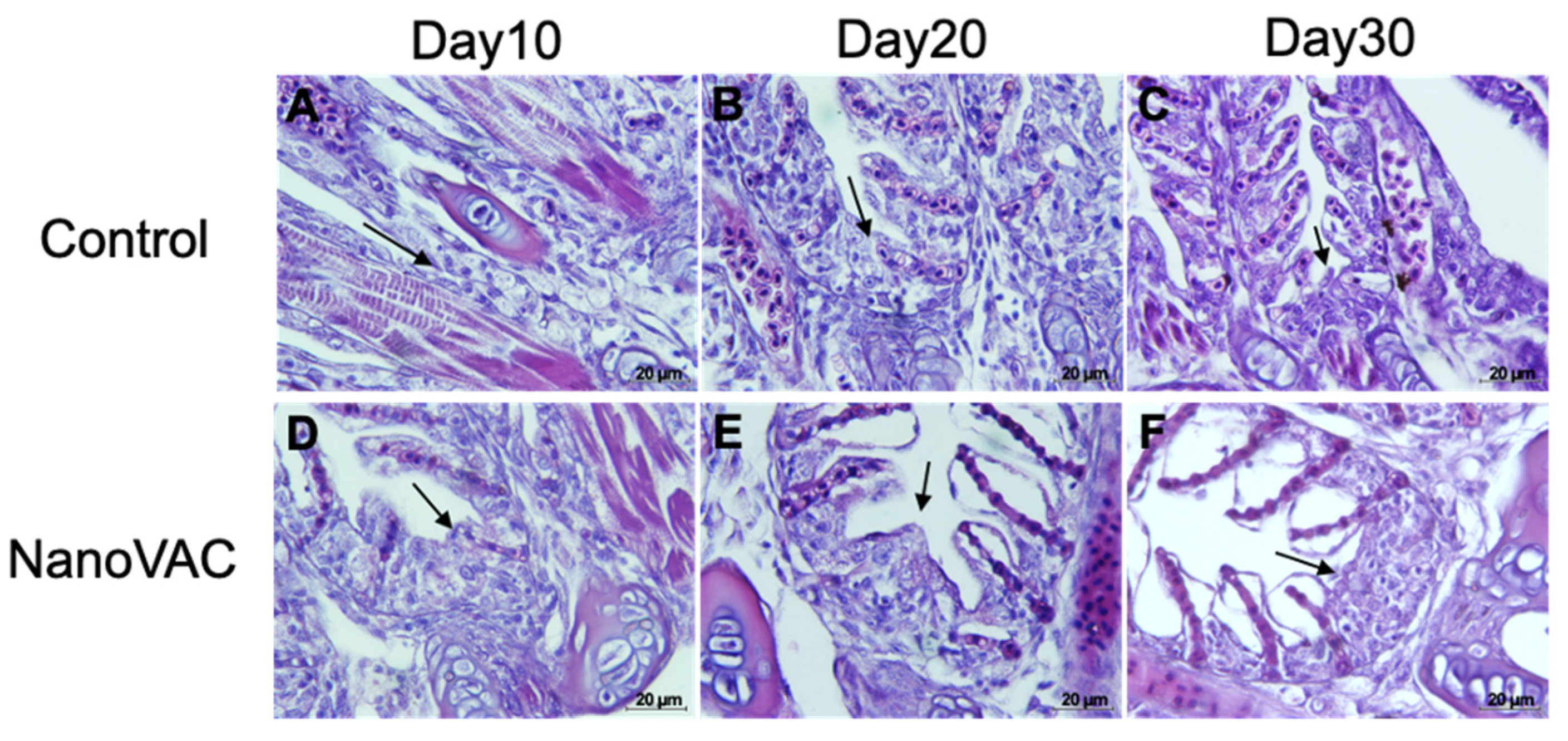
Histopathology intensively supported the surpassing local immune responses found in the above information. Our study demonstrates that bivalent nanovaccines could permeate the skin and gills, the main organs targeted by the immersion vaccination method [
45]. Interestingly, the interbranchial lymphoid tissue (ILT) in the gills of all vaccinated fish became elevated, with numerous immune-like cells related to the immune responses. However, this structure, the ILT, does not seem to have any equivalent among lymphoid tissues, and its function is still unknown, although it shares some properties with secondary lymphoid structures [
46]. Additionally, an elevation of the epithelium layer was observed in the gills of the vaccinated fish. These changes have never been reported previously in immersion vaccination trials, but similar characteristics can be observed in previous experiments, where fish were exposed to various factors [
47,
48,
49]. This mysterious observation requires further investigation.
Furthermore, histopathological changes in mucus cells on epithelial layers were also observed in the gut of all vaccinated fish after the second booster vaccination via oral administration at 30 DAV. This phenomenon strongly indicates that an effective oral vaccination route impacts immune responses in GALT, which is crucial for the local gastrointestinal tract resistant components of fish [
50,
51,
52]. Unfortunately, no available literature dealing with vaccination in fish using bivalent nanovaccines can be used for comparative analysis.
In the current study, research on combined nanovaccines using Gram-positive and Gram-negative bacteria for Asian seabass or other fish species is still scarce. Therefore, there is insufficient data to clearly analyze and compare the mechanism of combined nanovaccine responses in Asian seabass in the current study. Further research is needed to address these gaps in the future. However, previous studies in other fish species, especially tilapia, have demonstrated positive results, indicating the high impacts of nanovaccine application with various practical administrations.
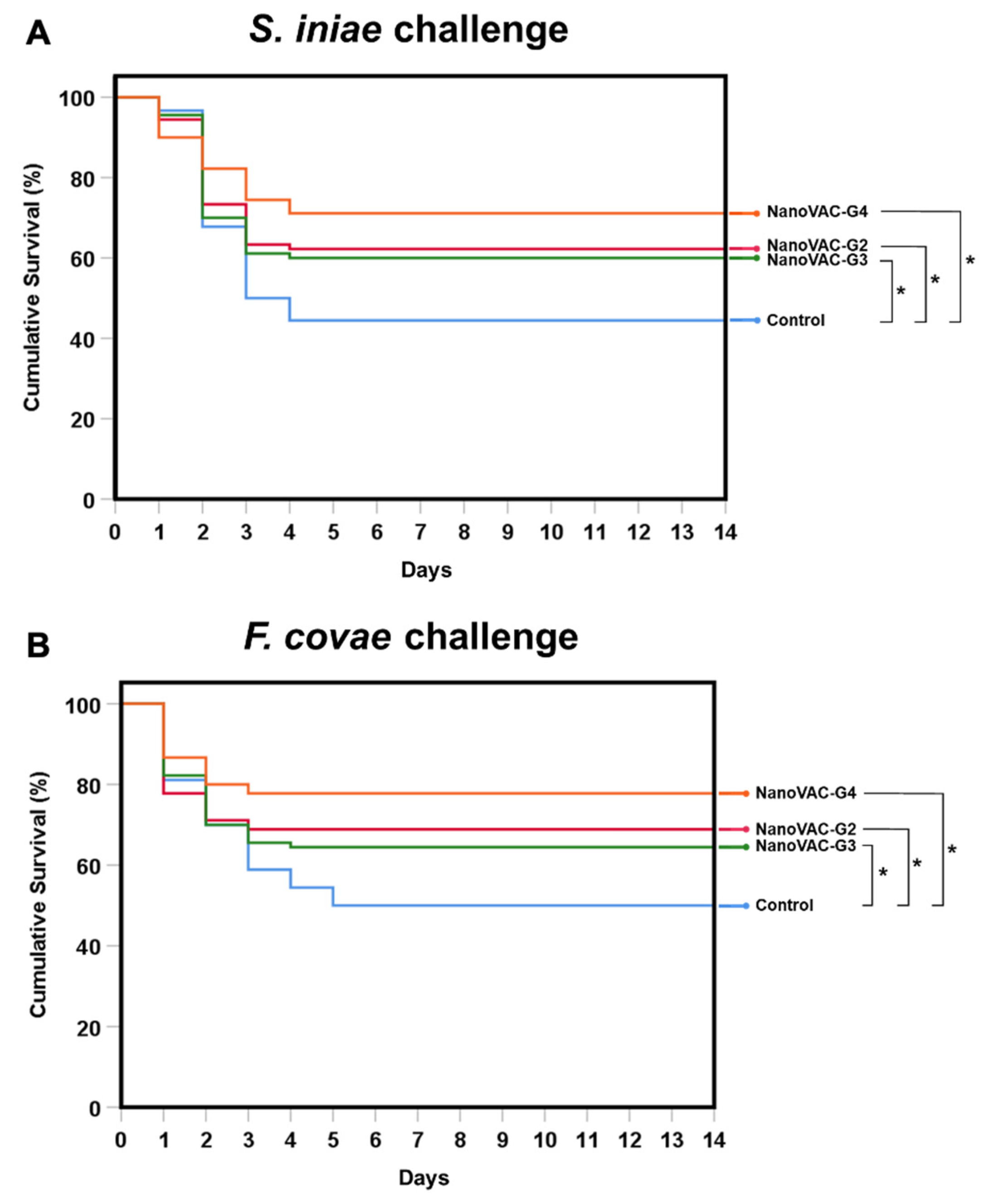
The high efficacy of chitosan-based nanovaccines indicated by the RPS or survival rate was found in various experiments. For example, chitosan-based monovalent nanovaccines against
Aeromonas veronii [
30],
Flavobacterium columnare [
13,
18],
Streptococcus agalactiae [
31,
32], and tilapia lake virus [
35] immunized with oral or immersion vaccinations showed high RPS or survival ranging from 52.2-100%. In our results, chitosan-based bivalent nanovaccines showed survival rates of 60.00-75.70% for
S. iniae challenge and 64.44-77.78% for
F. covae testing, similar to those of previous reports. This result suggested that the chitosan-based bivalent nanovaccines, which were immunized via the combination of immersion and oral administration in Asian seabass larvae to fingerlings, led to an additive action, dually protecting Asian seabass from columnaris and streptococcosis effectively.
Considerably, some immune parameters of the vaccinated groups 2-4 differed at some periods, which may be effectively due to different management methods of workers and should be optimized and considered when establishing a vaccination strategy for nursery farms.
Figure 1.
Total IgM (A) and IgM specific to S. iniae (B) and F. covae (C) in Asian seabass vaccinated with bivalent nanovaccines at 10, 20, and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05).
Figure 1.
Total IgM (A) and IgM specific to S. iniae (B) and F. covae (C) in Asian seabass vaccinated with bivalent nanovaccines at 10, 20, and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05).
Figure 2.
Lysozyme activity (A) and bactericidal activity (B) of Asian seabass vaccinated with bivalent nanovaccines at 10, 20, and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05).
Figure 2.
Lysozyme activity (A) and bactericidal activity (B) of Asian seabass vaccinated with bivalent nanovaccines at 10, 20, and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05).
Figure 3.
Relative gene expression levels of specific immune-related genes, including IgM (A), IgT (B), IgD (C), MHCIIα (D), TCRα (E), and CD4 (F), in the whole body of Asian seabass vaccinated with bivalent nanovaccines at 10, 20 and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05) or *** (p < 0.001).
Figure 3.
Relative gene expression levels of specific immune-related genes, including IgM (A), IgT (B), IgD (C), MHCIIα (D), TCRα (E), and CD4 (F), in the whole body of Asian seabass vaccinated with bivalent nanovaccines at 10, 20 and 30 DAV. All the values are presented as the means ± SDs (n = 8). Superscripted letters indicate differences among the treatment groups. The significant differences between the control and treatment groups are indicated by * (p < 0.05) or *** (p < 0.001).
Figure 4.
Histopathological changes in the skin of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Llp: lateral line tubules, Mu: muscle (H&E staining 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).
Figure 4.
Histopathological changes in the skin of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Llp: lateral line tubules, Mu: muscle (H&E staining 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).
Figure 5.
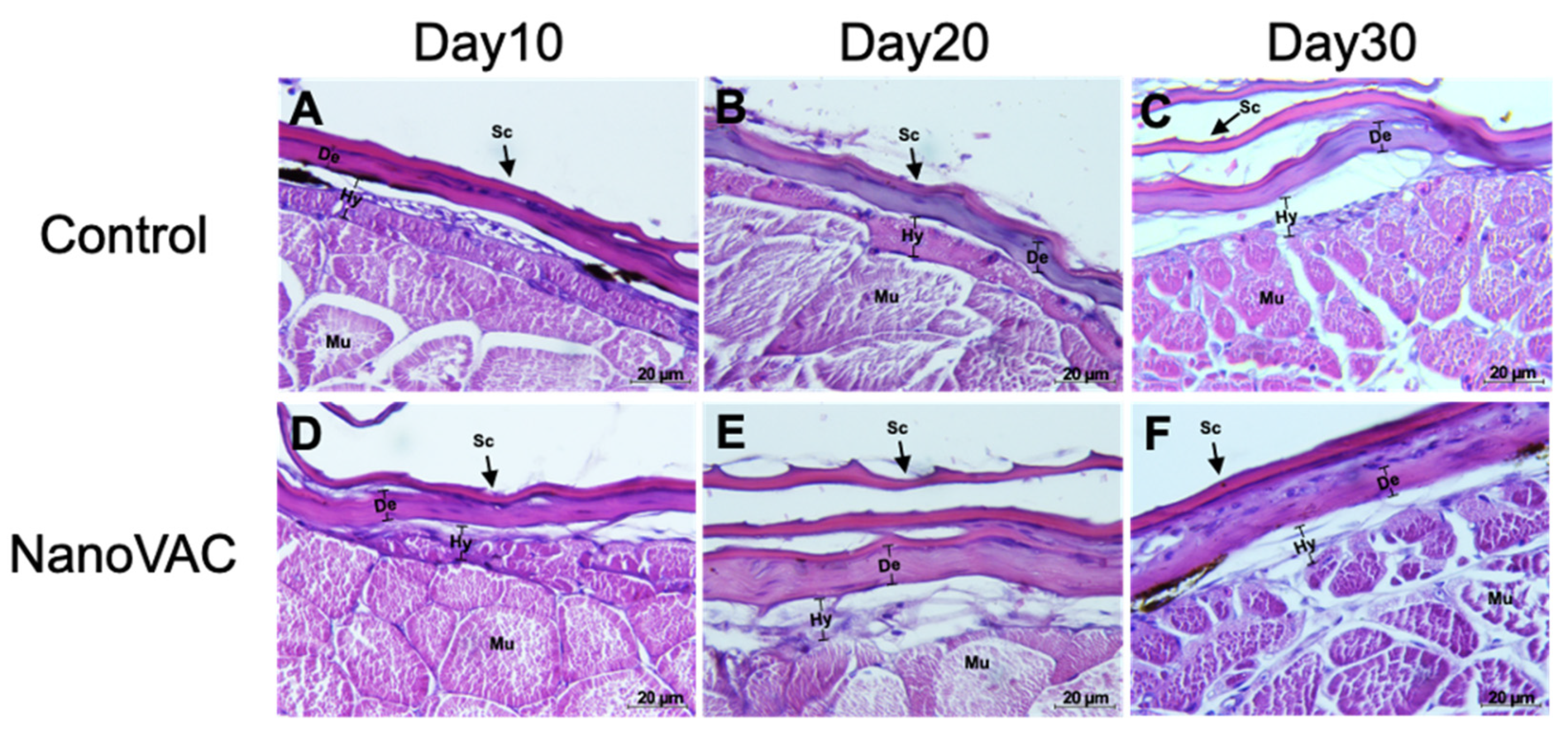
Histopathological changes in the skin of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Sc: scale, Hy: hypodermis layer, De: dermis, Mu: muscle (H&E staining, 100× magnification).
Figure 5.
Histopathological changes in the skin of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Sc: scale, Hy: hypodermis layer, De: dermis, Mu: muscle (H&E staining, 100× magnification).
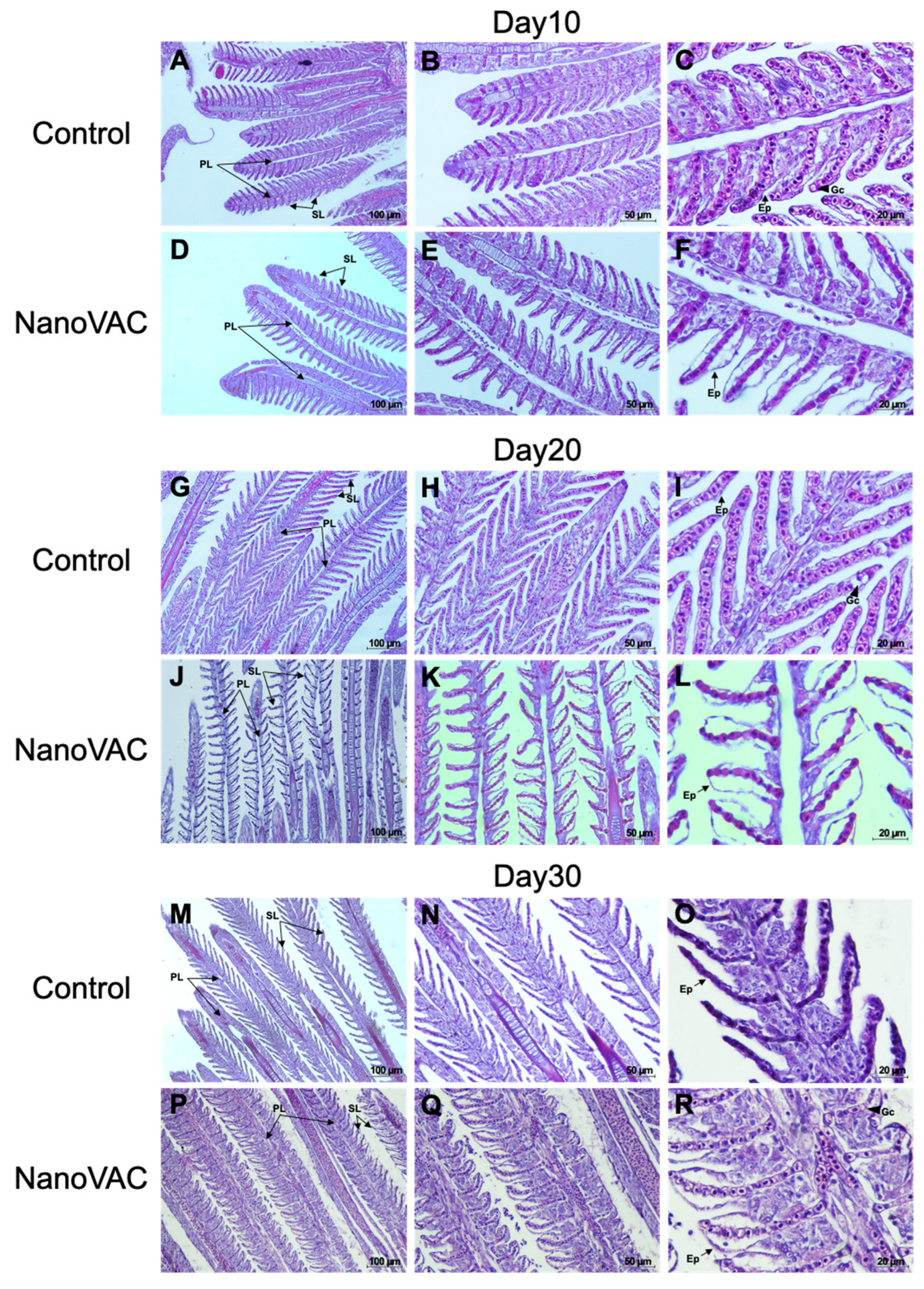
Figure 6.
Histopathological changes in the gills of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV; PL: Primary lamella, SL: Secondary lamella, Ep: Epithelium and Gc: Goblet cells (H&E staining, 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).
Figure 6.
Histopathological changes in the gills of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV; PL: Primary lamella, SL: Secondary lamella, Ep: Epithelium and Gc: Goblet cells (H&E staining, 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).
Figure 7.
Histopathological changes in the gills, focusing on interbranchial lymphoid tissues (ILTs), of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Black arrow: enlarged and cumulative immune-like cells of the ILTs (H&E staining, 100× magnification).
Figure 7.
Histopathological changes in the gills, focusing on interbranchial lymphoid tissues (ILTs), of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV. Black arrow: enlarged and cumulative immune-like cells of the ILTs (H&E staining, 100× magnification).
Figure 8.
Histopathological changes in the gut of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV; LU: Lumen, Ep: Epithelium, Lp: Laminar propria and Gc: Goblet cells (H&E staining, 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).
Figure 8.
Histopathological changes in the gut of Asian seabass after immunization with bivalent nanovaccines at 10, 20, and 30 DAV; LU: Lumen, Ep: Epithelium, Lp: Laminar propria and Gc: Goblet cells (H&E staining, 20× (A-P), 40× (B-Q), and 100× (C-R) magnification).
Figure 9.
Survival analysis of Asian seabass vaccinated with bivalent nanovaccines after challenge with the single pathogens S. iniae (A) and F. covae (B). Data and survival plots were generated using the Kaplan‒Meier method. The levels of statistical significance between the control and treatment groups are indicated by * (p < 0.05) (n = 30).
Figure 9.
Survival analysis of Asian seabass vaccinated with bivalent nanovaccines after challenge with the single pathogens S. iniae (A) and F. covae (B). Data and survival plots were generated using the Kaplan‒Meier method. The levels of statistical significance between the control and treatment groups are indicated by * (p < 0.05) (n = 30).
Table 1.
Primers used in this study for determining immune-related gene expression.
Table 1.
Primers used in this study for determining immune-related gene expression.
| Primer names |
Genes |
Nucleotide sequences (5’→3′) |
Annealing temperature (oC) |
Product size (bp) |
References |
| Lc_β-actin |
β-actin |
F-5′-TACCCCATTGAGCACGGTATTG-3′
R-5′-TCTGGGTCATCTTCTCCCTGTT-3′ |
60 |
160 |
XM_018667666.1 |
| Lc_IgM |
Immunoglobulin M (IgM)
(secreted form) |
F-5′-TGTCAAGGTAAACGAGGGAGC-3′
R-5′-TCCCCTGGATCCATTCGTCA-3′ |
60 |
152 |
ASM164080v1 |
| Lc_IgT |
Immunoglobulin T (IgT)
(secreted form) |
F-5′-GAGGCAACTTACAGAGGAACCATA-3′
R-5′-CTGGTCACTTCTCCATCAATTTCC-3′ |
60 |
194 |
ASM164080v1 |
| Lc_IgD |
Immunoglobulin D (IgD)
(membrane-bound form) |
F-5′-GAGTGTGAATGTTGCTGGGC -3′
R-5′-TTGGCCTGAAAGGTGACGTA -3′ |
60 |
150 |
ASM164080v1 |
| Lc_CD4 |
CD4 receptor (CD4) |
F-5′-AGTGCAATGGATTGGGGTAGATAA-3′
R-5′-GTTGCAGGCTCTGTAACTTTGATT-3′ |
60 |
156 |
XM_018672258 |
|
Lc_TCRα
|
T-cell receptor alpha (TCRα) |
F-5′-GGCCGTTCGGATAGAAGGAG-3′
R-5′-AGAGCCATTGTGTTCACCGT-3′ |
60 |
153 |
ASM164080v1 |
|
Lc_MHCIIα
|
Major histocompatibility complex class IIα (MHCIIα) |
F-5′-TTCCTACCTCCCTGATCTACCC-3′
R-5′-CTGAAGTCGCTGTTGGAGTAGT-3′ |
60 |
178 |
ASM164080v1 |
Table 2.
Characterization of S. iniae and F. covae nanovaccines.
Table 2.
Characterization of S. iniae and F. covae nanovaccines.
| Bacterial culture |
Formulation |
Average diameter (nm) |
Zeta potential (mV) |
| Streptococcus iniae |
Sonicated antigen (bacterial cells) |
203 ± 10 |
-36.87 ± 0.93 |
| |
Polymeric nanovaccine (solution form) |
246 ± 16 |
45.39 ± 1.31 |
| |
Polymeric nanovaccine (dry form) |
304 ± 25 |
47.60 ± 0.96 |
| Flavobacterium covae |
Sonicated antigen (bacterial cells) |
324 ± 6 |
-21.86 ± 0.89 |
| |
Polymeric nanovaccine (solution form) |
394 ± 14 |
38.25 ± 1.06 |
| |
Polymeric nanovaccine (dry form) |
426 ± 18 |
36.48 ± 1.02 |
Table 3.
Growth performance of Asian seabass 30 days after vaccination with bivalent nanovaccines (DAV).
Table 3.
Growth performance of Asian seabass 30 days after vaccination with bivalent nanovaccines (DAV).
| Growth parameters |
Treatments |
| Control |
NanoVAC-G2 |
NanoVAC-G3 |
NanoVAC-G4 |
| Weight gain (WG, g) |
0.542 ± 0.16 |
0.545 ± 0.17 |
0.552 ± 0.12 |
0.544 ± 0.15 |
| Specific growth rate (SGR, %/day) |
7.18 ± 0.75 |
7.18 ± 0.72 |
7.24 ± 0.79 |
7.19 ± 0.74 |
| Average daily gain (ADG, g/fish/day) |
0.0181 ± 0.0016 |
0.0182 ± 0.0018 |
0.0184 ± 0.0014 |
0.0181 ± 0.0011 |
| Feed conversion ratio (FCR) |
1.85 ± 0.44 |
1.83 ± 0.37 |
1.81 ± 0.39 |
1.84 ± 0.43 |