3.1. FIB-SEM Microscopy Study
The
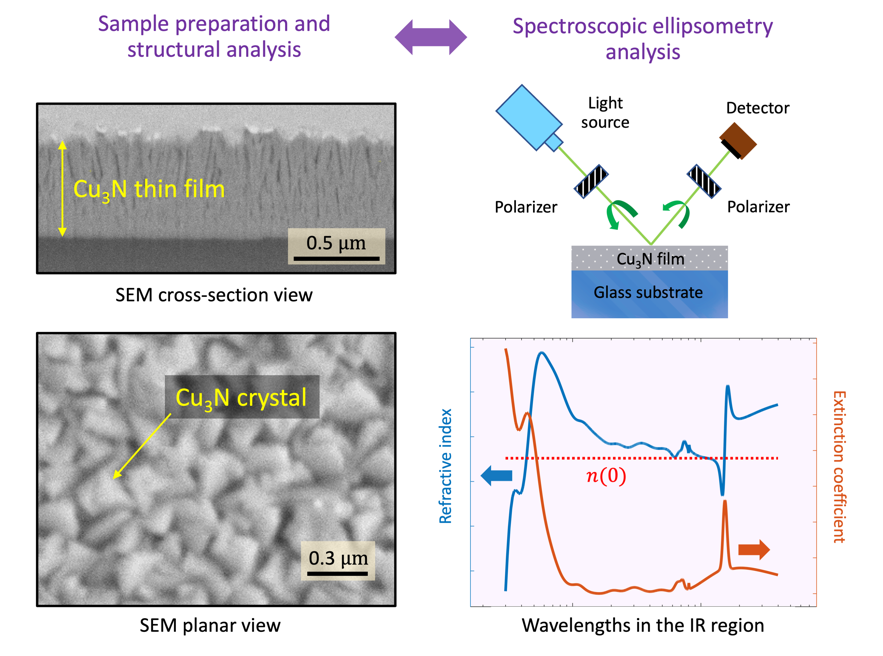
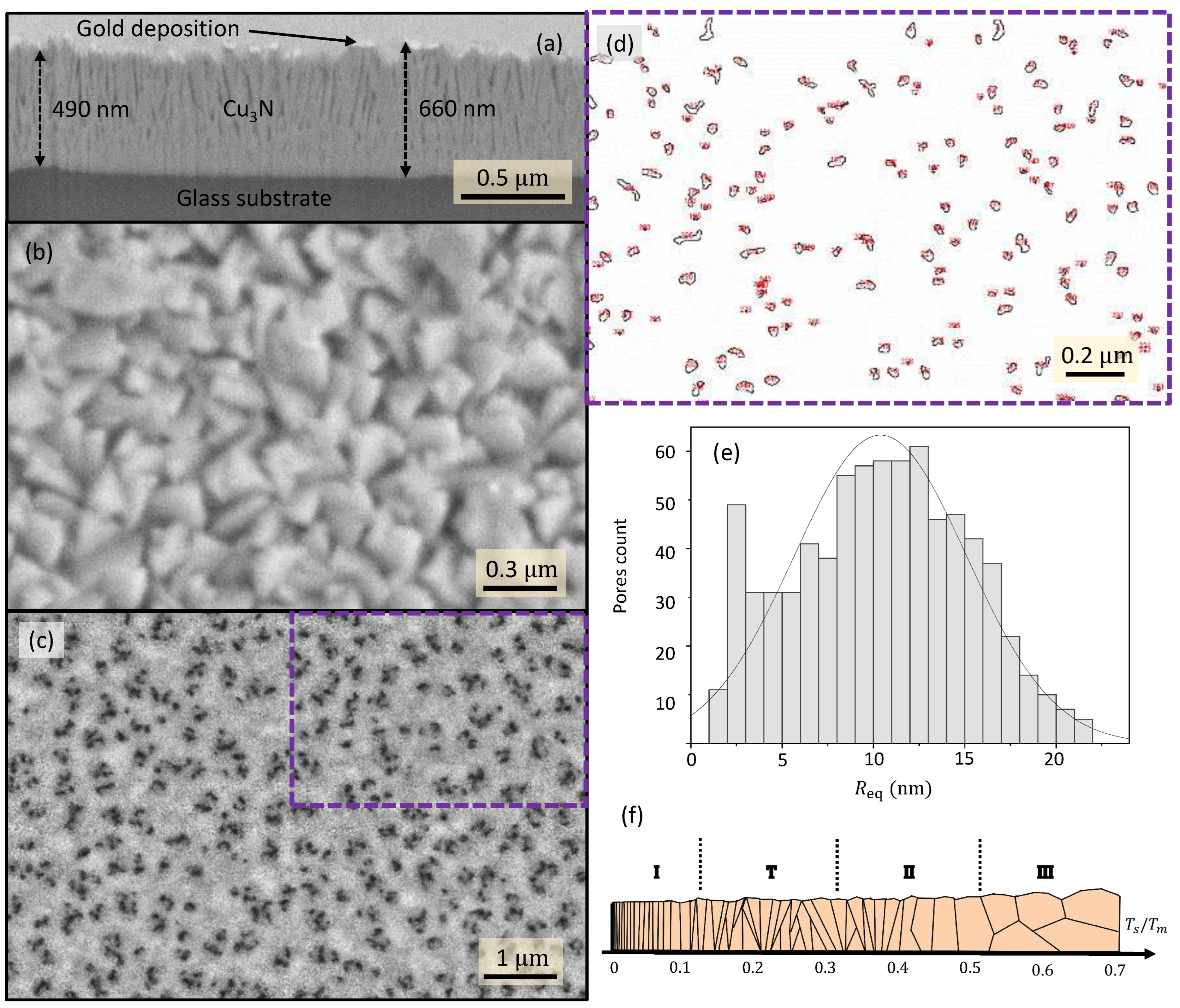
N thin films exhibited a columnar formation, as shown in
Figure 1a (the maximum and the minimum values of the film thickness are indicated in the micrograph). It is observed that the copper-nitride microstructure through about the first 100 nm (samples #1460 and #1490), or around the first 200 nm (sample #1360), from the glass surface, is undoubtedly compacted, while voided spaces between the
N-layer columns are clear in the rest of the layer thickness. This is the columnar-structure ‘zone’ 2 of the Thornton structural zone model [
13]; it consists of columnar and compact grains, with high density and smooth surfaces.
It is verified that the
ratio must obey the condition,
, in this particular zone 2, where
is the substrate temperature during deposition, and
is the coating-material melting point, which is well-known to be above 740 K, the decomposition temperature of
N. Hence, it is clear that the columnar structure of
N results from being grown at room temperature and low working-gas pressure. The value of the
ratio in our RF-sputtering depositions is found to be 0.41, certainly a value corresponding to the zone-2 category.
Figure 1b displays an SEM micrograph of the surface of sample #1460.
N pillars met at its surface, giving place to a conglomerated structure with many sealed ‘closed’ pores.
The FIB-SEM porosity of sample #1360 (see
Figure 2a) was quantified using images such as the one in
Figure 1c. The pore map in
Figure 1d, on the other hand, was obtained using the software
ImageJ from the region indicated by a dashed frame in
Figure 1c. This software is commonly utilized to measure particle sizes from images. It has similarly and successfully been used in this work to obtain the
equivalent pore radii.
Figure 1d shows the pore perimeter (black lines), and the numbering (in red) that
ImageJ uses to identify each particular pore. Pore areas,
’s, were automatically measured from the map, and the corresponding values of the equivalent pore radius,
, were obtained using the expression:
.
Figure 1d displays the corresponding histogram for the values calculated from the SEM image in
Figure 1c; the mean equivalent radius in this particular sample was found to be 10.4 ± 4.7 nm.
3.2. UV-MIR Ellipsometric Analysis
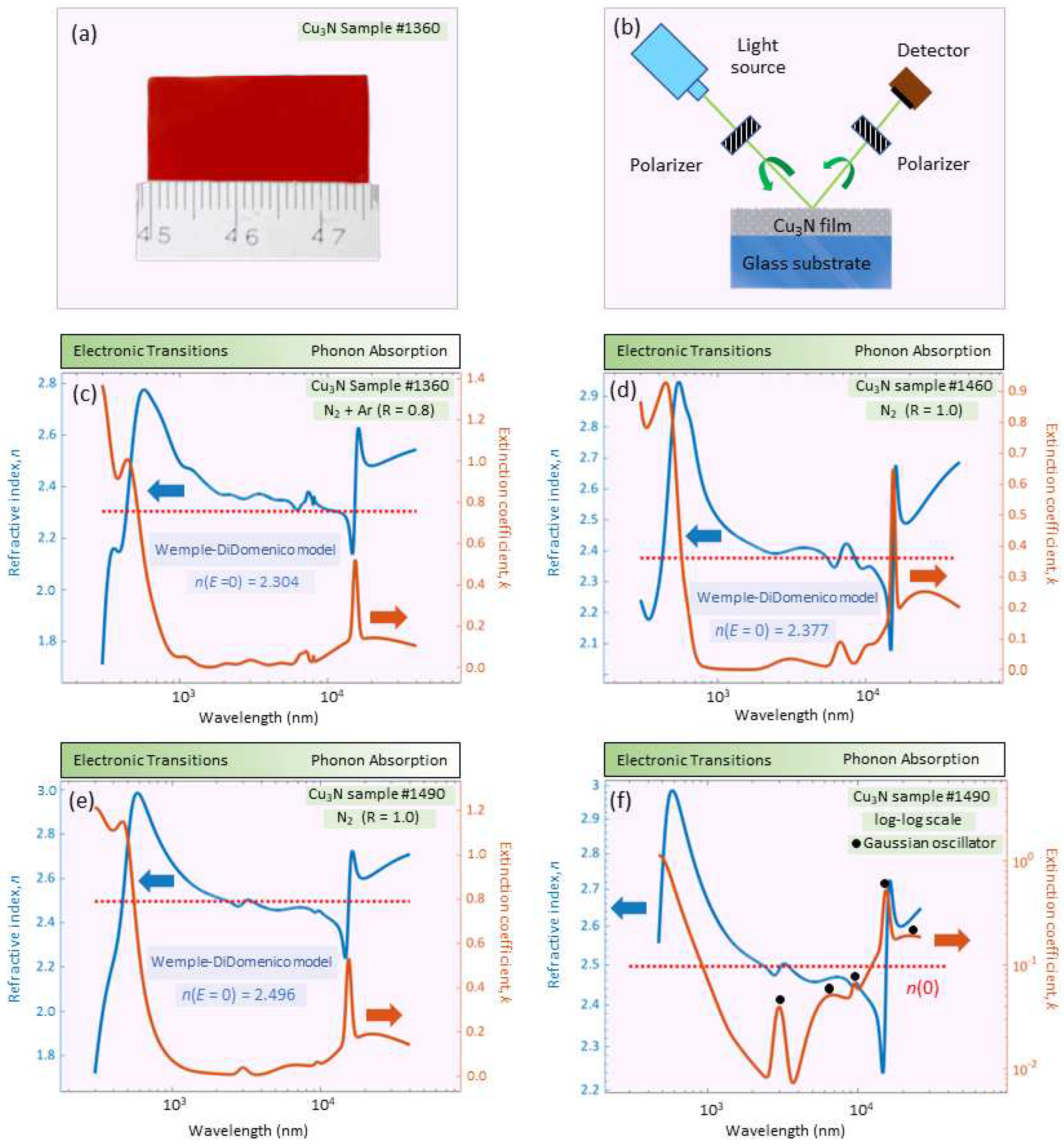
Optical and infrared ellipsometric data (see
Figure 2b) were fit over the ample range of
nm (
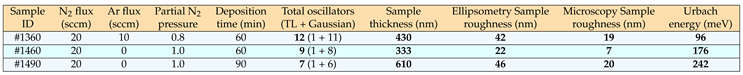
eV), simultaneously with normal-incidence optical transmission data (200-2500 nm, or 0.5-6.2 eV). The best-fit ellipsometric model required, in the case of sample #1490, the introduction of a 46-nm-thick surface-roughness layer (
Table 1).
Figure 2 shows the best-fit very-broadband optical constants,
n and
k, for the
N samples.
Table 1 gives information about the necessary number of oscillators in order to predict the dielectric functions contained in the so-called
GenOsc layer, for all
N samples. The excellent comparison between the surface roughness determined by both spectro-ellipsometry and atomic-force-microscopy is also shown in
Table 1. In addition, two major spectral features are seen in the optical constants of
N: A UV-Visible absorption edge with a peak at approximately 2.47 eV, and a second sharp resonant absorption in the infrared near a wavelength of 15,480 nm, both peaks for the particular case of #1490 layer. The specific UV-Visible absorption edge, with a clear peak, was modeled by combining a Gaussian and a Tauc-Lorentz oscillator [
14,
15,
16]. Gaussian oscillators were also added to fit the two ellipsometric angles,
and
, in the rest of the spectral range under study, for the #1490 sample. The extremely-sharp resonant absorption peak at 646
(
Figure 3a), on the other hand, determined by ellipsometry, and reported for the first time, suggests that this sample is of polycrystalline nature. This peak was modeled using a Gaussian oscillator, though it could also have been used instead a Lorentz oscillator.
Dielectrics and semiconductors are generally transparent at near-IR (NIR) wavelengths. These materials absorb light in the UV and visible ranges due to valence-electron transitions. Above the bandgap, we find interband transitions from the valence to the condition bands, with the corresponding absorption of photons, at energies higher than that of such a bandgap. Many will also show the IR absorption due to the presence of intraband transitions within the valence band, molecular vibrations, phonons, or free charge carriers. In our
N samples, the free-carrier Drude model clearly failed to accurately predict the IR response. Therefore, the associated free-charge-carrier density must be necessarily smaller than approximately
cm
−3, the corresponding detection limit for the IR-VASE ellipsometric technique. Lastly,
Figure 2c-f shows the very-broadband complex refractive index,
, for
N from the UV to the MIR, reported for the first time, and dearly illustrates the aforementioned UV-Visible absorption due to the valence-electron transitions; moreover, copper-nitride is quasi-transparent across the remaining visible and NIR regions, until the presence of phonon
does occur in the middle-infrared range of the spectra of the extinction coefficient,
k, displayed in
Figure 2c-f.
The crystal structure of Cu
3N belongs to the space group, Pm
m, where in the unit cell contains one formula unit. According to the space group theory there are 12 phonon modes (lattice vibrations) at the center of the first Brillouin zone (
critical point), among which nine are optic modes with irreducible representation:
. The
are infrared active, and the
modes are optically inactive (silent modes). Yu
et al. [
17] calculated the frequencies of
N.
-point optical modes, and found that a peak at around 651
corresponds to the Cu-N high-frequency stretching mode,
, while another band at about 154
corresponds to the Cu-N-Cu bending mode,
.
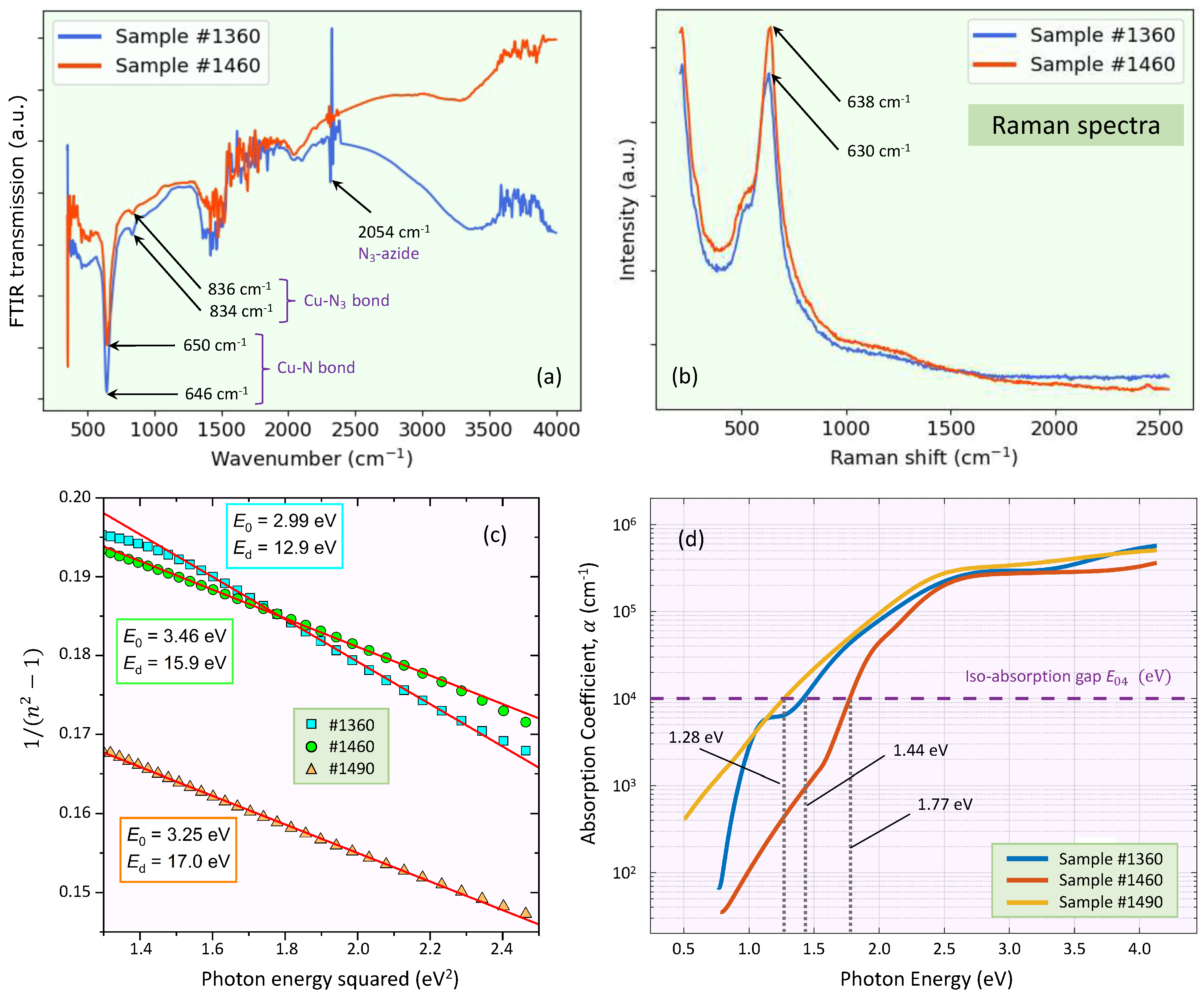
Concerning the previously mentioned UV-Visible absorption edge,
Figure 3d displays the graph of the absorption coefficient spectrum,
,
versus photon energy, for the three
N samples, calculated from both ellipsometric and intensity transmission measurements. These plots allow us to determine the
iso-absorption gap, the energy value at which
. The obtained values of structural-defect-related Urbach energy parameter
[
18] are also listed in
Table 1. According to the literature,
N shows
values ranging from 105 to 238 meV [
6], therefore, our obtained values of
are closely within this reported range. The iso-absorption gap,
, for being empirical, is less sensitive to interpretational difficulties associated to the optical bandgap, and, therefore, is in use as a common alternative practical definition of the optical bandgap in poly-crystalline and non-crystalline semiconductors.
It should be emphasized that the values of the index of refraction reported in our present ellipsometric study are clearly much higher than those measured for
N with the prism coupling technique [
19]. We considered that the values of the refractive index found with the latter technique, surprisingly around 1.5 at four wavelengths in the NIR region, are notably underestimated, taking into account that the values determined in our study are very consistent with those previously reported in the literature [
20], calculated making use of the popular Swanepoel transmission-envelope-method.
3.3. FTIR and Raman analysis
For an illustrative comparison, the
N thin-film samples were also analyzed by FTIR transmission spectroscopy. The representative FTIR transmission spectra are shown in
Figure 3a. The positions of the corresponding
N-phonon mode are all of them at around 645
, in excellent agreement indeed with those independently calculated by infrared ellipsometry (
i.e., this single band confirmed the creation of the Cu-N chemical bond). This would indicate that the amount of nitrogen contained within the sputtering gas atmosphere was adequate to form the
N phase. A weak peak around 835
, assigned to the Cu-
chemical bond, was also observed in the two cases. In addition, a peak at 2049
also appeared in the FTIR transmission spectra, corresponding to the stretching vibration of the
-azide.
Figure 3b displays the representative Raman spectra of two of the
N thin layers. Notably, we have observed Raman shifts at 638
and 630
, respectively, which are characteristic values of the Raman shift associated with
N. It has to be pointed out that, although the first-order Raman signal for a perfect crystalline
N is not allowed, Raman modes can be activated in the presence of structural disorder (
e.g., a small crystalline size and/or the presence of structural defects). The increase in Urbach energy,
, is primarily related to the increase of those structural defects (see
Table 1).
3.4. Using the sub-gap Wemple-didomenico single-oscillator dispersion model
We focus next on fitting the obtained
N refractive-index dispersion below the bandgap to the Wemple-DiDomenico single-effective-oscillator expression [
21]:
where
is the energy of the
effective dispersion oscillator, and
is the dispersion energy or oscillator strength. By plotting
versus
(
Figure 3c), the two parameters
and
were determined. The obtained values of these Wemple-DiDomenico dispersion parameters,
and
, are all indicated in
Figure 3c. The oscillator energy
is considered an ‘average’ energy gap. For the dispersion energy,
, on the other hand, a relationship was proposed [
21]:
where
is a two-valued constant, 0.37 ± 0.04 eV for covalent materials, and 0.26 ± 0.03 eV for more ionic materials.
is the coordination number of the cation nearest neighbor to the anion (copper in our case, with
),
is the formal valency of the anion (nitrogen in our binary compound, with
), and
is the
effective number of valence electrons per nitrogen anion. In the
N binary compound,
We are not including the Cu
d-electrons in our ‘electron count’ [
21]. For this
N material, we do
not expect the
-core electron contribution, as observed in Cu halides: It would imply that we have to necessarily add 10 more electrons to the present ‘electron count’,
. This would give rise to a clear disagreement between the experimental and calculated values of the dispersion-energy parameter,
, as will be shown next.
The particular value of
in copper nitride calculated by the use of Eq.
3, is found to be 17.8 eV. The small differences with the experimental values of
presented in
Figure 3c, and especially in the most-discrepant case of sample #1360, can reasonably be explained by the reported lack of stoichiometry of the studied sputtered
N films (the Cu/N-ratio was found to be smaller than the expected ratio of 3) [
4]. Moreover, the long-wavelength value of the refractive index,
, displayed in
Figure 2c-f, is given by the following expression:
Significantly, the values of these
static refractive indices are consistent with the data independently obtained by the IR-VASE ellipsometry (see the values of
in
Figure 2c-f). In addition, it also seems reasonable to propose that the determined values of
increase with the mass density of the
N samples. The less dense
N film is the specimen #1360, according to its value of
, 2.304, and the denser layer is the sample #1490, whose value of the static refractive index has been found to be 2.496. The ‘in-between’ case is the one of the film #1460, with
.
Furthermore, the existing correspondence between the Wemple-DiDomenico oscillator-energy parameter,
, and the so-called ‘Wemple-DiDomenico gap’,
, is generally expressed as
[
22]. For the
N compound, the value of
obtained from the dispersion parameter
goes from 1.50 eV, for sample #1360, to 1.73 eV for sample #1460, very close to the indicated values of the iso-absorption gap,
.