1. Introduction
Plants in natural habitats are surrounded by hostile environmental conditions, such as pathogen attacks, insect or animal predation, and excess light. They require secondary metabolites to protect against enemies and abiotic stresses [
1]. These metabolites can be found throughout the plant kingdom and are valuable for taxonomic information [
2,
3]. For example, secondary metabolites of two genera and nine species of Gentianaceae were employed for separations of both genus and species levels using chemometric analysis [
4]. In another case with
Paeonia, a genus of medicinal plants, root chromatographic profiles were analyzed using chemometrics to classify all species and three varieties of
Paeonia delavayi [
5]. Likewise, bryophytes contain many secondary metabolites. They are found on various kinds of substrates [
6]. Despite their small size, they are frequently faced with unsuitable environmental conditions. After all, they do not have rigid structures to protect themselves. The biochemicals are developed as a mechanism for survival [
7]. However, studies on bryophyte chemical composition are still limited, especially in mosses [
8].
The moss genus
Leucobryum Hampe is placed in the family Leucobryaceae [
9,
10]. About 80–100 species worldwide have predominantly Temperate and Tropical distribution [
11,
12,
13,
14]. In Thailand, eight species and two varieties were reported [
15]. It can be found on various substrates and throughout the country [
6,
15,
16]. However, species identification remains unclear due to morphological variation and taxonomic confusion of
Leucobryum in Thailand. Identifying small plants based on morphology and anatomy can be difficult, especially for bryophytes. There are many misidentifications in bryophytes because the morphological characters are often the result of parallelisms, reductions, and convergences [
17,
18]. In modern systematics, classification utilizes various types of comparative data, such as anatomy and physiology, chemistry, embryology, palynology, reproductive biology, and molecular genetics, for species separation and analysis [
19].
Plant chemotaxonomy is an approach that uses chemical constituents to improve the classification of plants. Over the years, many techniques have been developed for plant chemotaxonomy [
2,
3]. Chemometrics is one technique that analyzes multivariate datasets of chemical profiles to support taxonomic classification [
20]. This technique uses chemical profiles from thin-layer chromatography (TLC), high-performance thin-layer chromatography (HPTLC), or high-performance liquid chromatography (HPLC) for evaluating the similarity or difference between various plant species, including bryophytes [
21,
22]. The chemistry of
Leucobryum was first analyzed using thin-layer chromatography to detect flavonoids, but could not detect any significant amount of flavonoids [
23]. However, subsequent studies identified several chemicals from
Leucobryum. For example, Fatty acids, Sterols, Hydrocarbons, Phenolics, and Alkaloids [
24,
25,
26,
27]. Nevertheless, no detailed chemical profiles and chemometric reports have been conducted on
Leucobryum. Thus, the profile of compounds could provide an additional tool for species separation and classification of
Leucobryum species.
Therefore, the objective of this study was to use the chemical profiles and chemometrics with chromatographic data (TLC and HPLC) to support classification within the genus of Leucobryum found in Thailand. Two approaches, principal component analysis (PCA) and hierarchical clustering analysis (HCA), were used to determine differences in the chemical profiles among the studied Leucobryum species. The results showed the potential of a chemotaxonomic approach in bryophyte classification and future prospecting of bioactive compounds in bryophytes.
2. Materials and Methods
2.1. Plant Material and Extract Preparation
Plants of the genus
Leucobryum were obtained from a local plant market in Bangkok, Thailand with the origins from the north and northeastern part of Thailand. From these materials, eighteen samples from three
Leucobryum taxa and two outgroup taxa (
Table 1) were identified to the species level using available taxonomic keys and other related taxonomic literature [
12,
16,
28,
29]. These taxa were verified to ensure that they were previously reported from Thailand. Voucher specimens were kept in the Department of Botany, Faculty of Science, Kasetsart University.
The plant materials were dried under shade and extracted with methanol (MeOH) for seven days at room temperature in the dark. Subsequently, the solution was evaporated, and solid crude extracts were separated in purified water and chloroform (CHCl
3). The lipophilic extract was concentrated until dried using a rotary evaporator, weighed, and kept in the freezer at -34 °C for further analyses (
Table 1).
2.2. TLC Development
The lipophilic extract of
Leucobryum and outgroup taxa were analyzed using Thin-Layer Chromatography (TLC) on silica gel 60 F254 (0.25 mm thickness Merck) coated on the mirror plate at 25–30 °C. The concentration was adjusted to 10 mg/mL of the lipophilic extract. The solvent system was developed for the phytochemical screening using chloroform and hexane in the 7:3 (v/v) ratio. Each developed plate was sprayed with anisaldehyde-sulfuric acid and Dragendroff’s reagents to detect terpenoids and alkaloids in the plant extracts [
30].
2.3. HPLC Analysis
High-Performance Liquid Chromatography (HPLC) was used to analyze the lipophilic extract of studied plant samples. The concentration was adjusted to 10 mg/mL, similar to the TLC analysis. The analysis was conducted using an Agilent, 1100 series with UV photodiode array detector, eluted with MeOH in aq. Buffer (15 mM ortho-H3PO4 and 1.5 mM Bu4NOH, pH3) and used reversed-phase BDS hypersil C18 column (250 × 4.6 mm part number 28105–254630) with the run time of 30 minutes. The flow rate and injection volumes were 1.0 ml/min and 20 µl, respectively. The HPLC analysis was performed at the Scientific Equipment Center, Faculty of Science, Kasetsart University, Bangkok, Thailand.
2.4. Chemometric Analysis
The HPLC chromatograms from 230 nm wavelength at the retention time between 5–20 minutes were selected to represent the chemical profiles of the
Leucobryum taxa and the outgroups. The areas under the peaks with more than one percent of the total area from the selected part of the chromatogram were used as multivariate chemical profiles to compare among the studied samples. Statistical analyses, including principal component analysis (PCA) and hierarchical clustering analysis (HCA), were performed using the R program v.3.6.1 [
31]. Before PCA and HCA, the pairwise Bray-Curtis distance was calculated to emphasize the shared presence of detected chemicals. The PCA and HCA were performed on the distance matrix using the functions “prcomp” and “hclust” in the package “stats.” PERMANOVA was performed to determine if the chemical profiles are significantly different among taxa, using the function “adonis” in the package “vegan” [
32,
33].
3. Results and Discussion
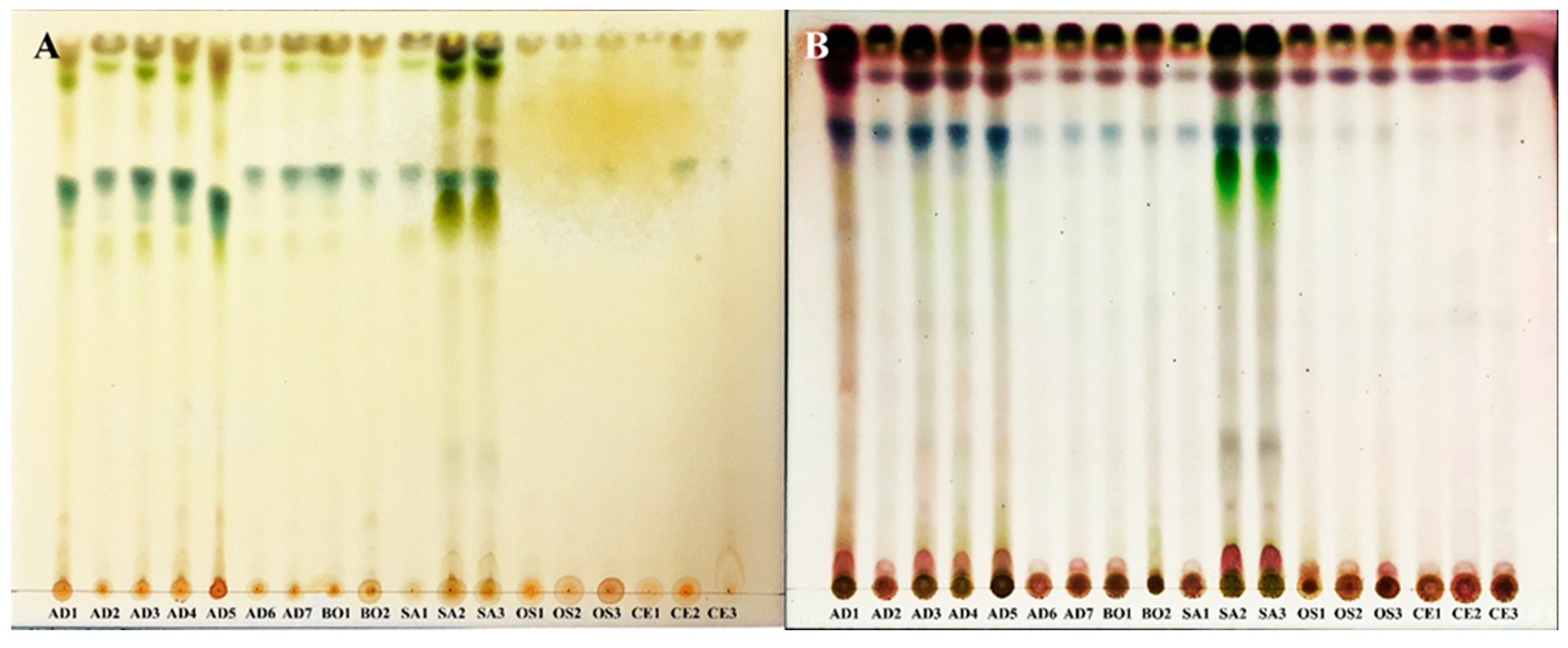
3.1. TLC Profiles
The lipophilic extracts were obtained from the 18 samples of three taxa of
Leucobryum and two outgroup taxa from Thailand (
Table 1). The extracts were analyzed using the TLC. Developed plates were sprayed using the anisaldehyde-sulfuric acid and Dragendroff’s reagents to detect potential terpenes and alkaloids, respectively (
Figure 1). Based on the band patterns, no differences within
Leucobryum taxa were detected with the two reagents. The TLC results showed the separation of the genus
Leucobryum from
Campylopus and
Ochrobryum with the anisaldehyde-sulfuric acid reagent. The results showed the difference in the chemical composition of
Leucobryum and the other genera, suggesting the potential use of TLC profiles for identifying
Leucobryum from the closely related genera. Several genera, such as
Leucophanes,
Ochrobryum,
Octoblepharum, share the “Leucobryoid” morphology of the short tufted whitish leaves. These genera are easily confused in the field and required detailed examinations of the leaf anatomy for identification. Having a TLC profile may help separate
Leucobryum from similar looking genera.
The inability to separate species within the genus
Leucobryum may result from an unsuitable solvent system. The TLC can separate compounds with varied polarity depending on the solvent system’s capabilities to generate chromatographic bands. The thick band at the end of each plate (
Figure 1B) showed that the current solvent system could not separate many compounds with low polarity in this study. Highly polar compounds such as alkaloids were not well separated either, as shown in the thick color bands in
Figure 1A. Therefore, we will have to develop additional solvent system for TLC if we need to discriminate among the species of
Leucobryum found in Thailand.
The result from TLC plates spaying with Dragendroff’s reagents showed that alkaloid compounds were found in all
Leucobryum extraction samples. It turned orange at the starting point (
Figure 1A). The result of this assay is consistent with the previous study that
Leucobryum javense contains at least four alkaloid compounds, including 2,4-dimethylbenzo[h]quinoline, N-hydroxy-N’-[2 (trifluoromethyl)phenyl]pyridine-3-carboximidamide, 2 Pyridinecarbohydrazonamide, N’-[(2,4-dimethoxyphenyl)methylidene]-, and Lochneridine [
27]. The anisaldehyde-sulfuric acid reagent was used to detect phenolic, terpene, sugar, and steroid compounds from the blue, red, grey, and green colors on the developed TLC plates, respectively. The result showed a high content of phenolic and terpene compounds in all
Leucobryum samples at RF values = 0.79 and 0.92, respectively. Some samples of
L. aduncum var.
aduncum and
L. sanctum showed steroids at RF values = 0.72 and 0.73, respectively (
Figure 1B). These groups of compounds were previously reported from the species of
Leucobryum. For example, in
L. javense, Azar, Rosleine, and Faizal [
27] reported chemicals in this group, including Butylated Hydroxytoluene (phenolics), cis-Pinane (terpenes), and 3-Deoxyestradiol (steroids). Additionally, 24-methyl-5,7,22-cholestatrienol (steroids) was reported in
L. glaucum [
25,
34]. Unfortunately, there has been no report of the secondary metabolites from the species in the current study. Our TLC data do not have enough resolution to identify the exact compounds in the moss extracts. The exact identity of these compounds will need to be verified in further chromatographic studies with reference compounds.
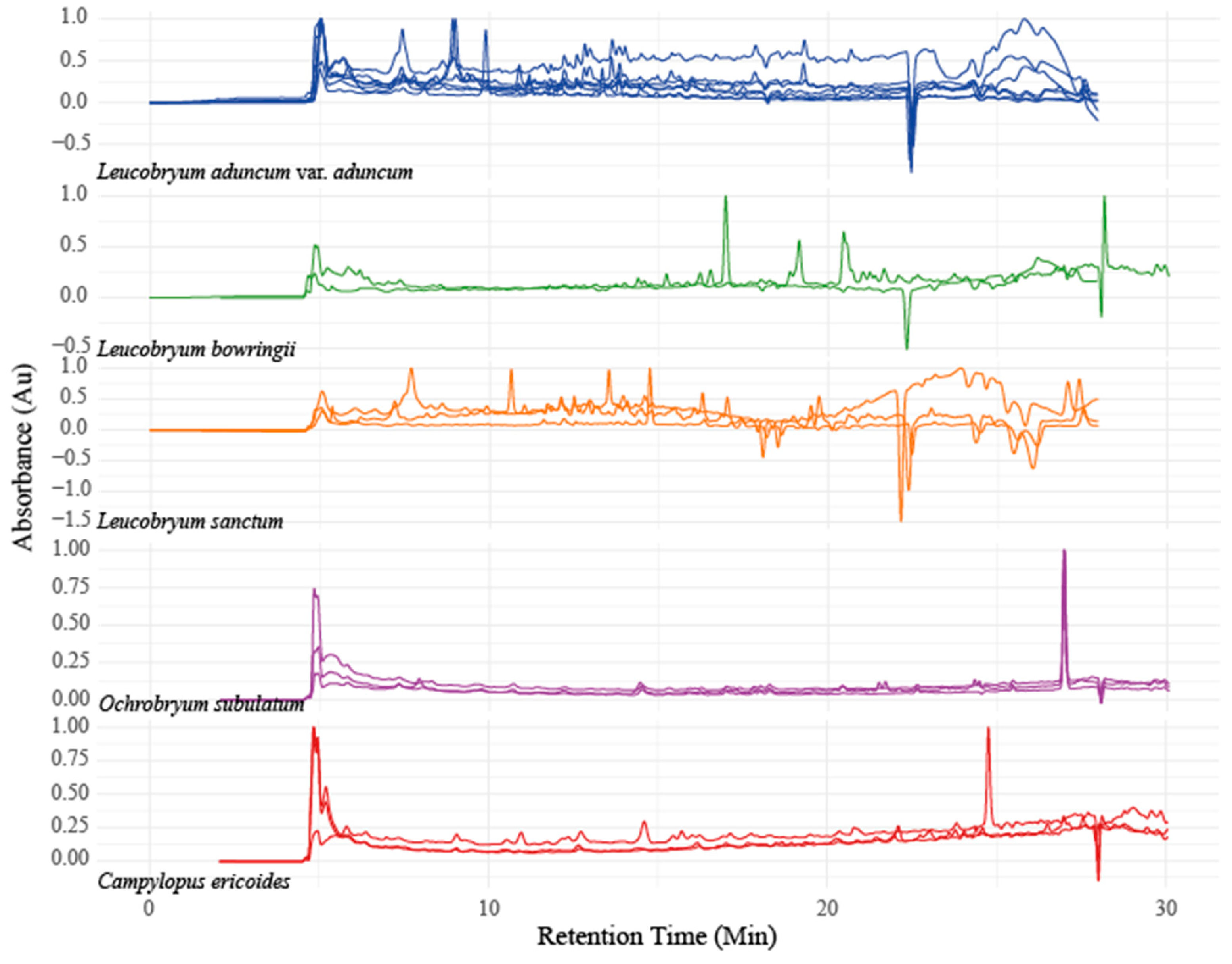
3.2. HPLC Profiles and Chemometrics Analysis
The same set of lipophilic extracts from TLC were also analyzed using HPLC. While TLC profiles showed no differences among
Leucobryum taxa, the HPLC profiles showed clear differences among the studied taxa. The chemical profiles individuals of the same taxa were similar to each other (
Figure 2). Both TLC and HPLC are chromatographic methods for separating the chemical constituents. However, their separation quality differs because HPLC uses simultaneous detection, making it more sensitive than the visual observation in TLC.
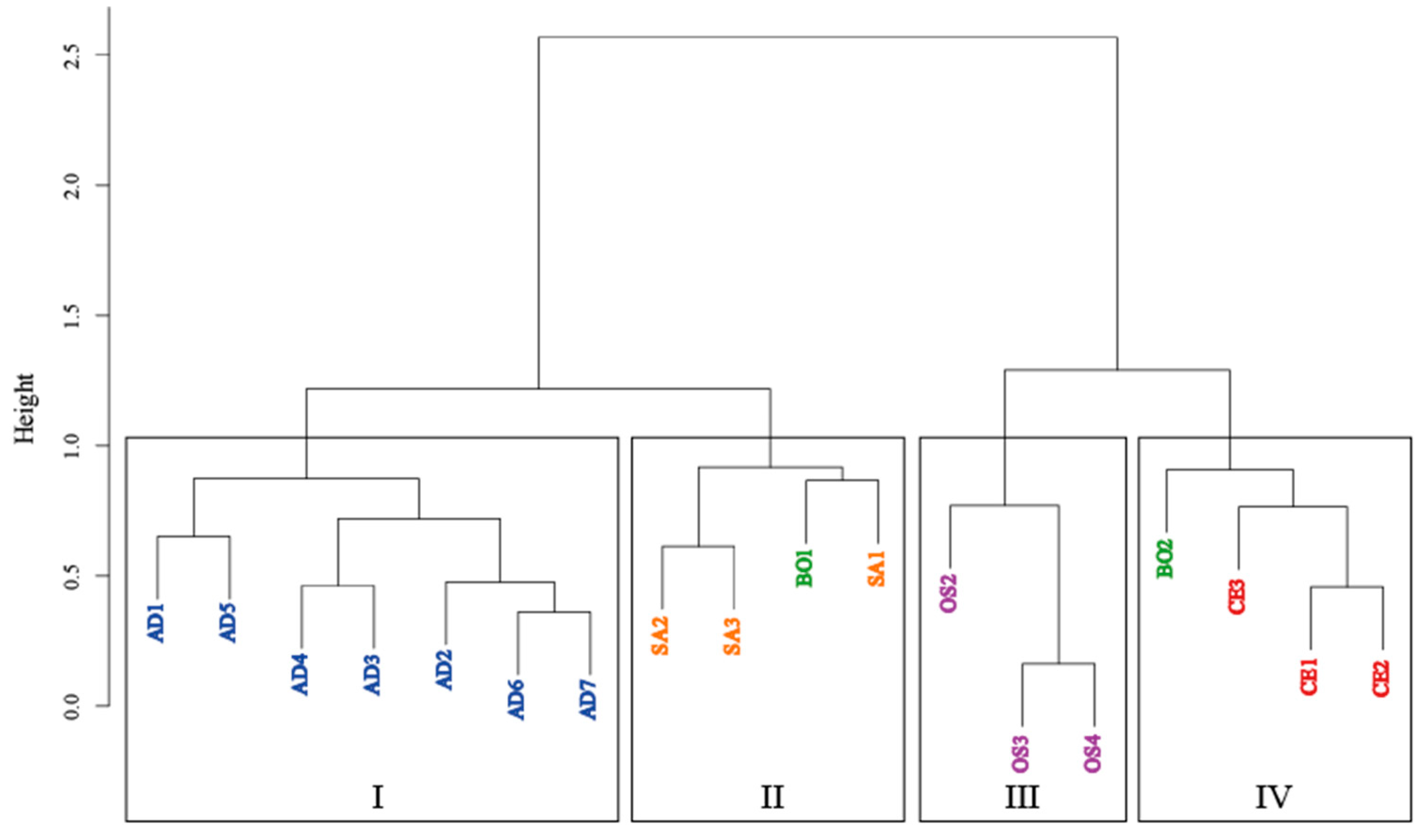
To confirm if the chemical compositions differed among the studied taxa, we analyzed the obtained HPLC profiles using HCA and PCA. The results from HCA showed that the majority of
Leucobryum samples were clustered together, separated from the outgroup (
Figure 3). According to the cluster dendrogram, 18 samples were divided into four clusters. All samples of
L. aduncum var.
aduncum were placed in cluster I. Cluster II contained all samples of
L. sanctum and one sample of
L. bowringii (B01). Cluster III contained only
Ochrobryum subulatum samples (outgroup). Finally, Cluster IV contained all samples of
Camplypolus ericoides, the outgroup, and one sample of
L. bowringii (B02).
These results were consistent with the classification of
Leucobryum species, with a noted exception of
L. bowringii. The HPLC profiles also showed different patterns of peaks between two samples of
L. bowringii around the retention times of 5-7 minutes and again at 15 minutes onward. As the lack of internal consistency within the species is likely to contribute to the clustering of
L. bowringii samples with the other taxa. The differences in chemical profiles within
L. bowringii may result from different environmental conditions where these plant samples were collected. In
Leucobryum, differences in chemical compounds among habitats of the same species have been reported in
L. javense [
27]. Our
L. bowringii samples also came from different venders at different times of purchase, suggesting the different localities, resulting in different chemical profiles. Increasing the number of samples within each species may help enhance the within-taxa consistency in chemical profiles compared to the current study.
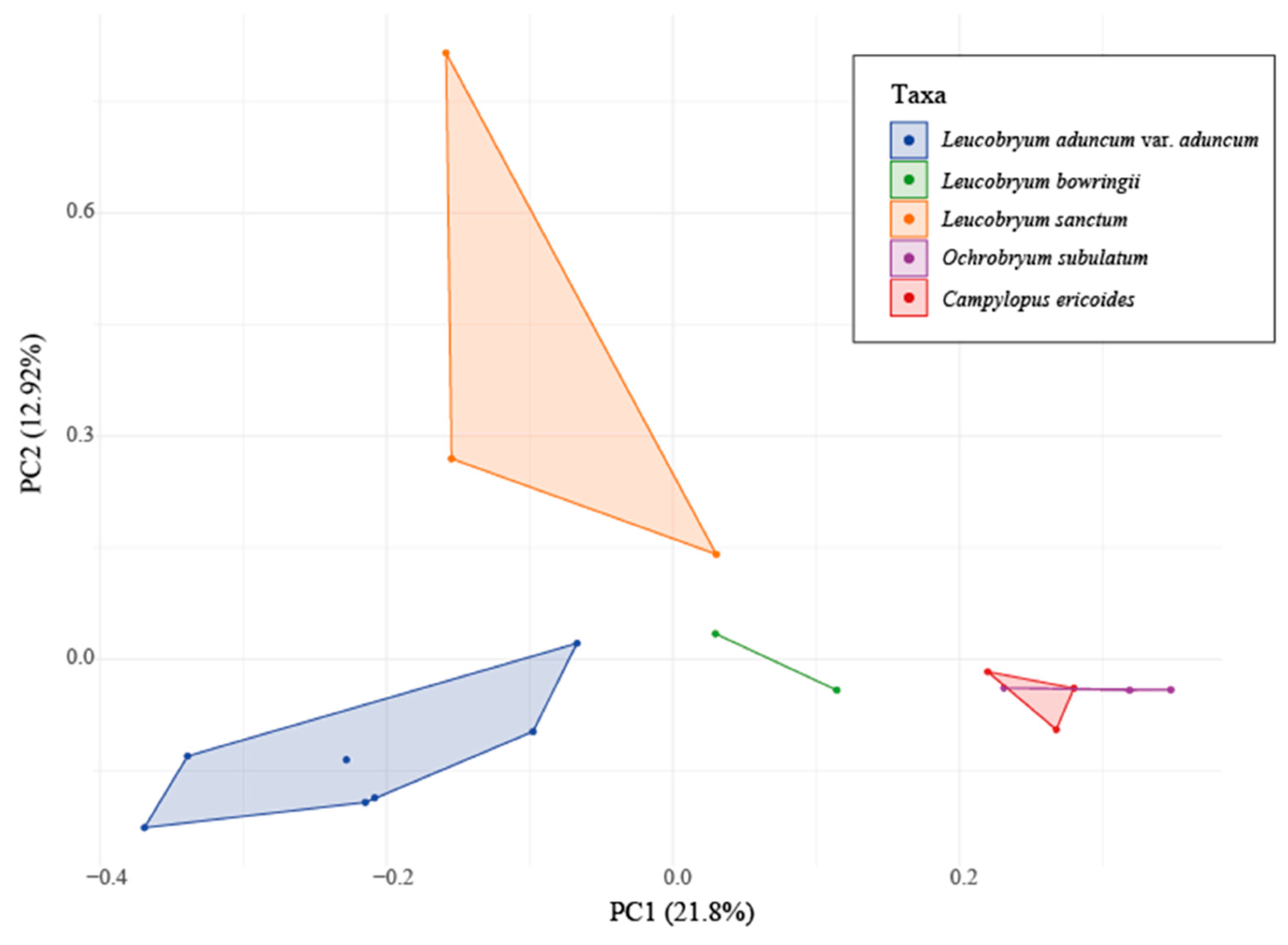
Overall, the PCA results confirmed the difference in the chemical profiles among the three
Leucobryum taxa (
Figure 4). Out of the overall variance explained (34.72%), the first component (PC1) accounted for 21.8%, and the second component (PC2) accounted for 12.92%. Three
Leucobryum taxa (
L. aduncum var.
aduncum,
L. bowringii, and
L. sanctum) showed three distinct, non-overlapping clusters, indicating that the three species were separated. The PERMANOVA showed significant differences among five studied taxa at
P-values = 0.002 and
F-values = 2.4973. This multivariate analysis showed that the studied
Leucobryum species differed markedly from each other and the outgroups in their HPLC chemical profiles.
While the HCA could not group the samples of L. bowringii together, the PCA readily distinguished this taxon from the others. The observed discrepancy highlights the importance of the choice for analytic tools in chemometrics. While Principal Component Analysis (PCA) and Hierarchical Clustering Analysis (HCA) are both powerful techniques for chemometric analysis, they were developed for different purposes and have different advantages. In the case of comparing chemical profiles, PCA uses the dimension reduction techniques, while HCA relies on clustering of the groups based on the distance matrix. PCA is inherently better at dealing with multicollinearity and highly correlated variables than HCA. Therefore, PCA might be able to position the slightly different chemical profiles of L. bowringii closer to each other than HCA. In our particular case, PCA was more effective at separating taxa within the genus Leucobryum, but the results could be different with other chemometric datasets. Therefore, it is advised that researchers use a variety of analytical techniques when analyzing chemical profile data, especially in the early stages of exploratory data analysis, in order to guarantee the validity and applicability of the findings for later research.
4. Future Directions
Our study shows that chemical profiles obtained from these analyses can effectively distinguish between different taxa of Leucobryum. There exist multiple directions for future research that have the potential to enhance our comprehension of Leucobryum species and their chemistry. First, a better identification of the secondary metabolites should be performed, using comparisons with the standards or a more powerful techniques, such as High-Resolution Liquid Chromatography-Mass Spectrometer Quadrupole Time of Flight (LC MS/MS Q-TOF). The latter technique allows the automatic identification of the observed compounds from the analysis, using an extensive database. Some these compounds can potentially be bioactive and should be test for their activities further.
5. Conclusions
The chemical profile studies of the moss genus Leucobryum from TLC and HPLC showed that the TLC profiles could only separate the genus from the outgroups. HPLC chromatograms provided a greater resolution than the TLC, allowing us to separate Leucobryum from the outgroup and classify species within the genus using PCA and Cluster Analysis. PERMANOVA showed significant separation of Leucobryum species by their chemical profiles. This work provides additional data for species delimitation in the moss genus Leucobryum and serves as an example of the use of chemometrics in the classification of bryophytes.
Author Contributions
sample collection, P.T.; extract preparation, P.T.; methodology, N.W. and S.V.; formal analysis, P.T.; data curation, P.T.; writing—original draft preparation, P.T.; writing—review and editing, E.K.; visualization, P.T.; supervision, E.K.; project suggestion, E.K. and S.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Graduate Scholarship of the Faculty of Science, Kasetsart University of the Year 2019.
Acknowledgments
The author would like to thank the researchers of the Biodiversity, Ecology, and Evolution Research Lab, the Department of Botany, Kasetsart University, and Patsara Nawabut, technical of Scientific Equipment Center, Faculty of Science, Kasetsart University.
References
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of Secondary Metabolites in Defense Mechanisms of Plants. Biology and Medicine 2011, 3, 232–249. [Google Scholar] [CrossRef]
- Stace, C.A. Plant taxonomy and biosystematics., 2nd ed.; Cambridge University Press: England, 1989. [Google Scholar]
- Singh, R. Chemotaxonomy: A tool for plant classification. Journal of Medicinal Plants Studies 2016, 4, 90–93. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Zhao, Y.-L.; Wang, Y.-Z.; Jin, H. Chemotaxonomic studies of nine Gentianaceae species from Western China based on liquid chromatography tandem mass spectrometry and fourier transform infrared spectroscopy. Phytochemical Analysis 2016, 27, 158–167. [Google Scholar] [CrossRef]
- He, C.; Peng, B.; Dan, Y.; Peng, Y.; Xiao, P. Chemical Taxonomy of Tree Peony Species from China Based on Root Cortex Metabolic Fingerprinting. Phytochemistry 2014, 107, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Gradstein, S.R.; Churchill, S.P.; Salazar-Allen, N. Guide to the bryophytes of tropical America. In Memoirs of the New York Botanical Garden, Buck, W. R.; Daniel, T.F., Reese, W.D., Eds.; The New York Botanical Garden Press: New York, 2001; Vol. 86, pp. 1–577. [Google Scholar]
- Xie, C.-F.; Lou, H.-X. Secondary metabolites in bryophytes: An ecological aspect. Chemistry & Biodiversity 2009, 6, 303–312. [Google Scholar] [CrossRef]
- Maksimova, V.; Klavina, L.; Bikovens, O.; Zicmanis, A.; Purmalis, O. Structural characterization and chemical classification of some bryophytes found in Latvia. Chemistry & Biodiversity 2013, 10, 1284–1294. [Google Scholar] [CrossRef]
- Goffinet, B.; Buck, W.R.; Shaw, A.J. Morphology, anatomy, and classification of the bryophyta. In Bryophyte biology, 2 ed.; Goffinet, B., Shaw, A.J., Eds.; Cambridge University Press: Cambridege, 2008; pp. 55–138. [Google Scholar]
- Goffinet, B.; Buck, W.R. Classification of the Bryophyta. Available online: http://bryology.uconn.edu/classification/ (accessed on January 12).
- Enroth, J. The Bryophytes of Sabah (North Borneo) with special reference to the BRYOTROP transect of Mount Kinabalu, IV. Leucobryaceae (Bryopsida). Wildenowia 1989, 18, 529–554. [Google Scholar]
- Eddy, A. Leucobryaceae. In A handbook of Malesian mosses; Eddy, A., Ed.; British Museum (Natural History): London, 1990; Vol. 2. [Google Scholar]
- Enroth, J. Bryophyte flora of the Huon Peninsula, Papua New Guinea. XXXVI. Leucobryaceae (Musci). Acta Botanica Fennica 1990, 139, 65–120. [Google Scholar]
- Klazenga, N. Australian Mosses Online. 35. Leucobryaceae: Leucobryum. Available online: http://www.anbg.gov.au/abrs/Mosses_online/Leucobryaceae_Leucobryum.pdf (accessed on January 16).
- He, S. An annotated checklist and atlas of the mosses of Thailand. Available online: http://www.mobot.org/MOBOT/moss/Thailand/welcome.shtml (accessed on August 6).
- Yamaguchi, T. A revision of the genus Leucobryum (Musci) in Asia. Journal of the Hattori Botanical Laboratory 1993, 73, 1–123. [Google Scholar]
- Renzaglia, K.S.; Schuette, S.; Duff, R.J.; Ligrone, R.; Shaw, A.J.; Mishler, B.D.; Duckett, J.G. Bryophyte phylogeny: Advancing the molecular and morphological frontiers. Bryologist 2007, 110, 179–213. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Shaw, J. The application of molecular data to the phylogenetic delimitation of species in bryophytes: A note of caution. Phytotaxa 2010, 9, 229–237. [Google Scholar] [CrossRef]
- Simpson, M. Plant systematics, 2nd ed.; Academic Press: Massachusetts, 2010. [Google Scholar]
- Vogt, N.B. Soft modelling and chemosystematics. Chemometrics and Intelligent Laboratory Systems 1987, 1, 213–231. [Google Scholar] [CrossRef]
- Hawryl, A.; Bogucka-Kocka, A.; Świeboda, R.; Hawryl, M.; Stebel, A. The reversed phase high performance liquid chromatography fingerprint profiles of thirty-nine mosses with chemometric. Journal of Liquid Chromatography & Related Technologies 2017, 40, 36–41. [Google Scholar] [CrossRef]
- Hawryl, A.; Bogucka-Kocka, A.; Świeboda, R.; Hawryl, M.; Stebel, A.; Waksmundzka-Hajnos, M. Thin-layer chromatography fingerprint and chemometric analysis of selected Bryophyta species with their cytotoxic activity. Journal of Planar Chromatography 2018, 31, 28–35. [Google Scholar] [CrossRef]
- Huneck, S. Chapter 1. Chemistry and Biochemistry of Bryophytes. In New Manual of Bryology; Schuster, R. M., Ed.; The Hattori Botanical Laboratory: Nichinan, Miyazaki, Japan, 1983; Vol. 1. [Google Scholar]
- Ichikawa, T.; Yamada, K.; Namikawa, M.; Sakai, K.; Kondo, K. New cyclopentenonyl fatty acids from Japanese mosses. Journal of the Hattori Botanical Laboratory 1984, 56, 209–213. [Google Scholar] [CrossRef]
- Chiu, P.-L.; Patterson, G.W.; Fenner, G.P. Sterols of bryophytes. Phytochemistry 1985, 24, 263–266. [Google Scholar] [CrossRef]
- Kojima, M.; Shiraki, H.; Ohnishi, M.; Ito, S. Two Diglycosyldiacylglycerol Isomers in Plant Leaves, Ferns, Mosses and Seaweeds. Phytochemistry 1990, 29, 1161–1163. [Google Scholar] [CrossRef]
- Azar, A.W.P.; Rosleine, D.; Faizal, A. Secondary metabolite profiles in the methanolic extract of Leucobryum javense isolated from tropical montane forest in West Java, Indonesia. In International Conference on Biology and Applied Science (ICOBAS); Romaidi, Wahyudi, D., Daryono, R.N.H., Yusnawan, E., Kikuchi, A., Eds.; Malang, Indonesia, 2019; Vol. 2120, pp. 030027–1–030027-8.
- Gangulee, H.C. Leucobryaceae. In Mosses of Eastern India and adjacent regions: Archidiales, Dicranales & Fissidentales; Gangulee, H.C., Ed.; Book & Allied Limited: Calcutta, 1971; Vol. 2. [Google Scholar]
- Chien, G.; Crosby, M.R.; He, S. Moss flora of China; Missouri Botanical Garden Press: St. Louis, 1999; Vol. 1. [Google Scholar]
- Merck, E. Dyeing reagents of thin-layer and paper chromatography; B. Grim Healthcare Co. LTD: Germany, 1980. [Google Scholar]
- R Core Team R: A language and environment for statistical computing. Available online: https://www.R-project.org/ (accessed on January 16).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecology 2001, 26, 32–46. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package. R package version 2.6–4. Available online: https://CRAN.R-project.org/package=vegan.
- Dembitsky, V.M. Lipids of bryophytes. Progress in Lipid Research 1993, 32, 281–356. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).