Submitted:
08 November 2023
Posted:
09 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumental Analysis
3. Results
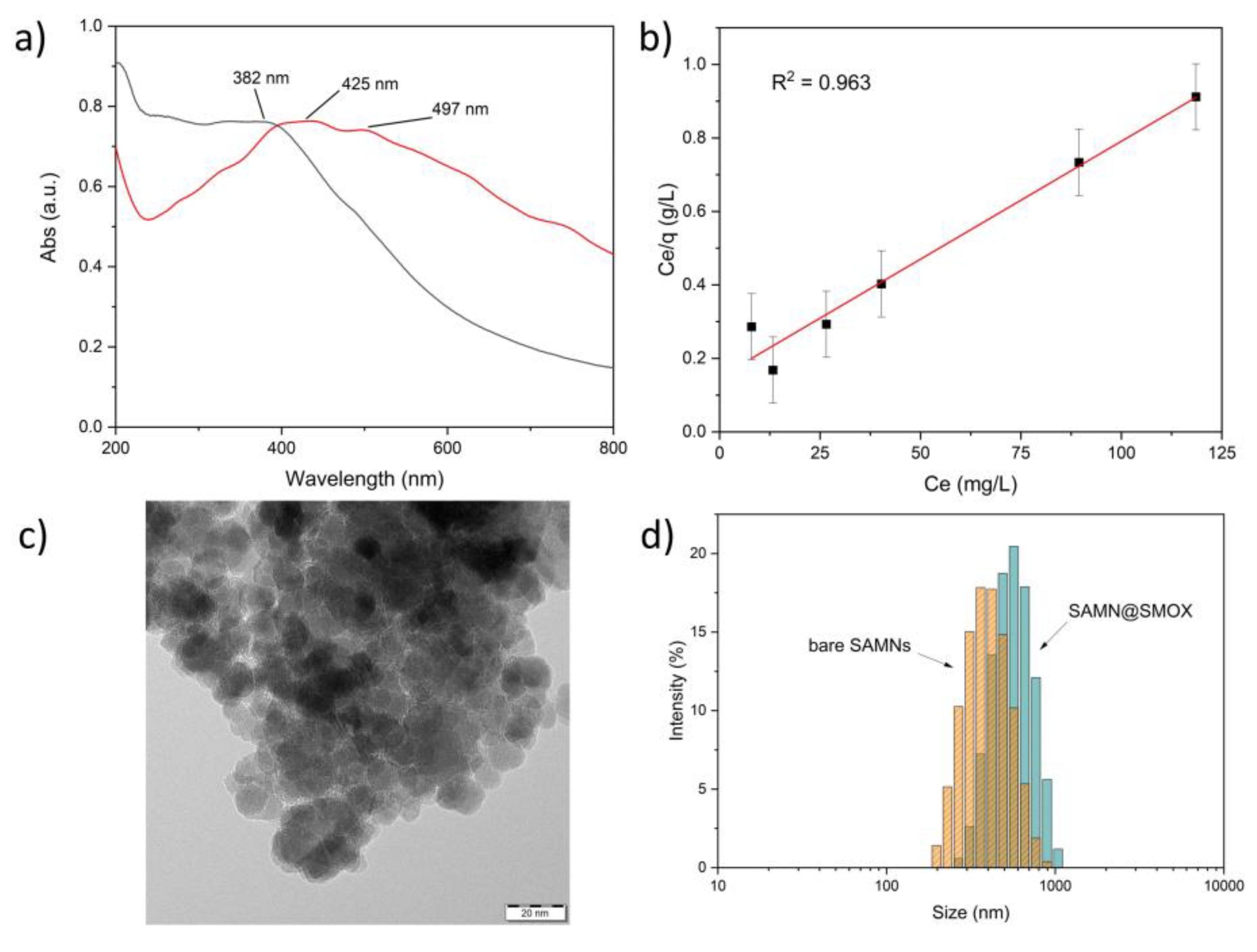
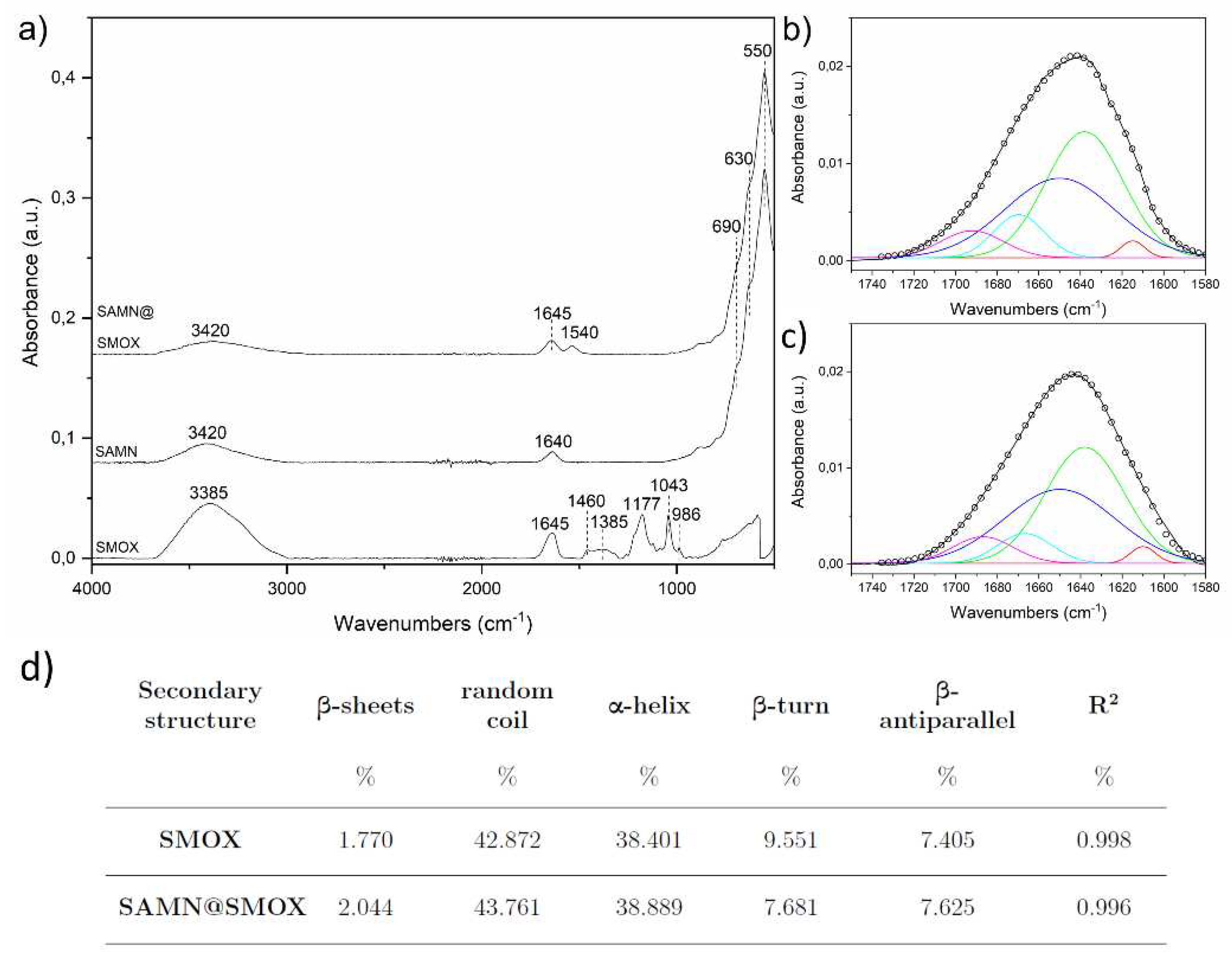
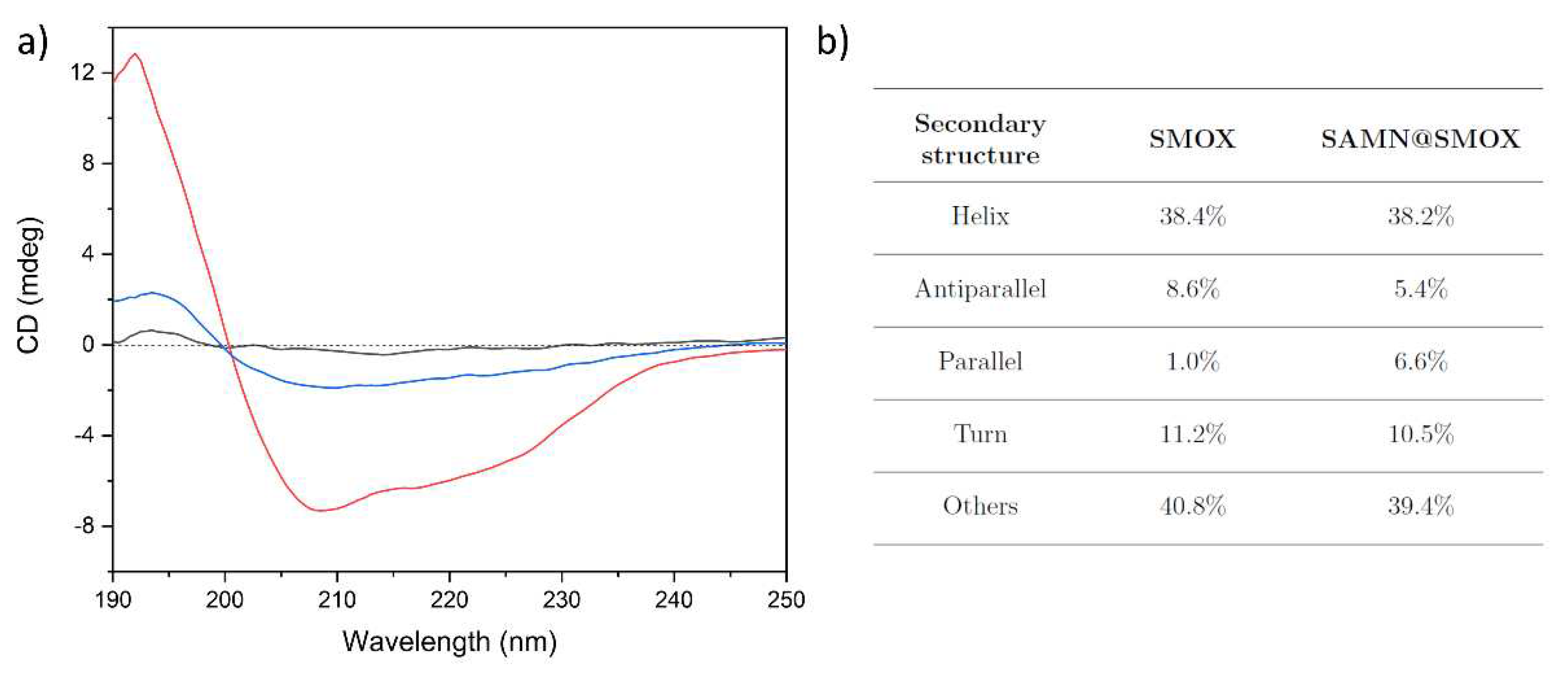
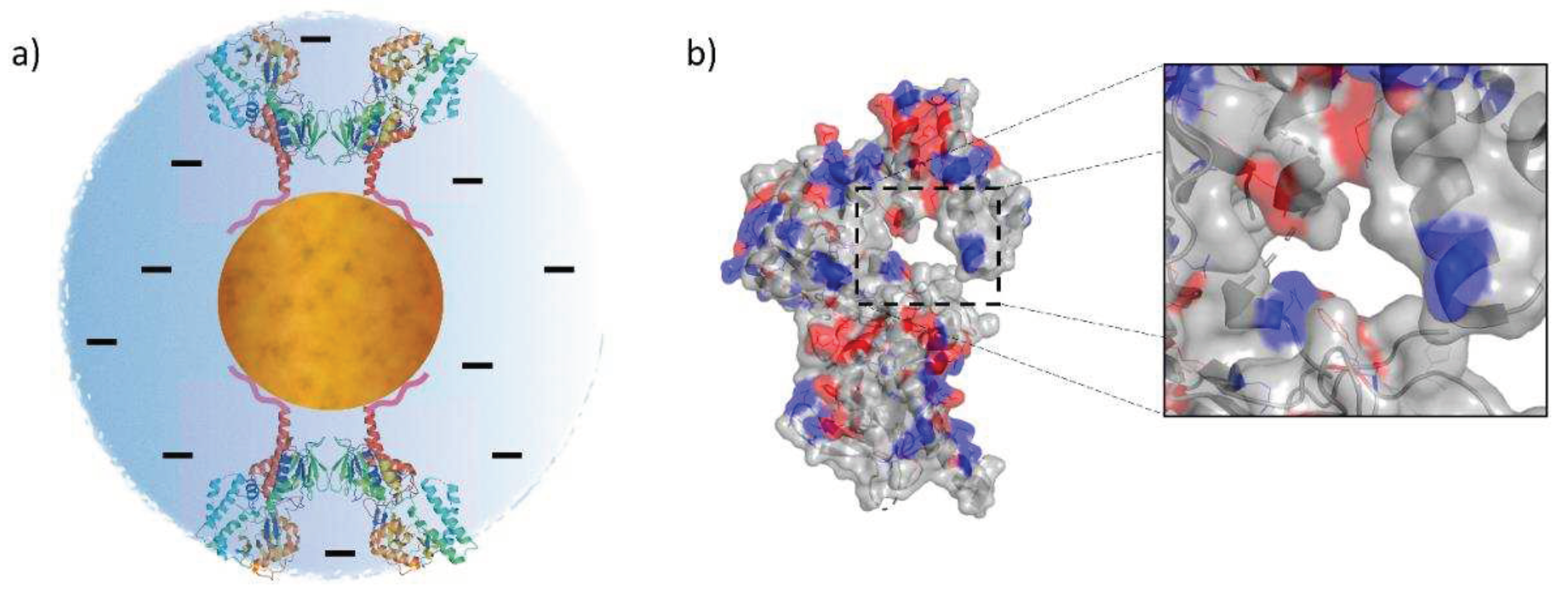
3.1. Chemical-Physical Characterization of the SAMN@SMOX Hybrid
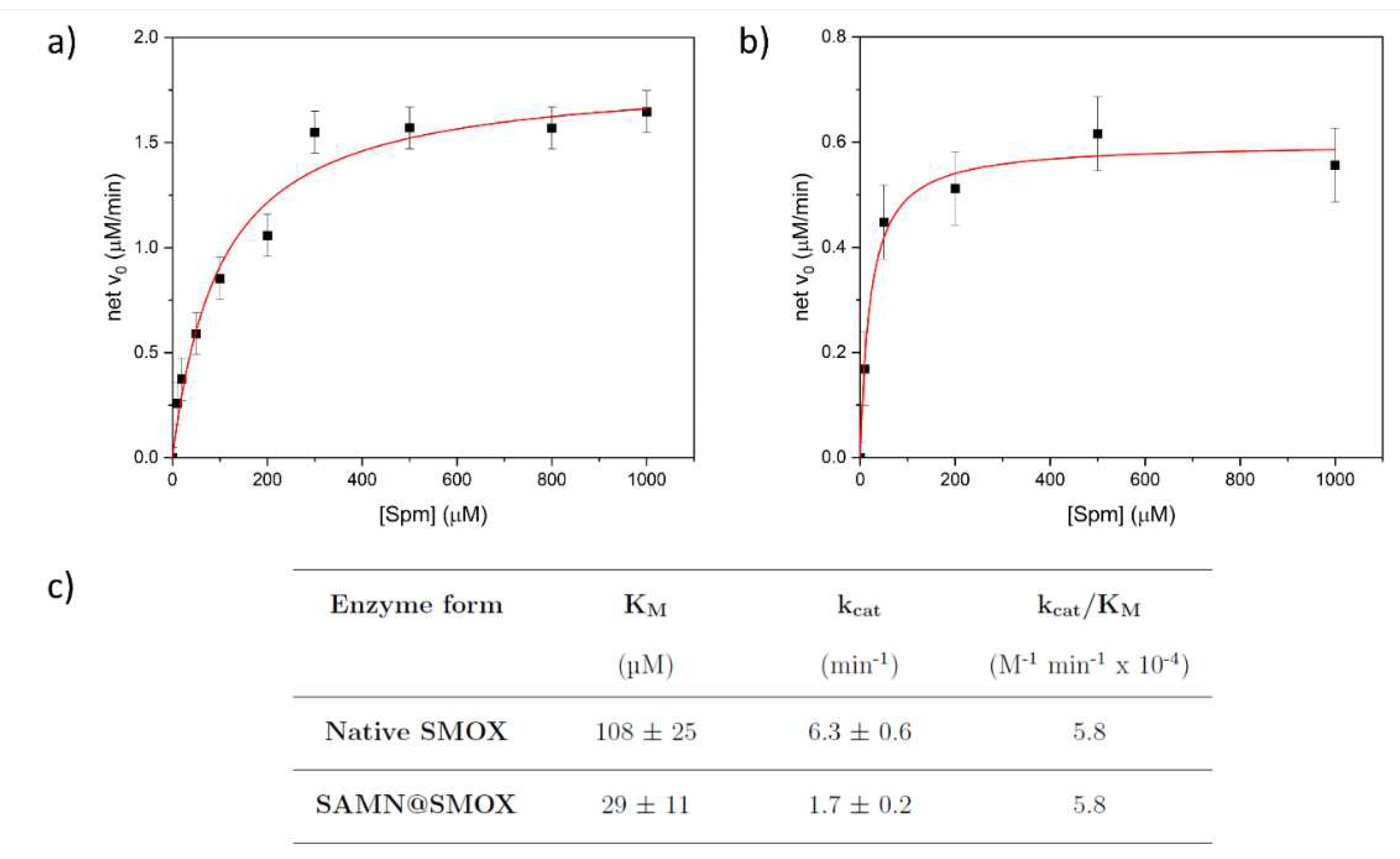
3.2. Comparison of the Activity of Native SMOX and of SAMN@SMOX Hybrid
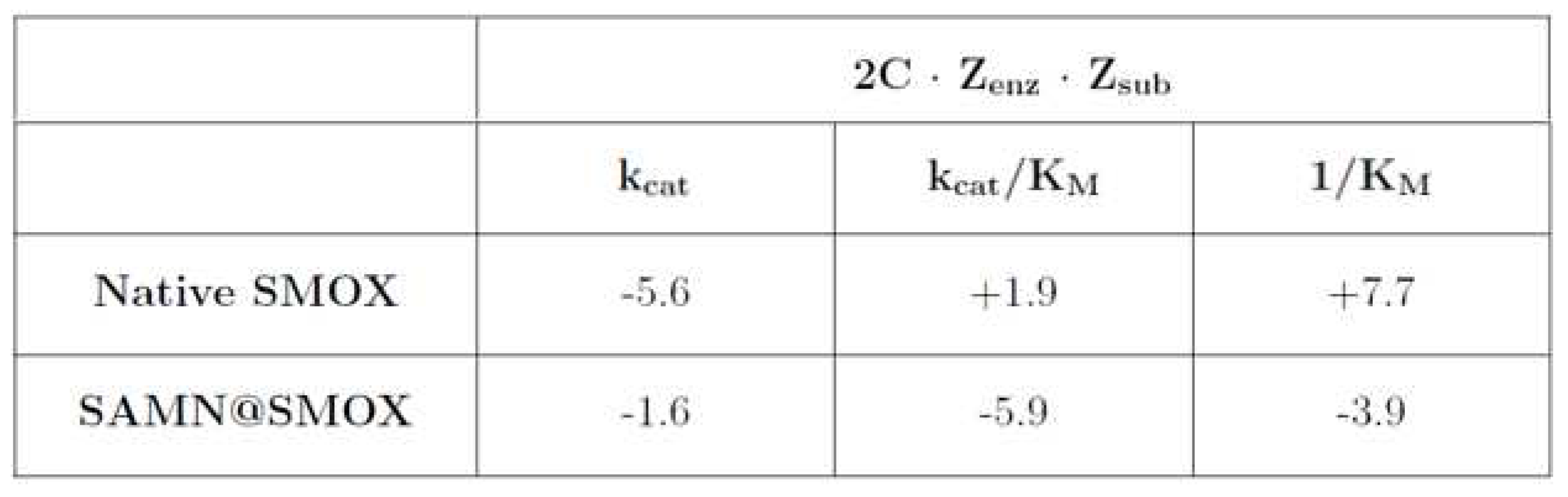 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Polticelli, F.; Salvi, D.; Mariottini, P.; Amendola, R.; Cervelli, M. Molecular Evolution of the Polyamine Oxidase Gene Family in Metazoa. BMC Evol. Biol. 2012, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mastrantonio, R.; Cervelli, M.; Pietropaoli, S.; Mariottini, P.; Colasanti, M.; Persichini, T. HIV-Tat Induces the Nrf2/ARE Pathway through NMDA Receptor-Elicited Spermine Oxidase Activation in Human Neuroblastoma Cells. PLoS One 2016, 11, e0149802. [Google Scholar] [CrossRef] [PubMed]
- Ceci, R.; Duranti, G.; Leonetti, A.; Pietropaoli, S.; Spinozzi, F.; Marcocci, L.; Amendola, R.; Cecconi, F.; Sabatini, S.; Mariottini, P.; et al. Adaptive Responses of Heart and Skeletal Muscle to Spermine Oxidase Overexpression: Evaluation of a New Transgenic Mouse Model. Free Radic. Biol. Med. 2017, 103, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, E.; Belli, F.; Dalla Vedova, L.; Marra, M.; Crateri, P.; Arancia, G. Hyperthermia Enhances Cytotoxicity of Amine Oxidase and Spermine on Drug-Resistant LoVo Colon Adenocarcinoma Cells. Int. J. Oncol. 2006, 28, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, E.; Vianello, F.; Magliulo, G.; Thomas, T.; Thomas, T.J. Nanoparticle Strategies for Cancer Therapeutics: Nucleic Acids, Polyamines, Bovine Serum Amine Oxidase and Iron Oxide Nanoparticles (Review). Int. J. Oncol. 2015, 46, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, S.; Mancinelli, R.; Miglietta, S.; Cona, A.; Angelini, R.; Canettieri, G.; Spandidos, D.A.; Gaudio, E.; Agostinelli, E. Maize Polyamine Oxidase in the Presence of Spermine/Spermidine Induces the Apoptosis of LoVo Human Colon Adenocarcinoma Cells. Int. J. Oncol. 2019, 54, 2080–2094. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Amendola, R.; Polticelli, F.; Mariottini, P. Spermine Oxidase: Ten Years After. Amino Acids 2012, 42, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Bellavia, G.; D’Amelio, M.; Cavallucci, V.; Moreno, S.; Berger, J.; Nardacci, R.; Marcoli, M.; Maura, G.; Piacentini, M.; et al. A New Transgenic Mouse Model for Studying the Neurotoxicity of Spermine Oxidase Dosage in the Response to Excitotoxic Injury. PLoS One 2013, 8, e64810. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Angelucci, E.; Stano, P.; Leboffe, L.; Federico, R.; Antonini, G.; Mariottini, P.; Polticelli, F. The Glu216/Ser218 Pocket Is a Major Determinant of Spermine Oxidase Substrate Specificity. Biochem. J. 2014, 461, 453–459. [Google Scholar] [CrossRef]
- Cervetto, C.; Vergani, L.; Passalacqua, M.; Ragazzoni, M.; Venturini, A.; Cecconi, F.; Berretta, N.; Mercuri, N.; D’Amelio, M.; Maura, G.; et al. Astrocyte-Dependent Vulnerability to Excitotoxicity in Spermine Oxidase-Overexpressing Mouse. NeuroMolecular Med. 2016, 18, 50–68. [Google Scholar] [CrossRef]
- de la Fuente, M.; Lombardero, L.; Gómez-González, A.; Solari, C.; Angulo-barturen, I.; Acera, A.; Vecino, E.; Astigarraga, E.; Barreda-gómez, G. Enzyme Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9181. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Venerando, A.; Macone, A.; Canettieri, G.; Agostinelli, E.; Vianello, F. Nanotechnology-Based Strategies to Develop New Anticancer Therapies. Biomolecules 2020, 10, 735. [Google Scholar] [CrossRef]
- Magro, M.; Vianello, F. Bare Iron Oxide Nanoparticles: Surface Tunability for Biomedical, Sensing and Environmental Applications. Nanomaterials 2019, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein-Nanoparticle Interactions: Opportunities and Challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Markiewicz, K.H.; Wilczewska, A.Z.; Car, H. Magnetic Nanoparticles as New Diagnostic Tools in Medicine. Adv. Med. Sci. 2012, 57, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Dawson, K.A. Protein-Nanoparticle Interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Johnson, B.J.; Russ Algar, W.; Malanoski, A.P.; Ancona, M.G.; Medintz, I.L. Understanding Enzymatic Acceleration at Nanoparticle Interfaces: Approaches and Challenges. Nano Today 2014, 9, 102–131. [Google Scholar] [CrossRef]
- Ding, S.; Cargill, A.A.; Medintz, I.L.; Claussen, J.C. Increasing the Activity of Immobilized Enzymes with Nanoparticle Conjugation. Curr. Opin. Biotechnol. 2015, 34, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.A.; Park, H.J.; Driscoll, A.J. Enzyme Nanoparticle Fabrication: Magnetic Nanoparticle Synthesis and Enzyme Immobilization. Methods Mol. Biol. 2011, 679, 183–191. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Magro, M.; Cozza, G.; Molinari, S.; Venerando, A.; Baratella, D.; Miotto, G.; Zennaro, L.; Rossetto, M.; Frömmel, J.; Kopečná, M.; et al. Role of Carboxylic Group Pattern on Protein Surface in the Recognition of Iron Oxide Nanoparticles: A Key for Protein Corona Formation. Int. J. Biol. Macromol. 2020, 164, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Rilievo, G.; Cecconello, A.; Molinari, S.; Venerando, A.; Rutigliano, L.; Govardhan, G.T.; Kariyawasam, D.H.; Arusei, R.J.; Zennaro, L.; Di Paolo, M.L.; et al. Acidic Shift of Optimum PH of Bovine Serum Amine Oxidase upon Immobilization onto Nanostructured Ferric Tannates. Int. J. Mol. Sci. 2022, 23, 12172. [Google Scholar] [CrossRef] [PubMed]
- Zanin, S.; Molinari, S.; Cozza, G.; Magro, M.; Fedele, G.; Vianello, F.; Venerando, A. Intracellular Protein Kinase CK2 Inhibition by Ferulic Acid-Based Trimodal Nanodevice. Int. J. Biol. Macromol. 2020, 165, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Polticelli, F.; Federico, R.; Mariottini, P. Heterologous Expression and Characterization of Mouse Spermine Oxidase. J. Biol. Chem. 2003, 278, 5271–5276. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Sinigaglia, G.; Nodari, L.; Tucek, J.; Polakova, K.; Marusak, Z.; Cardillo, S.; Salviulo, G.; Russo, U.; Stevanato, R.; et al. Charge Binding of Rhodamine Derivative to OH- Stabilized Nanomaghemite: Universal Nanocarrier for Construction of Magnetofluorescent Biosensors. Acta Biomater. 2012, 8, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Stevanato, R.; Mondovi’, B.; Sabatini, S.; Rigo, A. Spectrophotometric Assay for Total Polyamines by Immobilized Amine Oxidases. Anal. Chim. Acta 1990, 237, 391–397. [Google Scholar] [CrossRef]
- Hebia, C.; Bekale, L.; Chanphai, P.; Agbebavi, J.; Tajmir-Riahi, H.A. Trypsin Inhibitor Complexes with Human and Bovine Serum Albumins: TEM and Spectroscopic Analysis. J. Photochem. Photobiol. B Biol. 2014, 130, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Bienert, S.; Waterhouse, A.; De Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-New Features and Functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; De Paula, J. Atkins’ Physical Chemistry; 8th ed.; Oxford University Press: Oxford, 2006; ISBN 0198700725. [Google Scholar]
- Butterworth, P.J. The Chemical Kinetics of Enzyme Action (2nd Edition); Laidler, K.J., Bunting, P.S., Eds.; Portland Press, 1974; Vol. 2;
- Rajh, T.; Chen, L.X.; Lukas, K.; Liu, T.; Thurnauer, M.C.; Tiede, D.M. Surface Restructuring of Nanoparticles: An Efficient Route for Ligand-Metal Oxide Crosstalk. J. Phys. Chem. B 2002, 106, 10543–10552. [Google Scholar] [CrossRef]
- Magro, M.; Faralli, A.; Baratella, D.; Bertipaglia, I.; Giannetti, S.; Salviulo, G.; Zboril, R.; Vianello, F. Avidin Functionalized Maghemite Nanoparticles and Their Application for Recombinant Human Biotinyl-SERCA Purification. Langmuir 2012, 28, 15392–15401. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A General Treatment and Classification of the Solute Adsorption Isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using Circular Dichroism Spectra to Estimate Protein Secondary Structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Tavladoraki, P.; Cervelli, M.; Antonangeli, F.; Minervini, G.; Stano, P.; Federico, R.; Mariottini, P.; Polticelli, F. Probing Mammalian Spermine Oxidase Enzyme–Substrate Complex through Molecular Modeling, Site-Directed Mutagenesis and Biochemical Characterization. Amino Acids 2011, 40, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Fersht, A. Enzyme Structure and Mechanism, 2nd ed.; W.H. Freeman & Co.: New York, 1985; ISBN 9780716716143. [Google Scholar]
- Di Paolo, M.L.; Stevanato, R.; Corazza, A.; Vianello, F.; Lunelli, L.; Scarpa, M.; Rigo, A. Electrostatic Compared with Hydrophobic Interactions between Bovine Serum Amine Oxidase and Its Substrates. Biochem. J. 2003, 371, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, E.; Prantner, J.D.; Stellwagen, N.C. Do Zwitterions Contribute to the Ionic Strength of a Solution? Anal. Biochem. 2008, 373, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Ramezani-Dakhel, H. Simulations of Inorganic-Bioorganic Interfaces to Discover New Materials: Insights, Comparisons to Experiment, Challenges, and Opportunities. Chem. Soc. Rev. 2016, 45, 412–448. [Google Scholar] [CrossRef]
- Cervelli, M.; Leonetti, A.; Cervoni, L.; Ohkubo, S.; Xhani, M.; Stano, P.; Federico, R.; Polticelli, F.; Mariottini, P.; Agostinelli, E. Stability of Spermine Oxidase to Thermal and Chemical Denaturation: Comparison with Bovine Serum Amine Oxidase. Amino Acids 2016, 48, 2283–2291. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





