1. Introduction
Picobirnaviruses (PBVs) are small viruses approximately 40 nm of diameter, non-enveloped, icosahedral symmetry and have two genomic segments, the larger segment with 2.2-2.7 kbp size and the smaller segment with 1.2-1.9 kbp [
1]. The larger segment (segment I) plays a role in the coding of structural proteins, and the smaller (segment II) encodes the RNA-dependent viral RNA polymerase (RdRp) and determines PBV’s classification [
2].
The current PBV classification in Genogroup I and II, based on strains 1-CHN-97 (GI) and 4-GA-91 (GII) from humans infecting vertebrate animals, and the Genogroup III, recently proposed and unlike the others, affects invertebrate animals [
3].
PBVs are associated with gastroenteritis in both humans and animals and may present as an apparent symptom of diarrhea that may be directly linked to the virus as a single pathogen or other enteric pathogens, attributing to it the characteristic of a secondary pathogen in mixed infections. These viruses are further classified as opportunistic pathogens when detected in immunocompromised patients, and who have diarrheal conditions [
4,
5].
PBVs are considered emerging, opportunistic, and suggestive of zoonotic potential [
8]. The first report on PBV appeared from an outbreak of gastroenteritis in Brazil, where viruses were detected in human stool specimens and in a rat species [
9]. Since then, PBVs have been described as pathogens that infect a variety of animal species, such as mammals, reptiles, birds, humans, and is also present in sewage, showing a genetic diversity among circulating strains [
6]. Studies suggest that PBVs are bacteriophages of bacteria in the digestive tract of possible hosts of this pathogen [
7,
8].
Currently, several pathogens, including enteric viruses that affect humans and animals, have been estimated as responsible for 60% of zoonotic infections in humans [
9]. Zoonoses are responsible for 75% of emerging and reemerging diseases that affect humans and cause at least 20% loss in animal production [
9]. Thus, the investigation of PBV in samples from different hosts is important in relation to public health, due to their potential for zoonotic transmission [
10]. Therefore, it is imperative to monitor wild and domestic animals for the presence of different human pathogens which may trigger possible events of zoonotic transmission.
This study aimed to detect the presence of PBV in fecal specimens of wild and domestic animals collected in deforested areas of the Amazon biome.
2. Materials and Methods
2.1. Ethical aspects
The National Council for Animal Control and Experimentation (CONCEA), System of Authorization and Information in Biodiversity—SISBIO/ICMBIO/Ministry of the Environment approved this research under protocol No. 37174–1, and the Ethics Commission at the Evandro Chagas Institute (CEUA) under protocol number 35/2016.
2.2. Study area
The study area and the collection of biological samples comprised three cities: Santa Bárbara (Expedito Ribeiro settlement), Peixe-Boi (Vila do Ananin) and Viseu (Açaiteua-Centro Alegre), located in the North of Pará state. The respective area of the three municipalities comprises 278.15 km2, 450.29 km2, and 4.934.54 km2. The cities are part of a portion of the Brazilian Amazon, where the strong anthropic pressure increases the interaction between humans and animals. Also, the contact and handling of animals is becoming increasingly intense due to agribusiness, which is the main source of subsistence for families living there.
These areas have already been investigated for enteric virus, rotavirus, infected domestic and wild animals. Data concerning territorial extension and deforestation was published before [
11].
2.3. Sample collection
Sample collection was carried out from October 2014 to April 2016, comprising two annual visits to each city in the study area. The capture of wild animals (bats, birds, marsupials, and rodents) was done from areas of fragmented forest and adjacent urban areas, where a greater concentration of these species would be expected.
Different traps were used to capture wild animals. For birds and bats, fog nets were set up, at different times, from 4:00 am - 9:00 am, and 6:00 pm - 7:00 am, respectively, and inspected periodically. The other wild animals were captured using Tomahawk (45x16x16cm), Sherman (30x9x8cm), and Pitfall traps. The baits used to attract the animals to the traps were peanut butter, sardines, bacon, and fruit (pineapple, banana, and apple).
The biological material was collected by stimulation of the rectum using urethral probe (No 10) for the no-fly wild mammals and rectal swab “Zaragatoa” for wild birds and bats (small animals) through stimulation of the rectal ampulla. In relation to companion animals (dogs, cats, and poultries) and other mammals (pigs, horses, and cattle), sample collection and necessary information about the animals were carried out with authorization and contribution from their respective owners.
All samples were stored in sterile plastic vials and properly packed (-30ºC) to maintain the quality of the material before processing in the virology laboratory.
2.4. Samples
A total of 258 fecal samples were used in this study. The sample groups were composed of mammals (flying and non-flying) and birds (domestic and wild). The non-flying mammals include bovines (n = 23), canines (n = 34), equines (n = 41), felines (n = 33), rodents (n = 8), marsupials (n=25) and swine (n = 23). The flying mammals are the chiropters (n = 30). The bird group includes domestic birds (n = 3) and wild birds (n = 38).
2.5. Sample preparation
Fecal suspensions were prepared at 20% (w/v) in Tris/Ca2+ and clarified by centrifugation at 5000 rpm for 10 minutes at 4 ºC. Then, supernatants were collected and stored at -20 ºC for later use in the extraction, detection and characterization of viral genetic material.
The viral genome was extracted from the fecal suspensions using silica glass powder [
12]. During the extraction process, all contamination control measures were performed, including the use of positive (PBV positive sample) and negative control (ultrapure water). Polyacrylamide Gel Electrophoresis (PAGE) and silver staining were applied to analyze the electrophoretic profile of the samples, according method previously described [
13].
In the first moment, RT-PCR was used to amplify the genes using the PicoB25 and PicoB43 primers for Genogroup I, PicoB23 and PicoB24 for Genogroup II, which amplify 201 bp and 369 bp products, respectively [
14]. For this step of RT-PCR/nested, 4μL of extracted RNA and 1μL of each primer pair 20mM were denatured at 97ºC for 5 min in a thermocycler, followed by 5 min in an ice bath. cDNA was synthesized by adding 2 μL of dNTPs (20mM, Promega
®), 0.75μL of MgCl
2 (25mM, Promega
®), 2.5μL of buffer (5x, Promega
®), 0.5μL of Reverse Transcriptase enzyme (4U, Promega
®), and 13.25μL of RNAse/DNAse free H
2O up to 25μL of final volume. The synthesis reaction was carried out at 42°C for 1h.
cDNA was PCR-amplified by adding 25μL of cDNA to 3μL of dNTP (20mM, Promega®), 2.5μL of buffer (5x, Promega®), 0.75μL of MgCl2 (25mM, Promega®), 0.25μL of Taq Polymerase (5U, Promega®), and 18.50μL of RNAse/DNAse free H2O (Hyclone™). The tube with 50μL of final volume was submitted to PCR as follows: initial denaturation step at 95 °C for 2 min, 35 cycles at 95 °C for 1 min, 50 °C for 1 min, and 72 °C for 1 min, and a last extension step of 72 °C for 5 min.
This PCR amplified gene segment-2 (RdRp gene) by using the primer pairs PBV 1.2 FP, 5′AAGGTCGGKCCRATGT3′, and PBV RP,5′TTATCCCYTTTCATG CA3′ resulting in a ~1229 bp amplicon for the first stage (RT-PCR), according protocols described by (15).
Positive samples in the first PCR were then submitted to a second PCR using the primers Malik-2-FP (5’- TGG GWT GWT GGC GWG GAC ARG ARGG- 3, and Malik-2-RPreverse (5′-YSC AYT ACA TCC AC-3 ‘TCC) which amplified a ~ 580 bp RdRp fragment only for the Genogroup I [
23]. Conditions for this step were: initial denaturation at 95 °C for 5 min, followed by 35 cycles at 94 °C for 10 s, 48 °C for 25 s, and 72 °C for 45 s, and a last extension step of 72 °C for 10 min.
Subsequently, amplicons were purified (Promega Wizard PCR kit) and subjected to Sanger sequencing using the protocol of the Big Dye Terminator® v.3.1 kit (Applied Biosystems).
2.6. Sequence analysis
Obtained sequences were aligned and edited by the Geneious program (version 8.1.9), and compared with sequences available in the National Center for Biotechnology Information (NCBI) database (
www.ncbi.nlm.nih.gov) using the BLAST algorithm (version BLAST + 2.8.0-alpha).
Phylogenetic trees were built using the maximum likelihood method (Maximum Likelihood-ML) by the FastTree program. To determine the best nucleotide replacement model, the GTR (General Time Reversible) program was used. Bootstrap analysis (1,000 replicates) was used to give reliability to phylogenetic groups. The tree was visualized using the Evolview software [
15].
The construction of the phylogenetic tree included PBV sequences available from GenBank, belonging to different animal hosts and samples from previous studies from the same collection region, and belonging to PBV-GI.
2.7. Statistical analysis
Statistical analysis of the data was performed using R Statistical Software version 4.2.1 (RVAidemamoirre package). G and Chi-square tests were used to identify the frequency of pathogens in the cities, the period of collection, and the categorical variables: group of animals, gender (male and female) and city from where the samples originated. The significance value in the results was α = 0.05 (error probability of 5%).
3. Results
From September 2014 to March 2016, 258 biological samples were collected, from three different cities of Pará state, Brazil. The cities were Santa Bárbara, Peixe-Boi, and Viseu. The biological materials were from different groups of animals including 84.10% of mammals (217/258), and 15.90% of birds (41/258). The viral RNA of each sample was analyzed using firstly PAGE and then RT-PCR. No viral RNA could be detected by PAGE, however, RT-PCR assays showed positive results exclusively for Genogroup I (PBV GI).
In a total of 32 samples (12.4%) segment 2 (PBV-G1) could be amplified. No sample contained PBV GII. The frequency of PBV in this study (
Figure 1) concentrated in swine, which represented 28.13% of positivity, and felines which showed 28.13% (9/32) of positivity in cats, similar to swine. The other PBV-positive samples were 6.25% of avian (2/32), 9.37% of bovine (3/32), 14.7% of canine (5/32), and 9.37% of equine (3/32) origin. Only one positive sample was from a marsupial (3.13%, 1/32). No PBV was detected in the samples from bats and rodents.
A. Rplots for the positive proportion distributed among group of animals; B. Rplots for the positive proportion distributed among gender, female and male; C.- Rplots for the positive proportion distributed among the three cities of this study.
211 animal samples were gender-identified, of these, 47.40% samples were from males (100/211) and 52.60% from females (111/211), not being possible to identify the sex of 47 animals. Most positive samples were identified in Peixe-Boi followed by Santa Bárbara, and Viseu.
In statistical analysis, G and Chi-square tests were performed to assess the significance of positivity between the different groups of animals (birds, cattle, canines, equines, swine, felines, bats, marsupial and rodents). The results of this analysis demonstrated that the frequency of PBV was not significant for between gender (p>0.05). Therefore, the occurrence of PBV between animal groups and among the cities from the samples, p value was significant showing p < 0.05, in both variables. The data comparing groups of animals, gender (male and female), and cities from the samples (Santa Bárbara, Peixe-Boi and Viseu) are exposed in
Table 1.
For further phylogenetic analysis, 32 samples were amplified by RT-PCR for PBV GI. These samples were subjected to Nested-PCR since only seven samples (21.87%) showed satisfactory for sequencing. The PBV-positive samples sequenced were from five felines, one swine and one canine. Accession numbers in GenBank are MT872191 (PB089), MT872192 (PB097), PB100 MT872193 (PB100), PB103 MT872194 (PB103), PB106 MT872195 (PB106), PB088 MT872196 (PB088), MT872197 (PB091).
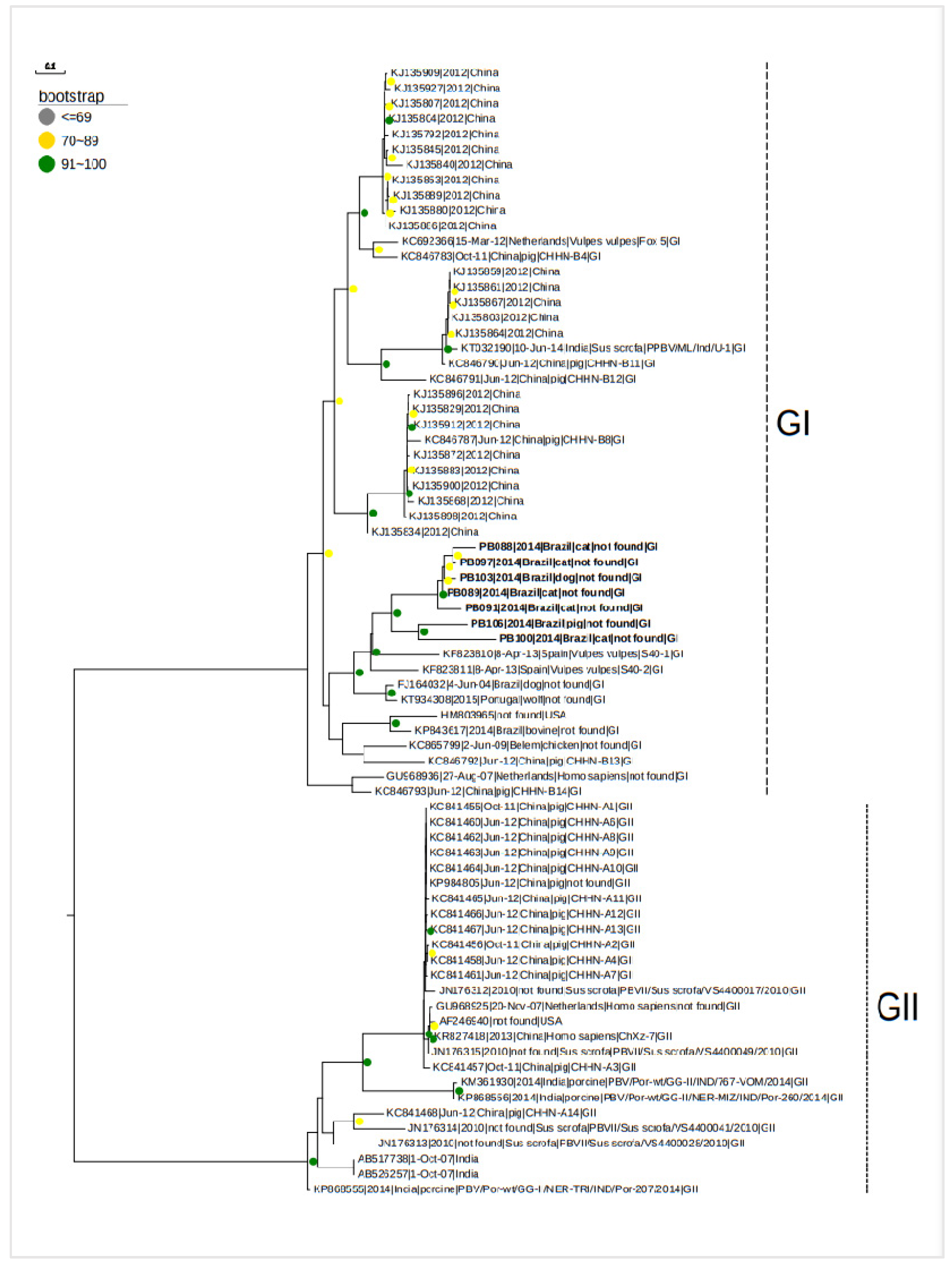
The samples in the phylogenetic three grouped heterogeneously, coinciding with clades of PBV reported from different countries and a variety of species. The genotype characterization identified all the sequences as part of PBV GI, as illustrated in
Figure 2.
The current study sequences are indicted in bold. The bootstrap values are indicated next to the nodes in three different color circles; GI – PBV Genogroup I, and GII – PBV Genogroup II.
Alignment and phylogenetic analysis showed a nucleotide identity from 70% to 89% identity among the samples in the study. All of the sequences from five felines (
Felis silvestris catus) of codes PB088, PB097, PB089, PB091 and PB100, one dog (
Canis lupus familiaris) of code PB103 and one swine of codes PB106 remained close together in the same clade with bootstrap values in the range of 70-100%. Interestingly, comparison of the identified RdRp gene sequences with other PBV showed that sequences were most closely related to picobirnaviruses detected previously in red fox samples from Spain [
16].
4. Discussion
Several pathogens, including enteric viruses, which affect humans and the most varied species of animals are important players of zoonotic infections in humans and also loss of productivity in life stock [
9].
Here, it was first tried to use the simple but accurate PAGE technique to screen the electrophoretical profile of infecting viruses in diarrheal infections as successfully done by others [
2]. In the present study, PAGE proved to exert a too low sensitivity of detection of PBV, probably due to limited sample quantity. For this reason, the RT-PCR based approach was employed, given its greater sensitivity and specificity [
5]. Additionally, fecal samples with low viral titers could be typed.
Clinical aspect of animals and fecal specimens were not considered, since detection may happen even though hosts, in many cases, remain asymptomatic [
2,
10]. In the present study, no specific effort was conducted to elucidate viral titers in samples. Previous studies carried out with fecal samples of broilers (
Gallus gallus) from the northeastern region of the state of Pará, reported PAGE-based PBV detection in 15.3% [
17] and 30% [
18] of samples, indicating that in comparison to fowl fezes, the herein examined samples indeed contain lower viral titers.
PBV were previously reported in several different hosts, being detected in 3.63% of cats from Portugal [
19], 14.28% in horses [
10], 11.15% in pigs [
20], and 0.73% in cattle in India [
21]. In Brazil, the positivity in swine was reported in 12.45% to 43.24% of animals [
22], 8.30% in cattle [
23], 1.84% in canines [
24], and 49.4% in broilers [
17]. Until now, no PBV had been detected in marsupials (
didelfideos family) and in wild birds. The present study corroborates the previously reported data, considering that the population with the highest frequency of PBVs was in swine showing 28.13% of positivity.
The counties of origin of samples in this study were strongly deforested during 2014 to 2016, due the use of the land for subsistent agricultural and livestock production [
25]. Clearly, the increase in the area of fragmentation, the alteration of the habitat and ecological niches of animals caused by anthropic pressure, and consequently the closer contact between humans and wild animals, contribute to the occurrence of infectious diseases, spread of emerging pathogens and new hosts [
26,
27]. This fact may have contributed to the wide interaction between the species, facilitated by the change in the environment where they live, and consequently higher viral spillover between species.
The dissemination of PBV among the most varied animal hosts in the present study was confirmed by phylogenetic analysis. The virus presence in canines, felines, cattle, poultry, swine, and in humans, shows a lack of host specificity, corroborating previous studies with PBV in animals [
6]. Phylogenetic analysis revealed that the virus circulated in several species of animals, regardless of time and geographic area of detection, as previously reported [
28], suggesting a strong potential of spreading that pathogen.
Rodents, birds and bats are among the wild animals in this study. Considering a fecal-oral infection route, and that the diet of marsupial (
Marmosa sp, family
Didelphidae) is composed of small vertebrates (little birds, chickens), fruit, decomposing organic matter (carrion) even invertebrates [
29], it is suggested that the feeding habits of these animals may have contributed to their infection by ingesting nutrients exposed in the contaminated environment.
Birds and chiropterans present great importance for public health. They are considered as potential reservoirs of pathogens that cause zoonotic diseases. Due to their ability to fly, they can act in the dispersion of viruses and thus establish new foci of emerging or re-emerging diseases along their paths. Chiropterans that participated in this study, except Desmodus rotundus species (blood-sucking habit), are frugivorous. Characteristics such as diet, ability to fly, seasonal migration, behavioral patterns, affinity to live in colonies, and other, include this group as a potential zoonotic host. Perhaps for their foraging habits, there was no PBV positivity for bats and birds in this study.
The strong anthropic pressure in Amazon has been an important factor of spreading zoonotic diseases, infecting new hosts, and expanding pathogens with wide-reaching potential through the interaction between species, including enteric pathogens such as PBV and RV [
11]. Pets (canines and felines) and livestock animals (cattle, horses, pigs and birds) sharing the same space and environment as humans, circulate in forest and home areas, and interact with each other and with humans turning the chances of viral transmission high, especially in cases of animals infected in a phase of viral excretion.
This study is the first to characterizing PBV (GI), in felines (Felis catus) in Brazil. These data suggest the possible interspecies transmission of PBV among the animals included in this study, considering that some animals were grouped with different taxa and some samples formed clusters with strains similar to strains isolated in porcine detected mainly in China.
5. Conclusion
The incidence of PBV G-I in this study occurred in different groups of sylvatic and domestic animals. Investigations to better characterize transmission, pathogenicity, and evolution, and increasing surveillance of Picobirnaviruses are essential to better understand their transmission to humans or other animals.
Author Contributions
Conceptualization, E.C., J.M, J.S., and Y.M; Methodology, E.C., J.J., D.B., B.B., and C.C.; Statics Analyze, H.P.; Phylogenetic Analyze, E.J., and F.S.; Writing - Original Draft, E.C.; Writing - Review and Editing, all authors.; Supervision, J.M. and Y.M.
Funding
Please add: “This research received no external funding” or “This research was funded by NAME OF FUNDER, grant number XXX” and “The APC was funded by XXX”. Check carefully that the details given are accurate and use the standard spelling of funding agency names at
https://search.crossref.org/funding. Any errors may affect your future funding.
Institutional Review Board Statement
The National Council for Animal Control and Experimentation (CONCEA), System of Authorization and Information in Biodiversity—SISBIO/ICMBIO/Ministry of the Environment approved this research under protocol No. 37174–1, and the Ethics Commission at the Evandro Chagas Institute (CEUA) under protocol number 35/2016.
Acknowledgments
This study was financially supported by the Brazilian National Council for Scientific and Technological Development (CNPq) process no. 137442/2016-5; 1666682/2017-9, and Evandro Chagas Institute, Ministry of Health, Ananindeua, Brazil. E.H.N.C, J.R.S. and J.W.B.D.J were recipients of CNPq fellowships; B.D.C.V.B. and C.M.O.C. were recipients of CAPES fellowships, and J.D.P.M. is recipients of CNPq fellowships. YSM acknowledges the help received from Education Division, Indian Council of Agricultural Research, New Delhi for National Fellowship.
Conflicts of Interest
The authors declare no conflict of interest regarding of this publication.
References
- Malik YS, Ghosh S. Etymologia: Picobirnavirus. Emerg Infect Dis. 2020;26. [CrossRef]
- Delmas B, Attoui H, Ghosh S, Malik YS, Mundt E, Vakharia VN. Ictv virus taxonomy profile: Picobirnaviridae. J Gen Virol. 2019;100: 133–134. [CrossRef]
- Perez LJ, Cloherty GA, Berg MG. Understanding the genetic diversity of picobirnavirus: A classification update based on phylogenetic and pairwise sequence comparison approaches. Viruses. 2021;13. [CrossRef]
- Li W, Qiang X, Qin S, Huang Y, Hu Y, Bai B, et al. Virome diversity analysis reveals novel enteroviruses and a human picobirnavirus in stool samples from African green monkeys with diarrhea. Infect Genet Evol. 2020;82: 104279. [CrossRef]
- Malik YS, Kumar N, Sharma K, Dhama K, Shabbir MZ, Ganesh B, et al. Epidemiology, Phylogeny, and Evolution of Emerging Enteric Picobirnaviruses of Animal Origin and Their Relationship to Human Strains. Luo M, editor. Biomed Res Int. 2014;2014: 780752. [CrossRef]
- Ghosh S, Malik YS. The True Host/s of Picobirnaviruses. Frontiers in Veterinary Science. Frontiers Media S.A.; 2021. [CrossRef]
- Krishnamurthy SR, Wang D. Extensive conservation of prokaryotic ribosomal binding sites in known and novel picobirnaviruses. Virology. 2018. [CrossRef]
- Neri U, Wolf YI, Roux S, Camargo AP, Lee BD, Kazlauskas D, et al. A Five-Fold Expansion of the Global RNA Virome Reveals Multiple New Clades of RNA Bacteriophages. SSRN Electron J. 2022. [CrossRef]
- Zanella JRC. Zoonoses emergentes e reemergentes e sua importância para saúde e produção animal. Pesqui Agropecuária Bras. 2016. [CrossRef]
- Ganesh B, Banyai K, Masachessi G, Mladenova Z, Nagashima S, Ghosh S, et al. Genogroup i picobirnavirus in diarrhoeic foals: Can the horse serve as a natural reservoir for human infection? Vet Res. 2011;42: 52. [CrossRef]
- Barros B de CV de, Chagas EN, Bezerra LW, Ribeiro LG, Duarte Júnior JWB, Pereira D, et al. Rotavirus A in wild and domestic animals from areas with environmental degradation in the Brazilian Amazon. Jin D-Y, editor. PLoS One. 2018;13: e0209005. [CrossRef]
- Boom R, Sol CJA, Salimans MMM, Jansen CL, Wertheim-Van Dillen PME, Van Der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990. 1990. [CrossRef]
- Pereira HG, Azeredo RS, Leite JP, Barth OM, Sutmoller F, de Farias V, et al. Comparison of polyacrylamide gel electrophoresis (PAGE), immuno-electron microscopy (IEM) and enzyme immunoassay (EIA) for the rapid diagnosis of rotavirus infection in children. Mem Inst Oswaldo Cruz. 1983. [CrossRef]
- Rosen BI, Fang ZY, Glass RI, Monroe SS. Cloning of human picobirnavirus genomic segments and development of an RT-PCR detection assay. Virology. 2000. [CrossRef]
- He Z, Zhang H, Gao S, Lercher MJ, Chen WH, Hu S. Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016. [CrossRef]
- Bodewes R, Ruiz-Gonzalez A, Schapendonk CME, Van Den Brand JMA, Osterhaus ADME, Smits SL. Viral metagenomic analysis of feces of wild small carnivores. Virol J. 2014;11: 1–13. [CrossRef]
- Ribeiro Silva R, Bezerra DAM, Kaiano JHL, Oliveira D de S, Silvestre RVD, Gabbay YB, et al. Genogroup I avian picobirnavirus detected in Brazilian broiler chickens: a molecular epidemiology study. J Gen Virol. 2014;95: 117–122. [CrossRef]
- Ribeiro AF, Silva RR da, Bezerra DAM, Bandeira R da S, Penha Junior ET da, Castro CMO de. Picobirnavirus genogroup 2 in broiler chickens of the metropolitan Belém mesoregion - PA – Brazil. Brazilian J Anim Environ Res. 2019;2: 1–8.
- Ng TFF, Mesquita JR, Nascimento MSJ, Kondov NO, Wong W, Reuter G, et al. Feline fecal virome reveals novel and prevalent enteric viruses. Vet Microbiol. 2014;171: 102–111. [CrossRef]
- Kylla H, Dutta TK, Roychoudhury P, Malik YS, Mandakini R, Subudhi PK. Prevalence and molecular characterization of porcine Picobirnavirus in piglets of North East Region of India. Trop Anim Health Prod. 2017;49: 417–422. [CrossRef]
- Malik YS, Chandrashekar KM, Sharma K, Haq AA, Vaid N, Chakravarti S, et al. Picobirnavirus detection in bovine and buffalo calves from foothills of Himalaya and Central India. Trop Anim Health Prod. 2011;43: 1475–1478. [CrossRef]
- Fregolente MCD, de Castro-Dias E, Martins SS, Spilki FR, Allegretti SM, Gatti MS V. Molecular characterization of picobirnaviruses from new hosts. Virus Res. 2009;143: 134–136. [CrossRef]
- Takiuchi E, Macedo R, Kunz AF, Gallego JC, Mello JL de, Otonel RAA, et al. Electrophoretic RNA genomic profiles of Brazilian Picobirnavirus (PBV) strains and molecular characterization of a PBV isolated from diarrheic calf. Virus Res. 2016. [CrossRef]
- Costa AP, Cubel Garcia RCN, Labarthe NV, Leite JPG. Detection of double-stranded RNA viruses in fecal samples of dogs with gastroenteritis in Rio de Janeiro, Brazil. Arq Bras Med Veterinária e Zootec. 2004;56: 554–557. [CrossRef]
- Assunção J, Lipscomb M, Mobarak AM, Szerman D. Agricultural productivity and deforestation in Brazil. Agric Environ Prot. 2016. Available: https://climatepolicyinitiative.org/wp-content/uploads/2017/06/Agricultural-Productivity-and-Deforestation-in-Brazil-CPI.
- Laurance WF, Vasconcelos HL. Consequências ecológicas da fragmentação florestal na amazônia. Oecologia Bras. 2009;13: 434–451. [CrossRef]
- Suzán G, Marcé E, Giermakowski JT, Armién B, Pascale J, Mills J, et al. The Effect of Habitat Fragmentation and Species Diversity Loss on Hantavirus Prevalence in Panama. Ann N Y Acad Sci. 2008;1149: 80–83. [CrossRef]
- Masachessi G, Ganesh B, Martinez LC, Giordano MO, Barril PA, Isa MB, et al. Maintenance of picobirnavirus (PBV) infection in an adult orangutan (Pongo pygmaeus) and genetic diversity of excreted viral strains during a three-year period. Infect Genet Evol. 2015. [CrossRef]
- Lessa LG, Geise L. Hábitos alimentares de masupiais didelfídeos brasileiros: análise do estado de conhecimento atual. Oecologia Aust. 2010;14: 901–910. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).