Submitted:
02 November 2023
Posted:
09 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Method
2.1. Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
References
- Centers for Disease Control and Prevention, Skin Cancer Statistics, U.S. Cancer StatisticsWorking Group. https://www.cdc.gov/ cancer/skin/statistics/.
- Sung, H, Ferlay, J, Siegel, RL, Laversanne M, Soerjomataram, I, Jemal, A, Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209-249. [CrossRef]
- Ferlay, J, Colombet, M, Soerjomataram, I, Parkin, D.M.; Piñeros, M, Znaor, A. et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021; Epub ahead of print.
- Bandarchi, B.; Ma, L.; Navab, R.; Seth, A Rasty, G. From Melanocyte to Metastatic Malignant Melanoma. Dermatology Research and Practice Volume 2010, Article ID 583748, 8 pages. [CrossRef]
- Stanga, A.; Hauschildc, A. Descriptive epidemiology of cutaneous melanoma. A treasure for generating hypotheses The Lancet Regional Health - Europe 2 (2021) 100040. [CrossRef]
- Forsea, A.M. Melanoma Epidemiology and Early Detection in Europe: Diversity and Disparities. Dermatol Pract Concept. 2020 Jun 29;10(3):e2020033. [CrossRef]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat,J.; Vaccarella, S; Meheus, F.; et al. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022 May; 158(5): 495–503. Published online 2022 Mar 30. [CrossRef]
- Australian Institute of Health and Welfare 2017. Cancer in Australia 2017. Cancer series no. 101. Cat. no. CAN 100. Canberra: AIHW.
- Lyth, J. Clinical-epidemiological studies on cutaneous malignant melanoma-A register approach. 2015. Linköping University Medical Dissertations No. 1428, Linköping 2015, Sweden.
- Leeneman, B.; Schreuder, K.; Carin, A. Uyl - de Groot , Alexander, C.J;. van Akkooi, D.; John, B.A.G.; Haanen, et al. Stage-specific trends in incidence and survival of cutaneous melanoma in the Netherlands (2003e2018): A nationwide population-based study. European Journal of Cancer 154 (2021) 111e119. [CrossRef]
- Howlader, N, Noone, A.M, Krapcho, M, Mille,r D, Brest, A, Yu, M et al. SEER Cancer Statistics Review, 1975-2018, National Cancer Institute. 2021 Apr 15 [cited 22 June 2021]. Bethesda, MD, based on November 2020 SEER data submission, posted to the SEER web sit. Available from: https://seer.cancer.gov/csr/1975_2018/.
- Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept 2017;7(2):1-6. [CrossRef]
- Grupta, K.A.; Bharadwaj, M.; Mehrotr R. Skin Cancer Concerns in People of Color: Risk Factors and Prevention. Asian Pac J Cancer Prev. 2016; 17(12): 5257–5264. [CrossRef]
- Leslie, K.D. Cumulative Sun Exposure and Melanoma in a Population-Based Case–Control Study: Does Sun Sensitivity Matter? Cancers 2022, 14, 1008. [CrossRef]
- Raimondi, S.; Suppa, M.; Gandini, S.. Melanoma Epidemiology and Sun Exposure. Epub ahead of print Apr 28, 2020. Acta Derm Venereol 2020; 100: adv00136.
- D’Orazio, J.; Jarret ,S.; Amaro-Ortiz, A.; Timothy, Scott. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222-12248; [CrossRef]
- Vogel, I.R.; Strayer, G.L.; Engelman, L.; Nelson, H.H.; Blaes, HA, Anderson, E.K. et al. Sun Exposure and Protection Behaviors among Long-term Melanoma Survivors and Population Controls. Cancer Epidemiol Biomarkers Prev. 2017 Apr; 26(4):. [CrossRef]
- Geller, L.M Pan. Update on indoor tanning legislation in the United States. ClinDermatol. 2015; 33(3):387-392. [CrossRef]
- Bowmanm, D.M.; Lewis, R.C.; Lee, M.S.; Yao, C.J. The Growing Public Health Challenges of Exposure to Ultraviolet Radiation From Use of Indoor Tanning Devices in the United States. New Solut. 2015 Aug; 25(2):164-71. [CrossRef]
- Thibault, C.; Sagar, P.; Nivatvongs, S.; Ilstrup, D.M.; Wolff, B.G. Anorectal melanoma-an incurable disease? Dis Colon Rectum. 1997; 40:661–668.
- Solaz Moreno, E.; Morales, V.; Silla Búrdalo, G.; Cervera Migue, J.I; Beveridge, D.; Rayón, J.M.; Primary melanoma of the rectum: an infrequent neoplasia with an atypical presentation. Clin Transl Oncol. 2005; 7:171–173.
- Morlino, A.; La Torre, G.; Vitagliano, G.; Cammarota, A. Malignant rectal melanoma. Case report. Ann Ital Chir. 2015; 86.
- Barbaric J, Sekerija M, Agius D, Coza D, Dimitrova N, Demetriou A, et al. Disparities in melanoma incidence and mortality in South-Eastern Europe: Increasing incidence and divergent mortality patterns. Is progress around the corner? Eur J Cancer. 2016 Mar;55:47-55. [CrossRef]
- Znaor, A.; van den Hurk, C.; Primic-Zakelj, M.; Agius, D.; Coz,a D.; Demetriou, A.; et al. Cancer incidence and mortality patterns in South Eastern Europe in the last decade: Gaps persist compared with the rest of Europe. European Journal of Cancer. 2013 May:49 (7):1683-169. [CrossRef]
- Institute for Public Health of Serbia Dr. Milan Jovanovic “Batut”: Malignant tumors in Republic of Serbia 2020; Belgrade, Serbia, 2022.
- Novaković, M.; Džodić, R.; Babović, N.; Kandolf Sekulović, L.; Brašanac, D.; Ferenc, V. National Guide: Melanoma-prevention, diagnostics and treatment. Belgrade, Serbia: Grafolik d.o.o., 2019.
- World Health Organization. International Classification of Diseases and Related Health Problems, 10th Revision. Available online: www.who.int/classifications/icd/en/.
- Segi, M. Cancer Mortality for Selected Sites in 24 Countries (1950–1957); Tohoku University School of Public Health: Sendai, Japan, 1960.
- Antonijevic, A.; Rancic, N.; Ilic, M.; Kocic, K.; Stevanovic, J.; Milic, M. Trends in incidence of non-melanoma and melanoma skin cancers in central Serbia. Srp Arh Celok Lek. 2018 Jul-Aug;146(7-8):391-395. [CrossRef]
- Memona, A.; Bannistera, P.; Rogersa, I.; Sundina, J.; Al-Ayadhyb, B.; Peter W. Jamesc,W.P. et al. Changing epidemiology and age-specific incidence of cutaneous malignant melanoma in England: An analysis of the national cancer registration data by age, gender and anatomical site, 1981-2018. The Lancet Regional Health - Europe 2 (2021) 100024. [CrossRef]
- Kandolf-Sekulovic, L.; Zivkovic-Perisic, S.; Radevic, T.; Rajović, M.;D inić, M.; Zolotarevski, L. et al. Melanoma in South-East Europe: epidemiological data from the central cancer registry and clinico pathological characteristics from the hospital-based registry in Serbia. Int J Dermatol. 2012;51(10):1186–1194. [CrossRef]
- Thrift, A.P.; Gudenkauf, F.J. Melanoma incidence among non-Hispanic whites in all 50 US states from 2001 through 2015. J Natl Cancer Inst 2019. [CrossRef]
- Paulson, K.G.; Gupta, D.; Kim, T.S. Age-Specific Incidence of Melanoma in the United States. JAMA Dermatol 2020;156(1):57-64. [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [CrossRef]
- de Winter, S.; Pavel, S. Zonnebanken: onduidelijk effect op huidkankerrisico [Tanning beds: effect on skin cancer risk unclear]. Ned Tijdschr Geneeskd. 2000 Mar 4;144(10):467-470.
- Bucchi, L.; Mancini, S.; Crocetti, E.; Dal Maso, L.; Baldacchini, F.; Vattiato, R.; et al. Mid-term trends and recent birth-cohort-dependent changes in incidence rates of cutaneous malignant melanoma in Italy. Int. J. Cancer. 2021;148:835–844. [CrossRef]
- Incidence of melanoma as defined by ICD-10 codes C43, D03, in the population aged under 55 years. FACT SHEET 4.2 z December 2009 z CODE: RPG4_UVrd_E1 www.euro.who.int/ENHIS.
- Lowe, C.G.; Saavedra, A.; Reed, B.K.; Velazquez, I.A.; Dronca, S.R. et al. Increasing Incidence of Melanoma among Middle-Aged Adults: An Epidemiologic Study in Olmsted County, Minnesota, Mayo Clin Proc. Author manuscript; available in PMC 2015 April 08. [CrossRef]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Ebell, M.; Epling, J.W. et al. Screening for skin cancer US preventive services task force recommendation statement. JAMA-J. Am. Med. Assoc. 2016, 22, 652–665. [CrossRef]
- Brunssen, A.; Waldmann, A.; Eisemann, N.; Katalinic, A. Impact of skin cancer screening and secondary prevention campaigns on skin cancer incidence and mortality: A systematic review. J. Am. Acad. Dermatol. 2017, 76, 129–139. [CrossRef]
- Hoorens, I.; Vossaert, K.; Pil, L.; Boone, B.; De Schepper, S.; Ongenae, K. et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016, 152, 27–34. [CrossRef]
- Weinstock, M.A.; Risica, P.M.; Martin, R.A.; Rakowski, W.; Smith, K.J.; Berwick, M. et al. Reliability of assessment and circumstances of performance of thorough skin self-examination for the early detection of melanoma in the Check-It-Out Project. Prev. Med. (Baltim) 2004, 38, 761–765. [CrossRef]
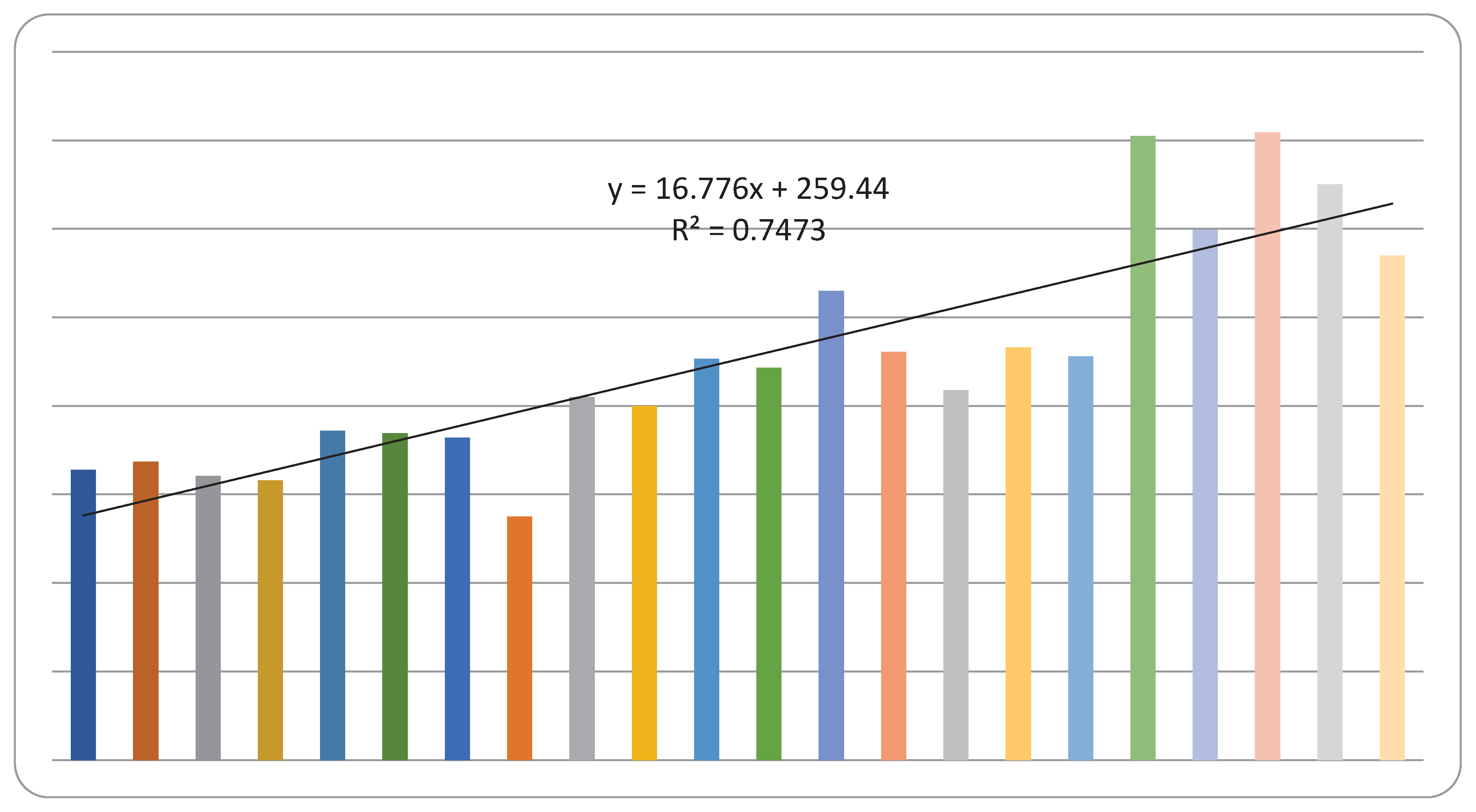
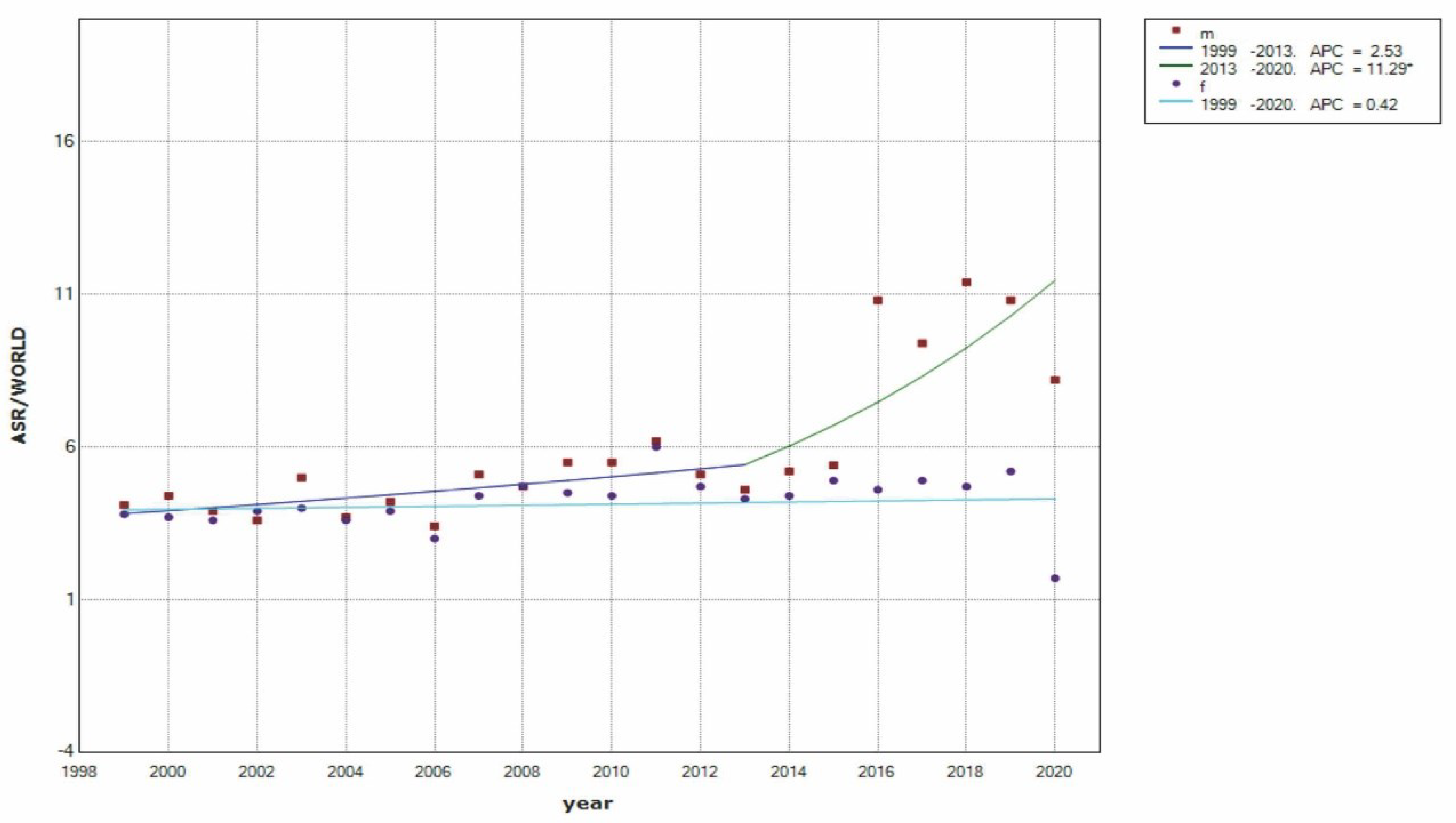
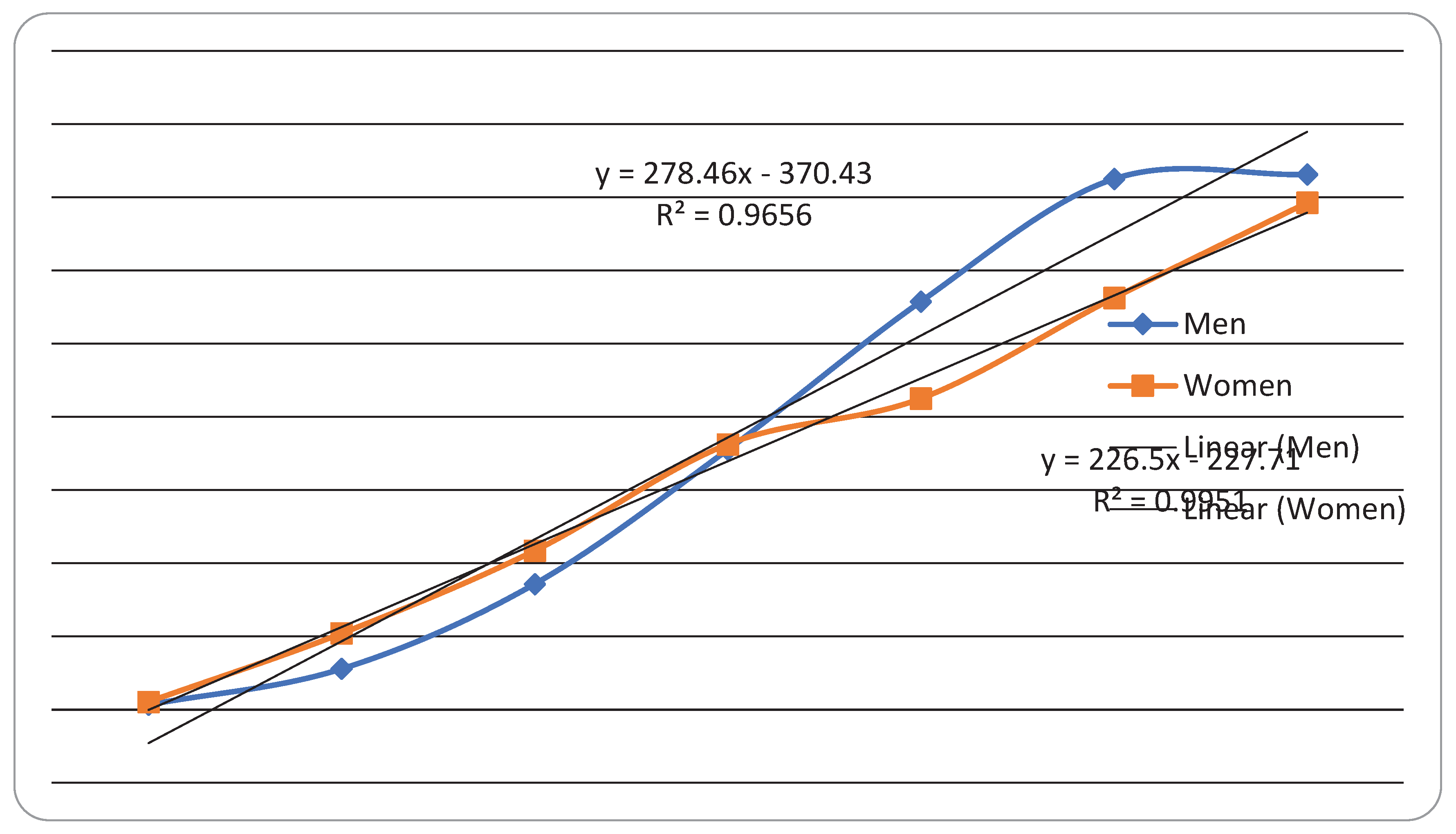
| Year | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| * No. of cases | Age-standardized incidence rate | No. of cases | Age-standardized incidence rate | No. of cases | ||
| 1999 | 154 | 4.1 | 174 | 3.8 | 328 | |
| 2000 | 175 | 4.4 | 162 | 3.7 | 337 | |
| 2001 | 161 | 3.9 | 160 | 3.6 | 321 | |
| 2002 | 152 | 3.6 | 164 | 3.9 | 316 | |
| 2003 | 197 | 5.0 | 175 | 4.0 | 372 | |
| 2004 | 215 | 3.7 | 154 | 3.6 | 369 | |
| 2005 | 185 | 4.2 | 179 | 3.9 | 364 | |
| 2006 | 145 | 3.4 | 130 | 3.0 | 275 | |
| 2007 | 214 | 5.1 | 196 | 4.4 | 410 | |
| 2008 | 197 | 4.7 | 203 | 4.7 | 400 | |
| 2009 | 241 | 5.5 | 212 | 4.5 | 453 | |
| 2010 | 233 | 5.5 | 210 | 4.4 | 443 | |
| 2011 | 266 | 6.2 | 264 | 6.0 | 530 | |
| 2012 | 241 | 5.1 | 220 | 4.7 | 461 | |
| 2013 | 206 | 4.6 | 212 | 4.3 | 418 | |
| 2014 | 239 | 5.2 | 227 | 4.4 | 466 | |
| 2015 | 231 | 5.4 | 225 | 4.9 | 456 | |
| 2016 | 372 | 10.8 | 333 | 9.2 | 705 | |
| 2017 | 322 | 9.4 | 277 | 7.7 | 599 | |
| 2018 | 389 | 11.4 | 320 | 8.9 | 709 | |
| 2019 | 364 | 10.8 | 286 | 8.0 | 650 | |
| 2020 | 305 | 8.2 | 265 | 6.8 | 570 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





