1. Introduction
According to the registries and mortality from the National Center for Health Statistics, in 2023, 153,020 individuals will be diagnosed with colorectal cancer (7.8 % of all new cancer cases), with 52,550 deaths (8.6% of all cancer deaths), which makes it the second most common cause of cancer mortality in the United States. The trends related to colorectal cancer morbidity and mortality show that although there was a decline in the annual incidence from 3%–4% during the 2000s to 1% during 2011-2019, the proportion of patients younger than 55 increased from 11% in 1995 to 20% in 2019. The proportion of rectal cancers within the colorectal group increased from 27% in 1995 to 31% in 2019 (1).
Over the past few years, organ-preserving treatment strategies, including nonoperative management (NOM) and additional local treatments, have emerged as viable and successful options (2–5), particularly since approximately 15–30% of patients with rectal cancer will achieve pathological complete response (pCR) (5–7). Organ preservation offers several potential advantages when compared to total mesorectal excision. It can help avoid surgical morbidity and mortality, including the need for a stoma, ultimately leading to a better quality of life and improved functional outcomes compared to surgery (4,8).
A multidisciplinary approach is used for evaluating treatment response following neoadjuvant therapy including digital rectal examination, endoscopy, and rectal MRI (9–13). These assessments are crucial not only for preoperative planning but are also pivotal factors in determining eligibility for NOM surveillance and organ-preserving strategies.
Rectal MRI has demonstrated superior accuracy in reassessing tumor response post-neoadjuvant treatment (12). Furthermore, it is a standardized approach for evaluating the rectal wall, mesorectum, sphincter complex, lymph nodes, and adjacent bones. This review aims to provide an update on total neoadjuvant treatment, outline a systematic approach to post-treatment MRI using the new terminology, discuss common pitfalls, and incorporate recent research on post-treatment assessment.
2. Overview of Neoadjuvant Therapy
Neoadjuvant therapy refers to any treatment administered before the standard definitive surgical resection (total mesorectal excision “TME”) and is usually indicated in locally advanced rectal cancer (LARC) (2,3). While the NCCN guidelines recommend it to any patient with T3-, T4-, T1-2 with N+, or locally unresectable or inoperable due to coexisting medical conditions (3), ESMO considers MRI features to stratify patients in different subgroups (2). Remarkably, it allows patients who achieve complete response to enter the protocol “watch-and-wait” (WW), also known as NOM, i.e., close surveillance, to avoid surgical complications (4). Extensive research has been done to propose different approaches to improve patient care. In the following paragraphs, we provide an insight into the strategies.
The traditional approach is the administration of capecitabine or infusional F-FU with long-course radiotherapy (RT) or a short-course of RT (3). While the long-course RT delivers 45–50 Gy over 25–30 fractions, the short-course provides 25 Gy over five fractions (commonly called 5x5) in just one week (3). It has been shown to lead to a reduced recurrence rate but is associated with increased local side effects, mainly due to RT (5–8).
Total neoadjuvant therapy (TNT) is an approach that delivers both systemic Chemotherapy (CT) and CRT before surgery, consisting of induction CT followed by CRT or consolidation CT administered after CRT (3,9). The goals are to decrease the rate of distant metastasis rate and increase the rates of clinical complete response, up to 40% in a recent meta-analysis (10). Some of the relevant trials on TNT include:
The Organ Preservation of Rectal Adenocarcinoma (OPRA) compared patients in INCT-CRT (Induction Chemotherapy with Chemoradiotherapy) and CRT-CNCT (Chemoradiotherapy with Consolidation Chemotherapy) and, remarkably, approximately 75% of both groups underwent the WW protocol. Both groups showed similar 3-year disease-free-survival (DFS) (76% and 76%, Log-rank P=.98), local recurrence-free survival (94% and 94%, Log-rank P=.78), distant metastasis-free survival (84% and 82%, log-rank P=.67), and similar local tumor regrowth for WW protocol (40% and 27%, Log-rank P=.03). Outstandingly, 60% (95%, 52 to 68) of CRT-CNCT versus 47% (95% CI, 39 to 56) of INCT-CRT preserved the rectum, which would justify initially providing induction CT with chemo-radiotherapy in cases where non-operative management is preferred (9).
Rectal cancer And Pre-operative Induction Therapy Followed by Dedicated Operation (RAPIDO) trial (11) showed decreased distant metastasis at 3 years of follow-up, reflected by the rate of disease-related treatment failure in patients treated with “short-course RT, CT, and TME” (approximately 24%) versus “chemo-radiotherapy long-course, TME, and adjuvant CT” (about 30%) (11). It also showed comparable rates of locoregional failure. Nevertheless, while the recently published 5-year follow-up of the trial showed similar results for distant metastasis, the rate of locoregional failure, reflected by the rate of locoregional recurrence, was higher for the "short-course RT, TNT, and TME" (approximately 12%) versus "TNT, TME, and optional adjuvant CT" (approximately 8%). These results highlight the necessity of refining this approach further (12).
The Unicancer Gastrointestinal Group and Pertenariat de Recherche en Oncologie Digestive (PRODIGE 23) group trial compared one group who received standard CRT, TME, and adjuvant FOLFOX (“standard-of-care”) and another who received neoadjuvant FOLFIRINOX therapy (“TNT”), CRT (radiotherapy and fluorouracil), TME, and adjuvant FOLFOX or capecitabine. The TNT group showed increased 3-year disease-free survival (76% vs 69%, HR 0.69, 95% CI 0.49-0.97) and increased 3-year rate of metastasis-free survival (79% vs. 72%, HR 0.64, 95% CI 0.44 – 0.93; p=0.017), and pCR rate (12% vs. 28%, P < 0.001) (13). The 7-year follow-up presented in the last ASCO meeting showed that the TNT group had an absolute increase of 7.6% for DFS, 6.9% for OS, 9.9% for Metastasis-Free Survival, and 5.7% for Cancer-Specific Survival as well as decreased locoregional relapse (5.3% vs. 8.1%, p=.38) (14).
Recently published Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients with Locally Advanced Rectal Cancer Undergoing Surgery (PROSPECT) trial aimed to evaluate the outcomes of those who only received neoadjuvant CT without RT only in patients with T2 node-positive, T3 node-negative, or T3 node-positive and candidates for sphincter-sparing surgery. They found that pre-operative FOLFOX was non-inferior to the patients who received the standard CRT with regards to DFS (HR for DSF, 0.92; 90.2% CI, 0.74 to 1.14; P = 0.005 for noninferiority) (15).
Finally, a novel approach with immunotherapy has been proposed for a subgroup of patients with LARC and deficiency in mismatch repair. As an example, Cercek et al. (16) conducted a prospective phase-2 study including 12 patients who completed treatment with dostarlimab (anti-PD-1 monoclonal antibody), and all of them had a complete clinical response and underwent WW at the time of the publication (16). Other recent published trials supported the results (17,18).
A consensus has been formulated to standardize the categorization of patients based on their treatment response (19). The core objective is to offer clinicians a well-defined framework for assessing and conveying the treatment's efficacy. This consensus categorizes patients into three specific groups based on the findings from Digital Rectal Examination (DRE), endoscopy, and MRI. The patients are classified into three groups, as follows:
Clinical Complete Response includes normal findings on DRE, no residual tumor with or without a flat scar, telangiectasia on endoscopy, and unremarkable rectal wall with or without fibrosis and no adenopathy on MRI.
Near Complete Response is associated with smooth indurations and/or minimal mucosal abnormalities on DRE, irregular or smooth mucosa irregularities, superficial ulcer, persistent erythema on endoscopy, and apparent decrease in size with predominant fibrosis and without or with borderline lymph nodes on MRI.
Incomplete Response refers to a palpable tumor on DRE, a visible tumor on endoscopy on MRI with or without a nodal regression.
3. Restaging MRI Protocol
Spasmolytic agents are usually part of the motion-mitigation strategy, but their use is not mandatory. The survey led by Gollub et al. found that 47% of academic institutions use spasmolytics (glucagon, 1 mg IV/IM/SC or hyoscyamine butyl bromide 20 mg IV) for rectal cancer MRI in the baseline or neoadjuvant setting (20). Spasmolytic agents reduce peristalsis-causing artifacts, especially on the Diffusion-weighted imaging (DWI) sequence of the upper rectum. Fasting for 2 hours before scanning decreases small bowel peristalsis (21).
Baseline MR protocol does not require a micro enema. However, it becomes crucial for MR preparation after treatment, to minimize gas-related artifacts, consequently enhancing the quality of DWI. It is recommended to apply it 15 minutes before the scan (22,23).
Numerous guidelines suggest a minimum field strength of 1.5 Tesla (T) for MRI in rectal cancer cases. The debate regarding the choice between 1.5 T and 3.0 T for rectal cancer imaging lacks a consensus. While 3T MRI offers advantages of faster image acquisition and improved spatial resolution with a higher signal-to-noise ratio, it comes at expense of higher susceptibility artifacts which might particularly impact DWI (21,24).
The patient should be positioned in a supine posture. Instead of endorectal coils, which can lead to discomfort and rectal distention while potentially affecting the measured distance between the tumor and circumferential resection margin (25), pelvic phased-array surface coils are recommended. These surface coils should be positioned with their lower edge just below the pubic bone or, for cases involving low rectal tumors, placed at least 10 cm below the symphysis pubis. The upper edge of the coil should align with the sacral promontory (21).
Large field-of-view (FOV) T2WI is essential for evaluating tumor extension beyond the mesorectal compartment, especially when assessing nodes outside the TME zone. These images should encompass the region from the origin of the superior mesenteric artery to the inguinal regions (21,26).
High-resolution T2WI should have a FOV of 16-20 cm, slice thickness of 2-3 mm, in-plane resolution less than 1 × 1 mm, with no gap between slices, and a TE (echo time) of 80–110 ms, depending on the field strength. High-resolution small FOV T2WI is crucial for assessing delicate structures that demand maximum contrast resolution, such as the rectal wall, mesorectal fat, mesorectal lymph nodes, and peritoneal reflection. This imaging is vital not only for T and N staging but also for detailed scrutiny of extramural vascular invasion (EMVI), differentiation between mesorectal tumor deposits and lymph nodes, and evaluating peritoneal reflection involvement (27,28). The imaging protocol includes high-resolution T2WI in the oblique axial, oblique coronal, and true sagittal planes relative to the tumor's location. This approach is critical to prevent volume averaging and to avoid misinterpretation of tumor extension (29). In cases of extensive tumors, acquiring oblique T2W axial and coronal images at different angles is sometimes necessary to assess orientation and extension at various levels. Oblique small FOV T2WI aligned with the anal canal assist in local staging of low rectal tumors and their relationship to the sphincter complex.
DWI is a functional imaging that relies on rate differences of random motion of the water molecules within the tissue of interest. A higher signal indicates the restricted ability of water molecules to diffuse within the microenvironment. Restricted diffusion applies to abscesses, hypercellular tumors, and benign and malignant lymphatics (lymph nodes, spleen). DWI adds to the accuracy of conventional morphological sequences, especially in the posttreatment setting. Even though DWI cannot distinguish benign and malignant lymph nodes, it is a helpful tool in detecting and differentiating them between common benign mimickers like varicose veins, phleboliths, among others (30). The use of multiple b values (50 s/mm2, 400 s/mm2, and 800 s/mm2) in a single acquisition not only saves time but also enhances diagnostic capabilities (21).
Small FOV DWI is essential in evaluating residual tumors to determine a clinical complete response (cCR) and further selection for nonoperative management (31). It is important to emphasize that DWI can detect recurrent tumors before they become apparent on endoscopy (32). Small FOV DWI provides better subjective image quality and higher accuracy for posttreatment reevaluation (33,34). Most protocols use b values ≥ 1000 s/mm2 to minimize false T2 shine through the effect of submucosal and luminal edema. Few studies dealt with the accuracy between the high b values, resulting in more conspicuity among b values of 2000s/mm2 compared to b = 1000 s/mm2 (35). Luminal gas is the main cause of susceptibility, potentially obscuring actual lesions or creating pseudo lesions (21).
Even though the reliability of the postcontrast sequences in the posttreatment setting is not as accurate, for example, because of the enhancement of the posttreatment and inflammatory changes, both the Society of Abdominal Radiology and the European Society of Gastrointestinal and Abdominal Radiology suggest that intravenous contrast may be considered as an optional tool in specific scenarios, for example in situations where image quality is compromised by artifacts. (20,36).
4. Rectal MRI Response Assessment
4.1. Why Assessment Matters
Conducting an MRI after neoadjuvant chemoradiotherapy is crucial for assessing cancer's response to treatment, detecting new disease sites, reevaluating the extent of the disease, and planning both surgical and NOM.
4.2. When to Evaluate
The issue of determining the optimal timing for the initial assessment of tumor response remains a point of contention within the field. Research suggests that the rate of achieving pCR may rise significantly after 12 weeks following radiotherapy (37). Nonetheless, some surgical groups express reservations about performing operations beyond the 8-week. Their concerns are rooted in fears of radiation-induced pelvic fibrosis and associated surgical complications (38). These apprehensions underscore the importance of early identification of poor or incomplete responders.
Efforts to shift the decision point from the conventional 6- to 8-week post-radiotherapy period to a later window of 10 to 12 weeks may not necessarily adversely impact surgery-related morbidity or mortality (39). Moreover, extending the surveillance window might prove advantageous since prolonged intervals may be useful to enhance response rates (40). It also offers the opportunity to initiate consolidation chemotherapy for metastatic high-risk patients, a decision that could potentially benefit from TNT (39). This strategic approach emphasizes the need for a more comprehensive understanding of when to assess tumor response and its potential implications for patient management.
4.3. Pre-Assessment Preparations
Before presenting findings from the restaging MRI of the rectum, it is crucial to delve into the patient's clinical history, including the results of digital rectal examinations and any conducted endoscopic procedures. It is equally important to consider the type of treatment administered, whether it is CRT or TNT, along with the time elapsed since the completion of the last treatment session (41). When the availability of prior clinical records is limited or absent, drawing substantiated conclusions becomes an intricate challenge.
Moreover, conducting an assessment of the baseline MRI scan, when available, assumes great significance as a preparatory step before embarking on the interpretation of the restaging rectal MRI. This initial examination serves to illuminate critical facets, encompassing tumor localization, characteristics, and the potential presence of mucinous components. Notably, post-neoadjuvant therapy, it's essential to recognize that the normal rectal wall near or opposite the treated tumor may experience edematous thickening, which could be mistakenly identified as residual tumor, often termed "pseudotumor," by some observers (42). Thus, establishing a correlation with the baseline rectal MRI stands as a highly beneficial practice, aiding in the precise determination of the tumor bed's location (43). Scar tissue should follow the same distribution and shape as the primary tumor (44). This integrated approach enhances the accuracy of interpretation and minimizes the potential for misinterpretations.
4.4. How to Evaluate the Tumor Response
In managing locally advanced rectal cancer, radiologists are pivotal in assessing treatment responses, specifically during the "Tumor Assessment" phase. Successful response to treatment in rectal tumors typically results in morphologic changes, including size reduction and fibrotic transformation.
Size Reduction-Based Assessment
Although literature suggests that a 60-80% reduction in tumor volume correlates with a positive treatment response (45), RECIST criteria are not commonly used in rectal cancer due to challenges in measuring irregularly shaped tumors.
Updates On Treatment Assessment
A new terminology has been introduced in 2023 by the Society of Abdominal Radiology Colorectal and Anal Cancer Disease-Focused Pane (SAR DFP) (54) to facilitate precise and comprehensible morphologic response characterization. It recommends treatment response at restaging to be categorized into complete response (CR), near complete response (nCR) or incomplete response (iCR) (
Figure 1). In this section, we delve deeply into the assessment process, unraveling the intricacies of T2 and DWI changes while elucidating each type of response.
a)
CR signifies the remarkable disappearance of T2 intermediate signal, indicating a significant reduction in tumor size and suggesting a highly favorable response to treatment. T2WI and DWI changes are described below and exemplified on
Figure 2:
T2-Weighted Imaging – On T2WI, complete response can be represented as a linear or crescent-shaped scar within the mucosal/ submucosal layers, or even the normalization of the rectal wall. It is known that rectal wall normalization can be seen in 5% of cases and is suggestive of complete response (55).
Diffusion-Weighted Imaging – Complete response on DWI is characterized by the absence of high signal intensity on high b-value DWI images (56-59). It is essential to compare with the baseline and use the normal rectum as references. This criterion can be especially valuable in identifying complete response in small, subcircumferential scars (60).
b)
nCR serves as a transitional state between CR and other responses, with substantial regression evident. The concept of a "near-complete response" has emerged more recently, driven by the observation that a significant proportion of patients initially displaying a very good yet incomplete response during the first assessment may ultimately achieve a complete clinical response when provided with a longer interval before re-assessment (26) (
Figure 3). However, it retains a trace of diffusion restriction post-neoadjuvant therapy, underscoring ongoing positive changes. In cases where tumor signal or diffusion restriction persists after one or two short-term follow-up evaluations, the case should be reclassified as iCR and considered unsuitable for observation.
c)
iCR characterizes a scenario where tumor volume experiences a reduction, but discernible residual tumor persists. This response type manifests through persistent diffusion restriction and the persistence of T2 intermediate signal within the tumor bed (
Figure 4).
It is important to note that the new terminology grouped CR and nCR patients together, as they can both benefit from vigilant monitoring, which may lead to improved treatment outcomes (49,61). The majority of cases classified as CR and nCR (ranging from 73% to 99%) typically transition to CR during short-term follow-up evaluations conducted within 6 to 12 weeks after neoadjuvant treatment (62).
Conversely, patients exhibiting iCR or no response on MRI are not ideal candidates for watch-and-wait management. These indicators are linked with suboptimal responses, potentially necessitating alternative therapeutic strategies.
Figure 1.
MRI-based morphologic response categories in rectal cancer post-treatment. This figure delineates the newly introduced terminology by the Society of Abdominal Radiology Colorectal and Anal Cancer Disease-Focused Panel (SAR DFP) for classifying morphologic responses in colorectal and anal cancers using MRI. The visual guide showcases:.
Figure 1.
MRI-based morphologic response categories in rectal cancer post-treatment. This figure delineates the newly introduced terminology by the Society of Abdominal Radiology Colorectal and Anal Cancer Disease-Focused Panel (SAR DFP) for classifying morphologic responses in colorectal and anal cancers using MRI. The visual guide showcases:.
- -
Complete Response: Characterized by the remarkable vanishing of intermediate signal intensity on T2WI, suggestive of substantial tumor size reduction, often seen as a scar in the mucosal or submucosal layers, or a normalized rectal wall structure.
- -
Near Complete Response: Indicative of significant tumor regression, nCR is a state that may evolve into CR with time. This stage may exhibit minimal but identifiable diffusion restriction
- -
Incomplete Response: Marked by a decrease in tumor volume with residual detectable tumor on imaging, signifying persistent diffusion restriction and intermediate T2 signal within the tumor bed, necessitating further treatment consideration.
Each category is supported by corresponding MRI scans displaying T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI) features, along with Apparent Diffusion Coefficient (ADC) map findings to aid in comprehensive evaluation and accurate classification of treatment response.
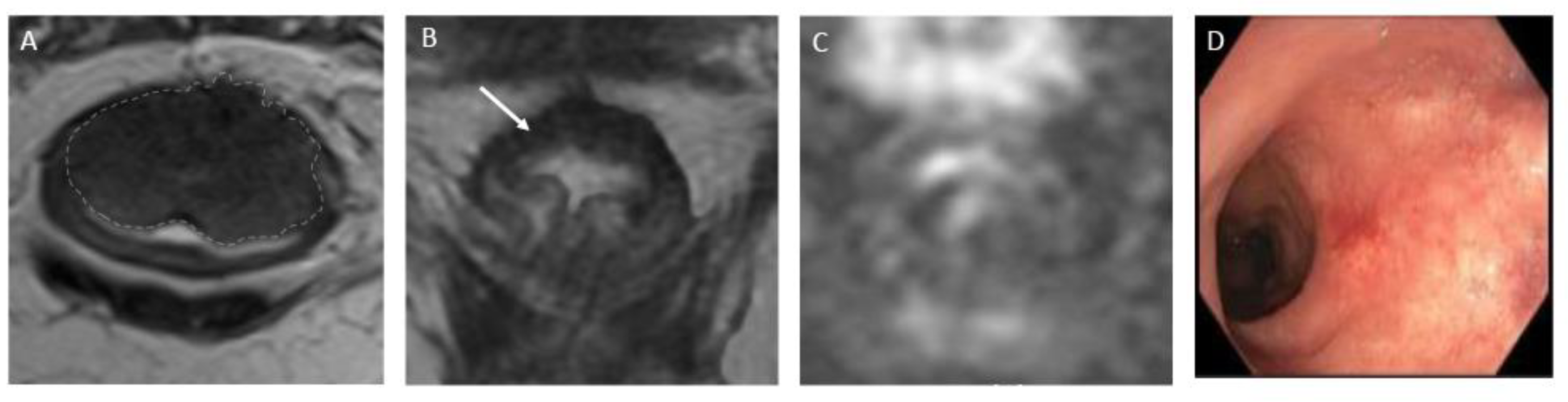
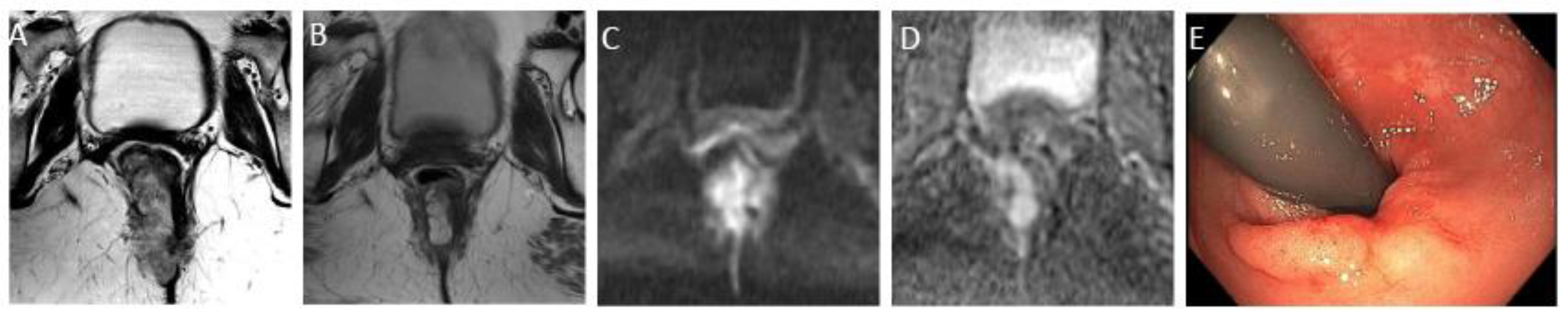
Figure 2.
Complete response after neoadjuvant chemotherapy in a 54-year-old man with low rectal adenocarcinoma. (A) Baseline axial T2-weighted MR image shows a low rectal tumor (dotted line). (B) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy shows a thick hypointense scar at the site of the treated tumor (arrow). No diffusion restriction was present on diffusion-weighted images (C), and no residual malignancy was identified at endoscopy (D). The patient was offered a watch-and-wait strategy and has been without evidence of disease for 36 months.
Figure 2.
Complete response after neoadjuvant chemotherapy in a 54-year-old man with low rectal adenocarcinoma. (A) Baseline axial T2-weighted MR image shows a low rectal tumor (dotted line). (B) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy shows a thick hypointense scar at the site of the treated tumor (arrow). No diffusion restriction was present on diffusion-weighted images (C), and no residual malignancy was identified at endoscopy (D). The patient was offered a watch-and-wait strategy and has been without evidence of disease for 36 months.
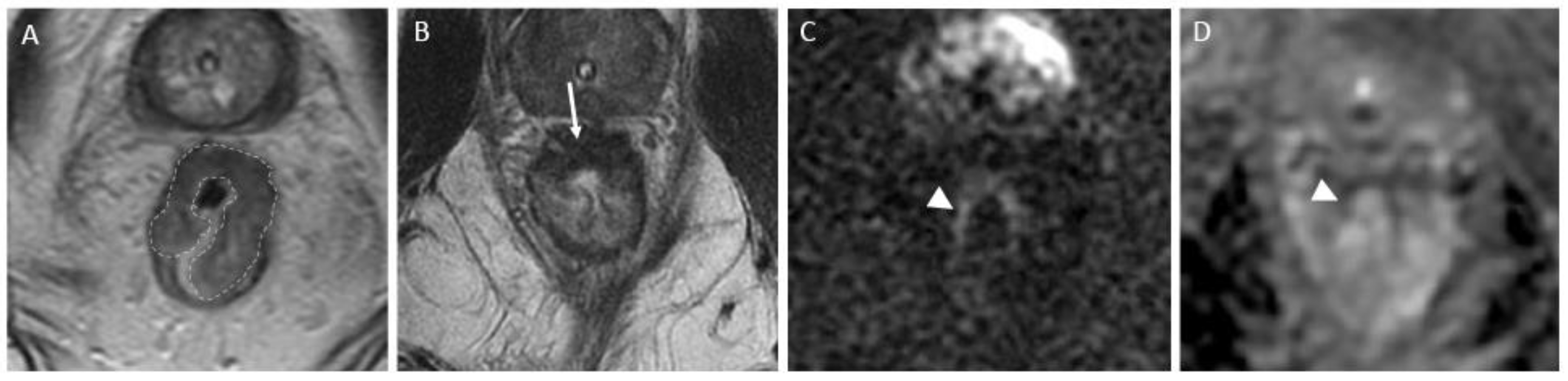
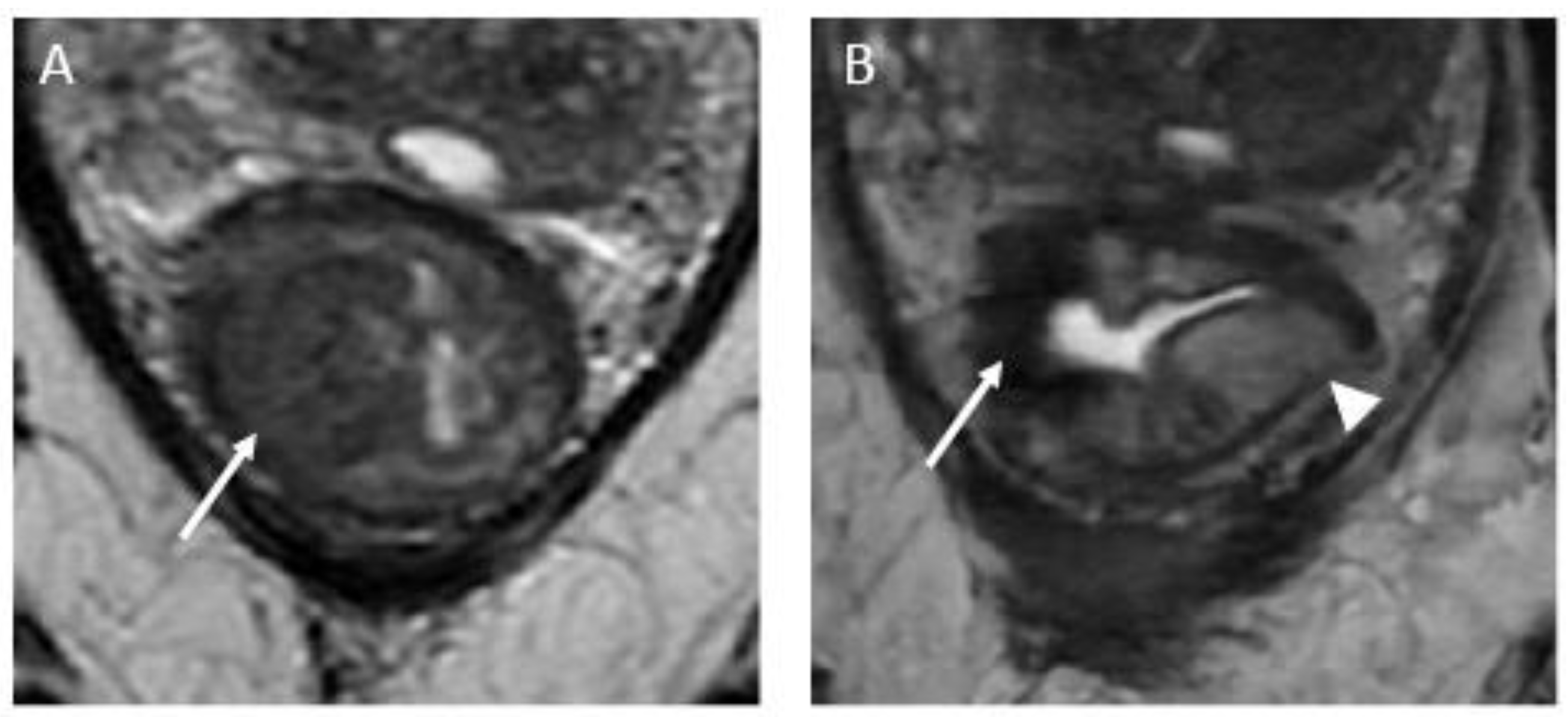
Figure 3.
Near complete response in a 65-year-old man with middle rectal adenocarcinoma. (A) Baseline axial T2-weighted MR image shows an intermediate-signal-intensity, near-circumferential low rectal tumor (dotted line). (B) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy shows a slight response with a small amount of fibrosis and residual tumor signal intensity (arrow). Axial diffusion-weighted image (C) shows high signal intensity (arrowhead), and axial ADC map (D) shows corresponding low signal intensity (arrowhead), in keeping with restricted diffusion at the nonfibrotic portion of the tumor.
Figure 3.
Near complete response in a 65-year-old man with middle rectal adenocarcinoma. (A) Baseline axial T2-weighted MR image shows an intermediate-signal-intensity, near-circumferential low rectal tumor (dotted line). (B) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy shows a slight response with a small amount of fibrosis and residual tumor signal intensity (arrow). Axial diffusion-weighted image (C) shows high signal intensity (arrowhead), and axial ADC map (D) shows corresponding low signal intensity (arrowhead), in keeping with restricted diffusion at the nonfibrotic portion of the tumor.
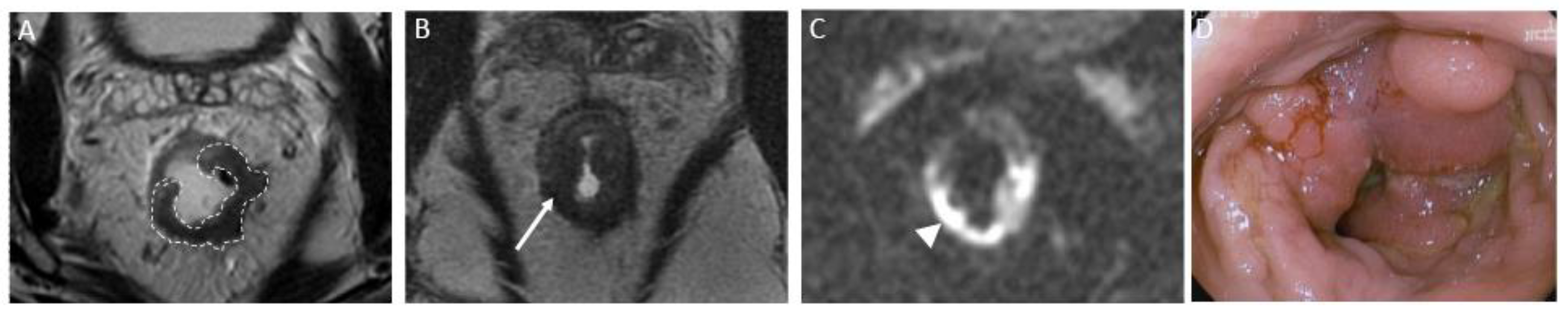
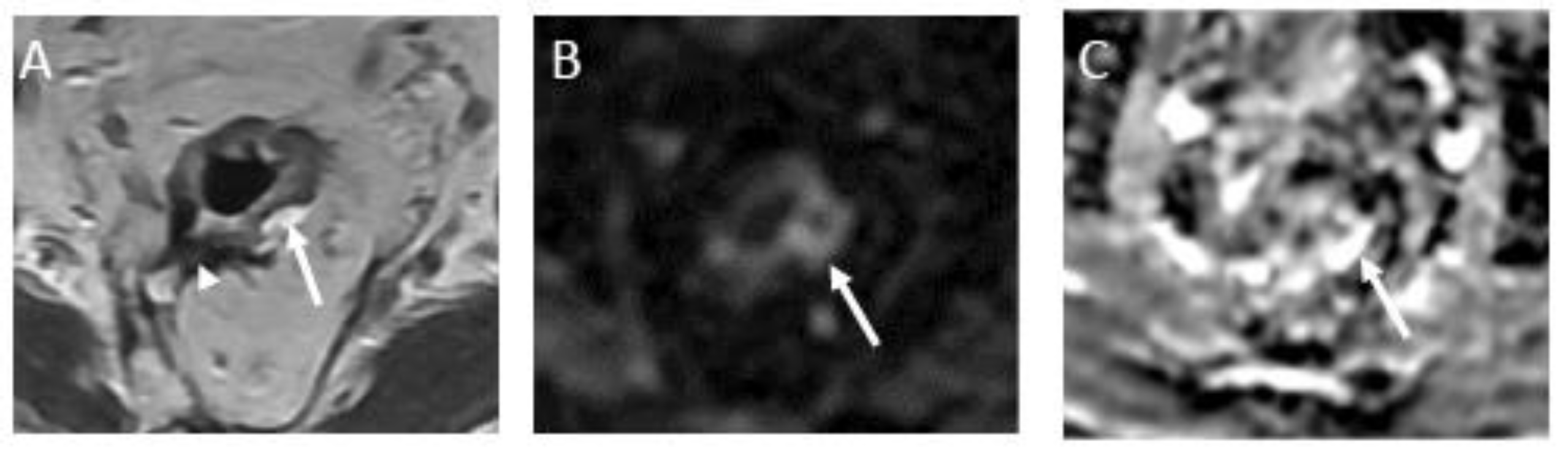
Figure 4.
Incomplete response in a 60-year-old man with low/middle rectal adenocarcinoma. (A) Baseline axial T2-weighted MR image shows an intermediate-signal-intensity, near-circumferential low rectal tumor (dotted line). (B) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy persistent intermediate signal intensity (arrow), representing incomplete response and residual tumor. (C) Axial diffusion-weighted image shows high signal intensity (arrow). (D) The residual malignancy was identified at endoscopy. The patient underwent total mesorectal excision.
Figure 4.
Incomplete response in a 60-year-old man with low/middle rectal adenocarcinoma. (A) Baseline axial T2-weighted MR image shows an intermediate-signal-intensity, near-circumferential low rectal tumor (dotted line). (B) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy persistent intermediate signal intensity (arrow), representing incomplete response and residual tumor. (C) Axial diffusion-weighted image shows high signal intensity (arrow). (D) The residual malignancy was identified at endoscopy. The patient underwent total mesorectal excision.
Mucinous Rectal Cancer
The assessment of mucinous rectal cancer post neoadjuvant therapy poses unique imaging challenges. There have been significant advances in the treatment of such patients that have greatly benefited the overall survival, particularly in the area of neoadjuvant immunotherapy (63). A decrease in tumor bulk is readily identifiable, but many of these cancers respond to therapy leaving residual areas of mucin. The limitation with MRI is its inability to distinguish between cellular and acellular mucinous components, which is a crucial distinction, particularly when considering a 'watch and wait' approach (64,65). This is particularly relevant in situations where a watch and wait scenario is considered.
Multiple MRI tumor regression grading systems (mrTRG) have been proposed for this purpose. One such grading system was developed by Park et al. (66) in 2017. This consists of an MRI staging of TRG 1-5 attempting to emulate the pathologic Mandard TRG stage. The common theme of the many MRI staging systems is that the likelihood of residual viable tumor cells is inversely proportional to the amount of intermediate T2 weighted soft tissue and/or fibrosis remaining.
Nevertheless, interpreting DWI remains complex due to the naturally high T2-weighted signal of mucin (as illustrated in
Figure 5). This is compounded when considering mucin pools distant from the primary tumor site and lymph nodes containing mucin, which further complicate the post-therapy diagnostic process
Pitfalls
There are a few commonly encountered pitfalls which the radiologist needs to be aware of when evaluating rectal tumor response on MRI. This includes the “T2 ‘shine-through’ effect which refers to the presence of high signal on diffusion weighted images without correlate low signal on ADC map, it will instead be high signal in ADC map since it related to absence of diffusion restriction. This phenomenon is usually a result of small amount of fluid content in the rectum which if a reader interprets based on DWI sequence alone will have it mistaken for incomplete response. Therefore, any focus of restricted diffusion seen at the tumor bed on DWI, should be correlated with low signal change on ADC map (
Figure 6). Additionally, studies have reported that the configuration of the signal on DWI should be considered. Since trace fluid will have a more trident appearance compared to mass-like shape resembling the tumor morphology of true diffusion restriction in for residual tumor. It is important to note that mucinous tumors commonly show T2-shine through due to high mucinous content.
On the other hand, “T2-dark through” is when extensive post treatment fibrosis results in low signal on ADC map. This is part results from the high collagen content of fibrosis. To avoid this, the reader should correlate the low signal seen on ADC map with that on DWI and T2-WI, if the signal is low on all three sequences and correlates to site of fibrosis low signal T2 WI signal, then it should be interpreted as fibrosis (
Figure 7) (64).
As previously mentioned, post-treatment changes include submucosal edema at the tumor bed particularly of the rectal wall opposite the treated mass. These changes will result in wall thickening with high to intermediate signal on T2WI, which can be mistaken as rectal mass, and commonly referred to as pseudotumor (
Figure 8). To avoid this, it is crucial to evaluate the baseline (pre-treatment) rectal MRI when available (67).
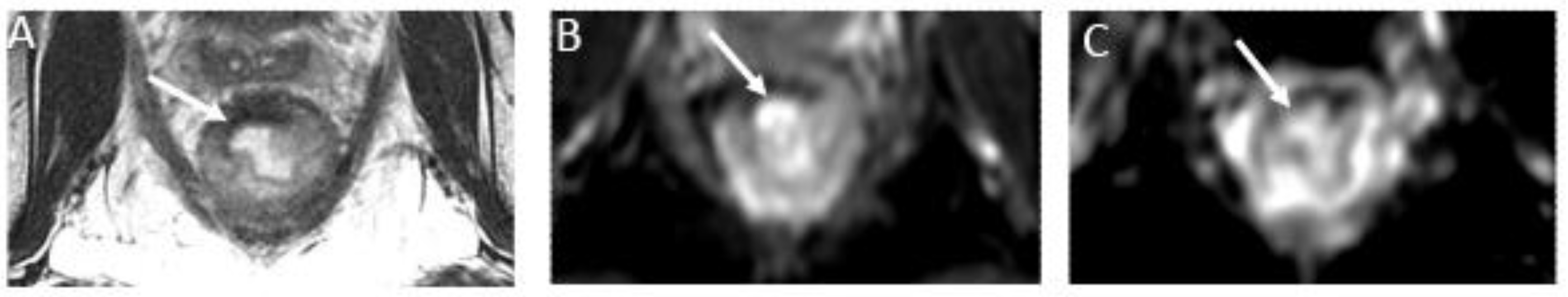
Figure 7.
T2 dark-through effect and rectal contents causing restricted diffusion and mimicking residual tumor in a 54-year-old man with rectal adenocarcinoma. (A) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy shows a thin hypointense scar at the tumor bed (arrow). (B) Axial diffusion-weighted image shows low signal intensity within tumor the tumor bed (arrow). (C) Corresponding axial ADC map shows low signal intensity at this site (arrow). T2 dark-through shows the rectal wall scar with low signal intensity on T2 imaging, DWI, and ADC due to fibrosis. Viable tumor should be considered only in cases with high signal intensity on DWI and low signal intensity on ADC map within the tumor bed. Comparison with baseline scans may also be of value.
Figure 7.
T2 dark-through effect and rectal contents causing restricted diffusion and mimicking residual tumor in a 54-year-old man with rectal adenocarcinoma. (A) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy shows a thin hypointense scar at the tumor bed (arrow). (B) Axial diffusion-weighted image shows low signal intensity within tumor the tumor bed (arrow). (C) Corresponding axial ADC map shows low signal intensity at this site (arrow). T2 dark-through shows the rectal wall scar with low signal intensity on T2 imaging, DWI, and ADC due to fibrosis. Viable tumor should be considered only in cases with high signal intensity on DWI and low signal intensity on ADC map within the tumor bed. Comparison with baseline scans may also be of value.
Figure 8.
Submucosal edema mimicking residual tumor in the uninvolved rectal wall in a 47-year-old man with rectal adenocarcinoma. (A) Baseline axial T2-weighted MR image shows a semicircumferential rectal tumor extending between the 7-o’clock and 12-o’clock positions (arrow). (B) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy shows a complete response appearance, characterized by a crescent-shaped scar within the mucosal/ submucosal layers (arrow). Note a high signal intensity at the uninvolved rectal wall (arrowhead), representing submucosal edema. Response assessment should not be based on the appearance of the uninvolved parts of the rectum.
Figure 8.
Submucosal edema mimicking residual tumor in the uninvolved rectal wall in a 47-year-old man with rectal adenocarcinoma. (A) Baseline axial T2-weighted MR image shows a semicircumferential rectal tumor extending between the 7-o’clock and 12-o’clock positions (arrow). (B) Axial T2-weighted MR image after completion of neoadjuvant chemoradiotherapy shows a complete response appearance, characterized by a crescent-shaped scar within the mucosal/ submucosal layers (arrow). Note a high signal intensity at the uninvolved rectal wall (arrowhead), representing submucosal edema. Response assessment should not be based on the appearance of the uninvolved parts of the rectum.
4.5. How to Evaluate Mesorectal Fascia Status
The most crucial element of all is the reassessment of the mesorectal fascia (MRF). Achieving clearance of the MRF on restaging high-resolution T2-weighted MRI holds a positive predictive value of up to 90% for a clear margin upon pathological examination, which may support a shift toward less invasive surgical approaches (68–70). Conversely, when the MRF is approached by dense hypointense fibrosis, the likelihood of tumor presence upon pathological assessment is lower as compared to when it is reached by an intermediate "tumor" signal intensity. Nevertheless, it remains significant and should be considered involved, since distinguishing purely fibrotic tissue from fibrosis with residual tumor cells is challenging (68–70).
The updated lexicon employs the term "MRF," which is an anatomical term, as opposed to circumferential resection margin (CRM), which relates to the operative surgical margin, dependent on the surgical approach (54).
The status of the MRF hinges on the shortest distance between the MRF and the outermost part of the rectal tumor, including extramural vascular invasion, tumor deposits, or disrupted capsule positive lymph nodes (71). Lymph nodes with an intact capsule are not considered involved, as they do not correlate with increased local recurrence rates (71,72). The SAR DFP template utilizes a three-tiered system for MRF status: "involved" for a distance less than 0.1 cm, "threatened" for 0.1–0.2 cm, and "clear" for more than 0.2 cm (73).
DWI may also play a role in predicting MRF status (74). However, it often overstimates the disease extent, particularly in anterior locations and in tumors close to the anal verge, as confirmed by a whole-mount study (75).
4.6. How to Evaluate Extra Rectal Disease
CR, nCR and iCR responses are also contingent on the assessment of extrarectal disease. When there are no lymph nodes, EMVI, or tumor deposits (TD), we categorize it as a complete response. In cases where any of these factors fall into borderline territory, the response is categorized as a near-complete response. However, if any of these factors raise suspicion, the response is deemed incomplete.
Lymph Nodes
When evaluating lymph nodes, it's important to note that after neoadjuvant therapy, most lymph nodes typically shrink or even vanish on MRI (76). MRI becomes more accurate after neoadjuvant therapy compared to the initial assessment, with the capability to identify patients without any signs of cancer in their lymph nodes, achieving up to 95% accuracy (77).
As this review will clarify, unlike the baseline assessment that utilizes morphological criteria, in restaging, the focus is solely on size.
There are different types of lymph nodes in the pelvic area. Some are close to the rectum and are considered "locoregional" nodes (N+ disease), which are mesorectal, obturator, and internal iliac nodes, while others are farther away and are labeled as "non-regional" nodes, suggesting the disease has spread (M1 disease). Common and external iliac nodes, as well as inguinal nodes are considered non-regional lymph nodes. There's an exception: if a lower rectal tumor goes below the dentate line (not visible on MRI but roughly at the middle of the anal canal), then the inguinal lymph nodes become "locoregional" again (78,79).
An additional update concerns N classification, as SAR DFP recommends using "N+" for abnormal locoregional lymph nodes or tumor deposits on MRI, and "N−" to indicate the absence of locoregional nodal disease, instead of specifying N0, N1a, N1b, N1c, or N2 (80).
Mesorectal Lymph Nodes
When assessing mesorectal lymph nodes, the most consistent correlation with negative mesorectal lymph node status upon pathological examination, was the absence of lymph node visualization on DWI (81). However, in general, mesorectal lymph nodes measuring less than 0.5 cm in the short axis should be considered indicative of a negative status (82-84), despite acknowledging the existence of false-positive and false-negative cases.
Lateral Pelvic Lymph Nodes
For lateral pelvic lymph nodes, morphologic criteria have not been shown to be helpful at baseline or restaging MRI. According to recommendations from the Lateral Node Consortium (83), during restaging, the persistence of an internal iliac lymph node measuring > 0.4 cm or an obturator lymph node measuring >0.6 cm after neoadjuvant chemoradiotherapy is regarded as suspicious, given their association with local and distant recurrence (85). As such, when positive on imaging, a boost of radiotherapy and/or a lymph node dissection may be indicated to improve the clinical outcome (85).
Non-Locoregional/Distant Lymph Nodes
Lymph nodes outside the regional area may raise suspicion if they measure more than 1.0 cm in the short axis. However, enlargement of the lymph node's short axis alone isn't a specific indicator of malignancy (26). It's essential to consider factors such as the tumor's location, expected drainage pattern, and malignant features like abnormal signal in the parenchyma, irregular node borders, asymmetry, and spherical shape.
Tumor Deposit/ EMVI
A TD is described as a tumoral lesion located within the mesorectum, disconnected from the primary tumor and devoid of lymphoid tissue upon pathological examination (86). On imaging, distinguishing a tumor deposit from a lymph node can be challenging; typically, tumor deposits manifest as irregular nodules closely associated with blood vessels, making separation difficult. When a tumor deposit is identified, it falls under the classification of N1c disease according to the 8th edition of the American Joint Committee on Cancer classification. The presence of TD often coexists with EMVI and they are linked to a worse oncological prognosis, with high incidence of liver and lung metastasis (87).
On MRI, EMVI is typically identified by prominent tubular structures exhibiting intermediate signal intensity on T2WI and diffusion restriction within the vessel, which originate from the tumor bed, with or without irregular contours. Regression of EMVI following neoadjuvant therapy is linked to enhanced survival compared to persistent EMVI (88), however the presence of edema, desmoplastic reaction, and inflammatory changes can limit MRI's effectiveness in identifying viable tumor within EMVI or tumor deposits. DWI serves as an additional tool to enhance EMVI detection (89).
A recent study has shown that DWI has high specificity and moderate sensitivity for the detection of viable TDs and EMVI after neoadjuvant therapy (90). Its presence, especially when detected using the DW imaging post-neoadjuvant therapy, is linked with a worse oncological prognosis, with an associated decrease in disease-free survival and overall survival.
5. Structured Report
There are a number of studies that have shown the value of structured reporting in rectal cancer (91–93). Furthermore, American and European societies have proposed lexicons and standardized reports (54,94,95).
A recent updated consensus statement from the SAR has proposed the following terminology in the evaluation of treatment response (54):
Complete response /near-complete response are grouped together because they can safely be closely monitored, and most cases of nCR will reach CR at 6-12 weeks after NAT (96). They imply that both T2-intermediate signal and restricted diffusion have resolved entirely or almost completely.
Incomplete response should be applied when, even though the tumor volume has decreased, there is residual T2-intermediate signal and/or restricted diffusion.
While recurrence should be used only after local excision or TME, the term regrowth is used after CT or RT treatment regimens. The latter applies when, after having documented CR, there is a new tumor in the bowel wall (local), adjacent structures (loco-regional), or lymph nodes. The bowel wall regrowth can be suspected when a prior low-signal intensity scar is a new area of T2-intermediate signal or restricted diffusion, thickening, or heterogeneity (54).
Also, on the SAR webpage, a reporting template to evaluate the response to treatment is available (97). It is a structured report that provides the features to evaluate the different possible scenarios:
Restricted diffusion and low ADC in tumor or tumor bed: present; absent; artifact/ equivocal/ not available (NA).
The T2-signal intensity in tumor or tumor bed: intermediate; mixed; entirely dark; nearly normalized appearance of rectal wall; bright mucin.
Distance of the inferior margin of the treated tumor to the anal verge and the top of the sphincter complex/anorectal junction.
Relationship of the treated tumor to the anterior peritoneal reflection: above; straddles; below.
Craniocaudal length and maximal wall thickening: current and pretreatment.
Extramural Vascular Invasion: no evident pretreatment; complete or partial regression; and unchanged from baseline.
The shortest distance of tumor/fibrosis to the Mesorectal Fascia or tumor deposit; LN; or EMVI threatening the MRF.
If it is a low-rectal tumor, is an invasion of the anal sphincter complex? No; extends into the internal sphincter; extends into intersphincteric space; extends into or through external sphincter.
Lymph nodes and/or tumor deposits: Mesorectal/superior rectal; or extra-mesorectal.
6. Future Directions
FDG-PET
FDG-PET serves as a functional imaging modality utilized to visualize and assess the metabolic activity within the body's tissues. Previous reports have established its utility, particularly in the realm of oncology clinical practice, and especially in evaluating treatment response. Nonetheless, the application of FDG-PET in rectal cancer lagged other cancers due to the impact of peristaltic movements, which had a detrimental effect on the quality of FDG-PET images. Recent advances in PET/CT technology have, however, mitigated these issues, enabling higher spatial resolutions and reduced imaging artifacts when visualizing rectal tumors. A recent meta-analysis by Lee et al., comprising 9 studies involving 427 patients, found that F-18 FDG PET/CT demonstrates comparable diagnostic performance to MRI in predicting pathologic responses following neoadjuvant chemotherapy in rectal cancer patients (98).
Furthermore, a report by Ince et al. highlights the potential of FDG-PET/MRI, achieving a remarkable 100% accuracy in assessing complete pathological responses. This accuracy is attributed to the ability of FDG-PET/MRI to detect residual disease that may be missed by MRI alone (99). A PET/MRI scanner, as a state-of-the-art medical imaging device, seamlessly integrates two potent imaging modalities: PET and MRI. This hybrid system facilitates the concurrent acquisition of functional and anatomical data within a single scan, offering the potential for more precise diagnostic assessments of treatment responses in rectal cancer patients.
Radiomics and Personalized Medicine
Advancements in the diagnosis and treatment of rectal cancer, particularly through the implementation of TME and neoadjuvant therapy, have led to a notable reduction in local recurrence rates. With the increasing intensity and broader utilization of neoadjuvant therapy, especially in patients with early tumor stages, for the purpose of enhancing organ preservation rates, the accurate evaluation of treatment response has become crucial in this context (100,101). In the context of future personalized medicine, the ability to predict treatment response prior to initiation is of paramount importance. This predictive capability is instrumental in identifying patients who are most likely to benefit from (intensified) neoadjuvant therapy while preventing unnecessary toxicity in individuals who are not suitable for active surveillance but still necessitate TME surgery.
Radiomics represents a state-of-the-art approach, involving the extraction and comprehensive analysis of numerous quantitative features from medical images. These features encompass a broad spectrum of information, encompassing aspects such as size, shape, texture, intensity, and spatial relationships within the image structures. Radiomics primarily transforms routine medical imaging modalities, including CT, MRI, and PET scans, into high-dimensional datasets amenable to advanced machine-learning techniques. A recent meta-analysis conducted by Tanaka et al., which incorporated findings from 16 studies, revealed that radiomics models designed for predicting neoadjuvant therapy response in rectal cancer demonstrate promising predictive potential. Of note, two models based on collagen features exhibited the most robust predictive performance, with AUCs ranging from 0.83 to 0.91 and favorable calibration (102). However, given the potential for bias in these studies, their utility should be assessed in the future through well-designed, prospective studies involving large population cohorts.
Author Contributions
Conceptualization, J.M.; methodology, J.M, P.C.A., J.N., T.B.D.C. and N.H.; formal analysis, J.M., P.C.A., J.N., N.H., L.d.P.G.d.F., H.K., Y.A., N.S. and M.C.F.; resources, J.M., J.M., P.C.A., J.N., L.d.P.G.d.F. and H.K.; writing—original draft preparation, J.M., P.C.A., J.N., N.H., L.d.P.G.d.F., H.K., Y.A., N.S. and N.H.; writing, review and editing: all authors; visualization, J.M. and L.d.P.Gd..F., supervision: T.B.D.C. and N.H.; project administration, N.H.; All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported in part by the National Cancer Institute Cancer Center Core Grant P30 CA008748 and the Society of MSK (PI: Natally Horvat); supported by RSNA Research & Education Foundation, through grant number RSD2302 (PI: Natally Horvat); supported by Colorectal Cancer Research Center at MSK; and supported by Co-ordination for the Improvement of Higher Education Personnel (CAPES).
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Rectal Cancer Version 5.2023 - September 21, N.C.C.N. Availabe online: (accessed on.
- Jayaprakasam, V.S.; Alvarez, J.; Omer, D.M.; Gollub, M.J.; Smith, J.J.; Petkovska, I. Watch-and-Wait Approach to Rectal Cancer: The Role of Imaging. Radiology 2023, 307, e221529. [Google Scholar] [CrossRef] [PubMed]
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. Jama 1990, 264, 1444–1450. [Google Scholar] [CrossRef]
- Peeters, K.C.; van de Velde, C.J.; Leer, J.W.; Martijn, H.; Junggeburt, J.M.; Kranenbarg, E.K.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Marijnen, C.A. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol 2005, 23, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Birgisson, H.; Påhlman, L.; Gunnarsson, U.; Glimelius, B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol 2005, 23, 6126–6131. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol 2022, 40, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open 2020, 3, e2030097. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Dijkstra, E.A.; Nilsson, P.J.; Hospers, G.A.P.; Bahadoer, R.R.; Meershoek-Klein Kranenbarg, E.; Roodvoets, A.G.H.; Putter, H.; Berglund, Å.; Cervantes, A.; Crolla, R.M.P.H.; et al. Locoregional Failure During and After Short-course Radiotherapy Followed by Chemotherapy and Surgery Compared With Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial. Annals of Surgery 2023, 278, e766–e772. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Etienne, P.-L.; Rio, E.; Evesque, L.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Boileve, A.; Delaye, M.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. Journal of Clinical Oncology 2023, 41, LBA3504–LBA3504. [Google Scholar] [CrossRef]
- Schrag, D.; Shi, Q.; Weiser, M.R.; Gollub, M.J.; Saltz, L.B.; Musher, B.L.; Goldberg, J.; Al Baghdadi, T.; Goodman, K.A.; McWilliams, R.R.; et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N Engl J Med 2023, 389, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jin, Y.; Guan, W.L.; Zhang, R.X.; Xiao, W.W.; Cai, P.Q.; Liu, M.; Lin, J.Z.; Wang, F.L.; Li, C.; et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol 2023, 8, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wu, T.; Yu, J.; Cai, X.; Li, G.; Li, X.; Huang, W.; Zhang, Y.; Wang, Y.; Yang, X.; et al. Locally advanced rectal cancer with dMMR/MSI-H may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: a multiple-center, cohort study. Front Immunol 2023, 14, 1182299. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Appelt, A.; Glynne-Jones, R.; Beets, G.; Perez, R.; Garcia-Aguilar, J.; Rullier, E.; Smith, J.J.; Marijnen, C.; Peters, F.P.; et al. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat Rev Clin Oncol 2021, 18, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Gollub, M.J.; Arya, S.; Beets-Tan, R.G.; dePrisco, G.; Gonen, M.; Jhaveri, K.; Kassam, Z.; Kaur, H.; Kim, D.; Knezevic, A.; et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY) 2018, 43, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Fraum, T.J.; Ma, J.; Jhaveri, K.; Nepal, P.; Lall, C.; Costello, J.; Harisinghani, M. The optimized rectal cancer MRI protocol: choosing the right sequences, sequence parameters, and preparatory strategies. Abdom Radiol (NY) 2023, 48, 2771–2791. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Rousset, P.; Lambregts, D.M.J.; Maas, M.; Gormly, K.; Lucidarme, O.; Brunelle, S.; Milot, L.; Arrive, L.; Salut, C.; et al. MRI restaging of rectal cancer: The RAC (Response-Anal canal-CRM) analysis joint consensus guidelines of the GRERCAR and GRECCAR groups. Diagn Interv Imaging 2023, 104, 311–322. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Bus, E.M.; Hauptmann, M.; Lahaye, M.J.; Maas, M.; Ter Beek, L.C.; Beets, G.L.; Bakers, F.C.H.; Beets-Tan, R.G.H.; Lambregts, D.M.J. Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T - Effect of applying a micro-enema to improve image quality. Eur J Radiol 2018, 99, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Jhaveri, K.; Kassam, Z.; Lall, C.; Kim, D.H. Rectal cancer MR staging: pearls and pitfalls at baseline examination. Abdom Radiol (NY) 2019, 44, 3536–3548. [Google Scholar] [CrossRef] [PubMed]
- Slater, A.; Halligan, S.; Taylor, S.A.; Marshall, M. Distance between the rectal wall and mesorectal fascia measured by MRI: Effect of rectal distension and implications for preoperative prediction of a tumour-free circumferential resection margin. Clin Radiol 2006, 61, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ernst, R.D.; Rauch, G.M.; Harisinghani, M. Nodal drainage pathways in primary rectal cancer: anatomy of regional and distant nodal spread. Abdom Radiol (NY) 2019, 44, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Lord, A.C.; D'Souza, N.; Shaw, A.; Rokan, Z.; Moran, B.; Abulafi, M.; Rasheed, S.; Chandramohan, A.; Corr, A.; Chau, I.; et al. MRI-Diagnosed Tumor Deposits and EMVI Status Have Superior Prognostic Accuracy to Current Clinical TNM Staging in Rectal Cancer. Ann Surg 2022, 276, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.S.; Hosseini-Nik, H.; Thipphavong, S.; Assarzadegan, N.; Menezes, R.J.; Kennedy, E.D.; Kirsch, R. MRI Detection of Extramural Venous Invasion in Rectal Cancer: Correlation With Histopathology Using Elastin Stain. AJR Am J Roentgenol 2016, 206, 747–755. [Google Scholar] [CrossRef]
- Furey, E.; Jhaveri, K.S. Magnetic resonance imaging in rectal cancer. Magn Reson Imaging Clin N Am 2014, 22, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, L.A.; Lambregts, D.M.; Mondal, D.; Martens, M.H.; Riedl, R.G.; Beets, G.L.; Beets-Tan, R.G. Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol 2013, 23, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Beets-Tan, R.G.; Lambregts, D.M.; Lammering, G.; Nelemans, P.J.; Engelen, S.M.; van Dam, R.M.; Jansen, R.L.; Sosef, M.; Leijtens, J.W.; et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011, 29, 4633–4640. [Google Scholar] [CrossRef] [PubMed]
- Gollub, M.J.; Das, J.P.; Bates, D.D.B.; Fuqua, J.L., 3rd; Golia Pernicka, J.S.; Javed-Tayyab, S.; Paroder, V.; Petkovska, I.; Garcia-Aguilar, J. Rectal cancer with complete endoscopic response after neoadjuvant therapy: what is the meaning of a positive MRI? Eur Radiol 2021, 31, 4731–4738. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, J.M.; Yoon, J.H.; Bae, J.S. Reduced field-of-view versus full field-of-view diffusion-weighted imaging for the evaluation of complete response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Abdom Radiol (NY) 2021, 46, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Z.; Tang, H.; Wang, Y.; Hu, X.; Shen, Y.; Hu, D. Comparison of reduced field-of-view diffusion-weighted imaging (DWI) and conventional DWI techniques in the assessment of rectal carcinoma at 3.0T: Image quality and histological T staging. J Magn Reson Imaging 2018, 47, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Delli Pizzi, A.; Caposiena, D.; Mastrodicasa, D.; Trebeschi, S.; Lambregts, D.; Rosa, C.; Cianci, R.; Seccia, B.; Sessa, B.; Di Flamminio, F.M.; et al. Tumor detectability and conspicuity comparison of standard b1000 and ultrahigh b2000 diffusion-weighted imaging in rectal cancer. Abdom Radiol (NY) 2019, 44, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018, 28, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Macchia, G.; Gambacorta, M.A.; Masciocchi, C.; Chiloiro, G.; Mantello, G.; di Benedetto, M.; Lupattelli, M.; Palazzari, E.; Belgioia, L.; Bacigalupo, A.; et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin Transl Radiat Oncol 2017, 4, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.A. Timing Is Everything: What Is the Optimal Duration After Chemoradiation for Surgery for Rectal Cancer? J Clin Oncol 2016, 34, 3724–3728. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, N.; Panteleimonitis, S.; Popeskou, S.; Cunha, J.F.; Qureshi, T.; Beets, G.L.; Heald, R.J.; Parvaiz, A. Delaying surgery after neoadjuvant chemoradiotherapy in rectal cancer has no influence in surgical approach or short-term clinical outcomes. Eur J Surg Oncol 2018, 44, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Sloothaak, D.A.; Geijsen, D.E.; van Leersum, N.J.; Punt, C.J.; Buskens, C.J.; Bemelman, W.A.; Tanis, P.J.; Dutch Surgical Colorectal, A. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013, 100, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.; Carlos Tavares Rocha, C.; Clemente Oliveira, B.; Petkovska, I.; Gollub, M.J. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 2019, 39, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.C.; Gollub, M.J.; Brown, G. The importance of MRI for rectal cancer evaluation. Surg Oncol 2022, 43, 101739. [Google Scholar] [CrossRef]
- Patel, U.B.; Blomqvist, L.K.; Taylor, F.; George, C.; Guthrie, A.; Bees, N.; Brown, G. MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience. AJR Am J Roentgenol 2012, 199, W486–495. [Google Scholar] [CrossRef]
- El Khababi, N.; Beets-Tan, R.G.H.; Tissier, R.; Lahaye, M.J.; Maas, M.; Curvo-Semedo, L.; Dresen, R.C.; Nougaret, S.; Beets, G.L.; Lambregts, D.M.J.; et al. Predicting response to chemoradiotherapy in rectal cancer via visual morphologic assessment and staging on baseline MRI: a multicenter and multireader study. Abdom Radiol (NY) 2023, 48, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.H.; van Heeswijk, M.M.; van den Broek, J.J.; Rao, S.X.; Vandecaveye, V.; Vliegen, R.A.; Schreurs, W.H.; Beets, G.L.; Lambregts, D.M.; Beets-Tan, R.G. Prospective, Multicenter Validation Study of Magnetic Resonance Volumetry for Response Assessment After Preoperative Chemoradiation in Rectal Cancer: Can the Results in the Literature be Reproduced? Int J Radiat Oncol Biol Phys 2015, 93, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.R.; Gormly, K.L.; Bhoday, J.; Balyansikova, S.; Battersby, N.J.; Chand, M.; Rao, S.; Tekkis, P.; Abulafi, A.M.; Brown, G. Interobserver agreement of radiologists assessing the response of rectal cancers to preoperative chemoradiation using the MRI tumour regression grading (mrTRG). Clin Radiol 2016, 71, 854–862. [Google Scholar] [CrossRef]
- Patel, U.B.; Taylor, F.; Blomqvist, L.; George, C.; Evans, H.; Tekkis, P.; Quirke, P.; Sebag-Montefiore, D.; Moran, B.; Heald, R.; et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011, 29, 3753–3760. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, F.; Brown, G.; Cunningham, D.; Wotherspoon, A.; Mendes, L.S.T.; Balyasnikova, S.; Evans, J.; Peckitt, C.; Begum, R.; Tait, D.; et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer 2017, 117, 1478–1485. [Google Scholar] [CrossRef]
- Jang, J.K.; Choi, S.H.; Park, S.H.; Kim, K.W.; Kim, H.J.; Lee, J.S.; Kim, A.Y. MR tumor regression grade for pathological complete response in rectal cancer post neoadjuvant chemoradiotherapy: a systematic review and meta-analysis for accuracy. Eur Radiol 2020, 30, 2312–2323. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Horvat, N.; Assuncao, A.N., Jr.; de, M.M.F.A.; Chakraborty, J.; Pandini, R.V.; Saraiva, S.; Nahas, C.S.R.; Nahas, S.C.; Nomura, C.H. MRI-based radiomic score increased mrTRG accuracy in predicting rectal cancer response to neoadjuvant therapy. Abdom Radiol (NY) 2023, 48, 1911–1920. [Google Scholar] [CrossRef]
- Patel, U.B.; Brown, G.; Rutten, H.; West, N.; Sebag-Montefiore, D.; Glynne-Jones, R.; Rullier, E.; Peeters, M.; Van Cutsem, E.; Ricci, S.; et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol 2012, 19, 2842–2852. [Google Scholar] [CrossRef] [PubMed]
- Achilli, P.; Magistro, C.; Abd El Aziz, M.A.; Calini, G.; Bertoglio, C.L.; Ferrari, G.; Mari, G.; Maggioni, D.; Peros, G.; Tamburello, S.; et al. Modest agreement between magnetic resonance and pathological tumor regression after neoadjuvant therapy for rectal cancer in the real world. Int J Cancer 2022, 151, 120–127. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, J.J.; van der Wolf, F.S.; Lahaye, M.J.; Heijnen, L.A.; Meischl, C.; Heitbrink, M.A.; Schreurs, W.H. Accuracy of MRI in Restaging Locally Advanced Rectal Cancer After Preoperative Chemoradiation. Dis Colon Rectum 2017, 60, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kassam, Z.; Baheti, A.D.; Hope, T.A.; Chang, K.J.; Korngold, E.K.; Taggart, M.W.; Horvat, N. Rectal cancer lexicon 2023 revised and updated consensus statement from the Society of Abdominal Radiology Colorectal and Anal Cancer Disease-Focused Panel. Abdom Radiol (NY) 2023, 48, 2792–2806. [Google Scholar] [CrossRef] [PubMed]
- Dresen, R.C.; Beets, G.L.; Rutten, H.J.; Engelen, S.M.; Lahaye, M.J.; Vliegen, R.F.; de Bruine, A.P.; Kessels, A.G.; Lammering, G.; Beets-Tan, R.G. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part I. Are we able to predict tumor confined to the rectal wall? Radiology 2009, 252, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Kim, S.H.; Lee, S.J.; Choi, J.Y.; Kim, M.J.; Rhim, H. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br J Radiol 2012, 85, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Sassen, S.; de Booij, M.; Sosef, M.; Berendsen, R.; Lammering, G.; Clarijs, R.; Bakker, M.; Beets-Tan, R.; Warmerdam, F.; Vliegen, R. Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur Radiol 2013, 23, 3440–3449. [Google Scholar] [CrossRef] [PubMed]
- Lambregts, D.M.; Vandecaveye, V.; Barbaro, B.; Bakers, F.C.; Lambrecht, M.; Maas, M.; Haustermans, K.; Valentini, V.; Beets, G.L.; Beets-Tan, R.G. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol 2011, 18, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, J.M.; Hong, S.H.; Kim, G.H.; Lee, J.Y.; Han, J.K.; Choi, B.I. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology 2009, 253, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Lambregts, D.M.J.; Delli Pizzi, A.; Lahaye, M.J.; van Griethuysen, J.J.M.; Maas, M.; Beets, G.L.; Bakers, F.C.H.; Beets-Tan, R.G.H. A Pattern-Based Approach Combining Tumor Morphology on MRI With Distinct Signal Patterns on Diffusion-Weighted Imaging to Assess Response of Rectal Tumors After Chemoradiotherapy. Dis Colon Rectum 2018, 61, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Sabbaga, J.; Gama-Rodrigues, J.; Sao Juliao, G.P.; Proscurshim, I.; Bailao Aguilar, P.; Nadalin, W.; Perez, R.O. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum 2013, 56, 1109–1117. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, Y.C.; Kim, H.; Kim, Y.W.; Hur, H.; Kim, J.S.; Min, B.S.; Kim, H.; Lim, J.S.; Seong, J.; et al. Tumor volume changes assessed by three-dimensional magnetic resonance volumetry in rectal cancer patients after preoperative chemoradiation: the impact of the volume reduction ratio on the prediction of pathologic complete response. Int J Radiat Oncol Biol Phys 2010, 76, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; White, M.G.; Peacock, O.; Kaur, H.; Palmquist, S.M.; You, N.; Taggart, M.; Salem, U.; Overman, M.; Kopetz, S.; et al. Pathological response following neoadjuvant immunotherapy in mismatch repair-deficient/microsatellite instability-high locally advanced, non-metastatic colorectal cancer. Br J Surg 2022, 109, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Lambregts, D.M.J.; Boellaard, T.N.; Beets-Tan, R.G.H. Response evaluation after neoadjuvant treatment for rectal cancer using modern MR imaging: a pictorial review. Insights Imaging 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Pinto, P.V.A.; Kinochita, F.; Garcia, C.M.; El Homsi, M.; Vilela de Oliveira, C.; Pandini, R.V.; Nahas, C.S.R.; Nahas, S.C.; Gollub, M.J.; et al. Mucinous Degeneration on MRI After Neoadjuvant Therapy in Patients With Rectal Adenocarcinoma: Frequency and Association With Clinical Outcomes. AJR Am J Roentgenol 2023, 221, 206–216. [Google Scholar] [CrossRef]
- Park, S.H.; Lim, J.S.; Lee, J.; Kim, H.Y.; Koom, W.S.; Hur, H.; Park, M.S.; Kim, M.J.; Kim, H. Rectal Mucinous Adenocarcinoma: MR Imaging Assessment of Response to Concurrent Chemotherapy and Radiation Therapy-A Hypothesis-generating Study. Radiology 2017, 285, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.D.B.; Homsi, M.E.; Chang, K.J.; Lalwani, N.; Horvat, N.; Sheedy, S.P. MRI for Rectal Cancer: Staging, mrCRM, EMVI, Lymph Node Staging and Post-Treatment Response. Clin Colorectal Cancer 2022, 21, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Vliegen, R.F.; Beets, G.L.; Lammering, G.; Dresen, R.C.; Rutten, H.J.; Kessels, A.G.; Oei, T.K.; de Bruine, A.P.; van Engelshoven, J.M.; Beets-Tan, R.G. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology 2008, 246, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, T.; Gollins, S.; Maw, A.; Hobson, P.; Byrne, R.; Widdowson, D. Magnetic resonance imaging in rectal cancer downstaged using neoadjuvant chemoradiation: accuracy of prediction of tumour stage and circumferential resection margin status. Colorectal Dis 2008, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beissbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol 2014, 32, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Beets, G.L.; Kim, M.J.; Kessels, A.G.; Beets-Tan, R.G. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004, 52, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; Goicoetxea, A.; Gomez, M.L.; Jimenez, G.; Llanos, M.C.; Jimenez, J.; Montes, B.; de Miguel, M. Impact of specific modes of circumferential resection margin involvement in rectal cancer local recurrence: A retrospective study. J Surg Oncol 2018, 118, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Kassam, Z.; Lang, R.; Bates, D.D.B.; Chang, K.J.; Fraum, T.J.; Friedman, K.A.; Golia Pernicka, J.S.; Gollub, M.J.; Harisinghani, M.; Khatri, G.; et al. SAR user guide to the rectal MR synoptic report (primary staging). Abdom Radiol (NY) 2023, 48, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Kim, S.H.; Lee, S.J.; Jang, K.M.; Rhim, H. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology 2011, 260, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Yuval, J.B.; Thompson, H.M.; Firat, C.; Verheij, F.S.; Widmar, M.; Wei, I.H.; Pappou, E.; Smith, J.J.; Weiser, M.R.; Paty, P.B.; et al. MRI at Restaging After Neoadjuvant Therapy for Rectal Cancer Overestimates Circumferential Resection Margin Proximity as Determined by Comparison With Whole-Mount Pathology. Dis Colon Rectum 2022, 65, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, L.A.; Maas, M.; Beets-Tan, R.G.; Berkhof, M.; Lambregts, D.M.; Nelemans, P.J.; Riedl, R.; Beets, G.L. Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? Int J Colorectal Dis 2016, 31, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.J.; Beets, G.L.; Engelen, S.M.; Kessels, A.G.; de Bruine, A.P.; Kwee, H.W.; van Engelshoven, J.M.; van de Velde, C.J.; Beets-Tan, R.G. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology 2009, 252, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Lambregts, D.M.J.; Bogveradze, N.; Blomqvist, L.K.; Fokas, E.; Garcia-Aguilar, J.; Glimelius, B.; Gollub, M.J.; Konishi, T.; Marijnen, C.A.M.; Nagtegaal, I.D.; et al. Current controversies in TNM for the radiological staging of rectal cancer and how to deal with them: results of a global online survey and multidisciplinary expert consensus. Eur Radiol 2022, 32, 4991–5003. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Anker, C.J.; Ashman, J.B.; Bhadkamkar, N.A.; Bradfield, L.; Chang, D.T.; Dorth, J.; Garcia-Aguilar, J.; Goff, D.; Jacqmin, D.; et al. Radiation Therapy for Rectal Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol 2021, 11, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kassam, Z.; Lang, R.; Bates, D.D.B.; Chang, K.J.; Fraum, T.J.; Friedman, K.A.; Golia Pernicka, J.S.; Gollub, M.J.; Harisinghani, M.; Khatri, G.; et al. Correction: SAR user guide to the rectal MR synoptic report (primary staging). Abdom Radiol (NY) 2023, 48, 200. [Google Scholar] [CrossRef] [PubMed]
- van Heeswijk, M.M.; Lambregts, D.M.; Palm, W.M.; Hendriks, B.M.; Maas, M.; Beets, G.L.; Beets-Tan, R.G. DWI for Assessment of Rectal Cancer Nodes After Chemoradiotherapy: Is the Absence of Nodes at DWI Proof of a Negative Nodal Status? AJR Am J Roentgenol 2017, 208, W79–W84. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Konishi, T.; Cunningham, C.; Garcia-Aguilar, J.; Iversen, H.; Toda, S.; Lee, I.K.; Lee, H.X.; Uehara, K.; Lee, P.; et al. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol 2019, 37, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Konishi, T.; Beets, G.L.; Cunningham, C.; Garcia-Aguilar, J.; Iversen, H.; Toda, S.; Lee, I.K.; Lee, H.X.; Uehara, K.; et al. Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated With Lateral Local Recurrence in Low Rectal Cancer After Neoadjuvant Chemoradiotherapy or Radiotherapy. JAMA Surg 2019, 154, e192172. [Google Scholar] [CrossRef] [PubMed]
- Beets-Tan, R.G.; Lambregts, D.M.; Maas, M.; Bipat, S.; Barbaro, B.; Caseiro-Alves, F.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2013, 23, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Schaap, D.P.; Boogerd, L.S.F.; Konishi, T.; Cunningham, C.; Ogura, A.; Garcia-Aguilar, J.; Beets, G.L.; Suzuki, C.; Toda, S.; Lee, I.K.; et al. Rectal cancer lateral lymph nodes: multicentre study of the impact of obturator and internal iliac nodes on oncological outcomes. Br J Surg 2021, 108, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Knijn, N.; Hugen, N.; Marshall, H.C.; Sugihara, K.; Tot, T.; Ueno, H.; Quirke, P. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol 2017, 35, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Sheedy, S.P.; Heiken, J.P.; Mohammadinejad, P.; Graham, R.P.; Lee, H.E.; Kelley, S.R.; Hansel, S.L.; Bruining, D.H.; Fidler, J.L.; et al. MRI-detected extramural venous invasion of rectal cancer: Multimodality performance and implications at baseline imaging and after neoadjuvant therapy. Insights Imaging 2021, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Carten, R.V.; Babiker, A.; Abulafi, M.; Lord, A.C.; Brown, G. Prognostic Importance of MRI-Detected Extramural Venous Invasion in Rectal Cancer: A Literature Review and Systematic Meta-Analysis. Int J Radiat Oncol Biol Phys 2021, 111, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Fornell-Perez, R.; Vivas-Escalona, V.; Aranda-Sanchez, J.; Gonzalez-Dominguez, M.C.; Rubio-Garcia, J.; Aleman-Flores, P.; Lozano-Rodriguez, A.; Porcel-de-Peralta, G.; Loro-Ferrer, J.F. Primary and post-chemoradiotherapy MRI detection of extramural venous invasion in rectal cancer: the role of diffusion-weighted imaging. Radiol Med 2020, 125, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Firat, C.; Thompson, H.M.; Gangai, N.; Zheng, J.; Capanu, M.; Bates, D.D.B.; Paroder, V.; Garcia-Aguilar, J.; Shia, J.; et al. Extramural Venous Invasion and Tumor Deposit at Diffusion-weighted MRI in Patients after Neoadjuvant Treatment for Rectal Cancer. Radiology 2023, 308, e230079. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.; Mangat, N.; Swift, I.R.; Brown, G. Proforma-based reporting in rectal cancer. Cancer Imaging 2010, 10 Spec no A, S142–150. [Google Scholar] [CrossRef]
- Al-Sukhni, E.; Messenger, D.E.; Charles Victor, J.; McLeod, R.S.; Kennedy, E.D. Do MRI Reports Contain Adequate Preoperative Staging Information for End Users to Make Appropriate Treatment Decisions for Rectal Cancer? Annals of Surgical Oncology 2013, 20, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Reinhold, C.; Mikhael, H.W.; Rouanet, P.; Bibeau, F.; Brown, G. The Use of MR Imaging in Treatment Planning for Patients with Rectal Carcinoma: Have You Checked the “DISTANCE”? Radiology 2013, 268, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Rousset, P.; Gormly, K.; Lucidarme, O.; Brunelle, S.; Milot, L.; Salut, C.; Pilleul, F.; Arrivé, L.; Hordonneau, C.; et al. Structured and shared MRI staging lexicon and report of rectal cancer: A consensus proposal by the French Radiology Group (GRERCAR) and Surgical Group (GRECCAR) for rectal cancer. Diagnostic and Interventional Imaging 2022, 103, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Kassam, Z.; Lang, R.; Bates, D.D.B.; Chang, K.J.; Fraum, T.J.; Friedman, K.A.; Golia Pernicka, J.S.; Gollub, M.J.; Harisinghani, M.; Khatri, G.; et al. SAR user guide to the rectal MR synoptic report (primary staging). Abdominal Radiology 2023, 48, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Hupkens, B.J.P.; Maas, M.; Martens, M.H.; van der Sande, M.E.; Lambregts, D.M.J.; Breukink, S.O.; Melenhorst, J.; Houwers, J.B.; Hoff, C.; Sosef, M.N.; et al. Organ Preservation in Rectal Cancer After Chemoradiation: Should We Extend the Observation Period in Patients with a Clinical Near-Complete Response? Annals of Surgical Oncology 2018, 25, 197–203. [Google Scholar] [CrossRef] [PubMed]
- https://abdominalradiology.org/wp-content/uploads/2021/03/Updated-MRI-pelvis-Rectal-Cancer-RESTAGING.pdf. Availabe online: https://abdominalradiology.org/wp-content/uploads/2021/03/Updated-MRI-pelvis-Rectal-Cancer-RESTAGING.
- Lee, S.W.; Jeong, S.Y.; Kim, K.; Kim, S.J. Direct comparison of F-18 FDG PET/CT and MRI to predict pathologic response to neoadjuvant treatment in locally advanced rectal cancer: a meta-analysis. Ann Nucl Med 2021, 35, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Ince, S.; Itani, M.; Henke, L.E.; Smith, R.K.; Wise, P.E.; Mutch, M.G.; Glasgow, S.C.; Silviera, M.L.; Pedersen, K.S.; Hunt, S.R.; et al. FDG-PET/MRI for Nonoperative Management of Rectal Cancer: A Prospective Pilot Study. Tomography 2022, 8, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.C.; Soucisse, M.; Michael, M.; Tie, J.; Ngan, S.Y.; Leong, T.; McCormick, J.; Warrier, S.K.; Heriot, A.G. Total Neoadjuvant Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Metaanalysis of Oncological and Operative Outcomes. Ann Surg Oncol 2021, 28, 7476–7486. [Google Scholar] [CrossRef]
- Miranda, J.; Tan, G.X.V.; Fernandes, M.C.; Yildirim, O.; Sims, J.A.; Araujo-Filho, J.A.B.; de, M.M.F.A.; Assuncao-Jr, A.N.; Nomura, C.H.; Horvat, N. Rectal MRI radiomics for predicting pathological complete response: Where we are. Clin Imaging 2022, 82, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.D.; Geubels, B.M.; Grotenhuis, B.A.; Marijnen, C.A.M.; Peters, F.P.; van der Mierden, S.; Maas, M.; Couwenberg, A.M. Validated Pretreatment Prediction Models for Response to Neoadjuvant Therapy in Patients with Rectal Cancer: A Systematic Review and Critical Appraisal. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).