Submitted:
09 November 2023
Posted:
09 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
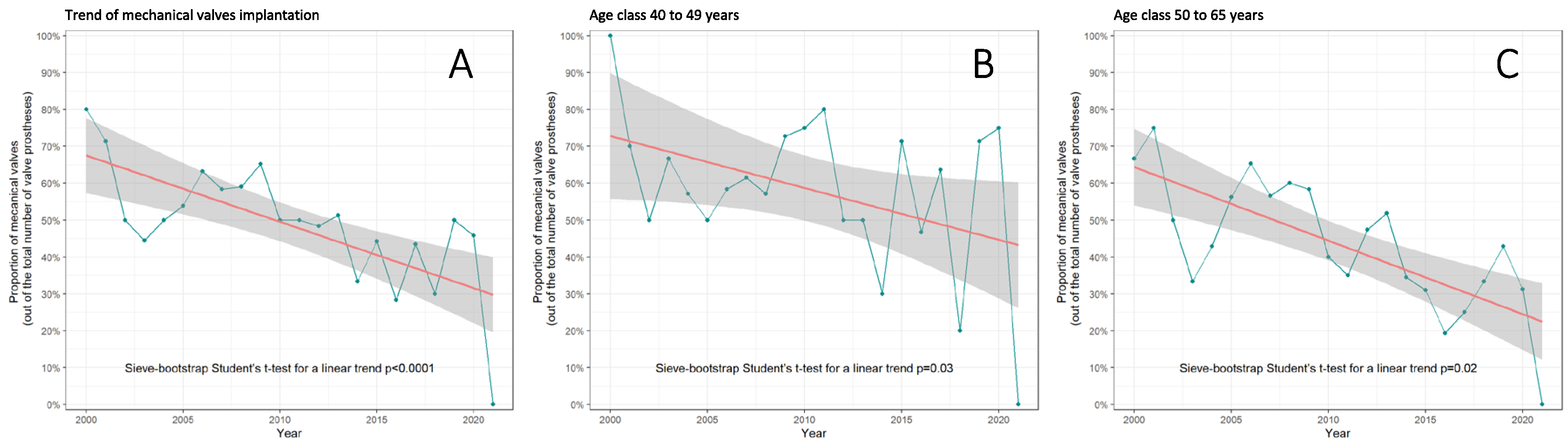
3.1. Trends in AVR with Mechanical vs. Biological Valves in Middle-Aged Patients
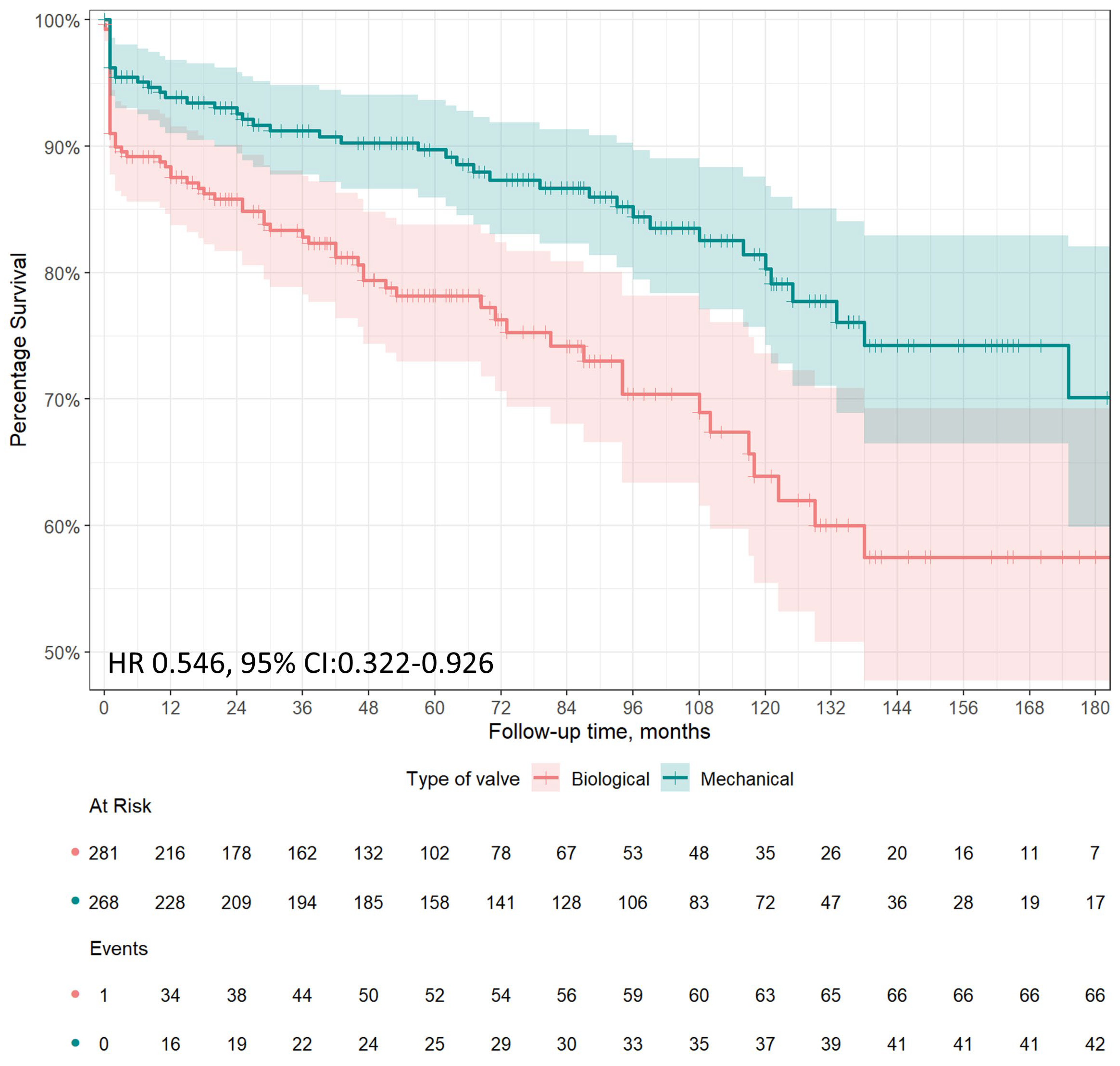
3.2. Long Term Survival
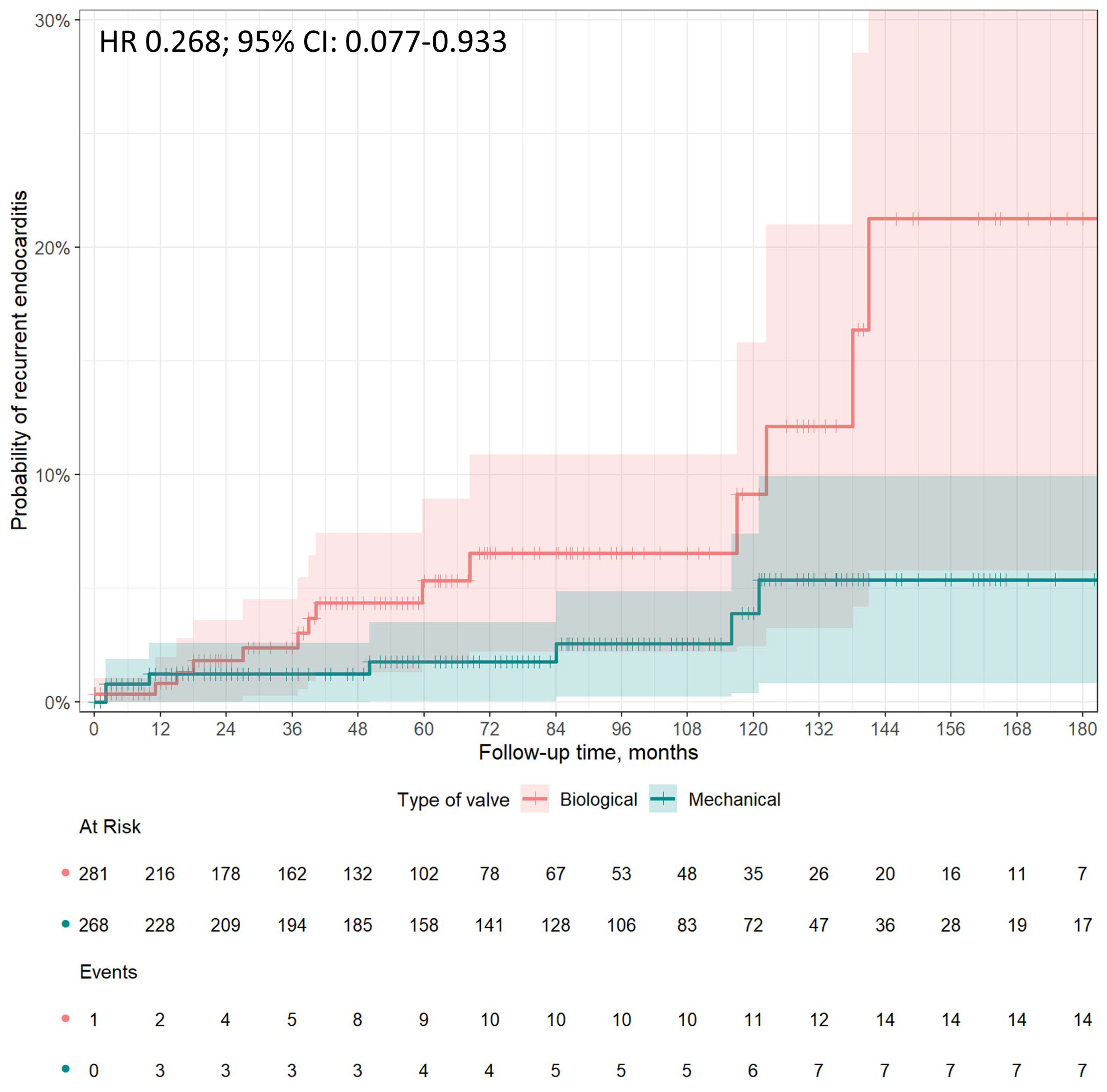
3.3. Recurrence of Endocarditis
3.4. Early Postoperative Complications
4. Discussion
Preference of Prosthetic Valve Types in Middle-Aged Patients
Perioperative Outcomes, Long-Term Survival and Recurrence of IE after AVR for IE in Middle-Aged Patients
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Citro R, Chan KL, Miglioranza MH, Laroche C, Benvenga RM, Furnaz S, Magne J, Olmos C, Paelinck BP, Pasquet A, Piper C, Salsano A, Savouré A, Park SW, Szymański P, Tattevin P, Vallejo Camazon N, Lancellotti P, Habib G; EURO ENDO Investigators group. Clinical profile and outcome of recurrent infective endocarditis. Heart 2022, 108:1729-1736. [CrossRef]
- Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, Caselli S, Doenst T, Ederhy S, Erba PA, Foldager D, Fosbøl EL, Kovac J, Mestres CA, Miller OI, Miro JM, Pazdernik M, Pizzi MN, Quintana E, Rasmussen TB, Ristić AD, Rodés-Cabau J, Sionis A, Zühlke LJ, Borger MA; ESC Scientific Document Group. 2023 ESC Guidelines for the management of endocarditis. Eur Heart J 2023, 25:ehad193. [CrossRef]
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022, 43:561-632. [CrossRef]
- Kytö V, Ahtela E, Sipilä J, Rautava P, Gunn J. Mechanical versus biological valve prosthesis for surgical aortic valve replacement in patients with infective endocarditis. Interact Cardiovasc Thorac Surg 2019, 29:386-392. [CrossRef]
- Delahaye F, Chu VH, Altclas J, Barsic B, Delahaye A, Freiberger T, Gordon DL, Hannan MM, Hoen B, Kanj SS, Lejko-Zupanc T, Mestres CA, Pachirat O, Pappas P, Lamas C, Selton-Suty C, Tan R, Tattevin P, Wang A; International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) Investigators. One-year outcome following biological or mechanical valve replacement for infective endocarditis. Int J Cardiol 2015, 78:117-23. [CrossRef]
- Toyoda N, Itagaki S, Tannous H, Egorova NN, Chikwe J. Bioprosthetic Versus Mechanical Valve Replacement for Infective Endocarditis: Focus on Recurrence Rates. Ann Thorac Surg 2018, 106:99-106. [CrossRef]
- Havers-Borgersen E, Butt JH, Østergaard L, Bundgaard H, Smerup M, Bruun NE, Gislason GH, Torp-Pedersen C, Køber L, Fosbøl EL. Recurrent infective endocarditis versus first-time infective endocarditis after heart valve surgery. Clin Res Cardiol 2020, 109:1342-1351. [CrossRef]
- Caus T, Chabry Y, Nader J, Fusellier JF, De Brux JL; EpiCard investigators. Trends in SAVR with biological vs. mechanical valves in middle-aged patients: results from a French large multi-centric survey. Front Cardiovasc Med 2023, 10:1205770. [CrossRef]
- J.S. Li, D.J. Sexton, N. Mick, et al., Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000, 30:633–638. [CrossRef]
- Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999, 16:9-13. [CrossRef]
- Zhao QY, Luo JC, Su Y, Zhang YJ, Tu GW, Luo Z. Propensity score matching with R: conventional methods and new features. Ann Transl Med 2021, 9:812. [CrossRef]
- Austin PC, Stuart EA. Estimating the effect of treatment on binary outcomes using full matching on the propensity score. Stat Methods Med Res 2017, 26:2505-2525. [CrossRef]
- Kong WKF, Salsano A, Giacobbe DR, Popescu BA, Laroche C, Duval X, Schueler R, Moreo A, Colonna P, Piper C, Calvo-Iglesias F, Badano LP, Srdanovic I, Boutoille D, Huttin O, Stöhr E, Timóteo AT, Vaskelyte JJ, Sadeghpour A, Tornos P, Abid L, Poh KK, Habib G, Lancellotti P. Outcomes of culture-negative vs. culture-positive infective endocarditis: the ESC-EORP EURO-ENDO registry. Eur Heart J 2022, 43:2770-2780. [CrossRef]
- Salsano A, Giacobbe DR, Sportelli E, Olivieri GM, Natali R, Prevosto M, Del Bono V, Viscoli C, Santini F. Aortic cross-clamp time and cardiopulmonary bypass time: prognostic implications in patients operated on for infective endocarditis. Interact Cardiovasc Thorac Surg 2018, 27:328-335. [CrossRef]
- Nappi F, Nenna A, Spadaccio C, Avtaar Singh SS, Almazil A, Acar C. The Use of the Cryopreserved Aortic Homograft for Aortic Valve Replacement: Is It Still an Option? J Cardiovasc Dev Dis. 2023,6:248. [CrossRef]
- Chiang YP, Chikwe J, Moskowitz AJ, Itagaki S, Adams DH, Egorova NN. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA 2014, 312:1323-9. [CrossRef]
- Caus T, Chabry Y, Nader J, Fusellier JF, De Brux JL; EpiCard investigators. Trends in SAVR with biological vs. mechanical valves in middle-aged patients: results from a French large multi-centric survey. Front Cardiovasc Med 2023,10:1205770. [CrossRef]
- Raschpichler M, de Waha S, Holzhey D, Schwarzer G, Flint N, Kaewkes D, Bräuchle PT, Dvir D, Makkar R, Ailawadi G, Abdel-Wahab M, Thiele H, Borger MA. Valve-in-Valve Transcatheter Aortic Valve Replacement Versus Redo Surgical Aortic Valve Replacement for Failed Surgical Aortic Bioprostheses: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2022, 11:e7965. [CrossRef]
- Savage EB, Saha-Chaudhuri P, Asher CR, Brennan JM, Gammie JS. Outcomes and prosthesis choice for active aortic valve infective endocarditis: analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2014, 98:806-14. [CrossRef]
- Formica, F.; Maestri, F.; Gripshi, F.; Gallingani, A.; Grossi, S.; Nicolini, F. Long-Term Outcome of Mechanical and Biological Prostheses in Patients with Left-Side Infective Endocarditis: A Systematic Review and Meta-Analysis. J. Clin. Med 2021, 10:4356. [CrossRef]
- Nguyen, D.T.; Delahaye, F.; Obadia, J.-F.; Duval, X.; Selton-Suty, C.; Carteaux, J.-P.; Hoen, B.; Alla, F. for the AEPEI Study Group. Aortic valve replacement for active infective endocarditis: 5-year survival comparison of bioprostheses, homografts and mechanical prostheses. Eur. J. Cardio-Thorac. Surg 2010, 37:1025–1032. [CrossRef]
- Rubino AS, Della Ratta EE, Galbiati D, Ashurov R, Galgano VL, Montella AP et al. Can prosthesis type influence the recurrence of infective endocar- ditis after surgery for native valve endocarditis? A propensity weighted comparison. Eur J Cardiothorac Surg 2021,60:1388–94. [CrossRef]
- Said SM, Abdelsattar ZM, Schaff HV, Greason KL, Daly RC, Pochettino A, Joyce LD, Dearani JA. Outcomes of surgery for infective endocarditis: a single-centre experience of 801 patients. Eur J Cardiothorac Surg 2018,53:435-439. [CrossRef]
- Moon MR, Miller DC, Moore KA, Oyer PE, Mitchell RS, Robbins RC, Stinson EB, Shumway NE, Reitz BA. Treatment of endocarditis with valve replacement: the question of tissue versus mechanical prosthesis. Ann Thorac Surg 2001,71:1164-71. [CrossRef]
- Potter DD, Sundt TM 3rd, Zehr KJ, Dearani JA, Daly RC, Mullany CJ, McGregor CG, Puga FJ, Schaff HV, Orszulak TA. Operative risk of reoperative aortic valve replacement. J Thorac Cardiovasc Surg 2005,129:94-103. [CrossRef]
- Attias D, Nejjari M, Nappi F, Dreyfus J, Eleid MF, Rihal CS. How to treat severe symptomatic structural valve deterioration of aortic surgical bioprosthesis: transcatheter valve-in-valve implantation or redo valve surgery? Eur J Cardiothorac Surg 2018, 54:977-985. [CrossRef]
- Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation 2010,121:2123-9. [CrossRef]
- Kossar AP, George I, Gordon R, Ferrari G. Bacterial infiltration and bioprosthetic valve failure: Emerging diagnostics for emerging therapies. J Thorac Cardiovasc Surg 2020,159:1279-1282. [CrossRef]
| Variables | Mechanical Valve (N=268) |
Biological Valve (N=281) |
p Value |
|---|---|---|---|
| Age (years, median [IQR]) | 52.00 [45.37, 57.56] | 57.00 [48.74, 62.00] | <0.001 |
| Female gender, n (%) | 64 (23.9) | 40 (14.2) | 0.006 |
| Hypertension, n (%) | 64 (23.9) | 75 (26.7) | 0.510 |
| Diabetes, n (%) | 23 (8.6) | 32 (11.4) | 0.341 |
| Obesity, n (%) | 18 (6.7) | 22 (7.8) | 0.736 |
| COPD, n (%) | 13 (4.9) | 16 (5.7) | 0.802 |
| Drug abuse, n (%) | 14 (5.2) | 13 (4.6) | 0.900 |
| Previous cardiac surgery, n (%) | 8 (3.0) | 14 (5.0) | 0.330 |
| LVEF (%, mean (SD)) | 54.94 (8.45) | 54.22 (9.47) | 0.346 |
| Peripheral arteriopathy, n (%) | 8 (3.0) | 13 (4.6) | 0.436 |
| Preoperative stroke, n (%) | 30 (11.2) | 34 (12.1) | 0.843 |
| Heart failure, n (%) | 28 (10.4) | 59 (21.0) | 0.001 |
| Cardiogenic shock, n (%) | 13 (4.9) | 27 (9.6) | 0.048 |
| Acute myocardial infarction (within 90 days), n (%) | 3 (1.1) | 4 (1.4) | 1.000 |
| Active endocarditis, n (%) | 205 (76.5) | 226 (80.4) | 0.309 |
| Preoperative MV, n (%) | 8 (3.0) | 28 (10.0) | 0.002 |
| Pulmonary hypertension (<50mmHg), n (%) | 15 (5.6) | 19 (6.8) | 0.697 |
| CKD, n (%) | 26 (9.7) | 28 (10.0) | 1.000 |
| Dialysis, n (%) | 11 (4.1) | 11 (3.9) | 1.000 |
| IABP, n (%) | 1 (0.4) | 5 (1.8) | 0.241 |
| Preoperative pacemaker implantation, n (%) | 2 (0.7) | 6 (2.1) | 0.317 |
| NVE, n (%) | 267 (99.6) | 281 (100) | 1.000 |
| PVE, n (%) | 1 (0.4) | 0 (0.0) | 1.000 |
| Abscess, n (%) | 27 (10.1) | 43 (15.3) | 0.088 |
| Large vegetation (>1 cm), n (%) | 121 (45.1) | 102 (36.3) | 0.043 |
| Leaflet perforation, n (%) | 29 (10.8) | 50 (17.8) | 0.027 |
| Paravalvular leak, n (%) | 1 (0.4) | 1 (0.4) | 1.000 |
| CABG, n (%) | 0.367 | ||
| 0 | 257 (95.9) | 265 (94.3) | |
| 1 | 9 (3.3) | 12 (4.3) | |
| 2 | 1 (0.4) | 4 (1.4) | |
| ≥3 | 1 (0.4) | 0 (0.0) | |
| Logistic Euroscore (median [IQR]) | 4.68 [2.78, 8.90] | 6.45 [3.62, 13.61] | 0.005 |
| Cardiopulmonary bypass time (min, mean (SD)) | 79.26 (33.47) | 90.94 (49.47) | 0.004 |
| Aortic cross-clamp time (min, mean (SD)) | 63.53 (23.62) | 73.39 (34.97) | 0.001 |
| Causative Agent | Mechanical Valve (N=268) |
Biological Valve (N=281) |
p Value |
|---|---|---|---|
| Culture negative endocarditis, n (%) | 84 (31.3) | 83 (29.5) | 0.714 |
| Streptococci, n (%) | 55 (20.5) | 61 (21.7) | 0.814 |
| Staphylococcus aureus, n (%) | 34 (12.7) | 37 (13.5) | 0.600 |
| Viridans group streptococci*, n (%) | 30 (11.2) | 28 (10.0) | 0.742 |
| Coagulase-negative staphylococci*, n (%) | 23 (8.6) | 20 (7.1) | 0.632 |
| Gram-negative bacteria (non HACEK), n (%) | 5 (1.9) | 9 (3.2) | 0.470 |
| Nutritionally variant streptococci*, n (%) | 2 (0.7) | 3 (1.1) | 1.000 |
| Candida spp, n (%) | 2 (0.7) | 1 (0.4) | 0.967 |
| HACEK group*, n (%) | 1 (0.4) | 0 (0.0) | 0.981 |
| Enterococcus faecalis, n (%) | 1 (0.004) | 1 (0.004) | 1.000 |
| Other organisms, n (%) | 32 (11.9) | 37 (13.2) | 0.591 |
| Variables | Overall Series | Estimation in Matched Cohort§ | ||||
|---|---|---|---|---|---|---|
| Mechanical Valve (N=268) |
Biological Valve (N=281) |
p Value | Odds Ratio | 95% CI | P value | |
| Early mortality, n (%) | 11 (4.1) | 23 (8.2) | 0.071 | 0.480 | 0.229-1.005 | 0.052 |
| Sepsis, n (%) | 9 (3.4) | 11 (3.9) | 0.898 | 0.849 | 0.346-2.084 | 0.722 |
| MOF, n (%) | 1 (0.4) | 7 (2.6) | 0.088 | 0.147 | 0.018-1.207 | 0.074 |
| Reoperation for bleeding, n (%) | 7 (2.6) | 11 (3.9) | 0.537 | 0.658 | 0.251-1.724 | 0.395 |
| Pacemaker implantation, n (%) | 4 (1.5) | 7 (2.5) | 0.592 | 0.591 | 0.171-2.042 | 0.406 |
| Atrial fibrillation, n (%) | 16 (6.3) | 42 (15.6) | 0.001 | 0.362 | 0.198-0.662 | <0.0001 |
| IABP, n (%) | 3 (1.1) | 6 (2.1) | 0.544 | 0.517 | 0.128-2.088 | 0.354 |
| Stroke, n (%) | 6 (2.2) | 3 (1.1) | 0.460 | 0.712 | 0.523-8.541 | 0.293 |
| Acute kidney injury, n (%) | 7 (2.6) | 20 (7.1) | 0.024 | 0.349 | 0.145-0.839 | 0.019 |
| Dialysis, n (%) | 1 (0.4) | 4 (1.5) | 0.401 | 0.261 | 0.029-2.350 | 0.231 |
| MV (hours, mean (SD)) | 23.74 (76.34) | 33.56 (82.75) | 0.315 | -9.819§ | 9.763§ | 0.315§ |
| ICU stay (days, median [IQR]) | 2.00 [1.00, 3.00] | 4.00 [2.00, 9.00] | <0.001 | -17.700§ | 13.599§ | 0.194§ |
| Hospital stay (median [IQR]) | 12.00 [8.50, 17.00] | 12.50 [8.00, 19.00] | 0.356 | -2.041§ | 1.367§ | 0.136§ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





