Submitted:
09 November 2023
Posted:
10 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
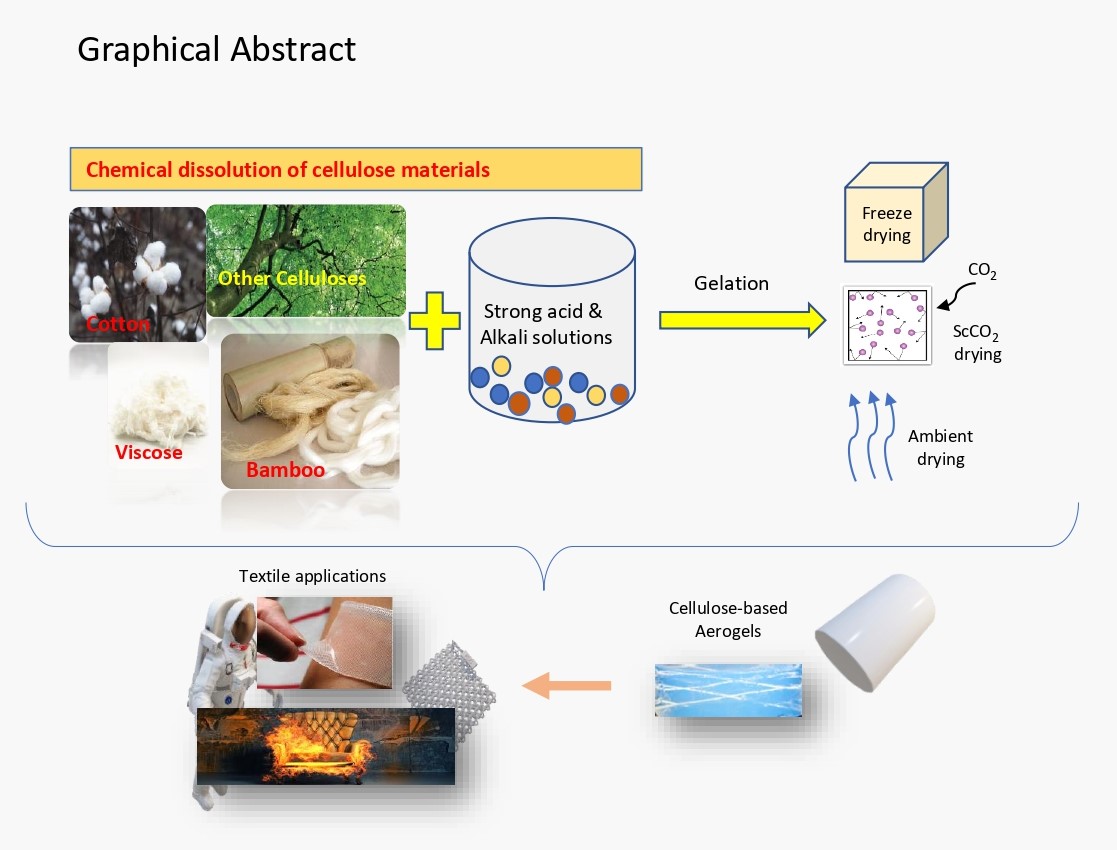
2. Creating Cellulose Aerogel
| Classification of Cellulose Aerogels | ||||||
|---|---|---|---|---|---|---|
| Cellulose -Aerogel Type | Starting material | Solvent | Surface chemistry | Drying method | Application | Ref. |
|
Pineapple leaf fiber, Cotton waste fiber | Poly (vinyl alcohol) (PVA) | - | Freeze-drying Freeze-drying |
Building towards sustainable development | [29] |
| Raw cotton fibers and cotton stalk | Tert-butyl alcohol | - | - | [57] | ||
| Softwood cellulose pulp | TEMPO | Monocomponent endoglucanase, cupriethylendiamine | Bio-fabrication of tissues, additional health and pharmacological uses | [58] | ||
| 1.a. Nano Cellulose | Cellulose nanofibers (CNFs), Graphite powder, concentrated sulfuric acid, concentrated acetic acid, solution hydrogen peroxide | Sodium hydroxide, sodium hypochlorite, MO (methyl orange), and potassium permanganate | NaOH | Freeze-drying | The treatment of domestic organic wastewater | [59] |
| 1.b. Bacterial Cellulose | Komagataeibacter sucrofermentans H-110, TEMPO, dextrose, protein hydrolysate, yeast concentrate, disodium phosphate | Sodium hydroxide solution | NaClO, NaBr | Freeze-drying | Bio-fabrication of tissues and preparation of injury treatment materials | [4] |
| Bacterial cellulose (BC) pellicles | - | Deionized water (DIW) | Pressure sensors, batteries and super-capacitors, substrates for catalysts, high-tech detectors | [60] | ||
|
Cotton and viscose-based regenerated cellulose | Imidazolium acetate ([EMIM], non-enium acetate ([DBNH][OAc]) | DMSO | Supercritical CO2, Lyophilization, ambitious drying | - | [61] |
| Bamboo pulp boards | NaOH/urea aqueous solutions | Methyl-pyrrolidone (NMP), potassium hydroxide (KOH) | Freeze-drying Freeze-drying |
Application of energy storage devices | [62] | |
| Bamboo cellulose nanofibrils (BCNF) | Polyvinyl alcohol (PVA) | Sodium tetraborate decahydrate (borax), N, N′-methylenebisacrylamide (MBA), Methyltrimethoxysilane (MTMS) | Eco-friendly wrapping in the refrigerated transportation of fresh produce | [63] | ||
|
Softwood kraft pulp sheets | 1,2-ethanediol, hydroxylammonium chloride monochloroacetic acid, poly-(1,4)-β-D-glucosamine |
Sodium (meta) periodate, sodium chlorite | Freeze-drying Freeze-drying |
The production of advanced bio-adsorbents | [64] |
| Softwood bleached kraft pulp (SBKP) | Water/tert-butyl alcohol (TBA) | (TEMPO)-oxidized cellulose nanofibril (TOCN) | High performance air filter | [65] | ||
| Cellulose acetate | Acetone | Polymethylene polyphenylpolyisocyanate (PMDI) | ScCO2 drying | Thermal insulation application | [50] | |
2.1. Sol–Gel Procedure
- A colloidal suspension is produced by dispersing solid nanoscale particles formed from a reactant in a liquid.
- Adding an acidic or basic catalyst initiates crosslinking and leads to the linkage and spreading of particles, forming an interlinked network configuration.
- Gel aging: To strengthen the gel’s backbone and material toughness, it is aged in its mother solution.
- To avoid gel fracture, the solvent is extracted from the pores of the gel during drying [68].
2.2. Drying Methods of Cellulose-Based Aerogels
2.2.1. Drying by Supercritical Carbon Dioxide
2.2.2. Vacuum Freezing and Drying
2.2.3. Ambient Drying
3. Characterization Methods of Cellulose-Based Aerogels
3.1. Characterization of Cellulose Aerogels’ Structure
3.1.1. Microscopic Analyses
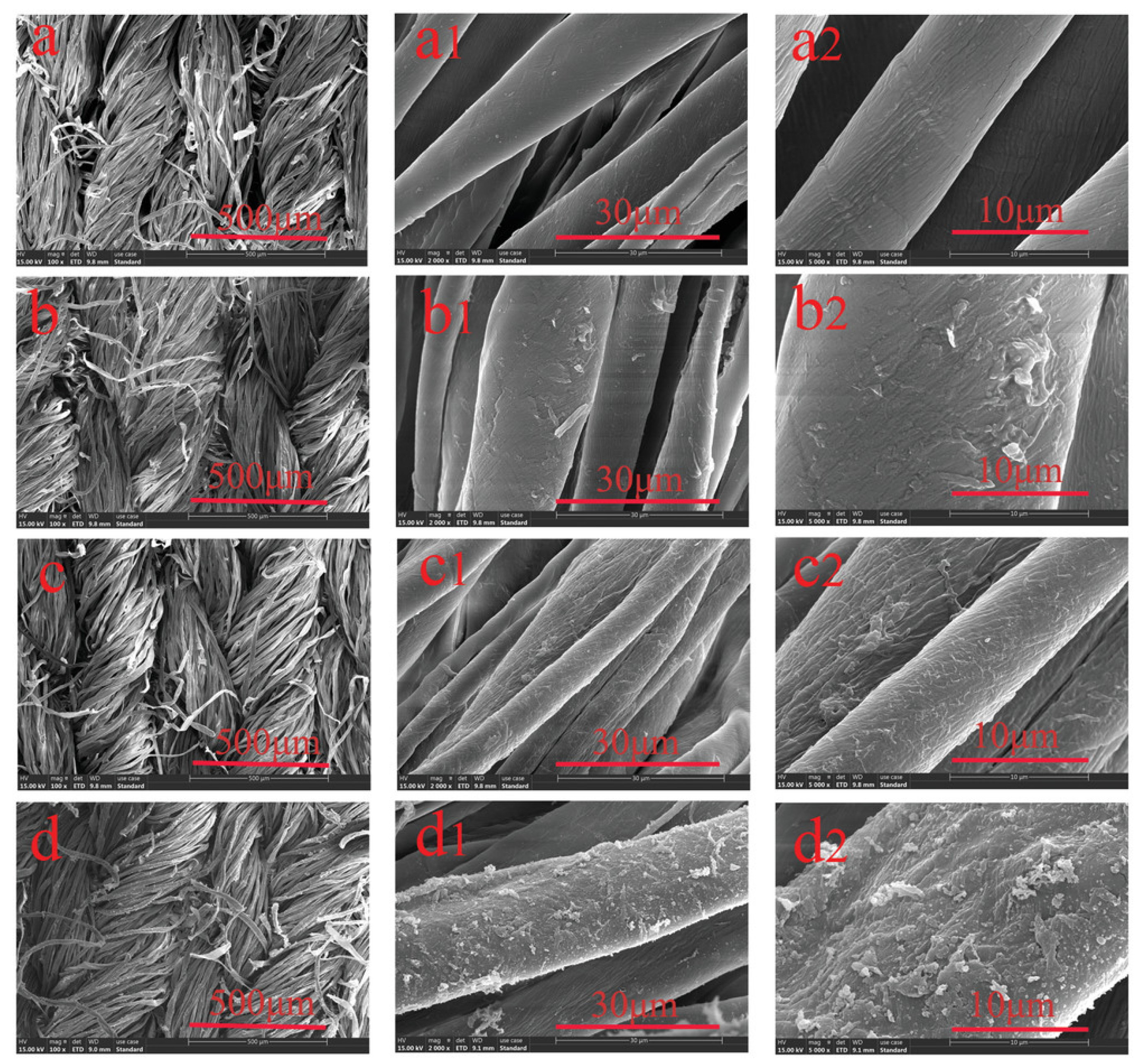
3.1.2. Scattering Techniques
3.1.3. Thermoporometry
3.1.4. Gas Sorption
- -
- Pressure-time curves were consistent with that of theoretical model created for pure Darcy flow, which was employed to fitting the data and get the permeability constant.
- -
- Permeability remained consistent regardless of the difference in pressure.
- -
- Choice of surfactant had an impact on the permeability.
3.1.5. Hg Porosimetry
3.2. Mechanical Characterization of Cellulose Aerogels
3.2.1. Differential Scanning Calorimetry (DSC), Dynamic Mechanical Analysis (DMA)
3.2.2. Tension, Compression
3.2.3. Sound Absorption and spreading
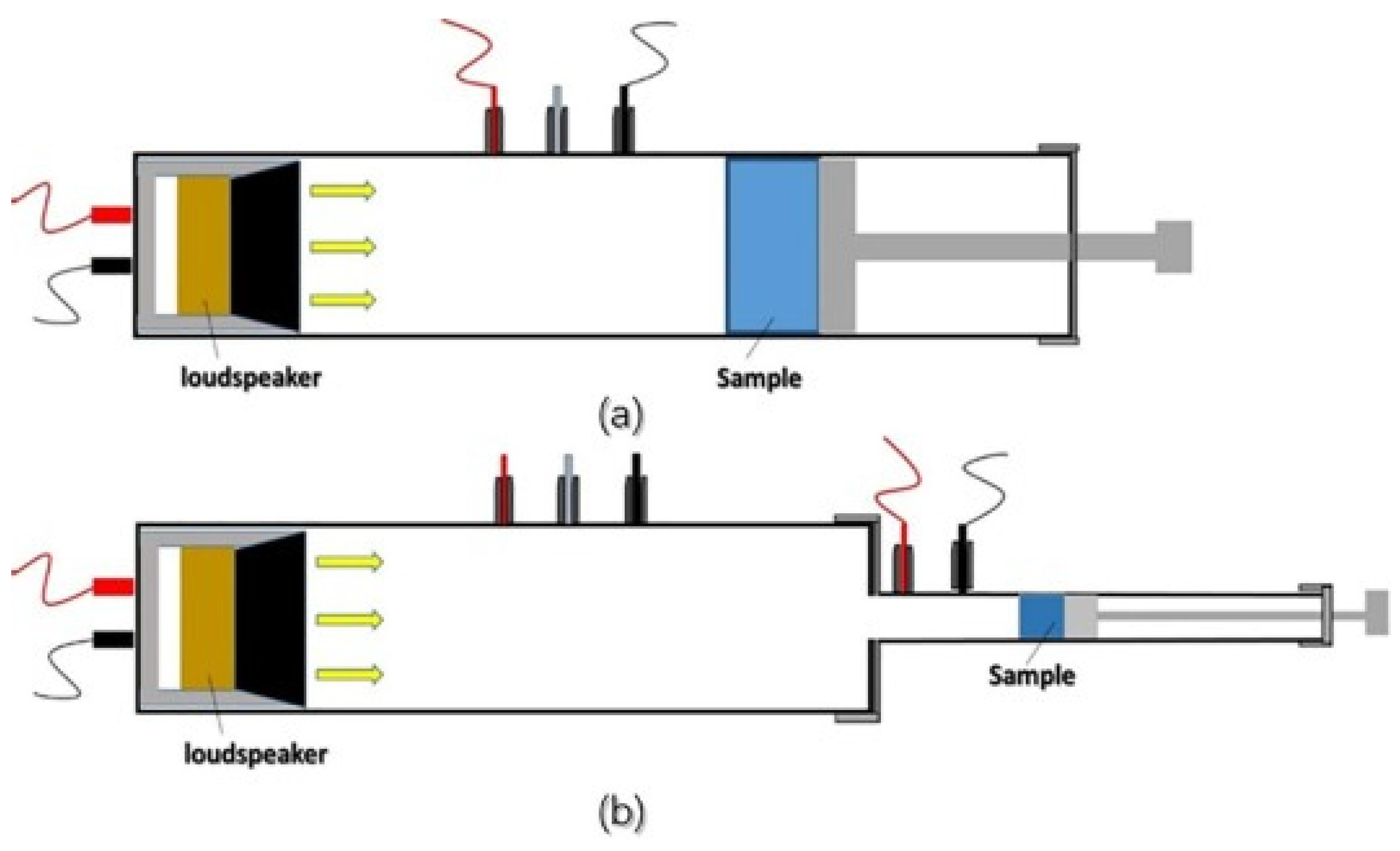
3.3. Thermal Characterization
4. Properties of Cellulose Aerogels
| Number | Aerogel Type | Main Properties | Application | Ref. |
|---|---|---|---|---|
| 1 | MXene composite aerogel (M−Aerogel) | Single-layered structure Conductive active material Three-dimensional porous structure Remarkable flexibility Superior compressive strength |
Flexible piezoresistive sensors | [162] |
| 2 | Holocellulose nanofibrils (HCNFs) Aerogel from Bamboo pulp and birch wood blocks | Fiber form aerogel properties Exceptional self-cleaning capabilities Outstanding thermal insulation performance Washability Impressive tensile strength Biodegradability Superb mechanical properties Potential for weaving into multifunctional textiles suitable for demanding environments |
Thermal management EMI shielding performance | [163] |
| 3 | Cellulose nanofibrils (CNFs) from rice straw cellulose | Amphiphilic - Hydrophobic and oleophilic nature High porosity Extremely lightweight |
Selective oil removal and recovery | [164] |
| 4 | Barley-straw cellulose aerogels | Highly porous and lightweight aerogel, large surface area, high concentration of cellulose content | Oil-spillage clean-up | [165] |
| 5 | Bio-inspired tubular cellulose aerogel from kapok fibers | Exceptionally high compressive strength of 32 MPa, self-extinguishing capabilities and exhibits excellent flame retardancy, cost-effective solution | Exterior wall insulation and vehicle interior | [166] |
| 6 | Bio-based aerogel (polysaccharide cryogel) from sodium alginate and chitosan | Eco-friendly and sustainable, excellent thermal insulation, bio-based flame-retardant, ultralight porous structure, practical mechanical properties, great flexibility, facilitating continuous flexing and rotating without fragmentation | Anti-flame apparel | [167] |
| 7 | Agar aerogels | substantial surface area per unit weight, significant acceleration in wound healing in vivo, the ability to be used for skin healing, in addition to its biocompatibility, renewability, and sustainability properties. | Wound dressing | [168] |
| 8 | Novel alginate-chitosan aerogel fibers | Highly porous structure reminiscent of cotton, non-cytotoxic, making it biocompatible, strong antibacterial activity, speeding wound closure in vitro design imitating injured life-unit monolayer healing | Wound healing applications | [169] |
| 9 | Aerogels made of tempo-oxidized cellulose nanofibers and sodium algin/chitosan | Serving as an interactive extracellular fabric, derived from biological sources and the capacity to degrade naturally, highly porous structure, creating an ideal microenvironment for various applications | Wound dressing, and injury tissue maturation | [170] |
| 10 | Alg-CaCO3 composite aerogels from Sodium alginate | Cost-effective, environmentally friendly, ultralight, and fireproof, characterized by high permeability and excellent structural properties, reduced heat transfer rate, and excellent hydrophobic characteristics | Green fireproof building insulation materials | [171] |
| 11 | Kapok aerogel | Lightweight, providing insulation and robustness, reusable and decomposable, and exceptional fire protection, high filling capacity, superior compressive resilience, and remarkable heat insulating abilities | Application in emerging fields | [172] |
| 12 | Chitosan aerogel | Elevated permeability and extensive superficial expanse, enabling rapid local administration of antibiotics, Infections are efficiently prevented early after wound debridement while cell viability is maintained, absorbing substantial amounts of aqueous fluids | The management of chronic wounds | [173] |
| 13 | A novel intelligent bio-aerogel using cellulose/Salep/anthocyanins | Maintaining structural integrity and allows for precise control over the porous structure, usage as intelligent aerogels in meat products, providing unique properties and benefits, serving as suitable matrices for pH-sensitive dyes, enabling their effective utilization | Application in beef packaging | [174] |
| 14 | Essential oil-loaded starch/cellulose aerogel | Aerogels with antimicrobial properties made from affordable materials | Application in cheese packaging | [175] |
| 15 | Hybrid bio-aerogel with green pectin (PML) and corn stalk nanofiber (CNF) | High porosity and low density, providing excellent elasticity. It exhibits a remarkable oil sorption capacity ranging from 82 to 161 g/g. | Applications to oil pollution treatment | [176] |
| 16 | Nanofibrillated cellulose/chitosan aerogel | Lightweight and flexible, having a well-defined three-dimensional linked cellular network structure, exhibiting outstanding mechanical properties both in air and underwater, high maximum adsorption capacity, rapid adsorption rate, and offers a low-cost solution with a long lifespan | Heavy metal pollution in agriculture | [177] |
| 17 | Aerogels comprising graphene oxide (EGO) and TEMPO-oxidized cellulose nanofibril (TOCNF) | Great promise as an environmentally friendly conductive ink suitable for printing 3D objects using the direct ink writing (DIW) method, the inks exhibit a high yield stress, improved electrical conductivity, uniform distribution of micro- and nano-scale fibrils, and efficient penetration, representing a sustainable approach to produce conductive carbon-based ink | Advanced applications (EMI shields) | [178] |
| 18 | Silica- cellulose nanoclaws hybrid aerogels | A biomimetic hybrid technique that is eco-friendly, cost-effective, outstanding formability and mechanical stability, as well as substantial surface area per unit weight, strength, Lightweight, and minimal heat transfer | Structures, industrial production, air transport, and cosmic space | [179] |
5. Multifunctional Application of Cellulose-Based Aerogels on Textile Structures
- -
- Other components can be added to the cellulose solution/suspension [11]. For example, the reaction of CNF with N-methylol-dimethyphospylpropionamide (MDPA) and further cross-linking by 1,2,3,4-butane tracarboxylic acid (BTCA) yields a flame retardant with good flexibility and self-extinguishment [180].
- -
- Coating or adding additional substances to the aerogel structure [11], such as the polyacrylonitrile-silica aerogel coating over viscose nonwoven fabric for protection and comfort [181]. Another area of study is the application of molecular layer by layer (m-LBL) technology. This technique enables the deposition of ultra-thin layers onto a surface through sequential covalent processes. As a consequence, a precise molecular-scale coating is generated, mostly by surface oligomerization, which is not possible with bulk synthesis techniques [182,183,184].
- -
5.1. Thermal Insulation Materials
| No. | Material | Drying Method | Thermal Conductivity | Pore Size | Density | Application | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Raw pineapple-leave fibers (PALF) | Freeze-drying | 0.030-0.034 W/mK | 1.38nm-2.21 nm | 0.04 g/cm3 | Heat and sound app. | [72] |
| 2 | Aerogels composed of bidirectional anisotropic polyimide/bacterial cellulose (b-PI/BC) | Freeze-drying | 23 mW/mK- 44 mW/mK (bidirectional PI/BC aerogels) 37 mW/mK -66 mK/mK (unidirectional PI/BC aerogels) |
10–20 μm | 46 mg/cm3 | Practical and complex thermal insulation applications in buildings and aerospace | [192] |
| 3 | Aerogels made of fibrous silica and bacterial cellulose (BC) | Ambient pressure drying | - | 13.7-15.5 nm | 0.164 g/cm3 | Wearable substances | [193] |
| 4 | Holocellulose nanofibrils/cellulose aerogel fiber (HCAFs) | ScCO2 drying | 0.048 W/mK | 265.4 ± 34.5 nm | 0.22 g/cm3 | Wearable substances | [163] |
| 5 | Multiscale nanocelluloses (NCs) | Freeze-drying | 25.4 mW/mK | 32 - 48 nm | 7.2 kg/m3 | Thermal insulation app. | [194] |
| 6 | Textile waste fibers (TWF) aerogel | Freeze-drying | 0.049 - 0.061 W/mK | - | 0.040-0.096 g/cm3 | Building insulation and oil spill cleanup. | [195] |
| 7 | Nanofibrous Kevlar Aerogel Threads | ScCO2 drying and Freeze-drying | 0.036 W/mK | 11-12.8 nm | 13 g/cm3 | Thermal insulation and thermal management. | [196] |
| 8 | Hydrophilic recycled cellulose aerogels | Freeze-drying | 0.029 -0.032 W/mK | 40-200 µm | 0.040 g/cm3 | Sorption of water/oil, resistance of water, and thermal insulation | [197] |
| 9 | Silk fibroin aerogel | Freeze-drying | 0.031 W/(mK) | 19.71 ± 8.53 | 0.21 g/cm3 | High performance thermal insulation | [198] |
| 10 | Aerogels made of nanofibrillated cellulose | Spray lyophilization | 0.018 W/(mK) | 10 to 100 nm | 0.012–0.033 g/cm3 | Thermal super insulating material | [110] |
5.2. Flame Retardancy
5.3. Medical Applications
6. Companies of Producing Cellulose Aerogels
7. Global Market Study Focused on Cellulose-based Aerogel and Their Future Aspects
8. Conclusion
9. List of Abbreviations
| Acronym | Description |
| DP | Degree of Polymerization |
| NaOH | Sodium Hydroxide |
| NMMO | N-methyl-morpholine N-oxide |
| 3D | Three dimensional |
| PVA | Polyvinyl Alcohol |
| TEMPO | 2,2,6,6-Tetramethylpiperidin-1-yl)oxyl |
| CNF | Cellulose Nanofibers |
| MO | Methyl Orange |
| NaClO | Sodium Hypochlorite |
| NaBr | Sodium bromide |
| BC | Bacterial Cellulose |
| DIW | Deionized Water |
| EMIM | Imidazolium acetate |
| ([DBNH][OAc]) | Non-enium acetate |
| DMSO | Dimethyl sulfoxide |
| SC CO2 | Supercritical Carbondioxide |
| NMP | Methyl-pyrrolidone |
| KOH | Potassium hydroxide |
| BCNF | Bamboo cellulose nanofibrils |
| MBA | N, N′-methylenebisacrylamide |
| MTMS | Methyltrimethoxysilane |
| SBKP | Softwood bleached kraft pulp |
| TBA | Tert-butyl alcohol |
| (TEMPO)- (TOCN) | 2,2,6,6-Tetramethylpiperidin-1-yl)oxyl, oxidized cellulose nanofibril |
| PMDI | Polymethylene polyphenylpolyisocyanate |
| PF | Pineapple Fiber |
| CO2 | Carbondioxide |
| HT | High temperature |
| LT | Low temperature |
| ESEM | Environmental Scanning electron Microscope |
| t-BuOH | Tert butyl alcohol |
| LS | Light Microscopy |
| AFM | Atomic force microscopy |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TA | Tannic acid |
| TA/B | Tannic acid/Borax |
| TA/B@PDA | Tannic acid/borax Polydopamine |
| SAS | Small-angle scattering |
| WAS | Wide-angle scattering |
| XRD | X-ray diffraction |
| USAXS | Ultra-low-angle scattering |
| PSD | Pore size distribution |
| N2 | Nitrogen |
| Hg | Mercury |
| DSC | Differential scanning calorimetry |
| DMA | Dynamic mechanical analysis |
| ASTM D638 | American Society for Testing and Materials- Standard Test Method for Tensile Properties of Plastics |
| ASTM D695 | American Society for Testing and Materials- Standard Test Method for Compressive Properties of Rigid Plastics |
| ASTM D3574 | American Society for Testing and Materials-Standard Test Methods for Flexible Cellular Materials—Slab, Bonded, and Molded Urethane Foams |
| ASTM E1050-10 | Standard Test Method for Impedance and Absorption of Acoustical Materials Using A Tube, Two Microphones and A Digital Frequency Analysis System |
| SW422, SW477, BSWA Technology Co. Ltd., China | SW series Impedance Tubes can accurately measure sound absorption coefficients and impedance |
| Amprobe SM-10, USA | Sound Meter, United States of America |
| Hz | Hertz |
| CC-BY license | Creative Commons Attribution |
| TGA | Thermogravimetric analysis |
| TPS | Transient plane source |
| DTG | Derivative thermogravimetric analysis |
| C, H, O | Carbon, Hydrogen, Oxygen |
| LOI | limiting oxygen index |
| CNF | Cellulose Nanofibril |
| UL-94 | The Standard Tests for Flammability -Vertical burning tests |
| MPa | Megapascal |
| HCNFs | Holocellulose nanofibrils |
| EMI | Electromagnetic Interference |
| MXene | Two-dimensional (2D) layered conductive nanomaterial, composed of transition metal carbide/nitride |
| CaCO3 | Calcium carbonate |
| PML | Premna Microphylla |
| EGO | Electrochemically synthesized graphene oxide |
| DIW | Direct Ink Writing |
| TEMPO- (TOCNF) | 2,2,6,6-tetramethylpiperidine-1-oxyl oxidized cellulose nanofibrils |
| MDPA | N-methylol-dimethyphospylpropionamide |
| BTCA | 1,2,3,4-butane tracarboxylic acid |
| m-LBL | Molecular layer by layer |
| PDMS | Poly(dimethyl siloxane |
| CFs | Cellulose Fibers |
| PALF | Pineapple-leave fibers |
| b-PI/BC | Bidirectional anisotropic polyimide/bacterial cellulose |
| HCAFs | Holocellulose nanofibrils/cellulose aerogel fibers |
| NCs | Nanocelluloses |
| TWF | Textile waste fibers |
| MH NPs | Magnesium hydroxide nanoparticles |
| FTIR | Fourier-transform infrared spectroscopy |
| AFM | Atomic force microscopy |
| MTT assay | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay |
| PCR | Polymerase chain reaction |
| SXAS | Small Angle X-Ray Scattering |
| CAGR | Compound annual growth rate |
| LiOH | Lithium hydroxide |
Declaration of Competing Interest
Acknowledgments
References
- Barrios, E.; Fox, D.; Li Sip, Y.Y.; Catarata, R.; Calderon, J.E.; Azim, N.; Afrin, S.; Zhang, Z.; Zhai, L. Nanomaterials in Advanced, High-Performance Aerogel Composites: A Review. Polymers 2019, 11, 726. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Revin, V.V.; Nazarova, N.B.; Tsareva, E.E.; Liyaskina, E.V.; Revin, V.D.; Pestov, N.A. Production of Bacterial Cellulose Aerogels With Improved Physico-Mechanical Properties and Antibacterial Effect. Front. Bioeng. Biotechnol. 2020, 8, 1392. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef]
- Bheekhun, N.; Abu Talib, A.R.; Hassan, M.R. Aerogels in Aerospace: An Overview. Adv. Mater. Sci. Eng. 2013, 2013, e406065. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Heavy metals in Iberian soils: Removal by current adsorbents/amendments and prospective for aerogels. Adv. Colloid Interface Sci. 2016, 237, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Stergar, J.; Maver, U. Review of aerogel-based materials in biomedical applications. J. Sol-Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef]
- Jiang, Y.; Chowdhury, S.; Balasubramanian, R. New insights into the role of nitrogen-bonding configurations in enhancing the photocatalytic activity of nitrogen-doped graphene aerogels. J. Colloid Interface Sci. 2019, 534, 574–585. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Meador, M.A.B.; Scheiman, D.; McCorkle, L. Polyimide Aerogels Using Triisocyanate as Cross-linker. ACS Appl. Mater. Interfaces 2017, 9, 27313–27321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- García-González, C.A.; Uy, J.J.; Alnaief, M.; Smirnova, I. Preparation of tailor-made starch-based aerogel microspheres by the emulsion-gelation method. Carbohydr. Polym. 2012, 88, 1378–1386. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, Flexible, and Superhydrophobic Functionalized Cellulose Aerogel for Selective and Versatile Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Novak, B.M.; Auerbach, D.; Verrier, C. Low-Density, Mutually Interpenetrating Organic-Inorganic Composite Materials via Supercritical Drying Techniques. Chem. Mater. 1994, 6, 282–286. [Google Scholar] [CrossRef]
- Fernandes, E.M.; Pires, R.A.; Mano, J.F.; Reis, R.L. Bionanocomposites from lignocellulosic resources: Properties, applications and future trends for their use in the biomedical field. Prog. Polym. Sci. 2013, 38, 1415–1441. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S. Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Jianan, C.; Shaoqiong, Y.; Jinyue, R. A study on the preparation, structure, and properties of microcrystalline cellulose. J. Macromol. Sci. Part Pure Appl. Chem. 1996, 33, 1851–1862. [Google Scholar] [CrossRef]
- Virtanen, T.; Svedström, K.; Andersson, S.; Tervala, L.; Torkkeli, M.; Knaapila, M.; Kotelnikova, N.; Maunu, S.L.; Serimaa, R. A physico-chemical characterisation of new raw materials for microcrystalline cellulose manufacturing. Cellulose 2012, 19, 219–235. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Properties and potential applications of natural cellulose fibers from the bark of cotton stalks. Bioresour. Technol. 2009, 100, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.; Luu, T.; Le, P.; Le, P.; Hoang Nguyen Do, N.; Chau, N.D.Q. Fabrication of cotton aerogels and its application in water treatment; 2020. [Google Scholar]
- Wang, X.; Li, H.; Cao, Y.; Tang, Q. Cellulose extraction from wood chip in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Bioresour. Technol. 2011, 102, 7959–7965. [Google Scholar] [CrossRef] [PubMed]
- Cara, C.; Ruiz, E.; Ballesteros, I.; Negro, M.J.; Castro, E. Enhanced enzymatic hydrolysis of olive tree wood by steam explosion and alkaline peroxide delignification. Process Biochem. 2006, 41, 423–429. [Google Scholar] [CrossRef]
- Abe, K.; Yano, H. Comparison of the characteristics of cellulose microfibril aggregates of wood, rice straw and potato tuber. Cellulose 2009, 16, 1017–1023. [Google Scholar] [CrossRef]
- Hoang Nguyen Do, N.; Tran, V.; Tran, Q.; Le, K.; Nguyen, P.; Duong, H.; Thai, Q.B.; Le, P. Recycling of Pineapple Leaf and Cotton Waste Fibers into Heat-insulating and Flexible Cellulose Aerogel Composites. J. Environ. Polym. Degrad. 2021, 29. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Zhao, H.; Sun, R.C. Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad. Stab. 2004, 84, 331–339. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.M.; Haafiz, M.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Hinterstoisser, B.; Salmén, L. Application of dynamic 2D FTIR to cellulose. Vib. Spectrosc. 2000, 22, 111–118. [Google Scholar] [CrossRef]
- Bochek, A.M. Effect of Hydrogen Bonding on Cellulose Solubility in Aqueous and Nonaqueous Solvents. Russ. J. Appl. Chem. 2003, 76, 1711–1719. [Google Scholar] [CrossRef]
- Lavanya, D.; Kulkarni, P.; Dixit, M.; Raavi, P.K.; Krishna, L.N.V. Sources of cellulose and their applications- A review. Int. J. Drug Formul. Res. 2011, 2, 19–38. [Google Scholar]
- Physical Chemistry of Non-aqueous Solutions of Cellulose and Its Derivatives | Wiley. Available online: https://www.wiley.com/en-us/Physical+Chemistry+of+Non+aqueous+Solutions+of+Cellulose+and+Its+Derivatives-p-9780471959243 (accessed on 28 December 2021).
- Biopolymers from Polysaccharides and Agroproteins, Copyright, Foreword. In Biopolymers from Polysaccharides and Agroproteins; ACS Symposium Series; American Chemical Society, 2001; Volume 786, pp. i–v. ISBN 978-0-8412-3645-5.
- Sözcü, S.; Venkataraman, M.; Tomkova, B.; Militky, J. Cellulose-Based Aerogels Understading Their Origin, Preparation and Multifunctional Effect for Potential Application. In Selected Topics in Fibrous Materials Science, TUL FT Liberec; Technicka Univerzita v Liberci, 2022; p. 93. [Google Scholar]
- Hon, D.N.-S.; Shiraishi, N. Wood and Cellulosic Chemistry, Second Edition, Revised, and Expanded; CRC Press, 2000; ISBN 978-0-8247-0024-9. [Google Scholar]
- Surapolchai, W.; Schiraldi, D.A. The effects of physical and chemical interactions in the formation of cellulose aerogels. Polym. Bull. 2010, 65, 951–960. [Google Scholar] [CrossRef]
- Ahmadi, M.; Madadlou, A.; Saboury, A.A. Whey protein aerogel as blended with cellulose crystalline particles or loaded with fish oil. Food Chem. 2016, 196, 1016–1022. [Google Scholar] [CrossRef]
- Seantier, B.; Bendahou, D.; Bendahou, A.; Grohens, Y.; Kaddami, H. Multi-scale cellulose based new bio-aerogel composites with thermal super-insulating and tunable mechanical properties. Carbohydr. Polym. 2016, 138, 335–348. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Cudjoe, E.; Douglas, A.; Scheiman, D.; McCorkle, L.; Meador, M.A.B.; Rowan, S.J. Polyimide Cellulose Nanocrystal Composite Aerogels. Macromolecules 2016, 49, 1692–1703. [Google Scholar] [CrossRef]
- Liebner, F.; Potthast, A.; Rosenau, T.; Haimer, E.; Wendland, M. Cellulose aerogels: Highly porous, ultra-lightweight materials. Holzforschung 2008, 62, 129–135. [Google Scholar] [CrossRef]
- Ratke, L. Monoliths and Fibrous Cellulose Aerogels. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Advances in Sol-Gel Derived Materials and Technologies; Springer: New York, NY, USA, 2011; pp. 173–190. ISBN 978-1-4419-7589-8. [Google Scholar]
- Innerlohinger, J.; Weber, H.K.; Kraft, G. Aerocellulose: Aerogels and Aerogel-like Materials made from Cellulose. Macromol. Symp. 2006, 244, 126–135. [Google Scholar] [CrossRef]
- Hoepfner, S.; Ratke, L.; Milow, B. Synthesis and characterisation of nanofibrillar cellulose aerogels. Cellulose 2008, 15, 121–129. [Google Scholar] [CrossRef]
- Sescousse, R.; Gavillon, R.; Budtova, T. Aerocellulose from cellulose–ionic liquid solutions: Preparation, properties and comparison with cellulose–NaOH and cellulose–NMMO routes. Carbohydr. Polym. 2011, 83, 1766–1774. [Google Scholar] [CrossRef]
- Tan, C.; Fung, B.; Newman, J.; Vu, C. Organic Aerogels with Very High Impact Strength. Adv. Mater. 2001, 13, 644–646. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloids Surf. Physicochem. Eng. Asp. 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Fischer, F.; Rigacci, A.; Pirard, R.; Berthon-Fabry, S.; Achard, P. Cellulose-based aerogels. Polymer 2006, 47, 7636–7645. [Google Scholar] [CrossRef]
- Fischer, S. Anorganische Salzhydratschmelzen. 2009. Available online: https://tubaf.qucosa.de/landingpage/?tx_dlf[id]=https%3A%2F%2Ftubaf.qucosa.de%2Fapi%2Fqucosa%253A22449%2Fmets (accessed on 6 June 2023).
- Fischer, S.; Leipner, H.; Thümmler, K.; Brendler, E.; Peters, J. Inorganic Molten Salts as Solvents for Cellulose. Cellulose 2003, 10, 227–236. [Google Scholar] [CrossRef]
- Frey, M.W.; Theil, M.H. Calculated phase diagrams for cellulose/ammonia/ammonium thiocyanate solutions in comparison to experimental results. Cellulose 2004, 11, 53–63. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Sadineni, S.B.; Madala, S.; Boehm, R.F. Passive building energy savings: A review of building envelope components. Renew. Sustain. Energy Rev. 2011, 15, 3617–3631. [Google Scholar] [CrossRef]
- Rahbar Shamskar, K.; Heidari, H.; Rashidi, A. Preparation and evaluation of nanocrystalline cellulose aerogels from raw cotton and cotton stalk. Ind. Crops Prod. 2016, 93, 203–211. [Google Scholar] [CrossRef]
- Pääkkö, M.; Vapaavuori, J.; Silvennoinen, R.; Kosonen, H.; Ankerfors, M.; Lindström, T.; Berglund, L.A.; Ikkala, O. Long and entangled native cellulose I nanofibers allow flexible aerogels and hierarchically porous templates for functionalities. Soft Matter 2008, 4, 2492–2499. [Google Scholar] [CrossRef]
- Nguyen, V.; Quoc Lam, H.; Nguyen, T.; Ly, P.; Nguyen, D.M.; Hoang, D. Nanocellulose and Graphene Oxide Aerogels for Adsorption and Removal Methylene Blue from an Aqueous Environment. ACS Omega 2021, XXXX. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Li, C.; Liang, H.-W.; Chen, J.-F.; Yu, S.-H. Ultralight, Flexible, and Fire-Resistant Carbon Nanofiber Aerogels from Bacterial Cellulose. Angew. Chem. Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef]
- Négrier, M.; Ahmar, E.E.; Sescousse, R.; Sauceau, M.; Budtova, T. Upcycling of textile waste into high added value cellulose porous materials, aerogels and cryogels. RSC Sustain. 2023, 1, 335–345. [Google Scholar] [CrossRef]
- Yang, X.; Fei, B.; Ma, J.; Liu, X.; Yang, S.; Tian, G.; Jiang, Z. Porous nanoplatelets wrapped carbon aerogels by pyrolysis of regenerated bamboo cellulose aerogels as supercapacitor electrodes. Carbohydr. Polym. 2018, 180, 385–392. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, G.; Wang, H.; Xu, D.; Zeng, K.; Wu, X.; Ren, D. Development of a novel bamboo cellulose nanofibrils hybrid aerogel with high thermal-insulating performance for fresh strawberry cold-chain logistics. Int. J. Biol. Macromol. 2023, 229, 452–462. [Google Scholar] [CrossRef]
- Yang, H.; Sheikhi, A.; van de Ven, T.G.M. Reusable Green Aerogels from Cross-Linked Hairy Nanocrystalline Cellulose and Modified Chitosan for Dye Removal. Langmuir 2016, 32, 11771–11779. [Google Scholar] [CrossRef]
- Nemoto, J.; Saito, T.; Isogai, A. Simple Freeze-Drying Procedure for Producing Nanocellulose Aerogel-Containing, High-Performance Air Filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Dervin, S.; Pillai, S. An Introduction to Sol-Gel Processing for Aerogels; 2017; pp. 1–22. ISBN 978-3-319-50142-0. [Google Scholar]
- Neacşu, I.A.; Nicoară, A.I.; Vasile, O.R.; Vasile, B.Ş. Chapter 9 - Inorganic micro- and nanostructured implants for tissue engineering. In Nanobiomaterials in Hard Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing, 2016; pp. 271–295. ISBN 978-0-323-42862-0. [Google Scholar]
- Carraher, C.E. General Topics. Polym. News 2005, 30, 386–388. [Google Scholar] [CrossRef]
- Do, N.H.N.; Luu, T.P.; Thai, Q.B.; Le, D.K.; Chau, N.D.Q.; Nguyen, S.T.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Heat and sound insulation applications of pineapple aerogels from pineapple waste. Mater. Chem. Phys. 2020, 242, 122267. [Google Scholar] [CrossRef]
- Duong, H.; Xie, Z.; Wei, K.; Nian, N.; Tan, K.; Lim, H.; Li, A.; Chung, K.-S.; Lim, W. Thermal Jacket Design Using Cellulose Aerogels for Heat Insulation Application of Water Bottles. Fluids 2017, 2, 64. [Google Scholar] [CrossRef]
- Karadagli, I.; Milow, B.; Ratke, L.; Schulz, B.; Seide, G.; Gries, T. Synthesis and characterization of highly porous cellulose aerogels for textiles applications. Proc. Cell. Mater. Cellmat 2012 2012. [Google Scholar]
- Han, Y.; Zhang, X.; Wu, X.; Lu, C. Flame Retardant, Heat Insulating Cellulose Aerogels from Waste Cotton Fabrics by in Situ Formation of Magnesium Hydroxide Nanoparticles in Cellulose Gel Nanostructures. ACS Sustain. Chem. Eng. 2015, 3, 1853–1859. [Google Scholar] [CrossRef]
- Bao, M.X.; Xu, S.; Wang, X.; Sun, R. Porous Cellulose Aerogels with High Mechanical Performance and their Absorption Behaviors. Bioresources 2016, 11, 8–20. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—Airy Materials: Chemistry, Structure, and Properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Quiño, J.; Ruehl, M.; Klima, T.; Ruiz, F.; Will, S.; Braeuer, A. Supercritical drying of aerogel: In situ analysis of concentration profiles inside the gel and derivation of the effective binary diffusion coefficient using Raman spectroscopy. J. Supercrit. Fluids 2016, 108, 1–12. [Google Scholar] [CrossRef]
- Fricke, J.; Tillotson, T. Aerogels: production, characterization, and applications. Thin Solid Films 1997, 297, 212–223. [Google Scholar] [CrossRef]
- Özbakır, Y.; Erkey, C. Experimental and theoretical investigation of supercritical drying of silica alcogels. J. Supercrit. Fluids 2015, 98, 153–166. [Google Scholar] [CrossRef]
- Sanz-Moral, L.M.; Rueda, M.; Mato, R.; Martín, Á. View cell investigation of silica aerogels during supercritical drying: Analysis of size variation and mass transfer mechanisms. J. Supercrit. Fluids 2014, 92, 24–30. [Google Scholar] [CrossRef]
- Griffin, J.S.; Mills, D.H.; Cleary, M.; Nelson, R.; Manno, V.P.; Hodes, M. Continuous extraction rate measurements during supercritical CO2 drying of silica alcogel. J. Supercrit. Fluids 2014, 94, 38–47. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Liebner, F.; Haimer, E.; Wendland, M.; Neouze, M.-A.; Schlufter, K.; Miethe, P.; Heinze, T.; Potthast, A.; Rosenau, T. Aerogels from Unaltered Bacterial Cellulose: Application of scCO2 Drying for the Preparation of Shaped, Ultra-Lightweight Cellulosic Aerogels. Macromol. Biosci. 2010, 10, 349–352. [Google Scholar] [CrossRef]
- Fabrication of mesoporous lignocellulose aerogels from wood via cyclic liquid nitrogen freezing–thawing in ionic liquid solution - Journal of Materials Chemistry (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2012/JM/c2jm31310c (accessed on 19 January 2022).
- Pircher, N.; Carbajal, L.; Schimper, C.; Bacher, M.; Rennhofer, H.; Nedelec, J.-M.; Lichtenegger, H.C.; Rosenau, T.; Liebner, F. Impact of selected solvent systems on the pore and solid structure of cellulose aerogels. Cellulose 2016, 23, 1949–1966. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Jiang, H.; Song, Y.; Zhou, Z.; Zhao, H. Fabrication and characterization of nano-cellulose aerogels via supercritical CO2 drying technology. Mater. Lett. 2016, 183. [Google Scholar] [CrossRef]
- Heath, L.; Thielemans, W. Cellulose nanowhisker aerogels. Green Chem. 2010, 12, 1448–1453. [Google Scholar] [CrossRef]
- Schestakow, M.; Karadagli, I.; Ratke, L. Cellulose aerogels prepared from an aqueous zinc chloride salt hydrate melt. Carbohydr. Polym. 2016, 137, 642–649. [Google Scholar] [CrossRef]
- Tajiri, K.; Igarashi, K.; Nishio, T. Effects of supercritical drying media on structure and properties of silica aerogel. J. Non-Cryst. Solids 1995, 186, 83–87. [Google Scholar] [CrossRef]
- Laudise, R.A.; Johnson, D.W. Supercritical drying of gels. J. Non-Cryst. Solids 1986, 79, 155–164. [Google Scholar] [CrossRef]
- Kocon, L.; Despetis, F.; Phalippou, J. Ultralow density silica aerogels by alcohol supercritical drying. J. Non-Cryst. Solids 1998, 225, 96–100. [Google Scholar] [CrossRef]
- Stolarski, M.; Walendziewski, J.; Steininger, M. Barbara Pniak Synthesis and characteristic of silica aerogels. Appl. Catal. Gen. 1999, 177, 139–148. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Lee, Y.K.; Chavan, N.K.; Mahadik, S.A.; Park, H.-H. Monolithic and shrinkage-free hydrophobic silica aerogels via new rapid supercritical extraction process. J. Supercrit. Fluids 2016, 107, 84–91. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.-D.; Cui, S. Direct synthesis of anatase TiO2 aerogel resistant to high temperature under supercritical ethanol. Mater. Lett. 2014, 117, 192–194. [Google Scholar] [CrossRef]
- Kirkbir, F.; Murata, H.; Meyers, D.; Chaudhuri, S.R. Drying of Large Monolithic Aerogels between Atmospheric and Supercritical Pressures. J. Sol-Gel Sci. Technol. 1998, 13, 311–316. [Google Scholar] [CrossRef]
- Kirkbir, F.; Murata, H.; Meyers, D.; Chaudhuri, S.R. Drying of aerogels in different solvents between atmospheric and supercritical pressures. J. Non-Cryst. Solids 1998, 225, 14–18. [Google Scholar] [CrossRef]
- Dowson, M.; Grogan, M.; Birks, T.; Harrison, D.; Craig, S. Streamlined life cycle assessment of transparent silica aerogel made by supercritical drying. Appl. Energy 2012, 97, 396–404. [Google Scholar] [CrossRef]
- van Bommel, M.J.; de Haan, A.B. Drying of silica aerogel with supercritical carbon dioxide. J. Non-Cryst. Solids 1995, 186, 78–82. [Google Scholar] [CrossRef]
- Sanz-Moral, L.M.; Rueda, M.; Nieto, A.; Novak, Z.; Knez, Ž.; Martín, Á. Gradual hydrophobic surface functionalization of dry silica aerogels by reaction with silane precursors dissolved in supercritical carbon dioxide. J. Supercrit. Fluids 2013, 84, 74–79. [Google Scholar] [CrossRef]
- Pajonk, G.M.; Venkateswara Rao, A.; Sawant, B.M.; Parvathy, N.N. Dependence of monolithicity and physical properties of TMOS silica aerogels on gel aging and drying conditions1Work supported by the Region Rhone-Alpes Foundation (France) and the Department of Atomic Energy (Project No. 34/12/90-G), Government of India.1. J. Non-Cryst. Solids 1997, 209, 40–50. [Google Scholar] [CrossRef]
- Masmoudi, Y.; Rigacci, A.; Ilbizian, P.; Cauneau, F.; Achard, P. Diffusion During the Supercritical Drying of Silica Gels. Dry. Technol. 2006, 24, 1121–1125. [Google Scholar] [CrossRef]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Gao, Y.-B.; Sun, Y.-H.; Chen, S.-Y. Effect of preparation parameters on the texture of SiO2 aerogels. Catal. Today 1996, 30, 171–175. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Jiang, H.; Song, Y.; Zhou, Z.; Zhao, H. Tert-butyl alcohol used to fabricate nano-cellulose aerogels via freeze-drying technology. Mater. Res. Express 2017, 4, 065006. [Google Scholar] [CrossRef]
- Pons, A.; Casas, L.; Estop, E.; Molins, E.; Harris, K.D.M.; Xu, M. A new route to aerogels: Monolithic silica cryogels. J. Non-Cryst. Solids 2012, 358, 461–469. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing–thawing. J. Mater. Chem. A 2013, 2, 350–359. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, Z.; Wang, H. The effect of freezing speed and hydrogel concentration on the microstructure and compressive performance of bamboo-based cellulose aerogel. J. Wood Sci. 2015, 61, 595–601. [Google Scholar] [CrossRef]
- Nakagaito, A.; Kondo, H.; Takagi, H. Cellulose nanofiber aerogel production and applications. J. Reinf. Plast. Compos. 2013, 32, 1547–1552. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Spray freeze-dried nanofibrillated cellulose aerogels with thermal superinsulating properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel Microspheres from Natural Cellulose Nanofibrils and Their Application as Cell Culture Scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose Aerogels from Aqueous Alkali Hydroxide–Urea Solution. ChemSusChem 2008, 1, 149–154. [Google Scholar] [CrossRef]
- Beaumont, M.; Kondor, A.; Plappert, S.; Mitterer, C.; Opietnik, M.; Potthast, A.; Rosenau, T. Surface properties and porosity of highly porous, nanostructured cellulose II particles. Cellulose 2017, 24, 435–440. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, R.; Xiao, S.; Yin, Y.; Liu, Q.; Li, J. Cellulose Based Aerogels: Processing and Morphology. In RSC Green Chemistry; 2018; pp. 25–41. ISBN 978-1-78262-765-4. [Google Scholar]
- Reichenauer, G. Structural Characterization of Aerogels. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Advances in Sol-Gel Derived Materials and Technologies; Springer: New York, NY, USA, 2011; pp. 449–498. ISBN 978-1-4419-7589-8. [Google Scholar]
- Luo, Y.; Wang, S.; Fu, X.; Du, X.; Wang, H.; Zhou, M.; Cheng, X.; Du, Z. Fabrication of a Bio-Based Superhydrophobic and Flame-Retardant Cotton Fabric for Oil–Water Separation. Macromol. Mater. Eng. 2021, 306, 2000624. [Google Scholar] [CrossRef]
- Walenta, E. Small angle x-ray scattering. Von O. GLATTER und O. KRATKY. London: Academic Press Inc. Ltd. 1982. ISBN 0-12-286280-5. X, 515 Seiten, geb. £ 43,60; US $ 81.00. Acta Polym. 1985, 36, 296. [Google Scholar] [CrossRef]
- Hunt, A.J. Light scattering for aerogel characterization. J. Non-Cryst. Solids 1998, 225, 303–306. [Google Scholar] [CrossRef]
- Fauziyah, M.; Widiyastuti, W.; Balgis, R.; Setyawan, H. Production of cellulose aerogels from coir fibers via an alkali–urea method for sorption applications. Cellulose 2019, 26. [Google Scholar] [CrossRef]
- Qiu, J.; Guo, X.; Lei, W.; Ding, R.; Zhang, Y.; Yang, H. Facile Preparation of Cellulose Aerogels with Controllable Pore Structure. Nanomaterials 2023, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Pirard, R.; Rigacci, A.; Maréchal, J.C.; Quenard, D.; Chevalier, B.; Achard, P.; Pirard, J.P. Characterization of hyperporous polyurethane-based gels by non-intrusive mercury porosimetry. Polymer 2003, 44, 4881–4887. [Google Scholar] [CrossRef]
- Rudaz, C.; Courson, R.; Bonnet, L.; Calas-Etienne, S.; Sallée, H.; Budtova, T. Aeropectin: Fully Biomass-Based Mechanically Strong and Thermal Superinsulating Aerogel. Biomacromolecules 2014, 15, 2188–2195. [Google Scholar] [CrossRef]
- Thomson, W. 4. On the Equilibrium of Vapour at a Curved Surface of Liquid. Proc. R. Soc. Edinb. 1872, 7, 63–68. [Google Scholar] [CrossRef]
- Gibbs, J.W. The Collected Works of J. Willard Gibbs: Thermodynamics; Yale University Press, 1948. [Google Scholar]
- Kaviany, M. Principles of Heat Transfer in Porous Media; Springer Science & Business Media, 2012; ISBN 978-1-4612-4254-3. [Google Scholar]
- Tanikawa, W.; Shimamoto, T. Klinkenberg effect for gas permeability and its comparison to water permeability for porous sedimentary rocks. Hydrol. Earth Syst. Sci. Discuss. 2006, 3, 1315–1338. [Google Scholar] [CrossRef]
- The Physics of Flow Through Porous Media (3rd Edition). Available online: https://www.degruyter.com/document/doi/10.3138/9781487583750/html (accessed on 26 June 2023).
- Mercury Porosimetry: A General (Practical) Overview - Giesche - 2006 - Particle & Particle Systems Characterization - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/ppsc.200601009 (accessed on 26 June 2023).
- Pirard, R.; Alie, C.; Pirard, J.-P. Characterization of porous texture of hyperporous materials by mercury porosimetry using densification equation. Powder Technol. - POWDER TECHNOL 2002, 128, 242–247. [Google Scholar] [CrossRef]
- Job, N.; Pirard, R.; Pirard, J.-P.; Alié, C. Non Intrusive Mercury Porosimetry: Pyrolysis of Resorcinol-Formaldehyde Xerogels. Part. Part. Syst. Charact. 2006, 23, 72–81. [Google Scholar] [CrossRef]
- Hossen, M.; Talbot, M.; Kennard, R.; Bousfield, D.; Mason, M. A comparative study of methods for porosity determination of cellulose based porous materials. Cellulose 2020, 27. [Google Scholar] [CrossRef]
- Lu, H.; Luo, H.; Leventis, N. Mechanical Characterization of Aerogels. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Advances in Sol-Gel Derived Materials and Technologies; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7589-8. [Google Scholar]
- Wingfield, C.; Baski, A.; Bertino, M.F.; Leventis, N.; Mohite, D.P.; Lu, H. Fabrication of Sol−Gel Materials with Anisotropic Physical Properties by Photo-Cross-Linking. Chem. Mater. 2009, 21, 2108–2114. [Google Scholar] [CrossRef]
- Dieter, G.E.; Bacon, D. Mechanical metallurgy; McGraw-hill New York, 1976; Volume 3. [Google Scholar]
- Ahmad, H.; Anguilano, L.; Fan, M. Microstructural architecture and mechanical properties of empowered cellulose-based aerogel composites via TEMPO-free oxidation. Carbohydr. Polym. 2022, 298, 120117. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Fricke, J. Ultrasonic velocity measurements in silica, carbon and organic aerogels. J. Non-Cryst. Solids 1992, 145, 217–222. [Google Scholar] [CrossRef]
- Calemczuk, R.; Goer, A.M.d.; Salce, B.; Maynard, R.; Zarembowitch, A. Low-Temperature Properties of Silica Aerogels. Europhys. Lett. 1987, 3, 1205. [Google Scholar] [CrossRef]
- Gross, J.; Reichenauer, G.; Fricke, J. Mechanical properties of SiO2 aerogels. J. Phys. Appl. Phys. 1988, 21, 1447. [Google Scholar] [CrossRef]
- Lemay, J.D.; Tillotson, T.M.; Hrubesh, L.W.; Pekala, R.W. Microstructural Dependence Of Aerogel Mechanical Properties. MRS Online Proc. Libr. 1990, 180, 321. [Google Scholar] [CrossRef]
- Woignier, T.; Phalippou, J.; Hdach, H.; Larnac, G.; Pernot, F.; Scherer, G.W. Evolution of mechanical properties during the alcogel-aerogel-glass process. J. Non-Cryst. Solids 1992, 147–148, 672–680. [Google Scholar] [CrossRef]
- Scherer, G.W. Crack-tip stress in gels. J. Non-Cryst. Solids 1992, 144, 210–216. [Google Scholar] [CrossRef]
- Zarzycki, J. Critical stress intensity factors of wet gels. J. Non-Cryst. Solids 1988, 100, 359–363. [Google Scholar] [CrossRef]
- Evans, A.G. Slow crack growth in brittle materials under dynamic loading conditions. Int. J. Fract. 1974, 10, 251–259. [Google Scholar] [CrossRef]
- Hafidi Alaoui, A.; Woignier, T.; Pernot, F.; Phalippou, J.; Hihi, A. Stress intensity factor in silica alcogels and aerogels. J. Non-Cryst. Solids 2000, 265, 29–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Xu, G.; Wang, G.; Zhao, M. Effect of Microstructure on Fatigue-Crack Propagation of 18CrNiMo7-6 High-Strength Steel. Int. J. Fatigue 2022, 163, 107027. [Google Scholar] [CrossRef]
- Buchtová, N.; Pradille, C.; Bouvard, J.-L.; Budtova, T. Mechanical properties of cellulose aerogels and cryogels. Soft Matter 2019, 15, 7901–7908. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, T.; Liu, H.; Cai, H.; Wang, C. Gain regulation of the microchannel plate system. Int. J. Mass Spectrom. 2017, 421, 234–237. [Google Scholar] [CrossRef]
- Cao, L.; Fu, Q.; Si, Y.; Ding, B.; Yu, J. Porous materials for sound absorption. Compos. Commun. 2018, 10, 25–35. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, P.; Ma, B.; Yuan, W.; Luo, J. Hierarchically Structured M13 Phage Aerogel for Enhanced Sound-Absorption. Macromol. Mater. Eng. 2020, 305. [Google Scholar] [CrossRef]
- Begum, H.; Horoshenkov, K.V.; Conte, M.; Malfait, W.J.; Zhao, S.; Koebel, M.M.; Bonfiglio, P.; Venegas, R. The acoustical properties of tetraethyl orthosilicate based granular silica aerogels. J. Acoust. Soc. Am. 2021, 149, 4149–4158. [Google Scholar] [CrossRef]
- Standard Test Method for Impedance and Absorption of Acoustical Materials Using a Tube, Two Microphones and a Digital Frequency Analysis System. Available online: https://www.astm.org/e1050-19.html (accessed on 29 June 2023).
- Kueh, A.; Razali, A.; Lee, Y.; Hamdan, S.; Yakub, I.; Suhaili, N. Acoustical and mechanical characteristics of mortars with pineapple leaf fiber and silica aerogel infills – Measurement and modeling. Mater. Today Commun. 2023, 35, 105540. [Google Scholar] [CrossRef]
- Wang, G.; Ma, B.; Yuan, W.; Luo, J. Acoustic and mechanical characterization of a novel polypropylene fibers based composite aerogel. Mater. Lett. 2023, 334, 133696. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Tu, W.; Su, Y.; Jiang, F.; Riffat, S. Sound absorption, structure and mechanical behavior of konjac glucomannan-based aerogels with addition of gelatin and wheat straw. Constr. Build. Mater. 2022, 352, 129052. [Google Scholar] [CrossRef]
- Feng, J.; Le, D.; Nguyen, S.T.; Tan Chin Nien, V.; Jewell, D.; Duong, H.M. Silica⿿cellulose hybrid aerogels for thermal and acoustic insulation applications. Colloids Surf. Physicochem. Eng. Asp. 2016, 506, 298–305. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Guo, W.; Niu, H.; Song, L.; Hu, Y. Eco-friendly thermally insulating cellulose aerogels with exceptional flame retardancy, mechanical property and thermal stability. J. Taiwan Inst. Chem. Eng. 2022, 131, 104159. [Google Scholar] [CrossRef]
- Ebert, H.-P. Thermal Properties of Aerogels; 2011; pp. 537–564. ISBN 978-1-4419-7477-8. [Google Scholar]
- Aerogels Handbook, 2011th edition.; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7477-8. [Google Scholar]
- Guo, W.; Chen, S.; Liang, F.; Jin, L.; Ji, C.; Zhang, P.; Fei, B. Ultra-light-weight, anti-flammable and water-proof cellulosic aerogels for thermal insulation applications. Int. J. Biol. Macromol. 2023, 125343. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Pan, Y.; Cheng, X.; Zhang, Z.; Deng, Y.; Lun, Z.; Gong, L.; Gao, M.; Zhang, H. “Robust–Soft” Anisotropic Nanofibrillated Cellulose Aerogels with Superior Mechanical, Flame-Retardant, and Thermal Insulating Properties. ACS Appl. Mater. Interfaces 2021, 13, 27458–27470. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Peng, H.; Yu, B.; Zhou, K.; Pan, H.; Zhang, L.; Li, M.; Liu, M.; Tian, A.; Fu, S. Biomimetic structural cellulose nanofiber aerogels with exceptional mechanical, flame-retardant and thermal-insulating properties. Chem. Eng. J. 2020, 389, 124449. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, X.; Lv, Y.; Zhao, B.; Fang, X.; Pan, K. Wearable and high-performance piezoresistive sensor based on nanofiber/sodium alginate synergistically enhanced MXene composite aerogel. Chem. Eng. J. 2023, 451, 138586. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Tao, S.; Chai, H.; Xu, D.; Li, X.; Qi, H. High-performance smart cellulose nanohybrid aerogel fibers as a platform toward multifunctional textiles. Chem. Eng. J. 2023, 466, 143153. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chem. A 2014, 2, 6337–6342. [Google Scholar] [CrossRef]
- Nguyen, H.S.H.; Phan, H.H.; Huynh, H.K.P.; Nguyen, S.T.; Nguyen, V.T.T.; Phan, A.N. Understanding the effects of cellulose fibers from various pre-treated barley straw on properties of aerogels. Fuel Process. Technol. 2022, 236, 107425. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Z.; An, B.; Ma, C.; Xu, L.; Zhang, Z.; Luo, S.; Li, W.; Liu, S. Thermal-insulating, flame-retardant and mechanically resistant aerogel based on bio-inspired tubular cellulose. Compos. Part B Eng. 2021, 220, 108997. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Yu, Z.; Liu, J.; Zhao, Y.; Ke, Y. Ecofriendly flame-retardant composite aerogel derived from polysaccharide: Preparation, flammability, thermal kinetics, and mechanism. Carbohydr. Polym. 2021, 269, 118291. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, T.; Hajnal, A.; Al-Najjar, M.A.A.; Alshweiat, A.; Obaidat, R.; Awad, A.A.; Al-Alwany, R.; Keitel, J.; Wu, D.; Kieserling, H.; et al. In vivo tests of a novel wound dressing based on agar aerogel. Int. J. Biol. Macromol. 2023, 239, 124238. [Google Scholar] [CrossRef]
- Batista, M.P.; Gonçalves, V.S.S.; Gaspar, F.B.; Nogueira, I.D.; Matias, A.A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef]
- Tripathi, G.; Park, M.; Lim, H.; Lee, B.-T. Natural TEMPO oxidized cellulose nano fiber/alginate/dSECM hybrid aerogel with improved wound healing and hemostatic ability. Int. J. Biol. Macromol. 2023, 243, 125226. [Google Scholar] [CrossRef]
- Han, X.; Ding, S.; Zhu, L.; Wang, S. Preparation and characterization of flame-retardant and thermal insulating bio-based composite aerogels. Energy Build. 2023, 278, 112656. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, C.; Du, C.; Xu, K.; Li, Y.; Xu, M.; Bourbigot, S.; Fontaine, G.; Li, B.; Liu, L. High-strength, thermal-insulating, fire-safe bio-based organic lightweight aerogel based on 3D network construction of natural tubular fibers. Compos. Part B Eng. 2023, 261, 110809. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mirmoeini, S.S.; Moradi, M.; Tajik, H.; Almasi, H.; Gama, F.M. Cellulose/Salep-based intelligent aerogel with red grape anthocyanins: Preparation, characterization and application in beef packaging. Food Chem. 2023, 425, 136493. [Google Scholar] [CrossRef] [PubMed]
- Mirmoeini, S.S.; Hosseini, S.H.; Lotfi Javid, A.; Esmaeili Koutamehr, M.; Sharafi, H.; Molaei, R.; Moradi, M. Essential oil-loaded starch/cellulose aerogel: Preparation, characterization and application in cheese packaging. Int. J. Biol. Macromol. 2023, 244, 125356. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Li, H.; Long, J.; Wang, Y.; Peng, D. Bio-aerogels derived from corn stalk and Premna Microphylla leaves as eco-friendly sorbents for oily water treatment: The role of microstructure in adsorption performance. J. Clean. Prod. 2023, 403, 136720. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, W.; Tian, Y.; Kong, L.; Cai, G.; Zhang, H.; Li, L.; Zhang, J. An ultralight and flexible nanofibrillated cellulose/chitosan aerogel for efficient chromium removal: Adsorption-reduction process and mechanism. Chemosphere 2023, 329, 138622. [Google Scholar] [CrossRef] [PubMed]
- Erfanian, E.; Moaref, R.; Ajdary, R.; Tam, K.C.; Rojas, O.J.; Kamkar, M.; Sundararaj, U. Electrochemically synthesized graphene/TEMPO-oxidized cellulose nanofibrils hydrogels: Highly conductive green inks for 3D printing of robust structured EMI shielding aerogels. Carbon 2023, 210, 118037. [Google Scholar] [CrossRef]
- Peng, Q.; Lu, Y.; Li, Z.; Zhang, J.; Zong, L. Biomimetic, hierarchical-ordered cellulose nanoclaw hybrid aerogel with high strength and thermal insulation. Carbohydr. Polym. 2022, 297, 119990. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Z.; Lyu, S.; Fu, F.; Wang, S. Highly flexible cross-linked cellulose nanofibril sponge-like aerogels with improved mechanical property and enhanced flame retardancy. Carbohydr. Polym. 2018, 179, 333–340. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.R.; Wang, L.; Shanks, R.A.; Ara, Z.A.; Saha, T. Electrospun polyacrylonitrile–silica aerogel coating on viscose nonwoven fabric for versatile protection and thermal comfort. Cellulose 2020, 27, 10501–10517. [Google Scholar] [CrossRef]
- Johnson: Molecular layer-by-layer deposition… - Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=Molecular%20layer-by-layer%20deposition%20of%20highly%20crosslinked%20polyamide%20films&publication_year=2012&author=P.M.%20Johnson&author=J.%20Yoon&author=J.Y.%20Kelly&author=J.A.%20Howarter&author=C.M.%20Stafford (accessed on 25 April 2023).
- Chan, E.P.; Lee, J.-H.; Chung, J.Y.; Stafford, C.M. An automated spin-assisted approach for molecular layer-by-layer assembly of crosslinked polymer thin films. Rev. Sci. Instrum. 2012, 83, 114102. [Google Scholar] [CrossRef]
- Atoufi, Z.; Reid, M.S.; Larsson, P.A.; Wågberg, L. Surface tailoring of cellulose aerogel-like structures with ultrathin coatings using molecular layer-by-layer assembly. Carbohydr. Polym. 2022, 282, 119098. [Google Scholar] [CrossRef]
- La, P.; Huynh, N.; Bui, K.; Pham, K.; Dao, X.-T.; Tran, T.; Nguyen, T.; Hoang, N.; Mai, P.; Nguyen, H.H. Synthesis And Surface Modification of Cellulose Aerogel from Coconut Peat for Oil Adsorption; 2021. [Google Scholar]
- Kaya, M. Super absorbent, light, and highly flame retardant cellulose-based aerogel crosslinked with citric acid. J. Appl. Polym. Sci. 2017, 134, 45315. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, D.; Carosio, F.; Camino, G.; Bergström, L. Tuning the Nanocellulose–Borate Interaction To Achieve Highly Flame Retardant Hybrid Materials. Chem. Mater. 2016, 28, 1985–1989. [Google Scholar] [CrossRef]
- Jelle, B.P. Traditional, state-of-the-art and future thermal building insulation materials and solutions – Properties, requirements and possibilities. Energy Build. 2011, 43, 2549–2563. [Google Scholar] [CrossRef]
- BuyAerogel.com | Airloy® X103 Strong Aerogel Large Panels.
- Antlauf, M.; Boulanger, N.; Berglund, L.; Oksman, K.; Andersson, O. Thermal Conductivity of Cellulose Fibers in Different Size Scales and Densities. Biomacromolecules 2021, 22, 3800–3809. [Google Scholar] [CrossRef] [PubMed]
- Thai, Q.B.; Nguyen, S.T.; Ho, D.K.; Tran, T.D.; Huynh, D.M.; Do, N.H.N.; Luu, T.P.; Le, P.K.; Le, D.K.; Phan-Thien, N.; et al. Cellulose-based aerogels from sugarcane bagasse for oil spill-cleaning and heat insulation applications. Carbohydr. Polym. 2020, 228, 115365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Xue, T.; Yang, F.; Fan, W.; Liu, T. Bidirectional anisotropic polyimide/bacterial cellulose aerogels by freeze-drying for super-thermal insulation. Chem. Eng. J. 2020, 385, 123963. [Google Scholar] [CrossRef]
- Sai, H.; Wang, M.; Miao, C.; Song, Q.; Wang, Y.; Fu, R.; Wang, Y.; Ma, L.; Hao, Y. Robust Silica-Bacterial Cellulose Composite Aerogel Fibers for Thermal Insulation Textile. Gels 2021, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, M.; Jiang, W.; Xu, Q.; Yu, J.; Liu, L.; Liu, L. Multiscale nanocelluloses hybrid aerogels for thermal insulation: The study on mechanical and thermal properties. Carbohydr. Polym. 2020, 247, 116701. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Hu, Y.; Zhu, Y.; Wang, J.; Jia, X.; Chen, J.; Li, J. Fabrication of Textile Waste Fibers Aerogels with Excellent Oil/Organic Solvent Adsorption and Thermal Properties. Gels 2022, 8, 684. [Google Scholar] [CrossRef]
- Liu, Z.; Lyu, J.; Fang, D.; Zhang, X. Nanofibrous Kevlar Aerogel Threads for Thermal Insulation in Harsh Environments. ACS Nano 2019, 13, 5703–5711. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Ng, S.K.; Wong, J.P.W.; Tan, V.B.C.; Duong, H.M. Advanced thermal insulation and absorption properties of recycled cellulose aerogels. Colloids Surf. Physicochem. Eng. Asp. 2014, 445, 128–134. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Liu, Z.; Cheng, H.; Li, C. Continuous, Strong, Porous Silk Firoin-Based Aerogel Fibers toward Textile Thermal Insulation. Polymers 2019, 11, 1899. [Google Scholar] [CrossRef]
- Song, M.; Jiang, J.; Qin, H.; Ren, X.; Jiang, F. Flexible and super thermal insulating cellulose nanofibril/emulsion composite aerogel with quasi-closed pores. ACS Appl. Mater. Interfaces 2020, 12, 45363–45372. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, T.; Han, D.; Ren, Q.; Siqueira, G.; Nyström, G. Ultralight, flexible, and biomimetic nanocellulose/silver nanowire aerogels for electromagnetic interference shielding. Acs Nano 2020, 14, 2927–2938. [Google Scholar] [CrossRef]
- Yu, Z.; Suryawanshi, A.; He, H.; Liu, J.; Li, Y.; Lin, X.; Sun, Z. Preparation and characterisation of fire-resistant PNIPAAm/SA/AgNP thermosensitive network hydrogels and laminated cotton fabric used in firefighter protective clothing. Cellulose 2020, 27, 5391–5406. [Google Scholar] [CrossRef]
- Yu: Thermal insulating and fire-retarding behavior… - Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=Thermal%20insulating%20and%20fire-retarding%20behavior%20of%20treated%20cotton%20fabrics%20with%20a%20novel%20high%20water-retaining%20hydrogel%20used%20in%20thermal%20protective%20clothing&publication_year=2021&author=Z.%20Yu&author=J.%20Liu&author=A.%20Suryawanshi&author=H.%20He&author=Y.%20Wang&author=Y.%20Zhao (accessed on 2 May 2023).
- Kim, S.J.; Kim, H.A. Effect of fabric structural parameters and weaving conditions to warp tension of aramid fabrics for protective garments. Text. Res. J. 2018, 88, 987–1001. [Google Scholar] [CrossRef]
- Cao, M.; Li, S.-L.; Cheng, J.-B.; Zhang, A.-N.; Wang, Y.-Z.; Zhao, H.-B. Fully bio-based, low fire-hazard and superelastic aerogel without hazardous cross-linkers for excellent thermal insulation and oil clean-up absorption. J. Hazard. Mater. 2021, 403, 123977. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, P.; Xu, Y.-J.; Jiang, Z.-M.; Dong, C.-H.; Liu, Y.; Zhu, P. Bio-based, nontoxic and flame-retardant cotton/alginate blended fibres as filling materials: Thermal degradation properties, flammability and flame-retardant mechanism. Compos. Part B Eng. 2020, 194, 108038. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Chen, M.; Chen, H.-B. Thermally insulating and flame-retardant polyaniline/pectin aerogels. ACS Sustain. Chem. Eng. 2017, 5, 7012–7019. [Google Scholar] [CrossRef]
- Chen, J.; Xie, H.; Lai, X.; Li, H.; Gao, J.; Zeng, X. An ultrasensitive fire-warning chitosan/montmorillonite/carbon nanotube composite aerogel with high fire-resistance. Chem. Eng. J. 2020, 399, 125729. [Google Scholar] [CrossRef]
- He, C.; Huang, J.; Li, S.; Meng, K.; Zhang, L.; Chen, Z.; Lai, Y. Mechanically Resistant and Sustainable Cellulose-Based Composite Aerogels with Excellent Flame Retardant, Sound-Absorption, and Superantiwetting Ability for Advanced Engineering Materials. ACS Sustain. Chem. Eng. 2018, 6, 927–936. [Google Scholar] [CrossRef]
- He, S.; Liu, C.; Chi, X.; Zhang, Y.; Yu, G.; Wang, H.; Li, B.; Peng, H. Bio-inspired lightweight pulp foams with improved mechanical property and flame retardancy via borate cross-linking. Chem. Eng. J. 2019, 371, 34–42. [Google Scholar] [CrossRef]
- Thanh, N.T.L. Investigation on the flame-retardant and physical properties of the modified cellulosic and polyurethane aerogel. Mater. Today Proc. 2022, 66, 2726–2729. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Dong, H.; Xie, Y.; Zeng, G.; Tang, L.; Liang, J.; He, Q.; Zhao, F.; Zeng, Y.; Wu, Y. The dual effects of carboxymethyl cellulose on the colloidal stability and toxicity of nanoscale zero-valent iron. Chemosphere 2016, 144, 1682–1689. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alzalouk, M.M.; Alfallous, K.A.; Abogmaza, A.F. Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed. Res. Ther. 2020, 7, 4032–4040. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef]
- Edwards, J.V.; Fontenot, K.; Liebner, F.; Pircher, N.D.n.; French, A.D.; Condon, B.D. Structure/Function Analysis of Cotton-Based Peptide-Cellulose Conjugates: Spatiotemporal/Kinetic Assessment of Protease Aerogels Compared to Nanocrystalline and Paper Cellulose. Int. J. Mol. Sci. 2018, 19, 840. [Google Scholar] [CrossRef]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.A.; Aquino, R.P.; Del Gaudio, P. Prilling and supercritical drying: A successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef]
- Lyu, W.; Li, J.; Zheng, L.; Liu, H.; Chen, J.; Zhang, W.; Liao, Y. Fabrication of 3D compressible polyaniline/cellulose nanofiber aerogel for highly efficient removal of organic pollutants and its environmental-friendly regeneration by peroxydisulfate process. Chem. Eng. J. 2021, 414, 128931. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Pan, D.; Yang, G.; Abo-Dief, H.M.; Dong, J.; Su, F.; Liu, C.; Li, Y.; Bin Xu, B.; Murugadoss, V.; Naik, N.; et al. Vertically Aligned Silicon Carbide Nanowires/Boron Nitride Cellulose Aerogel Networks Enhanced Thermal Conductivity and Electromagnetic Absorbing of Epoxy Composites. Nano-Micro Lett. 2022, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Dong, J.; Gui, Y.; Su, F.; Chang, B.; Liu, C.; Zhu, Y.-C.; Guo, Z. Ice template method assists in obtaining carbonized cellulose/boron nitride aerogel with 3D spatial network structure to enhance the thermal conductivity and flame retardancy of epoxy-based composites. Adv. Compos. Hybrid Mater. 2021, 5. [Google Scholar] [CrossRef]
- Cellulose Sciences International - Crunchbase Company Profile & Funding. Available online: https://www.crunchbase.com/organization/cellulose-sciences-international (accessed on 7 June 2023).
- INACELL: Cellulose aerogels for thermal insulation in buildings | Activos. Available online: https://www.tecnalia.com/en/technological-assets/inacell-cellulose-aerogels-for-thermal-insulation-in-buildings (accessed on 5 June 2023).
- Bioaerogels. Available online: https://www.aerogel-it.de/bioaerogels (accessed on 5 June 2023).
- info@orgo.ee, O.O. Aerogel-it and Fibenol Collaborate for Sustainable Superinsulation | Fibenol. Available online: https://fibenol.com/news/aerogel-it-and-fibenol-collaborate-for-sustainable-superinsulation (accessed on 6 June 2023).
- Guerrero-Alburquerque, N.; Zhao, S.; Adilien, N.; Koebel, M.M.; Lattuada, M.; Malfait, W.J. Strong, Machinable, and Insulating Chitosan–Urea Aerogels: Toward Ambient Pressure Drying of Biopolymer Aerogel Monoliths. ACS Appl. Mater. Interfaces 2020, 12, 22037–22049. [Google Scholar] [CrossRef] [PubMed]
- Empa - Building Energy Materials and Components - 3. Cellulose Aerogels. Available online: https://www.empa.ch/web/s312/3.-cellulose-aerogels (accessed on 7 June 2023).
- Electric Vehicle Solutions Singapore | JIOS Aerogel. Available online: https://jiosaerogel.com/ (accessed on 5 June 2023).
- Skogar®, super-insulating aerogel blankets. ENERSENS.
- Aerogel Blanket. Available online: https://www.cabotcorp.com/solutions/products-plus/aerogel/blanket (accessed on 5 June 2023).
- Spaceloft. Aspen Aerogels.
- Research, A.M. Aerogel Market to Garner $7.5 Billion, Globally, By 2032 at 19.4% CAGR, Says Allied Market Research. Available online: https://www.prnewswire.com/news-releases/aerogel-market-to-garner-7-5-billion-globally-by-2032-at-19-4-cagr-says-allied-market-research-301819591.html (accessed on 17 June 2023).
- Wood-based aerogels (AEROWOOD). Available online: https://blogs.helsinki.fi/aerowood-project/partners/ (accessed on 17 June 2023).
- Smirnova, I.; Gurikov, P. Aerogel production: Current status, research directions, and future opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Aerogel Market Size, Share, Growth & Trends Repot, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/aerogel-market (accessed on 19 June 2023).
- Sen, S.; Singh, A.; Bera, C.; Roy, S.; Kailasam, K. Recent developments in biomass derived cellulose aerogel materials for thermal insulation application: a review. Cellulose 2022, 29, 4805–4833. [Google Scholar] [CrossRef]
- Wu, J.; Cooper, D.; Miller, R. Virtual Impactor Aerosol Concentrator for Cleanroom Monitoring. J. Environ. Sci. 2006, 32, 52–56. [Google Scholar] [CrossRef]
- Komarneni, S.; Roy, R.; Selvaraj, U.; Malla, P.B.; Breval, E. Nanocomposite aerogels: The SiO2–Al2O3 system. J. Mater. Res. 1993, 8, 3163–3167. [Google Scholar] [CrossRef]
- Pajonk, G. ChemInform Abstract: Aerogel Catalysts. ChemInform 2010, 22. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Uetani, K.; Hatori, K. Thermal conductivity analysis and applications of nanocellulose materials. Sci. Technol. Adv. Mater. 2017, 18, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Kochumalayil, J.; Cuttica, F.; Camino, G.; Berglund, L. Oriented Clay Nanopaper from Biobased Components—Mechanisms for Superior Fire Protection Properties. ACS Appl. Mater. Interfaces 2015, 7, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Österberg, M.; Vartiainen, J.; Lucenius, J.; Hippi, U.; Seppälä, J.; Serimaa, R.; Laine, J. A Fast Method to Produce Strong NFC Films as a Platform for Barrier and Functional Materials. ACS Appl. Mater. Interfaces 2013, 5, 4640–4647. [Google Scholar] [CrossRef] [PubMed]
- Dorez, G.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.M. Thermal and fire behavior of natural fibers/PBS biocomposites. Polym. Degrad. Stab. 2013, 98, 87–95. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon Aerogel from Winter Melon for Highly Efficient and Recyclable Oils and Organic Solvents Absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Versatile Cellulose-Based Carbon Aerogel for the Removal of Both Cationic and Anionic Metal Contaminants from Water | ACS Applied Materials & Interfaces. Available online: https://pubs.acs.org/doi/abs/10.1021/acsami.5b08287 (accessed on 13 June 2023).
- Chen, H.; Wang, X.; Li, J.; Wang, X. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J. Mater. Chem. A 2015, 3, 6073–6081. [Google Scholar] [CrossRef]
- Facile synthesis of Fe3O4 nanoparticles decorated on 3D graphene aerogels as broad-spectrum sorbents for water treatment - ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0169433216301799 (accessed on 13 June 2023).
- Highly compressible anisotropic graphene aerogels fabricated by directional freezing for efficient absorption of organic liquids - ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0008622316300380 (accessed on 13 June 2023).
- Hong, J.-Y.; Sohn, E.-H.; Park, S.; Park, H.S. Highly-efficient and recyclable oil absorbing performance of functionalized graphene aerogel. Chem. Eng. J. 2015, 269, 229–235. [Google Scholar] [CrossRef]
- Suchithra, P.S.; Vazhayal, L.; Peer Mohamed, A.; Ananthakumar, S. Mesoporous organic–inorganic hybrid aerogels through ultrasonic assisted sol–gel intercalation of silica–PEG in bentonite for effective removal of dyes, volatile organic pollutants and petroleum products from aqueous solution. Chem. Eng. J. 2012, 200–202, 589–600. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Sreekumari, S.S. Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons. J. Environ. Sci. 2011, 23, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: a promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, T.; Liu, K.; Zhang, M.; Liu, W.; Li, H.; Du, H.; Si, C. Lignin-based electrodes for energy storage application. Ind. Crops Prod. 2021, 165, 113425. [Google Scholar] [CrossRef]
- Recent progress in carbon-based materials for supercapacitor electrodes: a review | SpringerLink. Available online: https://link.springer.com/article/10.1007/s10853-020-05157-6 (accessed on 13 June 2023).
- Wang, F.; Cheong, J.Y.; He, Q.; Duan, G.; He, S.; Zhang, L.; Zhao, Y.; Kim, I.-D.; Jiang, S. Phosphorus-doped thick carbon electrode for high-energy density and long-life supercapacitors. Chem. Eng. J. 2021, 414, 128767. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Xu, Z.; Zhang, H.; Ding, L.; Wang, S.; Zhang, G.; Hou, H.; Xu, W.; Yang, F.; et al. Comparison of the heteroatoms-doped biomass-derived carbon prepared by one-step nitrogen-containing activator for high performance supercapacitor. Diam. Relat. Mater. 2021, 114, 108316. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Xu, Z.; Gao, R.; Wang, S.; Zhang, G.; Jiang, S.; Xu, W.; Hou, H. Camellia Pollen-Derived Carbon with Controllable N Content for High-Performance Supercapacitors by Ammonium Chloride Activation and Dual N-Doping. ChemNanoMat 2021, 7, 34–43. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Li, H.; Duan, G.; He, S.; Zhang, L.; Zhang, G.; Zhou, Z.; Jiang, S. N-doped honeycomb-like porous carbon towards high-performance supercapacitor. Chin. Chem. Lett. 2020, 31, 1986–1990. [Google Scholar] [CrossRef]
- Liu, H.; Du, H.; Zheng, T.; Liu, K.; Ji, X.; Xu, T.; Zhang, X.; Si, C. Cellulose based composite foams and aerogels for advanced energy storage devices. Chem. Eng. J. 2021, 426, 130817. [Google Scholar] [CrossRef]
- Zaman, A.; Huang, F.; Jiang, M.; Wei, W.; Zhou, Z. Preparation, Properties, and Applications of Natural Cellulosic Aerogels: A Review. Energy Built Environ. 2020, 1, 60–76. [Google Scholar] [CrossRef]
- 3D network of cellulose-based energy storage devices and related emerging applications - Materials Horizons (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2000/mh/c6mh00500d/unauth (accessed on 13 June 2023).
- Ganesan: Review on the production of polysaccharide… - Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=Review%20on%20the%20production%20of%20polysaccharide%20aerogel%20particles&publication_year=2018&author=K.%20Ganesan&author=T.%20Budtova&author=L.%20Ratke&author=P.%20Gurikov&author=V.%20Baudron&author=I.%20Preibisch&author=P.%20Niemeyer&author=I.%20Smirnova&author=B.%20Milow (accessed on 13 June 2023).
- Mavelil-Sam, R.; Pothan, L.A.; Thomas, S. Polysaccharide and Protein Based Aerogels: An Introductory Outlook. RSC Green Chem. 2018, 2018-January, 1–8. [Google Scholar] [CrossRef]
- García-González, C.A.; Carenza, E.; Zeng, M.; Smirnova, I.; Roig, A. Design of biocompatible magnetic pectin aerogel monoliths and microspheres. RSC Adv. 2012, 2, 9816–9823. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. A novel hybrid material: An inorganic silica aerogel core encapsulated with a tunable organic alginate aerogel layer. RSC Adv. 2014, 4, 62362–62366. [Google Scholar] [CrossRef]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef]
- Kuttor, A.; Szalóki, M.; Rente, T.; Kerényi, F.; Bakó, J.; Fábián, I.; Lázár, I.; Jenei, A.; Hegedüs, C. Preparation and application of highly porous aerogel-based bioactive materials in dentistry. Front. Mater. Sci. 2014, 8, 46–52. [Google Scholar] [CrossRef]
- Scopus - Document details - Starch Aerogels: A Member of the Family of Thermal Superinsulating Materials | Signed in. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-85038214965&origin=inward&txGid=15941fa24b66c20859d4e60730430491 (accessed on 13 June 2023).
- Yan, N.; Zhou, Y.; Zheng, Y.; Qiao, S.; Yu, Q.; Li, Z.; Lu, H. Antibacterial properties and cytocompatibility of bio-based nanostructured carbon aerogels derived from silver nanoparticles deposited onto bacterial cellulose. RSC Adv. 2015, 5, 97467–97476. [Google Scholar] [CrossRef]
- Takeshita, S.; Yoda, S. Chitosan Aerogels: Transparent, Flexible Thermal Insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Wan, C.; Jiao, Y.; Sun, Q.; Li, J. Preparation, characterization, and antibacterial properties of silver nanoparticles embedded into cellulose aerogels. Polym. Compos. 2016, 37, 1137–1142. [Google Scholar] [CrossRef]
- Thomas, S.; Pothan, L.A.; Mavelil-Sam, R. Biobased Aerogels: Polysaccharide and Protein-based Materials; Royal Society of Chemistry, 2018; ISBN 978-1-78262-765-4. [Google Scholar]
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.A.A.P.; Silvestre, A.J.D.; Freire, C.S.R. Polysaccharide based hybrid materials. Polysacch. Based Hybrid Mater. 2018, 95–114. [Google Scholar]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Concha, M.; Vidal, A.; Giacaman, A.; Ojeda, J.; Pavicic, F.; Oyarzun-Ampuero, F.A.; Torres, C.; Cabrera, M.; Moreno-Villoslada, I.; Orellana, S.L. Aerogels made of chitosan and chondroitin sulfate at high degree of neutralization: Biological properties toward wound healing. J. Biomed. Mater. Res. - Part B Appl. Biomater. 2018, 106, 2464–2471. [Google Scholar] [CrossRef]
- Xin, G.; Yao, T.; Sun, H.; Scott, S.M.; Shao, D.; Wang, G.; Lian, J. Highly thermally conductive and mechanically strong graphene fibers. Science 2015, 349, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.-M. Fiber-based wearable electronics: A review of materials, fabrication, devices, and applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Li, G.; Hong, G.; Dong, D.; Song, W.; Zhang, X. Multiresponsive Graphene-Aerogel-Directed Phase-Change Smart Fibers. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, J.; Lee, P.S. Functional Fibers and Fabrics for Soft Robotics, Wearables, and Human–Robot Interface. Adv. Mater. 2021, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Liang, X.; Zhang, Y. Smart Fibers and Textiles for Personal Health Management. ACS Nano 2021, 15, 12497–12508. [Google Scholar] [CrossRef]
- Choi, H.W.; Shin, D.-W.; Yang, J.; Lee, S.; Figueiredo, C.; Sinopoli, S.; Ullrich, K.; Jovančić, P.; Marrani, A.; Momentè, R.; et al. Smart textile lighting/display system with multifunctional fibre devices for large scale smart home and IoT applications. Nat. Commun. 2022, 13. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Zhang, L.; Yang, B.; Shu, L.; Yang, Y.; Ren, M.; Wang, Y.; Chen, J.; Chen, W.; et al. Smart Textile-Integrated Microelectronic Systems for Wearable Applications. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef]
- Liu, X.; Miao, J.; Fan, Q.; Zhang, W.; Zuo, X.; Tian, M.; Zhu, S.; Zhang, X.; Qu, L. Smart Textile Based on 3D Stretchable Silver Nanowires/MXene Conductive Networks for Personal Healthcare and Thermal Management. ACS Appl. Mater. Interfaces 2021, 13, 56607–56619. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Z.; Ye, X.; He, W.; Qu, L.; Tian, M. A Smart Self-Powered Rope for Water/Fire Rescue. Adv. Funct. Mater. 2023, 33. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Ye, X.; Zhang, X.; Qu, L.; Tian, M. Tendril-Inspired 900% Ultrastretching Fiber-Based Zn-Ion Batteries for Wearable Energy Textiles. ACS Appl. Mater. Interfaces 2021, 13, 17110–17117. [Google Scholar] [CrossRef]
- Chen, B.; Wu, M.; Fang, S.; Cao, Y.; Pei, L.; Zhong, H.; Sun, C.; Lin, X.; Li, X.; Shen, J.; et al. Liquid Metal-Tailored PEDOT:PSS for Noncontact Flexible Electronics with High Spatial Resolution. ACS Nano 2022, 16, 19305–19318. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Yu, Z.; Zhu, T.; Kang, J.; Liu, K.; Li, Z.; Tan, S.C. In Situ Growth of a Stable Metal-Organic Framework (MOF) on Flexible Fabric via a Layer-by-Layer Strategy for Versatile Applications. ACS Nano 2022, 16, 14779–14791. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, G.; Wang, G.; Zhu, Y.; Cheng, W.; Zeng, S.; Zhou, J.; Xu, G.; Zhao, D. Cellulose-based functional gels and applications in flexible supercapacitors. Resour. Chem. Mater. 2023, 2, 177–188. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, W.; Bi, S.; Liu, S.; Huang, W.; Zhao, Q. Preparation and application of cellulose gel in flexible supercapacitors. J. Energy Storage 2021, 42, 103058. [Google Scholar] [CrossRef]
- Holocellulosic fibers and nanofibrils using peracetic acid pulping and sulfamic acid esterification - ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0144861722008074 (accessed on 14 June 2023).
- Zhang, C.; Wang, M.; Lin, X.; Tao, S.; Wang, X.; Chen, Y.; Liu, H.; Wang, Y.; Qi, H. Holocellulose nanofibrils assisted exfoliation of boron nitride nanosheets for thermal management nanocomposite films. Carbohydr. Polym. 2022, 291, 119578. [Google Scholar] [CrossRef]
- Yamada, S. A Transient Supercapacitor with a Water-Dissolvable Ionic Gel for Sustainable Electronics. ACS Appl. Mater. Interfaces 2022, 14, 26595–26603. [Google Scholar] [CrossRef]
- Nyström, G.; Marais, A.; Karabulut, E.; Wågberg, L.; Cui, Y.; Hamedi, M.M. Self-assembled three-dimensional and compressible interdigitated thin-film supercapacitors and batteries. Nat. Commun. 2015, 6, 7259. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ji, D.; Li, W.; Xiao, J.; Li, C.; Xiong, G.; Zhu, Y.; Wan, Y. Constructing a highly bioactive 3D nanofibrous bioglass scaffold via bacterial cellulose-templated sol-gel approach. Mater. Chem. Phys. 2016, 176. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Hiekkataipale, P.; Malm, J.; Karppinen, M.; Ikkala, O.; Ras, R.H.A. Inorganic Hollow Nanotube Aerogels by Atomic Layer Deposition onto Native Nanocellulose Templates. ACS Nano 2011, 5, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Melone, L.; Altomare, L.; Alfieri, I.; Lorenzi, A.; Nardo, L.D.; Punta, C. Ceramic aerogels from TEMPO-oxidized cellulose nanofibre templates: Synthesis, characterization, and photocatalytic properties. J. Photochem. Photobiol. Chem. 2013, Complete, 53–60. [Google Scholar] [CrossRef]
- Zhai, T.; Zheng, Q.; Cai, Z.; Turng, L.-S.; Xia, H.; Gong, S. Poly(vinyl alcohol)/Cellulose Nanofibril Hybrid Aerogels with an Aligned Microtubular Porous Structure and Their Composites with Polydimethylsiloxane. ACS Appl. Mater. Interfaces 2015, 7, 7436–7444. [Google Scholar] [CrossRef]
- Tian, L.; Luan, J.; Liu, K.-K.; Jiang, Q.; Tadepalli, S.; Gupta, M.K.; Naik, R.R.; Singamaneni, S. Plasmonic Biofoam: A Versatile Optically Active Material. Nano Lett. 2016, 16, 609–616. [Google Scholar] [CrossRef]
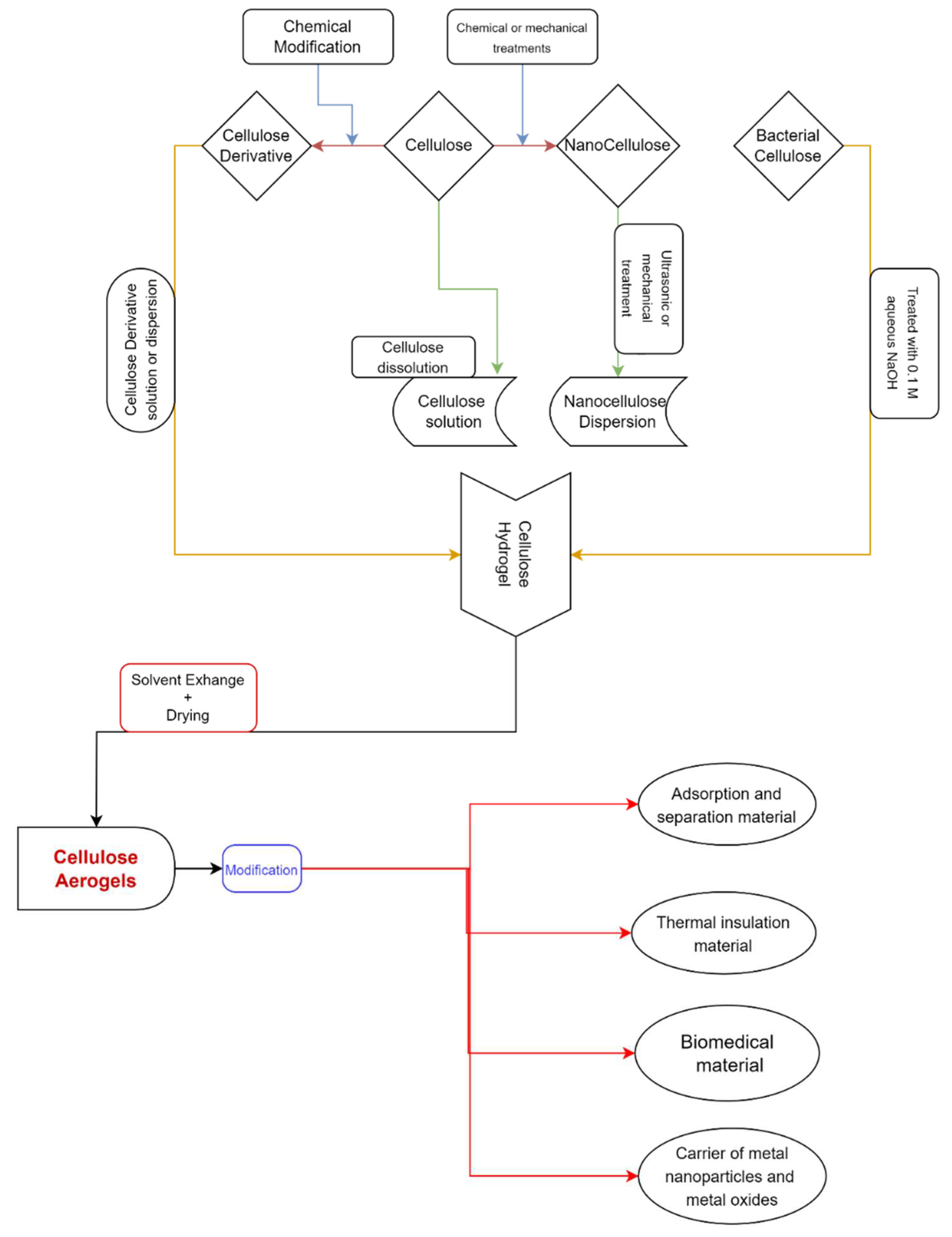
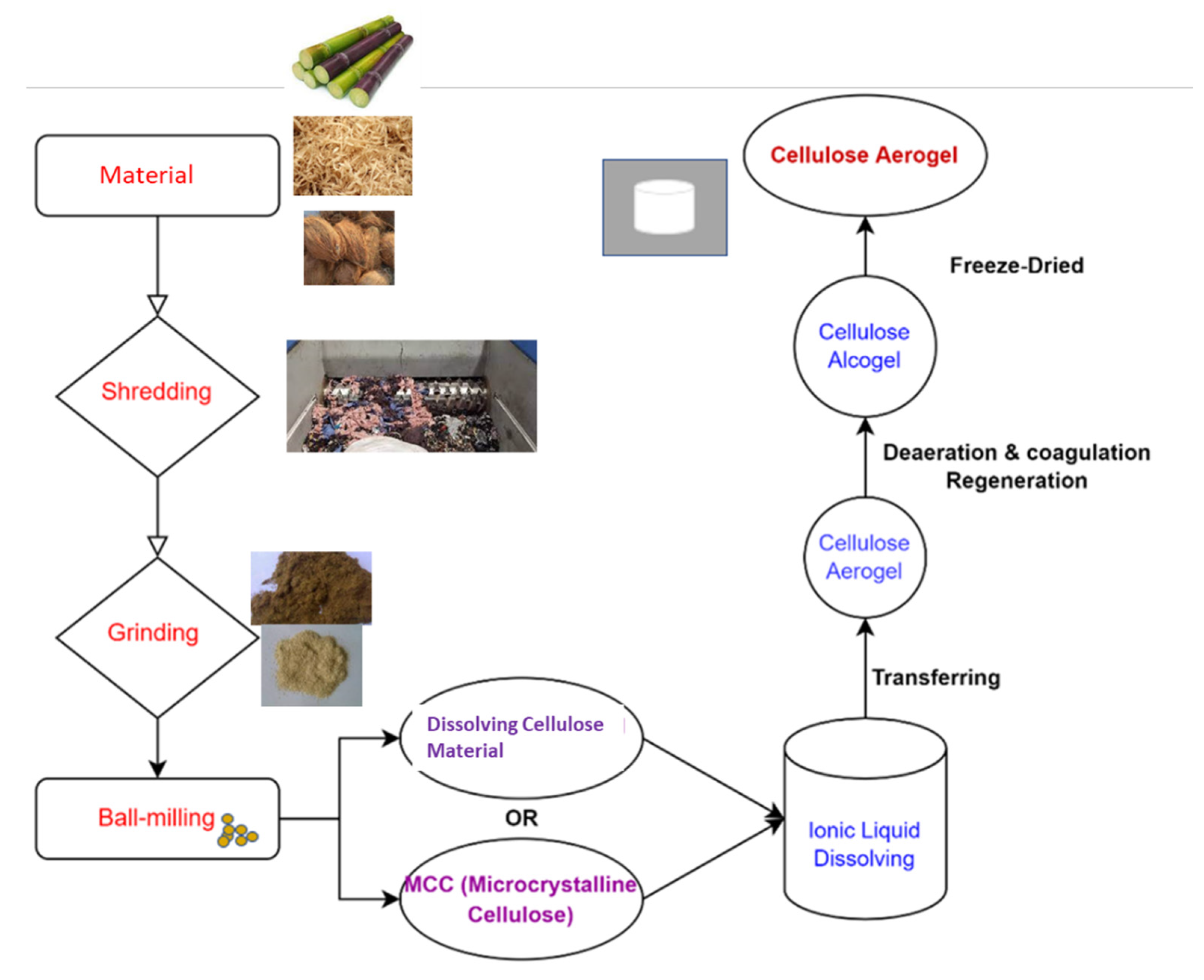
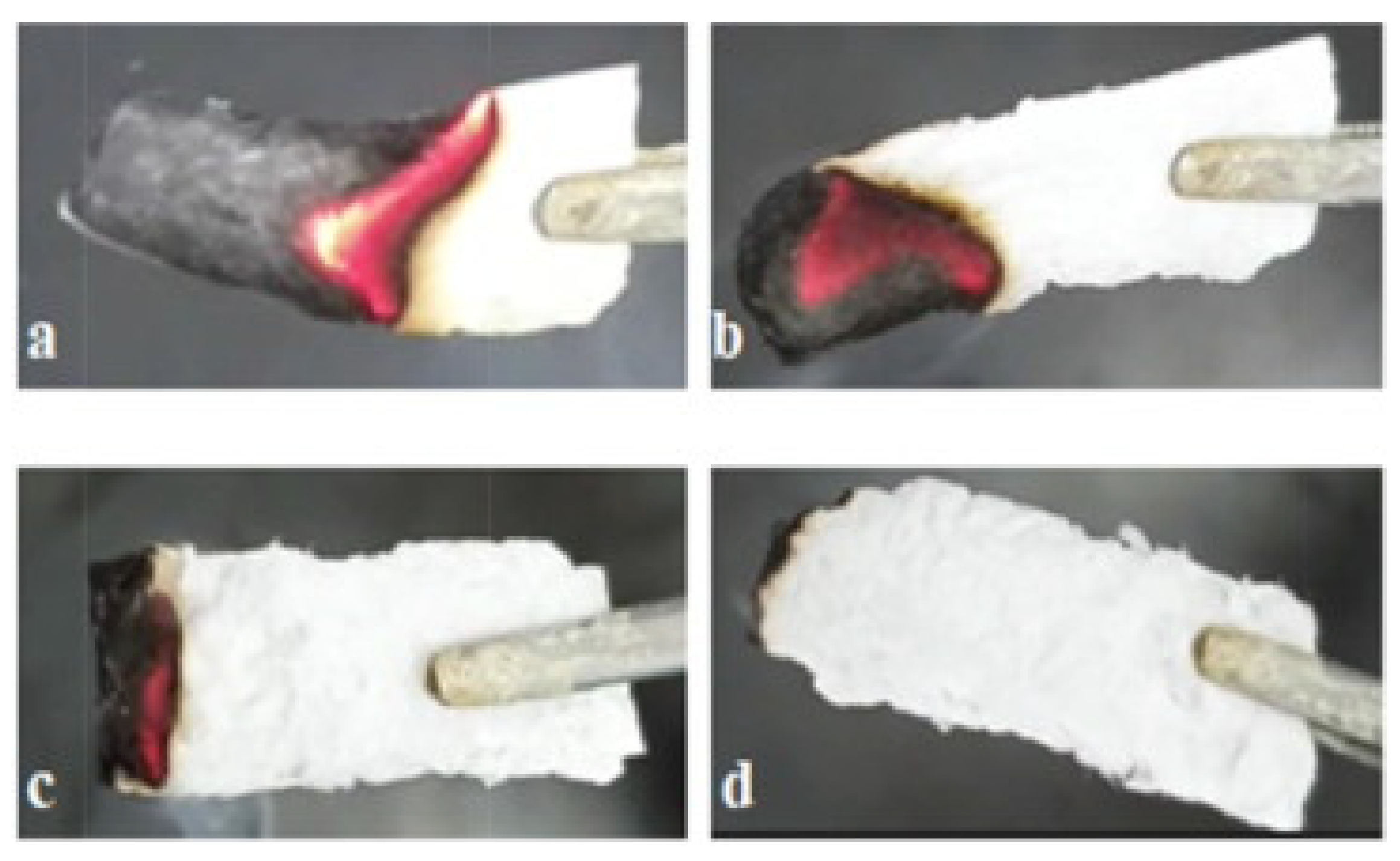
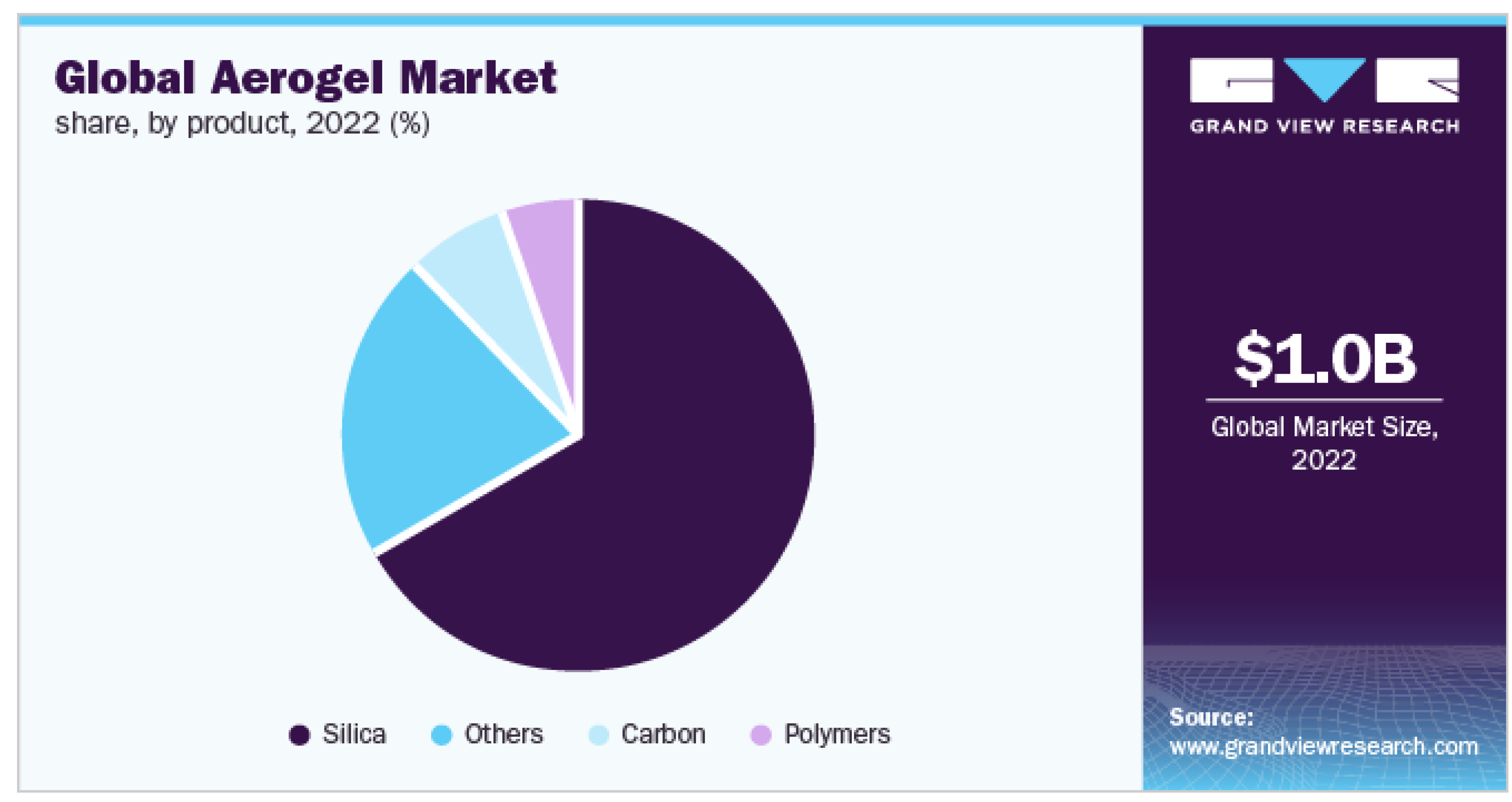
| Material | Drying Method | Applied Methods for Properties | Type of obtained aerogel | Application | Reference |
|---|---|---|---|---|---|
|
Freeze-drying | SEM, Shrinkage of aerogels, Porosity of aerogels, Thermal conductivity, TGA, FTIR, Antibacterial activity, AFM, Cytotoxicity Tests | Gel film (colorless, transparent) | Wound dressing | [4] |
|
Freeze-drying | XRD, FTIR Spectra, liquid substitution method, MTT assay |
Powdered dried and ultra-thin pellet | Wound bandage & Biological tissue platform | [214] |
|
Ambient drying leading to self-assembly (EISA) | TEM, SEM, XRD, SXAS and N2 physisorption, stimulated body fluids in vitro (SBF), PCR analysis, gram-negative bacteria, Escherichia.coli (for antibacterial properties) | Fine powder (combined with membrane structure later obtained composite aerogel) | Chronic wound healing dressing | [215] |
|
ScCO2 drying | Zeta potential for surface charge, Circular dichroism (CD), X-ray Diffraction (XRD), Rietveld method to determine a crystallite size | - | Wound bandage and Bactericidal activity | [216] |
|
ScCO2 drying | SEM, Sphericity coefficient (SC), UV–vis spectroscopy, Encapsulation efficiency (EE), DSC, FTIR spectrophotometer, simulated wound fluid (SWF) contact | Core-shell droplets gel (beads) | Wound healing process | [217] |
| Nation | Supplier | Chemical composition of the aerogel | Trade Name | Configuration of Aerogel | Reference |
|---|---|---|---|---|---|
| Spain | Technalia | Cellulose aerogels from wooden pulp | Inacell | Cellulosic sponge | [223] |
| Germany | Aerogel-it | Biomass and waste materials derived from agriculture, forestry, and marine ecosystems that are not intended for human consumption | Lignin Aerogel | Boards | [224] |
| Estonia | Fibenol | lignin, wood sugars, and specialty cellulose from wood residues. | Lignova | Fine and coarse ground | [225] |
| Switzerland | Empa | TEMPO-oxidized nanofibrillated cellulose (NFC), chitosan | - | Monolith | [226,227] |
| Singapore | Jios Aerogel | Ultra-light silica material into a fibrous material | Armacell/Armagel | Blankets | [228] |
| France | Enersens Absolute Insulation | Silica aerogel into nonwoven fibers | Skogar | Composite blankets | [229] |
| USA | Cabot corporation | Aerogel granules embedded in non-woven fibers | Thermal Wrap | Blankets | [230] |
| USA | Aspen Aerogel | Mainly silica, but also combining with reinforcing fiber | Spaceloft C | Blankets | [231] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





