Submitted:
09 November 2023
Posted:
10 November 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.3. DNA Analysis
2.3.1. DNA Extraction
2.3.2. CDDP and PBA Marker Assay
2.3.3. Microsatellite Markers Assay
2.3.4. Direct Sequencing
2.3.5. Data Analysis
3. Results and Discussion
4. Conclusions
- Regardless of the processing method, the highest levels of chlorophylls, β-carotene, and vitamin C were found in the frozen product from raw material collected in Limanowa and Sucha Beskidzka mountainous areas.
- The preservation method significantly impacted chlorophyll and vitamin C content and less on β-carotene content. The best way for preserving chlorophyll content was freezing; freeze-drying was best for β-carotene. In the case of vitamin C, air drying resulted in almost complete decomposition. In contrast, freezing and freeze-drying leaves only resulted in significant differences in the raw material collected in the Sucha Beskidzka (mountainous area), where freezing became the better choice.
- There was no difference in the total polyphenol content in the frozen leaves between those obtained in Krakow and Sucha Beskidzka. In contrast, the frozen leaves harvested from Limanowa and Ropa contained significantly more of these compounds. Convection drying of the leaves resulted in a slight increase in TP content in the leaves from Ropa and a decrease in the leaves from Sucha Beskidzka, compared to the frozen leaves. In contrast, lower TP content was found in the freeze-dried leaves regardless of the collection site. Drying contributed to a significant increase in antioxidant activity in comparison to freezing. The results were significantly higher for the air-dried leaves than the freeze-dried ones.
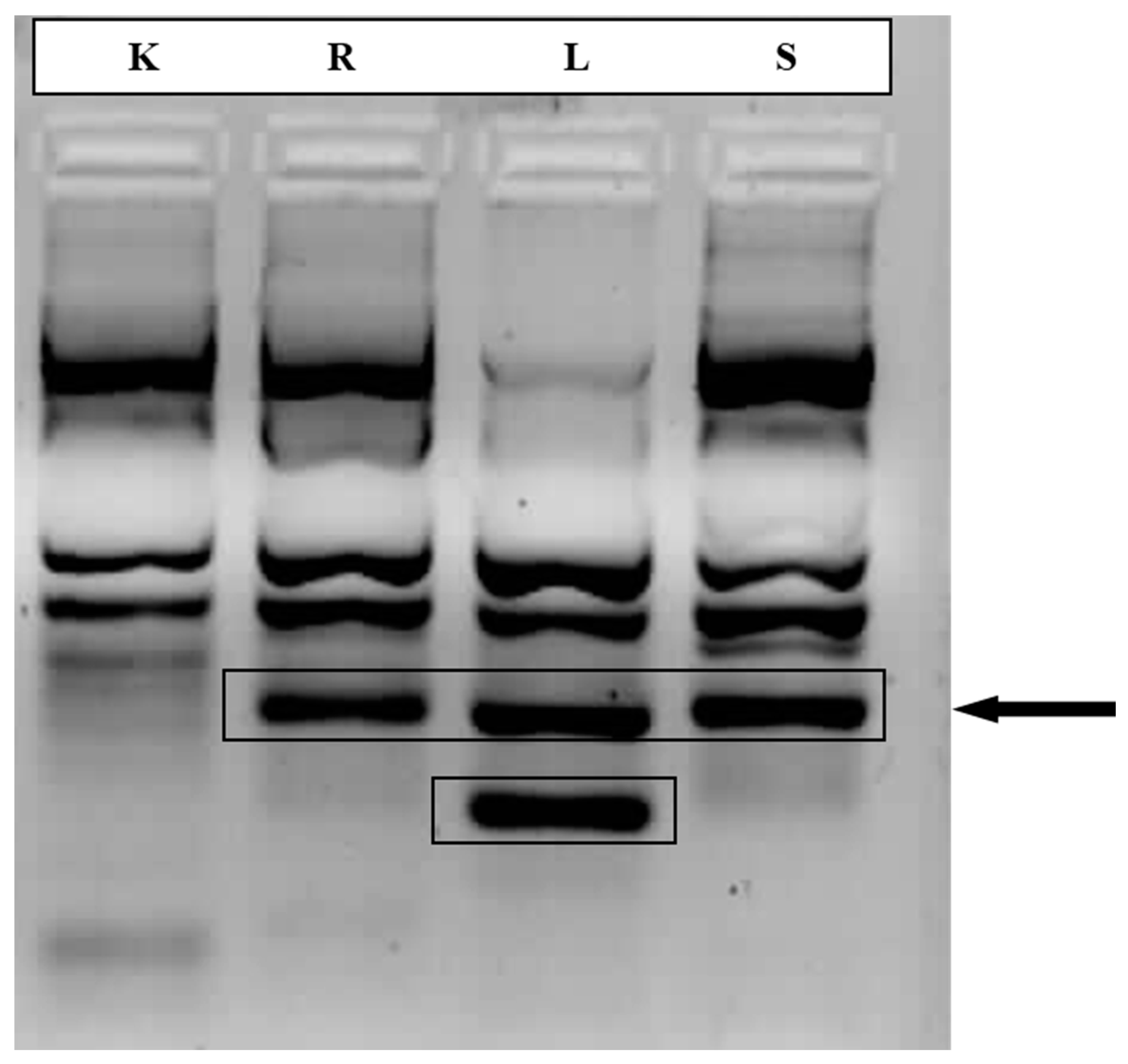
- Marker analysis of coding region-based CDDP and PBA techniques showed sequence variability in the samples from the mountainous areas – Ropa, Limanowa, and Sucha Beskidzka – compared to the sample from Krakow. One of the reasons for this variability might be environmental conditions.
- Taking into account the level of total polyphenols and antioxidant activity, the best preservation method was usually air drying; in the case of vitamin C and chlorophylls, it was freezing; and in the case of β-carotene, freeze-drying was best.
- The leaves collected in the Limanowa area were characterised by the highest level of total polyphenols, chlorophylls, β-carotene, and antioxidant activity (FRAP), and those picked in the Sucha Beskidzka area had the highest levels of vitamin C.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maurer, M.; Schueckler, A. Eds. Use and potential of wild plants in farm households, FAO, Rome, 1999. Available online: http://www.fao.org/3/W8801E/w8801e00.htm#toc_00.
- Bvenura, C.; Sivakumar, D. The role of wild fruits and vegetables in delivering a balanced and healthy diet. Food Res Int 2017, 99, 15. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.; Ahmad, M.; Haroon, M. Edible wild plants: A solution to overcome food insecurity. Springer, Cham, Switzerland, 2017. [CrossRef]
- Odeny, D.A.; Narina, S.S. Allium. In: Wild crop relatives: Genomic and breeding resources; Kole C. Ed.; Springer, Berlin, Heidelberg, Germany, 2011. pp. 1-10. [CrossRef]
- Khassanov, F.O. Taxonomical and ethnobotanical aspects of Allium species from Middle Asia with particular reference to subgenus Allium. In The Allium genomes. Compendium of plant genomes; Shigyo, M.; Khar, A.; Abdelrahman, M. Eds.; Springer, Cham, 2018, 11-21. [CrossRef]
- Rola, K. Taxonomy and distribution of Allium ursinum (Liliaceae) in Poland and adjacent countries. Biol 2012, 67, 1080–1087. [Google Scholar] [CrossRef]
- Peinado, M.; Aguirre, J.L.; Aparicio, A. The Iberian ranges and highlands. In: The vegetation of the Iberian peninsula. Plant and vegetation, vol. 12; Loidi, J. Ed.; Springer, Cham, Switzerland, 2017; pp. 439-512. [CrossRef]
- Bisen, P.S.; Emerald, M. Nutritional and therapeutic potential of garlic and onion (Allium sp.). Curr Nutr Food Sci 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursać Kovačević, D. An overview of organosulfur compounds from Allium spp.: from processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem 2019, 276, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Snopel, L.; Mlcek, J.; Planetova, T. Polyphenols and antioxidant capacity in different types of garlic. Potravinarstvo Slov J Food Sci 2018, 12, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Barla, G.; Poroch-Seritan, M.; Sanduleac (Tudosi), E.; Ciornei (Stefaroi), S.E. Antioxidant activity and total phenolic content in Allium ursinum and Ranunculus ficaria. Food Env Saf 2014, 13, 349. [Google Scholar]
- Apak, R. Current issues in antioxidant measurement. J Agric Food Chem 2019, 67, 9187. [Google Scholar] [CrossRef] [PubMed]
- MRiRW. Produkty regionalne i tradycyjne. (Polish Ministry of Agriculture and Rural Development, Local and traditional products database). Available online: https://www.gov.pl/web/rolnictwo/produkty-regionalne-i-tradycyjne (accessed on 10 May 2023).
- AOAC. Official methods of analysis, 14th ed.; Association of Official Chemists, Arlington, VA, USA, 1984.
- PN EN 14130:2004. Foodstuffs - determination of vitamin C by HPLC. Polish Commite of Normalisation, Warsaw, Poland.
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shok Kog Gakk 1999, 39, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pekkarinen, S.S.; Heinonen, I.M.; Hopia, A.I. Flavonoids, quercetin, myrcetin, kaempferol and (+) catechin and antioxidants in methyl linoleate. J Sci Food Agr 1999, 79, 499–506. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ”Antioxidant Power”: The FRAP assay. Anal Biochem 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Inui, H.; Komada, T.; Ohkawa, Y.; Ohkawa, H. Herbicide metabolism and cross-tolerance in transgenic potato plants co-expressing human CYP1A1, CYP2B6 and CYP2C19. Pestic Biochem Physiol 2000, 66, 116–129. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Z.L.; Zou, X.; Huang, J.; Ruas, P.; Thompson, D.; Shen, Q.J. Annotations and functional analyses of the rice WRKY gene superfamily reveals positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol 2005, 137, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Sliwinska, E. Genetic diversity and inbreeding level of Cotoneaster orbicularis Schltdl. in the Sinai Mountains revealed by microsatellite markers and flow cytometry. Egypt J Bot 2017, 57, 351–361. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications Innis, M.A.; Gelfand, D.H.; Sninsky, J.J.; White, T.J. Eds.; Academic Press, New York, USA, 1999, pp. 315–322.
- Jędrszczyk, E.; Kopeć, A.; Bucki, P.; Ambroszczyk, A.M.; Skowera, B. The enhancing effect of plants growth biostimulants in garlic cultivation on the chemical composition and level of bioactive compounds in the garlic leaves, stems and bulbs. Not Bot Horti Agrobot 2019, 47(1), 81. [Google Scholar] [CrossRef]
- Leahu, A.; Damian, C.; Oroian, M.; Juravle, L.; Ropciuc, S. Physico-chemical and antioxidant properties of two medicinal wild plants grown in Moldova region. Sci Pap Anim Sci Biotech 2015, 48(1), 382–388. [Google Scholar]
- Błażewicz-Woźniak, M.; Michowska, A. The growth, flowering and chemical composition of leaves of three ecotypes of Allium ursinum L. Acta Agrobot 2011, 64(4), 171–180. [Google Scholar] [CrossRef]
- Tomšik, A.; Radojčin, M.; Stamenković, Z.; Kevrešan, Ž.; Mastilović, J.; Pavkov, I.; Vidović, S. Convective drying and preservation of functional ingredients of wild garlic (Allium ursinum L.) in dependence of drying temperature. In: Preceding’s of the III International Congress on Food Technology, Quality and Safety, 25-27 October 2016, University of Novi Sad, Institute of Food Technology, Novi Sad, Serbia, pp. 646-650.
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci Tech 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C. Derivation of sufficiency values of a chlorophyll meter to estimate cucumber nitrogen status and yield. Comput Electron Agr 2017, 141, 54–64. [Google Scholar] [CrossRef]
- Çetİn, M. Changes in the amount of chlorophyll in some plants of landscape studies. Kastamonu Üniv J Forest Fac 2016, 16(1), 239-245.
- Marty-Audouin, C.; Leber, A.; Rocha-Mier, T. Influence of drying on the colour of plant products. In: Developments in drying: Food dehydration (Vol. 1); Mujumdar, A.S.; Sirikayala, S. Eds.; Kasetsart University Press, Bangkok, Thailand, 1999, pp. 207-233.
- Mishra, V.K.; Bacheti, R.K.; Husen, A. Medicinal uses of chlorophyll: a critical overview. In: Chlorophyll: Structure, function and medicinal uses. Le, H.; Salcedo, E. Eds.; Nova Science Publishers, Inc., Hauppauge, NY, USA, 2011, pp. 177-196.
- Srichaikul, B.; Bunsang, R.; Samappito, S.; Butkhup, S.; Bakker, G. Comparative study of chlorophyll content in leaves of Thai Morus alba Linn. species. Plant Sci Res 2011, 3, 17–20. [Google Scholar] [CrossRef]
- Luta, G.; Gherghina, E.; Balan, D.; Israel-Roming, F. Bioactive compounds and antioxidant properties of some wild plants with potential culinary uses. Rev Chim 2020, 71(2), 179–184. [Google Scholar] [CrossRef]
- Rocha, T.; Marty-Audouin, C.; Lebert, A. Effect of drying temperature and blanching on the degradation of chlorophyll a and b in mint (Mentha spicata Huds.) and basil (Ocimum basilicum): analysis by high performance liquid chromatography with photodiode array detection. Chromatographia 1993, 36(1), 152-156. [CrossRef]
- Štajner, D.; Milić, N.; Mimica-Dukić, N.; Lazić, B.; Igić, R. Antioxidant abilities of cultivated and wild species of garlic. Phytother Res 1998, 12(S1), S13–S14. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Baskaran, V. Carotenoid composition and retinol equivalent in plants of nutritional and medicinal importance. Efficacy of β-carotene from Chenopodium album in retinol-deficient rats. Food Chem 2010, 119, 1584–1590. [Google Scholar] [CrossRef]
- Žnidarčič, D.; Ban, D.; Šircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem 2011, 129(3), 1164–1168. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J.P. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agr 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Janda, K.; Kasprzak, M.; Wolska, J. Witamina C – budowa, właściwości, funkcje i występowanie (Vitamin C – structure, properties, occurrence and functions). Pom J Life Sci 2015, 61(4), 419. [Google Scholar]
- Wolska, J.; Czop, M.; Jakubczyk, K.; Janda, K. Influence of temperature and brewing time of nettle (Urtica dioica L.) infusions on vitamin C content. Rocz Panstw Zakl Hig.
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Tec 2000, 20(3), 207–220. [Google Scholar] [CrossRef]
- Lachowicz, S.; Kolniak-Ostek, J.; Oszmiański, J.; Wiśniewski, R. Comparison of phenolic content and antioxidant capacity of bear garlic (Allium ursinum L.) in different maturity stages. J Food Process Pres 2017, 41, e13089. [Google Scholar] [CrossRef]
- Pejatović, T.; Samardžić, D.; Krivokapić, S. Antioxidative properties of a traditional tincture and several leaf extracts of Allium ursinum L. (collected in Montenegro and Bosnia and Hercegovina). J Mater Environ Sci, 2017; 8, 1929–1934. [Google Scholar]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Brindza, J.; Vidović, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason Sonochem 2016, 29, 502–511. [Google Scholar] [CrossRef]
- Pavlović, D.R.; Veljković, M.; Stojanović, N.M.; Gočmanac-Ignjatović, M.; Mihailov-Krstev, T.; Branković, S.; Sokolović, D.; Marčetić, M.; Radulović, N.; Radenković, M. Influence of different wild-garlic (Allium ursinum) extracts on the gastrointestinal system: spasmolytic, antimicrobial and antioxidant properties. J Pharm Pharmacol 2017, 69(9), 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Krivokapic, M.Z.; Jakovljevic, V.L.; Sovrlic, M.M.; Bradic, J.V.; Petkovic, A.M.; Radojevic, I.D.; Brankovic, S.R.; Comic, L.R.; Andjic, M.M.; Kocovic, A.G.; Tomovic, M.T. Biological activities of different extracts from Allium ursinum leaves. Acta Pol Pharm Drug Res 2020, 77(1), 121–129. [Google Scholar]
- Gîtin, L.; Dinicâ, R.; Parnavel, R. The influence of extraction method on the apparent content of bioactive compounds in Romanian Allium spp. leaves. Not Bot Horti Agrobot 2012, 40(1), 93–97. [Google Scholar] [CrossRef]
- Pop, R.M.; Bocsan, I.C.; Buzoianu, A.D.; Chedea, V.S.; Socaci, S.A.; Pecoraro, M.; Popolo, A. Evaluation of the antioxidant activity of Nigella sativa L. and Allium ursinum extracts in a cellular model of doxorubicin-induced cardiotoxicity. Molecules. [CrossRef]
- Lachowicz, S.; Kolniak-Ostek, J.; . Oszmiański, J.; Wiśniewski, R. Comparison of phenolic content and antioxidant capacity of bear garlic (Allium ursinum L.) in different maturity stages. J Food Process Pres 2017, 41, e12921. [Google Scholar] [CrossRef]
- Kovarovič, J.; Bystrická, J.; Fehér, A.; Lenková, M. Evaluation and comparison of bioactive substances in selected species of the genus Allium. Potravinarstvo Slovak J Food Sci 2017, 11(1), 702–708. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.C.; Mackill, D.J. Conserved DNA-derived polymorphism (CDDP): a simple and novel method for generating DNA markers in plants. Plant Mol Biol Rep 2009, 27(4), 558–562. [Google Scholar] [CrossRef]
- Smolik, M.; Malinowska, K.; Smolik, B.; Pacewicz, K. Polymorphism in Random amplified and nuclear rDNA sequences assessed in certain apple (Malus x domestica Borkh.) cultivars. Not Bot Horti Agrobot, 2011; 39, 264–270. [Google Scholar]
- Álvarez, I.; Wendel, J.F. Ribosomal ITS sequences and plant phylogenetic inference. Mol Phylogenet Evol 2003, 29, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Nalini, E.; Bhagwat, S.G.; Jawali, N. Identification and characterization of some ITS variants from hexaploid wheat (Triticum aestivum L.). Plant Sci 2007, 173, 262–268. [Google Scholar] [CrossRef]
- Kumar, M.; Rakesh Sharma, V.; Kumar, V.; Sirohi, U.; Chaudhary, V.; Sharma, S.; Saripalli, G.; Naresh, K R.; Yadav, K.H.; Sharma, S. Genetic diversity and population structure analysis of Indian garlic (Allium sativum L.) collection using SSR markers. Physiol Mol Biol Plants 2019, 25, 377–386. [Google Scholar] [CrossRef]
- Barboza, K.; Beretta, V.; Kozub, P.C.; Salinas, C.; Morgenfeld, M.M.; Galmarini, C.R.; Cavagnaro, P.F. Microsatellite analysis and marker development in garlic: distribution in EST sequence, genetic diversity analysis, and marker transferability across Alliaceae. Mol Genet Genomics 2018, 293, 1091–1106. [Google Scholar] [CrossRef]
- da Cunha, CP,; Resende, FV.; Zucchi, MI.; Pinheiro, JB. SSR-based genetic diversity and structure of garlic accessions from Brazil. Genetica 2014, 142(5), 419-431. [CrossRef]

| Component | Product | Location | ||||
|---|---|---|---|---|---|---|
| Kraków | Limanowa | Ropa | Sucha Beskidzka |
mean | ||
|
Dry matter g/100 g FM |
frozen | 12.0b | 10.7a | 9.8a | 12.6b | 11.3A |
| air-dried | 91.3e | 87.5d | 92.7f | 85.5c | 89.2B | |
| freeze-dried | 94.5g | 94.3g | 96.9h | 95.4g | 95.3C | |
| mean | 65.9B | 64.2A | 66.4B | 64.5A | ||
|
Chlorophyll a mg/100 g DM |
frozen | 914e | 1081f | 936e | 641b | 893C |
| air-dried | 711c | 1078f | 830d | 450a | 767A | |
| freeze-dried | 817d | 1047f | 920e | 617b | 850B | |
| mean | 814B | 1069D | 895C | 569A | ||
|
Chlorophyll b mg/100 g DM |
frozen | 379fg | 430hi | 414h | 255c | 370B |
| air-dried | 342de | 454i | 324d | 188a | 327A | |
| freeze-dried | 329de | 401gh | 358ef | 220b | 327A | |
| mean | 350B | 428C | 365B | 221A | ||
|
Total chlorophyll (a+b) mg/100 g DM |
frozen | 1293e | 1511f | 1350e | 896b | 1263C |
| air-dried | 1053c | 1532f | 1154d | 638a | 1094A | |
| freeze-dried | 1146d | 1448f | 1278e | 837b | 1177B | |
| mean | 1164B | 1497D | 1261C | 790A | ||
|
β
-carotene mg/100 g DM |
frozen | 180bc | 213e | 155a | 187bcd | 184A |
| air-dried | 188bcd | 221e | 185bcd | 171ab | 191AB | |
| freeze-dried | 178bc | 220e | 204de | 193cd | 199B | |
| mean | 182A | 218B | 181A | 184A | ||
| Component | Product | Location | ||||
|---|---|---|---|---|---|---|
| Kraków | Limanowa | Ropa | Sucha Beskidzka | mean | ||
| L-ascorbic acid (AA) | frozen | 379cd | 473d | 441cd | 840f | 533C |
| air-dried | 15a | 50ab | 130b | 14a | 52A | |
| freeze-dried | 365c | 455cd | 445cd | 619e | 471B | |
| mean | 253A | 326B | 339B | 491C | ||
| L-dehydroascorbic acid (DHAA) | frozen | 462e | 497g | 542h | 833i | 584C |
| air-dried | 5a | 41b | 170c | 40b | 64A | |
| freeze-dried | 516g | 481f | 521g | 537h | 514B | |
| mean | 328A | 339B | 411C | 470D | ||
| Vitamin C | frozen | 841c | 969de | 983e | 1673g | 584C |
| air-dried | 20a | 91a | 300b | 54a | 64A | |
| freeze-dried | 881cd | 936de | 966de | 1156f | 514B | |
| mean | 328A | 339B | 411C | 470D | ||
| Component | Product | Location | ||||
|---|---|---|---|---|---|---|
| Kraków | Limanowa | Ropa | Sucha Beskidzka |
mean | ||
|
Total polyphenols g/100 g DM |
frozen | 1.64cd | 1.95e | 1.91e | 1.68d | 1.80B |
| air-dried | 1.73d | 2.01ef | 2.14f | 1.47ab | 1.84C | |
| freeze-dried | 1.59bcd | 1.51abc | 1.41a | 1.45ab | 1.49A | |
| mean | 1.65B | 1.83C | 1.82C | 1.53A | ||
|
ABTS ABTS µmol Trolox/g DM |
frozen | 347a | 511b | 418ab | 397ab | 418A |
| air-dried | 1860e | 1955e | 1889e | 1919e | 1905C | |
| freeze-dried | 1636d | 1434c | 1523c | 1725d | 1580B | |
| mean | 1281A | 1300AB | 1276A | 1347B | ||
|
DPPH ABTS µmol Trolox/g DM |
frozen | 20ab | 12a | 11a | 27bc | 18A |
| air-dried | 124f | 99e | 110e | 131f | 116C | |
| freeze-dried | 36cd | 48d | 48d | 42d | 43B | |
| mean | 60AB | 53A | 56A | 67B | ||
|
FRAP µmol Fe2+/g DM |
frozen | 707a | 798b | 717a | 716a | 734A |
| air-dried | 1295e | 1609g | 1379f | 1050c | 1333C | |
| freeze-dried | 1283e | 1205d | 1046c | 1248de | 1195B | |
| mean | 1095C | 1204D | 1047B | 1004A | ||
| CDDP analyse | ||
| Primer combination | Results of differentiation | Type of profile change |
| F/R1 | - | Monomorphic profile |
| F/R2 | + | Locus insertion in sample R |
| F/R2b | - | Monomorphic profile |
| F/R3 | + | Locus deletion in sample R |
| F/R3b | + | Locus deletion in sample R Locus insertion in sample L Locus insertion in sample S |
| PBA analyse | ||
| Primer combination | Results of differentiation | Type of profile change |
| CYP A1 F+R | + | Locus deletion in sample R Locus deletion in sample L Locus deletion in sample S |
| CYP 2B F+R | + | Locus deletion in sample R Locus deletion in sample L Locus deletion in sample S Locus insertion in sample R Locus insertion in sample L Locus insertion in sample S |
| CYP 2C F+R | + | Locus deletion in sample R Locus deletion in sample L Locus deletion in sample S Locus insertion in sample R Locus insertion in sample L Locus insertion in sample S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





