1. Introduction
Melanoma of the skin is an important public health related issue. In Romania Global Cancer Statistics 2020 estimates the 5-year prevalence at 25.04/100.000 inhabitants and places as the 20th cancer based on the number of new cases [
1]. The landscape of skin melanoma treatment has been forever changed with the introduction of anti-programmed death-1 antibodies, anti-cytotoxic T-lymphocyte antigen-4 antibodies and finally targeted therapies such as BRAF and MEK inhibitors [
2].
In the context of a pathology with such an impact on public health [
3] a serum tumoral marker that can help in the diagnostic process can prove invaluable. While being one of the first markers that proved useful in skin melanoma, lactate dehydrogenase (LDH) [
4], proves its usefulness time and time again in the diagnostic of skin melanoma. LDH is an enzyme responsible for the conversion of pyruvate to lactate [
4]. It can be found in the cytoplasm of melanoma cells that replicate and thrive through oxygen-low dependence mechanisms, such as anaerobic pathways [
5][
6]. The upregulation of this cytoplasmatic enzyme helps the cancers cells adapt to the low-oxygen environment that is created by the ever-expanding clone of cancer cells out demanding the supply of oxygenated blood supplied by the neoangiogenic blood vessels [
5]. The serum LDH levels raises when a large number of cells with high intracytoplasmic LDH levels spill their content into the bloodstream when cell death occurs. While in past research it was tough that high LDH levels were only associated with liver metastasis, newer data how this assumption to be false [
7][
8]. Now LDH level is associated with a multi-site skin melanoma spread [
9] and has diagnostic, prognostic, and predictive value [
10][
11].
The conceptual shift in the therapeutic approach took place with the release of the CheckMate studies, such as CheckMate 067 [
12] which concluded that that combination therapy with nivolumab and ipilimumab, or nivolumab alone in advanced melanoma yielded a better OS than ipilimumab alone. Another study, CheckMate 066 [
13] in which significant survival benefit, favorable safety profiles and a favorable quality of life was achieved when comparing nivolumab to dacarbazine. Subsequent changes in the guidelines placed check point inhibitors and targeted therapies at the forefront of advanced melanoma treatment.
These changes came in a context in which patients were treated beforehand in an adjuvant or metastatic setting with therapies such as interferon alfa-2b or different regimens of chemotherapy such as dacarbazine, temozolomide or platinum doublets [
14,
15]. This study retrospective evaluates stage IV melanoma patients treated with nivolumab and ipilimumab combination therapy with regards to survival data, incidence and severity of side effects based on real world data collected from The Oncology Institute “Prof. Dr. Ion Chiricuță” Cluj-Napoca, Romania, and The Regional Institute of Oncology, Iași, Romania between the years of 2019 up to the end of 2022.
While this type of novel agents has been adopted in day-to-day practice in Romania, local real-world data (RWD) regarding these types of therapies has not been published to the knowledge of the author. RWD can prove an invaluable source of information as some clinical scenarios that are encountered frequently in clinical practice have not been explored in approval clinical trials. Therefore, patients with characteristics omitted in such trials, for example patients with ECOG 2 performance status and patients with brain metastasis, can yield different results to these therapies than those reported in clinical trials. Also, the subgroup analyses provide invaluable information with regards to patients’ characteristics, disease related particularities and serological markers that can have predictive, prognostic and potentially even diagnostic value.
2. Materials and Methods
Patients and study design
We retrospectively enrolled patients with stage IV melanoma who underwent combined treatment with anti-programmed death-1 antibodies – Nivolumab and anti-cytotoxic T-lymphocyte antigen-4 antibodies – Ipilimumab. The patients were enrolled in the two centers from Romania between January 2019 until the end of December 2022. Inclusion criteria were histologically confirmed diagnosis of melanoma, tumor stage IV according to AJCC 2018 (8th edition) and eligibility for Nivolumab-Ipilimumab treatment according to national guidelines.
Medical data was collected from the local database of each institution, all patients that underwent Nivolumab-Ipilimumab combined treatment between the years mentioned above were investigated. After verifying inclusion criteria, we identified a total of 57 patients. From the identified subjects we excluded 4 patients due to incomplete medical data that prevented correct appreciation of OS, PFA or specific data used in subgroup analyses.
PFS was defined as the time from start of therapy to the date of the first progression of the disease, or death of the patient. For patients without disease progression at the time of analysis, PFS was censored on the date of last patient contact. OS was defined as the time from start of therapy until patient decease date. For patients alive at the date of analysis were censored. Adverse effects (AEs) were identified and rated by the center in which the patient underwent treatment in accordance with the Common Terminology Criteria for Adverse Events, Version 5 (CTCAE). Subgroup analyses included gender, BRAF-mutation status, LDH levels, previous therapies, and completion of Ipilimumab sequence. The study was carried out based on the approval of the Ethics Committees of The Oncology Institute "Prof. Dr. Ion Chiricuţă". All patients gave their consent in accordance with the Declaration of Helsinki.
Statistical analyses
The data were analyzed in SAS for Windows, V9.4 (SAS Inc., Cary, NC, USA). LDH means were stratified by the number of metastatic sites before treatment and compared using independent sample T-Test. OS was defined as the time from the start of the Nivolumab + Ipilimumab sequence of treatment until the date of death from any cause. The patients alive at the time of analysis were censored. PFS was defined as the time between the start of the Nivolumab + Ipilimumab sequence of treatment until the first record of disease progression or death from any cause. The survival curves were estimated using the Kaplan-Meier method, and survival distributions were compared with log-rank test. The effects of the main clinical and pathological variables on OS and PFS were investigated with Cox regression.
3. Results
Patients
A total of 53 patients receiving Nivolumab + Ipilimumab in metastatic setting were included from the two centers in Romania. The median-age of the patients was 54.1 ranging between 23 and 77 years old. The sex distribution was homogenous with 49.1% female and 50.9% male patients. ECOG status ranged between 0 and 2, with most patients 62.3% having ECOG 1 while 22.6% being ECOG 0 and 15.1% ECOG 2. LDH levels were withing normal ranges at the start of the treatment sequence for 56.6% of the patients. BRAF mutation status was evenly matched, 43.4% mutated and 47.2% wild type.
With regards to the distribution of metastasis at the start of combined anti PD-1 and anti CTLA-4 antibodies, non-regional lymph node metastases were present in 30 (56.6%) patients. Skin, and other soft tissue metastases, such as muscle or other visceral organs not mentioned e.g., spleen, were present in 19 patients (35.8%). Bone metastases were present in 18.9% of patients while the most frequent visceral sites of disease were lung metastases present in 50.9% of subjects and hepatic metastases present in 34% of subjects there was also an important precent of patients with central nervous system (CNS) metastases at the start of therapy, 9 (17%), as seen in table 1. Most frequent sites of distant disease progression observed during combined treatment were lung metastases closely followed by CNS and bone metastases while local progression occurred in only 1,9% of patients,
Table 1.
LDH levels a potential diagnostic tool for patients with skin melanoma in a high burden disease scenario
Analyzing mean LDH levels stratified by the number of metastatic sites showed a progressive grow of mean serum LDH levels as a higher number of metastatic sites were affected. For patients with one metastatic site, regardless of organ involved (this includes non-CNS visceral sites of metastasis, nonregional lymph node, CNS, distant skin metastasis and other soft tissue metastasis) a mean LDH level (U/L) (n=10) 196.2 (116-399) (SD=78.72). For patients with two metastatic sites (n=19) a mean LDH level of 307.36 (144-1643) (SD=338.19) and for patients with three or more metastatic sites (n=17) a mean LDH level of 357.23 (99-1159) (SD=264.04).
A comparison between the groups means was performed using Independent Samples T-Test, when comparing one metastatic site (M=196.2, SD=78.72) vs two metastatic sites (M=307.36, SD=338.19), statistical significance was not achieved t (21.44) =1.36, p=0.187. The comparison between the group with three or more metastatic sites (M=357.23, SD=264.04) with the group with only one metastatic site achieved statistical significance t (20.37) =2.34, p=0.02. Based on these results LDH levels have a high chance to be elevated in patients that present with three or more sites of metastatic disease.
Treatment and survival
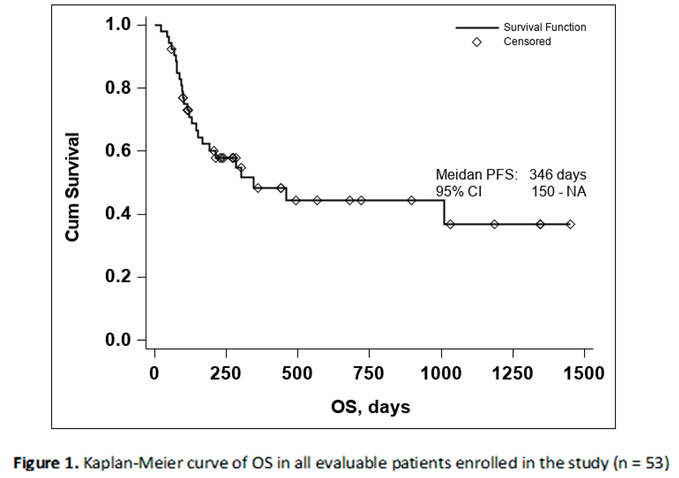
The Kaplan-Meier curve of OS in all evaluable patients enrolled in the study resulted in a median OS of 346 days, Figure 1
Due to the small number of events in the investigated group the upper confidence interval of the of the Kaplan-Meier estimator for OS is not available, thus the 95% CI for OS was 150-NA.
The lower median OS in comparison to Checkmate 067 [
2] is to be expected due to the different inclusion criteria. While the Checkmate study included previously untreated, unresectable, stage III or stage IV melanoma with a status performance of 0 or 1, in our study only stage IV patients were included and a significant number having central nervous system, lung and hepatic involvement,
Table 1. The differences between the two populations are to be further detailed in the Discussion section.
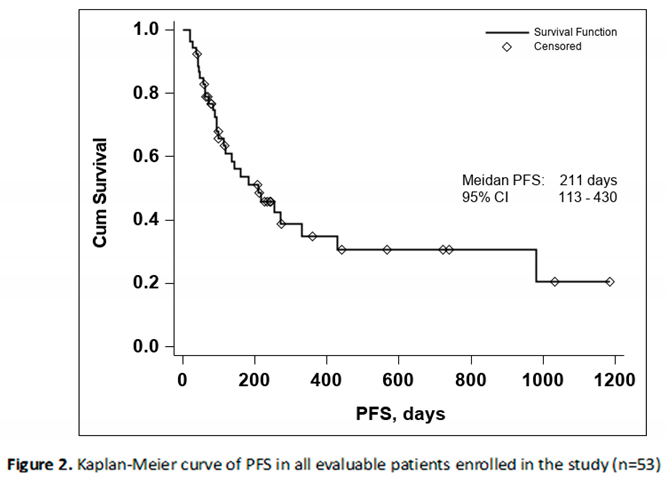
The median PFS was 211 days with a 95% CI (113-430), as seen in Figure 2. In both the Kaplan-Meier curves there can be noticed an aggregate of events occurring around the 250-day mark for OS and the 200-day mark for PFS, with and important number of subjects maintaining the response to treatment if no disease progression or death occurred until the aforementioned time marks. In conclusion, this data seems to indicate that if a response to treatment is observed past the 250-day mark a high chance of maintaining this response for an extended period of time exists.
Adverse events
45.3% of the patients experienced adverse events during the Nivolumab + Ipilimumab treatment with some of them having multiple organ systems involved,
Table 2. AEs above ≥3 were reported in 26.4% of patients with one death due to gastrointestinal complications occurring. 17% of patients had a discontinuation of the Ipilimumab sequence treatment due to AEs.
While most AEs occurring during the Nivolumab maintenance sequence were grade ≤ 2, one grade 5 AE occurred. Further investigation into that specific case revealed that the side effect occurred at the start of the Nivolumab maintenance sequence, immediately after the end of the Ipilimumab induction phase, thus no conclusion can be drawn as to the individual contribution that these agents had in this specific case. Most grade ≥3 were gastrointestinal disorders (colitis, including a case of ulcerative colitis, with the most common occurring symptom being diarrhea) and hepatic disorders (treatment related hepatitis frequently manifesting itself through raised liver enzymes and high bilirubin levels). Most grade ≤ 2 AEs consisted in gastroenterological, hepatic, and endocrine side effects with the most common gland disorders consisting in thyroiditis and subsequent sequalae,
Table 2.
Subgroup analyses
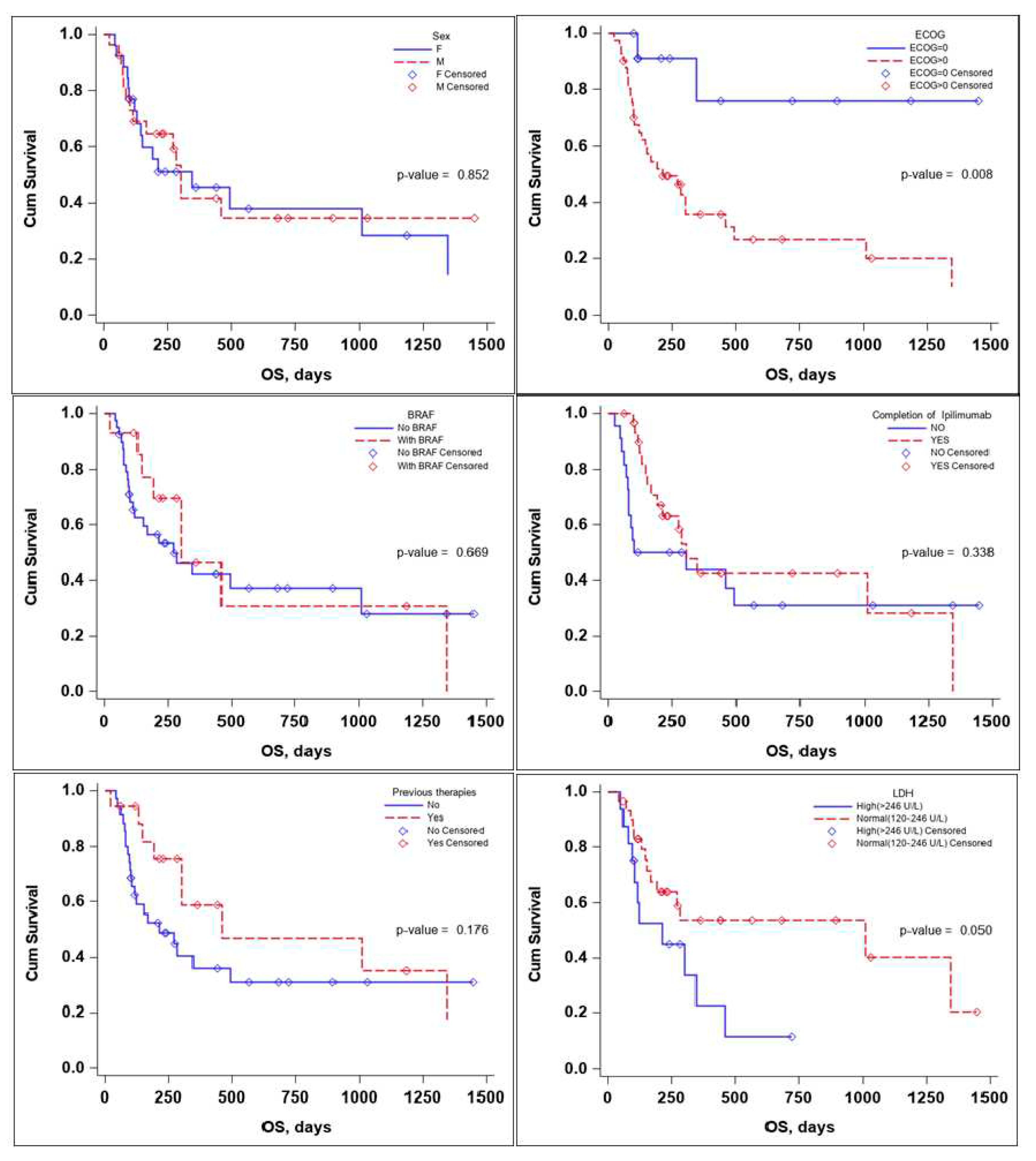
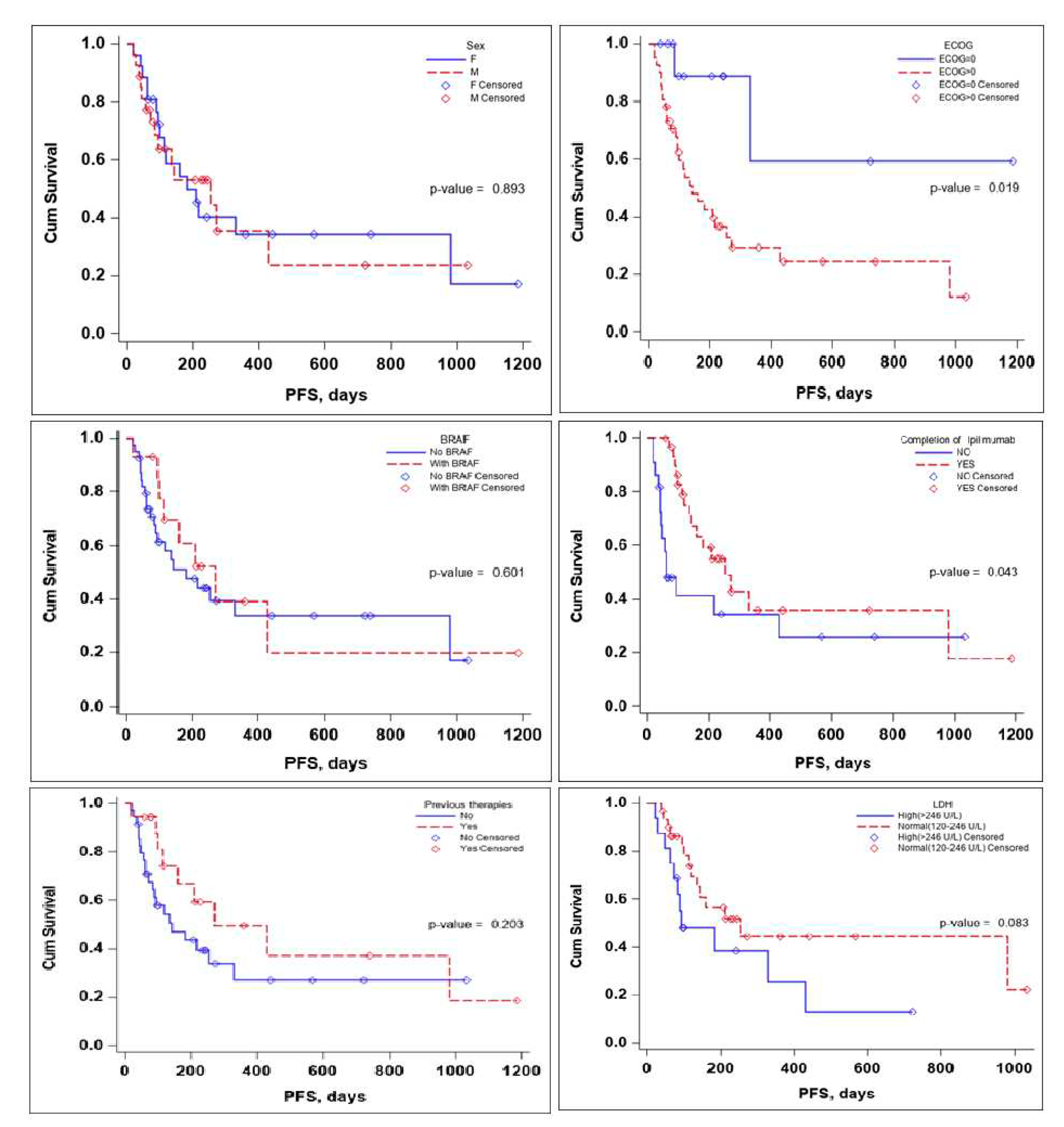
Several subgroup analyses were performed to evaluate the impact that sex, ECOG status, BRAF mutation status, the completion of the Ipilimumab sequence, previous therapies, and lactate dehydrogenase (LDH) levels would have on OS and PFS of the patients,
Figure 3 and
Figure 4.
The sex of the patient it did not influence the OS, with a median survival time of 346 days for female (95% CI: 130-1347) and 302 days for males (95% CI: 113-NA) with a p value that did not reach statistical significance neither on the log rang test, p-value 0.852, or the multivariate Cox regression analysis ,p-value 0.254, HR 95% CI: 1.74 (0.67-4.49),
Table 3.
ECOG status and LDH levels both influenced survival in a negative way. A patient’s ECOG Performance Status Scale score of 0 has a positive impact on OS. With a Kaplan-Meier curve for OS as seen in figure 3, the p-value on the log-rank test of 0.008 and a multivariate-Cox regression p-value of 0.031 HR (95% CI): 0.16 (0.03-0.84).
While LDH levels barely failed to meet the <0.05 significance value on the log-rank test, p-value = 0.050; median survival time LDH values > 246 U/L of 212 (95% CI: 94-346) and LDH values <246 U/L of 1011 (95% CI: 168-NA), the significance value was reached on the multivariate Cox regression analysis p-value 0.030 HR (95% CI): 2.90(1.11-7.55),
Table 3.
Receiving previous therapies, BRAF mutational status and completion of Ipilimumab induction sequence failed to reach statistical significance influencing OS,
Table 3.
With regards to PFS the sex of the patient and the BRAF mutation status did not seem to influence PFS in a significant manner. LDH levels seem to be associated with lower PFS, but the significance value was reached only in the multivariate Cox regression analysis, HR (95% CI) 0.10 (0.02-0.58), with a p-value of 0.010 – the scientific evidence does not allow for a definitive conclusion to be drawn due to the limited number of events in the group,
Table 4.
No definitive conclusion can be drawn with regards to the impact of previous therapies on PFS, while the log-rank test did not reach statistical significance (p-value 0.203) the number of events in the group was limited, thus no definitive statement can be made in this regard. While completion of the Ipilimumab induction sequence, 3mg/kg for 4 cycles, had a tendency of reaching statistical significance, in the Multivariate Cox regression analysis HR (95% CI) 0.92 (0.09-9.77) the p-value 0.945 failed to reach statistical significance,
Table 4.
4. Discussion
This study covers real-world data from 57 patients, out of which 4 were excluded due to lack of complete medical data. We retrospective analyzed the remaining 53 stage IV melanoma who received Nivolumab and Ipilimumab combination therapy with regards to survival data, incidence and severity of side effects based on real world data collected from The Oncology Institute “Prof. Dr. Ion Chiricuță” Cluj-Napoca, Romania, and The Regional Institute of Oncology, Iași, Romania between the years of 2019 up to the end of 2022.
LDH levels can be a useful tool in the initial diagnostic work-up for skin melanoma patients, as presented in our result patients that have three or more sites of metastasis have a higher chance of having higher serum LDH levels, our findings being in consensus with the literature on this matter [
9]. But this marker has its limitations, for example LDH levels do not associate as well as S-100B with metabolic active tumor volume (evaluated via 18F-FDG PET/CT scans)[
16].There is also the problem of racial disparities between the expression of this marker[
17,
18] and other confounding factor and comorbidities that can cause high serum LDH levels for example other malignancies: lymphomas, pancreatic carcinoma, various liver metastases etc. [
19] and other diseases myocardial infarction, obstructive jaundice, acute hepatitis etc. [
19,
20,
21,
22].
The OS values were lower than that reported in trials as the patients included in clinical trials are highly selected and most of them include also stage III patients who have a significantly better prognostic than stage IV patients. One solid evidence for the efficacy of the combined ipilimumab and nivolumab regimen is the CheckMate 067 study. In the long-term results reported [
23] for the ipilimumab-nivolumab combination an OS of 49%, a melanoma-specific survival (MSS) of 56%, with median MSS not reached at 6.5-years minimum follow up. With a minimum follow-up of 7.5 years, the results remained consistent, median OS of 72.1 months for the combination therapy, 36.9 months for the Nivolumab monotherapy and 19.9 months for the Ipilimumab monotherapy, median MSS were as followed: not reached, 49.4 months and 21.9 months. This study included stage III/IV previously untreated, unresectable with and ECOG performance status of 0-1and excluded patients with: ECOG ≥ 2, active brain metastases, uveal melanoma, and autoimmune diseases. When comparing the included population characteristics of the two studies,
Table 5 [
24] there are noticeable differences especially with regards to the ECOG status, number of patients with brain metastases and disease staging.
In comparison with results reported in the chemotherapy era of melanoma treatment, in which dacarbazine and paclitaxel + carboplatin regimens were staples of treatment in stage IV melanoma, a marked improvement can be noticed. Chemotherapy treatments in this scenario reported a median survival time of 7-9 months [25, 26, 27] and a one-year overall survival ranging between 30% and 65%, varying greatly based on the site of metastasis and LDH levels at diagnosis [
28]. Meanwhile our results reported an OS of 346 days with 17% of patients having CNS involvement and 30.2% of patients having high LDH levels at diagnosis.
With regards to side effects, most papers reported side events frequency varied: IMMUNED trial reported 71% (95% CI 57-82) grade 3-4 adverse events with combined therapy (28.4 months median follow-up: IQR 17.7-36.8) [
29]; CheckMate 915 [
30] at a minimum follow-up of approximately 23.7 months reported 32.6% grade 3-4 adverse events; Larkin et al. 2015 [
31] reported treatment-related adverse events of grade 3 or 4 occurred in 55% in the combination group ( median follow up ranged between 12.2 to 12.5 months across the three groups included). The frequency of treatment related adverse events in our study was 45.3% with AEs ≥ 3 being reported in 26.4% with one grade 5 event. Most ≥3 were gastrointestinal disorders (colitis, including a case of ulcerative colitis, with the most common occurring symptom being diarrhea) and hepatic disorders (treatment related hepatitis frequently manifesting itself through raised liver enzymes and high bilirubin levels),
Table 2.
Multivariate Cox regression analysis performed for LDH levels showed statistical significance in both OS and PFS thus LDH has both predictive and prognostic value. This serological marker also has the potential of being used as a complementary diagnostic tool as high LDH levels can be quite commonplace in a high burden disease scenario.
5. Conclusions
This study provides real-world insights into the survival data and safety profiles of combination therapy with anti-programmed death-1 antibodies (Nivolumab) and anti-cytotoxic T-lymphocyte antigen-4 antibodies (Ipilimumab), proving it to be an efficient treatment with a toxicity profile similar with other reporting. Also, LDH remains a serological marker useful in diagnostics as it associates with a high disease burden and its also useful due to also having prognostic and predictive value.
Author Contributions
ADAS contributed to literature search, figures, data collection, data analysis, data interpretation and writing; ACS was involved in data interpretation and writing; PD was involved in data collection, data interpretation and writing; VAA was involved in data collection, data analysis, data interpretation and writing; DS contributed to literature search, figures, data collection, data analysis, data interpretation and writing.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Oncology Institute “Prof. Dr. Ion Chiricuță” Cluj-Napoca, Romania.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
ADAS reports personal fees from Egis and Astra Zeneca outside the submitted work. No other conflicts of interest have been declared by the other authors.
References
- LI H. Global Health Estimates 2016: Deaths by cause, age, sex, by country and by region, 2000–2016. Geneva, World Health Organization; 2018.
- Michielin O, Van Akkooi AC, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Onc. 2019; 30:1884-901. [CrossRef]
- Seidler AM, Pennie ML, Veledar E, Culler SD, Chen SC. Economic burden of melanoma in the elderly population: population-based analysis of the Surveillance, Epidemiology, and End Results (SEER)–Medicare data. Archives of Dermatology. 2010 Mar 1;146(3):249-56.
- Weinstein D, Leininger J, Hamby C, Safai B. Diagnostic and prognostic biomarkers in melanoma. The Journal of clinical and aesthetic dermatology. 2014 Jun;7(6):13.
- Agarwala SS, Keilholz U, Gilles E, Bedikian AY, Wu J, Kay R, Stein CA, Itri LM, Suciu S, Eggermont AM. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951). European Journal of Cancer. 2009 Jul 1;45(10):1807-14. [CrossRef]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. British journal of cancer. 2003 Sep;89(5):877-85. [CrossRef]
- Finck SJ, Giuliano AE, Morton DL. LDH and melanoma. Cancer. 1983 Mar 1;51(5):840-3.
- Deichmann M, Benner A, Bock M, Jäckel A, Uhl K, Waldmann V, Näher H. S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. Journal of clinical oncology. 1999 Jun;17(6):1891-. [CrossRef]
- Fischer GM, Carapeto FC, Joon AY, Haydu LE, Chen H, Wang F, Van Arnam JS, McQuade JL, Wani K, Kirkwood JM, Thompson JF. Molecular and immunological associations of elevated serum lactate dehydrogenase in metastatic melanoma patients: A fresh look at an old biomarker. Cancer medicine. 2020 Nov;9(22):8650-61. [CrossRef]
- Deichmann M, Benner A, Bock M, Jäckel A, Uhl K, Waldmann V, Näher H. S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. Journal of clinical oncology. 1999 Jun;17(6):1891-. [CrossRef]
- Weide B, Elsässer M, Büttner P, Pflugfelder A, Leiter U, Eigentler TK, Bauer J, Witte M, Meier F, Garbe C. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. British journal of cancer. 2012 Jul;107(3):422-8. [CrossRef]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017; 377:1345-1356. [CrossRef]
- Long GV, Atkinson V, Ascierto PA, Robert C, Hassel JC, Rutkowski P, Savage KJ, Taylor F, Coon C, Gilloteau I, Dastani HB. Effect of nivolumab on health-related quality of life in patients with treatment-naïve advanced melanoma: results from the phase III CheckMate 066 study. Annals of Onc. 2016; 27:1940-6. [CrossRef]
- Wilson MA, Schuchter LM. Chemotherapy for melanoma. Melanoma. 2016:209-29.
- Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-α for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer treatment rev. 2003; 29:241-52. [CrossRef]
- Deckers EA, Kruijff S, Brouwers AH, Van Der Steen K, Hoekstra HJ, Thompson JF, García DV, Wevers KP. The association between active tumor volume, total lesion glycolysis and levels of S-100B and LDH in stage IV melanoma patients. European Journal of Surgical Oncology. 2020 Nov 1;46(11):2147-53. [CrossRef]
- Desai AD, Chinta S, Yeh C, Shah VP, Shah R, Paskhover B, Schwartz RA. An analysis of lactate dehydrogenase (LDH) levels in advanced stage IV melanoma of the skin: Prognostic capabilities and demographic variability. Archives of Dermatological Research. 2023 May;315(4):799-806. [CrossRef]
- Mokwatsi GG, Schutte AE, Kruger R. A biomarker of tissue damage, lactate dehydrogenase, is associated with fibulin-1 and oxidative stress in blacks: the SAfrEIC study. Biomarkers. 2016 Jan 2;21(1):48-55.
- Hsieh K, Blumenthal HT. Serum lactic dehydrogenase levels in various disease states. Proceedings of the Society for Experimental Biology and Medicine. 1956 Apr;91(4):626-30.
- Wu Y, Lu C, Pan N, Zhang M, An Y, Xu M, Zhang L, Guo Y, Tan L. Serum lactate dehydrogenase activities as systems biomarkers for 48 types of human diseases. Scientific Reports. 2021 Jun 21;11(1):12997. [CrossRef]
- AL-Janabi AA, Ali ZQ, Noree ZM. Lactate dehydrogenase as an indicator of liver, muscular and cancer diseases. Journal of Coastal Life Medicine. 2015;3(7):543-6.
- Al-Saadoon EA, Al-Naama LM, Hassan JK. Serum lactate dehydrogenase (LDH) activity in children with malignant diseases. Bahrain Medical Bulletin. 2003 Jun;25(2):1-7.
- Hodi FS, Chiarion-Sileni V, Lewis KD, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Queirolo P. Long-term survival in advanced melanoma for patients treated with nivolumab plus ipilimumab in CheckMate 067. [CrossRef]
- Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. The Lancet Onc. 2018; 19:1480-92. [CrossRef]
- Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, Dummer R. Improved survival with MEK inhibition in BRAF-mutated melanoma. NEJM. 2012; 367:107-14.
- Eigentler TK, Caroli UM, Radny P, Garbe C. Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. The lancet onc. 2003; 4:748-59. [CrossRef]
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. NEJM. 2004; 351: 998-1012.
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM. Final version of 2009 AJCC melanoma staging and classification. Journal of clinical onc. 2009 Dec; 27: 6199. [CrossRef]
- Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, Haferkamp S, Gutzmer R, Meier F, Mohr P, Hauschild A. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. 2020; 395: 1558-68. [CrossRef]
- Weber JS, Schadendorf D, Del Vecchio M, Larkin J, Atkinson V, Schenker M, Pigozzo J, Gogas H, Dalle S, Meyer N, Ascierto PA. Adjuvant therapy of nivolumab combined with ipilimumab versus nivolumab alone in patients with resected stage IIIB-D or stage IV melanoma (CheckMate 915). Journal of Clinical Onc. 2023; 41: 517. [CrossRef]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. NEJM. 2015; 373: 23-34. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).