1. Introduction
Prostate cancer represents today the most common malignancy in men, accounting for 23.2% of all new cancer cases in European countries [
1]. An early diagnosis is important for survival and complication rates [
2,
3]. Even though the diagnostic accuracy and specificity of an elevated serum prostate specific antigen (PSA) level is being questioned, PSA remains an important screening tool for prostate cancer and decision making before prostate biopsy [
3]. Indeed, making a decision on prostate biopsy in men with elevated age-related PSA findings and normal digital rectal examination (DRE) is one of most challenging issues in urology. Recently, multiparametric Magnetic Resonance Imaging (mpMRI) is increasingly used for detection, staging, targeted biopsies and treatment monitoring in prostate cancer [
3,
4,
5]. Furthermore, prostate biopsy is associated with an elevated risk of infectious complication such as acute prostatitis, urinary tract infections and sepsis which are arguments for avoiding unnecessary biopsies [
6,
7]. The situation caused a trend among urologists to prescribe antibiotics to see if an elevated PSA in a man with negative DRE findings might be due to infection. Recently, however Kayalı Y. et al. demonstrated that there is no significant difference in the diagnosis of cancer regardless of PSA decrease after antibiotic therapy, suggesting that the use of antibiotics to reduce high PSA levels in order to prevent unnecessary biopsies is not recommended [
8]. In this scenario, any therapeutic agent that is able to improve the diagnostic utility of PSA in decision making before prostate biopsy might help reduce the number of unnecessary biopsies. Moreover, in the era of antimicrobial stewardship, all use of unnecessary antibiotics should be avoided and an antimicrobial sparing approach should be preferred in the decision making before prostate biopsy [
9]. Several phytotherapeutic and nutraceuticals compounds show anti-inflammatory characteristics and are of interest in this field [
10]. In the last years, the use of phytotherapeutic compounds such as curcuma longa, boswellia, urtica dioica, pinus pinaster has been explored in the management of urological disease and have demonstrated minimal side-effects [
11,
12,
13,
14]. Based on the evidence above, we wanted to evaluated if a combination of Curcuma Longa, Boswellia, Pinus pinaster and Urtica dioica (Prostaflog
®) might have a role in decision making before prostate biopsy.
2. Materials and Methods
2.1. Study design and schedule
All consecutive patients with increased PSA value, attending two urological referred centers between June 2022 and May 2023 were enrolled in this exploratory, randomized, controlled phase III study. Patients in the treatment group received Prostaflog
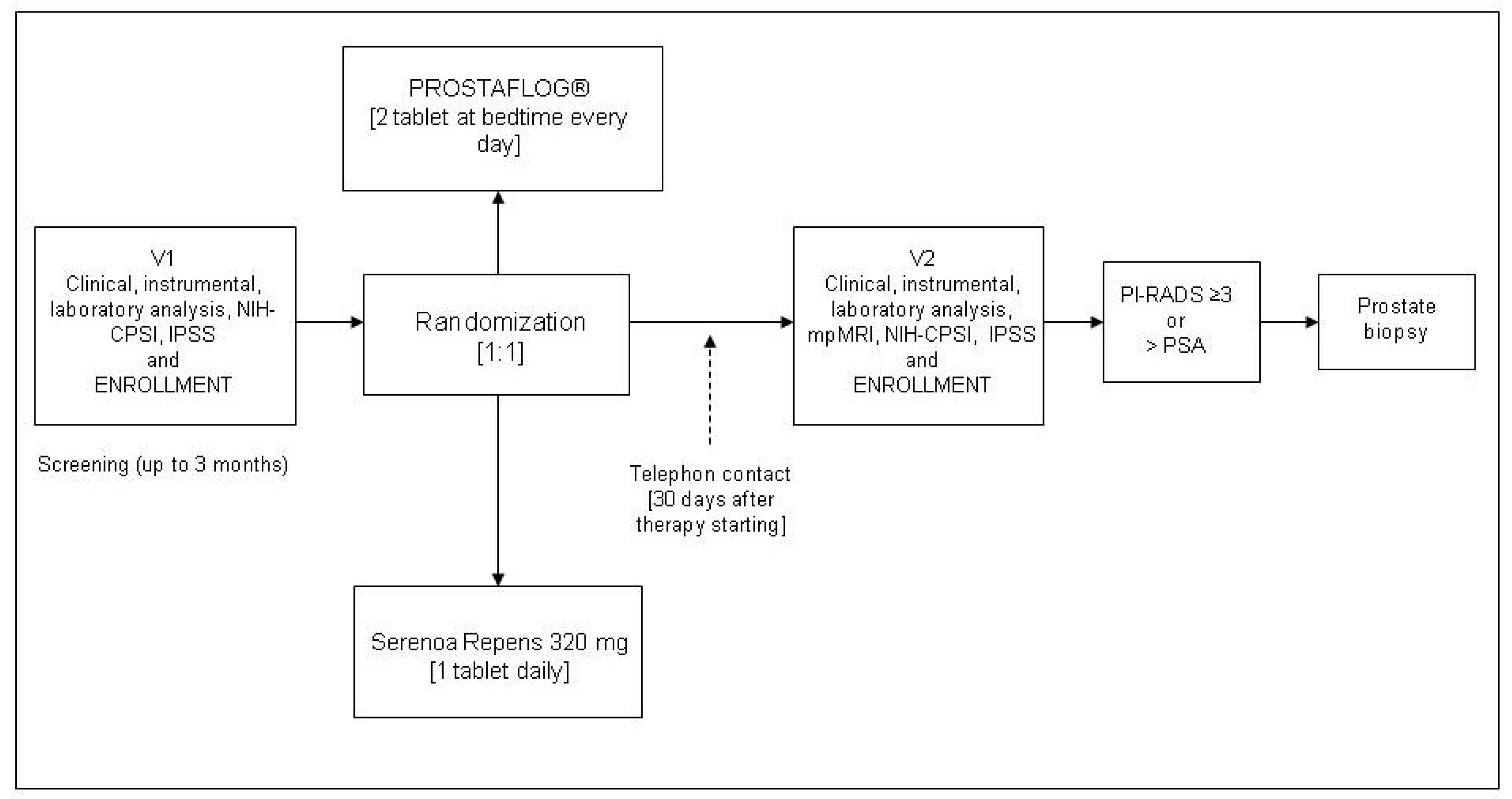
® two capsules at bedtime every day, while patients in the control group received Serenoa Repens 320 mg 1 tablet daily. Patients in both groups underwent 3 months of treatment. Upon arrival, all eligible patients gave written informed consent, provided the results of total and free/total PSA value, filled in baseline questionnaires, and underwent urological examination with DRE. All patients who met the inclusion criteria were randomized by using a computer-generated sequence of allocation. Patients were assigned to treatment groups according to a 1:1 randomization. Enrolled patients were not blinded. No placebo run-in period was considered necessary. All patients were contacted by telephone on day 30 of the therapy to ensure correct timing and dosing of treatment. At the end of the treatment, all patients were scheduled for (mpMRI), a urological follow-up visit with new total and free/total PSA value and repeat baseline questionnaires. Prostate biopsy was performed in patients with suspect prostate cancer at mpMRI (PI-RADS III or higher), in case total PSA had increased (>20% PSA level from the baseline), or suspect findings on DRE. The primary endpoint was the difference between groups in terms of change in PSA value between baseline and end of treatment (ΔPSA) and differences in histological findings. The study schedule is displayed in
Figure 1.
2.2. Inclusion and exclusion criteria
We enrolled patients with increased PSA value defined as a PSA value higher than 4.0 ng/ml at initial evaluation or as a PSA value higher than 75% of the previous PSA evaluation, in line with Leslie et al [
15]. We excluded patients <18 years of age and patients with; major concomitant diseases; a reported allergy to one or more components of the drugs; a history of chronic bacterial prostatitis or acute urinary tract infections; recent transurethral diagnostic or treatment approach; patients receiving 5-alpha reductase inhibitor therapy due to benign prostatic hyperplasia; and patients with indwelling catheter or intermittent catheterization. Moreover, we excluded all patients on active surveillance for prostate cancer or on hormonal treatment for prostate cancer.
2.3. Laboratory and instrumental considerations
No central laboratory was considered necessary. All patients were asked to come to the urological centers with results of total PSA and free/total PSA serum concentrations measured no more than 3 months earlier. PSA changes before and after treatment (ΔPSA) in were evaluated separately for all patients and between the groups, according to PI-RADS II-IV lesions on mpMRI, and findings of prostate cancer on biopsy. PSA reduction was defined as a reduction of 20% or more from the baseline, in line with De Nunzio et al [
16]. Multiparametric MRI was performed in line with international guidelines (17). MpMRI of the prostate combines the anatomic information from T1 and T2 weighted sequences with functional information from diffusion-weighted imaging and dynamic contrast enhancement. All mpMRI have been performed in line with the Prostate Imaging-Reporting and Data System (PI-RADS) v2.1 [
17]. A centralized radiological evaluation was not considered necessary.
2.4. Questionnaires
The validated Italian versions of the International Prostatic Symptom Score (IPSS) [
18] and NIH-Chronic Prostatitis Symptom Index (NIH-CPSI) [
19] score were filled in by each patient on the arrival to the urological outpatient clinics. The questionnaires were only used for the initial clinical assessment at baseline as part of routine clinical practice.
2.5. Composition and characterization of the extracts used
Each dose of Prostaflog® contained: Curcuma longa 500 mg, boswellia 300 mg, urtica dioica 240 mg, pinus pinaster 200 mg, as described in the manufacturer's instructions (Naturneed, 62100, Macerata, Italy). All patients in the control group received Serenoa Repens 320 mg/die.
2.6. Ethical and statistical considerations
This study was approved by the local Ethic Committee (approval protocol number 258, 2019) and its was conducted in compliance with the Institutional Review Board/Human Subjects Research Committee requirements and with the Declaration of Helsinki and the Guidelines for Good Clinical Trial Practice criteria. Written informed consent was obtained from all patients prior to treatment. We state that the study participation and the mpMRI performed within 3 months after referral would not cause delayed diagnosis in patients who turned out to have prostate cancer. Patients were informed accordingly. Randomization was based on a single sequence of random assignments (simple randomization) and performed using a pseudo-random number generator software (Research Randomizer Version 4.0, Social Psychology Network, Wesleyan University, Middletown, CT, USA). All data were presented as means with median and IQR range. χ2 test or Fisher exact test was used for categorical variables. Dependent, nonnormally distributed variables were compared with Wilcoxon Signed Rank test. Pearson’s correlation analysis was used. Data obtained were analyzed by testing the difference between two proportions for incidence of prostate cancer in each group, and evaluation of the ΔPSA. Sensitivity, specificity, positive and negative likelihood ratio, positive and negative predictive values were calculated. Statistical significance was achieved when p<0.05. All reported p-values were two-sided. Statistical analyses were performed using SPSS software, version 22.0 (SPSS, Inc., Chicago, IL, USA) for Apple-Mac.
3. Results
3.1. Patient populations
From an initial cohort of 182 patients attending our centers in the study enrollment period, 162 met the inclusion criteria and were randomly allocated with 74 to the group A and 88 to the group B. Twenty patients were excluded from analysis due to missing data at the follow-up examination. In the per-protocol analysis, data from 66 patients in the Prostaflog
® group and 76 in the Serenoa Repens group were analyzed.
Table 1 shows all demographic, anamnestic, clinical and laboratory data at enrollment.
3.2. mpMRI data
All patients underwent mpMRI according to schedule. Twenty-eight patients had a PI-RADS III or higher: 12 in the PROSTAFLOG
® group and 14 in the Serenoa Repens 320 mg group. The mpMRI findings displayed in
Table 2.
3.3. PSA values at the follow-up visit
Fifty patients in Prostaflog
® group (75.7%) showed a significant reduction of total PSA compared to 49 in Serenoa Repens group (64.4%) (p<0.001) (
Figure 2). No significant difference was found between the two groups in terms of free/total PSA ratio. All PSA values are displayed in
Table 2.
3.4. Decision on prostate biopsy and findings of prostate cancer
All patients with PI-RADS III ore higher at mpMRI underwent prostate biopsy. In the Prostaflog
® group 23 patients (34.8%) underwent prostate biopsy based on mpMRI evaluation and PSA kinetics compared to 59 (77.6%) in the Serenoa Repens group. Overall, prostate cancer was detected in 29 patients in both groups on histopathological analysis: 13 in the Prostaflog
® group (19.7%) and 16 in the Serenoa Repens group (21.0%). Three patients in the Prostaflog
® group showed a significant reduction of total PSA value and had positive findings on mpMRI (6%) while 9 in the Serenoa Repens group (19.5%) showed positive findings (p<0.001). Moreover, 7 patients in the Prostaflog
® group did not show any significant reduction of total PSA value and had negative findings on mpMRI (43%) while 22 in the Serenoa Repens group (81.4%) had negative findings. The ability of PSA reduction after Prostaflog
® therapy to predict positive findings on mpMRI showed a sensitivity of 75% (CI 42.8-94.5), specificity of 87% (CI 75.1-94.6), with a positive likelihood ratio of 5.7 (CI 2.2-12.4) and a negative likelihood ratio of 0.29 (CI 0.11-0.77). All correlations between PSA values and mpMRI findings between the two groups are displayed in
Table 3.
4. Discussion
4.1. Major findings
Here, we demonstrate for the first time that a three-month course of a combination of Curcuma Longa, Boswellia, Pinus pinaster and Urtica dioica in men with higher-than-normal PSA levels is superior to Serenoa Repens in reducing PSA values. Significantly fewer men in the combination group underwent prostate biopsy based on change in PSA value and findings on mpMRI. Our study demonstrates that Prostaflog® is a non-antibiotic option to enhance the utility of PSA in the decision making before prostate biopsy and might help avoiding unnecessary procedures.
4.2. Understanding study findings
Several studies have shown that some plants extract, such as isoflavones, exert a direct effect on the PSA level and prostate volume, both in patients with prostate cancer and benign prostatic enlargement. This is due to the direct inhibition of 5-alpha-reductase by isoflavones. Engelhardt PF et al. demonstrated that a daily dose of isoflavones caused a 10% reduction in the prostate volume after 1 year in patients with benign prostatic enlargement [27]. The difference between Prostaflog
® and Serenoa Repens in our study is probably due to the presence of compounds, such as curcuma, boswellia, urtica dioica and pinus pinaster. In particular, boswellia, urtica dioica and soybean extracts have been shown to have a positive effect on the prostate health [
11]. In particular, Cai et al. recently demonstrated that Prostaflog
® is able to decrease prostate inflammation by affecting the IL-8 level [28]. This means that phytotherapeutic compounds can reduce the PSA level through a hormonal as well as an anti-inflammatory pathway. For Serenoa Repens however, the anti-inflammatory effect seems limited.
4.3. Results in comparison with other studies
The positive relationship between prostate inflammation/infection and elevated PSA values is well established [22]. For this reason, many authors have been looking for ways to reduce elevated PSA that might be due to inflammation in order to reduce the number of unnecessary biopsies. With this aim, urologists have prescribed antibiotics for elevated PSA levels as an empirical treatment of subclinical infection or inflammation. This practice finds no support in international guidelines and violates antimicrobial stewardship principles [23,24,25]. Already in in 2015 Busato et al. demonstrated that empirical antibiotic treatment in asymptomatic male patients did not lead to PSA reduction and highlighted the risk of collateral damages in the form of increased antimicrobial resistance [25]. Moreover, they underlined that even a greater than 10% PSA reduction after antibiotic treatment of this population should not postpone prostate biopsy [25]. In the same way, Baltaci et al. reported that 5 out 17 patients who experienced a decreased PSA level to <4 ng/mL had prostate cancer on biopsy [26].
4.4. Clinical implications
No other studies have so far explored the role of phytotherapy in patients with elevated PSA before prostate biopsy. We found that only 3 patients in the Prostaflog
® group showed a significant reduction of total PSA value while having positive findings at mpMRI (6%) compared with 9 in the Serenoa Repens group (19.5%) (p<0.001), thereby showing that the risk of lowering PSA caused by prostate cancer by means of a hormonal pathway, is very low. After the introduction of mpMRI the number of unnecessary prostate biopsies have decreased and the role of PSA as diagnostic marker of prostate cancer is reduced [
2,
3,
20]. However, the use of mpMRI is not yet widespread in all countries and we therefore still need tools that enable us to differentiate if an elevated PSA is due to prostate cancer or inflammation [
21].
4.3. Strengths and limitations of the present study
A strength of this study is that a three-moth experimental study of a PSA lowering treatment could be performed in a country where it did not violate ethical or public health regulations. The study reports real life practice that was not changed by the protocol. Another strength is that the contents of the phytotherapeutic compounds studied are well known. Weaknesses of the study are related to the low number of patients, the diagnostic uncertainties of the PIRADs classification, the representativity of the prostate biopsies performed, and the lack of long term observational data, especially regarding PSA development and results of repeat biopsies.
5. Conclusions
In conclusion, a combination of Curcuma Longa, Boswellia, Pinus pinaster and Urtica dioica given to men with higher-than-normal PSA levels for three months, was superior to Serenoa Repens in reducing PSA values. After treatment, significantly fewer men in the combination group underwent prostate biopsy based on change in PSA value and findings on mpMRI. Phytotherapy seems an interesting tool to avoid unnecessary prostate biopsies in men with higher-than-normal PSA levels. Prostaflog® might be a good alternative for urologists who believe that an elevated PSA might be caused by inflammation, and who prefer an antibiotic-sparing approach as diagnostic tool before performing prostate biopsy. Further studies are, however, needed to confirm our data.
Author Contributions
Conceptualization, T.C. and L.G.; methodology, G.L.; software, T.C.; formal analysis, L.B.; data curation, I.T., M.P. and M.R.; writing—original draft preparation, T.C.; writing—review and editing, A.P.; supervision, editing and review, T.BJ. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Regione Calabria, approval protocol number 258, 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions in accordance to Italian bylaw.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; Morgan, T.M.; Carlsson, S.V. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur Urol 2023, 84(2),191-206. [CrossRef]
- Van Poppel, H.; Albreht, T.; Basu, P.; Hogenhout, R.; Collen, S.; Roobol, M. Serum PSA-based early detection of prostate cancer in Europe and globally: past, present and future. Nat Rev Urol 2022, 19(9):562-572. [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S., Fossati, N.; Gandaglia, G.; Gillessen, S.; Grivas, N.; Grummet, J., Henry, A.M.; van der Kwast, T.H.; Lam, T.B.; Lardas, M.; Liew, M.; Mason, M.D.; Moris, L.; Oprea-Lager, D.E.; van der Poel, H.G.; Rouvière, O.; Schoots, I.G.; Tilki, D.; Wiegel, T.; Willemse, P.M.; Cornford, P. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2021, 79(2):243-262. [CrossRef]
- Thompson, J.; Lawrentschuk, N.; Frydenberg, M., Thompson, L.; Stricker, P; USANZ. The role of magnetic resonance imaging in the diagnosis and management of prostate cancer. BJU Int 2013, 112 Suppl 2:6-20. PMID: 24127671. [CrossRef]
- Barret, E.; Turkbey, B.; Puech, P.; Durand, M.; Panebianco, V.; Fütterer, J.J.; Renard-Penna, R.; Rouvière, O. Update on the ICUD-SIU consultation on multi-parametric magnetic resonance imaging in localised prostate cancer. World J Urol 2019, 37(3):429-436. [CrossRef]
- Alidjanov, J.F.; Cai, T., Bartoletti, R.; Bonkat, G.; Bruyère, F.; Köves, B.; Kulchavenya, E.; Medina-Polo, J.; Naber, K.; Perepanova, T.; Pilatz, A.; Tandogdu, Z.; Bjerklund Johansen, T.E.; Wagenlehner, F.M. The negative aftermath of prostate biopsy: prophylaxis, complications and antimicrobial stewardship: results of the global prevalence study of infections in urology 2010-2019. World J Urol 2021, 39(9):3423-3432. [CrossRef]
- Pilatz, A.; Veeratterapillay, R.; Köves, B.; Cai, T.; Bartoletti, R.; Wagenlehner, F.; Bruyère, F.; Geerlings, S.; Bonkat, G.; Pradere, B. Update on Strategies to Reduce Infectious Complications After Prostate Biopsy. Eur Urol Focus 2019, 5(1):20-28. [CrossRef]
- Kayalı, Y.; Balbay, M.D.; İlktaç, A.; Ersöz, C.; Toprak, H.; Tarım, K.; Eden, A.B.; Akçay, M.; Doğan, B. PSA change after antibiotic treatment should not affect decisionmaking on performing a prostate biopsy. Turk J Med Sci 2023, 53(1):183-192. [CrossRef]
- Muratore, E.; Baccelli, F.; Leardini, D.; Campoli, C.; Belotti, T.; Viale, P.; Prete, A.; Pession, A.; Masetti, R.; Zama, D. Antimicrobial Stewardship Interventions in Pediatric Oncology: A Systematic Review. J Clin Med 2022 11(15):4545. [CrossRef]
- Cicero, A.F.G.; Allkanjari, O.; Busetto, G.M.; Cai, T.; Larganà, G.; Magri, V.; Perletti, G.; Robustelli Della Cuna, F.S.; Russo, G.I.; Stamatiou, K.; Trinchieri, A.; Vitalone, A. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch Ital Urol Androl 2019, 91(3). PMID: 31577095. [CrossRef]
- Milanese, G.; Agostini, E.; De Angelis, M.V.; Pretore, E.; Galosi, A.B.; Castellani, D. Efficacy of 1-Year Cavacurmin® Therapy in Reducing Prostate Growth in Men Suffering from Lower Urinary Tract Symptoms. J Clin Med 2023, 12(4):1689. [CrossRef]
- Cai, T.; Cocci, A.; Tiscione, D.; Puglisi, M.; Di Maida, F.; Malossini, G.; Verze, P.; Palmieri, A.; Mirone, V.; Bjerklund Johansen, T.E. L-Methionine associated with Hibiscus sabdariffa and Boswellia serrata extracts are not inferior to antibiotic treatment for symptoms relief in patients affected by recurrent uncomplicated urinary tract infections: Focus on antibiotic-sparing approach. Arch Ital. Urol. Androl. 2018, 90, 97–100.
- Cai, T.; Mazzoli, S.; Bechi, A.; Addonisio, P.; Mondaini, N.; Pagliai, R.C.; Bartoletti, R. Serenoa repens associated with Urtica dioica (ProstaMEV®) and curcumin and quercitin (FlogMEV®) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: Results from a prospective randomised study. Int. J. Antimicrob. Agents 2009, 33, 549–553. [CrossRef]
- Liu, J.-P.; Liang, S.-B.; Liang, N.; Bu, F.-L.; Lai, B.-Y.; Zhang, Y.-P.; Cao, H.-J.; Fei, Y.-T.; Robinson, N. The potential effects and use of Chinese herbal medicine pine pollen (Pinus pollen): A bibliometric analysis of pharmacological and clinical studies. World J. Tradit. Chin. Med. 2020, 6, 163–170. [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Anu, R. .I; Hussain Sajjad; Larry, E. Siref. Prostate Cancer. [Updated 2023 May 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470550/.
- De Nunzio, C.; Lombardo, R.; Nacchia, A.; Tema, G.; Tubaro, A. Repeat prostate-specific antigen (PSA) test before prostate biopsy: a 20% decrease in PSA values is associated with a reduced risk of cancer and particularly of high-grade cancer. BJU Int 2018, 122(1):83-88. [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; Thoeny, H.C.; Verma, S.; Barentsz, J.; Weinreb, J.C. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 2019, 76(3):340-351. [CrossRef]
- Badia, X.; Garcia-Losa, M.; Dal-Re, R. Ten-language translation and harmonization of the International Prostate Symptom Score: developing a methodology for multinational clinical trials. Eur Urol 1997, 31:129–40.
- Giubilei, G.; Mondaini, N.; Crisci, A.; Raugei, A.; Lombardi, G.; Travaglini, F.; Del Popolo, G.; Bartoletti, R. The Italian version of the National Institutes of Health Chronic Prostatitis Symptom Index. Eur Urol 2005, 47(6):805-11. [CrossRef]
- Corsi, A.; De Bernardi, E.; Bonaffini, P.A.; Franco, P.N.; Nicoletta, D.; Simonini, R.; Ippolito, D.; Perugini, G.; Occhipinti, M.; Da Pozzo, L.F.; Roscigno, M.; Sironi, S. Radiomics in PI-RADS 3 Multiparametric MRI for Prostate Cancer Identification: Literature Models Re-Implementation and Proposal of a Clinical-Radiological Model. J Clin Med 2022, 11(21):6304. [CrossRef]
- Krimphove, M.J.; Fletcher, S.A.; Trinh, Q.D. Multiparametric magnetic resonance imaging for prostate cancer detection: do clinical trial findings reflect real-world practice? BJU Int 2019, 123(2):197-198. [CrossRef]
- Kryvenko, O.N.; Wang, Y.; Sadasivan, S.; Gupta, N.S.; Rogers, C.; Bobbitt, K.; Chitale, D.A.; Rundle, A.; Tang, D.; Rybicki, B.A. Potential effect of anti-inflammatory drug use on PSA kinetics and subsequent prostate cancer diagnosis: Risk stratification in black and white men with benign prostate biopsy. Prostate 2019, 79(10):1090-1098. [CrossRef]
- Greiman, A.; Shah, J.; Bhavsar, R.; Armeson, K.; Caulder, S.; Jones, R.; Keane, T.E.; Clarke, H.S.; Savage, S.J. Six Weeks of Fluoroquinolone Antibiotic Therapy for Patients With Elevated Serum Prostate-specific Antigen Is Not Clinically Beneficial: A Randomized Controlled Clinical Trial. Urology 2016, 90:32-7. [CrossRef]
- Eggener, S.E.; Large, M.C.; Gerber, G.S.; Pettus, J.; Yossepowitch, O.; Smith, N.D.; Kundu, S.; Kunnavakkam, R.; Zorn, K.; Raman, J.D. Empiric antibiotics for an elevated prostate-specific antigen (PSA) level: a randomised, prospective, controlled multi-institutional trial. BJU Int 2013, 112(7):925-9. [CrossRef]
- Busato, W.F.; Almeida, G.L.; Geraldo, J.; Busato, F.S. Does PSA reduction after antibiotic therapy permits postpone prostate biopsy in asymptomatic men with PSA levels between 4 and 10 ng/mL? Int Braz J Urol 2015, 41(2):329-36. [CrossRef]
- Baltaci, S.; Aksoy, H.; Türkölmez, K.; Elhan, A.H.; Ozden, E.; Göğüş, O. Use of percent free prostate-specific antigen density to improve the specificity for detecting prostate cancer in patients with normal rectal examinations and intermediate prostate-specific antigen levels. Urol Int 2003, 70(1):36-41. [CrossRef]
- Engelhardt, P.F.; Riedl, C.R. Effects of one-year treatment with isoflavone extract from red clover on prostate, liver function, sexual function, and quality of life in men with elevated PSA levels and negative prostate biopsy findings. Urology 2008, 71(2):185-90; discussion 190. [CrossRef]
- Cai, T.; Anceschi, U.; Tamanini, I.; Verze, P.; Palmieri, A. Soybean Extracts (Glycine Max) with Curcuma, Boswellia, Pinus and Urtica Are Able to Improve Quality of Life in Patients Affected by CP/CPPS: Is the Pro-Inflammatory Cytokine IL-8 Level Decreasing the Physiopathological Link? Uro 2022, 2, 40-48. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).