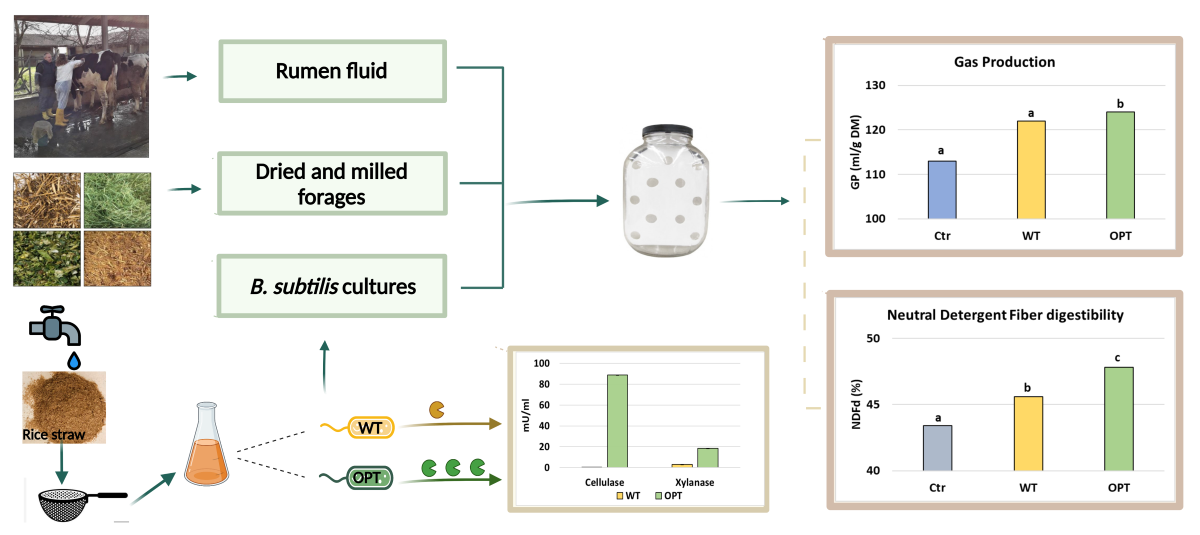
1. Introduction
Current challenges for the livestock sector are the identification of technologies able to boost animal performances through sustainable farming systems [
1,
2]. Sustainability can be met by raising animal productivity through the improvement of the nutritional properties of current forages or sub-optimal feeds, thereby avoiding further exploitation of natural resources. Forage fibers are mainly constituted by cellulose and hemicellulose, and animal productivity has been often correlated with their degradability by the rumen microorganisms [
3,
4]. A higher fiber degradability translates into a lower amount of feed necessary to produce a unit of meat or milk, reducing the impact of animal production on environmental resources. Another non-negligible advantage of incrementing fiber degradability is the possibility of using lower nutritional biomasses that, differently from grains, are not in competition with human nutrition.
To increase the fiber surface area accessible to rumen microbial activity, several forage treatments have been considered, ranging from physicochemical approaches, e.g., treatments with strong alkalis, ammonium, urea, or steam, to more environmentally friendly processes based on the application of exogenous hydrolytic enzymes to deconstruct the lignocellulosic biomass [
5,
6]. However, the latter strategy heavily impacts on farm profitability due to the high costs of industrial enzymes and, importantly, enzymatic activity is often rapidly lost within the rumen environment [
2,
7].
The direct supplementation of probiotic microorganisms producing degradative enzymes into forages and diets may overcome such limits, guaranteeing continuous secretion and turnover of hydrolytic enzymes to deconstruct the biomass, eventually facilitating its fermentation in the rumen.
Numerous microorganisms are known to secrete hydrolytic enzymes that attack the lignocellulosic matrix of vegetable materials, mainly represented by fungi and soil bacteria [
8]. One of the best-characterized bacterial species known to produce degradative enzymes is
Bacillus subtilis. Its attractiveness not only derives from its propensity to secrete massive amounts of enzymes directly into the growth medium (up to 20-25 g/L), but also from the ease of cultivation, and its recognized safety in human and animal nutrition [
9,
10]. Dwelling mainly in the upper layers of soil and in the plant rhizosphere,
B. subtilis genome has evolutionarily accumulated a large set of degradative enzymes [
11]. This species, listed in the Qualified Presumption of Safety (QPS) recommended microorganisms intentionally added to food or feed [
12], was shown to colonize the gastrointestinal tract of mammals and poultry, in the form of vegetative cells (spores) or by forming biofilms, thereby acting as probiotic [
13,
14,
15]. In particular,
B. subtilis spores added to dairy cattle feed consistently exerted a positive influence on ruminal fermentation, growth, lactation performance, and milk composition [
16,
17,
18,
19,
20,
21,
22,
23].
Remarkably, the bacterial characteristics that play a key role on the beneficial effect exerted by B. subtilis-based probiotics still await elucidation.
In this work, we sought to determine whether cellulases and xylanases, crucial enzymes for fiber degradation, are important players in fermentation of feeds in the ruminal environment.
From a common laboratory strain of
B. subtilis, we recently derived an isogenic strain in which the production of endogenous cellulase and xylanase was optimized [
24,
25], while the synthesis of additional hydrolytic enzymes, such as amylase, pullulanase and proteases, remained unaltered. Several types of common cattle feeds were treated in parallel with the two strains (parent vs optimized strain) to evaluate whether
in vitro degradability of the vegetable fibers and gas production, fundamental parameters in feed quality [
26], could be improved by the enhanced cellulolytic and xylanolytic activities.
Moreover, to preserve the affordability of forage treatment,
B. subtilis was conveniently grown in a salt medium in which the carbon source derived from minimally processed rice straw, which is a low-cost, annually renewable, agricultural waste and the third most abundant world biomass, not in competition with the food or feed industry [
27]. Besides, fresh cultures of
B. subtilis were directly applied to forages, without the need of complex and expensive purification steps.
The comparison of feed quality after treatment with the two isogenic strains enabled us to unambiguously establish that cellulases and xylanases play a significant role in the properties of B. subtilis on dairy cows feed fermentation and might represent a valuable characteristic for animal probiotics.
2. Results
2.1.
An inexpensive procedure for the production of affordable
B. subtilis-based feed additives was set up, through a simplified protocol that could be carried out at the farm site. Rice straw was selected as carbon source because it represents an inexpensive and abundant agricultural waste and contains significant amounts of water-soluble carbohydrates, i.e., fructose, sucrose and β-1,3-1,4-glucan [
28,
29]. To extract those sugars through an environmentally friendly and simple process, dried rice straw was vigorously washed with tap water at room temperature. HPLC analyses of the washing liquid (WL) recovered demonstrated the presence of monomeric sugars; to verify whether complex sugars were also present in WL, enzymatic hydrolysis of the WL liquid was carried out with commercial enzymes (see Material & Methods). As shown in
Table 1, the monomeric sugars increased substantially after hydrolysis (300% and 40% for glucose and xylose, respectively), demonstrating that complex sugars could also be recovered by rice straw washing.
The non-hydrolyzed WL fraction was thus used as carbon source for the growth of
Bacillus subtilis in the low-cost medium. Two isogenic laboratory strains were used: PB5700, as wild-type strain (WT), and the engineered PB2OPT strain, only differing from the former for the optimized secretion of cellulases and xylanases [
25]. Both strains were able to grow in shake flask conditions on the WL fraction in a comparable manner, without the requirement of preliminary saccharification of the medium, reaching a final optical density at 600 nm (OD
600) of 2.85 ± 0.14 for the WT and of 3.83 ± 0.14 for PB2OPT at 24 h.
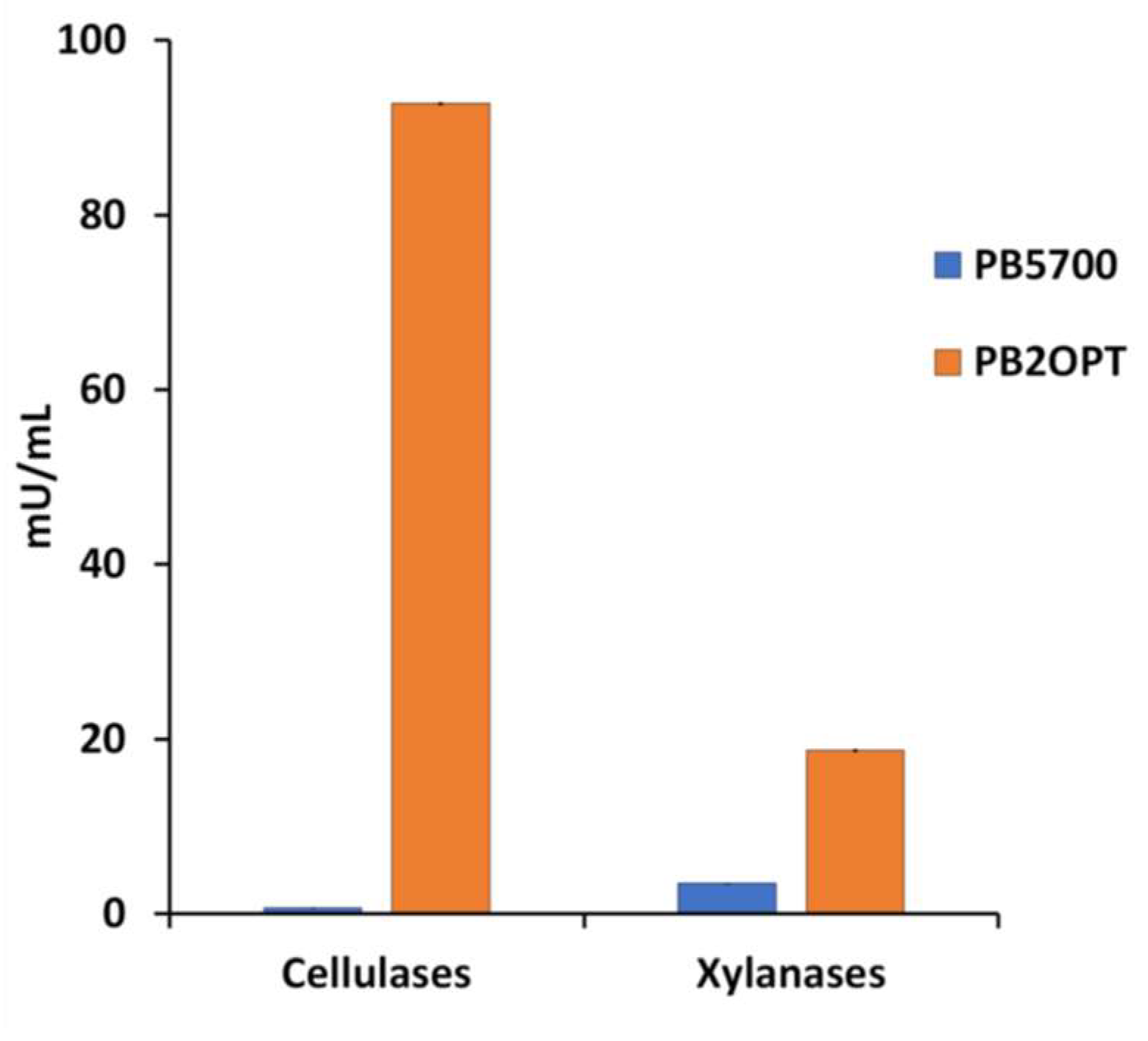
Cellulases and xylanases, released from both strains in the growth medium, were quantified at the end of the incubation. As expected, secretion of both enzymes by the optimized strain was much higher (
Figure 1). Cellulase activity in the supernatant of PB2OPT was 142-fold higher than for PB5700 (92.8 ± 1.6 mU/mL vs 0.7 ± 0.2 mU/mL, respectively). Xylanase activity was also significantly higher in PB2OPT, albeit by only 5.4-fold (18.7 ± 2.2 mU/mL vs 3.5 ± 0.4 mU/mL).
Through the simple process described, a low-cost medium was successfully obtained that supported the 24-h growth of the two strains to be used for feed treatments.
Each bacterial culture was directly applied to common feed ingredients for dairy cows, such as meadow hay, alfalfa hay, corn silage and total mixed ration (TMR), and incubated with feeds in a simulated in vitro rumen environment. Two different sources of each feed type were subjected to identical treatments. Raw cultures of the two B. subtilis strains, which included bacteria and the growth medium in which degradative enzymes had accumulated, were used without any preliminary enzyme or bacterial purification steps, to preserve the economic affordability of the treatment. Sterile WL growth medium was used as control treatment.
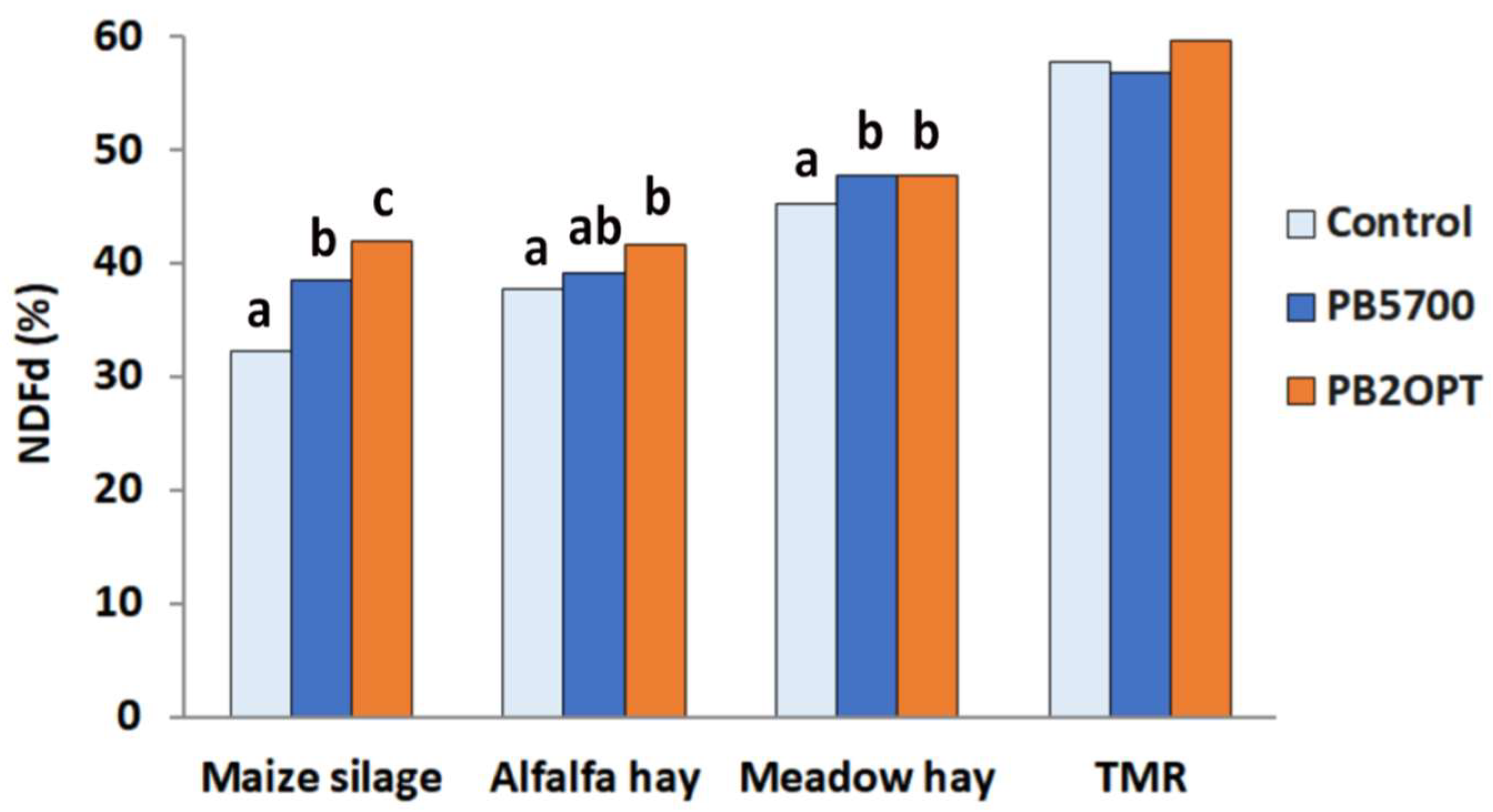
The degradability of the Neutral Detergent Fiber content of each feed (NDFd) was analyzed in all the conditions described (two strains and control treatment, with 8 feeds). NDFd is a parameter that defines the percentage of fiber that can be degraded by ruminal microorganisms, which is in turn positively associated with forage quality [
26,
30]. Analyzing the pool of feed ingredients as a whole, a significant increment in NDFd was observed for both strains with respect to the control and also between each other (p<0.001) (
Table 2). In the samples treated with the WT
B. subtilis strain, NDFd attained to 45.6%, showing a 5% increase compared to the control (43.4% NDFd). Ddegradability showed a remarkable 10% increment (47.8%) with respect to the control when the treatment was carried out with the PB2OPT strain, which secretes higher amounts of degradative enzymes. The degradative enzymes present in the whole cultures at the beginning of the treatment as well as those secreted over time during the treatment may contribute to the described NDFd trend in which PB2OPT-treated feeds showed the highest degradability index.
NDFd data also revealed a significant interaction between Treatment x Feed (p=0.028). Particularly, the NDF content of maize silages (on average around 48% in our heterogeneous samples) was significantly more sensitive to enzymatic digestion and the degradation efficiency correlated with the amount of degradative enzymes released by the strains (PB2OPT>PB5700>control) (
Figure 2); alfalfa hay showed a significant increment in NDFd only in the presence of the optimized strain, although a gradual trend was appreciable, whereas for meadow hay both strains equally contributed to the increase in NDFd as compared to the control (
Figure 2). Unsurprisingly, no effect of the bacterial treatment was observed on TMR forage, characterized by a lower NDF content (on average 34% in our samples).
Indeed, the sensitivity to the treatment was inversely related to the intrinsic degradability of the fiber, represented by the NDFd of the untreated control samples, which is in turn associated with the chemical composition of the substrates. Maize silage, which had the most recalcitrant fiber to ruminal activity among the substrates tested (in terms of digestion rate), benefited the most from the support of bacterial enzymes.
Although the increment given by the PB2OPT treatment compared to the WT is apparently not dramatic, accounting for 5% of NDFd, it is worth recalling that 1% increase in forage NDFd is associated with a 0.17 kg/d increase in DMI and 0.23 kg/d of milk produced [
3,
4]. This result demonstrates that
B. subtilis cellulases and xylanases effectively contribute to increase fiber degradation in the rumen and can thus be responsible, at least in part, for the 14% increase in DMI and 6.94% higher milk production observed upon supplementation of
Bacillus subtilis to lactating cows [
20].
2.2. Gas Production and ruminal fermentative profile
Gas production (GP), an indirect measurement of rumen microbial fermentation, is often used to complement NDFd analyses in the evaluation of the nutritive value of feeds, as it positively correlates with the degradability and nutritional value of the forage [
31].
The gas produced from
in vitro ruminal fermentation of the experimental feeds upon
B. subtilis treatment demonstrated that both strains were able to improve GP compared to the control (
Table 2): regardless of the strain, gas was released more effectively in the presence of
B. subtilis. Although differences among the two strains just showed a trend, the treatment with the PB2OPT strain produced the highest gas volume both at 24 and 48 h of incubation. These data confirmed that
B. subtilis enzymes, as a whole, improved the degradability of the feeds. Indeed,
B. subtilis secretes a large number of degradative enzymes in addition to cellulases and xylanases (e.g., proteases, amylases, pullulanases, pectinases and others) which do not differ between the two strains. Therefore, the limited difference observed between the two strains could be ascribed to the fact that the enzymatic mixture produced by the WT strain is already sufficient to increase the degradation of the feeds within the 48-h incubation.
Moreover, as observed for the NFDd, also in GP the effectiveness of the enzymatic pool is linked to the different chemical composition of the individual substrates, as shown by the interaction between Treatment x Feed (p<0.10). The analyses of single feed ingredients indicated that for meadow hays, the feed with the highest fiber content, PB2OPT was more effective than the WT strain in improving GP (GP 48 h/g DM was, respectively, 95.3, 113 and 125 mL for control, PB5700 and PB2OPT), underlying the significance of cellulase and xylanase activities for this type of substrate. The data presented in
Table 2 also show that GP was higher during the first 24 h of incubation rather than in the following 24 h; this effect was most likely due to rapid fermentation of the easily degradable fractions, such as simple sugars and non-structural carbohydrates (such as starch). Remarkably, the positive effect of the
B. subtilis treatments on GP, which was immediately appreciable (p<0.001 for GP at 24 h), was maintained until the end of the incubation (p<0.001 for GP at 48 h).
Concerning the fermentative profile, N-NH3 concentration was only affected by the feed (p<0.001) and not by the treatment: it was higher for alfalfa and TMR samples (on average 50.5 mg/dL) than for meadow and corn silage samples (45.9 mg/dL) due to their higher Crude Protein content. Similarly, acetic acid production was also mainly affected by the feed (p<0.001), showing a lower concentration in TMR and corn silage samples (on average 55.3% vs 59.3% in alfalfa and meadow). There was a trend towards a lower propionic acid content (% total VFA) (p=0.063) in the samples treated with either PB5700 or PB2OPT (on average 20.2%) as compared to the untreated control (21.4%). The slightly lower propionic acid release in those samples, although not significant, can be related to a higher fiber degradability; the proportion and type of volatile fatty acids produced depend on the substrate metabolized and in this regard fiber fermentation usually promotes a higher acetic production and lower propionate formation.
3. Discussion
The application of
Bacillus spp. to forages included in ruminant diets as probiotics was shown to have a positive impact on animal productivity both
in vivo and
in vitro [
20,
21,
23,
32]. The novelty of our work was the demonstration that the cellulases and xylanases secreted by this microorganism are important determinants in the increase in NDFd and GP observed
in vitro.
The possibility to draw such conclusion was made possible by the availability of two isogenic strains just differing for the production of these two specific enzymes. The comparison of their relative efficacy as feed treatments allowed to confidently ascribe to these two enzymes a relevant role in supporting the ruminal flora in the recovery of nutrients from fibrous material, excluding any confounding effect.
We also designed a convenient, sustainable and simplified production process through which Bacillus subtilis feed additives can be obtained, based on raw cultures grown on an abundant agricultural waste biomass such as rice straw. Based on the acquired data, the raw cultures of Bacillus subtilis obtained with this simplified procedure can be adopted to increase the degradability of different types of feeds.
New strains, each engineered for the over-expression of a different set of enzymes, e.g., proteases, amylases, pullulanases, pectinases and/or others, might allow to verify whether each of them plays a role in the feed degradability and, in the long run, might pave the way for developing the ideal strain to be used as feed additive.
Following the designed procedure, Bacillus subtilis-based feed additives could be produced on site, and possibly be integrated into local biorefineries, creating a virtuous circular-economy process.
4. Materials and Methods
4.1. Bacillus subtilis strains
Bacillus subtilis strains used in this study are the wild-type (WT) PB5700 strain and its engineered version PB5703 (PB2OPT). PB5700 is a spontaneous
swrA+ derivative of the JH642 strain (GenBank accession no. CM000489.1) in which the auxotrophies caused by the
trpC2 pheA1 mutations have been cured as described [
33]. PB2OPT was derived from PB5700 by genetic optimization without the introduction of any exogenous DNA sequence and is similar to strain OS58 [
25]. A patent application is under preparation for this strain; for this reason, additional details on the engineered strain are herein undisclosed.
4.2. Bacterial medium preparation and its characterization
Open-air dried rice straw (Oryza sativa L.) was collected from two local farmers in the Pavia area (northwestern part of Italy) and stored in burlap sacks. Upon use, straw was minced using a kitchen blender (Moulinex AY45 Easy Power Blender) and dried to constant weight in a 60 °C oven. One liter tap water was added to 25 grams of straw and the suspension was vigorously stirred at room temperature for 2 hours with a magnetic stirrer. Straw was removed through a 1 mm mesh-strainer covered with two sheets of gauze. The washing liquid (WL) was collected and subjected to low-pressure sterilization (5 psi/~0.35 bar), to avoid sugar caramelization, for 20 minutes and then stored at -20 °C.
To quantify the sugars extracted, 300 µL of WL were brought to a final volume of 500 µL with 50 mM Na-Acetate buffer pH 5 and incubated at 50 °C for 64 h with shaking, either alone or in the presence of 22 µL- enzymes cocktail constituted by 1U Cellulase (product no 22178, Millipore-Sigma), 1U Beta-glucosidase (product no 49290, Millipore-Sigma), 1U Xylanase (product no X2629, Millipore-Sigma) and 0.013 U Cellobiohydrolase I (product no E6412, Millipore-Sigma). A mock reaction was carried out with the enzymatic cocktail in the absence of substrate. After incubation, samples were centrifuged at 16.873 × g at room temperature for 5 min, filtered through sterile 0.2 µm polyethersulfone (PES) filters and stored at -20 °C until HPLC analysis. Twenty-five microliter samples were injected through an automatic injector and analyzed in an LC-2000 HPLC system (Jasco) equipped with a Supelco C-610H 30 cm x 7.8 mm column (59320-U, Sigma Aldrich) and a RID 10A detector (Shimadzu). For chromatography, 0.1% H
3PO
4 was used as mobile phase with 0.5 mL/min flow rate. Column temperature was kept at 30 °C. Chromatograms were analyzed by the ChromNAV 2.0 software (Jasco). The column was previously calibrated with standard solutions of glucose, xylose, galactose, mannose, arabinose and cellobiose, serially diluted in mobile phase to prepare calibration curves based on at least three replicates for each sugar. The lower limit of quantification for all sugars was 0.3 g/L. Xylose, galactose, and mannose showed the same retention time and the same concentration-peak area calibration lines, thereby enabling the precise quantification of their sum. However, since the rice straw content in galactose and mannose is negligible compared with the one of xylose, we assumed that the concentration of co-eluted xylose, galactose and mannose detected was only due to xylose [
34,
35]. No chromatogram peaks corresponding to arabinose or cellobiose were detected. Background sugars present in the enzymatic cocktail were subtracted from each sample. For control samples with values below the limit of quantification (LOQ) of the calibration curve (0.3 mg/mL), the LOQ/2 value (i.e., 0.15 mg/mL) was used as background [
36]. Sugar quantification values are the mean of two independent experiments.
4.3. Bacillus subtilis cultures
The growth medium was prepared by adding to WL the following components: 7 g/L K2HPO4; 2 g/L KH2PO4; 0.5 g/L Na citrate; 0.1 g/L MgSO4; 1 g (NH4)2SO4; 1 g/L asparagine. PB5700 and PB2OPT spores were revitalized over-night on LB plates. Few single colonies were inoculated in 3 mL of Antibiotic Medium 3 (BD-Difco, New Jersey, U.S) and grown at 37 °C with orbital shaking until culture density was appreciable by visual inspection. Pre-cultures were used to inoculate 20 mL of fresh Antibiotic Medium 3 containing with 5 mg/mL glucose which was grown at 37 °C over-night. Fermentation was carried out in flasks for 24 hours in the WL-based medium at 37 °C with 150 rpm agitation, starting with an optical density at 600 nm (OD600) of 0.2. Each fermentation was independently repeated at least three times.
4.4. Cellulase and xylanase activity assays
At the end of 24-h fermentations, cellulase (endo-1,4-β-glucanase; EC 3.2.1.4) and endo-xylanase (endo-1,4-β-xylanase; EC 3.2.1.8) activities were assayed in the culture supernatants, after acidification, using the CellG5 and XylX6 kits (Megazyme, Wicklow, Ireland) as previously described [
37].
4.5. In vitro analyses
The effect of the enzymes produced by PB5700 and PB2OPT on the degradability of feeds was analyzed using two different in vitro incubation methods aimed to evaluate ruminal degradability of neutral detergent fiber (NDFd) and gas production (GP). For both methods, ruminal fluid was collected from three rumen-cannulated cows (non-lactating Holstein–Friesian). The cows were fed a total mixed ration (TMR) composed by 66% hay and 34% concentrate twice daily to achieve a dry matter intake (DMI) of 8 kg/d. Rumen liquor was collected two hours after the morning feeding, immediately strained through four layers of cheesecloth into a pre-warmed (39 °C) flask, flushed with CO2 and used within one hour from sampling. The donor animals were handled as outlined by the Directive 2010/63/EU on animal welfare for experimental animals and all animal procedures were conducted under the approval of the University of Milan Ethics Committee for animal use and care and with the authorization of the Italian Ministry of Health (authorization n. 904/2016-PR).
For both NDFd and GP experiments, three different treatments were analyzed: i) the control treatment, constituted by the WL-based growth medium, in the absence of bacteria; ii) the treatment, constituted by PB5700 culture; iii) the treatment, constituted by PB2OPT culture.
The incubations were conducted on 8 different feed ingredients, representing a wide range of NDF concentrations: 2 different meadow hays, 2 different alfalfa hays, 2 different corn silages, and 2 different TMRs for lactating cows. All feeds were dried at 60 °C for 48 h in a forced-air oven and ground to pass a 1-mm Fritsch mill (Fritsch Pulverisette, Idar-Oberstein, Germany). All the experiments (three treatments x 8 feed ingredients) were independently repeated three times (three incubation runs for each method).
4.5.1. In vitro NDF degradability
The NDF degradability was determined at 48 h using the Ankom Daisy II incubator (Ankom Technology, Macedon, NY, USA) as previously reported [
38] modified as follows: each jar, containing 250 mg of each feed weighted in triplicate in Ankom F57 bags, was inoculated with 665 mL of fresh bacterial culture from one of the three experimental treatments (control, PB5700, PB2OPT), 133 mL of salt mix solution (90 g/L KH
2PO
4; 5 g/L NaCl; 5 g/L Urea; 4.5 g/L MgSO
4*7H
20; 1 g/L CaCl
2*2H
20), 532 mL of distilled water, 266 mL of reducing solution (Na
2CO
3 15 g/L; Na
2S*9H
2O 1 g/L), and 400 mL of rumen fluid. The NDF content of feed ingredients at the end of incubation was determined using the Ankom 200 Fiber Analyzer (Ankom Technology, Macedon, NY, USA) following the procedure previously reported [
39].
4.5.2. In vitro gas production
Gas production (GP) was analyzed for 48 hours by incubating 200 mg of each feed in individual 120 mL serum bottles for each of the three treatments (control, PB5700, PB2OPT) as previously described [
40]. Each bottle was inoculated with 30 mL of final solution made of 8.33 mL fresh bacterial culture, 1.67 mL of salt mix solution (above-described), 6.7 mL distilled water, 3.33 mL reducing solution (above-described) and 10 mL rumen fluid; for the control treatment (un-inoculated culture), deionized water replaced the WL fraction as, alone, the sugars contained in the WL, and not consumed in the absence of
B. subtilis, could be fermented by the rumen bacteria developing a high level of gas. For each treatment, 2 blanks (bottles containing just the buffered rumen fluid, prepared accordingly to the corresponding treatment, but lacking the substrate feed), were also included, as a measure of the gas produced in absence of any feed. Gas production was determined measuring the headspace pressure in the incubation vials [
41]. The pressure was recorded after 24 and 48 hours of incubation using a digital manometer (model 840082, Sper Scientific, Scottsdale, AZ, USA). The values of pressure obtained from the
in vitro gas production experiment were converted into volume (mL) of gas produced at standard temperature (0 °C) and pressure (1 bar) using the ideal gas law. The gas production of each treatment was corrected with the respective blank before the statistical analyses.
At the end of the incubation, fermentation was stopped by putting all vials into an ice bath and the pH was recorded. The content of each vial was individually transferred into 50 mL Falcon tubes and centrifuged at 10,000 g for 15 min. After centrifugation, 5 mL and 30 mL of supernatant were sampled for subsequent VFA analysis as previously reported [
42]. The NH
3 concentration was determined with a Raypa NP-1500-MP Kjeldahl distiller (Raypa, Terrassa, Spain).
4.6. Calculations and statistical analyses
Data were statistically analyzed by the PROC MIXED procedure of SAS 9.4 (SAS Institute Inc., Cary, NC, USA), with the following model:
where Y
ijk = dependent variable, μ = overall mean, T
i = fixed effect of the treatment (i = 1 to 3), F
j = fixed effect of the feed (j = 1 to 8), R
k = random effect of the fermentation run (k = 1 to 3), T x F
ij = interaction treatment x feed, and ε
ijk is the random error. The least-square means were reported. For all statistical analyses, significance was declared at p-value (p) ≤ 0.05 and trends at p ≤ 0.10.
Author Contributions
Conceptualization, C.C. and S.C.; methodology, C.C., S.C. and G.G.; formal analysis, C.C., L.P., M.B., G.G. and S.C.; investigation, V.B., M.B., E.R., M.C., G.G. and S.C.; data curation, S.C.; writing—original draft preparation, V.B., C.C.; writing—review and editing, C.C., L.P.; visualization, V.B.; supervision, C.C. All authors have read and agreed to the published version of the manuscript. .
Funding
This research was funded by FONDAZIONE CARIPLO, Bando Economia Circolare: ricerca per un futuro sostenibile, grant number 2018-1011.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of the University of Milan with the authorization of the Italian Ministry of Health (authorization n. 904/2016-PR).
Data Availability Statement
All data generated or analyzed during this study are included.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Capper, J.L.; Bauman, D.E. The Role of Productivity in Improving the Environmental Sustainability of Ruminant Production Systems. Annual Review of Animal Biosciences 2013, 1, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Adesogan, A.T.; Arriola, K.G.; Jiang, Y.; Oyebade, A.; Paula, E.M.; Pech-Cervantes, A.A.; Romero, J.J.; Ferraretto, L.F.; Vyas, D. Symposium review: Technologies for improving fiber utilization. Journal of Dairy Science 2019, 102, 5726–5755. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Allen, M.S. Evaluation of the Importance of the Digestibility of Neutral Detergent Fiber from Forage: Effects on Dry Matter Intake and Milk Yield of Dairy Cows. Journal of Dairy Science 1999, 82, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.; Leonardi, C.; Hoffman, P.C.; Combs, D.K. Intake and milk production of cows fed diets that differed in dietary neutral detergent fiber and neutral detergent fiber digestibility. Journal of Dairy Science 2009, 92, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.M.; de Resende, L.C.; Cook, D.E.; Atalla, R.H.; Combs, D.K. Technical note: A comparison of alkali treatment methods to improve neutral detergent fiber digestibility of corn stover. Journal of Dairy Science 2018, 101, 9058–9064. [Google Scholar] [CrossRef] [PubMed]
- Mor, P.; Bals, B.; Tyagi, A.K.; Teymouri, F.; Tyagi, N.; Kumar, S.; Bringi, V.; VandeHaar, M. Effect of ammonia fiber expansion on the available energy content of wheat straw fed to lactating cattle and buffalo in India. Journal of Dairy Science 2018, 101, 7990–8003. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.G.; Azzoni, A.R.; Freitas, S. On the production cost of lignocellulose-degrading enzymes. Biofuels, Bioproducts and Biorefining 2021, 15, 85–99. [Google Scholar] [CrossRef]
- Himmel, M.E.; Xu, Q.; Luo, Y.; Ding, S.-Y.; Lamed, R.; Bayer, E.A. Microbial enzyme systems for biomass conversion: emerging paradigms. Biofuels 2010, 1, 323–341. [Google Scholar] [CrossRef]
- van Dijl, J.M.; Hecker, M. Bacillus subtilis: from soil bacterium to super-secreting cell factory. Microb Cell Fact 2013, 12, 3. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: a universal cell factory for industry, agriculture, biomaterials and medicine. Microbial Cell Factories 2020, 19, 173. [Google Scholar] [CrossRef]
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends in Microbiology 2008, 16, 269–275. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K. ; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; de Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the list of qualified presumption of safety (QPS) recommended microorganisms intentionally added to food or feed as notified to EFSA. EFSA Journal 2023, 21, e07747. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J Food Sci Technol 2017, 54, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-K.; Kim, W.-S.; Paik, H.-D. Bacillus strains as human probiotics: characterization, safety, microbiome, and probiotic carrier. Food Science and Biotechnology 2019, 28, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Mun, D.; Kyoung, H.; Kong, M.; Ryu, S.; Jang, K.B.; Baek, J.; Park, K., II; Song, M.; Kim, Y. Effects of Bacillus-based probiotics on growth performance, nutrient digestibility, and intestinal health of weaned pigs. Journal of Animal Science and Technology 2021, 63, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, J.-Q.; Zhang, H.-T. Effects of supplementation of Bacillus subtilis natto Na and N1 strains on rumen development in dairy calves. Animal Feed Science and Technology 2011, 164, 154–160. [Google Scholar] [CrossRef]
- Sun, P.; Li, J.; Bu, D.; Nan, X.; Du, H. Effects of Bacillus subtilis natto and Different Components in Culture on Rumen Fermentation and Rumen Functional Bacteria In Vitro. Current Microbiology 2016, 72, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.L.; Lopes, N.M.; Zacaroni, O.F.; Silveira, V.A.; Pereira, R.A.N.; Freitas, J.A.; Almeida, R.; Salvati, G.G.S.; Pereira, M.N. Lactation performance and diet digestibility of dairy cows in response to the supplementation of Bacillus subtilis spores. Livestock Science 2017, 200, 35–39. [Google Scholar] [CrossRef]
- Choonkham, W.; Schonewille, J.T.; Bernard, J.K.; Suriyasathaporn, W. Effects of on-farm supplemental feeding of probiotic Bacillus subtilis on milk production in lactating dairy cows under tropical conditions. Journal of Animal and Feed Sciences 2020, 29, 199–205. [Google Scholar] [CrossRef]
- Jia, P.; Tu, Y.; Liu, Z.; Li, F.; Yan, T.; Ma, S.; Dong, L.; Diao, Q. Diets supplementation with Bacillus subtilis and Macleaya cordata extract improve production performance and the metabolism of energy and nitrogen, while reduce enteric methane emissions in dairy cows. Animal Feed Science and Technology 2022, 294, 115481. [Google Scholar] [CrossRef]
- Pan, L.; Harper, K.; Queiroz, O.; Copani, G.; Cappellozza, B.I. Effects of a Bacillus-based direct-fed microbial on in vitro nutrient digestibility of forage and high-starch concentrate substrates. Translational Animal Science 2022, 6, txac067. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Cui, Y.; Gao, B.; Zhang, H.; Jiang, Q.; Loor, J.J.; Deng, Z.; Xu, C. Bacillus subtilis Produces Amino Acids to Stimulate Protein Synthesis in Ruminal Tissue Explants via the Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Beta–Serine/Threonine Kinase–Mammalian Target of Rapamycin Complex 1 Pathway. Frontiers in Veterinary Science 2022, 9. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.Q.; Deng, L.F. Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal 2013, 7, 216–222. [Google Scholar] [CrossRef]
- Srivatsan, A.; Han, Y.; Peng, J.; Tehranchi, A.K.; Gibbs, R.; Wang, J.D.; Chen, R. High-Precision, Whole-Genome Sequencing of Laboratory Strains Facilitates Genetic Studies. PLOS Genetics 2008, 4, e1000139. [Google Scholar] [CrossRef]
- Doria, E.; Buonocore, D.; Marra, A.; Bontà, V.; Gazzola, A.; Dossena, M.; Verri, M.; Calvio, C. Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower. Plants 2022, 11. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Chen, Y.; Solomon, R. The quality of commercial wheat silages in Israel1. Journal of Dairy Science 2009, 92, 638–644. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Das, P.; Ragauskas, A.J. Rice straw as a feedstock for biofuels: Availability, recalcitrance, and chemical properties. Biofuels, Bioproducts and Biorefining 2018, 12, 83–107. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass and Bioenergy 2011, 35, 3094–3103. [Google Scholar] [CrossRef]
- Park, J.-y.; Seyama, T.; Shiroma, R.; Ike, M.; Srichuwong, S.; Nagata, K.; Arai-Sanoh, Y.; Kondo, M.; Tokuyasu, K. Efficient Recovery of Glucose and Fructose via Enzymatic Saccharification of Rice Straw with Soft Carbohydrates. Bioscience, Biotechnology, and Biochemistry 2009, 73, 1072–1077. [Google Scholar] [CrossRef]
- Nave, R.L.G.; Sulc, R.M.; Barker, D.J. Relationships of Forage Nutritive Value to Cool-Season Grass Canopy Characteristics. Crop Science 2013, 53, 341–348. [Google Scholar] [CrossRef]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: a crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef]
- Romero, J.J.; Zarate, M.A.; Arriola, K.G.; Gonzalez, C.F.; Silva-Sanchez, C.; Staples, C.R.; Adesogan, A.T. Screening exogenous fibrolytic enzyme preparations for improved in vitro digestibility of bermudagrass haylage. Journal of Dairy Science 2015, 98, 2555–2567. [Google Scholar] [CrossRef]
- Smith Janet, L.; Goldberg Jonathan, M.; Grossman Alan, D. Complete Genome Sequences of Bacillus subtilis subsp. subtilis Laboratory Strains JH642 (AG174) and AG1839. Genome Announcements 2014, 2, e00663–00614. [Google Scholar]
- Belal, E.B. Bioethanol production from rice straw residue. Brazilian Journal of Microbiology 2013, 44, 225–234. [Google Scholar] [CrossRef]
- Krishania, M.; Kumar, V.; Sangwan, R.S. Integrated approach for extraction of xylose, cellulose, lignin and silica from rice straw. Bioresource Technology Reports 2018, 1, 89–93. [Google Scholar] [CrossRef]
- Bergstrand, M.; Karlsson, M.O. Handling data below the limit of quantification in mixed effect models. AAPS J 2009, 11, 371–380. [Google Scholar] [CrossRef]
- Ermoli, F.; Bontà, V.; Vitali, G.; Calvio, C. SwrA as global modulator of the two-component system DegSU in Bacillus subtilis. Research in Microbiology 2021, 172, 103877. [Google Scholar] [CrossRef]
- Spanghero, M.; Chiaravalli, M.; Colombini, S.; Fabro, C.; Froldi, F.; Mason, F.; Moschini, M.; Sarnataro, C.; Schiavon, S.; Tagliapietra, F. Rumen Inoculum Collected from Cows at Slaughter or from a Continuous Fermenter and Preserved in Warm, Refrigerated, Chilled or Freeze-Dried Environments for In Vitro Tests. Animals 2019, 9. [Google Scholar] [CrossRef]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: collaborative study. J AOAC Int 2002, 85, 1217–1240. [Google Scholar] [CrossRef]
- Pirondini, M.; Colombini, S.; Malagutti, L.; Rapetti, L.; Galassi, G.; Zanchi, R.; Crovetto, G.M. Effects of a selection of additives on in vitro ruminal methanogenesis and in situ and in vivo NDF digestibility. Animal Science Journal 2015, 86, 59–68. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Animal Feed Science and Technology 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Colombini, S.; Rota Graziosi, A.; Parma, P.; Iriti, M.; Vitalini, S.; Sarnataro, C.; Spanghero, M. Evaluation of dietary addition of 2 essential oils from Achillea moschata, or their components (bornyl acetate, camphor, and eucalyptol) on in vitro ruminal fermentation and microbial community composition. Animal Nutrition 2021, 7, 224–231. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).