1. Introduction
The humoral response is pivotal in adaptive immunity against numerous viral infections [
1]. In COVID-19, alpha and gamma immunoglobulins (Ig) derived from infected individuals or those who have received vaccinations contribute to viral neutralization. However, their roles in immunity differ across various infection stages and specific anatomical sites [
2,
3,
4]. Among these, IgA, predominant in mucous membranes, is the most abundantly produced Ig in humans (66 mg/kg/day), while IgG is the primary isotype in blood and reaches most tissues by diffusion [
2,
5]. The distribution of IgA on mucosal surfaces that encounter infectious agents positions it uniquely for intervening in transmission.
The principal target of human IgG and IgA against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the integral spike (S) protein, common to all currently employed vaccines [
6,
7]. The timeline of IgA and IgG responses induced by mRNA vaccines during trials was recently published [
8,
9]. Notably, IgA against the SARS-CoV-2 spike protein emerges earlier in both infected [
7,
8,
10,
11] and vaccinated patients [
12,
13], demonstrating superior antiviral potency to IgG, not only against SARS-CoV-2 but also influenza [
4,
11,
14].
The serum IgA isotype proves to be seven times more effective in viral neutralization than IgG [
12], and the IgA dimers, the primary form in the nasopharynx, are approximately 15 times more potent than IgA monomers [
15]. Thus, secretory IgA (sIgA) responses may be particularly valuable for protection against SARS-CoV-2 and for vaccine efficacy. This antiviral protective immunity is also evident in the temporal dynamics of circulating IgA+ plasmablasts equipped with mucosal homing receptors and in the presence of neutralizing IgA in airway fluid and saliva [
10,
11]. Conversely, the overall levels of immunoglobulins (IgA, IgG, and IgM) and complement proteins (C3 and C4) in COVID-19 patients have been found within the normal range [
11]. However, significant differences in their persistence in the serum after infection [
12,
16,
17] and COVID-19 vaccination [
12,
18] have been demonstrated. Elevated IgG or IgM antibody levels targeting SARS-CoV-2 spike protein or receptor-binding domain (RBD) appear ten days after symptom onset. The average antibody response pattern is an early IgM increase followed by IgG development. Although different seroconversion types exist, such as synchronous seroconversion of IgG-IgM, earlier IgM seroconversion, and delayed IgM seroconversion [
19,
20,
21,
22], the clinical value of antibody testing has yet to be fully demonstrated.
The role played by serical and mucosal IgA responses, the total IgA generation rate from this response, and the involvement of its epitopes in COVID-19 severity and/or vaccination are largely poor explored areas [
3,
4,
23], aside from notable instances such as frequent thromboembolisms in severe COVID-19 cases [
24,
25,
26,
27].
Davis et al. [
28] characterized the IgA immune response regarding neutralization and Fc-effector function. They found that the plasma IgA response contributed to the neutralization antibody response of wild-type SARS-CoV-2 RBA and various RBD mutations despite displaying greater heterogeneity and was less potent than IgG.
Several other investigators have also examined various aspects of the IgA immune response to the S protein in the context of COVID-19. They have utilized various techniques, such as studying fragments of peptides with different sizes [
29], exploring antibody affinity maturation in relation to clinical outcomes in hospitalized COVID-19 patients [
30], employing microarray analysis of peptides to investigate the disease severity over time in a small cohort of patients [
23], and using microarray of peptides technology to analyze the humoral response profile to COVID-19 [
31]. In this context, some lateral flow rapid tests, enzyme-linked immunosorbent assay (ELISA) kits, and IgA-based assays have been regarded as more sensitive yet less specific than IgG-based counterparts [
32,
33]. However, cross-reactivity has been detected with both tests [
34,
35,
36,
37]. However, the continuous emergence of new kits in the market and the potential to develop in-house kits based on the quality of SARS-CoV-2 antigen plates presents various options.
It can be challenging to differentiate viral infections caused by COVID from arbovirus infections, like DENV and CHYV [
34,
39,
40,
41], due to their similar clinical and laboratory characteristics. This similarity can lead to the consideration of COVID-19 when, in fact, a false positive dengue serology result may result in a misdiagnosis with potentially serious concerns.
Parte superior do formulário
Consequently, this study aimed to map the IgA linear B cell epitopes of the COVID-19 spike protein, encompassing both native and mutated variants. Furthermore, identify the cross-reactive, pinpoint specific epitopes, and assess these peptides using ELISA, employing sera from COVID-19 and dengue virus-infected patients before the pandemic.
2. Materials and Methods
2.1. Patient Samples and Ethical Approval
A panel comprising 33 serum samples (
Table S1) was obtained from symptomatic patients with confirmed positive PCR diagnostic tests at the pandemic’s beginning and 25 serum from vaccinated patients. Serum samples from individuals with confirmed cases of dengue (n=24) were collected before the onset of the COVID-19 pandemic and generously provided by the Laboratory of Flavivirus of the Oswaldo Cruz Institute of FIOCRUZ in Rio de Janeiro and the Laboratory Central of Public Health of Ceará state, Brazil. Serum from healthy donors, also collected before the pandemic, was provided by HEMORIO, a centralized network of blood donor facilities in Rio de Janeiro, Brazil. To ensure patient privacy, all samples were provided without identifying information.
2.2. Synthesis of the cellulose-membrane-bound peptide array
The entire sequence (1273 aa) of the S protein from SARS-CoV (P0DTC2) deposited in the UniProt database (
http://www.uniprot.org/: Accessed 27 January 2020) was covered with the synthesis of 14-residues-long peptides with overlapping of 9 residues, semiautomatically prepared on cellulose membranes (Amino-PEG 500-UC540) according to the standard SPOT synthesis protocol using an Auto-Spot Robot ASP-222 (Intavis Bioanalytical Instruments AG, Köln, Germany). Peptide library contained 258 sequences for P0DTC2. Coupling, blocking, and deprotection until the expected desired peptide was generated and carried out as described previously [
38]. Membranes containing the synthetic peptides were probed immediately. Peptides as negative controls were included in each assay.
2.3. Screening of SPOT membranes and measurement of spot signal intensities
SPOT membranes were washed with TTBS (50 mM Tris, 136 Mm NaCl, two mM KCl, 0.05% Tween, pH 7.4) and then blocked with TTBS containing 1.5% casein (TTBS-C) under agitation overnight at 8°C. After extensive washing with TTBS, membranes were incubated for 90 min with a pool (n=10) of patients sera (symptomatic collected during the pandemic and with the PCR+;
Table S1) diluted 1:100 in TBS-CT and then washed again with TTBS-T. After that, membranes were incubated with goat anti-human IgA alkaline phosphatase labeled (diluted 1:5000; Kirkegaard & Perry Lab (KPL)., Inc, Maryland, U.S.A) prepared in TTBS-C for 1h, and then washed with TTBS containing 0.5 M NaCl followed by CBS (50 mM citrate-buffer saline). Finally, Chemiluminescent CDP-Star
®Substrate (0.25 mM) with Nitro-Block-II™ Enhance (Applied Biosystems, Waltham, MA, USA) was added to complete the reaction. Chemiluminescent signals were detected on an Odyssey FC (LI-COR Bioscience, Lincoln, NE, USA) using previously described conditions [
43]. Briefly, a digital image file was generated at a resolution of 5 MP, and the signal intensities were quantified for 2 min using the TotalLab TL100 (v2009, Nonlinear Dynamics, USA) software. An epitope was considered the minor resultant sequence of two or more positive contiguous spots with a signal intensity (SI) greater than or equal to 30% of the highest value obtained from the set of spots on the respective membrane. The signal intensity (SI) used as a background was a set of negative controls spotted in each membrane.
A comparative analysis of the reactivity index of the Spots normalized on a dimensional hierarchical level was realized according to the approach described previously [
44].
2.4. Structural localization of the epitopes
Spike protein 3D structures were retrieved from I-Tasser (
https://zhanggroup.org/I-TASSER/, accessed on 22 July 2022) and visualized and analyzed using the PyMOL Molecular Graphics System, Version 2.0, Schrödinger, LLC. The models were chosen according to the best C-score and TM-score (topological evaluation value) [
45]. The prediction of the secondary structure of the proteins by the PSIPRED server [
http://bioinf. cs.ucl.ac.uk/ inspired /] and CDM [
http://gor.bb. iastate.edu/cdm/].
For comparative analysis, the receptor-binding domain motif (RBD) sequences from all reported SARS-CoV-2 variants of concern were retrieved from the viralzone database (
https://viralzone.expasy. org/; Accessed on 22 July 2022). The sequences of identified IgA epitopes were aligned using CustalW on MEGA11 software. IgA epitope sequences from all concern variants were further analyzed by sequence logo using Weblogo (
https://weblogo.threeplusone.com/; Accessed on 22 July 2022) to evaluate physicoche mical and biochemical changes in mutated sequences.
2.5. Preparation of the MAP peptides
The dendrimer multi-antigen peptides (MAP4) were used for preparation using the tetrameric Fmoc2-Lys-B-Ala Wang resin (CEM, Corp, Charlotte, NC, USA). The peptides possessed the sequence GSYADSFVIRDGSGS (pep-248; epitope #SC/14; aa395-404), GSNNSNN DSKVGSGS (pep-249; epitope #SC/16; aa 436-449), and GSLKPFERDISTGSG (pep-250; epitope #SC/17; aa459-468). In each sequence of the peptides, the dipeptide GS in the N-terminus, the tetrapeptide GSGS (#SC/14 and SC/#15), and the tripeptide GSG (#SC/17) in the C-terminus to complete fifteen amino acids. The synthesis was conducted on an automated peptide synthesizer (MultiPep-1 CEM Corp, Charlotte, NC, USA), with F-moc-amino acids and protecting groups as required. The peptides were then cleaved, deprotected, and precipitated, and each MAP4 was analyzed as described previously [
42]. In most cases, the peptides were used as prepared.
2.6. In house ELISA
The peptide ELISA (pELISA) was conducted as described previously [
46]. Immunolon 2HB plates (Immunochemistry Technologies, Bloomington, MN, USA) were coated overnight at 4
oC with 500 ng of peptides per well in coating buffer (50 mM Na2CO3–NaHCO3, pH 9.6). After each incubation step, the plates were washed three times using PBS-T washing buffer (PBS with 0.1% Tween 20 adjusted to pH 7.2), blocked (200 µl) with 1% BSA, and incubated for 1 h at 37
oC. Next, the patient’s sera were diluted (1:25) in coating buffer, and 100 µl were applied onto immunosorbent plates and incubated for 1 h at 37
oC. Following several washes with PBS-T, the plates were incubated with 100 µl goat anti-human IgA-HRP (1:5000 dilutions at blocking buffer; Sigma-Aldrich, St Louis, MO, USA) for 1 h at 37
oC. Finally, 3,3′,5,5′-tetramethylbenzidine (1-Step™ Ultra TMB-ELISA, Scienco Biotech Ltd.a, Lages, SC, Brazil) was added for 15 min, and the reaction was stopped by adding 0.5M sulfuric acid. The absorbance values at 405 nm were read using an ELX800 Microplate Reader (Bio-Tek Instruments Inc, Winooski, USA). The plate was read within 2 h of adding the Stop Solution. Values of blank wells, which contained only peptides, were subtracted from the sample’s optic density.
2.7. Statistical analysis
Data were analyzed using GraphPad Prism software (GraphPad version 6, San Diego, CA, USA). The reactivity index (RI) reflected the absorbance divided by the cutoff (mean of negative samples + 2 times the standard deviation) determined by each peptide. All results >1 was considered positive and <0.99 negative. Kruskal–Wallis’s test was applied to identify statistical differences, followed by Dunn’s multiple comparisons tests. Significant differences were considered with p < 0.05.
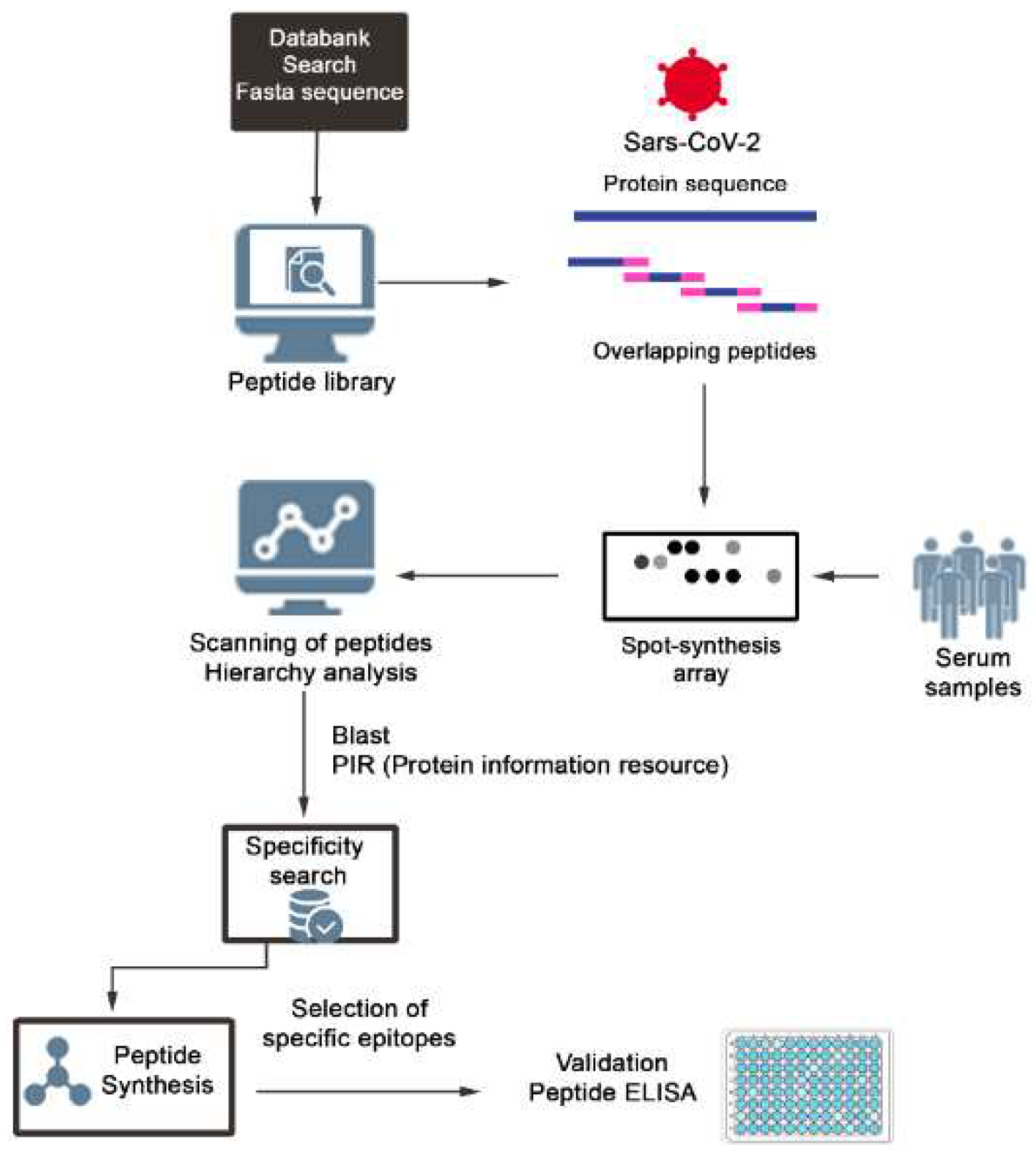
Figure 1.
Schematic representation of the strategy used to identify and select SARS-CoV-2 IgA epitopes. Peptide libraries of 15 mer with overlapping of 10 residues were constituted using a software and the positive peptides revealed with patients sera and secondary labelled antibodies. The chemiluminescent posive spots were quantified using a scanner and those with the best performance listed accordinng with the hierarquic position using a software. The possible cross reactivity of the epitopes was cheked in databanks and those considered specifics were synthesized as single or multi epitopes peptides and validated by ELISA.
Figure 1.
Schematic representation of the strategy used to identify and select SARS-CoV-2 IgA epitopes. Peptide libraries of 15 mer with overlapping of 10 residues were constituted using a software and the positive peptides revealed with patients sera and secondary labelled antibodies. The chemiluminescent posive spots were quantified using a scanner and those with the best performance listed accordinng with the hierarquic position using a software. The possible cross reactivity of the epitopes was cheked in databanks and those considered specifics were synthesized as single or multi epitopes peptides and validated by ELISA.
3. Results
3.1. Identification of IgA Epitopes in the COVID-19 Spike protein
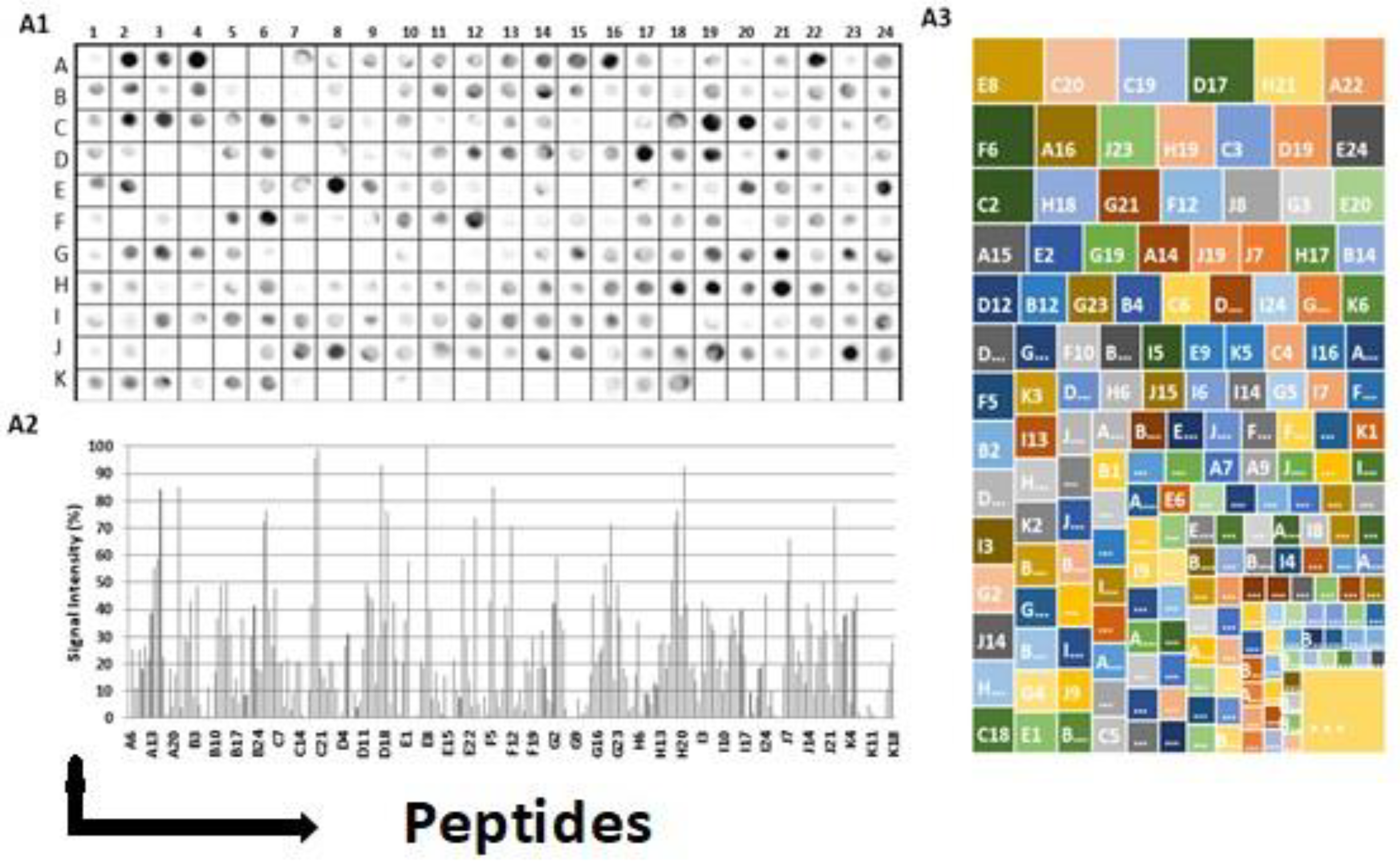
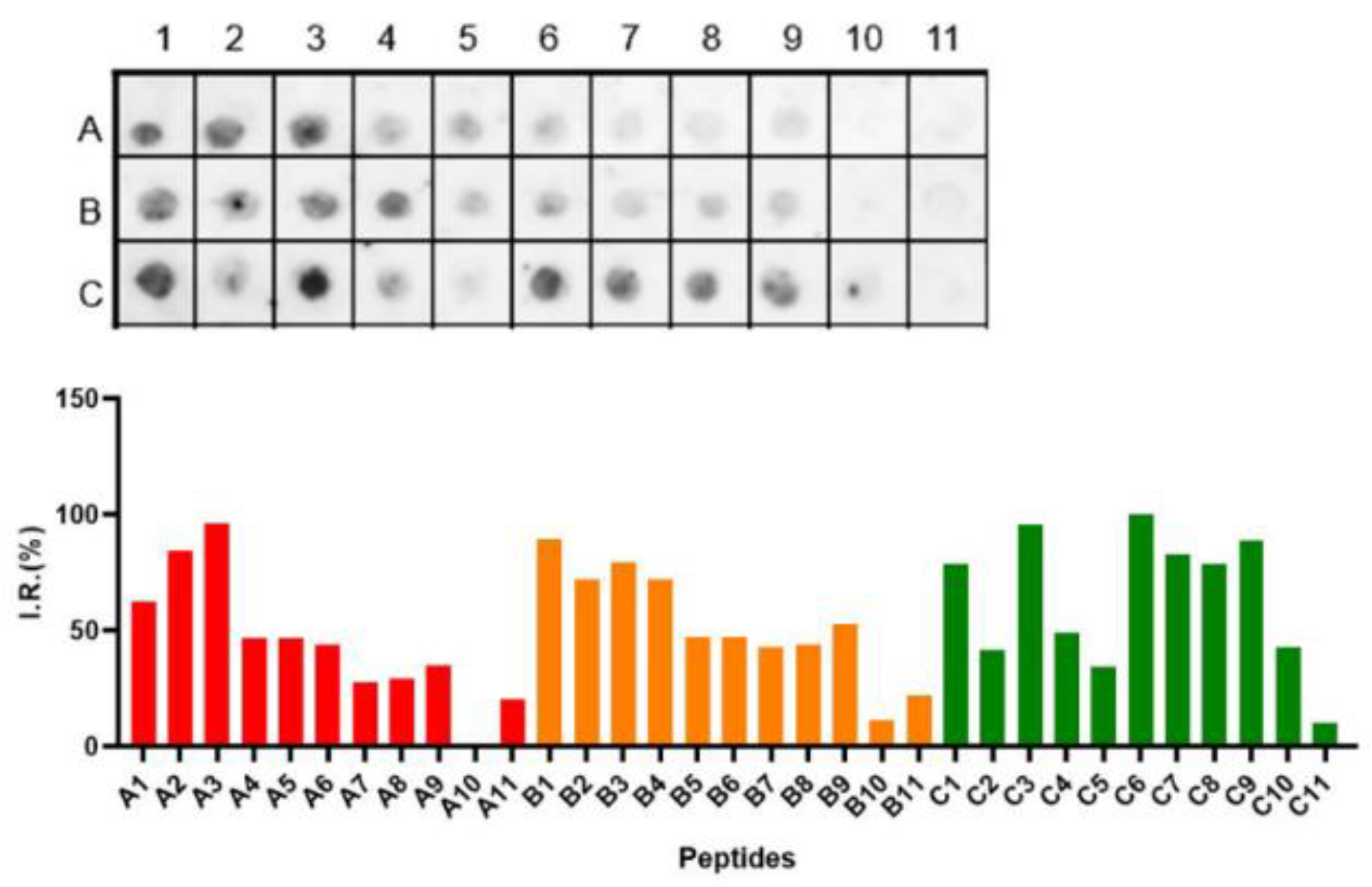
IgA epitopes within the COVID-19 Spike protein (1273 aa) were identified through the recognition of peptides in a synthesized library (144 peptides) by sera from COVID-19 patients (Material and Methods).
Figure 2A1 depicts the chemiluminescent signal image obtained from each library peptide, showing their reactivity with human IgA antibodies in pooled sera from infected patients.
Figure 2A2 displays the measured intensity and the position of each peptide. Intensities were normalized to 100%, as established by the positive control. A list of the synthesized peptides and their corresponding positions on the membranes is provided in
Table S2. The antibody reactivity pattern generated in infected patients demonstrated the recognition of many peptides (
Figure 2A). An analysis of the sequences comprising the synthesized peptides in reactive regions defined forty major epitopes recognized by patient sera (
Table 1).
Figure 2A3 shows the results of the hierarchic comparison of the reactivity index of the Spots-peptides normalized on a dimensional hierarchical level. The hierarchical levels sort the vertices based on their distance from the initial subgraphs and sort the vertices based on their distance. The graph’s layout shows its overall hierarchical structure, with the epitope reactivity index being the epitope with the highest values highlighted in the upper left portion of the image [
45]. Thus, the peptide E8, followed by C20 and F6, was the most reactive.
3.2. Structural localization of the IgA epitopes in the subunits and RBD of the S protein
The S protein of SARS-CoV-2 has 1273 aa and plays a key role in the receptor recognition and cell membrane fusion process. It is composed of a signal peptide (aa 1-13) located at the N-terminus, and two subunits, S1 (aa 14-685) and S2 (aa 686-1273). The S1 subunit contains the N-terminal domain (aa14-305) and the receptor binding domain (RBD, aa319-541) that recognizes and binds to the host receptor ACE-2. In the S2 subunit, there is the fusion peptide (FP, aa788-806), heptapeptide repeat sequence (HR1, aa912-984), HR2 (aa1163-1213), TM domain (aa1213-1237), and cytoplasm domain (aa1237-1273) [
47,
48].
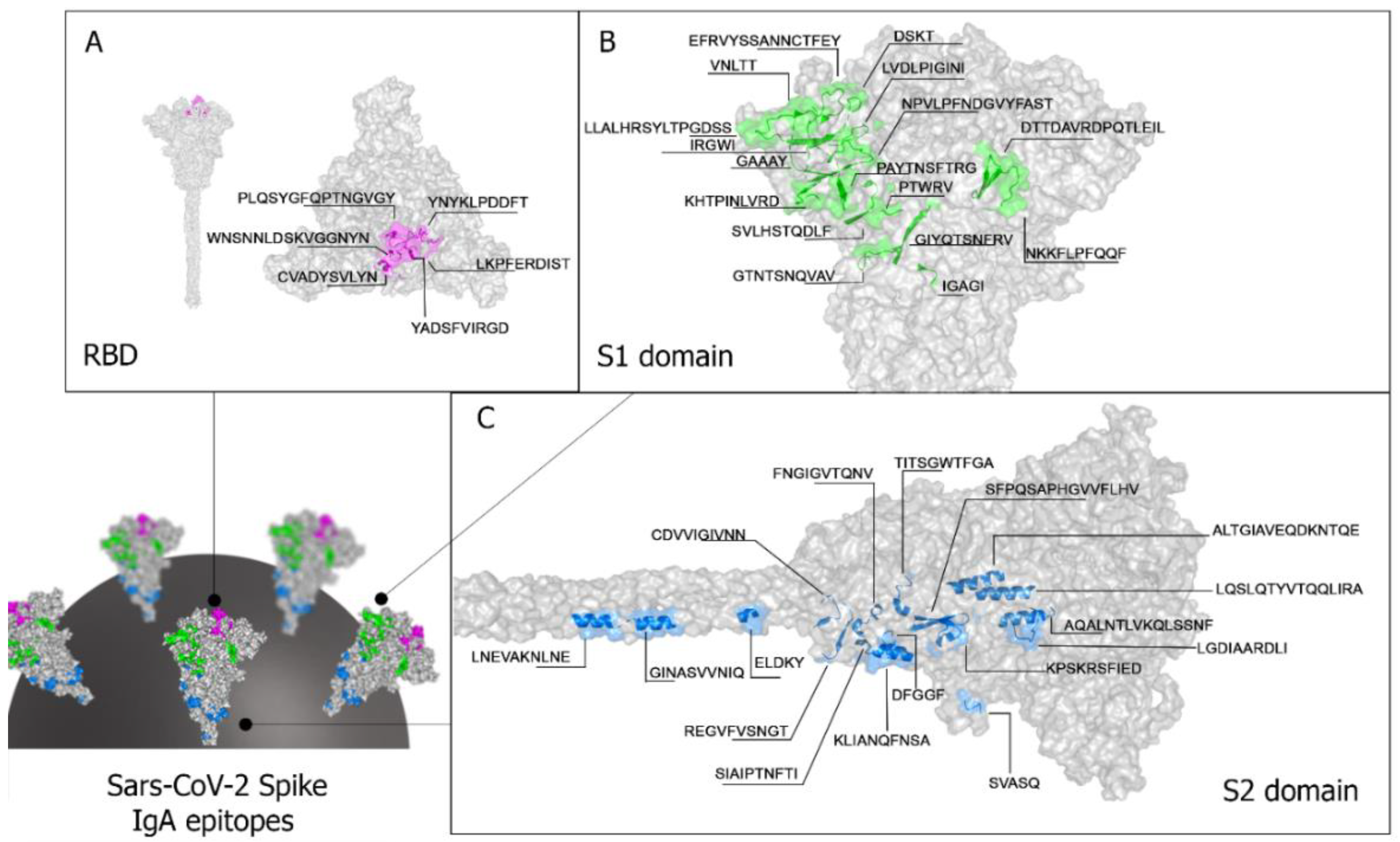
The crystallographic structure available in PDB (PDB: 1xdt) was used to analyze the location of the epitopes in S protein. It displays the spatial location of the forty IgA reactive epitopes identified by the Spot-synthesis array experiments (
Table 1). Most of the identified epitopes were in loop/coil structures, present on the protein surface, and accessible to the solvent. Twenty-three epitopes were placed in the S1 subunit (
Table 1,
Figure 3), six (SC/13huA to SC/18huA) situated in the RBD of the S protein (
Table 1,
Figure 3A). Seventeen epitopes (SC/24huA to SC/40huA) were positioned in the S2 subunit (
Figure 3D), one in the fusion peptide (SC/27huA), three in the HR1 (SC/31huA, SC/32hua, and SC/33huA) and two in HR2 (SC/39huA and SC/40huA) (
Table 1). No epitope was detected in the transmembrane region (aa1213-1237) and the CyD (aa 1237-1273).
Blastp analysis against non-redundant sequences deposited in diverse databases and containing amino acids aligning without gaps showed twenty-four DENV cross-reactive epitopes (
Table 1). These cross-reactive epitopes were distributed in the S1 (17) and S2 (15). The S1/RBD contained three cross-reactive epitopes (SC/13, SC/15, and SC/18), the S2/HR1 all three (SC/31, SC/32, and SC/33), and the S2/HR2 one (SC/40).
Beta-coronaviruses (beta-CoVs) share striking similarities in genome structure and exhibit immunological relatedness. Consequently, we delved into investigating IgA epitope correlations among different beta-CoVs. Our study unveiled that twelve IgA epitopes within the S1 domain demonstrated cross-reactivity with SARS-CoV. Notably, these epitopes encompassed SC/7, SC/9, SC/11, SC/12, SC/19, SC/20, and SC/23. Furthermore, the cross-reactivity extended to encompass all six epitopes located in the S1/RBD region (SC/13 to SC/18) and the twelve within the S2 domain (SC/25, SC/27, SC/30 to SC/38, and SC/40). Remarkably, this cross-reactivity was observed not only with SARS-CoV but also with MERS-CoV and MERS-OC43. Additionally, epitope SC/32 displayed cross-reactivity with HKU1 (
Figure S2).
3.3. Evolutionary analysis of the RBD IgA Epitopes in the spike protein of SARS-CoV-2 variants (from Alpha to Omicron)
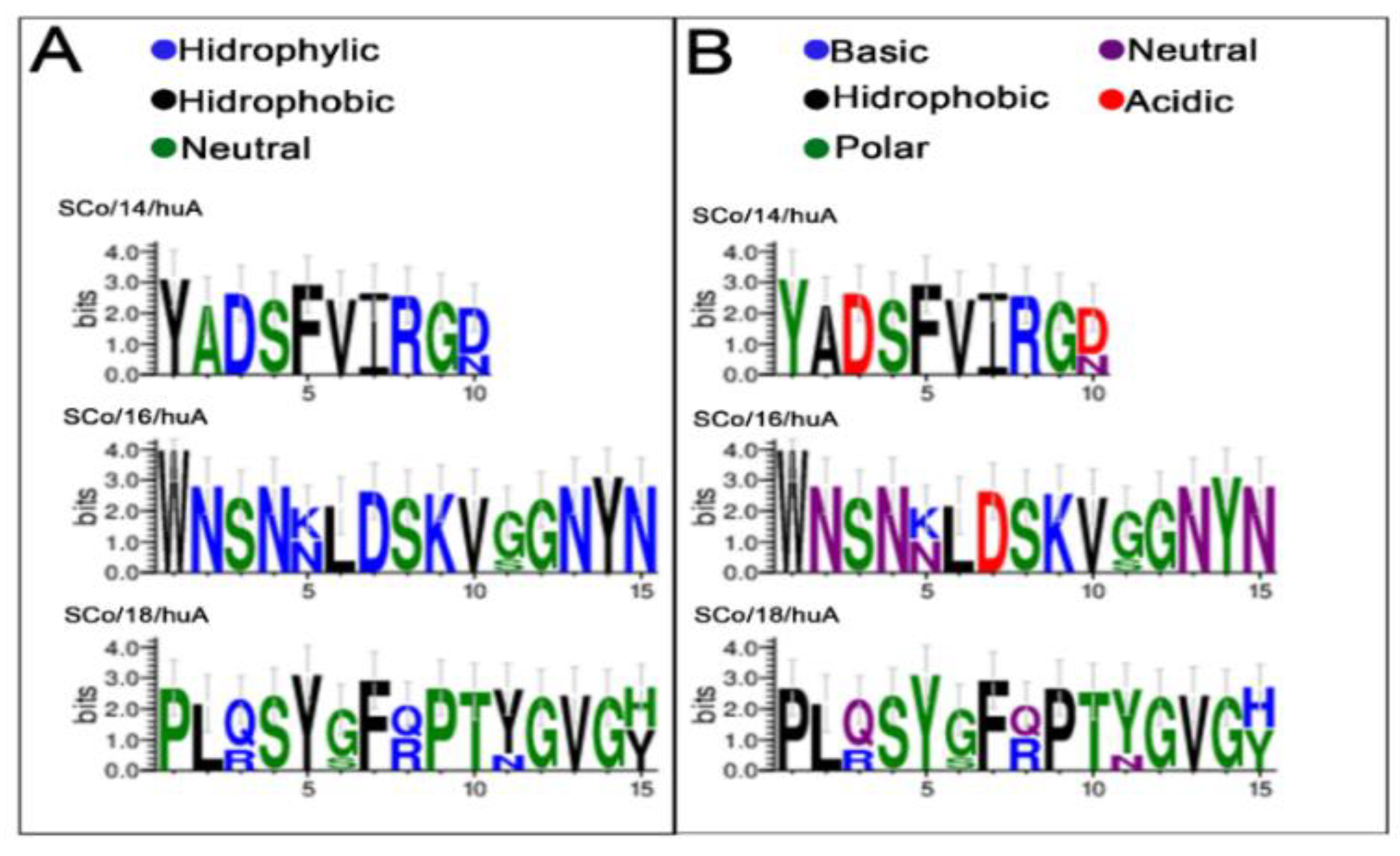
The protection of the vaccines against variants of the SARS-CoV-2 is dependent on the common sequences of the epitopes. However, the phylogenetic analysis identified that at least one epitope presented significant structural divergence (
Table 2). Therefore, these experiments had two objectives: (1) to validate the six identified IgA epitopes of the RBD and (2) to demonstrate the absence of cross-reactivity of the mutated SC/18huA epitope (Omicron Ba.4 and Omicron Ba.5).
As seen in
Table 2, the SC/18huA epitope was the one that showed the greatest divergence, especially in the Omicron variants, followed by the SC/16 and SC/14 epitopes. The SC/13 and SC/17 epitopes remained constant in all variants and the wild-type virus (Wuhan). Multiple alignments also showed a significant charge and amino acid composition change in these epitopes (
Figure 4).
Thus, to validate and confirm the immunological importance of the mutated epitopes of the new variants, six IgA-epitope peptides (#SC/13huA to #SC/18huA) were synthesized covalently attached on cellulose support (Spot-synthesis) as described before and evaluated using a pool (n=10;
Table S1) of sera from the hospitalized patient who had been independently confirmed with COVID-19 by PCR. Only the shortest epitope sequence was used in the synthesis. However, to maintain a reactive sequence in the assays, all peptides were synthesized with 15 amino acids in length using glycines at the N and C terminals as spacing. Negative controls included collections both before and after the COVID-19 pandemic were included. The list of synthesized peptides is shown in the
Table S3.
The immunoreactivity of the patients’ sera collected during the different steps of the pandemic (1st phase, vaccinated, and 2nd phase-mutated COVID) against the epitopes of the non-mutated and mutated epitopes of the RBD is shown in
Figure 5. This approach revealed that from the six IgA-RBD epitopes, only the SC/18 of the Omicron variants (Ba.1, BA.2, BA2.12.1, BA.4, and BA.5) presented a single IgA epitope reactive with sera from patients from the second wane (Omicron).
3.4. Validation by pELISA of the three RBD-specific epitopes
Before undertaking this analysis, we assessed the potential for cross-reactivity of a commercial IgA-ELISA assay (SARS-CoV-2 IgA; Serion Immunomat, Würzburg, Germany) with our collection of DENV patient sera gathered before the pandemic. The results indicated that 70% of the DENV patient sera (n=24) and 90% of the sera from hospitalized SARS-CoV-2 patients (n=33) tested positive for IgA antibodies (
Figure S3). It’s important to note that this assay is designed based on the entire S structural membrane protein.
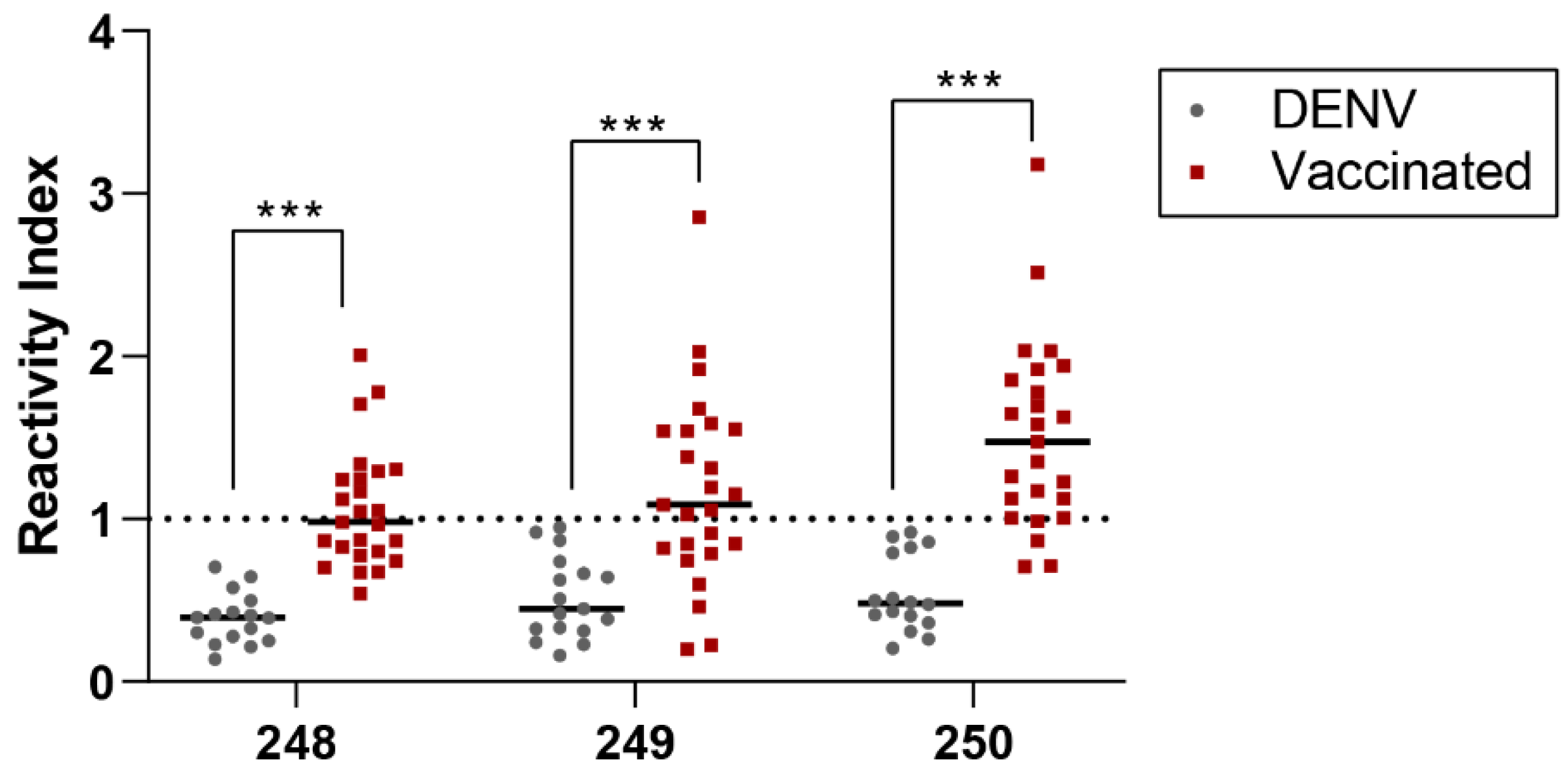
To validate our chosen RBD epitopes, we compared the same group of DENV patient sera (n=16) and sera from individuals who received COVID-19 vaccinations (n=25). The results of this comparison are displayed in
Figure 6.
Significant differences between pre-pandemic DENV-positive and vaccinated samples were evidenced for all peptides. Higher reactivity index and positive samples were evidenced for vaccinated individuals for all three tested peptides 248 (GSYADSF VIRDGGSG; RI mean = 1.0±0.3, positive samples=11), 249 (GSNNSNNDSKVGSGS; RI mean= 1.1±0.5; positive samples=15) and 250 (GSLKPFERDISTGSG; RI mean=1.5±0.5; positive samples= 21). Pre-pandemic DENV-positive sera did not significantly cross-react for all three tested peptides.
4. Discussion
The human immune system can remember various target antigens (epitopes). It can effectively respond to these antigens upon subsequent encounters, provided it has been previously primed. Coronaviruses share enough common features to elicit cross-reactive immune responses despite their genetic diversity. Additionally, the distribution of IgA and IgM on mucosal surfaces exposed to infectious agents uniquely positions the immune system to intervene in transmission. IgA1 is the dominant immunoglobulin in the respiratory tract, the primary entry point for many microorganisms. Consequently, IgA1, including secretory IgA, plays a pivotal role in defending against respiratory pathogens by neutralizing or preventing their attachment to the mucosal epithelium [
49].
In this study, we have identified forty IgA epitopes within the Spike protein of SARS-CoV-2, which were recognized by patients’ sera through a peptide microarray analysis. This discovery highlights the Spike protein’s potent mucosal immunostimulatory adjuvant properties, capable of eliciting robust mucosal and immune responses. Notably, all existing vaccines primarily target the Spike protein. However, recent research has shown that the SARS-CoV-2-neutralizing IgA response occurs earlier than the IgG response but is modest and diminishes more rapidly [
50].
Among these IgA epitopes, eight appeared specific to SARS-CoV-2, while thirty-two exhibited cross-reactivity with proteins from the dengue virus. All forty epitopes were exposed on the molecular surface and accessible to the immune system (
Figure 3).
HCoV generally infects target cells through the action of S proteins, which exhibit characteristics resembling class I fusion proteins [
51,
52]. These S proteins are trimeric integral membrane proteins, structured into subdomains crucial for engaging the receptor angiotensin-converting enzyme 2 (ACE2) (S1/RBD) and harbor an S2/HR1 and HR2 responsible for facilitating membrane fusion [
53]. Due to this, the S2 region has been considered an intriguing therapeutic target for addressing COVID-19. Although HR1 and HR2 have been observed to be transiently exposed during the fusion process, notable antibody responses against these S2 regions have yet to be previously reported. Our study successfully identified five IgA epitopes within this region: SC/31, SC/32, and SC/33 in the S2/HR1 segment and epitopes SC/39 and SC/40 in the S2/HR2 segment. All these epitopes exhibited reactivity with DENV, except for SC/39, which was specific to COVID-19 (
Table 1).
The variability of COVID-19 suggests that an individual’s immune response to SARS-CoV-2 plays a crucial role in determining the clinical course, ranging from asymptomatic to severe disease. In response to pathogens with no pre-existing immunity, our bodies rapidly engage the innate immune response to generate highly specific and effective tools: high-affinity antibodies, B cells, and T cells. Extensive analysis of the antibody response has shown that SARS-CoV-2 induces virus-specific antibodies across all immunoglobulin isotypes, including IgM, IgG, and IgA [
54,
55].
Our study demonstrates that the RBD of all SARS-CoV-2 variants contains six IgA epitopes [three SARS-CoV-2 specific (SC/14, SC/16, and SC/17) and three DENV cross-reactive], with only the Omicron BA.2 and BA.2.12.1 variants possessing a critical epitope capable of inducing a particular IgA response (
Figure 4). The other six variants (Alpha, Delta, Gamma, Omicron BA.1, BA.4, and BA.5) exhibit mutation points in the S1/RBD. Still, these alterations do not interfere with IgA binding induced by the ancestral variant (
Figure 5 and Table 3).
Phylogenetic analysis conducted on human beta-CoV reveals a notable observation: many IgA epitopes from SARS-CoV-2 display cross-reactivity with SARS-CoV. This includes all six epitopes within the S1/RBD (SC/13 to SC/18). Furthermore, cross-reactivity was also identified with MERS-CoV and MERS-OC43 (
Figure S2).
Regarding cross-reactivity with other organisms, we have identified thirty-two cross-reactive epitopes, with twenty-eight interacting with DENV proteins and four with proteins from various organisms (
Table 1). Previous studies using rapid IgM and IgG diagnostic assays and ELISAs have shown serological cross-reactivity between DENV, ZIKA, other flaviviruses, and SARS-CoV-2 [
56,
57,
58,
59]. Cross-reactivity has also been observed with diagnostic tests for IgA [
34,
35,
36,
37,
38]. Furthermore, the presence of cross-reactive IgA in the S1 subunit of the spike protein has been detected in the saliva of uninfected individuals living in areas of DENV [
60].
As demonstrated in our study, these findings underscore the importance of identifying and selecting specific epitopes to develop more accurate diagnostic tests for SARS-CoV-2. Depending on its purpose, specificity in serological testing is essential, whether for prevalence screening or diagnosing individual patients. High sensitivity (≥ 95%) is critical for diagnosing individual patients, while high specificity (≥98%) is preferred for seroprevalence studies to minimize false-positive results. The choice of test characteristics depends on the pretest probability of the disease.
In assumption, the results of our study highlight the potential of three selected epitopes (#SC/14, #SC/16, and #SC/17) to effectively distinguish between negative and positive samples in serological diagnosis. These epitopes are suitable for further evaluation in phase IIA studies and support their continued use in chimeric multiepitope protein constructs for more sensitive and rapid IgA diagnostic tests [
61].
5. Conclusions
While some prior studies have identified IgA epitopes of the spike protein from SARS-CoV-2 [
23,
29,
31], this is the first study to provide a comprehensive list of the IgA epitome. Out of the total epitopes identified, most (32) exhibited cross-reactivity with proteins from DENV and other organisms, including coronaviruses. Specifically, only eight epitopes proved to be exclusive to SARS-CoV-2. Among these, six were located within the RBB, and notably, the Omicron variants (BA.1, BA.2, and BA.2.12.1) presented a distinct IgA epitope (SCo/18/huA) compared to the ancestral strain. Furthermore, the study outlines the development of a chimeric polypeptide with multiple epitopes tailored to target SARS-CoV-2 IgA specifically. Leveraging the high precision of our RBD-based pELISA, it emerges as an invaluable tool for investigating the IgA response in SARS-CoV-2 infections, seroepidemiological research, and assessing vaccine coverage. Finally, the carefully selected epitopes identified in this study can serve as a foundation for designing an intranasal multiepitope vaccine [
62]. The peptides we describe are currently undergoing optimization and adaptation for high-throughput platforms [
63]. This development shows promise for establishing reliable antibody detection methods to support informed decision-making for clinicians, the public health community, policymakers, and industry stakeholders.
6. Patents
The antigenic peptides described in this study are protected under Brazilian and US provisional patent applications BR 10.2019.017792.6 and PCT/BR2020/ 050341, respectively, filed by FIOCRUZ. They may serve as a future source of funding.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Clinical characteristics of COVID-19 patients sera used in the study. 1Mild Illness: Individuals presenting with various signs and symptoms of COVID-19, such as cough, fever, sore throat, malaise, headache, myalgias, nausea, vomiting, gastrointestinal symptoms, and loss of taste and smell. However, they do not exhibit shortness of breath, dyspnea, or abnormal chest imaging. 2Moderate Illness: Individuals who display evidence of lower respiratory disease during clinical assessment or imaging and maintain an oxygen saturation measured by pulse oximetry (SpO2) of ≥94% while breathing room air at sea level. 3Severe Illness: Individuals with a SpO2 measurement of <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) below 300 mm Hg, a respiratory rate exceeding 30 breaths per minute, or lung infiltrates covering more than 50% of their lung field. Table S2: List of synthesized peptides covering the entire sequence of spike protein (P0DTC2, Uniprot database) from SARS-CoV-2. Positive controls (F3, F4, F5, F11, F12, F13) and negative controls (F9, F17). The overlapping of positive peptides defined by the epitopes is labeled in red. Table S3: List synthesized peptides covering the entire sequence of RBD mutated spike proteins from SARS-CoV-2 variants. Positive controls: YPGEFADYEELREQL (Influenza virus), spots 09 (A, B, C, D, E e F); negative controls: QEVRKY (Cowpox vírus); spot 11 (A, B, C, D, E and F); KEVPALTAVETGATN (Poliovirus) spot 10 (A, B, C, D, E and F). Figure S1: Multiple sequence alignment of the six-coronaviruses spike proteins deposited in the database with a higher degree of identity (≥97%) with SARS-CoV-2. BLAST was carried out in the UniProt (Universal Protein) database of the EBI (European Bioinformatics Institute), using the P0DTC2 protein from the SARS-Cov-2 as input. HCoV-NL63 (YP_003767.1), HCoV-229E l(NP_073551.1), HCoV-HKU1 (Q14EB0.1), HCoV-OC43 (P36334.1), MERS-CoV (YP_007188579.1), SARS-CoV-2 (P0DTC2), and SARS-CoV (P59594.1). Figure S2: Analysis of pre-pandemic sera from patients with DENV (n=24) and sera from hospitalized patients with SARS-CoV-2 (first wave) using a commercial enzyme-linked immunosorbent assay for IgA-SARS-CoV-2. The methodology described by the manufacturer (Serion Immunomat, Würzburg, Germany).
Author Contributions
Conceptualization, S.G.D-S.; C.M.M.; methodology, P.N-P.; G.C.L.; M.E.M.; J.P.R.S.; software, M.E.M.; G.C.L.; formal analysis and data curation, S.G.D.-S.; writing—original draft preparation, S.G.D-S.; writing—review and editing, D.W.P. and S.G.D.-S.; project administration, C.M.M.; S.G.D.-S.; funding acquisition, C.M.M. and S.G.D.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro/FAPERJ (#110.198-13) and the Brazilian Council for Scientific Research (CNPq, #467.488/2014-2 and 301744/2019-0). Funding was also provided by FAPERJ (#210.003/ 2018) through the National Institutes of Science and Technology Program (INCT) to Carlos M. Morel (INCT-IDPN).
Institutional Review Board Statement
The study was approved by the Human Research Ethical Committee of the Oswaldo Cruz Institute/FIOCRUZ (CAAE:49971421.8.0000.5248), the University of Estacio de Sá (CAAE: 3309 0820.8. 0000.5284) and UNIGRANRIO (CAAE: 21362220.1.0000.5283) study center ethics committee and conducted under good clinical practice and applicable regulatory requirements, including the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study except those sera donated by the Laboratory of Flavivirus from the Oswaldo Cruz Institute.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
FIOCRUZ/Biomanguinhos (Rio de Janeiro, Brazil) for the purification of the peptides. P.N-P. and G.C.L. are Post-doc fellows from the INOVA-FIOCRUZ Program. M.E.M. and JPRS are CAPES/DSc fellows from the Post-Graduation Program on Parasitic Biology from FIOCRUZ and Science and Biotechnology, Federal Fluminense University, respectively.
Conflicts of Interest
The authors declare no dispute involving the research reported. The funding agencies had no role in the study design, data collection, data analysis, publication decision, or manuscript preparation.
References
- Drosten, C.; Günther, S.; Preiser, W.; van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003, 348, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Baid, K.; Mossman, K. Molecular pathogenesis of Middle East Respiratory Syndrome (MERS) coronavirus. Curr Clin Microbiol Rep 2019, 6, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. . Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef] [PubMed]
- Olas, K.; Butterweck, H.; Teschner, W.; Schwarz, H.P.; Reipert, B. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol 2005, 140, 478–90. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Manfredi, M.; Grossi, V.; Lari, B.; Fabbri, S.; Benucci, M.; Fortini, A.; Damiani, A.; Mobilia, E.M.; Panciroli, M.; et al. Closing the serological gap in the diagnostic testing for COVID-19: The value of anti-SARS-CoV-2 IgA antibodies. J Med Virol 2021, 93, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Tré-Hardy, M.; Wilmet, A.; Beukinga, I.; Favresse, J.; Dogné, J.M.; Douxfils, J.; Blairon, L. Analytical and clinical validation of an ELISA for specific SARS-CoV-2 IgG, IgA, and IgM antibodies. J Med Virol 2021, 93, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Wisnewski, A.V.; Campillo Luna, J.; Redlich, C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS One 2021, 16, e0249499. [Google Scholar] [CrossRef]
- Yu, H.Q.; Sun, B.Q.; Fang, Z.F.; Zhao, J.C.; Liu, X.Y.; Li, Y.M.; Sun, X.Z.; Liang, H.F.; Zhong, B.; Huang, Z.F.; et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J 2020, 56, 2001526. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Li, D.; Calderone, R.; Nsouli, T.M.; Reznikov, E.; Bellanti, J.A. Salivary and serum IgA and IgG responses to SARS-CoV-2-spike protein following SARS-CoV-2 infection and after immunization with COVID-19 vaccines. Allergy Asthma Proc 2022, 43, 419–430. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Salivary, and serum IgA and IgG after COVID-19 and after immunization with COVID-19 vaccines. Allergy Asthma Proc 2023, 44, e1. [Google Scholar] [CrossRef]
- Guerra, E.N.S.; de Castro, V.T.; Amorim Dos Santos, J.; Acevedo, A.C.; Chardin, H. Saliva is suitable for SARS-CoV-2 antibodies detection after vaccination: A rapid systematic review. Front Immunol. 2022, 13, 1006040. [Google Scholar] [CrossRef]
- Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Viant, C.; Gaebler, C.; Cipolla, M.; Hoffmann, H.H.; Oliveira, T.Y.; Oren, D.A.; et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021, 13, eabf1555. [Google Scholar] [CrossRef]
- Padoan, A.; Sciacovelli, L.; Basso, D.; Negrini, D.; Zuin, S.; Cosma, C.; Faggian, D.; Matricardi, P.; Plebani, M. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin Chim Acta 2020, 507, 164–166. [Google Scholar] [CrossRef]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef]
- Carvalho, Á.; Henrique, A.R.; Queirós, P.; Rodrigues, J.; Mendonça, N.; Rodrigues, A.M.; Canhão, H.; de Sousa, G.; Antunes, F.; Guimarães, M. Persistence of IgG COVID-19 antibodies: A longitudinal analysis. Front Public Health 2023, 10, 1069898. [Google Scholar] [CrossRef]
- Zuiani, A.; Wesemann, D.R. Antibody dynamics and durability in Coronavirus disease-19. Clin Lab Med 2022, 42, 85–96. [Google Scholar] [CrossRef]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020, 15, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, X.; Yang, G.; Fan, J.; Tang, Y.; Zhao, J.; Long, X.; Guo, S.; Zhao, Z.; Liu, Y.; et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect 2020, 81, e28–e32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, R.H.; Li., B.; Zheng, X.S.; Yang, X.L.; Hu, B.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; Shi, Z.L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect 2020, 9, 386–389. [Google Scholar] [CrossRef]
- Fu, Y.; Pan, Y.; Li, Z.; Li., Y. The utility of specific antibodies against SARS-CoV-2 in laboratory diagnosis. Front Microbiol 2021, 11, 603058. [Google Scholar] [CrossRef]
- Heidepriem, J.; Dahlke, C.; Kobbe, R.; Santer, R.; Koch, T.; Fathi, A.; Seco, B.M.S.; Ly, M.L.; Schmiedel, S.; Schwinge, D.; et al. The Id-Uke COVID-study group. Longitudinal development of antibody responses in COVID-19 patients of different severity with ELISA, peptide, and glycan arrays: An immunological case series. Pathogens 2021, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka A, Borovac JA, Guerreiro RA, Giustozzi M, Parker W, Caldeira D, Chiva-Blanch G. Thrombotic complications in patients with COVID-19: pathophysiological mechanisms, diagnosis, and treatment. Cardiovasc Drugs Ther. 2021, 35, 215-229. [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann Intern Med 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Loo, J.; Spittle, D.A.; Newnham, M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021, 76, 412–420. [Google Scholar] [CrossRef]
- Marvi, T.K.; Stubblefield, W.B.; Tillman, B.F.; Tenforde, M.W.; Patel, M.M.; Lindsell, CJ; Self, WH; Grijalva, C.G.; Rice, TW Serial thromboelastography and the development of venous thromboembolism in critically Ill patients with COVID-19. Crit Care Explor 2022, 4, e0618. [CrossRef]
- Davis, S.K.; Selva, J.; Lopez, E.; Haycroft, E.R.; Lee, W.S.; Wheatley, A.K.; Juno, J.A.; Adair, A.; Pymm, P.; Redmond, S.J.; et al. Heterologous SARS-CoV-2 IgA neutralizing antibody responses in convalescent plasma. Clin Transl Immunology. 2022, 11, e1424. [Google Scholar] [CrossRef]
- Camerini, D.; Randall, A.Z.; Trappl Kimmons, K.; Oberai, A.; Hung, C.; Edgar, J.; Shandling, A.; Huynh, V.; Teng, A.A.; Hermanson, G.; Pablo, J.V.; et al. Mapping SARSCoV-2 antibody epitopes in COVID-19 patients with a multi-coronavirus protein microarray. Microbiol Spectr 2021, 9, e0141621. [Google Scholar] [CrossRef]
- Tang J, Ravichandran S, Lee Y, Grubbs G, Coyle EM, Klenow L, Genser H, Golding H, Khurana S. Antibody affinity maturation and plasma IgA associate with clinical outcome in hospitalized COVID-19 patients. Nat Commun 2021,12,1221. [CrossRef]
- Acharjee, A.; Ray, A.; Salkar, A.; Bihani, S.; Tuckley, C.; Shastri, J.; Agrawal, S.; Duttagupta, S.; Srivastava, S. Humoral immune response profile of COVID-19 reveals severity and variant-specific epitopes: lessons from SARS-CoV-2 peptide microarray. Viruses 2023, 15, 248. [Google Scholar] [CrossRef]
- Amellal, H.; Assaid, N.; Charoute, H.; Akarid, K.; Maaroufi, A.; Ezzikouri, S.; Sarih, M. Kinetics of specific anti-SARS-CoV-2 IgM, IgA, and IgG responses during the first 12 months after SARS-CoV-2 infection: A prospective longitudinal study. PLoS One 2023, 18, e0288557. [Google Scholar] [CrossRef]
- Needle, R.; Gilbert, L.; Zahariadis, G.; Yu, Y.; Dalton-Kenny, H.; Russell, R.S.; Wang, P.; Donovan, C.; Hookey, S.; Jiao, L. Serological evaluation of human antibodies of the immunoglobulin class A and G against SARS-CoV-2 in serum collected in Newfoundland and Labrador. Viral Immunol. 2021, 34, 182–189. [Google Scholar] [CrossRef]
- Shrock E, Fujimura E, Kula T, Timms RT, Lee IH, Leng Y, Robinson ML, Sie BM, Li MZ, Chen Y, et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science. 2020,370,:eabd4250. [CrossRef]
- Santoso MS, Masyeni S, Haryanto S, Yohan B, Hibberd ML, Sasmono RT. Assessment of dengue and COVID-19 antibody rapid diagnostic tests cross-reactivity in Indonesia. Virol J. 2021,18, 54. [CrossRef]
- Vanroye F, Bossche DVD, Brosius I, Tack B, Esbroeck MV, Jacobs J. COVID-19 Antibody detecting rapid diagnostic tests show high cross-reactivity when challenged with pre-pandemic malaria, schistosomiasis, and dengue samples. Diagnostics (Basel) 2021, 11, 1163. [CrossRef]
- Nutalai, R.; Zhou, D.; Tuekprakhon, A.; Ginn, H.M.; Supasa, P.; Liu, C.; Huo, J.; Mentzer, A.J.; Duyvesteyn, H.M.E.; Dijokaite-Guraliuc, A.; et al. Potent cross-reactive antibodies following Omicron breakthrough in vaccinees. Cell. 2022, 185, 2116–2131.e18. [Google Scholar] [CrossRef]
- Hunsawong, T.; Buddhari, D.; Rungrojcharoenkit, K.; Suthangkornkul, R.; Mongkolsirichaikul, D.; Lohachanakul, J.; Tayong, K.; Sirikajornpan, K.; Rodpradit, P.; Poolpanichupatam, Y.; et al. Anti-arbovirus antibodies cross-react with Severe Acute Respiratory Syndrome Coronavirus 2. Microbiol Spectr. 2022, 10, e0263922. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Keler, S.; Kolodny, R.; Ben-Tal, N.; Atias-Varon, D.; Shlush, E.; Gerlic, M.; Munitz, A.; Doolman, R.; Asraf, K.; et al. Cross-reactivity between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and dengue viruses. Clin Infect Dis. 2021, 73, e2444–e2449. [Google Scholar] [CrossRef]
- Chen, M.; Qin, R.; Jiang, M.; Yang, Z.; Wen, W.; Li, J. Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review. Int J Infect Dis 2021, 104, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Ghosh, A.; Dutta, C.; Sukla, S.; Biswas, S. Cross-reactivity of SARS-CoV-2 with other pathogens, especially dengue virus: A historical perspective. J Med Virol 2023, 95, e28557. [Google Scholar] [CrossRef] [PubMed]
- De-Simone, S.G.; Gomes, L.R.; Napoleão-Pêgo, P.; Lechuga, G.C.; de Pina, J.S.; da Silva, F.R. Epitope mapping of the diphtheria toxin and development of an ELISA-specific diagnostic assay. Vaccines (Basel) 2021, 9, 313. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Napoleão-Pêgo, P.; Gonçalves, P.S.; Provance, D.W., Jr.; Morel, C.M.; Silva, F.R.B. Cell epitope mapping of the Vibrio cholerae toxins A, B and P. Int. J. Mol. Sci. 2023, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Moutsinas, G.; Shuaib, C.; Guo, W.; Jarvis, S. Graph hierarchy: a novel framework to analyze hierarchical structures in complex networks. Sci Rep. 2021, 11, 13943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 2008, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhu, Y.; Liu, M.; Lan, Q.; Xu, W.; Wu, Y.; Ying, T.; Liu, S.; Shi, Z.; Jiang, S.; et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol 2020, 17, 765–767. [Google Scholar] [CrossRef]
- Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin 2020, 41, 1141-1149. [CrossRef]
- Barrett CT, Neal HE, Edmonds K, Moncman CL, Thompson R, Branttie JM, Boggs KB, Wu CY, Leung DW, Dutch RE. Effect of clinical isolate or cleavage site mutations in the SARS-CoV-2 spike protein on protein stability, cleavage, and cell-cell fusion. J Biol Chem 2021, 297, 100902. [CrossRef]
- Dhama, K.; Dhawan, M.; Tiwari, R.; Emran, T.B.; Mitra, S.; Rabaan, A.A.; Alhumaid, S.; Alawi, Z.A.; Al Mutair, A. COVID-19 intranasal vaccines: current progress, advantages, prospects, and challenges. Hum Vaccin Immunother. 2022, 18, 2045853. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol 2021, 6, eabi6950. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Khatri, R.; Lohiya, B.; Kaur, G.; Maithil, V.; Goswami, A.; Sarmadhikari, D.; Asthana, S.; Samal, S. Understanding the role of conserved proline and serine residues in the SARS-CoV-2 spike cleavage sites in the virus entry, fusion, and infectivity. 3 Biotech. 2023, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Coco, A.; Patamia, V.; Zagni, C.; Floresta, G.; Rescifina, A. Targeting the SARS-CoV-2 HR1 with small molecules as inhibitors of the fusion process. Int J Mol Sci. 2022, 23, 10067. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Omata, K.; Shimizu, Y.; Kinoshita-Iwamoto, N.; Terada, M.; Suzuki, T.; Morioka, S.; Uemura, Y.; Ohmagari, N.; Maeda, K.; Mitsuya, H. SARS-CoV-2-neutralizing humoral IgA response occurs earlier but is modest and diminishes faster than IgG response. Microbiol Spectr 2022, 10, e0271622. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qin, R.; Jiang, M.; Yang, Z.; Wen, W.; Li, J. Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review. Int J Infect Dis 2021, 104, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Medina FA, Vila F, Premkumar L, Lorenzi O, Paz-Bailey G, Alvarado LI, Rivera-Amill V, de Silva A, Waterman S, Muñoz-Jordán J. Capacity of a multiplex IgM antibody capture ELISA to differentiate zika and dengue virus infections in areas of concurrent endemic transmission. Am J Trop Med Hyg. 2021,106, 585-592. [CrossRef]
- Munoz-Jordan J, Cardona J, Beltrán M, Colón C, Schiffer J, Stewart-Clark E, Zellner B, Semenova V, Li Y, Jia LT, et al. Evaluation of serologic cross-reactivity between dengue virus and SARS-CoV-2 in patients with acute febrile illness - United States and Puerto Rico, April 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2022, 71, 375-377. [CrossRef]
- Hunsawong, T.; Buddhari, D.; Rungrojcharoenkit, K.; Suthangkornkul, R.; Mongkolsirichaikul, D.; Lohachanakul, J.; Tayong, K.; Sirikajornpan, K.; Rodpradit, P.; Poolpanichupatam, Y. Anti-arbovirus antibodies cross-react with Severe Acute Respiratory Syndrome Coronavirus 2. Microbiol Spectr. 2022, 10, e0263922. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Castro, Y.; Troyes-Rivera, L.; Martins-Luna, J.; Cabellos-Altamirano, F.; Aguilar-Luis, M.A.; Carrillo-Ng, H.; Del Valle, L.J.; Kym, S.; Miranda-Maravi, S.; Silva-Caso, W.; et al. Detection of SARS-CoV-2 antibodies in febrile patients from an endemic region of dengue and chikungunya in Peru. PLoS One. 2022, 17, e0265820. [Google Scholar] [CrossRef]
- Tsukinoki, K.; Yamamoto, T.; Handa, K.; Iwamiya, M.; Saruta, J.; Ino, S.; Sakurai, T. Detection of cross-reactive immunoglobulin A against the severe acute respiratory syndrome-coronavirus-2 spike 1 subunit in saliva. PLoS One. 2021, 16, e0249979. [Google Scholar] [CrossRef]
- Russell MW, Mestecky J. Mucosal immunity: The missing link in comprehending SARS-CoV-2 infection and transmission. Front Immunol. 2022, 13, 57107. [CrossRef]
- Napoleão-Pêgo, P.; Carneiro, F.R.G.; Durans, A.M.; Gomes, L.R.; Morel, C.M.; Provance, D.W., Jr.; De-Simone, S.G. Performance assessment of multiepitope chimeric antigen for serodiagnosis of the acute phase of Mayaro fever. Sci. Rep. 2021, 11, 15374. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).