Introduction
A significant health threat is typhoid fever, which is a great concern. The clinical presentation is similar to many other febrile diseases, making it difficult to determine the true impact (World Health Organization WHO Background Document: The Diagnosis, Treatment and Prevention of Typhoid Fever Communicable Disease Surveillance and Response Vaccines and Biologicals, 2003). In endemic countries where the disease burden is assessed by governments and hospitals using an undefined denominator, a significant hurdle to diagnosing typhoid fever is the absence of laboratory personnel and equipment (Khanam et al., 2022). Salmonella Typhi (S. Typhi) causes typhoid fever (Masuet-Aumatell & Atouguia, 2021). The bacterium is human-specific, and the most common way to contract typhoid fever is to consume food or water contaminated with S. Typhi through fecal or urinary transmission (Parry et al., 2002a). After ingesting S. Typhi, it moves from the gut into the blood, where it proceeds to multiply in the intestinal lymph nodes, liver, and spleen (Milligan et al., 2018). ). High fever is the main sign of the infection, and other adverse consequences include nausea, abdominal discomfort, and irregular bowel movements (Crump et al., 2015a). The urgency of an effective vaccine against this pathogen arises from the limitations of current preventive measures. The literature review aims to critically analyze the existing research on vaccine candidates targeting S. Typhi while also exploring the pathogen's structural components, emphasizing outer membrane proteins, transcriptional factors or regulators, and virulent factors. This will help us to discover new immunological targets of the bacteria, which will be applied to develop novel vaccines.
Vaccines against S. Typhi
Typhoid vaccination has been reported to be a successful preventive approach, especially if combined with other preventive measures, including treating household water, hand washing, and providing proper sanitation (Sahastrabuddhe & Saluja, 2019). Vaccines have been produced against S. Typhi, with some administered to people in endemic countries, while others are in various phases of clinical trials yet to be used due to the rise in multidrug-resistant strains that make treatment of typhoid fever more difficult. This is because antibiotic resistance makes it difficult to treat the disease effectively (Wain et al., 2015). There are two typhoid vaccines commonly available, Ty21a(oral) and Vi polysaccharide (parenteral), whereas newer typhoid conjugate vaccines (TCV) are at varying phases of development and usage (Milligan et al., 2018).
A study conducted by Chakraborty and colleagues revealed that intranasal administration of a subunit vaccine, which contains T2544 and the cholera toxin B subunit, protected a mouse model against S. Typhi. The researchers noted that the immunization of CTB-T2544 boosted the gut-homing receptor's expression in lymphocytes. It inhibited the growth of bacteria and their attachment to epithelial tissues, and it stimulated the development of antibodies against the pathogens. The researchers noted that the immunization with CTB-T2544 significantly increased the circulating Th1 and Th2 cytokines. It also doubled the number of T-FH cells in the lymph nodes. The researchers noted that the immunization with CTB-T2544 resulted in significantly higher IgG, IgA, and IgG1 antibody titers than the unimmunized mice. It also recovered significant numbers of T2544-specific IgA and IgG antibodies in various parts of the body, such as the spleen, lymph nodes, and Peyer's patch (Suparna Chakraborty et al., 2023).
In the formulation of a vaccine for S. Typhi, it is imperative to take into account the inherent characteristics and composition of S. Typhi, including its pathogenicity, mode of infection, epidemiology, and drug resistance. Multiple elements of S. Typhi, such as Vi-polysaccharides, O-antigens, flagellar antigens, full-length outer membrane proteins (OMPs), and short peptides derived from OMPs, have been employed in the design of vaccines against typhoid fever. Within the realm of in-silico research, there has been a particular focus on the development of outer membrane vesicles (OMVs) derived from S. Typhi as a potential means of immunization against typhoid fever (Haque et al., 2021).
The paper emphasized the immediate necessity for efficacious immunizations to avert typhoid fever, given its severity as an infection with considerable rates of resistance to antibiotics and its primary transmission through deficient food hygiene and inadequate sanitation. The examination imparts knowledge regarding the characteristics and constitution of S. Typhi, its capacity to cause disease, method of infection, prevalence, and resistance to drugs, which can inform the development of targeted immunizations. The investigation suggests employing outer membrane vesicles (OMVs) derived from S. Typhi as a potential vaccine for safeguarding against typhoid fever. This puts forth a novel approach to designing immunizations that utilize specific elements of the pathogen. The discoveries presented in this paper can serve as a guide to researchers and vaccine developers in devising more efficacious immunizations against typhoid fever, potentially resulting in enhanced preventive strategies and diminished disease burden (Haque et al., 2021). Other authors argues that there is a need to identify vaccines or agents to prevent the spread of Salmonella Typhi, which can cause fever and other health issues in developing nations. Currently, the only available vaccines are ineffective and have limitations such as age restrictions and poor efficacy. According to the study, the STIV vaccine induces a robust and immunogenic response in mice. It also produces high serum levels of various immunoglobulin subclasses. The rSTIV vaccine protected them from Salmonella enterica and S. Paratyphi A infections in mice. It also reduced the bacterial load in their organs. These findings support the development of a potential new vaccine candidate for the prevention of enteric fever (Das et al., 2019).
Currently, no animal model accurately reproduces the pathogenesis of S. Typhi infection, as both the bacteria and its virulence factors are specifically adapted to humans. However, despite these challenges, various animal models have been utilized to investigate specific aspects of typhoid illnesses and S. Typhi infection. For instance, higher primates like chimpanzees can be infected with S. Typhi, but they do not display the symptoms of typhoid fever, suggesting that crucial host factors found in humans are absent in this model. One such host factor is a glycan known as N-acetylneuraminic acid (Neu5Ac), the host cell receptor for typhoid toxin (Edsall et al., 1960). While chimpanzees primarily express a different glycan called sialic acid N-glycolylneuraminic acid (Neu5Gc), which prevents toxin binding, mice naturally express Neu5Ac despite the presence of a functional enzyme that converts it to Neu5Gc (Chou et al., 2002). This enzyme is the CMP-N-acetylneuraminic acid hydroxylase (CMAH), notwithstanding the presence of CMAH in mice, mice do not support the replication of S. Typhi. They, however, express the Neu5Ac receptor, a glycan receptor for the toxin. Mice with low expression of this glycan receptor can be administered with purified typhoid toxin to play the role of a surrogate model for investigating the function of the toxin in typhoid patients (Y.-A. Yang et al., 2017). Additionally, mice that exclusively express Neu5Ac, like humans, have been developed and can be used for pre-clinical testing of preventative and therapeutic strategies against typhoid toxin-mediated symptoms and pathogenesis (Y.-A. Yang et al., 2018).
Typ21a Vaccine: A live attenuated, oral-route vaccine was first developed in the 1970s (Shams et al., 2020). The vaccine can protect against S. Typhi by increasing cellular and humoral responses and immunity in the intestinal mucosa, providing defense against the pathogen (Pennington et al., 2016). Typ21a vaccine is the first oral vaccine against S. Typhi and was developed in Switzerland by chemical mutagenesis of wild-type strain of the bacterium (Levine et al., 1989). This strain does not have the galactose-epimerase gene and Vi antigen; hence, it is highly attenuated. Both a liquid and an enteric-coated capsule form of this vaccination are available. Over seven years, various clinical trials have indicated efficacy up to 67%. Ty21a has some risks, such as the need for high bacterial concentrations for the oral dose to produce appropriate immunity; therefore, its use is only advised for children older than five years (Sahastrabuddhe & Saluja, 2019).
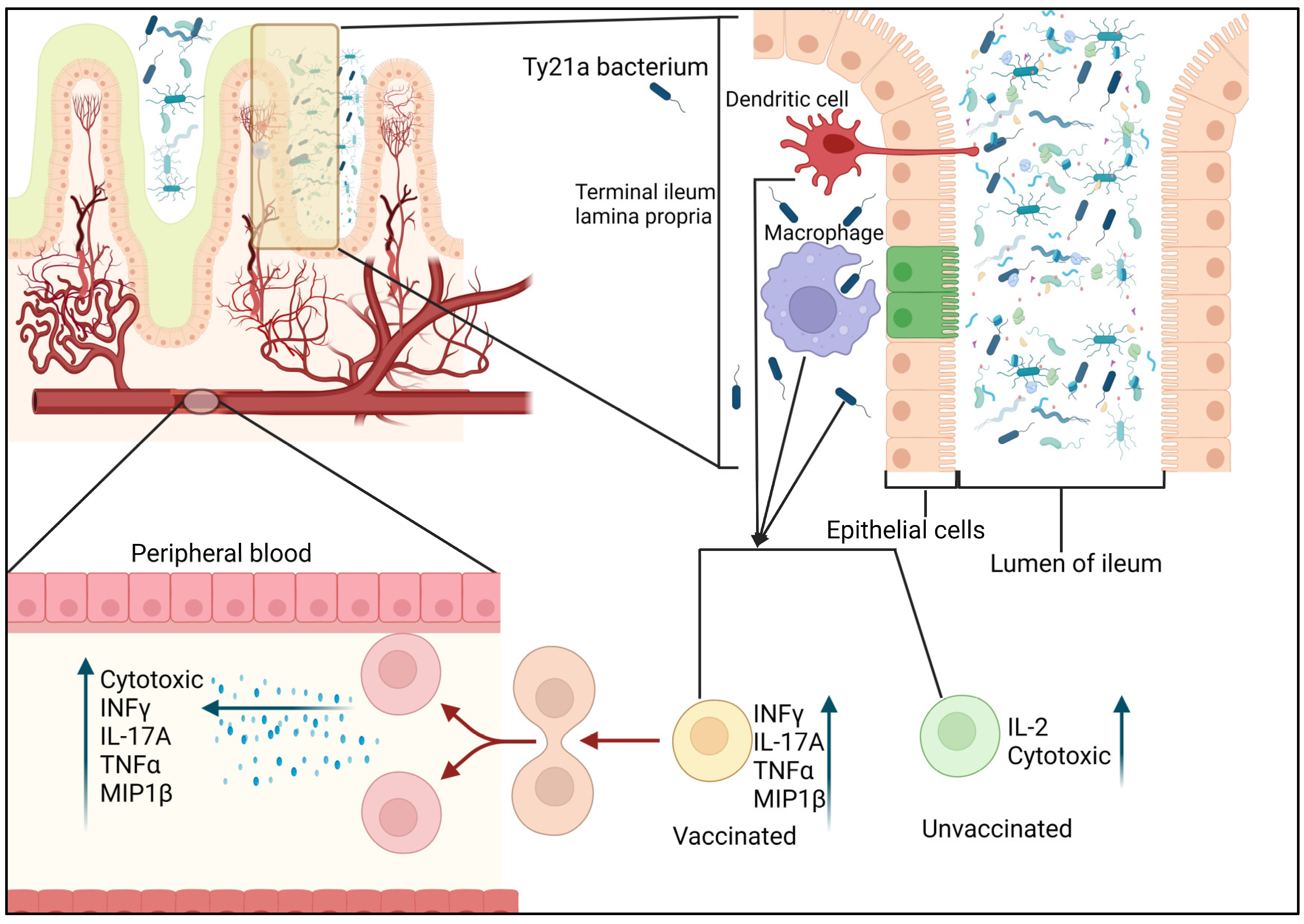
CD4 T cells are essential in generating immune responses induced by vaccines, which are likely to provide adequate protection against various pathogens. Additionally, it is becoming evident that immune responses differ between tissues and peripheral blood (Sharma et al., 2013). Therefore, assessing T-cell-mediated responses at the infection site is crucial as part of vaccine development efforts. Booth et al. investigated the impact of oral immunization with the attenuated oral typhoid vaccine Ty21a on CD4+ T memory cells and evaluated the specific responses to S. Typhi in human terminal ileum lamina propria mononuclear cells (TI-LPMC) and peripheral blood mononuclear cells (PBMC). Booth et al. investigated the impact of oral immunization with the attenuated oral typhoid vaccine Ty21a on CD4+ T memory cells and evaluated the specific responses to S. Typhi in human terminal ileum lamina propria mononuclear cells (TI-LPMC) and peripheral blood mononuclear cells (PBMC) (Booth et al., 2019). Their findings revealed that oral Ty21a immunization at the terminal ileum mucosa, which is the preferred site of S. Typhi infection, resulted in (i) increased frequencies of CD4+ T cells, (ii) modulation of the homing and accumulation of effector cells, and (iii) induction of multifunctional CD4+ T cells that respond to S. Typhi (producing IL-17A, IL-2, and/or MIP1β) in TI-LPMC. Specifically, they observed activation of all major subsets of CD4+ memory T cells (effector memory, central memory, and terminally differentiated effector memory) in the TI mucosa following Ty21a immunization, and each subset displayed distinct response profiles. Their effector memory T cells showed trends of increased production of IFNγ and IL-17A, central memory T cells exhibited significant increases in IFNγ and MIP1β production, and terminally differentiated effector memory T cells showed trends of increased IL-2 production. Furthermore, they demonstrated that CD4+ T memory cell responses in TI-LPMC specific to S. Typhi were multifunctional and distinct from those in the systemic circulation following Ty21a immunization. Taken together, their findings provided valuable new insights into the immune responses elicited by oral Ty21a immunization in the TI mucosa of humans, which have important implications for future vaccine design and development. The initial evidence indicates that the oral Ty21a vaccine stimulates the specific T cells of the LPMC CD4+ T multifunctional responses (IL-17A, IL-2, and/or MIP1β), which indicates that the response of the human intestine to this type of immunization is compartmentalized.
Fine granularity is essential in characterizing immune responses and studying the differences between different immune compartments. For instance, using specific subsets of the immune system is crucial in identifying the mechanisms by which the Ty21a vaccine can stimulate the T cells' response to the TI-LPMC. This study shows that the oral Ty21A vaccine can elicit the local TI-LPMC T cells to respond to the S. Typhi antigens, which can protect against the pathogen. Unvaccinated individuals exhibited high baseline T responses to the S. Typhi antigen. Thus, suggesting that gut microbiota might trigger memory responses related to previous exposure to other strains of Salmonella. Other genetic factors can also affect the development and maintenance of immune responses. For example, specific genetic variants, such as the DRB1*04:10 allele, have been linked to the protection against S. Typhi. It is also believed that the higher levels of multifunctional CD4+ T cells, capable of responding to S. Typhi, contribute to disease protection.
As shown in
Figure 1, after the vaccine is administered, the CD4+ T cells are activated by macrophages or dendritic cells to generate higher levels of chemokines and cytokines. They also develop increased cytotoxicity. Following the immunization of Ty21a, the various CD4+ T cells involved in the Terminal ileum - lamina propria (TI-LP), such as the multifunctional cells (MF) and effector cells, acquire unique characteristics. Following the Ty21a immunization, the peripheral blood mononuclear cells (PBMC's) CD4+ T is modified to produce MIP1β, IL-17A, IL-2, TNFα and IFNγ. Compared to the single-producing cells, the responses exhibited by the various CD4 T cells following the Ty21a immunization are mainly multifunctional (MF) cells. The magnitude of these responses is lower than that of the lamina propria mononuclear cells (LPMC) CD4+ T cells. This demonstrates that the effector cells that are stimulated by the Ty21a immunization can only move between the peripheral blood and Terminal ileum (TI) mucosa. They also become MF once they have become part of the gut. The figure depicts MF cells that exhibited higher responses to the Ty21a vaccine compared to the unvaccinated. The trends in the number of responses that the Ty21a vaccine elicits against unvaccinated and the vaccinated are indicated by the black arrow, which shows the higher levels of responses in the TI-LPMC compared to the PBMC. Red arrows indicate the opposite trend.
Vi Polysaccharide (Vi-PS) vaccine: It is a subunit vaccine that was developed based on the Vi capsular polysaccharide antigen of S. Typhi, and it is introduced parenterally into an individual (Crump et al., 2015b). The Vi polysaccharide, a homopolymer of ([alpha]-4),2-deoxy-2-N-acetyl galacturonic acid, partially O-acetylated at carbon 3 (Stone & Szu, 1988). Vi Polysaccharide vaccine usually is delivered as a single dose intramuscularly or via deep subcutaneous injection. Revaccination is advised for people still at risk of infection after three years. In most nations, it is recommended for use in adults and children older than 24 months (Hessel et al., 1999). The Vi vaccine, like the majority of polysaccharide vaccinations, does not cause a booster response or levels of antibodies that are protective in young children (Lin et al., 2001a).
Aside from toxins, the only vaccines used against bacterial infections are capsular polysaccharide antigens. Hence, it is crucial to comprehend how capsular polysaccharides induce protection to understand bacterial vaccinology more broadly. The ability of conjugate vaccines like typhoid conjugate vaccine (TCV) to elicit immune responses in infants is a significant reason for their use. However, they also serve as valuable tools for evaluating how immune responses differ depending on the host's encounter with the same antigen, specifically as a conjugated or unconjugated antigen containing a limited number of carbohydrate epitopes, as seen in the current study (Patel et al., 2021; Shakya et al., 2019). The differences in outcomes resulting from this variation in presentation are significant, including a notable change in the longevity of the IgG antibody response and the frequency of plasma cells in niches such as the bone marrow. The approval of TCV, its success in challenge and field studies, reports of reduced responsiveness after Vi-PS, and the intention to explore the impact of boosted immune responses on protection in children all emphasize the importance of studying the immunology of responses to different capsular polysaccharide vaccines (Jin et al., 2021). These studies aid in maximizing the clinical benefits of TCV for infants and adults throughout their lives. Several factors can influence the responses elicited by different conjugates, and it is necessary to consider these factors when extrapolating findings from one type of conjugate vaccine to another. The Vi-PS and TCV can be produced using well-established methodologies to ensure manufacturing consistency. Both unconjugated Vi-PS and TCV induced early IgM and IgG responses and classical extrafollicular responses (Jha & Janoff, 2019; Jossi et al., 2023). However, only mice immunized with TCVs exhibited germinal center responses, as expected. Anti-Vi IgA may contribute to the protection provided by Vi vaccines. In our studies, minimal IgA was detected and only consistently shortly after immunization, so the role of IgA could not be explored (Andersen et al., 2019). Despite the differences in antibody responses to the conjugated and unconjugated vaccines, bacterial burdens in challenged mice that were immunized for a short duration were similar, regardless of the vaccine type or number of doses administered. Similar vaccine efficacies were observed for unconjugated Vi-PS and TCV vaccines in a human challenge study of adult volunteers who were challenged one month after vaccination. Therefore, while the network of activities associated with producing high-affinity antibodies to Vi antigen could potentially enhance protection, it is not essential in adults. Thus, germinal center-derived antibody responses may offer other benefits, such as promoting longer-lasting responses. This overlap between the contribution of antibody type and persistence may complicate the identification of simple correlates of protection for Vi-based vaccines. The contribution of T cells to the different immune responses and levels of protection observed after immunization with the different vaccines was not evaluated in this study.
Conjugate Polysaccharide Vaccine (Vi-rEPA)
This is the first typhoid conjugate polysaccharide vaccine was developed by Szu et al. The heterobifunctional cross-linking reagent N-succinimidyl-3-(2-pyridyldithio)-propionate or adipic acid di-hydrazide was used in the conjugation process created by researchers at the US National Institute of Child Health and Disease to bind Vi to proteins (Szu et al., 1987). The resulting conjugate (Vi-rEPA) was more immunogenic in mice and young Rhesus monkeys than the Vi alone, using a nontoxic recombinant protein that is antigenically similar to Pseudomonas aeruginosa exotoxin A as a carrier protein (Szu et al., 1987). This synthesis method proved repeatable, yielded large quantities of Vi-protein conjugates, and worked with a variety of therapeutically significant proteins, including diphtheria toxoids (DT) and tetanus toxoids (TT) (Sahastrabuddhe & Saluja, 2019). Most of these vaccines are in development and early manufacturing (Mohan et al., 2015). The vaccine's strong immunogenicity was shown in field trials, including 13,766 children aged 2-4 years in Vietnam. Based on the data, all children had at least ten times higher levels of Vi antibodies. The immunization showed an effectiveness of 93% at 27 months and 89% at 46 months of age when given in two doses spaced six weeks apart. 91% of people responded well to the vaccine, even after only one dosage, and those who had typhoid after receiving the shot reported less severe symptoms (Ajay Kalra et al, 2010; Lin et al., 2001b).
TypBar-TCV vaccine
The vaccine comprises a conjugate of Vi polysaccharide and tetanus toxoid (Wain et al., 2015). The usage of this vaccine was authorized as of January 2021 in nine nations, including Ghana, Tanzania, Kenya, and Zambia (Haselbeck et al., 2021). Bharat Biotech produced it in Hyderabad, India, and was recently prequalified by WHO in January 2018. This typhoid conjugate vaccine has demonstrated strong and persistent immunogenicity. It may be safely administered to infants as young as six months old and can be incorporated into national immunization programs. When Salmonella typhi's capsular polysaccharide is conjugated to a protein carrier, foreign peptide antigens are presented to the immune system, triggering antigen-specific CD4+ Th cells and T-dependent antibody responses. However, the polysaccharide alone does not cause B-cell responses. Higher-affinity antibodies and long-term immunological memory are two features of T-dependent responses, which are also triggered by toxoids (Bharat Biotech, n.d.).
PedaTyph (Vi-TT) vaccine
The first TCV to receive an Indian license was PedaTyph (World Health Organization, 2017). It was advised that children older than three months receive a single dose of 0.5 mL, followed by booster doses between the ages of 2 and 3 years (Sahastrabuddhe & Saluja, 2019). The vaccine is made up of 5 g of S. Typhi's Vi polysaccharide coupled to 5 g of tetanus toxoid protein in isotonic saline. Following many trials, the vaccine's effectiveness was 95% in the first year of follow-up, with few post-vaccination side effects (Mitra et al., 2016).
According to the study results from Mitra et al., children can be effectively protected against typhoid fever by the Vi-TT conjugate typhoid vaccine. The vaccine also triggered Strong immune responses, as evidenced by the high levels of anti-Vi IgG antibodies found in the children after vaccination. After 12 months of vaccination, the anti-Vi IgG antibody levels were still high, suggesting that the vaccine can offer sustained protection against typhoid fever (Mitra et al., 2016).
Vi-CRM197 vaccine
This vaccine has two components: Vi polysaccharide part and CRM197 as a carrier protein. The carrier protein, CRM197, is a diphtheria toxin (nontoxic mutant) acquired from Bacillus's virulence gene mutant strain (Xiong et al., 2021). In both phase 1 and 2 trials of the vaccine, its safety and immunogenicity against S. Typhi were tested in European adults. A phase 2 trial with 320 participants, including adults, children, and infants, was conducted in India, Pakistan, and the Philippines; it concluded that Vi-CRM197 was safe and immunogenic in endemic populations of all ages, although the responses were short-lived (Sahastrabuddhe & Saluja, 2019).
In a study with animals, the glycoconjugate vaccine Vi-CRM197 promotes the development of serum IgG1 titers specific to Vi that lasted for over 60 days after immunization. The investigated animals' intestinal washes also included Vi-specific IgG antibodies. Additionally, the study revealed that Vi-CRM197 stimulated T-cell response, which increased the production of antibodies by Vi-specific B cells (Fiorino et al., 2012). Thuluva et al. investigation demonstrated that a single-dose Vi-CRM197 conjugate vaccination elicited a robust anti-Vi immunological response, as evidenced by the 99% seroconversion rate among participants in the age range of ≥6 to <64 years (Thuluva et al., 2022). This result was consistent with another research that found adults, toddlers, and newborns aged 9 to 11 months could all develop robust anti-Vi immune responses with a 100% seroconversion rate after receiving a single dose of the Vi-CRM197 conjugate vaccine (Bhutta et al., 2014).
Vi-DT vaccine
This vaccine is made up of Vi-polysaccharide conjugated to Diphtheria toxoid (Vi-DT). It was examined in a clinical trial in the Philippines to determine its immunogenicity and safety. Their phase I trial involved participants aged 2 to 45, while their phase II trial involved 6 to 23 months. These two clinical study phases demonstrated that Vi-DT is immune-stimulating, well-tolerated, and safe for the aforementioned age ranges (Medise et al., 2020). In their study, Medise et al. compared the Vi-DT vaccine to Vi-PS using a randomized, observer-blind, superiority design. Two groups of 200 children, ages 2 to 11, were formed; half of the groups received Vi-DT, and the other half received Vi-PS. After the study, Anti-Vi IgG antibody titers were assessed 28 days after vaccination and before. The Geometric mean titer (GMT) increased dramatically 28 days after vaccination, with a significantly higher value in Vi-DT compared to Vi-PS (p < 0.001). At 28 days after vaccination, 93% of the subjects in the Vi-PS group and 100% of the subjects in the Vi-DT group, respectively, experienced an antibody increment of at least four times. This confirms how the Vi-DT vaccine against typhoid fever works more effectively than Vi-PS in this study (Medise et al., 2020).
Overview of structure and function of OMPs as probable target-receptors for anti-Salmonella Typhi compounds
Structure of S. Typhi – Outer Membrane Proteins (OMPs)
The Gram-negative bacteria cell wall consists of the peptidoglycan layer and the Outer membrane layer. The outer membrane contains lipopolysaccharide (LPS), proteins, and phospholipids, mainly phosphatidylethanolamine (Smit et al., 1975). There are groups of proteins known as Porins, and they facilitate the influx and efflux of low molecular mass substances across the cell wall into the cell's cytoplasm (Shams et al., 2020). The OMPs are often produced in the cytoplasm before they cross the plasma membrane into the outer membrane's surface and serve as immunogenic targets to antibodies and lymphocytes (Singh et al., 2017). OMPs and lipoproteins of their cell membrane serve various purposes, including maintaining and stabilizing membranes, active and passive ion and solute transport, signal transduction, defense, and catalysis (Khalid et al., 2008).
OmpA
The N-terminal domain of OmpA proteins is an eight-stranded, anti-parallel barrel embedded in the outer membrane. In contrast, the C-terminal domain is globular and found in the periplasm (Findlay et al., 2005). OmpA serves structural and ion-permeable porin functions, and the electrostatic gating mechanism that regulates its ionic pore under the effect of salt enables bacterial survival under osmotic stress (Hong et al., 2006). These proteins actively participate in a range of pathogenic activities in numerous organ systems, most notably the digestive, respiratory, urogenital, and neurological systems, due to the high copy number and surface exposure of OmpA proteins in pathogenic bacteria (Krishnan & Prasadarao, 2012). OmpA proteins play significant pathogenic functions in a variety of pathogenic bacteria like S. Typhi that adhere to epithelial surfaces, invade cells, evade host defenses, or stimulate the production of proinflammatory cytokines. Additionally, OmpA family proteins might be immune system targets due to their immunogenicity in relation to the molecule's exposed surface loops. OmpA proteins of S. Typhi are considered possible vaccine candidates (Confer & Ayalew, 2013).
OmpC
In typhoid patients, OmpC, a significant surface antigen and porin protein of S. Typhi, is produced throughout the infection phase. It is a suitable candidate for heterologous epitopes to be shown on the cell surface, and it is expressed in both low and high osmolarity (Arockiasamy & Krishnaswamy, 2000; Puente et al., 1995). OmpC is a homotrimer that is mature and functional. OmpC monomer has a molecular mass of 39 kDa and 357 a without the signal peptide. According to estimates, it is a barrel protein with a pore radius of 1.1 nm (Sundara Baalaji et al., 2006). The protein is evolutionarily conserved in 11 Salmonella serovars; it has eight variable regions compared to other strains; they are presented to B-cells, eliciting an immune response (Arockiasamy & Krishnaswamy, 1995). In inducing hyperimmune sera in Swiss Albino mice, ELISA was used to assess the immunogenicity of the recombinant porin protein (Verma, 2009). These justifications imply that OmpC might be a candidate for creating a Salmonella r-DNA vaccine. Additionally, it can serve as a potential antigen for immunization and diagnosis of Typhoid fever (Immunol et al., n.d.).
OmpF
The homotrimer protein OmpF, a key porin in S. Typhi, is connected to the translocation of bacterial survival under osmotic stress. A barrel structure with 16 membrane-spanning -strands is formed by each monomer of molecular weight 37 kDa. Small hydrophilic molecules, including nutrients, antibiotics, and waste materials, can diffuse across the outer bacterial membrane through the OmpF porin's three sizable water-filled channels per trimer (Hong et al., 2006). Drugs like quinolones, tetracyclines, and lactams can pass through OmpF (Cohen et al., 1989; Tavío et al., 1999). To develop novel typhoid vaccines, it is crucial to comprehend the link between S. Typhi OmpF's structure and function. The S. Typhi OmpF mutant displays reduced pathogenicity than the wild type (Chatfield et al., 1991). Recombinant OmpC and OmpF of S. Typhi have been demonstrated to be immunogenic proteins in mouse models in their denatured forms. These two OMPs are recommended as potential vaccination candidates. However, there was no in vivo challenge investigation done on the various porin types (Secundino et al., 2006).
YshA/OmpL
YshA is a 230-residue protein with a high degree of conservation that is projected to have ten transmembrane -strands and an N-terminal signal sequence. It is predicted that YshA is a trans based on all of the physicochemical and spectroscopic characteristics it demonstrated. When produced in S. Typhi, recombinant YshA localizes explicitly to the outer membrane (Freeman et al., 2011). In the research, OmpL was chosen as a candidate protein due to its location on the bacterial surface and the abundance of its expression (Freeman et al., 2011; Y. Yang et al., 2013a). OmpL of S. Typhimurium is highly immunogenic, OmpL might elicit humoral and cell-mediated immune responses, and OmpL confers 100% protection to immunized mice against challenges with extremely high doses of S. Typhimurium. Additionally, mice that received the vaccination showed adequate bacterial clearance from their reticuloendothelial systems. OmpL, therefore, presents a viable target for creating a potential vaccination against S. Typhi infection (Y. Yang et al., 2013b).
Transcription factors or regulators of S. Typhi
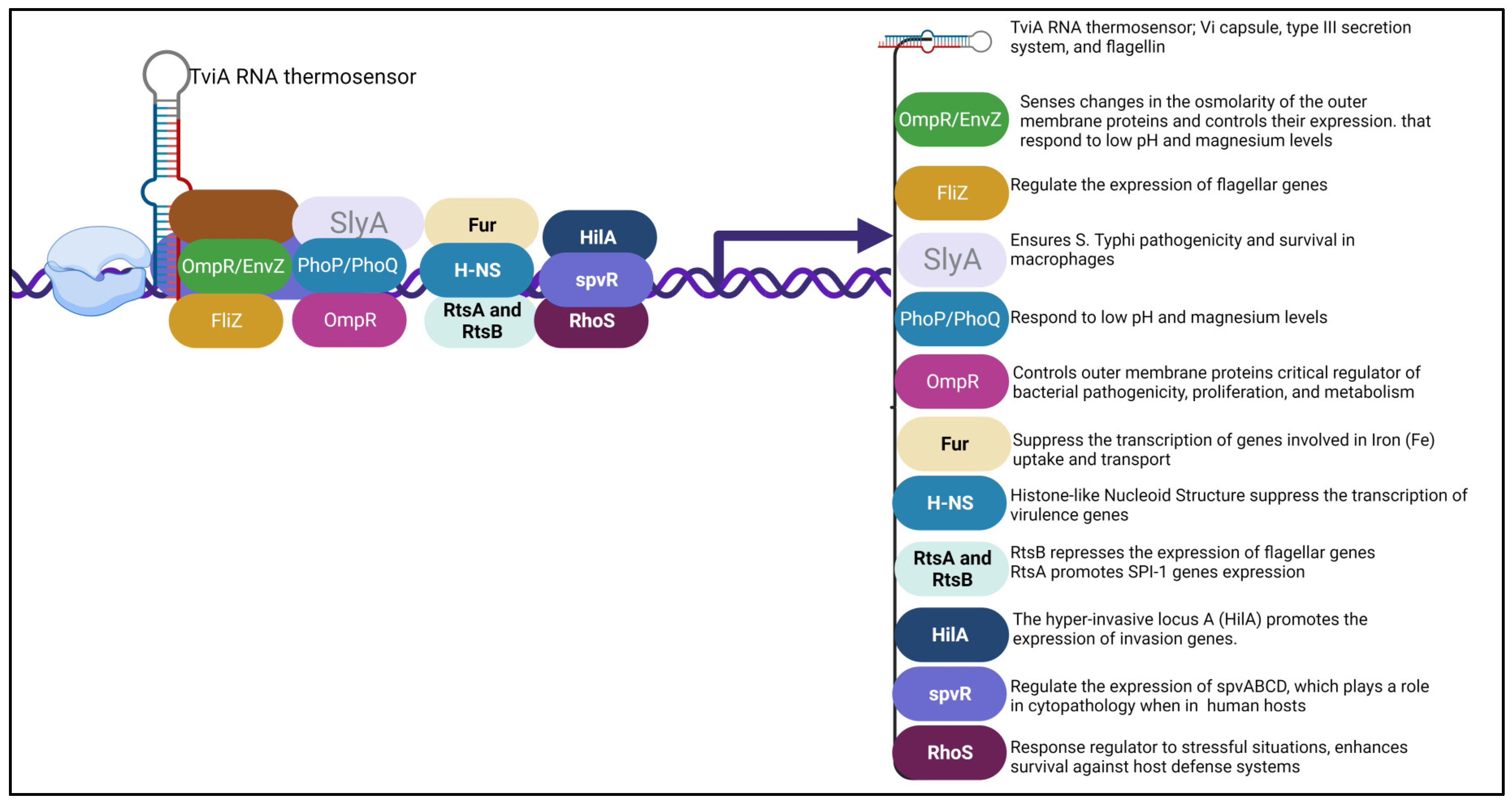
Bacteria can sense their surroundings and change how genes that aid in their survival are expressed. This is made possible by transcription factors controlling gene expression for positive or adverse effects. There are several types of transcription regulators in S. Typhi.
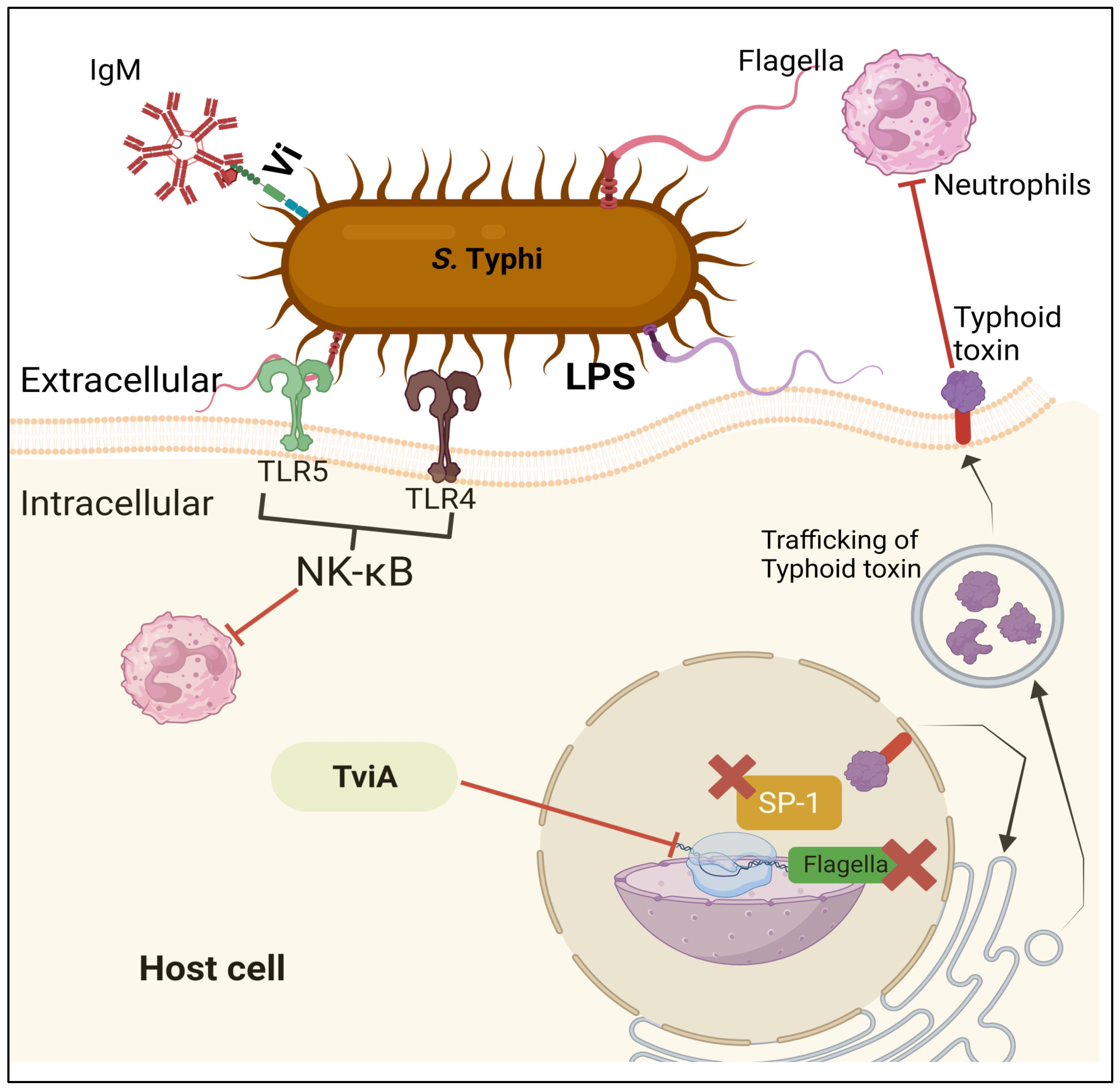
The TviA RNA thermosensor is a transcriptional factor that regulates the expression of the Vi capsule, type III secretion system and flagellin. It helps the bacteria survive and invade other organisms. The ability of bacterial pathogens to sense and respond to environmental signals is very important for their survival and spread. Temperature can be used as an indication that they have entered an unfamiliar host, and these pathogens must alter their expression to elude the body's immune system. In a study, the authors identified a thermosensor in the tviA region that is encoded by the Salmonella enterica var Typhi bacterium. They demonstrated that the tviA gene is a key component of the development of the Vi capsule, type III secretion system-1, and flagellin. It is also responsible for the regulation of other important factors such as the growth of the bacteria. In order to understand the function of this gene, they introduced point mutations to its secondary structure. These changes allow them to identify a functional RNAT that can be utilized in vitro and in the binding of ribosomes. The development of mutations in the RNAT in S. Typhi led to the aberrant expression of certain virulent factors. Thus, they showed that this bacterium controls the expression of these most virulent factors through the RNAT in the tviA region. Their findings suggest that the presence of this RNAT in the host's body can help limit inflammation (Brewer et al., 2021).
The FliZ system is responsible for the positive expression of flagellar genes, essential for the bacteria's ability to move and attach to host cells. In a study conducted on the fliZ gene, tehy discovered that it has a positive regulatory effect on the growth and proliferation of flagellar operon species in Salmonella enterica. The researchers also found that the mutation reduced the amount of protein that can be excreted. They showed that the lacZ gene is associated with a subset of genes that are known to be involved in the development of a variety of infectious diseases. The hilA gene is a positive regulator of invasion proteins. In addition, the fliZ mutation decreased the ability of the S. enterica species to invade HEp-2 cells. In a mouse model of typhoid, the fliZ mutation attenuated its ability to produce full-scale virulent behavior. The results of the study suggest that the product of the gene is responsible for regulating the invasion genes and for the efficient expression of full virulent behavior. (Iyoda et al., 2001).
SlyA is a virulence-associated transcriptional regulator (Treg) discovered as a member of a family of low-molecular-weight Treg. It is necessary for S. Typhi's pathogenicity and survival in macrophages. It is required for resistance to oxidative stress and is often expressed in the intracellular environment of macrophages. The SlyA regulon in bacteria is active during host infection and is necessary to resist harmful reticuloendothelial system oxidative products (Buchmeier et al., 1997).
Outer membrane protein regulator (OmpR) controls outer membrane proteins and is a critical regulator of bacterial pathogenicity, proliferation, and metabolism. OmpR is triggered when osmotic and acidic stress in the cytoplasm of S. Typhi is changed (Chakraborty & Kenney, 2018). In S. Typhi, OmpR inhibits (Sigma S) RpoS, an alternate stationary phase sigma factor, releasing yghA from RpoS's repression. It is hypothesized that the putative oxidoreductase YghA will generate protons. Also, at high osmolality, OmpR-mediated repression of the speF gene inhibits cell recovery (Chakraborty et al., 2017). The OmpR/EnvZ system senses changes in the osmolarity of the outer membrane proteins and controls their expression.
Histone-like Nucleoid Structure (H-NS): It is known that this nucleoprotein (histone-like protein) directly binds DNA-targeted areas in an oligomerized state to suppress the transcription of numerous genes in Gram-negative enteric bacteria, including virulence genes acquired by horizontal transfer (Stoebel et al., 2008). The H-NS protein represses nearly 10% of the genes, particularly the central virulence genes on the chromosome and virulence plasmid. By attaching to the DNA regions harboring these genes, H-NS can silence or knock down these genes (Hurtado-Escobar et al., 2019).
RtsA and RtsB: In S. Typhi, the regulatory proteins RtsA and RtsB have opposing impacts on gene expression. RtsB represses the expression of flagellar genes, including the flagellar master operon D&C (flhDC) promoter, while RtsA promotes the expression of genes connected to the Salmonella pathogenicity Island 1 (SPI-1), which encodes for type III secretion system (TTSS). These proteins are crucial for coordinating the expression of particular gene sets in response to environmental signals or circumstances when in the host (Ellermeier & Slauch, 2003).
Ferric uptake regulator (Fur): It is a regulatory protein that suppresses the transcription of genes involved in Iron (Fe) uptake and transport and is active in the presence of Fe. In addition, when Fe levels are high, the ion uptake is inhibited by Fur, with excess Fe stored by storage proteins such as FtnA, FtnB, and Bfr (Leclerc et al., 2013). Fur also regulates genes involved in acid stress and adaptation, oxidative stress resistance, and virulence (Foster, 1991).
PhoPQ system: S. Typhi has a two-part regulatory system called PhoPQ that regulates transcription. It comprises the transcription factor PhoP and the sensor kinase PhoQ. When activated, PhoQ recognizes environmental cues and phosphorylates PhoP. The subsequent binding of phosphorylated PhoP to particular DNA sequences either activates or inhibits the transcription of the target genes. Genes that are controlled by phoPQ are involved in pathogenicity, nutritional adaptability, and stress response. PhoPQ enables bacteria to modify their gene expression in response to environmental changes, which helps them adapt and survive (García Véscovi et al., 1994). The PhoP/PhoQ system responds to low pH and magnesium levels. It modulates genes involved in the development of antimicrobial resistance and the maintenance of intracellular survival.
HilA regulator: In response to both genetic and environmental regulatory inputs, the HilA regulator produces a transcriptional regulator of the OmpR/ToxR family, which promotes the expression of invasion genes (Jones, 2005). The hyper-invasive locus A (hilA) gene was discovered to be a locus that, when overexpressed, renders bacteria resistant to high-oxygen inhibition of invasion (Lee et al., 1992). The Salmonella pathogenicity island I (SPI-1) contains the hilA gene, which encodes the type III secretion system's structural elements, chaperones, and secreted effectors required for invasion (Bajaj et al., 1995; Darwin & Miller, 1999).
Sigma S (RhoS) regulator: In S. Typhi and Escherichia coli, RpoS is a crucial response regulator to stressful situations. RhoS's effects on pathogenesis differ greatly depending on the species, in contrast to its conserved and well-understood involvement in stress response. The late exponential phase is the time when the Sigma S regulator transcribes. It regulates the stationary phase genes and is a central player in the stress response. In addition to helping cells endure environmental challenges, it also prepares them for the subsequent stresses (Hengge-Aronis, 2002). In infections caused by S. Typhi, RhoS enhances survival against host defense systems or directly regulates the expression of virulence components, while in others, RhoS is either dispensable or even antagonistic to virulence (Dong & Schellhorn, 2010).
Salmonella plasmid virulence R protein (spvR): The spv genes in S. Typhi are grouped as an operon, with spvR protein regulating the expression of spvABCD. SpvR is included in the LysR family of transcriptional activators, hence its function. These genes control virulence and are needed for cytopathology when in humans (Libby et al., 2000).
Figure 3.
Diagrammatic illustration of some of the possible transcriptional regulators that can be targeted by anti-Salmonella typhi compounds and their role in S. Typhi pathogenesis.
Figure 3.
Diagrammatic illustration of some of the possible transcriptional regulators that can be targeted by anti-Salmonella typhi compounds and their role in S. Typhi pathogenesis.
Possible virulent factors that anti-Salmonella typhi compounds can target.
Pathogenic bacteria can colonize host niches with virulence factors, as shown in
Figure 4, which leads to tissue damage and local and systemic inflammation. These elements are crucial for pathogens to establish an infection and cover a broad spectrum. Thus, they directly and indirectly affect disease processes (de Nies et al., 2021). The complicated trait known as virulence is only fully exhibited in vivo during host-pathogen interactions (M Tsolis et al., 1999).
S. Typhi virulence factors, including the genes for adhesion, invasion, and toxin, are concentrated mainly in regions of the chromosome called "
Salmonella pathogenicity islands" (SPI) (R. L. Santos et al., 2003). Host invasion and intracellular proliferation, the two characteristics of Salmonella pathogenicity, have a direct relationship with genes in SPI. While SPI-2 is essential for intracellular pathogenesis and plays a crucial role in systemic
S. enterica infections, SPI-1 contains genes involved in invasion (Hansen-Wester & Hensel, 2001).
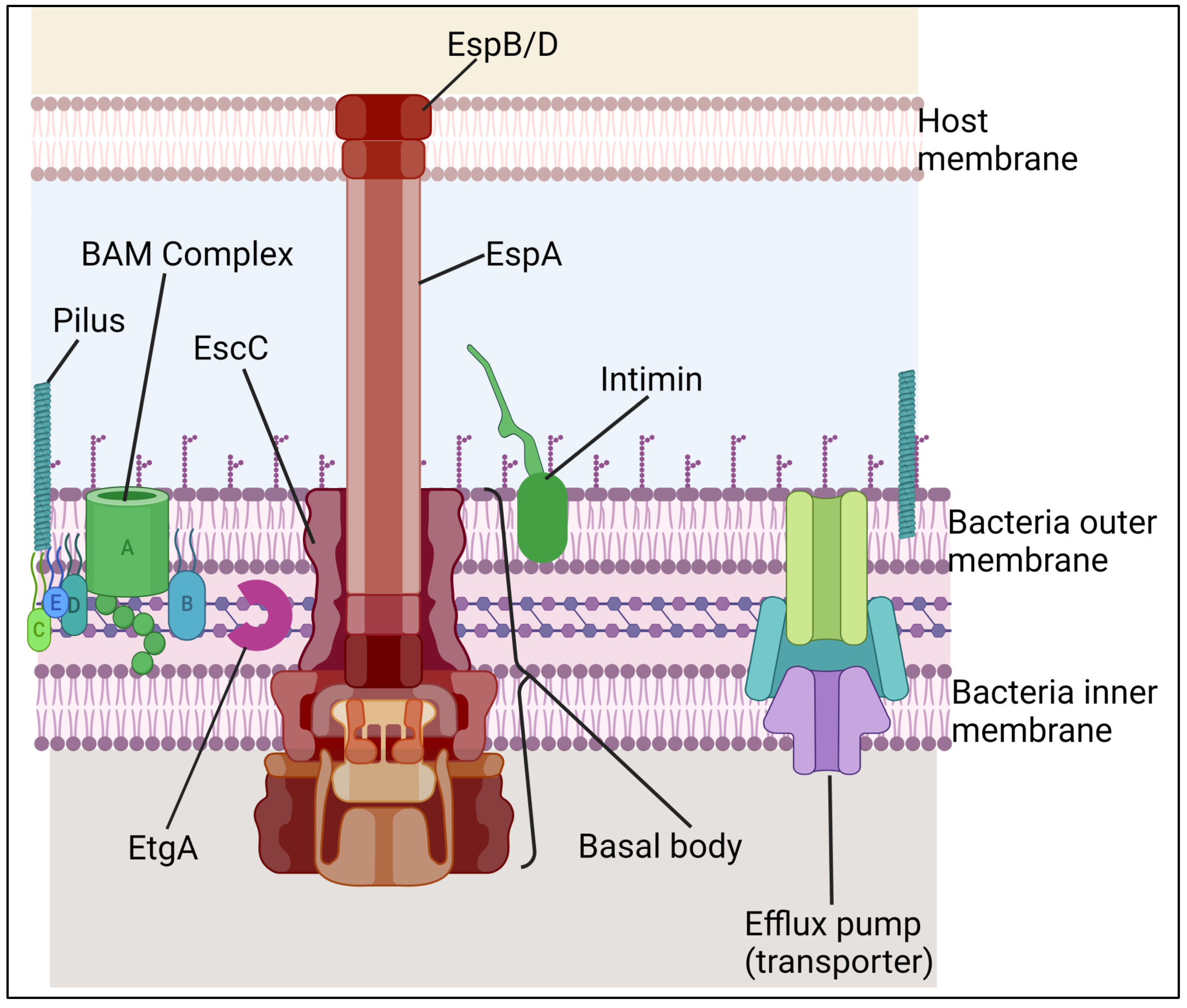
Type III Secretion System (T3SS): It is a molecular mechanism that
S. Typhi has, which is in charge of the ability of these microbes to deliver its effector proteins into the cytoplasm of host cells and modify host cells' signaling cascades for the benefit of bacteria (A. M. P. dos Santos et al., 2019). The T3SS protein complex (
Figure 5) enables the direct transport of virulence factors into host cells (Marlovits et al., 2004) hence acting as "Molecular syringes" (Hueck, 1998). Once within the cell, these effectors can change cellular processes such as cytoskeleton structure, membrane transport, signal transduction, and cytokine production, promoting host cell invasion (Haraga et al., 2008). Salmonella enterica has two unique T3SSs (T3SS-1 and T3SS-2), which are encoded by SPI-1 and SPI-2, which are found in several
S. Typhi DNA clusters (Hansen-Wester & Hensel, 2001). T3SS-2 is active within the phagosome and translocate effectors into the vacuolar space, whereas T3SS-1 is active when the bacterium encounters the host cell membrane and translocate effector proteins into its cytoplasm (Haraga et al., 2008).
S. Typhi possesses a Type III Secretion System (T3SS). The T3SS in
S. Typhi is involved in the pathogenesis and invasion of host cells. The T3SS allows
S. Typhi to inject effector proteins into host cells, manipulating host cell functions and promoting bacterial survival and replication. The T3SS in
S. Typhi is a crucial virulent factor for developing typhoid fever. Understanding the T3SS in
S. Typhi is vital for developing effective vaccines and therapeutics against typhoid fever. For instance, a study by Truong et al. showed that the Type 3 secretion system (T3SS) in
Salmonella plays a role in the pathogenesis and invasion of host cells (Truong et al., 2018). They further showed that the
Salmonella Type 3 secretion systems (T3SSs) recruit SopB as its effector, which is also known to have lipid phosphatase activity. However, a study by Chowdhury et al. showed that
S. Typhi can invade host cells independent of the T3SS-1 pathway (Chowdhury et al., 2018). They further demonstrated that targeting the STIV-Met interaction may restrict pathogenesis, and inhibition of Met tyrosine kinase can also limit infection.
The three main parts of the T3SS are the extracellular appendages, composed of a translocation pore, a filament, and a needle. The basal body is made up of a set of membrane rings that are connected through a periplasmic rod. The three IM rings house the components of the export apparatus. These include the ATPase complex, gatekeeper protein, and C-ring. This section shows the structures of the outer membrane proteins of T3SS. The illustration also shows the outer membrane protein intimin. The T3SS is a complex nanomolecular device comprising around 3.5 MDa of protein. It is characterized by a global architecture that is similar to the flagellar system. This is a syringe-shaped structure composed of a central channel approximately 2 to 3 nm in diameter and multiple ring structures located in the outer and inner membranes of the bacterium. These structures are connected to a periplasmic inner rod. The T3SS components can be grouped depending on the external structures they form. These include the extracellular appendages, the cytoplasm, and the basal body. A diagram of the structures of the T3SS is shown in
Figure 5. The needle complex's primary structural features are similar in most bacteria.
Virulence factor of Salmonella typhi
Fimbriae
It is important to note that the initial attachment to the host intestinal mucosa after oral infection is a critical stage during bacterial pathogenesis. One of the most common adhesive structures in members of the family Enterobacteriaceae, including Salmonella typhi, are Type 1 fimbriae (T1F), which are crucial for the bacterium's pathogenicity. T1F is essential for virulence and consists of FimA, a helical structural component held together by disulfide linkages. These fimbriae are flexible and not rigidly attached to the cell surface, allowing them to adapt and attach to host cells even in dynamic or crowded environments. The balance between stiffness and flexibility enables T1F to adhere to host cells effectively, making them essential for Salmonella typhi's pathogenicity(Kolenda et al., 2019; Knight & Bouckaert, 2009).
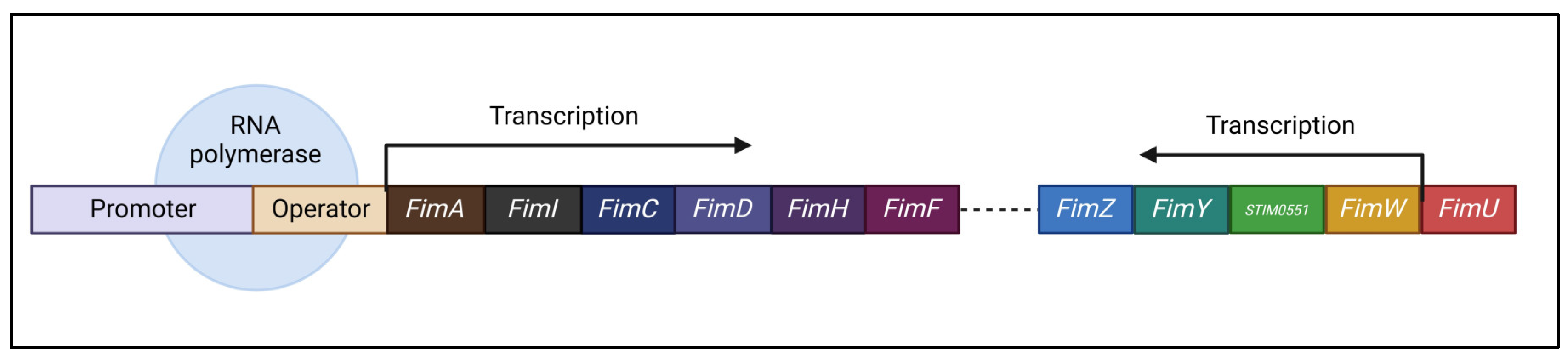
The
Salmonella fim cluster (as shown in
Figure 6) consists of a tRNA-Arg and ten genes: fimA, fimI, fimC, fimD, fimH, fimF, fimZ, fimY, fimW, and stm0551 (Purcell et al., 1987). These include fimA, fimI, fimC, fimD, fimH, and fimF, all controlled by the fimA promoter region (PfimA) and function inside a single operon. This operon encodes proteins essential for Type 1 fimbriae (T1F) development and structure. Furthermore, tRNA-Arg controls T1F expression at the translational level, whereas FimW, FimY, FimZ, and the STM0551 open reading frame contribute to the regulation of T1F transcription(Kolenda et al., 2019; Saini et al., 2009).
T1F is characterized by rod-shaped structures that are mostly made of 500–3000 FimA monomers. FimH, a protein that resembles lectin, is located at the top of the fimbrial shaft. FimF places FimH at the summit of the fimbrial structure, and FimH is essential for binding high-mannose oligosaccharides found on the surface glycoproteins of eukaryotic cells (Hahn et al., 2002).
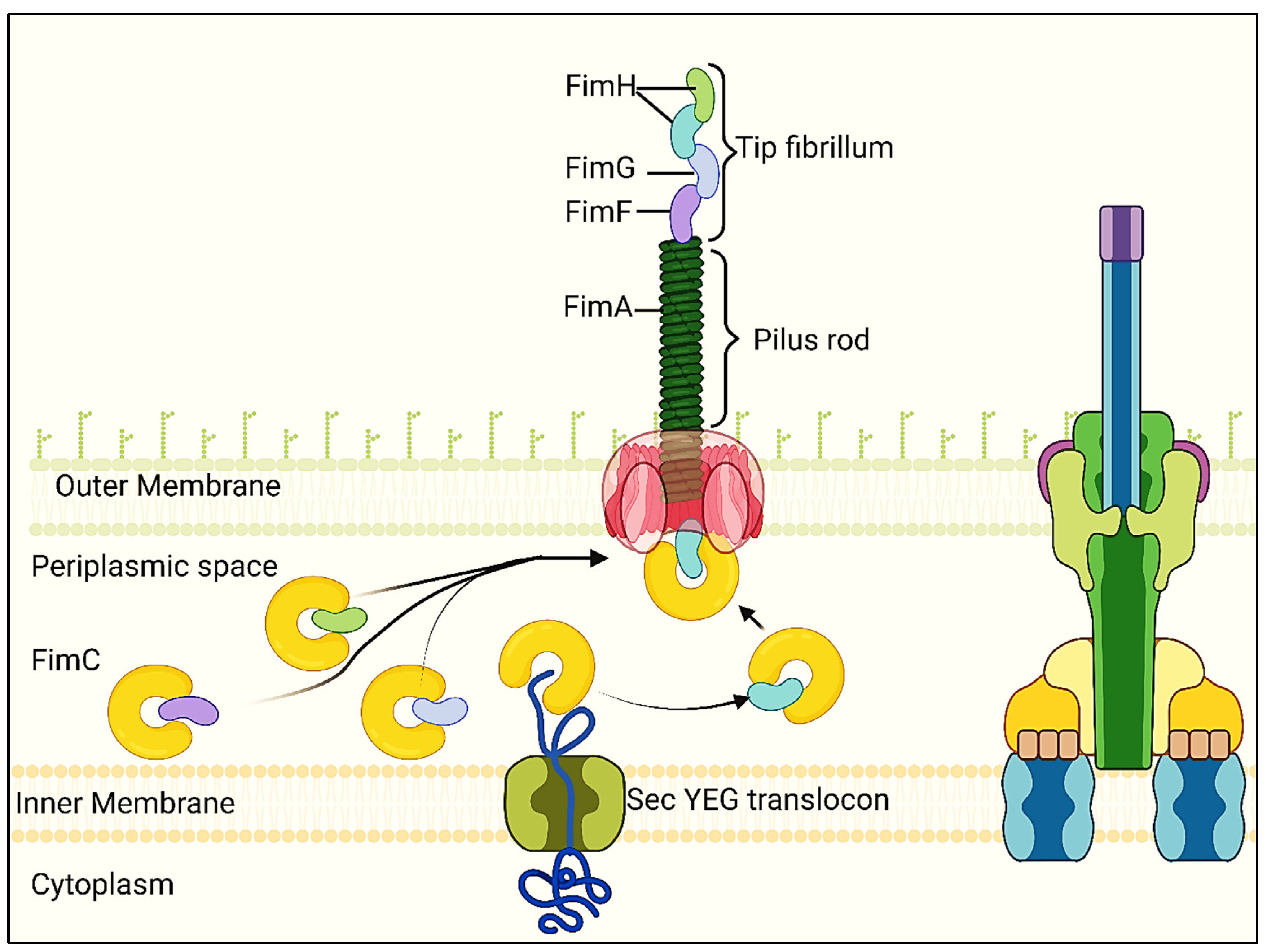
Type 1 fimbriae assemble according to the chaperone-usher route. Signal peptides are present in all proteins needed for assembly. In the periplasm, FimC acts as a chaperone for FimA, FimF, and FimH, avoiding premature polymerization and aiding in folding and assembly. Hydrophobic N- and C-terminal extensions of FimA, FimF, and FimH interact with a corresponding hydrophobic groove in FimC. FimD is an example of an outer-membrane usher protein that facilitates the assembly of fimbrial subunits by facilitating the passage of fimbrial proteins through the outer membrane. Donor strand exchange is the mechanism by which the proteins that makeup T1F are joined together via N- and C-terminal extensions (Remaut et al., 2006).
When the FimC-FimH complex binds to the FimD usher protein, T1F begins to assemble as shown in
Figure 7. The FimC-FimF complex is then moved into the FimD pocket, where the FimF N-terminal extension replaces the FimC linked to the FimH C-terminal extension, forming the FimH-FimF complex (Kolenda et al., 2019). FimA is the next step in this process, which results in the fimbrial shaft extending even farther. The lack of fimbriae is caused by deleting any of the fimA, fimF, or fimH genes, highlighting their combined function in pilus formation (Zeiner, 2012). Though the exact process is yet unknown, there is conjecture that fimI is involved in controlling fimbrial length and adhesion (Rossolini et al., 1993).
Table 1.
The Table shows the various virulence factors of S. Typhi that can be targeted for vaccine development and the roles they play.
Table 1.
The Table shows the various virulence factors of S. Typhi that can be targeted for vaccine development and the roles they play.
| |
Virulence factor |
Function |
| 1 |
Vi antigen |
Inhibit phagocytosis and complement binding (Robbins & Robbins, 1984) |
| 2 |
Somatic O antigen (LPS) |
It produces OMPs, which are immunogenic (Benz, 1988) |
| 3 |
H antigen - Flagella |
Flagella activate innate immune responses by engaging with TLR5 and NAIP receptors through the detection of monomeric flagellin (Hayashi et al., 2001; Kortmann et al., 2015) |
| 4 |
Fimbriae and Pili |
Essential adhesion elements that aid in numerous cellular contacts during infection and host colonization (Berrocal et al., 2015) |
| 5 |
Salmonella Virulence Plasmid |
SVP is necessary for reticuloendothelial system bacteria multiplication (Rotger & Casadesús, 1999) |
| 6 |
Invasiveness |
S. Typhi proteins to defeat both oxygen-dependent and oxygen-independent phagocytic cell defenses is what allows the pathogen to survive in macrophages (Achouri et al., 2015) |
| 7 |
Biofilm formation |
Proteinaceous compounds produced by biofilm cells enable synergistic growth and protect potentially harsh surroundings like acidity (Corcoran, 2013) |
| 8 |
Endotoxin |
Lethal toxicity, pyrogenicity, and tissue necrotizing activity are among the harmful effects of S. Typhi endotoxin (Mahamuni & Ghosh, 2017) |
|
SPI-1 associated T3SS effector proteins: modified from (Phoebe & Lee, 2001) |
| 9 |
SipA |
Rearrangement of cytoskeleton in host cells |
| |
SipB |
Actin nucleation and translocation of other effector proteins via T3SS |
| 10 |
SipC |
Translocate other effectors |
| 11 |
SopA |
Fluid secretion in host gut which leads to diarrhea |
| 12 |
SopB |
Involved in cytoskeleton rearrangement, neutrophils recruitment, and fluid secretion as in SopA |
| 13 |
SopC and SopD |
Neutrophils recruitment and fluid secretion |
| 14 |
SopE and SptP |
Cytoskeleton rearrangement and suppression of innate immunity |
|
SPI-2 associated T3SS effector proteins: modified from (Waterman & Holden, 2003) |
| 15 |
SpiC |
Alter vesicular transport in host cell |
| 16 |
SseF and SseG |
Helps in Salmonella-induced filament formation |
| 17 |
SifA and SseJ |
Salmonella – containing vacuole membrane integrity |
| 18 |
SifB |
Targeting to Salmonella induced filaments |
| 19 |
SspH2 and SseI |
Rearrangement of host cells cytoskeleton |
| 20 |
SrfT |
Results in cell death (Apoptosis) |
| 21 |
ttr genes |
Located on SPI-2 and responsible for producing tetrathionate reductase which serve as terminal electron acceptor in anaerobic conditions (Fàbrega & Vila, 2013) |
| 22 |
SseB, SseC, SseD |
They are located on SPI-2 and are all responsible for the formation of macromolecular structures which serves as translocon in T3SS system (Lan et al., 2008) |
| 23 |
MisL |
Located on SPI-3 and responsible for long term persistence in host cells (Dorsey et al., 2005) |
| 24 |
MgtCB |
Helps the bacteria to survive within Macrophages. Also helps regulate magnesium homeostasis (Blanc-Potard, 1997) |
| 25 |
SiiE |
Found on SPI-4 and responsible for adhesion to epithelium (Gerlach et al., 2007) |
| 26 |
SopB |
Located on SPI-5 and prevents apoptosis of epithelial cells. This allows S. Typhi to establish a stable intracellular niche within the host cells (Knodler et al., 2002) |
| 27 |
SpvR |
Found on pSLT Plasmid of S. Typhi and is controls the expression of spv operon which have several genes involved in virulence (Guiney & Fierer, 2011) |
| 28 |
SpvB |
An effector protein that prevents actin polymerization in host cells (Guiney & Fierer, 2011) |
| 29 |
SpvC |
Inhibits mitogen-activated protein kinase and immune signaling (Silva et al., 2017) |
DRUGS USED TO TREAT TYPHOID FEVER
Antimicrobial resistance to the first-line medicines used to treat enteric fever is a concern that makes managing the condition more difficult even though some drugs show some level of effectiveness against Typhoid fever(Parry et al., 2002b). These drugs may be bactericidal (kill the bacteria) or bacteriostatic (inhibit growth), which help to treat (Ocampo et al., 2014).
Table 2.
The Table below indicate some drugs used against S. Typhi.
Table 2.
The Table below indicate some drugs used against S. Typhi.
| DRUG |
GROUP |
MODE OF ACTION |
REFERENCE |
| Chloramphenicol |
Chloraphenicols |
It attaches to the 50S ribosomal subunit, thus inhibiting protein synthesis, leading to the bacteria's death. |
(Dinos et al., 2016) |
| Amoxicillin (an amino-penicillin) |
Penicillin antibiotics |
It attaches the β-lactam ring to Penicillin-Binding Proteins (PBPs), which hinder the cross-linking procedure (transpeptidation), and activate autolytic enzymes to prevent the production of the bacterial cell wall. The bacterial cell wall is compromised by this disturbance, which results in cell lysis and, eventually, bacterial annihilation. |
(Bernatová et al., 2013) |
| Co-trimoxazole (Trimethoprim-Sulfamethoxazole,TMP-SMX) |
Sulfonamide
antibiotics |
By competitively inhibiting bacterial dihydropteroate synthetase, SMX prevents the incorporation of p-aminoben zoic acid into dihydrofolic acid. TMP prevents the biologically active cofactor for producing purines, thymidine, and DNA, dihydrofolate reductase, from converting dihydrofolate to tetrahydrofolic acid. |
(Smilack, 1999) |
| Ampicillin |
Penicillin antibiotics |
The drug binds to and suppresses membrane-bound penicillin-binding proteins, which are critical players in synthesizing cell wall peptidoglycan.
The integrity of the cell wall, which exists in a hypotonic environment, is maintained by peptidoglycan; when it is disrupted, lysis and cell death result. |
(Ghooi & Thatte, 1995; Peechakara & Gupta, 2023) |
| Azithromycin |
Macrolides |
It lowers the formation of biofilm and mucus, inhibits bacterial quorum sensing, and broadens the spectrum of its antibacterial effects.
Additionally, it blocks the formation of the 50S big ribosomal subunit and the expansion of the nascent polypeptide chain by attaching to them and interfering with their functions. |
(Champney & Burdine, 1998; Parnham et al., 2014) |
| Clarithromycin |
Macrolides |
It causes the gastrointestinal pathogen S. Typhi to produce less of the rdar biofilm activator CsgD and consistently downregulate the biofilm-related genes csgB and adrA transcription. |
(Zafar et al., 2020) |
| Ceftriaxone |
Cephalosporins |
It binds to one or more penicillin-binding proteins and prevents the final trans-peptidoglycan step during peptidoglycan synthesis in bacterial cell walls. This prevents production and stops the sequencing of cell wall elaboration during bacterial cell death. |
(Hartman et al., 2021) |
| Cefixime |
Cephalosporins |
It is a third-generation cephalosporin antibiotic having bactericidal activity. It works by inhibiting penicillin-binding proteins, which damages the peptidoglycan production pathway and the bacterial cell wall. |
(Ramdhani et al., 2021) |
| Aztreonam |
Monobactams |
It was discovered to interact with specific penicillin-binding proteins of bacteria, preventing the formation of bacterial cell walls. This reduces the virulence of the bacteria imposed by the cell wall. |
(Sykes & Bonner, 1985) |
| Ciprofloxacin |
Fluoroquinolones |
Influence DNA metabolism by preventing DNA topoisomerase and DNA gyrase from functioning, which prevents S. Typhi cell replication. |
(Campoli-Richards et al., 1988) |
| Ofloxacin |
Fluoroquinolones |
It significantly reduces DNA gyrase's ability to form supercoils, disrupting bacterial DNA processes. This is detrimental to bacterial growth and results in the death of the bacterial cell. |
(Sato et al., 1986) |
| Levofloxacin |
Fluoroquinolones |
It encourages DNA strand breaks by preventing DNA-gyrase in susceptible organisms, which prevents supercoiled DNA from relaxing. |
(Podder & Sadiq, 2023) |
| Moxifloxacin |
Fluoroquinolones |
It binds to the DNA gyrase and topoisomerase IV in bacteria, preventing DNA replication, repair, and transcription. |
(Saravolatz & Leggett, 2003) |
Secreted Virulence Factors of S. Typhi
The presence of bacterial exotoxins can play a significant role in the development and maintenance of a disease. In S. Typhi, for instance, the toxin is known to play a role in patients' symptoms and chronic infection. Multiple strategies can be used to prevent the toxin's action during an infection.
One of the most effective ways to prevent the spread of a disease is by using therapeutic monoclonal antibodies (mAbs). These are highly specific to the target toxins and can only be used to attack them without affecting the beneficial microbes and host cells. For instance, the toxin known as Shiga-toxin IIB is responsible for the organ damage caused by gastrointestinal bleeding and hemorrhagic colitis in patients infected with EHEC. MAbs designed to neutralize Shiga toxin prevented the infection's early onset in healthy volunteers. Moreover, these drugs were well-tolerated in subjects with no apparent side effects. In addition, these methods can be utilized against other AB toxins, such as ricin, anthrax, and botulinum. In convalescent typhoid patients, antibodies related to the CdtB toxin were detected in their sera. In a study, the researchers noted that mice given typhoid toxoid had high levels of anti-CddtB antibodies. These antibodies protected the animals from a lethal dose of the active toxin. Based on these findings, it was concluded that developing therapeutic mAbs designed to target the toxin could be a promising strategy for treating typhoid. This could be especially useful when there are increasing cases of antibiotic-resistant strains (Y.-A. Yang et al., 2018).
Among the promising strategies for tackling bacterial exotoxins is one that involves the interaction between the toxins and their host cells. This could result in minimal or no toxin delivery. Most bacterial AB toxins such as Shiga-toxin IIB (Stx2B), exotoxin by E. coli O157:H7, which causes organ damage and hemolytic uremic syndrome, including those related to typhoid, use a type of glycan. In a study, the researchers discovered that a higher affinity glycan can be useful in treating typhoid by interacting with the toxin's B subunit, which has five binding pockets. In another study, the researchers discovered that a higher-affinity version of the carbohydrate receptor of Shiga-like toxins inhibited the clinical symptoms of the disease. High affinity glycans that have been terminated with Neu5Ac effectively compete with natural ligands and prevent certain biological processes, such as axon outgrowth (Y.-A. Yang et al., 2018) In addition, small molecules that can prevent the endocytosis of toxins can be used to prevent their exposure to host cells (Wu et al., 2017).
These findings suggest that the development of membrane trafficking agents can be combined with other drugs to enhance the degradation of toxins. For instance, researchers discovered that specific small molecules can be used as transport inhibitors. They were able to block the transport of Cholera and Shiga toxin. These studies show the potential of small cell trafficking inhibitors to treat typhoid.
Conclusions
The spread of multidrug-resistant (MDR), extensively drug-resistant S. Typhi poses a significant threat to human health. Although the development of vaccines has dramatically improved the protection against this disease, it continues to spread. This is because there are no effective strategies to control the disease in populations usually asymptomatic for long periods. Understanding how S. Typhi causes chronic and persistent infections is essential to eradicating this disease. Currently, there are several types of anti-S. Typhi drugs in market and in clinical trials. The development of safer and more effective anti- S. Typhi drugs is urgently needed. The pathways that are involved in the pathogenesis, development, and maturation of S. Typhi, such as protein synthesis, S. Typhi structures involved in attachment, and transcriptional factors involved in transcription, fimbriae biogenesis, flagella, the S. Typhi secretion systems have been identified as promising targets for developing anti- S. Typhi drugs. Targeting persistent S. Typhi has been regarded as a major focus area of research. The development of new and safer anti- S. Typhi drugs will require finding more effective and reasonable targets. Currently, there are still a lot of unanswered questions regarding the development of these drugs. This review summarizes current knowledge of S. Typhi that could help develop new vaccines and other treatment methods against this pathogen.
References
- Achouri, S., Wright, J. A., Evans, L., Macleod, C., Fraser, G., Cicuta, P., & Bryant, C. E. (2015). The frequency and duration of Salmonella –macrophage adhesion events determines infection efficiency. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1661), 20140033. [CrossRef]
- Ajay Kalra, & Premasish Mazumdar. (2010). Journal of Pediatric Sciences. Typhoid Vaccines - Newer Developments , 5-e52.
- Andersen, T. K., Huszthy, P. C., Gopalakrishnan, R. P., Jacobsen, J. T., Fauskanger, M., Tveita, A. A., Grødeland, G., & Bogen, B. (2019). Enhanced germinal center reaction by targeting vaccine antigen to major histocompatibility complex class II molecules. Npj Vaccines, 4(1), 9. [CrossRef] [PubMed]
- Arockiasamy, A., & Krishnaswamy, S. (1995). Prediction of B-cell epitopes forSalmonella typhi OmpC. Journal of Biosciences, 20(2), 235–243. [CrossRef]
- Arockiasamy, A., & Krishnaswamy, S. (2000). Homology Model of Surface Antigen OmpC From Salmonella typhi and its Functional Implications. Journal of Biomolecular Structure and Dynamics, 18(2), 261–271. [CrossRef] [PubMed]
- Bajaj, V., Hwang, C., & Lee, C. A. (1995). hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Molecular Microbiology, 18(4), 715–727. [CrossRef] [PubMed]
- Benz, R. (1988). Structure and Function of Porins from Gram-Negative Bacteria. Annual Review of Microbiology, 42(1), 359–393. [CrossRef]
- Bernatová, S., Samek, O., Pilát, Z., Šerý, M., Ježek, J., Jákl, P., Šiler, M., Krzyžánek, V., Zemánek, P., Holá, V., Dvořáčková, M., & Růžička, F. (2013). Following the Mechanisms of Bacteriostatic versus Bactericidal Action Using Raman Spectroscopy. Molecules, 18(11), 13188–13199. [CrossRef] [PubMed]
- Berrocal, L., Fuentes, J. A., Trombert, A. N., Jofré, M. R., Villagra, N. A., Valenzuela, L. M., & Mora, G. C. (2015). stg fimbrial operon from S. Typhi STH2370 contributes to association and cell disruption of epithelial and macrophage-like cells. Biological Research, 48(1), 34. [CrossRef] [PubMed]
- Bharat Biotech. (n.d.). About Typbar TCV. Journal of Clinical Infectious Diseases.
- Bhutta, Z. A., Capeding, M. R., Bavdekar, A., Marchetti, E., Ariff, S., Soofi, S. B., Anemona, A., Habib, M. A., Alberto, E., Juvekar, S., Khan, R. M. Q., Marhaba, R., Ali, N., Malubay, N., Kawade, A., Saul, A., Martin, L. B., & Podda, A. (2014). Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. The Lancet Infectious Diseases, 14(2), 119–129. [CrossRef]
- Blanc-Potard, A.-B. (1997). The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. The EMBO Journal, 16(17), 5376–5385. [CrossRef]
- Booth, J. S., Patil, S. A., Goldberg, E., Barnes, R. S., Greenwald, B. D., & Sztein, M. B. (2019). Attenuated Oral Typhoid Vaccine Ty21a Elicits Lamina Propria and Intra-Epithelial Lymphocyte Tissue-Resident Effector Memory CD8 T Responses in the Human Terminal Ileum. Frontiers in Immunology, 10. [CrossRef] [PubMed]
- Brewer, S. M., Twittenhoff, C., Kortmann, J., Brubaker, S. W., Honeycutt, J., Massis, L. M., Pham, T. H. M., Narberhaus, F., & Monack, D. M. (2021). A Salmonella Typhi RNA thermosensor regulates virulence factors and innate immune evasion in response to host temperature. PLOS Pathogens, 17(3), e1009345. [CrossRef] [PubMed]
- Buchmeier, N., Bossie, S., Chen, C.-Y., Fang, F. C., Guiney, D. G., & Libby, S. J. (1997). SlyA, a Transcriptional Regulator of Salmonella typhimurium, Is Required for Resistance to Oxidative Stress and Is Expressed in the Intracellular Environment of Macrophages. In INFECTION AND IMMUNITY (Vol. 65, Issue 9). https://journals.asm.org/journal/iai.
- 16. Campoli-Richards, D. M., Monk, J. P., Price, A., Benfield, P., Todd, P. A., & Ward, A. (1988). Ciprofloxacin. Drugs, 35(4), 373–447. [CrossRef]
- Chakraborty, S., & Kenney, L. J. (2018). A new role of OmpR in acid and osmotic stress in salmonella and E. coli. Frontiers in Microbiology, 9(NOV). [CrossRef] [PubMed]
- Chakraborty, S., Winardhi, R. S., Morgan, L. K., Yan, J., & Kenney, L. J. (2017). Non-canonical activation of OmpR drives acid and osmotic stress responses in single bacterial cells. Nature Communications, 8(1), 1587. [CrossRef] [PubMed]
- Champney, W. S., & Burdine, R. (1998). Azithromycin and Clarithromycin Inhibition of 50S Ribosomal Subunit Formation in Staphylococcus aureus Cells. Current Microbiology, 36(2), 119–123. [CrossRef] [PubMed]
- Chatfield, S. N., Dorman, C. J., Hayward, C., & Dougan, G. (1991). Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both ompC and ompF are attenuated in vivo. Infection and Immunity, 59(1), 449–452. [CrossRef] [PubMed]
- Chou, H.-H., Hayakawa, T., Diaz, S., Krings, M., Indriati, E., Leakey, M., Paabo, S., Satta, Y., Takahata, N., & Varki, A. (2002). Inactivation of CMP- N -acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proceedings of the National Academy of Sciences, 99(18), 11736–11741. [CrossRef] [PubMed]
- Chowdhury, R., Das, S., Ta, A., & Das, S. (2018). Epithelial invasion by <scp> Salmonella Typhi </scp> using <scp>STIV</scp> – <scp>Met</scp> interaction. Cellular Microbiology, e12982. [CrossRef]
- Cohen, S. P., McMurry, L. M., Hooper, D. C., Wolfson, J. S., & Levy, S. B. (1989). Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrobial Agents and Chemotherapy, 33(8), 1318–1325. [CrossRef]
- Confer, A. W., & Ayalew, S. (2013). The OmpA family of proteins: Roles in bacterial pathogenesis and immunity. Veterinary Microbiology, 163(3–4), 207–222. https://doi.org/10.1016/j.vetmic.2012.08.019 Authors. [CrossRef]
- Corcoran, M. (2013). Title Salmonella enterica-biofilm formation and survival of disinfection treatment on food contact surfaces. http://hdl.handle.net/10379/3515.
- Crump, J. A., Sjölund-Karlsson, M., Gordon, M. A., & Parry, C. M. (2015a). Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. In Clinical Microbiology Reviews (Vol. 28, Issue 4, pp. 901–937). American Society for Microbiology. [CrossRef]
- Crump, J. A., Sjölund-Karlsson, M., Gordon, M. A., & Parry, C. M. (2015b). Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. In Clinical Microbiology Reviews (Vol. 28, Issue 4, pp. 901–937). American Society for Microbiology. [CrossRef] [PubMed]
- Darwin, K. H., & Miller, V. L. (1999). Molecular Basis of the Interaction of Salmonella with the Intestinal Mucosa. Clinical Microbiology Reviews, 12(3), 405–428. [CrossRef]
- Das, S., Chowdhury, R., Pal, A., Okamoto, K., & Das, S. (2019). Salmonella Typhi outer membrane protein STIV is a potential candidate for vaccine development against typhoid and paratyphoid fever. Immunobiology, 224(3), 371–382. [CrossRef] [PubMed]
- de Nies, L., Lopes, S., Busi, S. B., Galata, V., Heintz-Buschart, A., Laczny, C. C., May, P., & Wilmes, P. (2021). PathoFact: a pipeline for the prediction of virulence factors and antimicrobial resistance genes in metagenomic data. Microbiome, 9(1). [CrossRef]
- Dinos, G., Athanassopoulos, C., Missiri, D., Giannopoulou, P., Vlachogiannis, I., Papadopoulos, G., Papaioannou, D., & Kalpaxis, D. (2016). Chloramphenicol Derivatives as Antibacterial and Anticancer Agents: Historic Problems and Current Solutions. Antibiotics, 5(2), 20. [CrossRef]
- Dong, T., & Schellhorn, H. E. (2010). Role of RpoS in Virulence of Pathogens. Infection and Immunity, 78(3), 887–897. [CrossRef]
- Dorsey, C. W., Laarakker, M. C., Humphries, A. D., Weening, E. H., & Bäumler, A. J. (2005). Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Molecular Microbiology, 57(1), 196–211. [CrossRef]
- dos Santos, A. M. P., Ferrari, R. G., & Conte-Junior, C. A. (2019). Virulence Factors in Salmonella Typhimurium: The Sagacity of a Bacterium. In Current Microbiology (Vol. 76, Issue 6, pp. 762–773). Springer New York LLC. [CrossRef]
- Edsall, G., Gaines, S., Landy, M., Tigertt, W. D., Sprinz, H., Trapani, R.-J., Mandel, A. D., & Benenson, A. S. (1960). STUDIES ON INFECTION AND IMMUNITY IN EXPERIMENTAL TYPHOID FEVER. The Journal of Experimental Medicine, 112(1), 143–166. [CrossRef] [PubMed]
- Ellermeier, C. D., & Slauch, J. M. (2003). RtsA and RtsB Coordinately Regulate Expression of the Invasion and Flagellar Genes in Salmonella enterica Serovar Typhimurium. Journal of Bacteriology, 185(17), 5096–5108. [CrossRef]
- Fàbrega, A., & Vila, J. (2013). Salmonella enterica Serovar Typhimurium Skills To Succeed in the Host: Virulence and Regulation. Clinical Microbiology Reviews, 26(2), 308–341. [CrossRef]
- Findlay, H. E., McClafferty, H., & Ashley, R. H. (2005). Surface expression, single-channel analysis and membrane topology of recombinant Chlamydia trachomatis Major Outer Membrane Protein. BMC Microbiology, 5(1), 5. [CrossRef]
- Fiorino, F., Ciabattini, A., Rondini, S., Pozzi, G., Martin, L. B., & Medaglini, D. (2012). Immunization with the conjugate vaccine Vi-CRM197 against Salmonella Typhi induces Vi-specific mucosal and systemic immune responses in mice. Vaccine, 30(43), 6111–6114. [CrossRef]
- Foster, J. W. (1991). Salmonella acid shock proteins are required for the adaptive acid tolerance response. Journal of Bacteriology, 173(21), 6896–6902. [CrossRef] [PubMed]
- Freeman, T. C., Landry, S. J., & Wimley, W. C. (2011). The prediction and characterization of YshA, an unknown outer-membrane protein from Salmonella typhimurium. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1808(1), 287–297. [CrossRef]
- García Véscovi, E., Soncini, F. C., & Groisman, E. A. (1994). The role of the PhoP/PhoQ regulon in Salmonella virulence. Research in Microbiology, 145(5–6), 473–480. [CrossRef]
- Gerlach, R. G., Jäckel, D., Stecher, B., Wagner, C., Lupas, A., Hardt, W.-D., & Hensel, M. (2007). Salmonella Pathogenicity Island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cellular Microbiology, 9(7), 1834–1850. [CrossRef]
- Ghooi, R. B., & Thatte, S. M. (1995). Inhibition of cell wall synthesis — is this the mechanism of action of penicillins? Medical Hypotheses, 44(2), 127–131. [CrossRef]
- Guiney, D. G., & Fierer, J. (2011). The Role of the spv Genes in Salmonella Pathogenesis. Frontiers in Microbiology, 2. [CrossRef] [PubMed]
- Hahn, E., Wild, P., Hermanns, U., Sebbel, P., Glockshuber, R., Häner, M., Taschner, N., Burkhard, P., Aebi, U., & Müller, S. A. (2002). Exploring the 3D Molecular Architecture of Escherichia coli Type 1 Pili. Journal of Molecular Biology, 323(5), 845–857. [CrossRef]
- Hansen-Wester, I., & Hensel, M. (2001). Salmonella pathogenicity islands encoding type III secretion systems. Microbes and Infection, 3(7), 549–559. [CrossRef] [PubMed]
- Haque, S., Swami, P., & Khan, A. (2021). S. Typhi derived vaccines and a proposal for outer membrane vesicles (OMVs) as potential vaccine for typhoid fever. Microbial Pathogenesis, 158, 105082. [CrossRef] [PubMed]
- Haraga, A., Ohlson, M. B., & Miller, S. I. (2008). Salmonellae interplay with host cells. Nature Reviews Microbiology, 6(1), 53–66. [CrossRef] [PubMed]
- Hartman, S. J. F., Upadhyay, P. J., Hagedoorn, N. N., Mathôt, R. A. A., Moll, H. A., van der Flier, M., Schreuder, M. F., Brüggemann, R. J., Knibbe, C. A., & de Wildt, S. N. (2021). Current Ceftriaxone Dose Recommendations are Adequate for Most Critically Ill Children: Results of a Population Pharmacokinetic Modeling and Simulation Study. Clinical Pharmacokinetics, 60(10), 1361–1372. [CrossRef] [PubMed]
- Haselbeck, A. H., Tadesse, B. T., Park, J., Gibani, M. M., Espinoza, L. M. C., Abreu, A., Van Rensburg, C., Owusu-Ansah, M., Twuamsi-Ankrah, S., Owusu, M., Aguna, I., Picot, V., Jeon, H., Higginson, E., Park, S., Mojares, Z. R., Im, J., Carey, M. E., Khanam, F., … Marks, F. (2021). Evaluation of Typhoid Conjugate Vaccine Effectiveness in Ghana (TyVEGHA) Using a Cluster-Randomized Controlled Phase IV Trial: Trial Design and Population Baseline Characteristics. Vaccines, 9(3), 281. [CrossRef] [PubMed]
- Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., Eng, J. K., Akira, S., Underhill, D. M., & Aderem, A. (2001). The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature, 410(6832), 1099–1103. [CrossRef] [PubMed]
- Hengge-Aronis, R. (2002). Signal Transduction and Regulatory Mechanisms Involved in Control of the σ S (RpoS) Subunit of RNA Polymerase. Microbiology and Molecular Biology Reviews, 66(3), 373–395. [CrossRef]
- Hessel, L., Debois, H., Fletcher, M., & Dumas, R. (1999). Experience With Salmonella typhi Vi Capsular Polysaccharide Vaccine. European Journal of Clinical Microbiology & Infectious Diseases, 18(9), 609–620. [CrossRef]
- Hong, H., Szabo, G., & Tamm, L. K. (2006). Electrostatic couplings in OmpA ion-channel gating suggest a mechanism for pore opening. Nature Chemical Biology, 2(11), 627–635. [CrossRef] [PubMed]
- Hueck, C. J. (1998). Type III Protein Secretion Systems in Bacterial Pathogens of Animals and Plants. Microbiology and Molecular Biology Reviews, 62(2), 379–433. [CrossRef] [PubMed]
- Hurtado-Escobar, G. A., Grépinet, O., Raymond, P., Abed, N., Velge, P., & Virlogeux-Payant, I. (2019). H-NS is the major repressor of Salmonella Typhimurium Pef fimbriae expression. Virulence, 10(1), 849–867. [CrossRef] [PubMed]
- Immunol, V. J., Singh, Y., Saxena, A., Kumar, R., Bhatt, P., Saxena, M., & Kumar Saxena, M. (n.d.). Virology & Immunology Journal Immunogenic Outer membrane Proteins (Omps) of Salmonella: Potential Candidate for sub-unit vaccine Immunogenic Outer membrane Proteins (Omps) of Salmonella: Potential Candidate for sub-unit vaccine.
- yoda, S., Kamidoi, T., Hirose, K., Kutsukake, K., & Watanabe, H. (2001). A flagellar gene regulates the expression of invasion genes and virulence phenotype in serovar Typhimurium. Microbial Pathogenesis, 30(2), 81–90. [CrossRef] [PubMed]
- Jha, V., & Janoff, E. N. (2019). Complementary Role of CD4+ T Cells in Response to Pneumococcal Polysaccharide Vaccines in Humans. Vaccines, 7(1), 18. [CrossRef] [PubMed]
- Jin, C., Hill, J., Gunn, B. M., Yu, W.-H., Dahora, L. C., Jones, E., Johnson, M., Gibani, M. M., Spreng, R. L., Alam, S. M., Nebykova, A., Juel, H. B., Dennison, S. M., Seaton, K. E., Fallon, J. K., Tomaras, G. D., Alter, G., & Pollard, A. J. (2021). Vi-specific serological correlates of protection for typhoid fever. Journal of Experimental Medicine, 218(2). [CrossRef] [PubMed]
- Jones, B. D. (2005). Salmonella Invasion Gene Regulation: A Story of Environmental Awareness. In The Journal of Microbiology (Vol. 43).
- Jossi, S. E., Arcuri, M., Alshayea, A., Persaud, R. R., Marcial-Juárez, E., Palmieri, E., Di Benedetto, R., Pérez-Toledo, M., Pillaye, J., Channell, W. M., Schager, A. E., Lamerton, R. E., Cook, C. N., Goodall, M., Haneda, T., Bäumler, A. J., Jackson-Jones, L. H., Toellner, K.-M., MacLennan, C. A., … Cunningham, A. F. (2023). Vi polysaccharide and conjugated vaccines afford similar early, IgM or IgG-independent control of infection but boosting with conjugated Vi vaccines sustains the efficacy of immune responses. Frontiers in Immunology, 14. [CrossRef] [PubMed]
- Khalid, S., Bond, P. J., Carpenter, T., & Sansom, M. S. P. (2008). OmpA: Gating and dynamics via molecular dynamics simulations. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1778(9), 1871–1880. [CrossRef] [PubMed]
- Khanam, F., Ross, A. G., McMillan, N. A. J., & Qadri, F. (2022). Toward Typhoid Fever Elimination. In International Journal of Infectious Diseases (Vol. 119, pp. 41–43). Elsevier B.V. [CrossRef]
- Knight, S. D., & Bouckaert, J. (2009). Structure, Function, and Assembly of Type 1 Fimbriae (pp. 67–107). [CrossRef]
- Knodler, L. A., Celli, J., Hardt, W.-D., Vallance, B. A., Yip, C., & Finlay, B. B. (2002). Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Molecular Microbiology, 43(5), 1089–1103. [CrossRef]
- Kolenda, R., Ugorski, M., & Grzymajlo, K. (2019). Everything You Always Wanted to Know About Salmonella Type 1 Fimbriae, but Were Afraid to Ask. Frontiers in Microbiology, 10. [CrossRef] [PubMed]
- Kortmann, J., Brubaker, S. W., & Monack, D. M. (2015). Cutting Edge: Inflammasome Activation in Primary Human Macrophages Is Dependent on Flagellin. The Journal of Immunology, 195(3), 815–819. [CrossRef] [PubMed]
- Krishnan, S., & Prasadarao, N. V. (2012). Outer membrane protein A and OprF: versatile roles in Gram-negative bacterial infections. FEBS Journal, 279(6), 919–931. [CrossRef]
- Lan, Y., Wang, S., Yin, Y., Hoffmann, W. C., & Zheng, X. (2008). Using a Surface Plasmon Resonance Biosensor for Rapid Detection of Salmonella Typhimurium in Chicken Carcass. Journal of Bionic Engineering, 5(3), 239–246. [CrossRef]
- Leclerc, J. M., Dozois, C. M., & Daigle, F. (2013). Role of the Salmonella enterica serovar Typhi Fur regulator and small RNAs RfrA and RfrB in iron homeostasis and interaction with host cells. Microbiology (United Kingdom), 159(PART3), 591–602. [CrossRef]
- Lee, C. A., Jones, B. D., & Falkow, S. (1992). Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proceedings of the National Academy of Sciences, 89(5), 1847–1851. [CrossRef] [PubMed]
- Levine, M. M., Ferreccio, C., Black, R. E., Tacket, C. O., & Germanier, R. (1989). Progress in Vaccines Against Typhoid Fever. Clinical Infectious Diseases, 11(Supplement_3), S552–S567. [CrossRef] [PubMed]
- Libby, S. J., Lesnick, M., Hasegawa, P., Weidenhammer, E., & Guiney, D. G. (2000). The salmonella virulence plasmid spv genes are required for cytopathology in human. Cellular Microbiology, 2(1), 49–58. [CrossRef]
- Lin, F. Y. C., Ho, V. A., Khiem, H. B., Trach, D. D., Bay, P. Van, Thanh, T. C., Kossaczka, Z., Bryla, D. A., Shiloach, J., Robbins, J. B., Schneerson, R., Szu, S. C., Lanh, M. N., Hunt, S., Trinh, L., & Kaufman, J. B. (2001a). The Efficacy of a Salmonella typhi Vi Conjugate Vaccine in Two-to-Five-Year-Old Children. New England Journal of Medicine, 344(17), 1263–1269. [CrossRef]
- Lin, F. Y. C., Ho, V. A., Khiem, H. B., Trach, D. D., Bay, P. Van, Thanh, T. C., Kossaczka, Z., Bryla, D. A., Shiloach, J., Robbins, J. B., Schneerson, R., Szu, S. C., Lanh, M. N., Hunt, S., Trinh, L., & Kaufman, J. B. (2001b). The Efficacy of a Salmonella typhi Vi Conjugate Vaccine in Two-to-Five-Year-Old Children. New England Journal of Medicine, 344(17), 1263–1269. [CrossRef]
- M Tsolis, R. E., Garry Adams, L., Ficht, T. A., & Ba, A. J. (1999). Contribution of Salmonella typhimurium Virulence Factors to Diarrheal Disease in Calves. In INFECTION AND IMMUNITY (Vol. 67, Issue 9). https://journals.asm.org/journal/iai.
- Mahamuni, P. P., & Ghosh, *. (2017). Proteolytic and lipolytic properties of endotoxins (enterotoxins) produced by Salmonella typhi NCIM 5255, Salmonella typhimurium NCIM 2501and Shigella flexneri NCIM 5265 Proteolytic and lipolytic properties of endotoxins (enterotoxins) produced by Salmonella typhi NCIM 5255, Salmonella typhimurium NCIM 2501 and Shigella flexneri NCIM 5265. In Article in International Food Research Journal (Vol. 24, Issue 6).
- Marlovits, T. C., Kubori, T., Sukhan, A., Thomas, D. R., Galán, J. E., & Unger, V. M. (2004). Structural Insights into the Assembly of the Type III Secretion Needle Complex. Science, 306(5698), 1040–1042. [CrossRef]
- Masuet-Aumatell, C., & Atouguia, J. (2021). Typhoid fever infection – Antibiotic resistance and vaccination strategies: A narrative review. In Travel Medicine and Infectious Disease (Vol. 40). Elsevier Inc. [CrossRef]
- Medise, B. E., Soedjatmiko, S., Gunardi, H., Sekartini, R., Satari, H. I., Hadinegoro, S. R., Wirahmadi, A., Puspita, M., Sari, R. M., Yang, J. S., Sil, A., Sahastrabuddhe, S., & Bachtiar, N. S. (2020). A novel Vi-diphtheria toxoid typhoid conjugate vaccine is safe and can induce immunogenicity in healthy Indonesian children 2–11 years: a phase II preliminary report. BMC Pediatrics, 20(1), 480. [CrossRef] [PubMed]
- Milligan, R., Paul, M., Richardson, M., & Neuberger, A. (2018). Vaccines for preventing typhoid fever. In Cochrane Database of Systematic Reviews (Vol. 2018, Issue 5). John Wiley and Sons Ltd. [CrossRef]
- Mitra, M., Shah, N., Ghosh, A., Chatterjee, S., Kaur, I., Bhattacharya, N., & Basu, S. (2016). Efficacy and safety of vi-tetanus toxoid conjugated typhoid vaccine (PedaTyphTM) in Indian children: School based cluster randomized study. Human Vaccines & Immunotherapeutics, 12(4), 939–945. [CrossRef]
- Mohan, V. K., Varanasi, V., Singh, A., Pasetti, M. F., Levine, M. M., Venkatesan, R., & Ella, K. M. (2015). Safety and Immunogenicity of a Vi Polysaccharide–Tetanus Toxoid Conjugate Vaccine (Typbar-TCV) in Healthy Infants, Children, and Adults in Typhoid Endemic Areas: A Multicenter, 2-Cohort, Open-Label, Double-Blind, Randomized Controlled Phase 3 Study. Clinical Infectious Diseases, 61(3), 393–402. [CrossRef]
- Ocampo, P. S., Lázár, V., Papp, B., Arnoldini, M., Abel zur Wiesch, P., Busa-Fekete, R., Fekete, G., Pál, C., Ackermann, M., & Bonhoeffer, S. (2014). Antagonism between Bacteriostatic and Bactericidal Antibiotics Is Prevalent. Antimicrobial Agents and Chemotherapy, 58(8), 4573–4582. [CrossRef] [PubMed]
- Parnham, M. J., Haber, V. E., Giamarellos-Bourboulis, E. J., Perletti, G., Verleden, G. M., & Vos, R. (2014). Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacology & Therapeutics, 143(2), 225–245. [CrossRef]
- Parry, C. M., Hien, T. T., Dougan, G., White, N. J., & Farrar, J. J. (2002a). Typhoid Fever. New England Journal of Medicine, 347(22), 1770–1782. [CrossRef]
- Parry, C. M., Hien, T. T., Dougan, G., White, N. J., & Farrar, J. J. (2002b). Typhoid Fever. New England Journal of Medicine, 347(22), 1770–1782. [CrossRef] [PubMed]
- Patel, P. D., Patel, P., Liang, Y., Meiring, J. E., Misiri, T., Mwakiseghile, F., Tracy, J. K., Masesa, C., Msuku, H., Banda, D., Mbewe, M., Henrion, M., Adetunji, F., Simiyu, K., Rotrosen, E., Birkhold, M., Nampota, N., Nyirenda, O. M., Kotloff, K., … Neuzil, K. M. (2021). Safety and Efficacy of a Typhoid Conjugate Vaccine in Malawian Children. New England Journal of Medicine, 385(12), 1104–1115. [CrossRef] [PubMed]
- Peechakara, B. V., & Gupta, M. (2023). Ampicillin/Sulbactam.
- Pennington, S. H., Thompson, A. L., Wright, A. K. A., Ferreira, D. M., Jambo, K. C., Wright, A. D., Faragher, B., Gilmour, J. W., Gordon, S. B., & Gordon, M. A. (2016). Oral Typhoid Vaccination With Live-Attenuated Salmonella Typhi Strain Ty21a Generates Ty21a-Responsive and Heterologous Influenza Virus–Responsive CD4 + and CD8 + T Cells at the Human Intestinal Mucosa. Journal of Infectious Diseases, 213(11), 1809–1819. [CrossRef]
- Phoebe Lostroh, C., & Lee, C. A. (2001). The Salmonella pathogenicity island-1 type III secretion system. Microbes and Infection, 3(14–15), 1281–1291. [CrossRef] [PubMed]
- Podder, V., & Sadiq, N. M. (2023). Levofloxacin.
- Puente, J., Juárez, D., Bobadilla, M., Arias, C. F., & Calva, E. (1995). The Salmonella ompC gene: Structure and use as a carrier for heterologous sequences. Gene, 156(1), 1–9. [CrossRef] [PubMed]
- Purcell, B. K., Pruckler, J., & Clegg, S. (1987). Nucleotide sequences of the genes encoding type 1 fimbrial subunits of Klebsiella pneumoniae and Salmonella typhimurium. Journal of Bacteriology, 169(12), 5831–5834. [CrossRef] [PubMed]
- Ramdhani, D., Kusuma, S. A. F., Sediana, D., Bima, A. P. H., & Khumairoh, I. (2021). Comparative study of cefixime and tetracycline as an evaluation policy driven by the antibiotic resistance crisis in Indonesia. Scientific Reports, 11(1), 18461. [CrossRef] [PubMed]
- Remaut, H., Rose, R. J., Hannan, T. J., Hultgren, S. J., Radford, S. E., Ashcroft, A. E., & Waksman, G. (2006). Donor-Strand Exchange in Chaperone-Assisted Pilus Assembly Proceeds through a Concerted β Strand Displacement Mechanism. Molecular Cell, 22(6), 831–842. [CrossRef]
- Robbins, J. D., & Robbins, J. B. (1984). Reexamination of the Protective Role of the Capsular Polysaccharide (Vi antigen) of Salmonella typhi. Journal of Infectious Diseases, 150(3), 436–449. [CrossRef]
- Rossolini, G. M., Muscas, P., Chiesurin, A., & Satta, G. (1993). Analysis of the Salmonella fim gene cluster: Identification of a new gene ( fimI ) encoding a fimbrin-like protein and located downstream from the fimA gene. FEMS Microbiology Letters, 114(3), 259–265. [CrossRef]
- Rotger, R., & Casadesús, J. (1999). The virulence plasmids of Salmonella. International Microbiology: The Official Journal of the Spanish Society for Microbiology, 2(3), 177–184.
- Sahastrabuddhe, S., & Saluja, T. (2019). Overview of the Typhoid Conjugate Vaccine Pipeline: Current Status and Future Plans. Clinical Infectious Diseases, 68, S22–S26. [CrossRef] [PubMed]
- Saini, S., Pearl, J. A., & Rao, C. V. (2009). Role of FimW, FimY, and FimZ in Regulating the Expression of Type I Fimbriae in Salmonella enterica Serovar Typhimurium. Journal of Bacteriology, 191(9), 3003–3010. [CrossRef] [PubMed]
- Santos, R. L., Tsolis, R. M., Bäumler, A. J., & Adams, L. G. (2003). Pathogenesis of Salmonella-induced enteritis. Brazilian Journal of Medical and Biological Research, 36(1), 03–12. [CrossRef] [PubMed]
- Saravolatz, L. D., & Leggett, J. (2003). Gatifloxacin, Gemifloxacin, and Moxifloxacin: The Role of 3 Newer Fluoroquinolones. Clinical Infectious Diseases, 37(9), 1210–1215. [CrossRef] [PubMed]
- Sato, K., Inoue, Y., Fujii, T., Aoyama, H., & Mitsuhashi, S. (1986). Antibacterial activity of ofloxacin and its mode of action. Infection, 14(S4), S226–S230. [CrossRef]
- Secundino, I., Lopez-Macias, C., Cervantes-Barragan, L., Gil-Cruz, C., Rios-Sarabia, N., Pastelin-Palacios, R., Villasis-Keever, M. A., Becker, I., Puente, J. L., Calva, E., & Isibasi, A. (2006). Salmonella porins induce a sustained, lifelong specific bactericidal antibody memory response. Immunology, 117(1), 59–70. [CrossRef]
- Shakya, M., Colin-Jones, R., Theiss-Nyland, K., Voysey, M., Pant, D., Smith, N., Liu, X., Tonks, S., Mazur, O., Farooq, Y. G., Clarke, J., Hill, J., Adhikari, A., Dongol, S., Karkey, A., Bajracharya, B., Kelly, S., Gurung, M., Baker, S., … Pollard, A. J. (2019). Phase 3 Efficacy Analysis of a Typhoid Conjugate Vaccine Trial in Nepal. New England Journal of Medicine, 381(23), 2209–2218. [CrossRef]
- Shams, N., Shakarami Gandabeh, Z., Nazifi, N., Forouharmehr, A., Jaydari, A., & Rashidian, E. (2020). Computational Design of Different Epitope-Based Vaccines Against Salmonella typhi. International Journal of Peptide Research and Therapeutics, 26(3), 1527–1539. [CrossRef]
- Sharma, R. K., Yolcu, E. S., Srivastava, A. K., & Shirwan, H. (2013). CD4+ T Cells Play a Critical Role in the Generation of Primary and Memory Antitumor Immune Responses Elicited by SA-4-1BBL and TAA-Based Vaccines in Mouse Tumor Models. PLoS ONE, 8(9), e73145. [CrossRef]
- Silva, C., Puente, J. L., & Calva, E. (2017). Salmonella virulence plasmid: pathogenesis and ecology. Pathogens and Disease, 75(6). [CrossRef]
- Smilack, J. D. (1999). Trimethoprim-Sulfamethoxazole. Mayo Clinic Proceedings, 74(7), 730–734. [CrossRef] [PubMed]
- Smit, J., Kamio, Y., & Nikaido, H. (1975). Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. Journal of Bacteriology, 124(2), 942–958. [CrossRef]
- Stoebel, D. M., Free, A., & Dorman, C. J. (2008). Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology (Reading, England), 154(Pt 9), 2533–2545. [CrossRef]
- Stone, A. L., & Szu, S. C. (1988). Application of optical properties of the Vi capsular polysaccharide for quantitation of the Vi antigen in vaccines for typhoid fever. Journal of Clinical Microbiology, 26(4), 719–725. [CrossRef]
- Sundara Baalaji, N., Mathew, M. K., & Krishnaswamy, S. (2006). Functional assay of Salmonella typhi OmpC using reconstituted large unilamellar vesicles: a general method for characterization of outer membrane proteins. Biochimie, 88(10), 1419–1424. [CrossRef]
- Suparna Chakraborty, Pujarini Dutta, Ananda Pal, Swarnali Chakraborty, George Banik, Prolay Halder, Animesh Gope, Shin-ichi Miyoshi, & Santasabuj Das. (2023). Intranasal Immunization of Mice with a Chimeric Antigen of Cholera Toxin B and Salmonella Typhi outer membrane protein T2544 elicits protective antibodies and T cell response at the intestinal mucosa. [CrossRef]
- Sykes, R. B., & Bonner, D. P. (1985). Aztreonam: The first monobactam. The American Journal of Medicine, 78(2), 2–10. [CrossRef]
- Szu, S. C., Stone, A. L., Robbins, J. D., Schneerson, R., & Robbins, J. B. (1987). Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. The Journal of Experimental Medicine, 166(5), 1510–1524. [CrossRef]
- Tavío, M. del M., Vila, J., Ruiz, J., Ruiz, J., Martín-Sánchez, A. M., & de Anta, M. T. J. (1999). Mechanisms involved in the development of resistance to fluoroquinolones in Escherichia coli isolates. Journal of Antimicrobial Chemotherapy, 44(6), 735–742. [CrossRef]
- Thuluva, S., Paradkar, V., Matur, R., Turaga, K., & GV, S. R. (2022). A multicenter, single-blind, randomized, phase-2/3 study to evaluate immunogenicity and safety of a single intramuscular dose of biological E’s Vi-capsular polysaccharide-CRM 197 conjugate typhoid vaccine (TyphiBEV TM ) in healthy infants, children, and adults in comparison with a licensed comparator. Human Vaccines & Immunotherapeutics, 18(5). [CrossRef]
- Truong, D., Boddy, K. C., Canadien, V., Brabant, D., Fairn, G. D., D’Costa, V. M., Coyaud, E., Raught, B., Pérez-Sala, D., Park, W. S., Heo, W. Do, Grinstein, S., & Brumell, J. H. (2018). Salmonella exploits host Rho GTPase signalling pathways through the phosphatase activity of SopB. Cellular Microbiology, 20(10), e12938. [CrossRef] [PubMed]
- Verma, S. K. (2009). Overexpression, Purification, and Immunogenicity of Recombinant Porin Proteins of Salmonella enterica Serovar Typhi (S. Typhi). Journal of Microbiology and Biotechnology, 19(9), 1034–1040. [CrossRef] [PubMed]
- Wain, J., Hendriksen, R. S., Mikoleit, M. L., Keddy, K. H., & Ochiai, R. L. (2015). Typhoid fever. The Lancet, 385(9973), 1136–1145. [CrossRef]
- Waterman, S. R., & Holden, D. W. (2003). Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cellular Microbiology, 5(8), 501–511. [CrossRef]
- World Health Organization. (2017, October). WHO Strategic Advisory Group of Experts (SAGE) on Immunization. Http://Www.Who.Int/Imunization/Sage/En/Index.Html.
- World Health Organization WHO Background document: The diagnosis, treatment and prevention of typhoid fever Communicable Disease Surveillance and Response Vaccines and Biologicals. (2003). www.who.int/vaccines-documents/.
- Wu, Y., Pons, V., Goudet, A., Panigai, L., Fischer, A., Herweg, J.-A., Kali, S., Davey, R. A., Laporte, J., Bouclier, C., Yousfi, R., Aubenque, C., Merer, G., Gobbo, E., Lopez, R., Gillet, C., Cojean, S., Popoff, M. R., Clayette, P., … Barbier, J. (2017). ABMA, a small molecule that inhibits intracellular toxins and pathogens by interfering with late endosomal compartments. Scientific Reports, 7(1), 15567. [CrossRef]
- Xiong, A.-W., Fang, J.-M., Ren, S.-X., Li, W., Wang, J., Zhao, Y., Chen, G.-Y., Xu, Q., & Zhou, C.-C. (2021). A Novel Combined Conjugate Therapeutic Cancer Vaccine, Recombinant EGF-CRM197, in Patients With Advanced Solid Tumors: A Phase I Clinical Study. Frontiers in Oncology, 11. [CrossRef]
- Yang, Y., Wan, C., Xu, H., & Wei, H. (2013a). Identification and characterization of OmpL as a potential vaccine candidate for immune-protection against salmonellosis in mice. Vaccine, 31(28), 2930–2936. [CrossRef]
- Yang, Y., Wan, C., Xu, H., & Wei, H. (2013b). Identification and characterization of OmpL as a potential vaccine candidate for immune-protection against salmonellosis in mice. Vaccine, 31(28), 2930–2936. [CrossRef]
- Yang, Y.-A., Chong, A., & Song, J. (2018). Why Is Eradicating Typhoid Fever So Challenging: Implications for Vaccine and Therapeutic Design. Vaccines, 6(3), 45. [CrossRef]
- Yang, Y.-A., Lee, S., Zhao, J., Thompson, A. J., McBride, R., Tsogtbaatar, B., Paulson, J. C., Nussinov, R., Deng, L., & Song, J. (2017). In vivo tropism of Salmonella Typhi toxin to cells expressing a multiantennal glycan receptor. Nature Microbiology, 3(2), 155–163. [CrossRef]
- Zafar, M., Jahan, H., Shafeeq, S., Nimtz, M., Jänsch, L., Römling, U., & Choudhary, M. I. (2020). Clarithromycin Exerts an Antibiofilm Effect against Salmonella enterica Serovar Typhimurium rdar Biofilm Formation and Transforms the Physiology towards an Apparent Oxygen-Depleted Energy and Carbon Metabolism. Infection and Immunity, 88(11). [CrossRef] [PubMed]
- Zeiner, S. A. (2012). Type 1 fimbrial structure and regulation in Salmonella enterica serovar Typhimurium [University of Iowa]. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).