1. Introduction
Frailty is a distinctive health state related to acceleration of the biological ageing process in which multiple body systems disproportionately lose their in-built reserves; as a result, older people living with frailty are at higher risk of adverse health outcomes when their physiology is suddenly challenged [
1,
2]. Frailty is increasingly recognized as an important construct in medicine because it has significant health implications for older adults’ wellbeing at multiple levels, including the physical, cognitive, social, and emotional [
3]. Many studies have shown that older people living with frailty are at higher risk of falls, hospitalization, worsening disability, and premature mortality [
4,
5].
The central tenet of frailty is the potential for serious adverse outcomes after a seemingly minor stressor event or challenge [
6]. Early detection of frailty is therefore of paramount importance because evidence has shown that targeted interventions may be able to increase physiological resilience in older adults [
7]. While the full assessment and individualized management of frailty typically encompass a comprehensive multidisciplinary evaluation of an individual's physical, cognitive, and social function [
8,
9], many tools have been developed to rapidly identify frailty and hence help decide who might need priority for a comprehensive geriatric assessment [
10]. Since different frailty identification tools incorporate different elements of the geriatric assessment, they have been shown to capture different morbidity and functional profiles (e.g., degree of disability), and long-term risks (e.g., mortality) [
11,
12], but there is paucity of data as to how different frailty tools capture differences in continuous physiological signals during stressor challenges [
13].
A physiological stressor that people experience multiple times on a daily basis is the orthostatic challenge, which requires the individual’s ability to counteract the physiological demands imposed by the act of standing up quickly from lying or sitting [
14]. Upon transiting to an upright posture, gravity causes redistribution of blood (i.e., blood pooling) in the compliant distensible veins of the abdomen and lower extremities [
15]. The consequent reduction in central venous pressure leads to a decline in venous return, stroke volume and arterial pressure [
16]. The primary mechanism in response to orthostatic stress is the baroreflex-mediated vagal withdrawal and activation of the sympathetic nervous system, which lead to increased heart rate and blood pressure [
17]. In addition, there are dynamic cerebrovascular changes to ensure that oxygen supply to the brain remains as constant as possible [
18]. Some of these complex real-time cardiovascular and neurovascular adaptations can be continuously measured using non-invasive biosensors, e.g., as regards fluctuations in blood pressure and heart rate, and degree of brain tissue oxygenation.
Referred to as neurocardiovascular instability (NCVI) [
19], abnormal physiological adaptations to standing have been described in older people using a single frailty identification tool [
20]. However, a knowledge gap remains as to how different frailty tools capture differences in continuous physiological signals during an orthostatic stress test. In this study, we utilized data from The Irish Longitudinal Study on Ageing (TILDA) to compare frailty by three different identification criteria in their continuous cardiovascular and neurovascular responses to an active stand test, compared to the absence of frailty.
2. Materials and Methods
2.1. Study population
TILDA is a landmark prospective cohort study of community-dwelling adults aged 50 years or older in Ireland. Data was collected at each wave via computer-aided personal interviewing and self-completed questionnaire, and at waves 1, 3 and 6 by a comprehensive centre- or home-based health assessment. In this study, we used the data of TILDA participants who completed the health centre assessment at wave 3 (2014-2015). Of note, the active stand test was not available in the home health assessment [
21]. Ethical approval was granted from the Faculty of Health Sciences Research Ethics Committee at Trinity College Dublin, Ireland. All participants provided written informed consent. All research was performed in accordance with the Declaration of Helsinki.
2.2. Frailty identification tools
For comparison of orthostatic physiologies, we identified four mutually exclusive groups: frail only by one of the following (but not the others): physical Frailty Phenotype (FP) [
22], Frailty Index (FI) [
23], and Clinical Frailty Scale (CFS) classification tree [
24]; and a fourth group where participants were not frail by any of these tools. The FP, CFS [
25], and a 32-item FI [
26] were previously operationalized and adapted to TILDA survey contents, and those adaptations were used in the present study.
Briefly, as per TILDA FP classification, individuals were considered to be frail when they met three or more of: measured slowness (by timed-up-and go test), weakness (by handgrip strength); and self-reported exhaustion, unintentional weight loss, and low physical activity. Non-frail referred to participants without any criteria [
25].
The CFS was adapted to TILDA utilizing the previously published classification tree, which takes into account recorded levels of help required with activities of daily living, overall number of chronic conditions, and self-reported general health, exhaustion (everything is an effort) and level of physical activity [
27]. CFS scores were dichotomized into non-frail (1 to 3) and frail (5 to 9).
The 32 self-reported items composing the TILDA FI can be seen in
Table S1. The FI expresses the proportion of deficits present in each participant out of the 32 deficits considered. Participants were dichotomized as non-frail (FI < 0.10) vs. frail (FI ≥ 0.25).
Regarding the intermediate category (pre-frail) for each frailty classification, rather than combining numbers with the non-frail or frail categories, they were excluded from the analyses as the primary focus was to compare established frailty vs. absence of frailty.
2.3. Active Stand
The active stand test is a standardized procedure for assessing the spectrum of abnormal cardiovascular and neurovascular responses to standing and evaluating causes of orthostatic intolerance. In the TILDA wave 3 setup [
28], six continuous non-invasive physiological signals were collected during the active stand test; three in the cardiovascular domain: systolic (sBP), diastolic (dBP) blood pressure, and heart rate (HR), using a digital artery photoplethysmography device; and three in the neurovascular domain: frontal lobe cerebral oxygenation (Oxy), deoxygenation (Deoxy) and tissue saturation index (TSI), using near-infrared spectroscopy (NIRS). All measurements were carried out at an ambient temperature of 21 to 23
◦C in a comfortably lit assessment room. Participants laid supine for ≈10 minutes before transitioning to a standing position and remained standing still for 3 minutes while data was continuously recorded using the instrumentation detailed below. Participants were instructed to stand as quick as possible and those with mobility difficulties were assisted by a research nurse if required.
2.4. Instrumentation
2.4.1. Continuous cardiovascular signals
A Finometer device (Finometer MIDI, Finapres® Medical Systems, Amsterdam, The Netherlands) was used to measure reconstructed brachial artery pressure noninvasively on a beat-to-beat basis. This is a photoplethysmography-based device and measures the arterial pressure waveform at the finger at 200 Hz using the volume-clamp method. The volume of the finger artery is measured with optical sensors and is automatically maintained constant with the finger cuff connected to a pneumatic control system [
29]. The volume-clamp method showed good agreements with intra-arterial monitoring [
30] and the auscultatory method [
31]. It corrects for the hydrostatic height of the finger with respect to the heart level through a position sensor mounted to the finger.
2.4.2. Continuous neurovascular signals
NIRS provides non-invasive and non-ionizing technology that has been used to measure variations in oxygenated and deoxygenated haemoglobin concentrations in various human tissues [
32,
33,
34]. It has been shown that NIRS provides consistent readings with other measurement modalities in various applications, including cerebral blood flow [
35] and skeletal muscle contractions [
36]. With capabilities in time-resolved, frequency-domain, and continuous wave spectroscopic implementations, NIRS has a potential to be widely used in both research and clinical settings due to its versatility and high temporal resolution [
37].
Based on optical sensing technology, NIRS measurements record absorbance of light at several wavelengths, where changes in absorbance in the region near 850 nm are ascribed to Oxy, and absorbance in the region near 760 nm is attributed to Deoxy [
38]. Combinations of Oxy and Deoxy are often reported, e.g., TSI expressed as 100*Oxy/(Oxy + Deoxy) [
39].
In this study, we used the PortaLite® device (Artinis Medical Systems, The Netherlands), which is a wireless NIRS device capable of transmitting data in real-time via Bluetooth® at a maximum sampling frequency of 50 Hz. Oxysoft v3.0.53 was the user interface for the setup, recording and exporting of NIRS data. The NIRS sensor was affixed approximately 2 cm above the left eye (approximately the FP1 (left frontal) position of the 10 to 20 electrode system (3 cm lateral and 3.5 cm superior to the nasion) [
40] and the sampling frequency was set at 50 Hz for all participants. The influence of ambient light was minimized via a black headband covering the sensor [
28].
2.5. Signal acquisition, synchronization and preprocessing
A one-minute section of the active stand data, from 20 seconds before to 40 seconds after standing, was used in this study. The beat-to-beat cardiovascular signals from the Finapres® MIDI were interpolated at 5 Hz. The neurovascular signals recorded by NIRS were downsampled to 5 Hz. All signals were synchronized via multiple manual markers throughout the recordings. The onset of the stand (i.e., the moment participants started standing up from the supine position) was determined via the height sensor data using an algorithm previously described in detail by O’Connor et al [
41]. Baseline cardiovascular and neurovascular values were established by averaging readings from 60 to 30 seconds prior to standing, in keeping with previous investigations [
14,
28,
42].
2.6. Statistical parametric mapping
Statistical parametric mapping (SPM) is a method that utilises Random Field Theory [
43] to perform whole-trajectory analysis using topological inference. With a primary application in neuroimaging [
44], SPM can be applied to any spatiotemporally registered and smooth signal. In the context of one-dimensional trajectories, like cardiovascular and neurovascular signals measured during the active stand test, SPM can be used to quantify the differences between multiple groups and identify the precise regions where significant differences are found in a temporal manner [
20]. One-Dimensional Statistical Parametric Mapping (SPM1D) [
45] is a Python/MATLAB package that has been employed in the analysis of a variety of physiological traces [
46,
47,
48]. While several other software packages implementing the SPM methodology are available across different platforms (e.g., spmR, SPM12 and NIPY), SPM1D is currently the only package specifically designed for analysing one-dimensional data, like the time series measured during the active stand test [
49].
2.7. Statistical Analyses
Descriptive statistics of the cohort and temporal analyses of cardiovascular and neurovascular measures were conducted in R (version 4.0.5) using RStudio 2022.07.1+554 (Boston, MA, USA). Cohort characterization variables included:
- -
Age and sex.
- -
Number of chronic conditions counted from the following list: heart attack or heart failure or angina, cataracts, hypertension, high cholesterol, stroke, diabetes, lung disease, asthma, arthritis, osteoporosis, cancer, Parkinson’s disease, peptic ulcer and hip fracture [
50]. From this, we extracted the list of cardiovascular diseases.
- -
Number of regular medications excluding supplements.
- -
Number of physical limitations, counted from the following list: walking 100 meters (100 yards); running or jogging about 1.5 kilometres (1 mile); sitting for about two hours; getting up from a chair after sitting for long periods; climbing several flights of stairs without resting; climbing one flight of stairs without resting; stooping, kneeling, or crouching; reaching or extending arms above shoulder level; pulling or pushing large objects like a living room chair; lifting or carrying weights over 10 pounds/5 kilos, like a heavy bag of groceries; and picking up a small coin from a table (
https://tilda.tcd.ie/data/documentation/; Accessed on
10/11/2023).
- -
Baseline cardiovascular and neurovascular parameters.
- -
Self-reported dizziness during the entirety of the 3-minute standing phase in the active stand test (yes or no).
For overall comparisons across groups, the independent-samples Kruskal-Wallis test and the Chi-square test were used for non-normal continuous variables and categorical variables, respectively. Each of the three frail groups were also compared with the non-frail group using the unadjusted pairwise Wilcoxon rank-sum test.
The open-source package SPM1d 0.4 (
http://www.spm1d.org/), which is dependent primarily on SPM8 (
https://www.fil.ion.ucl.ac.uk/spm/), was used in MATLAB environment (R2020b, The MathWorks, Inc., Natick, MA, USA) for the analyses. Independent t tests were conducted within SPM1d, which returns regions of significance in the form of p values. These are contiguous values over which the curve is determined to be not consistent with random sampling.
The statistical significance level was set at p<0.05 throughout.
3. Results
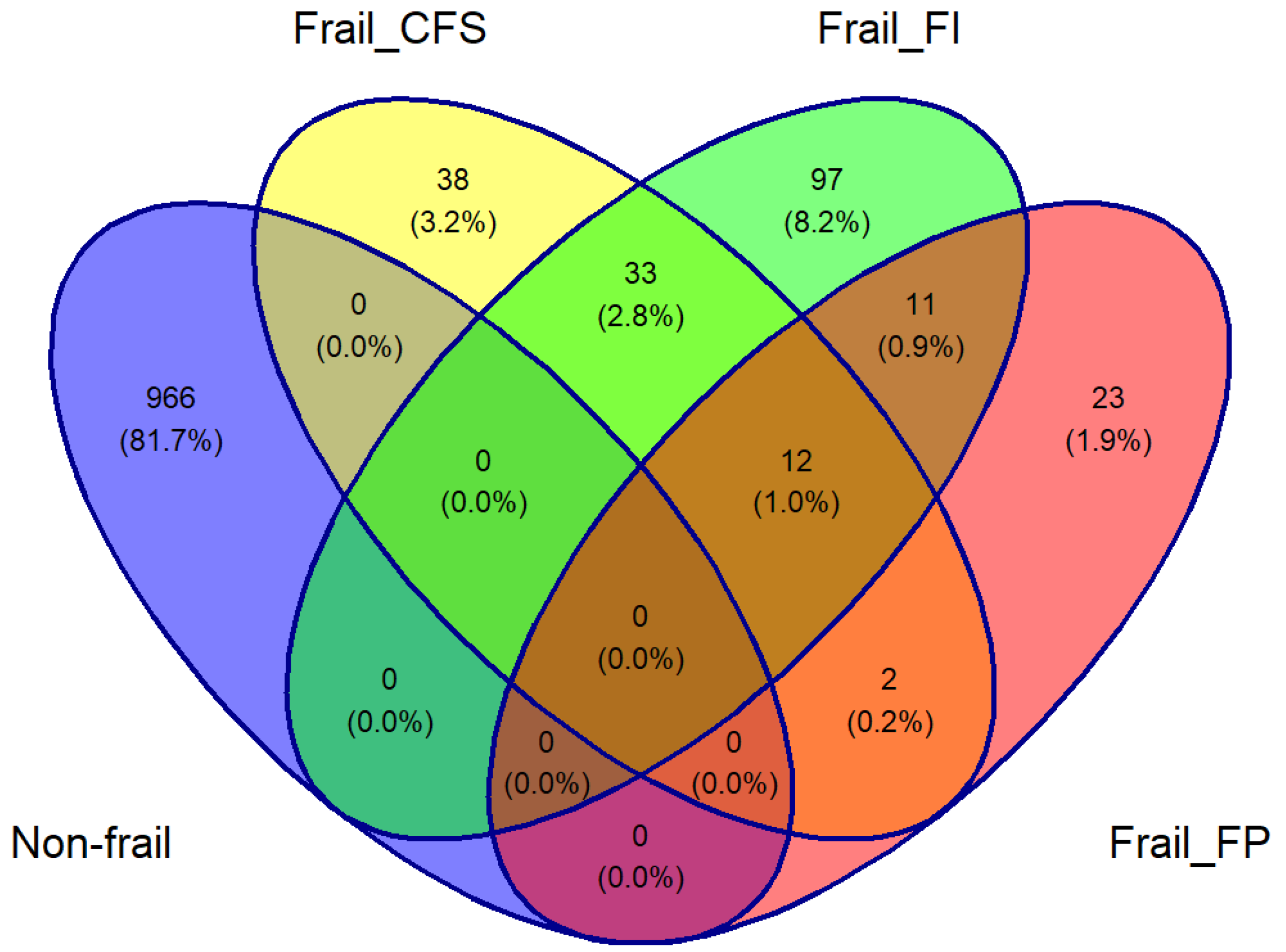
There were 2133 participants aged ≥50 years in TILDA at wave 3 with complete Finometer and NIRS data. The overlaps between those who were identified as frail by the three tools and those who were not frail by any tool are depicted in
Figure 1’s Venn diagram. Of this total of 1182 participants, 1124 (mean age 63.5 years, 50.2% women) were included: 23 frail only by FP, 97 by FI, 38 by CFS, and 966 by none.
The characterization of all four groups included in the study is summarised in
Table 1. As expected, all frail participants were more comorbid, more medicated, and more physically limited than non-frail participants, but these differences were most accentuated for the FI classification. In the pairwise comparison, only the frail by FI reported a significantly higher proportion of post-stand dizziness compared to the non-frail group (p=0.014).
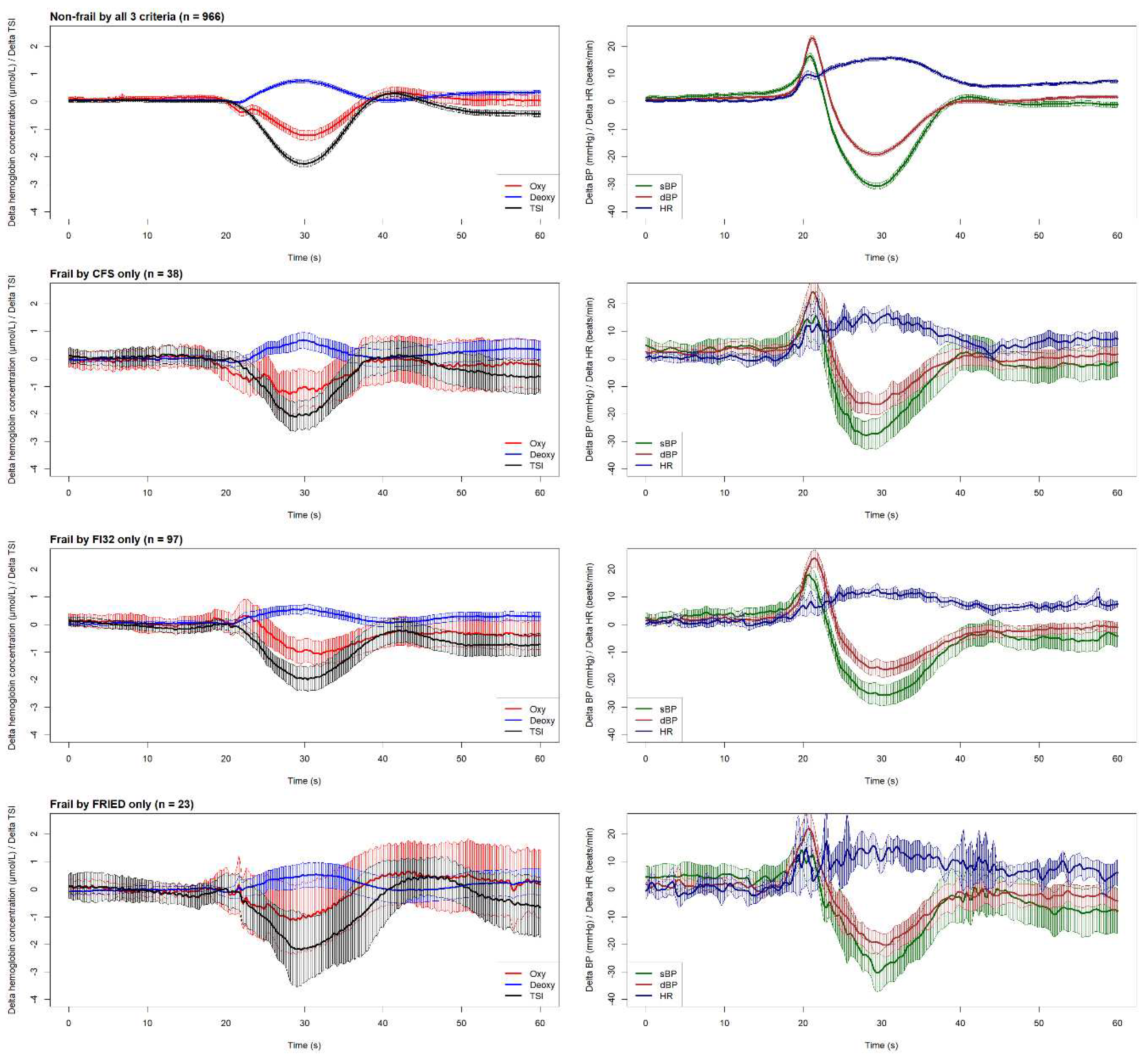
The graphical overview of cardiovascular and neurovascular signals shown in
Figure 2 illustrates the physiological responses upon standing across all four groups included in this study.
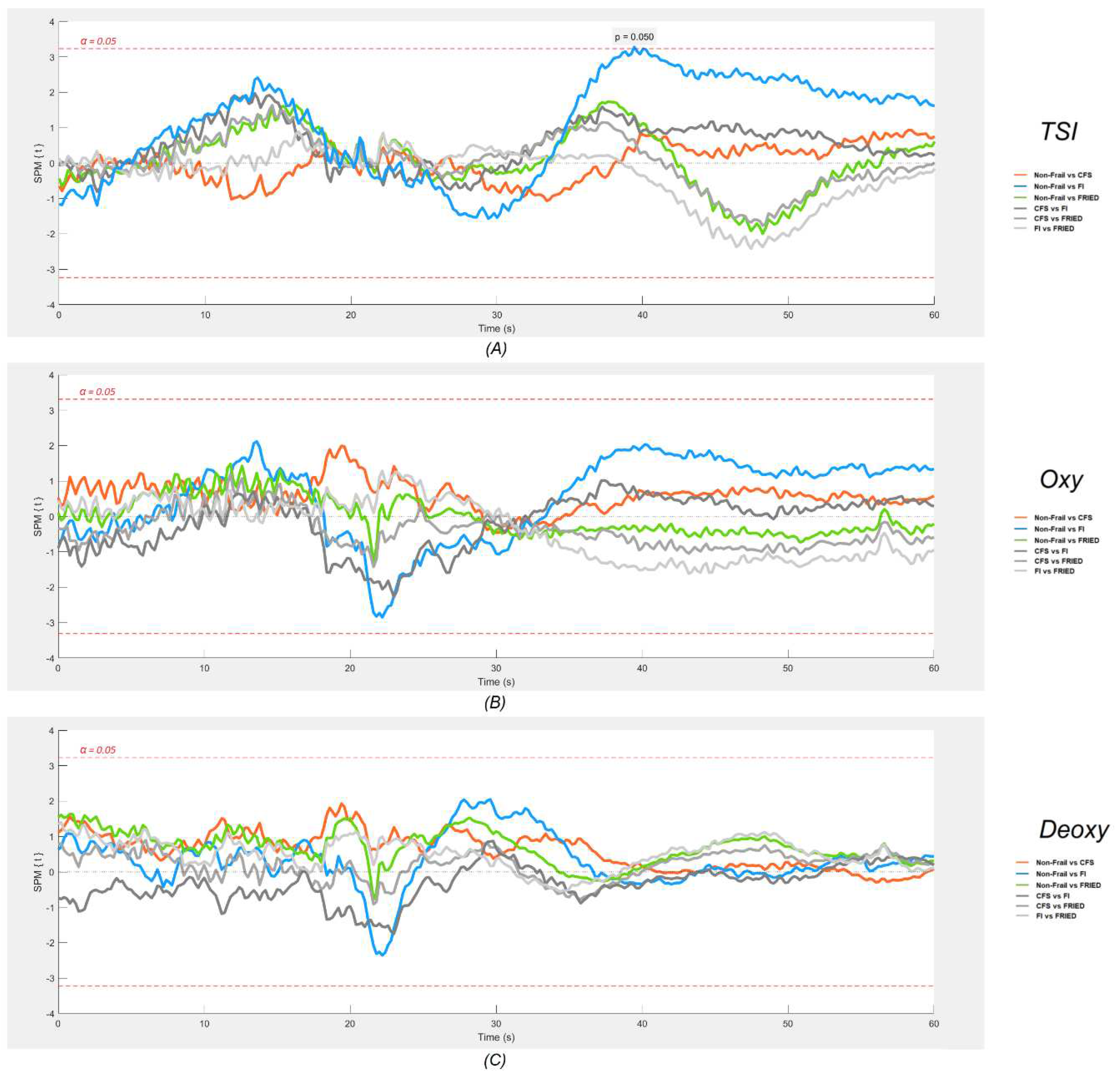
Figure 3 (neurovascular) and 4 (cardiovascular signals) show the results of the SPM analyses. As regards neurovascular signals (
Figure 3), with a p value of <0.05, SPM results indicated a small region, approximately between 19 to 20 s post-stand, where the TSI signal was significantly lower in those classified as frail by FI compared to the non-frail group. No other statistically significant differences were found in this analysis.
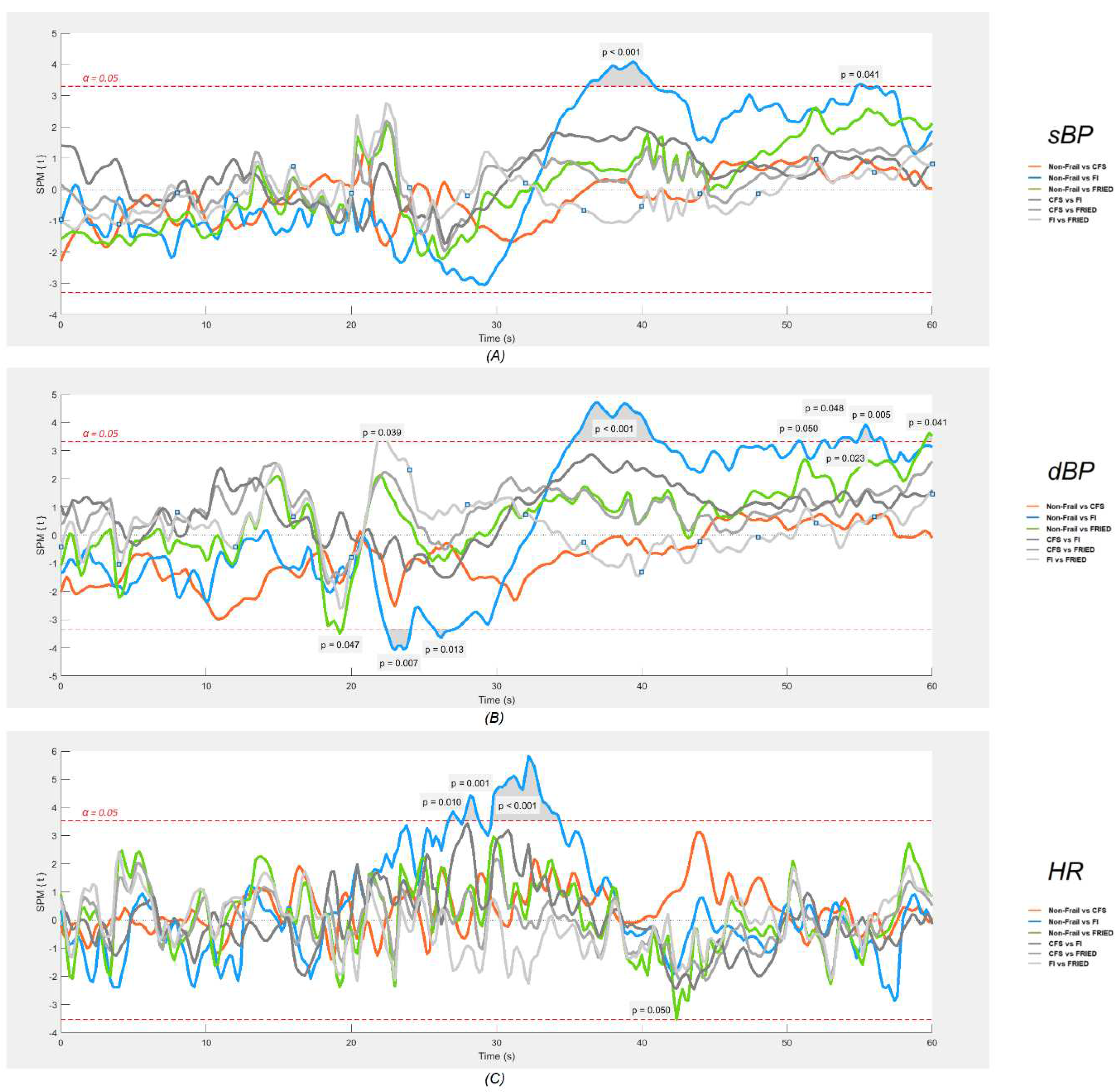
As regards cardiovascular signals (
Figure 4), SPM analyses revealed that only the frail by FI had significantly different post-stand signals (p<0.05) compared to the non-frail group, namely an attenuated gain in HR between approximately 5-15 s post-stand and larger deficits in both sBP and dBP between 15-40 s post-stand. Little significant differences in post-stand cardiovascular signals were found for other frail classifications.
4. Discussion
The aim of this study was to compare frailty by three different identification criteria in their continuous cardiovascular and neurovascular responses to an active stand test, compared to the absence of frailty. We used data from wave 3 of TILDA and identified four mutually exclusive groups: frail only by physical Frailty Phenotype (FP), 32-item Frailty Index (FI), and the Clinical Frailty Scale (CFS) classification tree; and a fourth group where participants were not frail by any of these tools. As expected, all frail participants were more comorbid, more medicated, and more physically limited than non-frail participants, but these differences were most accentuated for the FI classification. In the pairwise comparison (
Table 1), when compared to the non-frail group, only the group classified as frail by FI exhibited a lower mean baseline Oxy, higher baseline sBP, lower dBP, and reported a significantly higher proportion of post-stand dizziness. SPM analyses revealed that only the frail by FI had significantly different signals compared to the non-frail group: an attenuated gain in HR between 5-15 s post-stand, larger deficits in sBP and dBP between 15-40 s post-stand, and possibly lower TSI around 20 s post-stand.
As regards cardiovascular signatures, it is possible that frailty, encapsulating problems in multiple orthostatic compensatory mechanisms, may lead to a blunted primary cardiac pump response (indicated by a lesser increase in HR) in the initial post-stand period and/or impaired blood pressure stabilization during the early recovery period (20 – 40 s). Although this remains causally unproven, it has been seen across many studies [
51,
52,
53,
54,
55,
56,
57,
58,
59,
60]. Another interesting characteristic of our frail by FI group is the fact that on pairwise comparison analyses, it was the only frail group where baseline sBP was significantly higher than the non-frail group, which is reminiscent of the clinically challenging and risky syndrome of supine hypertension with concomitant orthostatic hypotension [
61].
As regards neurovascular (NIRS) signals, the literature has much less information. Our study complements a previous TILDA investigation, in which Maguire et al. [
20] pioneered the application of the one-dimensional SPM methodology to the cardiovascular and neurovascular comparisons of internal FI categories (i.e., non-frail, pre-frail and frail) during the active stand test. In keeping with the present results, that study showed that a higher degree of FI frailty was associated with lower orthostatic HR around 10 s post-stand, and lower TSI around 25 s post-stand. Our findings are also consistent with those of a clinical investigation by Perez-Denia et al., showing that multimorbidity in 303 falls clinic attendees was associated with poorer recovery of TSI at 30 s after standing, as well as impaired dBP recovery at 30 s [
62]. The potential reasons as to why the TSI signal of FI frailty was only transiently different post-stand and not sustained up to 40 s as seen for blood pressure are not known, but could potentially involve the activation of specific cerebral autoregulatory mechanisms by 20 s post-stand, which in the case of the frail by FI, might be less vigorous due to underlying cardiovascular/cerebrovascular disease.
Consequently, we hypothesize that the significant effect of the FI frailty classification and the lack of significant post-stand effects for the FP and CFS classifications may be due to the more direct capture of specific cardiovascular and neurovascular morbidities in the FI definition. This is evident in the cross-sectional characterization of the frailty groups shown in
Table 1, where the FI is the most dominant frailty criterion for discriminating the number of cardiovascular diseases.
Technically, in this study we demonstrated that plotting six continuous physiological signals in a synchronised fashion, as shown in
Figure 2, provides not only an efficient way to visually inspect the physiological responses of different groups during the active stand, but also makes it possible to postulate possible connections between cardiovascular and neurovascular responses, allowing for the generation of hypotheses to be tested in further studies. The results of the one-dimensional SPM analyses suggested that this is quite a sensitive tool for detection of signal differences, as judged by very minor and transient pre-stand differences that are unlikely to be of clinical significance (e.g., CFS vs. non-frail differences in dBP seen in
Figure 4). In this regard, the post-stand TSI difference FI vs. non-frail seemed modest in comparison with the more obvious and temporally sustained sBP and dBP signal differences.
To our knowledge, our study is the first to utilise the SPM methodology to compare more than one frailty measure across active stand dynamics in mutually exclusive groups. Another strength of our design is the large population-based sample from which the physiological data was collected, although the need for mutually exclusive groups and the exclusion of pre-frail groups to maximize non-frail vs. frail differences reduced the size of the study population. This precluded subanalysis by sex, which could be of potential interest [
62]. Finally, another limitation is that our FP definition in TILDA is an adaptation of the original criteria, and the CFS is based on a retrospective classification tree rather than contemporaneous face-to-face scoring in TILDA participants. Self-report limitations may also apply to the frailty tools, and it is to be noted that the 32-item TILDA FI was entirely self-reported.
5. Conclusions
In our analysis, different frailty identification tools captured different continuous cardiovascular and neurovascular responses to an orthostatic stress test. The FI had better discrimination than FP and CFS possibly because of better capture of cardiovascular morbidities and may therefore have better clinical applicability.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.Org Author Contributions
Conceptualization, Feng Xue and Roman Romero-Ortuno; Data curation, Feng Xue, Silvin Knight, Aisling M. O’Halloran, Morgana Shirsath, Eoin Duggan and Roman Romero-Ortuno; Formal analysis, Feng Xue, Aisling M. O’Halloran and Roman Romero-Ortuno; Funding acquisition, Rose Anne Kenny and Roman Romero-Ortuno; Investigation, Feng Xue, Rose Anne Kenny and Roman Romero-Ortuno; Methodology, Feng Xue, Silvin Knight, Aisling M. O’Halloran and Roman Romero-Ortuno; Project administration, Rose Anne Kenny and Roman Romero-Ortuno; Resources, Rose Anne Kenny and Roman Romero-Ortuno; Software, Feng Xue and Aisling M. O’Halloran; Supervision, Roman Romero-Ortuno; Validation, Feng Xue, Silvin Knight, Aisling M. O’Halloran, Eoin Duggan and Roman Romero-Ortuno; Visualization, Feng Xue; Writing – original draft, Feng Xue and Roman Romero-Ortuno; Writing – review & editing, Feng Xue, Silvin Knight, Emma Connolly, Aisling M. O’Halloran, Eoin Duggan, Rose Anne Kenny and Roman Romero-Ortuno. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from Science Foundation Ireland [18/FRL/6188]. TILDA is funded by Atlantic Philanthropies, the Health Research Board, and Irish Life. Emma Connolly is funded by a grant from the Irish Research Council [GOIPD/2021/702].
Acknowledgments
The authors greatly appreciate the opportunity provided by TILDA for granting access to their data and all the TILDA participants for making this work possible.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Campbell, A.J.; Buchner, D.M. Unstable disability and the fluctuations of frailty. Age Ageing 1997, 26, 315–318. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Ahmed, N.; Mandel, R.; Fain, M.J. Frailty: an emerging geriatric syndrome. Am. J. Med. 2007, 120, 748–753. [Google Scholar] [CrossRef]
- Kojima, G.; Liljas, A.E.M.; Iliffe, S. Frailty syndrome: implications and challenges for health care policy. Risk Manag. Healthc. Policy 2019, 12, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Chang, S.F.; Ho, H.Y. Adverse Health Effects of Frailty: Systematic Review and Meta-Analysis of Middle-Aged and Older Adults With Implications for Evidence-Based Practice. Worldviews Evid. Based Nurs. 2021, 18, 282–289. [Google Scholar] [CrossRef]
- Fried, L.P.; Cohen, A.A.; Xue, Q.L.; Walston, J.; Bandeen-Roche, K.; Varadhan, R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 2021, 1, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- van Kan, G.A.; Rolland, Y.; Houles, M.; Gillette-Guyonnet, S.; Soto, M.; Vellas, B. The assessment of frailty in older adults. Clinics in geriatric medicine 2010, 26, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Cella, A.; Pilotto, A.; Daragjati, J.; Veronese, N.; Musacchio, C.; Mello, A.M.; Logroscino, G.; Padovani, A.; Prete, C.; et al. Three Decades of Comprehensive Geriatric Assessment: Evidence Coming From Different Healthcare Settings and Specific Clinical Conditions. J. Am. Med. Dir. Assoc. 2017, 18, 192–e191. [Google Scholar] [CrossRef]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef]
- Oviedo-Briones, M.; Laso, A.R.; Carnicero, J.A.; Cesari, M.; Grodzicki, T.; Gryglewska, B.; Sinclair, A.; Landi, F.; Vellas, B.; Checa-Lopez, M.; et al. A Comparison of Frailty Assessment Instruments in Different Clinical and Social Care Settings: The Frailtools Project. J. Am. Med. Dir. Assoc. 2021, 22, 607–e607. [Google Scholar] [CrossRef]
- Romero-Ortuno, R.; Hartley, P.; Kenny, R.A.; O'Halloran, A.M. Frail by different measures: a comparison of 8-year mortality in The Irish Longitudinal Study on Ageing (TILDA). Eur. Geriatr. Med. 2022, 13, 279–284. [Google Scholar] [CrossRef]
- Cohen, H.J. In search of the underlying mechanisms of frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M706–708. [Google Scholar] [CrossRef] [PubMed]
- Finucane, C.; van Wijnen, V.K.; Fan, C.W.; Soraghan, C.; Byrne, L.; Westerhof, B.E.; Freeman, R.; Fedorowski, A.; Harms, M.P.M.; Wieling, W.; et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin. Auton. Res. 2019, 29, 427–441. [Google Scholar] [CrossRef]
- Tansey, E.A.; Montgomery, L.E.A.; Quinn, J.G.; Roe, S.M.; Johnson, C.D. Understanding basic vein physiology and venous blood pressure through simple physical assessments. Adv. Physiol. Educ. 2019, 43, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.M. Mechanisms of sympathetic regulation in orthostatic intolerance. J. Appl. Physiol. (1985) 2012, 113, 1659–1668. [Google Scholar] [CrossRef]
- Biaggioni, I.; Shibao, C.A.; Diedrich, A.; Muldowney, J.A.S., 3rd; Laffer, C.L.; Jordan, J. Blood Pressure Management in Afferent Baroreflex Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- van Beek, A.H.; Claassen, J.A.; Rikkert, M.G.; Jansen, R.W. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J. Cereb. Blood Flow. Metab. 2008, 28, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Kenny, R.A.; Kalaria, R.; Ballard, C. Neurocardiovascular instability in cognitive impairment and dementia. Ann. N. Y Acad. Sci. 2002, 977, 183–195. [Google Scholar] [CrossRef]
- Maguire, F.; Romero-Ortuno, R.; O'Connor, J.D.; Reilly, R.B.; Knight, S.P.; Kenny, R.A. One-Dimensional Statistical Parametric Mapping Identifies Impaired Orthostatic Cerebrovascular and Cardiovascular Response in Frailty Index. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 885–892. [Google Scholar] [CrossRef]
- Donoghue, O.A.; McGarrigle, C.A.; Foley, M.; Fagan, A.; Meaney, J.; Kenny, R.A. Cohort Profile Update: The Irish Longitudinal Study on Ageing (TILDA). Int. J. Epidemiol. 2018, 47, 1398–1398l. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–156. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Theou, O.; Perez-Zepeda, M.U.; van der Valk, A.M.; Searle, S.D.; Howlett, S.E.; Rockwood, K. A classification tree to assist with routine scoring of the Clinical Frailty Scale. Age Ageing 2021, 50, 1406–1411. [Google Scholar] [CrossRef]
- O'Donoghue, P.; O'Halloran, A.M.; Kenny, R.A.; Romero-Ortuno, R. Do the frail experience more adverse events from intensive blood pressure control? A 2-year prospective study in the Irish Longitudinal Study on Ageing (TILDA). EClinicalMedicine 2022, 45, 101304. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuno, R.; Hartley, P.; Knight, S.P.; Kenny, R.A.; O'Halloran, A.M. Frailty index transitions over eight years were frequent in The Irish Longitudinal Study on Ageing. HRB Open Res. 2021, 4, 63. [Google Scholar] [CrossRef]
- O'Halloran, A.M.; Hartley, P.; Moloney, D.; McGarrigle, C.; Kenny, R.A.; Romero-Ortuno, R. Informing patterns of health and social care utilisation in Irish older people according to the Clinical Frailty Scale. HRB Open Res. 2021, 4, 54. [Google Scholar] [CrossRef]
- Knight, S.P.; Newman, L.; O'Connor, J.D.; Davis, J.; Kenny, R.A.; Romero-Ortuno, R. Associations between Neurocardiovascular Signal Entropy and Physical Frailty. Entropy (Basel) 2020, 23. [Google Scholar] [CrossRef] [PubMed]
- Cho, J. Current Status and Prospects of Health-Related Sensing Technology in Wearable Devices. J. Healthc. Eng. 2019, 2019, 3924508. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Casadei, R.; Groppelli, A.; Di Rienzo, M.; Mancia, G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 1989, 13, 647–655. [Google Scholar] [CrossRef]
- Schutte, A.; Huisman, H.; Van Rooyen, J.; Malan, N.; Schutte, R. Validation of the Finometer device for measurement of blood pressure in black women. Journal of human hypertension 2004, 18, 79–84. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 2014, 85 Pt. 1, 6–27. [Google Scholar] [CrossRef]
- Cortese, L.; Zanoletti, M.; Karadeniz, U.; Pagliazzi, M.; Yaqub, M.A.; Busch, D.R.; Mesquida, J.; Durduran, T. Performance Assessment of a Commercial Continuous-Wave Near-Infrared Spectroscopy Tissue Oximeter for Suitability for Use in an International, Multi-Center Clinical Trial. Sensors (Basel) 2021, 21. [Google Scholar] [CrossRef]
- Pham, T.; Tgavalekos, K.; Sassaroli, A.; Blaney, G.; Fantini, S. Quantitative measurements of cerebral blood flow with near-infrared spectroscopy. Biomed. Opt. Express 2019, 10, 2117–2134. [Google Scholar] [CrossRef]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4577–4590. [Google Scholar] [CrossRef]
- Boas, D.A.; Elwell, C.E.; Ferrari, M.; Taga, G. Twenty years of functional near-infrared spectroscopy: introduction for the special issue. Neuroimage 2014, 85 Pt. 1, 1–5. [Google Scholar] [CrossRef]
- Kröger, H.; Donner, I.; Skiello, G. Influence of a new virostatic compound on the induction of enzymes in rat liver. Arzneimittelforschung 1975, 25, 1426–1429. [Google Scholar]
- Newman, L.; Nolan, H.; Carey, D.; Reilly, R.B.; Kenny, R.A. Age and sex differences in frontal lobe cerebral oxygenation in older adults-Normative values using novel, scalable technology: Findings from the Irish Longitudinal Study on Ageing (TILDA). Arch. Gerontol. Geriatr. 2020, 87, 103988. [Google Scholar] [CrossRef]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar]
- O'Connor, J.D.; O'Connell, M.D.L.; Nolan, H.; Newman, L.; Knight, S.P.; Kenny, R.A. Impact of Standing Speed on the Peripheral and Central Hemodynamic Response to Orthostasis: Evidence From the Irish Longitudinal Study on Ageing. Hypertension 2020, 75, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Duggan, E.; Knight, S.P.; Romero-Ortuno, R. Relationship between sarcopenia and orthostatic blood pressure recovery in older falls clinic attendees. Eur. Geriatr. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.J.; Taylor, J.E. Random fields and geometry; Springer, 2007; Volume 80. [Google Scholar]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. Statistical parametric mapping: the analysis of functional brain images; Elsevier, 2011. [Google Scholar]
- Pataky, T.C. One-dimensional statistical parametric mapping in Python. Computer methods in biomechanics and biomedical engineering 2012, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Schuermans, J.; Danneels, L.; Van Tiggelen, D.; Palmans, T.; Witvrouw, E. Proximal neuromuscular control protects against hamstring injuries in male soccer players: a prospective study with electromyography time-series analysis during maximal sprinting. The American journal of sports medicine 2017, 45, 1315–1325. [Google Scholar] [CrossRef]
- Alizadeh, S.; Mattes, K. How anterior pelvic tilt affects the lower extremity kinematics during the late swing phase in soccer players while running: A time series analysis. Human. Movement Science 2019, 66, 459–466. [Google Scholar] [CrossRef]
- Sánchez-Sixto, A.; McMahon, J.J.; Floría, P. Verbal instructions affect reactive strength index modified and time-series waveforms in basketball players. Sports biomechanics 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pataky, T.C. rft1d: Smooth One-Dimensional Random Field Upcrossing Probabilities in Python. Journal of Statistical Software 2016, 71, 1–22. [Google Scholar] [CrossRef]
- Romero-Ortuno, R.; Scarlett, S.; O’Halloran, A.M.; Kenny, R.A. Is phenotypical prefrailty all the same? A longitudinal investigation of two prefrailty subtypes in TILDA. Age Ageing 2020, 49, 39–45. [Google Scholar] [CrossRef]
- Romero-Ortuno, R.; Cogan, L.; O'Shea, D.; Lawlor, B.A.; Kenny, R.A. Orthostatic haemodynamics may be impaired in frailty. Age Ageing 2011, 40, 576–583. [Google Scholar] [CrossRef]
- Romero-Ortuno, R.; Cogan, L.; Foran, T.; Kenny, R.A.; Fan, C.W. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J. Am. Geriatr. Soc. 2011, 59, 655–665. [Google Scholar] [CrossRef]
- Rockwood, M.R.; Howlett, S.E.; Rockwood, K. Orthostatic hypotension (OH) and mortality in relation to age, blood pressure and frailty. Arch. Gerontol. Geriatr. 2012, 54, e255–260. [Google Scholar] [CrossRef]
- O'Connell, M.D.; Savva, G.M.; Fan, C.W.; Kenny, R.A. Orthostatic hypotension, orthostatic intolerance and frailty: The Irish Longitudinal Study on Aging-TILDA. Arch. Gerontol. Geriatr. 2015, 60, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Chen, X.J.; Lee, W.J.; Chen, L.K. Association between Orthostatic Hypotension and Frailty in Hospitalized Older Patients: a Geriatric Syndrome More Than a Cardiovascular Condition. J. Nutr. Health Aging 2019, 23, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, S.E.; Soysal, P.; Bulut, E.A.; Aydin, A.E.; Dokuzlar, O.; Isik, A.T. What is the relationship between frailty and orthostatic hypotension in older adults? J. Geriatr. Cardiol. 2019, 16, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.H.; Borrel, D.; Sabbaghan, K.; Kum, C.; Yang, Y.; Robinovitch, S.N.; Claydon, V.E. Relationships between orthostatic hypotension, frailty, falling and mortality in elderly care home residents. BMC Geriatr. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Toba, A.; Ishikawa, J.; Suzuki, A.; Tamura, Y.; Araki, A.; Harada, K. Orthostatic blood pressure rise is associated with frailty in older patients. Geriatr. Gerontol. Int. 2019, 19, 525–529. [Google Scholar] [CrossRef]
- van Twist, D.J.L.; Mostard, G.J.M.; Sipers, W. Delayed recovery from initial orthostatic hypotension: an expression of frailty in the elderly. Clin. Auton. Res. 2020, 30, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Mol, A.; Slangen, L.R.N.; van Wezel, R.J.A.; Maier, A.B.; Meskers, C.G.M. Orthostatic blood pressure recovery associates with physical performance, frailty and number of falls in geriatric outpatients. J. Hypertens. 2021, 39, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuno, R.; O’Connell, M.D.; Finucane, C.; Soraghan, C.; Fan, C.W.; Kenny, R.A. Insights into the clinical management of the syndrome of supine hypertension–orthostatic hypotension (SH-OH): the Irish Longitudinal Study on Ageing (TILDA). BMC geriatrics 2013, 13, 1–14. [Google Scholar] [CrossRef]
- Perez-Denia, L.; Claffey, P.; Byrne, L.; Rice, C.; Kenny, R.A.; Finucane, C. Increased multimorbidity is associated with impaired cerebral and peripheral hemodynamic stabilization during active standing. J. Am. Geriatr. Soc. 2022, 70, 1973–1986. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).