You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Review
Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration Within the One Health Approach
Altmetrics
Downloads
181
Views
108
Comments
0
A peer-reviewed article of this preprint also exists.
Abstract
This comprehensive review elucidates the profound relationship between the human microbiome and breast cancer managrement. Recent findings highlight the significance of microbial alterations in tissues, such as the gut and the breast, and their role in influencing breast cancer risk, development, progression, and treatment outcomes. We delve into how the gut microbiome can modulate systemic inflammatory responses and estrogen levels, thereby impacting cancer initiation and therapeutic drug efficacy. Furthermore, we explore the unique microbial diversity within breast tissue, indicating potential imbalances brought about by cancer and highlighting specific microbes as promising therapeutic targets. Emphasizing a holistic One Health approach, this review underscores the importance of integrating insights from human, animal, and environmental health to gain a deeper understanding of the complex microbe-cancer interplay. As the field advances, strategic manipulation of the microbiome and its metabolites presents innovative prospects for enhancing cancer diagnostics and therapeutics. However, rigorous clinical trials remain essential to confirm the potential of microbiota-based interventions in breast cancer management.
Keywords:
Subject: Biology and Life Sciences - Biochemistry and Molecular Biology
1. Introduction
Early detection of cancer is associated with a decrease in adverse outcomes and an increase in the success rate of treatments. Presently, cancer diagnostic methods range in their levels of invasiveness, and many still necessitate invasive, biopsies for verification. Therefore, research has been geared towards discovering methods that are less invasive yet retain high sensitivity (identifying actual cancer cases) and specificity (excluding non-cancerous cases). This effectiveness is sometimes measured using the AUROC score, where a score of 1.0 symbolizes an ideal classification with 100% specificity and sensitivity. Recent studies have centered around utilizing microbiomes as a more non-invasive diagnostic approach, either directly or indirectly related to cancer. Samples such as saliva, stool, and plasma, which are easier to collect, in comparison to biopsies are being considered as alternatives to the more invasive diagnostic methods currently in practice. Microorganisms play an indirect role in affecting the emergence, natural course and/or severity of various cancers. For instance, it has been demonstrated that microbes in the gut influence cancer stem cells, which are dormant cancer cells believed to cause disease recurrence. Research has also indicated that metabolites from certain archaea, mainly from the Euryarchaeota phylum and the TACK superphylum, are linked to numerous cancers, even though these microbes are typically overlooked due to their rarity. Predominantly, research connecting microbes and cancer has concentrated on bacteria and their involvement in different cancers, with a few studies also exploring potential links with fungi and viruses [1].
The presence of a unique microbiome in breast tissue, previously unacknowledged, has gained recognition through recent research. Microbes are found in both healthy and cancerous breast tissues. Interestingly, breast tumor tissues showed a decrease in total bacterial DNA, and an inverse relationship was observed between bacterial DNA load and advanced cancer stages [2]. This implies that cancer may disturb the natural balance of microbes in this area. Over 60% of breast cancer samples harbor bacterial expression, with these bacteria found both inside breast cancer cells and immune cells. The microbial diversity in breast tumor samples outpaced that in other cancers and exhibited specific variations based on receptor status. From these samples, live bacteria from phyla like Proteobacteria, Firmicutes, and Actinobacteria were isolated. Fusobacterium nucleatum, previously linked to colorectal cancer, was also found to be more prevalent in breast tissue and colonize mammary tissue where is enhances tumor growth and metastasis. An increase of Methylobacterium radiotolerans and a decrease of Sphingomonas yanoikuyae in tumor tissue was also noted [3]. Moreover, the fungal genus Malassezia was abundantly found in breast tumors [4]. Mouse studies have demonstrated the role of local breast tissue microbiome in breast cancer. A decline in bacteria within tumors was linked with fewer lung metastases. Staphylococcus spp. and Lactobacillus spp., in particular, were related to an upsurge in metastatic tumors. Furthermore, gut microbes influenced the onset and advancement of breast cancer. In specific breast cancer mouse models, introducing Helicobacter hepaticus increased the presence of tumors in the mammary glands, mammary tissue inflammation, and the load of neutrophils. Comprehending the typical microbiome of breast tissue and its transformation during breast cancer might unveil microbes as potential therapeutic or preventive targets. However, more research is crucial to ascertain the universality of these microbial alterations across diverse patient groups [5,6].
In this review, we emphasize the ways in which the microbiome can both directly and indirectly influence various facets of cancer management, ranging from disease onset and progression to diagnostics and treatment options. We delve into recent studies that have reshaped our understanding of these interactions and their potential implications for cancer care.
2. Microbiota-Based Therapies for Breast Cancer Treatment and Prevention
Microbial therapy is also termed as oncolytic virotherapy or bacterial therapy. Alterations of certain microbial communities increase the risk of breast cancer as they alter tissue metabolism on multiple levels, including the trigger and progression of cancer in the host [7]. This form of treatment may involve immune regulation, influencing the efficacy of anti-tumor drugs, targeted therapy of engineered pro- and prebiotics fecal microbiota transplantation, and administration of antitumor drugs [8]. The human microbiome is capable of affecting an array of human biological, including hormonal and metabolic pathways and may also trigger initiation, proliferation, and genetic instability of cancer within the host cell or even trigger apoptosis [9].
Evidence supports that gut microbiota dysbiosis affects the development, progression, and prognosis of Breast cancer (BC) both through direct and indirect signaling pathways. Treating breast cancer through microbial therapy may involve managing microbiota that will target the immune response. Staphylococcus genus for example is negatively associated with expression of TRAF4, an oncogene that triggers a cascade of events favouring cancer survival through NF-κB [10]. Increasing expression of Staphylococcus genus in breast cancer tissue eliminates TRAF4 activity and increases activation of T-cell genes associated with the microbe. Equally, Propionibacterium expression is positively correlated with activation of T-cells through NFAT2, NFIL3 and IFNGR, but its expression in breast cancer tissue is reduced [10]. This study also presents the co-existence of Methylibium with multiple genes including ICOS, MRC1 and Toll-like receptors but this fact requires further investigation. Administration of Lactobacillus acidophilus, an anaerobic microbe, increases cytokine expression such as IFN-γ from splenocyte [11]. This cytokine in turn increases proliferation of lymphocytes such as NK cells, promoting Type 1 T helper activity expressing anti-tumour immunity and has anti-angiogenic effects [11]. TGF-β is an additional cytokine, involved in blocking T-cell production, that tumors produce in high concentrations to ensure they are unaffected by immune surveillance. Expression of this cytokine is hindered by L. acidophilus, as a result reduces tumour growth rate and increases lymphocyte proliferation.
Transfering Micrococcus luteus in in vivo models hinders 4TI tumor growth and this may be due to the microbiota upregulating M1-macrophage-related genes which include among others, IL-6IL,-8 and IL12 genes [12]. Potentially introducing Micrococcus luteus peritoumorally can improve patient outcome, however before reaching this stage further safety and effective evaluations are necessary. Sphingomonas yanoikuyae affect the immune response through the proliferation of invariant NKT cells involved in immunosurveillance as well as the expression of TLR2,5, and 9 [2]. Toll-like receptor 5 (TLR5) expression is also associated with microbial expression, e.g., Salmonella typhimurium flagellin, and activates the innate immune response in breast cancer patients, marking TLR5 as a therapeutic target [13]. Absence of inflammation in breast cancer patients is also instigated by the absence of Roseburia inulinivorans microbiota. A case common in post-menopausal breast cancer patients than premenopausal breast cancer patients [14].
Escherichia Coli produce antimicrobial peptides that are activated under cellular stress conditions and affect cell survival through cell cycle arrest and DNAse activity, [15,16]. Colicin A for example increases apoptosis in multiple breast cancer cell lines (MCF7, MDA-MD-231, MRC5 and Osteosarcoma cell line HOS) by 7–28%, while Colicin E1 increases apoptosis even more in MCF7 (58%) and in HS913T cells (14%) whilst Colicin A was ineffective [16]. Anti-cancer expression also involves membrane disintegration of breast cancer cells following expression by a defensin peptide expressed by Brevibacillus sp. [17].
Focus, evolves around Bifidobacterium, because of their anaerobic status is ideal for successfully delivering therapeutic genetic material to the necrotic center of tumours. A plasmid has been assembled carrying a DNA binding protein and E.coli as a source of cytosine deaminase required to convert the prodrug 5-Fluorocytosine to the tumor toxic 5-Fluorouracil [18]. Bifidobacterium sp. also tackle cancer indirectly, through metabolism of lapachol, synthesizing cytotoxic metabolites against breast cancer, as proven in SKBR-3 cell lines [19]. Bifidobacterium is also effective in synergy with Bacteroides. The mixture of the two-microbiota exibit anti-breast cancer properties as they suppress angiogenesis, proliferation and apoptosis against breast cancer cells [20]. The combination of these two-microbiota increased secretion of interferon γ, inducing tumor cell lysis.
Overall, modifying or eliminating certain communities of microbes can reverse the environmental conditions favouring cancer existence and triggering apoptosis. Complementary to traditional breast cancer therapies, restoring breast microbiota to their local natural concentrations does not only ensure a functional immune response but can also prevent host microenvironment interference to the cancer drugs administered or the metabolites they react.
2.1. Bacterial therapeutics for tumor treatment and immune modulation
Recent scientific discourse has highlighted the significance of microbiota in mitigating breast cancer via its anti-tumor activities [21]. Contemporary studies assert that specific intestinal bacteria can obstruct oncogenesis and promote tumor regression. Nonetheless, indiscriminate modulation of the gut microbiota can manifest unforeseen consequences. Precise targeting of tumor-associated bacteria is thus crucial for ensuring the safety and effectiveness of therapeutic modalities [22,23]. Current research underscores the imperative of regulating specific tumor-inducing bacteria through methodologies such as bacteria-mediated tumor therapy (BMTT), fecal bacterial and bacteriophage transplantation, prebiotic enhancement, and the utilization of bacterial toxins and enzymes [24].
However, a salient concern in harnessing bacterial agents for cancer therapeutics pertains to their potential cytotoxicity and pathogenic manifestations. To mitigate these risks, the scientific community has explored genetic engineering as a solution, allowing the excision of virulence-inducing genes while preserving therapeutic features [25]. Yet, the selection of bacteria is paramount: an ideal bacterial candidate should effectively permeate tumors, possess a minimal infectious propensity, and remain amenable to antibiotic-mediated elimination post-intervention. Numerous bacterial candidates await rigorous clinical evaluations, leaving certain aspects of their metabolic functions ambiguous. Challenges encompass inadvertent cytotoxic effects on healthy cells, incomplete tumor eradication, and unpredictable bacterial genome mutations. The transient existence of bacterial peptides in the human body further perplexes their therapeutic potential. As such, there is an emergent demand for exhaustive clinical trials to elucidate these bacteria-tumor cell dynamics and the potential ramifications of such interventions [26].
Conventional cancer treatments, primarily radiotherapy and chemotherapy, remain central to breast cancer intervention paradigms. Despite their dominance, the associated adverse effects and non-selective toxicity of these treatments necessitate the exploration of more targeted therapeutic options. In this context, genetically engineered bacteria, which exhibit a selective affinity for cancer cells while sparing healthy tissues, have emerged as promising agents in cancer research [27].
These bacteria inherently possess the ability to produce and excrete proteinaceous toxins that can inhibit specific cellular functions, a property that may be harnessed for therapeutic purposes. These agents can target cancer cells, thereby minimizing damage to surrounding healthy cells. Studies have suggested a potential symbiotic relationship between certain bacterial strains and tumor regression observed in animal models. Nevertheless, there is an unequivocal need for additional research to validate and understand these associations further [28].
While the exclusive use of bacterial agents might not secure complete tumor elimination, their combination with conventional drugs presents a promising avenue for cancer treatment. This integrated approach—incorporating drug-laden bacteria—appears especially propitious for overcoming the limitations of existing treatments, primarily their inability to effectively penetrate the tumor microenvironment [29,30].
The utilization of bacteria, particularly Listeria, Clostridium, and Salmonella, offers a solution to this challenge as they can navigate and infiltrate the tumor’s environment. For instance, Salmonella typhimurium has demonstrated remarkable anti-tumor properties, showing efficiency in invading and eliminating various cancer cells in in vivo studies. This strain has yielded significant results as a monotherapy against pancreatic, prostate, and breast cancers in animal models [31,32].
Furthermore, Clostridium perfringens has been acknowledged for producing an enterotoxin that, upon interaction with specific transmembrane proteins, can induce tumor regression. The FDA, recognizing the potential of BMTT, approved the use of Bacillus-Calmette-Guerin, a weakened strain of Mycobacterium bovis, for a specific bladder cancer treatment in the late 1970s, a testament to the enduring clinical relevance of this approach [33,34].
Despite these promising advances, bacterial-mediated therapies are not without challenges. Concerns like potential antibiotic resistance and infection risks associated with the use of live bacteria in treatments need careful consideration and resolution. Hence, while the paradigm of BMTT isn’t new in oncology, its widespread application and implementation remain subjects of ongoing debate and investigation. The preliminary findings, although promising, underscore the importance of continued and expanded research to fully comprehend and validate the potential symbiosis between bacterial strains and tumor regression.
2.2. Bacteriotherapy approaches
Bacteriotherapy, an evolving field in the domain of anticancer therapies, employs a diverse range of bacterial forms, inclusive of genetically modified organisms (GMOs), in both their living and attenuated states. These bacterial entities function via promoting apoptosis or disrupting cell membrane that primarily targets cancer cells through a range of anticancer agents, including bacteriocins, spores, and bacterial peptides [35].
Historical records indicate the rudimentary utilization of bacteriotherapy as far back as 1550 BC. A seminal advancement in cancer immunotherapy can be attributed to Dr. William Coley, an American orthopedic surgeon. His pioneering work involved the development of a heat-inactivated concoction of Streptococcus pyogenes and Serratia marcescens, subsequently referred to as “Coley’s toxin.” Administering this therapeutic concoction to patients with inoperable cutaneous carcinoma yielded substantial clinical outcomes, notably tumor regression and complete remission in a significant proportion of patients [36].
Various bacterial strains, encompassing the likes of Vibrio, Shigella, Salmonella, Listeria, and Bifidobacteria, have manifested profound efficacy in tumor invasion, colonization, and subsequent eradication. However, the relationship between bacteria and cancer remains multifaceted. Certain bacterial strains possess the potential to instigate carcinogenesis or induce malignancies. A case in point is Helicobacter pylori, which, through the secretion of specific cytokines and chemokines, can incite chronic inflammatory responses with detrimental cellular implications. Pertinently, the cytotoxin associated gene A (cagA) has emerged as a pivotal bacterial protein with profound implications in oncogenesis, primarily by compromising the function of the tumor suppressor protein, p53 [37,38].
Though historically malignancies were often associated with pathogenic bacterial infections, contemporary research is increasingly highlighting the anticarcinogenic potentials of certain bacteria. This paradigm shift can be epitomized by the nuanced understanding of how tumor cells proliferate by subverting host immunologic defenses. Recent studies accentuate the anticarcinogenic activities of Salmonella, which appear to operate through a multifaceted mechanism involving both adaptive and innate immune response activations.
Bacteriocins, intricate peptides or proteins synthesized ribosomally, first garnered attention in 1920, courtesy of the seminal work by the Belgian scientist André Gratia and the discovery of colicin (the first bacteriocin) from E. coli [39]. Today, their applications transcend in clinical domains, with their antimicrobial properties finding utility in food preservation as well. Based on their molecular weight, they are categorized into four classes. Significant bacteriocins include Bovicin HC5 from S. bovis and Nisin A from Lactococcus lactis. Some research indicates that the enterotoxin (TcdA) and cytotoxin (TcdB) produced by Clostridioides difficile could be pivotal in treating colorectal cancer [40].
Bacteria, in their myriad forms, are increasingly being recognized as potent immunotherapeutic agents. Their ability to modulate tumor antigenicity holds promise in augmenting immune responses. The novel insights into bacterial interactions, particularly infections with Clostridium novyi, underscore the profound potential in harnessing bacteria for therapeutic advancements in oncological properties. Therefore, bacteria are hailed as promising immunotherapeutic agents due to their ability to amplify the antigenicity of tumor cells, thus bolstering immune responses. By using bacterial cancer immunotherapy, tumor cells are identified as infected cells rather than mere cancer cells, elevating the chances of their elimination. For instance, infections with C. novyi can trigger the formation of heat shock proteins like Hsp70, promoting the maturation of professional dendritic and antigen-presenting cells, leading to potent antigen-specific immune responses [41,42].
2.3. Oncolytic virotherapy and phage-based immunotherapies in cancer treatment
Oncolytic virotherapy employs either naturally occurring or genetically engineered viruses with an affinity for tumor cells, leading to their selective targeting and replication. This process culminates in tumor regression attributed not only to direct cytotoxicity but also the stimulation of anti-tumor immune responses. Remarkably, this occurs without detriment to healthy cells and tissues [43].
In both in vitro and in vivo preclinical investigations, the third-generation oncolytic herpes simplex virus-1 (HSV-1) vector, G47Δ, exhibited amplified cytotoxic effects across multiple BC cell lines, inclusive of MCF-7, MDA-MB-468, and the tamoxifen-resistant variant MCF-7/TAM-R [44,45,46]. Notably, a synergistic cytotoxic effect was observed in breast cancer cells when G47Δ was combined with the chemotherapeutic agent, paclitaxel. This potentiated the anti-tumor efficacy of paclitaxel, resulting in a five-fold dosage reduction to achieve equivalent tumor reduction in vivo[46], a shift that could minimize chemotherapy-associated adversities.
In 2015, the U.S. Food and Drug Administration (FDA) granted approval for talimogene laherparepvec (T-VEC) as the inaugural oncolytic agent for melanoma treatment. Derived from the genetically modified HSV-1, its utility was further highlighted in a phase 2 clinical trial, which revealed that, in conjunction with neoadjuvant chemotherapy (NAC), it enhanced pathological complete response rates in triple-negative breast cancer (TNBC) patients, resulting in an 89% 2-year disease-free rate [47]. It warrants mention that observed adverse effects, though generally mild, ranged from injection site pain and headaches to low-grade fevers. This was notably in contrast to the more severe immune-mediated toxicities associated with TNBC treatment involving both chemotherapy and pembrolizumab [48] .
Likewise, phage therapy is emerging as a promising strategy in breast cancer immunotherapies. It utilizes the ability of phages to invoke anti-tumor immune responses. Specifically, in phage display immunotherapy, antigens (proteins or peptides) fused to phage coat-proteins, function as protective vaccines against cancer. Certain peptides, including E75, AE37, and GP2, have shown potential in breast cancer tests on BALB/c mice. Phages offer two main vaccine delivery techniques: (i) showcasing immunogenic peptides through modified phage coat proteins, and (ii) acting as delivery mediums for DNA vaccines by inserting a eukaryotic promoter-driven vaccine gene within their genome. These vaccines, presenting numerous antigen copies on immunogenic phage particles, incite strong immune reactions. They are also stable, cost-effective, and potent. Experiments have validated their effectiveness in mice and rabbits. Vaccines against Human Papilloma Viruses (HPV), such as Gardasil-9, demonstrate the applications beyond breast cancer. Another anti-breast cancer development is a phage-based anti-HER2 vaccine, designed to bypass immune tolerance. Other studies have developed vaccines for prostate cancer and utilized inovirus-associated vector vaccines for antibody production. Furthermore, a dual anthrax-plague vaccine and a lambda phage-based vaccine for hepatocellular carcinoma underscore the expansive potential of phage-based therapies.
Additionally, in a recent study by Catala and colleagues (2021), Protein-Lipid Particles (PLPs) have been innovatively crafted using bacteriophage lambda to display a fluorescent probe and the therapeutic antibody trastuzumab (Trz), leading to the formation of Trz-PLPs [49]. These are designed to target HER2-positive breast cancer cells. By increasing Trz’s density on PLPs, more prolonged inhibition of cell growth is achieved compared to using free Trz. Trz-PLPs have impacts on numerous cellular pathways, influencing amino acid metabolism, mitochondrial function, and more. They modulate the phosphorylation of key signaling proteins, such as Akt and mTOR, influencing the vital PI3K/Akt/mTOR signaling pathway in cancer cells [49]. Dong and colleagues (2022) have also spotlighted the potential of the M13 phage-based vaccines [50]. By combining M13 phage with a cationic polymer, PEI, a hybrid platform (M13@PEI) was designed capable of efficiently absorbing negatively charged antigens. This resulted in the MPO vaccine, combined with the Ovalbumin (OVA) antigen, which improved the maturation of antigen-presenting cells (APCs) and boosted antigen presentation. The M13 phage genome’s CpG regions and PEI’s role as a TLR5 agonist facilitated this. Enhanced antigen processing and uptake were further confirmed through in vitro assays, emphasizing the robust cytotoxic T lymphocyte (CTL) response. In vivo studies also confirmed the MPO vaccine’s ability to deliver antigens effectively and enhance antigen-specific T-cell mediated responses. When combined with α-PD1 treatment, the vaccine showcased powerful anti-tumor effects, improved survival rates, and enhanced immune memory responses, proving the significant potential of M13 phage-based vaccines in anti-tumor immunotherapy [50].
2.4. Probiotics and breast cancer pathogenesis
Emerging data suggests a nexus between microbial dysbiosis in breast and gut regions and the onset and progression of BC [51]. BC pathogenesis is frequently linked to sustained inflammation, incited by intestinal bacteria that activate NF-κB, subsequently releasing pro-inflammatory cytokines such as TNF-alpha [52,53,54]. Variations in the gut microbiome have been observed across different BC stages [55,56]. These alterations can influence therapeutic efficacy and potential toxicities. Interventions aimed at modifying the gut microbiome, employing probiotics and prebiotics, could be instrumental in attenuating systemic inflammation and alleviating treatment-associated toxicities. Specific strains such as Bifidobacterium and Lactobacillus have shown promise due to their immunomodulatory and antigenotoxic characteristics [57]. Recent research by Yazdi et al. (2012) revealed that the prophylactic administration of Lactobacillus plantarum augmented with selenium nanoparticles led to a significant reduction in tumor volume and elevated survival rates in a murine model of advanced human BC [58]. Furthermore, milk fermented with Lactobacillus casei CRL 431 administered to the same model demonstrated a marked decrease in tumor invasiveness and metastatic potential [59].
In addition, there have been a multitude of clinical trials in oncology field investigating the efficacy of probiotics alongside standard anti-cancer regimens. These studies predominantly indicate a beneficial reduction in gastrointestinal adversities that often arise from conventional cancer therapies [60,61,62,63,64,65]. A significant finding is seen in RCC patients who received the bacterial supplement, CBM588, alongside immunotherapy, reporting improved progression-free survival rates and response outcomes [66]. Probiotics play a crucial role in enhancing the immune system, significantly elevating immunoglobulin A (IgA) levels in the gut, which is vital for immune function. Specific probiotic bacteria, including L. casei and Sphingomonas yanoikuyae, have been noted to bolster the production of natural killer (NK) cells, playing a pivotal role in regulating cancer progression by actively participating in the body’s defence against cancer. Moreover, probiotics not only foster immune defenses but also produce compounds instrumental in protecting against DNA damage and breaking down carcinogens, thereby potentially preventing cancer. For example, L. casei strain Shirota (BLS) has been studied for its probable cancer-preventive properties, showing an inverse correlation between its consumption and the incidence of BC [67]. Furthermore, probiotics have shown promise in reducing chemotherapy-related cognitive impairment (CRCI) in BC patients and alleviating various other chemotherapy-induced side effects [68,69].
Species like L. casei Shirota and Bifidobacterium Bb12 have exhibited antigenotoxic activity, a crucial component in preventing genetic mutations that may lead to cancer, although the degree of this effect varies between bacterial species and is dependent on long-term exposure. Furthermore, the effectiveness of immune cells activation by probiotics varies and is based on dosage and bacterial strain.
However, despite the optimistic preliminary findings, it is essential to acknowledge that the effects of probiotics tend to diminish over time. This diminishing effect underscores the need for ongoing research to understand the long-term role and potential benefits of probiotics in both cancer prevention and treatment, and in mitigating side effects associated with cancer therapies. Given the initial promising results predominantly based on in vivo experiments, there is a palpable need for enhanced, comprehensive research, including in vitro exploration to overcome challenges observed in existing studies, such as inefficient mucosal adhesion and reduced gastrointestinal activity.
Current clinical trials are sparse, especially those focusing on probiotics specific to BC. Nevertheless, the initial findings are promising, and the results from ongoing clinical trials are anticipated to provide a clearer understanding of the role probiotics play in potentially enhancing BC treatment outcomes and in cancer prevention more broadly.
2.5. Microbial polysaccharides
Microbial polysaccharides (MPs) play roles in various cellular processes, including signal transduction and immune response modulation [70]. Glycans and Basidiomycetes-derived MPs, like krestin, schizophyllan, and lentinan, exhibit anticancer properties through immunostimulation, downregulation of NF-κB responses, and induction of tumor cell apoptosis [71,72].
Polysaccharide peptides from the mushroom YunZhi (Coriolus versicolor/Trametes versicolor) have been used in combination with chemotherapy to treat BC patients in Asian countries for decades. These peptides exhibit anti-proliferative properties, as they significantly reduce BC cell (MDA-MB-231) proliferation by upregulating the p21 gene expression [73]. Moreover, a meta-analysis by L.Y. Eliza et al. (2012) concluded that Coriolus versicolor can increase survival rates in cancer patients, including those with BC [74].
Additional beneficial MPs include Levan, which induces apoptotic cell death in MCF-7 BC cells, and the proteoglucan D-Fraction from Grifola frondosa, which reduces mammary tumor cell migration and lung metastases [75,76]. A clinical trial on D-Fraction in breast and lung cancer patients (stage II–IV) found that it hindered cancer progression, metastasis, and increased NK cell activity [77]. This suggests MPs’ potential in enhancing anti-tumor immunity in BC patients without significant toxicities. However, there’s a need for additional clinical trials to study D-fraction’s efficacy in combination with chemotherapeutics in a larger patient population.
3. Cancer Immunotherapy and Microbiome Immunomodulation
Immunotherapy represents a prominent emerging therapeutic approach for certain haematological and solid malignancies, including BC. Bacterial metabolites directly influence the activities of local immune cells. These effects include modulation of immunoglobulin secretion, the promotion of lymphocyte differentiation into regulatory T-lymphocytes and T-helper 17 cells, the generation of immunomodulatory cytokines, and even the epigenetic regulation of histone deacetylase enzymes.
Concerning the connection between the human microbiota and breast cancer, various metabolites have been identified as potential risk factors or modifiers. These include substances such as estrogens, active phytestrogens, short-chain fatty acids, lithocholic acid, and cadaverine. In particular, the gut microbiota’s production of estrogens, largely driven by the enzyme β-glucuronidase from specific intestinal bacteria, can result in the deconjugation of xenobiotics and sex hormones like estrogens. This process increases the reabsorption of estrogens into the systematic circulation, potentially elevating the risk of hormone-dependent breast cancer in women. Conversely, some metabolites such as phytestrogens, lithocholic acid, and cadaverine have been associated with a protective or risk-reducing influence on breast cancer development [78].
Gut bacteria can also promote breast cancer through the induction of chronic inflammation, which is closely linked to tumorigenesis. These bacteria, via pathogen-associated molecular patterns, can upregulate toll-like receptors and activate NF-κB, a critical regulator of inflammation and cancer. NF-κB activation leads to the release of several cytokines, including IL-6, IL-12, IL-17, IL-18, and tumor necrosis factor-alpha, contributing to persistent inflammation within the tumor microenvironment [79]. Secondary metabolites released by intestinal bacteria, along with pro-inflammatory molecules reaching the liver via the portal vein, may further promote carcinogenesis. For instance, butyrate, a microbial metabolite, can enhance the antitumor cytotoxic CD8 T cell response by modulating the ID2-dependent IL-12 signaling pathway [80].
The gut microbiome also plays a role in epigenetic deregulation, potentially affecting tumor development. Microorganisms produce bioactive substances with low molecular weight, such as folates, short-chain fatty acids, and biotin, which can participate in epigenetic processes by altering substrates used for methylation or influencing the activity of epigenetic enzymes [81].
Immune checkpoint inhibitors are a class of therapies that leverage the immune system to combat tumors by blocking inhibitory interactions between T-lymphocyte receptors and ligands on malignant cells. While BC is typically not considered highly immunogenic compared to other malignancies like lung cancer or melanoma, recent data have shown benefits of immunotherapy, particularly in the triple-negative subtype (ER/PR and HER2 negative).
Clinical trials have demonstrated varying response rates with different immunotherapies, alone or in combination with chemotherapy, in these patients. The KEYNOTE-012 trial investigated pembrolizumab monotherapy in previously treated triple-negative breast patients, showing an overall response rate (ORR) of 18.5% and a median time to response of 17.9 weeks [82]. The KEYNOTE-086 trial tested pembrolizumab as a first-line therapy for metastatic triple-negative breast, achieving an ORR of 23% [83]. Other trials, such as NCT01375842 and JAVELIN, assessed atezolizumab and avelumab, with observed ORRs of 10% and 5.2%, respectively [84,85].
Combinations of immunotherapy with chemotherapy were also explored, with atezolizumab combined with nab-paclitaxel yielding an ORR of 67% in first-line treatment [86]. The IMpassion 130 trial examined the combination of atezolizumab and nab-paclitaxel in untreated metastatic triple-negative breast patients, revealing a progression-free survival (PFS) benefit. The ENHANCE-1/KEYNOTE-150 trial evaluated eribulin combined with pembrolizumab, showing a higher ORR in PD-L1-positive breast cancer patients [87]. The KEYNOTE-355 trial combined pembrolizumab with chemotherapy, demonstrating a significant progression-free survival benefit in patients with high PD-L1 values [88]. These trials collectively indicate the potential of immune checkpoint inhibitors in improving outcomes for triple-negative breast patients, particularly in cases with high PD-L1 expression.
4. Treatment Outcomes and Therapy Resistance
The dynamic nature of the gut microbiome, combined with tumor heterogeneity, can lead to varied observations across scientific studies. Numerous reports highlight the alterations in both alpha and beta diversity of the gut microbiome across various cancers, including BC.
A study by Aarnoutse et al., (2022) identified a significant reduction in species richness (p = 0.042) within the gut microbiome of estrogen receptor-positive BC patients during treatment with (neo)adjuvant chemotherapy drugs [89]. A similar trend in decreased gut microbiome richness during chemotherapy was documented by Bilenduke et al., (2022), which later returned to baseline post-treatment [90]. Conversely, Horigome et al. (2019) found no notable shifts in gut microbiome composition between chemotherapy-treated BC patients and untreated counterparts [91]. This disparity was attributed to the fact that the patients had completed their chemotherapy two years before the study’s commencement, suggesting any chemotherapy-induced dysbiosis might have reverted. Various other studies, such as the CANTO trial, reported increased α diversity post-chemotherapy. Wu et al., (2022) made a similar observation for neoadjuvant chemotherapy patients [92].
A depleted microbial richness is linked to intensified depression symptoms, heightened fear of cancer recurrence, and the onset of diarrhea in BC patients undergoing chemotherapy [89,90,93]. Chemotherapy-associated cognitive impairment (CACI) is a widely documented side effect in BC patients, with deficits manifesting in memory retention, processing speed, and visuospatial ability [92,94,95,96]. An array of chemotherapeutic drugs has been implicated in inducing oxidative stress within the brain and promoting inflammation markers within the central nervous system.
Another possible mechanism underlying CACI involves the gut-brain interaction mediated by the peripheral immune system. The “Gut-Immune-Brain Axis” (GBA) theory proposes a communication triad between the intestinal microbiome, immune system, and the brain. Chemotherapy-induced dysbiosis might disrupt the intestinal barrier, leading to immune cell infiltration and the release of pro-inflammatory cytokines into the bloodstream [92]. These cytokines can penetrate the blood-brain barrier (BBB), inducing neuroinflammation, resulting in cognitive disturbances [97]. Several studies support this theory by identifying a correlation between elevated pro-inflammatory cytokines and CACI in BC patients [95,98].
On a positive note, certain bacteria, known for producing butyrate, demonstrate potential anti-inflammatory and anti-cancer properties. The abundance of such beneficial bacteria, including Coprococcus, Ruminococcus, and Faecalibacterium, was noted in BC patients devoid of neurotoxicity post-chemotherapy, pointing to their possible role in curbing neuroinflammation [92].
4.1. Radiotherapy
Radiation therapy, or radiotherapy, primarily functions by damaging the DNA of cancer cells to induce cell death. Typically administered locally, it can also be combined with other treatments. Notably, it’s a well-recorded observation that radiation therapy frequently causes gastrointestinal discomfort as a side effect, even if the gastrointestinal region isn’t directly treated. This indicates a possible involvement of gut microbes in this side effect [99].
Few studies have delved into the impacts of Radiotherapy (RT) or Chemoradiation (CRT) on cancer patients’ gut microbiomes. Specifically, for BC, clinical studies detailing RT’s influence on the gut microbiome are scant, even though RT is a standard treatment for a majority of BC patients. Gynecological cancer patients undergoing RT show a marked decline in gut microbiota richness, exemplified by the decrease in beneficial gut commensal Firmicutes and an increase in opportunistic pathogens like Fusobacterium [100]. Several studies corroborate this finding, revealing that reduced alpha diversity and gut microbiome dysbiosis post-RT and CRT often accompany gastrointestinal side effects or fatigue in various cancers.
In cases of RT, it’s noteworthy that higher alpha gut diversity has been associated with increased tumor infiltration of activated CD4+ T-cells, subsequently leading to better recurrence-free and overall survival rates in cancer patients. While animal studies are foundational in understanding the intricate interactions of radiation, bacteria, and fungi, only one study, as of now, has delved into these interactions in a BC mouse model [101]. This study accentuated the importance of both bacteria and fungi in modulating the outcomes of RT.
Furthermore, studies have shown a connection between gut bacteria and the effectiveness of radiation therapy in treating various cancers. Eradicating Gram-positive bacteria with antibiotics improved radiation’s antitumor effects in melanoma, lung, and cervical cancer mouse models. However, reintroducing the metabolite sodium butyrate, produced by these bacteria, negated this benefit [102]. Complete removal of gut bacteria reduced radiation effectiveness in some cancer models, while gut fungi depletion enhanced it [101]. Fungal sensor Dectin-1’s higher concentrations correlated with poorer breast cancer survival rates. Mice’s response to radiation varied based on their microbiota, with Enterococcaceae and Lachnospiraceae being prominent in responsive mice. Leukemia patients with these bacterial families experienced fewer gastrointestinal symptoms post-radiation. Metabolites from these bacteria were linked to radioprotection. Though research is limited, gut microbiota’s role in radiation response emphasizes the need for more studies on this relationship [103].
4.2. Chemotherapy
Chemotherapy primarily involves the use of medicinal drugs to chemically treat cancer by interfering with cell division (mitosis). The effectiveness of chemotherapy differs across various types of cancer. Its main objective is to harm or overwhelm cancer cells to induce programmed cell death (apoptosis), and some chemotherapy drugs can also stimulate immune reactions. Since chemotherapy is administered throughout the body, it may also impact normal cells that undergo rapid division. The gut microbiome was initially identified as a factor influencing the effectiveness of chemotherapy because systemic administration is likely to affect these microbial communities [104].
Administering antibiotics to neutralize the gut microbiome decreased the effectiveness of chemotherapy drugs like cisplatin and oxaliplatin in treating lymphoma and colon cancer in mice, attributed to reduced reactive oxygen species (ROS) production. This indicates the necessity of a functional commensal microbiome for the success of these platinum-based treatments. Antibiotics also lowered oxaliplatin’s effectiveness in a specific colon cancer mouse model. Subsequent experiments revealed associations between certain microbes and the drug’s effectiveness. For instance, the presence of Paraprevotella clara was linked with a lack of response to oxaliplatin, while B. fragilis correlated with a positive response. In terms of the chemotherapy drug cyclophosphamide, it resulted in the movement of particular gut microbes like Lactobacillus johnsonii and Enterococcus hirae to the spleen and lymph nodes, initiating an immune response in melanoma and sarcoma mouse models. Additional research showed that the existence of E. hirae and Barnesiella intestinihominis in the gut could enhance cyclophosphamide activity and rejuvenate its effectiveness post-antibiotic administration.
Additionally, the CANTO trial documented significant changes in the gut microbiome composition throughout chemotherapy [92]. Favourable commensals like Dorea formicigenerans and Methanobrevibacter smithii archaea, commonly found in healthy individuals, increased in abundance in BC patient’s post-chemotherapy. Coprococcus and members of the Ruminococcaceae and Eubacteriaceae families were positively linked with a good prognosis and the absence of axillary lymph node metastasis. Conversely, harmful bacterial species such as certain Klebsiella and Bacteroides species, along with members of the Lachnospiraceae and Clostridiaceae families, were associated with axillary lymph node invasion and advanced BC stages after chemotherapy. In particular, B. uniformis was prominently abundant in non-metastatic BC of advanced stage but reduced post-chemotherapy. High abundance of Bacteroides also correlated with non-responsiveness to trastuzumab treatment in HER2-positive BC patients [105].
A metabolite from the gut microbiome, indole-3-acetic acid, has been observed to enhance the effectiveness of FOLFIRINOX, a combination chemotherapy used in treating metastatic PDAC. This enhancement is due to the accumulation of ROS and a reduction in the cancer cells’ autophagy. However, influences on chemotherapy effectiveness are not limited to the gut microbiome alone; bacteria in and around the tumor also play a significant role. There’s a growing interest in exploring how these local bacteria impact the effectiveness of chemotherapy and can contribute to drug resistance in cancer patients.
Studies have shown that the commensal bacteria E. coli has the ability to modify the effectiveness of various chemotherapy drugs. It increased the toxicity of some drugs, while reducing the toxicity of others like gemcitabine, doxorubicin, and mitoxantrone. Further experiments involving E. coli and gemcitabine in a mouse model indicated a decrease in the drug’s ability to combat tumors. Likewise, both Mycoplasma hyorhinis and E. coli, have been found to metabolize gemcitabine into an inactive form, making cancer cells resistant. In another study, the commensal F. nucleatum, when combined with colon cancer cells, reduced the cells’ apoptosis when exposed to certain chemotherapy drugs, leading to increased drug resistance. It implies that the local microbes and those within the tumor microenvironment can substantially impact chemotherapy’s effectiveness. On the other hand, manipulating the lung microbiota using aerosolized antibiotics and specifically probiotics has shown improvement in the efficacy of a chemotherapeutic drug, dacarbazine. Such findings underscore the potential of further exploring microbial interactions in various body regions and within the tumor to enhance the outcomes of chemotherapy treatments.
5. Integrating One Health Approach in Cancer Ecology
The holistic approach to health promoted by the One Health principles mainly focuses on infectious diseases and zoonoses [106]. However, the mechanisms underlying oncogenesis and novel management strategies in oncology recognize the interrelations between human health, animal health and the environment, thus placing emphasis to the transdisciplinary and multisectoral relevance of One Health. The connection between environmental, animal and human health and oncogenesis be supported by several observations [107,108]. The transmissibility of specific malignancies between animals: although rare, transmission of cancer between individuals of the same or related species (such as the canine transmissible venereal sarcoma) has been documented [109]. In addition, the transmission mode in several cases remains undefined (e.g., in the case of a leukemia-like disease among marine bivalves, such as clams and mussels [109]. Similarly, from an evolutionary perspective, transmission of cancer between different species remains a possibility [109,110]. The evolutionary cancer suppression mechanisms detected in animal species: one such example is the development of immune-modulating resistance against devil facial tumour disease (DFTD) reported in Tasmanian devils (Sarcophilus harrisii), which probably contributed to survival of the species over this fatal type of transmissible cancer [111]. Another example is that of the myxoma virus (MYXV) and the European rabbit (Oryctolagus cuniculus), which co-evolved, selecting for viruses of attenuated virulence that caused extended (over fatal) disease and in parallel, for rabbits that developed resistance to myxomatosis [112] through genetic alterations that facilitated an enhanced immune response the disease [113]. Such recorded cases provide evidence that transmissible cancers can exert selection pressure on affected species, promoting the domination of organisms with genetic alterations and immune mechanisms that confer a survival benefit. The mutagenetic and oncogenetic ability of human activities to animals: the extent, intensity and nature of human activity have significant impact on the diversity of the environment and on the health and habits of different animal species, resulting in variable contact patterns between animals, arthropods and humans and facilitating the emergence and re-emergence of infectious diseases [114]. Simultaneously, it is well established that environmental alterations resulting from urbanization, air, soil noise, light, and water pollution, environmental degradation, and dietary interventions, precipitate ongogenic processes in humans, thus increasing the risk of cancer. Accumulating literature suggests that these conditions also affect the health of animals, including wildlife, through various pathways, including endocrine and immune dysregulation, chronic inflammation, nutritional disruptions, oncogenic infections, epigenetic changes, chromosomal alterations, and microbiome changes [115,116,117].
The shared environment between animals and humans, affecting microbiota composition and microbiome and unraveling possible causative relationships with cancer development. An organisms’ homeostasis and physiology are closely intercalated with its microbiome. Similarly, environmental perturbations, anthropogenic or not, can influence the microbiota of both humans and animals, with health implications across different organs and systems [118,119,120].
In animal studies, westernized diet-associated bacteria exerted transgenerational effects in utero resulting in chronic disease and cancer in mice [121]. Furthermore, antibiotic-affected microbial communities in bees led to significant alterations in gene expressions in the host, which could also be passed across generations [122]. Such findings signify the close relationship between microbiome, normal host functions, genetic changes and the ability to pass these changes across generations.
Taken together, these observations advocate for collaborative efforts to address the various mechanisms underlying cancer development by acknowledging similarities that exist among animals and humans in a shared ecosystem. Acknowledging that the environment and disease can cause disruptions and imbalances in the host microbiota composition, predisposing to physiologic and homeostatic changes, a One Health approach to cancer comprises of the following:
Surveillance: cancer surveillance across human medicine, veterinary medicine, and environmental science. Areas and species can serve as sentinel targets for early detection of carcinogens in the environment and for cancer patterns that might reflect eco-environmental exposures or cancer types that warrant further research.
Detection, including early warning systems: interdisciplinary collaboration to create diagnostic tools (e.g., biomarkers, screening protocols, geographic information systems). Employment of artificial intelligence and machine learning tools can help combine surveillance, diagnosis and warning systems to facilitate early diagnosis and prompt detection.
Elucidating and addressing common risk factors: several risk factors for cancer, such as exposure to carcinogens and lifestyle choices (e.g., nutrition, physical activity), affect both humans and animals [123]. Understanding the presence and pathophysiologic mechanisms of shared risk factors can help elucidate oncogenetic pathways or design and implement preventive strategies. Surveillance and prompt detection can facilitate the design of future studies in order to elucidate unidentified risks for cancer development in different species [124].
Environmental health: the association between environment and disease is bidirectional. The behavior of animal species can increase cancer risk, through the induction of cancer-associated environmental exposures. Spatial, temporal, ecological and population changes can have documented and unpredicted effects on the emergence, re-emergence and frequency of diseases [114,123]. These are not limited to communicable diseases, but can span across endocrine conditions, malignancies, cardiovascular disease, metabolic conditions, skin conditions, toxin exposures, etc [125]. Up to one fourth of global deaths and one-fifth of disability-adjusted life years (DALYs) in humans are associated to environmental exposures [125]. Conversely, environment-attributable diseases can affect the behavior of animals, leading to further environmental changes. For example, poor living conditions in rural areas drive urbanization, further increasing air and noise pollution in urban areas. In wildlife, diseased animals are more prone to fall to prey, thus increasing the transmission risk of communicable diseases, while representing an inferior nutritional source for preying [89] species. Animals and humans who are malnourished or suffer from chronic disease, have a higher risk of other diseases after exposure, either to environmental factors, pollutants or transmissible agents, increasing the incidence of environment-associated diseases and affecting the growth, behavior and health of animal populations [123]. Cumulatively, environmental degradation increases risk for disease and diseased populations can affect the environment, creating a vicious cycle of eco-environmental health [126].
Cancer prevention: prevention strategies span over different levels, including primordial, primary, secondary, tertiary and quaternary [126]. Health promotion, a principle focused on humans, concerns empowering people to increase control over their own health [127]. In the context of One Health, cancer prevention extends beyond individual and populational levels of health, to the broad geospatial and interspecies framework of ecosystems [123].
Antimicrobial stewardship: an important and emerging aspect of One Health, the ecological effects of antimicrobials at the population and environmental level are globally recognized. Exposure to antimicrobials facilitates the dominance of resistant bacterial populations in the environment, acquiring genes which have the potential to transmit in the environment, from organism to organisms, and between species [128]. Antimicrobial usage has been found to be directly related to the number of resistance genes in the gut microbiome of humans, even in the absence of direct antimicrobial administration [129]. Water and soil constitute significant environmental reservoirs of antimicrobial resistant genes [130]. Antimicrobial resistant genes can spread in the environment to such an extent that can even reach air masses in areas of high antimicrobial usage [131]. These observations raise the imminent need for application stringent efforts to control transmission of antimicrobial resistance in the community. The strongest risk for emergence of antimicrobial resistance in community settings is inappropriate use of antimicrobials in humans, animals and agriculture [130]. Indeed, the volume of inappropriate use of antimicrobials in animal husbandry, veterinary medicine and agriculture, significantly exceeds their use in human medicine, representing a key target for stewardship interventions[132]. Antibiotic-associated tissue dysbiosis has been associated with tumor development, further supporting the need for prudent use of antimicrobials in the animals [133]. In addition, disruption of normal immune responses to malignant cells, induced by antimicrobials, can have detrimental outcomes in organisms with cancer, by affecting their responses both to cancer and to therapeutic agents [134].
Ethical considerations: considerations related to ethical issues surrounding animal welfare, humane treatment, healthy livelihood, access to optimal healthcare and experimental studies, for all living organisms are integrated into the One Health approach, ensuring that research, medical interventions and environmental health are addressed under the same moral boundaries [127,135,136].
6. Conclusion
Employing agents involved in multiple human biological reactions such as immune response and metabolism may be key to developing tumour drugs with higher selectivity and specificity hence reducing adverse side effects for patients. These drugs could be prescribed in synergy with traditional cancer treatments which includes radiation, immunotherapy or chemotherapy. These forms of treatment can potentially diminish drug resistance. A microbiome screen test can pinpoint potential target that may help improve patient prognosis, as previously described, but may also avert drug resistance. Additionally, standardised protocols need to be composed to ensure clinical guidelines and protocols are available. Such a uniform system will also allow for collection of efficiency and safety evidence. Breast tumours can be accessed by adjusting the gut microbiota or by means of microbiota transport vectors introduced peritoumorally. Ultimately the relationship between gut and breast microbiome colonies needs to be scrutinised prior to designing new drugs that work in this way and evaluate what effects these microbiomes will have on the immune system.
6.1. Integration of microbial therapy within the one health approach
The concept of ONE Health describes a transdisciplinary approach considering the interaction between plants, animals, people, and the environment they share. The basis of this concept is to recognise and eliminate zoonotic diseases, water contaminants, antimicrobial-resistant germs, vector-borne diseases and more. Evidence so far supports that there is a strong link between microbiome and cancer development, cancer proliferation and apoptosis. Specific human microbiome also serve as biomarkers for certain cancers. Shifting the microbiome (expression) accordingly reverses these affects and it does so through a more holistic approach [137]. Manipulating the gut microbiota through such an approach makes for more personalised treatments with fewer side effects and potentially greater health outcomes. This could also improve patients mental position as they may appreciate the concept being offered a more holistic treatment option as to chemical drugs.
Interdisciplinary collaboration between human and animal researchers can advance microbial research by providing epidemiological insights of the disease. A partnership between veterinary medicine and human medicine can increase the amount of data available on cancer and behaviour of microbiota and provide valuable information that can help progress therapeutic methods. As comparative and spontaneous oncology emerge, more information becomes available as to how an animals’ immune system fights off neoplasms. Extrapolation of this information can increase understanding on the behaviour of a cancer and hence provide novel therapeutic targets and treatment schemes [138]. More importantly as ownership of both domestic and exotic pets is increasing, the need to survey ONE Health elements it crucial to monitor and potentially prevent outbreaks.
Transmissible cancers are emerging, accelerating the loss of biodiversity across ecosystems redirecting research as to why these cancers arise and how they unfold and prevent any potential pandemics [108]. An additional aspect that’s requires further evaluation is the degree to which malignancies in animals, such as breast cancer, are transmissible to humans.
Identifying the exact microbiome species through genetic analysis can uncover their environmental origin, this could be animal or other food products. Chemical agents also disturb microbiome. Fertilisers provide crops with nonspecific nutrients meaning that microbiota populations are also nourished hence proliferate, once produce is consumed by human the gut microbiota is subsequently increased [139]. Infant feeding, infections, medication and diet are among the environmental factors that need to be further assessed to further understand the impact they could have on breast cancer. An ongoing study (NCT03885648) is evaluating the hypothesis that risk of breast cancer for humans could be due to environmental contaminants affecting the mammary/gut microbiota.
6.2. Regulatory considerations and ethical implications
Despite emerging microbiome-based therapies presenting promising therapeutic results against BC therapy both regulatory and ethical considerations must be thoughtfully evaluated. This is to ensure patient safety and diagnosis. Prior to marketing a drug for microbiome-based therapy, clinical trials are essential to evaluate drug safety and efficacy followed by evaluation of the results from the Food and Drug Administration (FDA) and European Medicines Agency (EMA). Following the marketing of drugs, off-label use by clinicians may also be a factor to consider in terms of both regulatory and ethical concerns, ensuring the patient is constantly informed of the efficacy and limitations of the treatment they are receiving. It is critical to ensure that with informed consent patients are fully aware of the limited knowledge available on long-term effects of administering microbiome therapies [140]. Microbiome can express both tumour suppressor and promoter properties, therefore interventions causing dysbiosis must be investigated to identify how genes and lifestyle (e.g., diet) affect the natural biodiversity of microbiome. This information will also affect patient response to microbial therapy. Access to microbiome therapies is also of ethical concern, to ensure individuals from different populations and socioeconomical groups all have access to these drugs. Utilisation of big data and machine learning for precision medicine is already being discussed. This raises data privacy concerns as use of omics technologies will involve databases that store and constantly analyse sensitive data on the gut microbiome of multiple individuals. Setting up such data sets calls for adherence to ethical guidelines and approval from ethical committees and requires completion of informed consent forms [141].
6.3. Future directions
Suitable regulation of gastrointestinal microbiome by antibiotics or dietary regimes restrains estrogen effects and regulates bacterial activities which in turn can prevent breast cancer or breast cancer reoccurrence. As microbes translocate to mammary tissue from the gut they also affect how the inflammatory response is triggered [142]. Further gene analysis is required to map all commensal microorganisms and other wild-type microorganisms naturally expressed in the human body that contribute to additional genetic material necessary for human metabolism. The focal aspects that need to be determined to ensure a microbiome can be labelled as therapeutic agents are to first identify the species naturally present in the human body under ‘healthy’ conditions, determine the ‘healthy’ concentrations of these microbiota and finally the levels at which these microorganisms activate harmful responses.
Mapping out all microbes that inhabit a patient’s body can provide insights of the treatment plan that should be administrated. This is important as microbiota in both the gut and within the tumour environment can affect drug metabolism and disturb activity of chemotherapeutic agents [143]. By reversing dysbiosis the microbiome can be manipulated to reverse microbiota to ‘health’ levels avoiding tumour drug resistance and subsequently harmful effects instigated by these drugs. Dietary plans may also accompany drug administration for more efficient results, for instance in vivo results presented that hyperbaric oxygen therapy accompanied by a ketogenic diet express significant antitumor effect [144].
Proceeding with microbial therapies will also require supplementary investigation to understand how microbiome-to-microbiome interaction can affect patient prognosis. This is necessary as the same drug can shape up differently in every human individual due to the unique composition of their microbiome [145]. Additionally, the shift age exerts on microbiome concentrations is also vital as it may affect a patient’s diagnosis and prognosis. The stage of the cancer should also be considered as this affects the concentration of the microbiome but also other conditions such as whether the patient is in a pre- or postmenopausal state [146]. Evidently, Lachnospiraceae and Clostridium are abundant in stage II and III breast cancer patient gut microbiome as compared to stage 0 and stage I. The level of dysbiosis is also affected by the menopausal state of breast cancer. Post-menopausal breast cancer patients express a higher presence of pathogenic bacteria than pre-menopausal patients proposing a non-invasive approach as these concentrations can be rereferred to as markers for detection, prevention and potentially therapeutic targets [147].
Fucobacterium nucleatum is an anaerobe that suppresses T cell accumulation while promoting tumour growth and stepping up metastasis [148]. Additional evaluation as to whether eliminating the microbiota will have the opposite effect and allow for anticancer cells to react effectively. An additional pathway requiring further exploration is the servicing of E.coli secretome as biomarkers for breast cancer as they disrupt key metabolic pathways of MCF-7 cells [75]. As microbial settlements are volatile, continuous monitoring is essential to ensure suitable adjustments are made to increase treatment efficacy. Clinical trials are necessary to further evaluate the effectiveness and safety of these therapies both short and long-term. The latter is especially crucial given that some microbiota are key for normal biological functions thus altering their concentrations or introducing them in other parts of the human body than their normal environment can have unintentional consequences.
6.4. Promising avenues for further research and development
Bacteriotherapy has been a possibility since 1988 and involves implantation of gut flora from a healthy individual to a patient reversing gut dysbiosis to improve diagnosis [149]. Bacteria have been enlisted in the treatment regime of multiple cancers; Salmonella Typhimurium for colon breast carcinoma, Escherichia coli for nasopharyngeal carcinoma, Klebsiella pneumoniae for cervical cancer and pseudomonas aeruginosa in multiple cancers, C. botulinum in breast cancer [150,151]. Due to their anaerobic properties’ microbiota can enter a tumour microenvironment without being affected by its necrotic and hypoxic properties. One mode of action is utilising the microbiome as a vector to deliver chemotherapeutic drugs deep into the tumour so that they may exert their apoptotic tumour effects [152]. An alternative mechanism is one where microbiome expression is tuned to ‘healthy’ levels permitting chemotherapeutic drugs to perform their effects successfully within the human body without interference and with limited adverse side effects. Having determined the fluctuations that microbial community concentrations undergo due to the presence of a tumour, has marked novel biomarkers to be targeted for tumour diagnosis. Future diagnoses of cancer may involve completion of genomic profile screening on a patient biopsy to identify the dysregulated microbes that need to be targeted. These findings can then be utilised to establish an effective patient-specific cancer-microbial therapy plan with the least side effects.
Assuming microbial therapy where fecal microbiota transplantation is involved will require detailed screening of the donor to ensure the material that will be retrieved is free from alternate diseases or even infectious agents that could maltreat the patient. As materials are extracted from another human donor this may also lead to unexpected immunological side effects within the patient. Finally, similarly to other novel treatments, long-term safety is unknown.
In this review, we explore the influence of both gut and local microbiomes on various aspects of cancer care, encompassing its development, progression, diagnosis, and treatment. Recent advancements have seen groups harnessing synthetic biology to modify commensal microbes, triggering specific immune responses against diseases, including cancer. As research techniques and analytical models evolve, our understanding of the microbiomes within and around us will deepen. We foresee a marked rise in the discovery of microbes associated with cancer, going beyond just the easily cultured ones. These newly discovered microbes present promising avenues for refining diagnostic and therapeutic strategies, setting the stage for enhanced cancer prevention and more effective treatment outcomes. Additionally, interdisciplinary collaborations between human, animal, and environmental health researchers will provide invaluable insights into cancer-microbiome interplay. Comprehending the identities, distributions, and activities of human microbes in health and disease through a One Health approach is essential for strategically modulating the microbiome and harnessing its intricate ties with breast cancer for improved patient outcomes.
Figure 1.
One health Conceptual Diagram. One health includes Human Health and Well-being, Animal Health, Environment health as well as the Microbial and biochemical Ecology of Soils and Plants. The interconnectivity and dynamic interaction between all of them emphasizes the level of each can affect the other by stressing the need of their balance.
Figure 1.
One health Conceptual Diagram. One health includes Human Health and Well-being, Animal Health, Environment health as well as the Microbial and biochemical Ecology of Soils and Plants. The interconnectivity and dynamic interaction between all of them emphasizes the level of each can affect the other by stressing the need of their balance.
Figure 2.
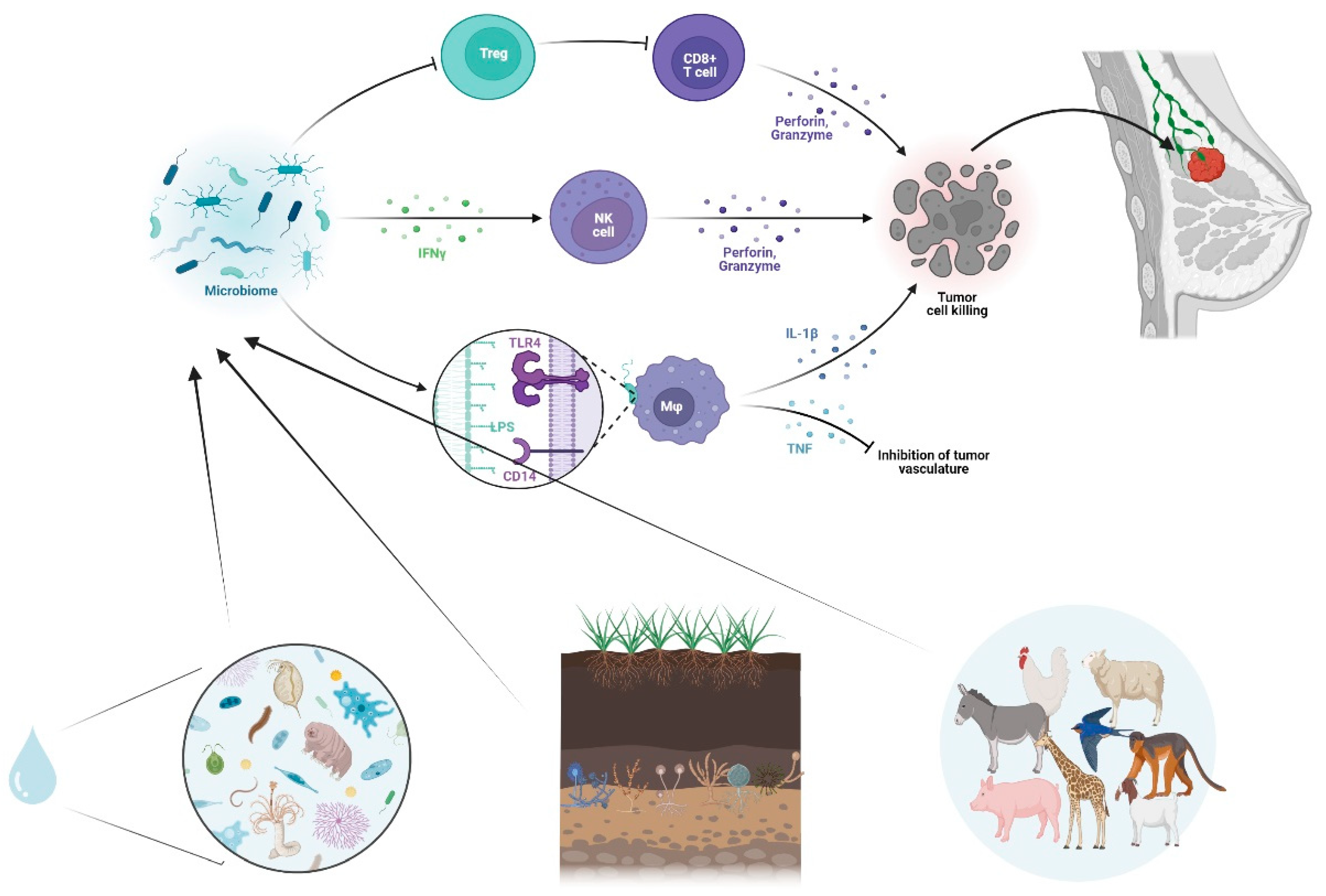
Microbiome and Tumor Microenvironment interplay. Schematic illustration of the Microbiome –mediated modulation of the immune system, including Toll-Like Receptor activation, Cytokine release, anti-tumor responses, inhibition of tumor vasculature and immune cell activation.
Figure 2.
Microbiome and Tumor Microenvironment interplay. Schematic illustration of the Microbiome –mediated modulation of the immune system, including Toll-Like Receptor activation, Cytokine release, anti-tumor responses, inhibition of tumor vasculature and immune cell activation.
Author Contributions
Conceptualization, A.Y. and C.F.; data curation, A.Y, C.F, S.C.T., G.M., Y.P., M.F.; writing—original draft preparation, A.Y., C.F., S.C.T., C.A.K., Z.-D.P. and C.T.; writing—review and editing, A.Y., C.F., S.C.T., C.A.K, Z.-D.P. and E.O.J.All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Artusa, V. , Calabrone, L., Mortara, L., Peri, F., Bruno, A.: Microbiota-Derived Natural Products Targeting Cancer Stem Cells: Inside the Gut Pharma Factory. IJMS. 24, 4997 (2023). [CrossRef]
- Xuan, C. , Shamonki, J.M., Chung, A., DiNome, M.L., Chung, M., Sieling, P.A., Lee, D.J.: Microbial Dysbiosis Is Associated with Human Breast Cancer. PLoS ONE. 9, e83744 (2014). [CrossRef]
- Nejman, D. , Livyatan, I., Fuks, G., Gavert, N., Zwang, Y., Geller, L.T., Rotter-Maskowitz, A., Weiser, R., Mallel, G., Gigi, E., Meltser, A., Douglas, G.M., Kamer, I., Gopalakrishnan, V., Dadosh, T., Levin-Zaidman, S., Avnet, S., Atlan, T., Cooper, Z.A., Arora, R., Cogdill, A.P., Khan, M.A.W., Ologun, G., Bussi, Y., Weinberger, A., Lotan-Pompan, M., Golani, O., Perry, G., Rokah, M., Bahar-Shany, K., Rozeman, E.A., Blank, C.U., Ronai, A., Shaoul, R., Amit, A., Dorfman, T., Kremer, R., Cohen, Z.R., Harnof, S., Siegal, T., Yehuda-Shnaidman, E., Gal-Yam, E.N., Shapira, H., Baldini, N., Langille, M.G.I., Ben-Nun, A., Kaufman, B., Nissan, A., Golan, T., Dadiani, M., Levanon, K., Bar, J., Yust-Katz, S., Barshack, I., Peeper, D.S., Raz, D.J., Segal, E., Wargo, J.A., Sandbank, J., Shental, N., Straussman, R.: The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science. 368, 973–980 (2020). [CrossRef]
- Dohlman, A.B. , Klug, J., Mesko, M., Gao, I.H., Lipkin, S.M., Shen, X., Iliev, I.D.: A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell. 185, 3807-3822.e12 (2022). [CrossRef]
- Fu, A. , Yao, B., Dong, T., Chen, Y., Yao, J., Liu, Y., Li, H., Bai, H., Liu, X., Zhang, Y., Wang, C., Guo, Y., Li, N., Cai, S.: Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 185, 1356-1372.e26 (2022). [CrossRef]
- Lakritz, J.R. , Poutahidis, T., Mirabal, S., Varian, B.J., Levkovich, T., Ibrahim, Y.M., Ward, J.M., Teng, E.C., Fisher, B., Parry, N., Lesage, S., Alberg, N., Gourishetti, S., Fox, J.G., Ge, Z., Erdman, S.E.: Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. 6, 9387–9396 (2015). [CrossRef]
- Liu, M. , Jia, S., Dong, T., Zhao, F., Xu, T., Yang, Q., Gong, J., Fang, M.: Metabolomic and Transcriptomic Analysis of MCF-7 Cells Exposed to 23 Chemicals at Human-Relevant Levels: Estimation of Individual Chemical Contribution to Effects. Environ Health Perspect. 128, 127008 (2020). [CrossRef]
- Xu, J.-Y. , Liu, M.-T., Tao, T., Zhu, X., Fei, F.-Q.: The role of gut microbiota in tumorigenesis and treatment. Biomedicine & Pharmacotherapy. 138, 111444 (2021). [CrossRef]
- Roy, S. , Trinchieri, G.: Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 17, 271–285 (2017). [CrossRef]
- Tzeng, A. , Sangwan, N., Jia, M., Liu, C.-C., Keslar, K.S., Downs-Kelly, E., Fairchild, R.L., Al-Hilli, Z., Grobmyer, S.R., Eng, C.: Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 13, 60 (2021). [CrossRef]
- Maroof, H. , Hassan, Z.M., Mobarez, A.M., Mohamadabadi, M.A.: Lactobacillus acidophilus Could Modulate the Immune Response Against Breast Cancer in Murine Model. J Clin Immunol. 32, 1353–1359 (2012). [CrossRef]
- Bernardo, G. , Le Noci, V., Ottaviano, E., De Cecco, L., Camisaschi, C., Guglielmetti, S., Di Modica, M., Gargari, G., Bianchi, F., Indino, S., Sartori, P., Borghi, E., Sommariva, M., Tagliabue, E., Triulzi, T., Sfondrini, L.: Reduction of Staphylococcus epidermidis in the mammary tumor microbiota induces antitumor immunity and decreases breast cancer aggressiveness. Cancer Letters. 555, 216041 (2023). [CrossRef]
- Cai, Z. , Sanchez, A., Shi, Z., Zhang, T., Liu, M., Zhang, D.: Activation of Toll-like Receptor 5 on Breast Cancer Cells by Flagellin Suppresses Cell Proliferation and Tumor Growth. Cancer Research. 71, 2466–2475 (2011). [CrossRef]
- Zhu, J. , Liao, M., Yao, Z., Liang, W., Li, Q., Liu, J., Yang, H., Ji, Y., Wei, W., Tan, A., Liang, S., Chen, Y., Lin, H., Zhu, X., Huang, S., Tian, J., Tang, R., Wang, Q., Mo, Z.: Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 6, 136 (2018). [CrossRef]
- Lakey, J.H., Slatin, S.L.: Pore-Forming Colicins and Their Relatives. In: Van Der Goot, F.G. (ed.) Pore-Forming Toxins. pp. 131–161. Springer Berlin Heidelberg, Berlin, Heidelberg (2001). 2001. [CrossRef]
- Kaur, S. , Kaur, S.: Bacteriocins as Potential Anticancer Agents. Front. Pharmacol. 6, (2015). [CrossRef]
- Baindara, P. , Gautam, A., Raghava, G.P.S., Korpole, S.: Anticancer properties of a defensin like class IId bacteriocin Laterosporulin10. Sci Rep. 7, 46541 (2017). [CrossRef]
- Hidaka, A. , Hamaji, Y., Sasaki, T., Taniguchi, S., Fujimori, M.: Exogeneous Cytosine Deaminase Gene Expression in Bifidobacterium breve I-53-8w for Tumor-Targeting Enzyme/Prodrug Therapy. Bioscience, Biotechnology, and Biochemistry. 71, 2921–2926 (2007). [CrossRef]
- Silva, E.O. , De Carvalho, T.C., Parshikov, I.A., Dos Santos, R.A., Emery, F.S., Furtado, N.A.J.C.: Cytotoxicity of lapachol metabolites produced by probiotics. Lett Appl Microbiol. 59, 108–114 (2014). [CrossRef]
- Karami, P. , Goli, H.R., Abediankenari, S., Chandani, S.R., Jafari, N., Ghasemi, M., Ahanjan, M.: Anti-tumor effects of Bacteroides fragilis and Bifidobacterium bifidum culture supernatants on mouse breast cancer. Gene Reports. 33, 101815 (2023). [CrossRef]
- Bernardo, G. , Le Noci, V., Di Modica, M., Montanari, E., Triulzi, T., Pupa, S.M., Tagliabue, E., Sommariva, M., Sfondrini, L.: The Emerging Role of the Microbiota in Breast Cancer Progression. Cells. 12, 1945 (2023). [CrossRef]
- Zhu, R. , Lang, T., Yan, W., Zhu, X., Huang, X., Yin, Q., Li, Y.: Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy. Advanced Science. 8, (2021). [CrossRef]
- Mendes, I. , Vale, N.: How Can the Microbiome Induce Carcinogenesis and Modulate Drug Resistance in Cancer Therapy? International Journal of Molecular Sciences. 24, 11855 (2023). [CrossRef]
- Trivanović, D. , Pavelić, K., Peršurić, Ž.: Fighting Cancer with Bacteria and Their Toxins. Int J Mol Sci. 22, 12980 (2021). [CrossRef]
- Khoshnood, S. , Fathizadeh, H., Neamati, F., Negahdari, B., Baindara, P., Abdullah, M.A., Haddadi, M.H.: Bacteria-derived chimeric toxins as potential anticancer agents. Frontiers in Oncology. 2022; 12. [Google Scholar] [CrossRef]
- Browne, K. , Chakraborty, S., Chen, R., Willcox, M.D., Black, D.S., Walsh, W.R., Kumar, N.: A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int J Mol Sci. 21, 7047 (2020). [CrossRef]
- Patyar, S. , Joshi, R., Byrav, D.P., Prakash, A., Medhi, B., Das, B.: Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 17, 21 (2010). [CrossRef]
- Nandi, D. , Parida, S., Sharma, D.: The gut microbiota in breast cancer development and treatment: The good, the bad, and the useful! Gut Microbes. 15, 2221452. [CrossRef]
- Allemailem, K.S. : Innovative Approaches of Engineering Tumor-Targeting Bacteria with Different Therapeutic Payloads to Fight Cancer: A Smart Strategy of Disease Management. International Journal of Nanomedicine. 16, 8159 (2021). [CrossRef]
- Liang, S. , Wang, C., Shao, Y., Wang, Y., Xing, D., Geng, Z.: Recent advances in bacteria-mediated cancer therapy. Frontiers in Bioengineering and Biotechnology. 2022; 10. [Google Scholar] [CrossRef]
- Duong, M.T.-Q. , Qin, Y., You, S.-H., Min, J.-J.: Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 51, 152 (2019). [CrossRef]
- Wei, X. , Du, M., Chen, Z., Yuan, Z.: Recent Advances in Bacteria-Based Cancer Treatment. Cancers (Basel). 14, 4945 (2022). [CrossRef]
- Freedman, J.C. , Shrestha, A., McClane, B.A.: Clostridium perfringens Enterotoxin: Action, Genetics, and Translational Applications. Toxins (Basel). 8, 73 (2016). [CrossRef]
- Cardillo, F. , Bonfim, M., da Silva Vasconcelos Sousa, P., Mengel, J., Ribeiro Castello-Branco, L.R., Pinho, R.T.: Bacillus Calmette–Guérin Immunotherapy for Cancer. Vaccines (Basel). 9, 439 (2021). [CrossRef]
- Nallar, S.C. , Xu, D.-Q., Kalvakolanu, D.V.: Bacteria and genetically modified bacteria as cancer therapeutics: Current advances and challenges. Cytokine. 89, 160–172 (2017). [CrossRef]
- McCarthy, E.F. : The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop J. 2006; 26, 154–158. [Google Scholar]
- Forbes, N.S. : Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 10, 785–794 (2010). [CrossRef]
- Hatakeyama, M. : Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 15, 306–316 (2014). [CrossRef]
- Felgner, S. , Kocijancic, D., Frahm, M., Heise, U., Rohde, M., Zimmermann, K., Falk, C., Erhardt, M., Weiss, S.: Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology. 7, e1382791 (2018). [CrossRef]
- Field, D. , Cotter, P.D., Ross, R.P., Hill, C.: Bioengineering of the model lantibiotic nisin. Bioengineered. 6, 187–192 (2015). [CrossRef]
- Roberts, N.J. , Zhang, L., Janku, F., Collins, A., Bai, R.-Y., Staedtke, V., Rusk, A.W., Tung, D., Miller, M., Roix, J., Khanna, K.V., Murthy, R., Benjamin, R.S., Helgason, T., Szvalb, A.D., Bird, J.E., Roy-Chowdhuri, S., Zhang, H.H., Qiao, Y., Karim, B., McDaniel, J., Elpiner, A., Sahora, A., Lachowicz, J., Phillips, B., Turner, A., Klein, M.K., Post, G., Diaz, L.A., Riggins, G.J., Papadopoulos, N., Kinzler, K.W., Vogelstein, B., Bettegowda, C., Huso, D.L., Varterasian, M., Saha, S., Zhou, S.: Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 6, 249ra111 (2014). [CrossRef]
- Bhardwaj, N. , Gnjatic, S., Sawhney, N.B.: TLR AGONISTS: Are They Good Adjuvants? Cancer journal (Sudbury, Mass.). 16, 382 (2010). [CrossRef]
- Kaufman, H.L. , Kohlhapp, F.J., Zloza, A.: Erratum: Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 15, 660–660 (2016). [CrossRef]
- Fan, J. , Jiang, H., Cheng, L., Liu, R.: The oncolytic herpes simplex virus vector, G47Δ, effectively targets tamoxifen-resistant breast cancer cells. Oncology Reports. 35, 1741–1749 (2016). [CrossRef]
- Wang, L.-J. , Han, S.-X., Bai, E., Zhou, X., Li, M., Jing, G.-H., Zhao, J., Yang, A.-G., Zhu, Q.: Dose-dependent effect of tamoxifen in tamoxifen-resistant breast cancer cells via stimulation by the ERK1/2 and AKT signaling pathways. Oncology Reports. 29, 1563–1569 (2013). [CrossRef]
- Zeng, W.-G. , Li, J.-J., Hu, P., Lei, L., Wang, J.-N., Liu, R.-B.: An oncolytic herpes simplex virus vector, G47Δ, synergizes with paclitaxel in the treatment of breast cancer. Oncology Reports. 29, 2355–2361 (2013). [CrossRef]
- Soliman, H. , Hogue, D., Han, H., Mooney, B., Costa, R., Lee, M.C., Niell, B., Williams, A., Chau, A., Falcon, S., Soyano, A., Armaghani, A., Khakpour, N., Weinfurtner, R.J., Hoover, S., Kiluk, J., Laronga, C., Rosa, M., Khong, H., Czerniecki, B.: Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial. Nat Med. 29, 450–457 (2023). [CrossRef]
- Schmid, P. , Cortes, J., Dent, R., Pusztai, L., McArthur, H., Kümmel, S., Bergh, J., Denkert, C., Park, Y.H., Hui, R., Harbeck, N., Takahashi, M., Untch, M., Fasching, P.A., Cardoso, F., Andersen, J., Patt, D., Danso, M., Ferreira, M., Mouret-Reynier, M.-A., Im, S.-A., Ahn, J.-H., Gion, M., Baron-Hay, S., Boileau, J.-F., Ding, Y., Tryfonidis, K., Aktan, G., Karantza, V., O’Shaughnessy, J.: Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med. 386, 556–567 (2022). [CrossRef]
- Catala, A. , Dzieciatkowska, M., Wang, G., Gutierrez-Hartmann, A., Simberg, D., Hansen, K.C., D’Alessandro, A., Catalano, C.E.: Targeted Intracellular Delivery of Trastuzumab Using Designer Phage Lambda Nanoparticles Alters Cellular Programs in Human Breast Cancer Cells. ACS Nano. 15, 11789–11805 (2021). [CrossRef]
- Dong, X. , Pan, P., Ye, J.-J., Zhang, Q.-L., Zhang, X.-Z.: Hybrid M13 bacteriophage-based vaccine platform for personalized cancer immunotherapy. Biomaterials. 289, 121763 (2022). [CrossRef]
- Wu, H. , Ganguly, S., Tollefsbol, T.O.: Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment. Microorganisms. 10, 1727 (2022). [CrossRef]
- Rutkowski, M.R. , Stephen, T.L., Svoronos, N., Allegrezza, M.J., Tesone, A.J., Perales-Puchalt, A., Brencicova, E., Escovar-Fadul, X., Nguyen, J.M., Cadungog, M.G., Zhang, R., Salatino, M., Tchou, J., Rabinovich, G.A., Conejo-Garcia, J.R.: Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell. 27, 27–40 (2015). [CrossRef]
- Schwabe, R.F. , Jobin, C.: The microbiome and cancer. Nat Rev Cancer. 13, 800–812 (2013). [CrossRef]
- Garrett, W.S. : Cancer and the microbiota. Science. 348, 80–86 (2015). [CrossRef]
- Eslami-S, Z. , Majidzadeh-A, K., Halvaei, S., Babapirali, F., Esmaeili, R.: Microbiome and Breast Cancer: New Role for an Ancient Population. Front. Oncol. 10, 120 (2020). [CrossRef]
- Luu, T.H. , Michel, C., Bard, J.-M., Dravet, F., Nazih, H., Bobin-Dubigeon, C.: Intestinal Proportion of Blautia sp. is Associated with Clinical Stage and Histoprognostic Grade in Patients with Early-Stage Breast Cancer. Nutrition and Cancer. 69, 267–275 (2017). [CrossRef]
- Malik, S.S. , Saeed, A., Baig, M., Asif, N., Masood, N., Yasmin, A.: Anticarcinogenecity of microbiota and probiotics in breast cancer. International Journal of Food Properties. 21, 655–666 (2018). [CrossRef]
- Yazdi, M. , Mahdavi, M., Kheradmand, E., Shahverdi, A.: The Preventive Oral Supplementation of a Selenium Nanoparticle-enriched Probiotic Increases the Immune Response and Lifespan of 4T1 Breast Cancer Bearing Mice. Arzneimittelforschung. 62, 525–531 (2012). [CrossRef]
- Aragón, F. , Carino, S., Perdigón, G., De Moreno De LeBlanc, A.: Inhibition of Growth and Metastasis of Breast Cancer in Mice by Milk Fermented With Lactobacillus casei CRL 431. Journal of Immunotherapy. 38, 185–196 (2015). [CrossRef]
- Chitapanarux, I. , Chitapanarux, T., Traisathit, P., Kudumpee, S., Tharavichitkul, E., Lorvidhaya, V.: Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol. 5, 31 (2010). [CrossRef]
- Mego, M. , Chovanec, J., Vochyanova-Andrezalova, I., Konkolovsky, P., Mikulova, M., Reckova, M., Miskovska, V., Bystricky, B., Beniak, J., Medvecova, L., Lagin, A., Svetlovska, D., Spanik, S., Zajac, V., Mardiak, J., Drgona, L.: Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complementary Therapies in Medicine. 23, 356–362 (2015). [CrossRef]
- Hibberd, A.A. , Lyra, A., Ouwehand, A.C., Rolny, P., Lindegren, H., Cedgård, L., Wettergren, Y.: Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 4, e000145 (2017). [CrossRef]
- Theodoropoulos, G.E. , Memos, N.A., Peitsidou, K., Karantanos, T., Spyropoulos, B.G., Zografos, G.: Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann Gastroenterol. 2016; 29, 56–62. [Google Scholar]
- Demers, M. , Dagnault, A., Desjardins, J.: A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clinical Nutrition. 33, 761–767 (2014). [CrossRef]
- Österlund, P. , Ruotsalainen, T., Korpela, R., Saxelin, M., Ollus, A., Valta, P., Kouri, M., Elomaa, I., Joensuu, H.: Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 97, 1028–1034 (2007). [CrossRef]
- Dizman, N. , Meza, L., Bergerot, P., Alcantara, M., Dorff, T., Lyou, Y., Frankel, P., Cui, Y., Mira, V., Llamas, M., Hsu, J., Zengin, Z., Salgia, N., Salgia, S., Malhotra, J., Chawla, N., Chehrazi-Raffle, A., Muddasani, R., Gillece, J., Reining, L., Trent, J., Takahashi, M., Oka, K., Higashi, S., Kortylewski, M., Highlander, S.K., Pal, S.K.: Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 28, 704–712 (2022). [CrossRef]
- Toi, M. , Hirota, S., Tomotaki, A., Sato, N., Hozumi, Y., Anan, K., Nagashima, T., Tokuda, Y., Masuda, N., Ohsumi, S., Ohno, S., Takahashi, M., Hayashi, H., Yamamoto, S., Ohashi, Y.: Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-control Study. CNF. 9, 194–200 (2013). [CrossRef]
- Juan, Z. , Chen, J., Ding, B., Yongping, L., Liu, K., Wang, L., Le, Y., Liao, Q., Shi, J., Huang, J., Wu, Y., Ma, D., Ouyang, W., Tong, J.: Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomised, double-blind, and placebo-controlled trial. European Journal of Cancer. 161, 10–22 (2022). [CrossRef]
- Khazaei, Y. , Basi, A., Fernandez, M.L., Foudazi, H., Bagherzadeh, R., Shidfar, F.: The effects of synbiotics supplementation on reducing chemotherapy-induced side effects in women with breast cancer: a randomized placebo-controlled double-blind clinical trial. BMC Complement Med Ther. 23, 339 (2023). [CrossRef]
- Shanmugam, M. , Abirami, R.G.: Microbial Polysaccharides - Chemistry and Applications. Journal of Biologically Active Products from Nature. 9, 73–78 (2019). [CrossRef]
- Ullah, S. , Khalil, A.A., Shaukat, F., Song, Y.: Sources, Extraction and Biomedical Properties of Polysaccharides. Foods. 8, 304 (2019). [CrossRef]
- Lemieszek, M. , Rzeski, W.: Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. wo. 4, 285–289 (2012). [CrossRef]
- Chow, L.W.C. , Lo, C.S.Y., Loo, W.T.Y., Hu, X., Sham, J.S.T.: Polysaccharide Peptide Mediates Apoptosis by Up-regulating p21 Gene and Down-regulating Cyclin D 1 Gene. Am. J. Chin. Med. 31, 1–9 (2003). [CrossRef]
- L.Y. Eliza, W., K. Fai, C., P. Chung, L.: Efficacy of Yun Zhi (Coriolus versicolor) on Survival in Cancer Patients: Systematic Review and Meta-Analysis. IAD. 6, 78–87 (2012). [CrossRef]
- Queiroz, E.A.I.F. , Fortes, Z.B., Da Cunha, M.A.A., Sarilmiser, H.K., Barbosa Dekker, A.M., Öner, E.T., Dekker, R.F.H., Khaper, N.: Levan promotes antiproliferative and pro-apoptotic effects in MCF-7 breast cancer cells mediated by oxidative stress. International Journal of Biological Macromolecules. 102, 565–570 (2017). [CrossRef]
- Alonso, E.N. , Ferronato, M.J., Fermento, M.E., Gandini, N.A., Romero, A.L., Guevara, J.A., Facchinetti, M.M., Curino, A.C.: Antitumoral and antimetastatic activity of Maitake D-Fraction in triple-negative breast cancer cells. Oncotarget. 9, 23396–23412 (2018). [CrossRef]
- Kodama, N. , Komuta, K., Nanba, H.: Effect of Maitake ( Grifola frondosa ) D-Fraction on the Activation of NK Cells in Cancer Patients. Journal of Medicinal Food. 6, 371–377 (2003). [CrossRef]
- Alpuim Costa, D. , Nobre, J.G., Batista, M.V., Ribeiro, C., Calle, C., Cortes, A., Marhold, M., Negreiros, I., Borralho, P., Brito, M., Cortes, J., Braga, S.A., Costa, L.: Human Microbiota and Breast Cancer—Is There Any Relevant Link?—A Literature Review and New Horizons Toward Personalised Medicine. Front. Microbiol. 12, 584332 (2021). [CrossRef]
- Laborda-Illanes, A. , Sanchez-Alcoholado, L., Dominguez-Recio, M.E., Jimenez-Rodriguez, B., Lavado, R., Comino-Méndez, I., Alba, E., Queipo-Ortuño, M.I.: Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment. Cancers. 12, 2465 (2020). [CrossRef]
- Wu, M. , Bai, J., Ma, C., Wei, J., Du, X.: The Role of Gut Microbiota in Tumor Immunotherapy. Journal of Immunology Research. 2021, 1–12 (2021). [CrossRef]
- Haque, S. , Raina, R., Afroze, N., Hussain, A., Alsulimani, A., Singh, V., Mishra, B.N., Kaul, S., Kharwar, R.N.: Microbial dysbiosis and epigenetics modulation in cancer development – A chemopreventive approach. Seminars in Cancer Biology. 86, 666–681 (2022). [CrossRef]
- Nanda, R. , Chow, L.Q.M., Dees, E.C., Berger, R., Gupta, S., Geva, R., Pusztai, L., Pathiraja, K., Aktan, G., Cheng, J.D., Karantza, V., Buisseret, L.: Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. JCO. 34, 2460–2467 (2016). [CrossRef]
- Adams, S. , Schmid, P., Rugo, H.S., Winer, E.P., Loirat, D., Awada, A., Cescon, D.W., Iwata, H., Campone, M., Nanda, R., Hui, R., Curigliano, G., Toppmeyer, D., O’Shaughnessy, J., Loi, S., Paluch-Shimon, S., Tan, A.R., Card, D., Zhao, J., Karantza, V., Cortés, J.: Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Annals of Oncology. 30, 397–404 (2019). [CrossRef]
- Dirix, L.Y. , Takacs, I., Jerusalem, G., Nikolinakos, P., Arkenau, H.-T., Forero-Torres, A., Boccia, R., Lippman, M.E., Somer, R., Smakal, M., Emens, L.A., Hrinczenko, B., Edenfield, W., Gurtler, J., Von Heydebreck, A., Grote, H.J., Chin, K., Hamilton, E.P.: Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 167, 671–686 (2018). [CrossRef]
- AACR Annual Meeting 2017 Online Proceedings and Itinerary Planner | Presentation, https://www.abstractsonline.com/pp8/#!/4292/presentation/1296.
- Emens, L.A. , Adams, S., Loi, S., Schneeweiss, A., Rugo, H.S., Winer, E.P., Barrios, C.H., Dieras, V., de la Haba-Rodriguez, J., Gianni, L., Chui, S.Y., Schmid, P.: IMpassion130: a Phase III randomized trial of atezolizumab with nab-paclitaxel for first-line treatment of patients with metastatic triple-negative breast cancer (mTNBC). JCO. 34, TPS1104–TPS1104 (2016). [CrossRef]
- Tolaney, S.M. , Kalinsky, K., Kaklamani, V.G., D’Adamo, D.R., Aktan, G., Tsai, M.L., O’Regan, R., Kaufman, P.A., Wilks, S., Andreopoulou, E., Patt, D.A., Yuan, Y., Wang, G., Xing, D., Kleynerman, E., Karantza, V., Diab, S.: A phase Ib/II study of eribulin (ERI) plus pembrolizumab (PEMBRO) in metastatic triple-negative breast cancer (mTNBC) (ENHANCE 1). JCO. 38, 1015–1015 (2020). [CrossRef]
- Cortes, J. , Cescon, D.W., Rugo, H.S., Nowecki, Z., Im, S.-A., Yusof, M.M., Gallardo, C., Lipatov, O., Barrios, C.H., Holgado, E., Iwata, H., Masuda, N., Otero, M.T., Gokmen, E., Loi, S., Guo, Z., Zhao, J., Aktan, G., Karantza, V., Schmid, P., Luis, F., Gonzalo, G.A., Diego, K., Ruben, K., Matias, M., Mirta, V., Sally, B.-H., Stephen, B., Philip, C., Sherene, L., Dhanusha, S., Andrea, G., Donatienne, T., Carlos, B., Leandro, B., Fabiano, C., Ruffo, D.F.J., Roberto, H., Domicio Carvalho, L., Fernando Cezar Toniazzi, L., Roberto Odebrecht, R., Antonio Orlando, S.N., Felipe, S., David, C., Danielle, C., Cristiano, F., Xinni, S., Joanne, Y., Alejandro, A., Carlos, G., Claudio, S., Cesar, S., Eduardo, Y., Alvaro, G.D., Jesus, S., Petra, H., Zdenek, K., Bohuslav, M., Katarina, P., Jana, P., Vesna, G., Erik, J., Jeanette, J., Soren, L., Tamas, L., Herve, B., Isabelle, D., Anthony, G., Anne-Claire, H.-B., Luis, T., Jens-Uwe, B., Peter, F., Dirk, F., Nadia, H., Jens, H., Anna, K.F.D.S., Christian, K., Sibylle, L., Diana, L., Tjoung-Won, P.-S., Raquel Von, S., Pauline, W., Louis, C., Ava, K., Kai Cheong Roger, N., Peter, A., Tibor, C., Zsuzsanna, K., Laszlo, L., Karoly, M., Gabor, R., John, C., Catherine, K., Seamus, O., Saverio, C., Antonietta, Da., Enrico, R., Tomoyuki, A., Takaaki, F., Kenichi, I., Takashi, I., Yoshinori, I., Tsutomu, I., Hiroji, I., Yoshimasa, K., Koji, M., Yasuo, M., Hirofumi, M., Seigo, N., Naoki, N., Shoichiro, O., Akihiko, O., Yasuaki, S., Eiji, S., Masato, T., Yuko, T., Kenji, T., Koichiro, T., Junichiro, W., Naohito, Y., Yutaka, Y., Teruo, Y., Anita, B., Mastura, M.Y., Angel, G.V., Alejandro, J.R., Jorge, M.R., Flavia, M.-V., Jessica, R.C., Karin, B., Vivianne, T.-H., David, P., Ewa, C., Ewa, N.-Z., Zbigniew, N., Barbara, R., Joanna, S., Cezary, S., Rafal, T., Bogdan, Z., Alexander, A., Natalia, F., Oleg, L., Andrey, M., Vladimir, M., Guzel, M., Jin Hee, A., Seock-Ah, I., Keun Seok, L., Kwong Hwa, P., Yeon Hee, P., Begona, B.D.L.H., Javier, C., Josefina, C.J., Luis, D.L.C.M., Jose, G.S., Maria, G., Esther, H., Esther, Z.A., Chien-Ting, L., Mei-Ching, L., Chiun-Sheng, H., Chao-Jung, T., Ling-Ming, T., Cagatay, A., Gul, B., Irfan, C., Erhan, G., Seyda, G., Nil, M.M., Mustafa, O., Ozgur, O., Sinan, Y., Steve, C., Janine, G., Iain, M., Peter, S., Nicholas, T., Mark, T., Christopher, T., Duncan, W., Hryhoriy, A., Oleksandr, B., Igor, B., Oleksii, K., Olena, K., Hanna, K., Anna, K., Iurii, L., Alla, N., Natalya, O., Olga, P., Andrii, R., Sergii, S., Yaroslav, S., Dmytro, T., Grygorii, U., Ihor, V., Sibel, B., Madhu, C., Michael, C., Patrick, C., Scott, C., Jennifer, D., Keerthi, G., Jeffrey, H., Kent, H., William, I., Randa, L., Janice, L., Raul, M., Susan, M., Rita, N., Ira, O., Coral, O., Timothy, P., Amit, P., Brian, P., Hope, R., Irina, R., Michael, S., Robert, S., Michael, S., Laura, S., Bradley, S., Michaela, T., Frances, V.-A.: Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. The Lancet. 396, 1817–1828 (2020). [CrossRef]
- Aarnoutse, R. , Ziemons, J., Hillege, L.E., De Vos-Geelen, J., De Boer, M., Bisschop, S.M.P., Vriens, B.E.P.J., Vincent, J., Van De Wouw, A.J., Le, G.N., Venema, K., Rensen, S.S., Penders, J., Smidt, M.L.: Changes in intestinal microbiota in postmenopausal oestrogen receptor-positive breast cancer patients treated with (neo)adjuvant chemotherapy. npj Breast Cancer. 8, 89 (2022). [CrossRef]
- Bilenduke, E. , Sterrett, J.D., Ranby, K.W., Borges, V.F., Grigsby, J., Carr, A.L., Kilbourn, K., Lowry, C.A.: Impacts of breast cancer and chemotherapy on gut microbiome, cognitive functioning, and mood relative to healthy controls. Sci Rep. 12, 19547 (2022). [CrossRef]
- Horigome, A. , Okubo, R., Hamazaki, K., Kinoshita, T., Katsumata, N., Uezono, Y., Xiao, J.Z., Matsuoka, Y.J.: Association between blood omega-3 polyunsaturated fatty acids and the gut microbiota among breast cancer survivors. Beneficial Microbes. 10, 751–758 (2019). [CrossRef]
- Terrisse, S. , Derosa, L., Iebba, V., Ghiringhelli, F., Vaz-Luis, I., Kroemer, G., Fidelle, M., Christodoulidis, S., Segata, N., Thomas, A.M., Martin, A.-L., Sirven, A., Everhard, S., Aprahamian, F., Nirmalathasan, N., Aarnoutse, R., Smidt, M., Ziemons, J., Caldas, C., Loibl, S., Denkert, C., Durand, S., Iglesias, C., Pietrantonio, F., Routy, B., André, F., Pasolli, E., Delaloge, S., Zitvogel, L.: Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 28, 2778–2796 (2021). [CrossRef]
- Okubo, R. , Kinoshita, T., Katsumata, N., Uezono, Y., Xiao, J., Matsuoka, Y.J.: Impact of chemotherapy on the association between fear of cancer recurrence and the gut microbiota in breast cancer survivors. Brain, Behavior, and Immunity. 85, 186–191 (2020). [CrossRef]
- Rodríguez Martín, B. , Fernández Rodríguez, E.J., Rihuete Galve, M.I., Cruz Hernández, J.J.: Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer. IJERPH. 17, 8896 (2020). [CrossRef]
- Williams, L.J. , Fletcher, E., Douglas, A., Anderson, E.D.C., McCallum, A., Simpson, C.R., Smith, J., Moger, T.A., Peltola, M., Mihalicza, P., Sveréus, S., Zengarini, N., Campbell, H., Wild, S.H.: Retrospective cohort study of breast cancer incidence, health service use and outcomes in Europe: a study of feasibility. European Journal of Public Health. 28, 327–332 (2018). [CrossRef]
- Jim, H.S.L. , Phillips, K.M., Chait, S., Faul, L.A., Popa, M.A., Lee, Y.-H., Hussin, M.G., Jacobsen, P.B., Small, B.J.: Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated With Standard-Dose Chemotherapy. JCO. 30, 3578–3587 (2012). [CrossRef]
- Wu, D. , Chen, Q., Chen, X., Han, F., Chen, Z., Wang, Y.: The blood–brain barrier: structure, regulation, and drug delivery. Sig Transduct Target Ther. 8, 217 (2023). [CrossRef]
- Cheung, Y.T. , Ng, T., Shwe, M., Ho, H.K., Foo, K.M., Cham, M.T., Lee, J.A., Fan, G., Tan, Y.P., Yong, W.S., Madhukumar, P., Loo, S.K., Ang, S.F., Wong, M., Chay, W.Y., Ooi, W.S., Dent, R.A., Yap, Y.S., Ng, R., Chan, A.: Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Annals of Oncology. 26, 1446–1451 (2015). [CrossRef]
- Shadad, A.K. : Gastrointestinal radiation injury: Symptoms, risk factors and mechanisms. WJG. 19, 185 (2013). [CrossRef]
- Nam, Y.-D. , Kim, H.J., Seo, J.-G., Kang, S.W., Bae, J.-W.: Impact of Pelvic Radiotherapy on Gut Microbiota of Gynecological Cancer Patients Revealed by Massive Pyrosequencing. PLoS ONE. 8, e82659 (2013). [CrossRef]
- Shiao, S.L. , Kershaw, K.M., Limon, J.J., You, S., Yoon, J., Ko, E.Y., Guarnerio, J., Potdar, A.A., McGovern, D.P.B., Bose, S., Dar, T.B., Noe, P., Lee, J., Kubota, Y., Maymi, V.I., Davis, M.J., Henson, R.M., Choi, R.Y., Yang, W., Tang, J., Gargus, M., Prince, A.D., Zumsteg, Z.S., Underhill, D.M.: Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell. 39, 1202-1213.e6 (2021). [CrossRef]
- Uribe-Herranz, M. , Rafail, S., Beghi, S., Gil-de-Gómez, L., Verginadis, I., Bittinger, K., Pustylnikov, S., Pierini, S., Perales-Linares, R., Blair, I.A., Mesaros, C.A., Snyder, N.W., Bushman, F., Koumenis, C., Facciabene, A.: Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. Journal of Clinical Investigation. 130, 466–479 (2019). [CrossRef]
- Guo, H. , Chou, W.-C., Lai, Y., Liang, K., Tam, J.W., Brickey, W.J., Chen, L., Montgomery, N.D., Li, X., Bohannon, L.M., Sung, A.D., Chao, N.J., Peled, J.U., Gomes, A.L.C., Van Den Brink, M.R.M., French, M.J., Macintyre, A.N., Sempowski, G.D., Tan, X., Sartor, R.B., Lu, K., Ting, J.P.Y.: Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 370, eaay9097 (2020). [CrossRef]
- Alexander, J.L. , Wilson, I.D., Teare, J., Marchesi, J.R., Nicholson, J.K., Kinross, J.M.: Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 14, 356–365 (2017). [CrossRef]
- Di Modica, M. , Gargari, G., Regondi, V., Bonizzi, A., Arioli, S., Belmonte, B., De Cecco, L., Fasano, E., Bianchi, F., Bertolotti, A., Tripodo, C., Villani, L., Corsi, F., Guglielmetti, S., Balsari, A., Triulzi, T., Tagliabue, E.: Gut Microbiota Condition the Therapeutic Efficacy of Trastuzumab in HER2-Positive Breast Cancer. Cancer Research. 81, 2195–2206 (2021). [CrossRef]
- One Health, https://www.who.int/news-room/questions-and-answers/item/one-health.
- Kleber, K.T. , Iranpur, K.R., Perry, L.M., Cruz, S.M., Razmara, A.M., Culp, W.T.N., Kent, M.S., Eisen, J.A., Rebhun, R.B., Canter, R.J.: Using the canine microbiome to bridge translation of cancer immunotherapy from pre-clinical murine models to human clinical trials. Front. Immunol. 13, 983344 (2022). [CrossRef]
- M. Dujon, A., Brown, J.S., Destoumieux-Garzón, D., Vittecoq, M., Hamede, R., Tasiemski, A., Boutry, J., Tissot, S., Alix-Panabieres, C., Pujol, P., Renaud, F., Simard, F., Roche, B., Ujvari, B., Thomas, F.: On the need for integrating cancer into the One Health perspective. Evolutionary Applications. 14, 2571–2575 (2021). [CrossRef]
- Kattner, P. , Zeiler, K., Herbener, V.J., Ferla-Brühl, K.L., Kassubek, R., Grunert, M., Burster, T., Brühl, O., Weber, A.S., Strobel, H., Karpel-Massler, G., Ott, S., Hagedorn, A., Tews, D., Schulz, A., Prasad, V., Siegelin, M.D., Nonnenmacher, L., Fischer-Posovszky, P., Halatsch, M.-E., Debatin, K.-M., Westhoff, M.-A.: What Animal Cancers teach us about Human Biology. Theranostics. 11, 6682–6702 (2021). [CrossRef]
- Dujon, A.M. , Gatenby, R.A., Bramwell, G., MacDonald, N., Dohrmann, E., Raven, N., Schultz, A., Hamede, R., Gérard, A.-L., Giraudeau, M., Thomas, F., Ujvari, B.: Transmissible Cancers in an Evolutionary Perspective. iScience. 23, 101269 (2020). [CrossRef]
- Epstein, B. , Jones, M., Hamede, R., Hendricks, S., McCallum, H., Murchison, E.P., Schönfeld, B., Wiench, C., Hohenlohe, P., Storfer, A.: Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nat Commun. 7, 12684 (2016). [CrossRef]
- Kerr, P. , Liu, J., Cattadori, I., Ghedin, E., Read, A., Holmes, E.: Myxoma Virus and the Leporipoxviruses: An Evolutionary Paradigm. Viruses. 7, 1020–1061 (2015). [CrossRef]
- Di Giallonardo, F. , Holmes, E.C.: Viral biocontrol: grand experiments in disease emergence and evolution. Trends in Microbiology. 23, 83–90 (2015). [CrossRef]
- Spernovasilis, N. , Tsiodras, S., Poulakou, G.: Emerging and Re-Emerging Infectious Diseases: Humankind’s Companions and Competitors. Microorganisms. 10, 98 (2022). [CrossRef]
- Giraudeau, M. , Sepp, T., Ujvari, B., Ewald, P.W., Thomas, F.: Human activities might influence oncogenic processes in wild animal populations. Nat Ecol Evol. 2, 1065–1070 (2018). [CrossRef]
- Sepp, T. , Ujvari, B., Ewald, P.W., Thomas, F., Giraudeau, M.: Urban environment and cancer in wildlife: available evidence and future research avenues. Proc. R. Soc. B. 286, 20182434 (2019). [CrossRef]
- Pesavento, P.A. , Agnew, D., Keel, M.K., Woolard, K.D.: Cancer in wildlife: patterns of emergence. Nat Rev Cancer. 18, 646–661 (2018). [CrossRef]
- White, J. , Amato, K.R., Decaestecker, E., McKenzie, V.J.: Editorial: Impact of anthropogenic environmental changes on animal microbiomes. Front. Ecol. Evol. 11, 1204035 (2023). [CrossRef]
- Sonnenburg, E.D. , Sonnenburg, J.L.: The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol. 17, 383–390 (2019). [CrossRef]
- Moustafa, M.A.M. , Chel, H.M., Thu, M.J., Bawm, S., Htun, L.L., Win, M.M., Oo, Z.M., Ohsawa, N., Lahdenperä, M., Mohamed, W.M.A., Ito, K., Nonaka, N., Nakao, R., Katakura, K.: Anthropogenic interferences lead to gut microbiome dysbiosis in Asian elephants and may alter adaptation processes to surrounding environments. Sci Rep. 11, 741 (2021). [CrossRef]
- Poutahidis, T. , Varian, B.J., Levkovich, T., Lakritz, J.R., Mirabal, S., Kwok, C., Ibrahim, Y.M., Kearney, S.M., Chatzigiagkos, A., Alm, E.J., Erdman, S.E.: Dietary Microbes Modulate Transgenerational Cancer Risk. Cancer Research. 75, 1197–1204 (2015). [CrossRef]
- Kowallik, V. , Das, A., Mikheyev, A.S.: Experimental inheritance of antibiotic acquired dysbiosis affects host phenotypes across generations. Front. Microbiol. 13, 1030771 (2022). [CrossRef]
- Dujon, A.M. , Ujvari, B., Thomas, F.: Cancer risk landscapes: A framework to study cancer in ecosystems. Science of The Total Environment. 763, 142955 (2021). [CrossRef]
- Efird, J.T. , Davies, S.W., O’Neal, W.T., Anderson, E.J.: Animal Viruses, Bacteria, and Cancer: A Brief Commentary. Front. Public Health. 2, (2014). [CrossRef]
- Prüss-Üstün, A. , Wolf, J., Corvalán, C.F., Bos, R., Neira, M.P.: Preventing disease through healthy environments: a global assessment of the burden of disease from environmental risks. World Health Organization, Geneva (2016).
- AbdulRaheem, Y. : Unveiling the Significance and Challenges of Integrating Prevention Levels in Healthcare Practice. J Prim Care Community Health. 14, 21501319231186500 (2023). [CrossRef]
- About: Health promotion and disease prevention through population-based interventions, including action to address social determinants and health inequity, http://www.emro.who.int/about-who/public-health-functions/health-promotion-disease-prevention.html.
- Grenni, P. , Ancona, V., Barra Caracciolo, A.: Ecological effects of antibiotics on natural ecosystems: A review. Microchemical Journal. 136, 25–39 (2018). [CrossRef]
- Lee, K. , Raguideau, S., Sirén, K., Asnicar, F., Cumbo, F., Hildebrand, F., Segata, N., Cha, C.-J., Quince, C.: Population-level impacts of antibiotic usage on the human gut microbiome. Nat Commun. 14, 1191 (2023). [CrossRef]
- Wang, W. , Weng, Y., Luo, T., Wang, Q., Yang, G., Jin, Y.: Antimicrobial and the Resistances in the Environment: Ecological and Health Risks, Influencing Factors, and Mitigation Strategies. Toxics. 11, 185 (2023). [CrossRef]
- Rossi, F. , Péguilhan, R., Turgeon, N., Veillette, M., Baray, J.-L., Deguillaume, L., Amato, P., Duchaine, C.: Quantification of antibiotic resistance genes (ARGs) in clouds at a mountain site (puy de Dôme, central France). Science of The Total Environment. 865, 161264 (2023). [CrossRef]
- Lloyd, D.H. , Page, S.W.: Antimicrobial Stewardship in Veterinary Medicine. Microbiol Spectr. 6, 6.3.03 (2018). [CrossRef]
- Roderburg, C. , Loosen, S.H., Joerdens, M.S., Demir, M., Luedde, T., Kostev, K.: Antibiotic therapy is associated with an increased incidence of cancer. J Cancer Res Clin Oncol. 149, 1285–1293 (2023). [CrossRef]
- Ibragimova, S. , Ramachandran, R., Ali, F.R., Lipovich, L., Ho, S.B.: Dietary Patterns and Associated Microbiome Changes that Promote Oncogenesis. Front. Cell Dev. Biol. 9, 725821 (2021). [CrossRef]
- emhj: One Health: perspectives on ethical issues and evidence from animal experiments, http://www.emro.who.int/emhj-volume-18-2012/issue-11/article-15.html.
- Van Herten, J. , Bovenkerk, B., Verweij, M.: One Health as a moral dilemma: Towards a socially responsible zoonotic disease control. Zoonoses and Public Health. 66, 26–34 (2019). [CrossRef]
- Ursell, L.K. , Metcalf, J.L., Parfrey, L.W., Knight, R.: Defining the human microbiome. Nutrition Reviews. 70, S38–S44 (2012). [CrossRef]
- Baba, A.I. , Câtoi, C.: COMPARATIVE ONCOLOGY. In: Comparative Oncology. The Publishing House of the Romanian Academy (2007).
- Dincă, L.C. , Grenni, P., Onet, C., Onet, A.: Fertilization and Soil Microbial Community: A Review. Applied Sciences. 12, 1198 (2022). [CrossRef]
- Woodworth, M.H. , Sitchenko, K.L., Carpentieri, C., Friedman-Moraco, R.J., Wang, T., Kraft, C.S.: Ethical Considerations in Microbial Therapeutic Clinical Trials. The New Bioethics. 23, 210–218 (2017). [CrossRef]
- Cammarota, G. , Ianiro, G., Ahern, A., Carbone, C., Temko, A., Claesson, M.J., Gasbarrini, A., Tortora, G.: Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat Rev Gastroenterol Hepatol. 17, 635–648 (2020). [CrossRef]
- Yang, J. , Tan, Q., Fu, Q., Zhou, Y., Hu, Y., Tang, S., Zhou, Y., Zhang, J., Qiu, J., Lv, Q.: Gastrointestinal microbiome and breast cancer: correlations, mechanisms and potential clinical implications. Breast Cancer. 24, 220–228 (2017). [CrossRef]
- Yin, B. , Wang, X., Yuan, F., Li, Y., Lu, P.: Research progress on the effect of gut and tumor microbiota on antitumor efficacy and adverse effects of chemotherapy drugs. Front. Microbiol. 13, 899111 (2022). [CrossRef]
- Poff, A.M. , Ari, C., Seyfried, T.N., D’Agostino, D.P.: The Ketogenic Diet and Hyperbaric Oxygen Therapy Prolong Survival in Mice with Systemic Metastatic Cancer. PLoS ONE. 8, e65522 (2013). [CrossRef]
- Gilbert, J.A. , Blaser, M.J., Caporaso, J.G., Jansson, J.K., Lynch, S.V., Knight, R.: Current understanding of the human microbiome. Nat Med. 24, 392–400 (2018). [CrossRef]
- Soto-Pantoja, D.R. , Gaber, M., Arnone, A.A., Bronson, S.M., Cruz-Diaz, N., Wilson, A.S., Clear, K.Y.J., Ramirez, M.U., Kucera, G.L., Levine, E.A., Lelièvre, S.A., Chaboub, L., Chiba, A., Yadav, H., Vidi, P.-A., Cook, K.L.: Diet Alters Entero-Mammary Signaling to Regulate the Breast Microbiome and Tumorigenesis. Cancer Research. 81, 3890–3904 (2021). [CrossRef]
- Hou, M.-F. , Ou-Yang, F., Li, C.-L., Chen, F.-M., Chuang, C.-H., Kan, J.-Y., Wu, C.-C., Shih, S.-L., Shiau, J.-P., Kao, L.-C., Kao, C.-N., Lee, Y.-C., Moi, S.-H., Yeh, Y.-T., Cheng, C.-J., Chiang, C.-P.: Comprehensive profiles and diagnostic value of menopausal-specific gut microbiota in premenopausal breast cancer. Exp Mol Med. 53, 1636–1646 (2021). [CrossRef]
- Parhi, L. , Alon-Maimon, T., Sol, A., Nejman, D., Shhadeh, A., Fainsod-Levi, T., Yajuk, O., Isaacson, B., Abed, J., Maalouf, N., Nissan, A., Sandbank, J., Yehuda-Shnaidman, E., Ponath, F., Vogel, J., Mandelboim, O., Granot, Z., Straussman, R., Bachrach, G.: Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 11, 3259 (2020). [CrossRef]
- Bennet, JustinD., Brinkman, M.: TREATMENT OF ULCERATIVE COLITIS BY IMPLANTATION OF NORMAL COLONIC FLORA. The Lancet. 333, 164 (1989). [CrossRef]
- Mills, H. , Acquah, R., Tang, N., Cheung, L., Klenk, S., Glassen, R., Pirson, M., Albert, A., Hoang, D.T., Van, T.N.: The Use of Bacteria in Cancer Treatment: A Review from the Perspective of Cellular Microbiology. Emergency Medicine International. 2022, 1–6 (2022). [CrossRef]
- Pang, Z. , Gu, M.-D., Tang, T.: Pseudomonas aeruginosa in Cancer Therapy: Current Knowledge, Challenges and Future Perspectives. Front. Oncol. 12, 891187 (2022). [CrossRef]
- Kasinskas, R.W. , Forbes, N.S.: Salmonella typhimurium Lacking Ribose Chemoreceptors Localize in Tumor Quiescence and Induce Apoptosis. Cancer Research. 67, 3201–3209 (2007). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
10 November 2023
Posted:
13 November 2023
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
Submitted:
10 November 2023
Posted:
13 November 2023
You are already at the latest version
Alerts
Abstract
This comprehensive review elucidates the profound relationship between the human microbiome and breast cancer managrement. Recent findings highlight the significance of microbial alterations in tissues, such as the gut and the breast, and their role in influencing breast cancer risk, development, progression, and treatment outcomes. We delve into how the gut microbiome can modulate systemic inflammatory responses and estrogen levels, thereby impacting cancer initiation and therapeutic drug efficacy. Furthermore, we explore the unique microbial diversity within breast tissue, indicating potential imbalances brought about by cancer and highlighting specific microbes as promising therapeutic targets. Emphasizing a holistic One Health approach, this review underscores the importance of integrating insights from human, animal, and environmental health to gain a deeper understanding of the complex microbe-cancer interplay. As the field advances, strategic manipulation of the microbiome and its metabolites presents innovative prospects for enhancing cancer diagnostics and therapeutics. However, rigorous clinical trials remain essential to confirm the potential of microbiota-based interventions in breast cancer management.
Keywords:
Subject: Biology and Life Sciences - Biochemistry and Molecular Biology
1. Introduction
Early detection of cancer is associated with a decrease in adverse outcomes and an increase in the success rate of treatments. Presently, cancer diagnostic methods range in their levels of invasiveness, and many still necessitate invasive, biopsies for verification. Therefore, research has been geared towards discovering methods that are less invasive yet retain high sensitivity (identifying actual cancer cases) and specificity (excluding non-cancerous cases). This effectiveness is sometimes measured using the AUROC score, where a score of 1.0 symbolizes an ideal classification with 100% specificity and sensitivity. Recent studies have centered around utilizing microbiomes as a more non-invasive diagnostic approach, either directly or indirectly related to cancer. Samples such as saliva, stool, and plasma, which are easier to collect, in comparison to biopsies are being considered as alternatives to the more invasive diagnostic methods currently in practice. Microorganisms play an indirect role in affecting the emergence, natural course and/or severity of various cancers. For instance, it has been demonstrated that microbes in the gut influence cancer stem cells, which are dormant cancer cells believed to cause disease recurrence. Research has also indicated that metabolites from certain archaea, mainly from the Euryarchaeota phylum and the TACK superphylum, are linked to numerous cancers, even though these microbes are typically overlooked due to their rarity. Predominantly, research connecting microbes and cancer has concentrated on bacteria and their involvement in different cancers, with a few studies also exploring potential links with fungi and viruses [1].
The presence of a unique microbiome in breast tissue, previously unacknowledged, has gained recognition through recent research. Microbes are found in both healthy and cancerous breast tissues. Interestingly, breast tumor tissues showed a decrease in total bacterial DNA, and an inverse relationship was observed between bacterial DNA load and advanced cancer stages [2]. This implies that cancer may disturb the natural balance of microbes in this area. Over 60% of breast cancer samples harbor bacterial expression, with these bacteria found both inside breast cancer cells and immune cells. The microbial diversity in breast tumor samples outpaced that in other cancers and exhibited specific variations based on receptor status. From these samples, live bacteria from phyla like Proteobacteria, Firmicutes, and Actinobacteria were isolated. Fusobacterium nucleatum, previously linked to colorectal cancer, was also found to be more prevalent in breast tissue and colonize mammary tissue where is enhances tumor growth and metastasis. An increase of Methylobacterium radiotolerans and a decrease of Sphingomonas yanoikuyae in tumor tissue was also noted [3]. Moreover, the fungal genus Malassezia was abundantly found in breast tumors [4]. Mouse studies have demonstrated the role of local breast tissue microbiome in breast cancer. A decline in bacteria within tumors was linked with fewer lung metastases. Staphylococcus spp. and Lactobacillus spp., in particular, were related to an upsurge in metastatic tumors. Furthermore, gut microbes influenced the onset and advancement of breast cancer. In specific breast cancer mouse models, introducing Helicobacter hepaticus increased the presence of tumors in the mammary glands, mammary tissue inflammation, and the load of neutrophils. Comprehending the typical microbiome of breast tissue and its transformation during breast cancer might unveil microbes as potential therapeutic or preventive targets. However, more research is crucial to ascertain the universality of these microbial alterations across diverse patient groups [5,6].
In this review, we emphasize the ways in which the microbiome can both directly and indirectly influence various facets of cancer management, ranging from disease onset and progression to diagnostics and treatment options. We delve into recent studies that have reshaped our understanding of these interactions and their potential implications for cancer care.
2. Microbiota-Based Therapies for Breast Cancer Treatment and Prevention
Microbial therapy is also termed as oncolytic virotherapy or bacterial therapy. Alterations of certain microbial communities increase the risk of breast cancer as they alter tissue metabolism on multiple levels, including the trigger and progression of cancer in the host [7]. This form of treatment may involve immune regulation, influencing the efficacy of anti-tumor drugs, targeted therapy of engineered pro- and prebiotics fecal microbiota transplantation, and administration of antitumor drugs [8]. The human microbiome is capable of affecting an array of human biological, including hormonal and metabolic pathways and may also trigger initiation, proliferation, and genetic instability of cancer within the host cell or even trigger apoptosis [9].
Evidence supports that gut microbiota dysbiosis affects the development, progression, and prognosis of Breast cancer (BC) both through direct and indirect signaling pathways. Treating breast cancer through microbial therapy may involve managing microbiota that will target the immune response. Staphylococcus genus for example is negatively associated with expression of TRAF4, an oncogene that triggers a cascade of events favouring cancer survival through NF-κB [10]. Increasing expression of Staphylococcus genus in breast cancer tissue eliminates TRAF4 activity and increases activation of T-cell genes associated with the microbe. Equally, Propionibacterium expression is positively correlated with activation of T-cells through NFAT2, NFIL3 and IFNGR, but its expression in breast cancer tissue is reduced [10]. This study also presents the co-existence of Methylibium with multiple genes including ICOS, MRC1 and Toll-like receptors but this fact requires further investigation. Administration of Lactobacillus acidophilus, an anaerobic microbe, increases cytokine expression such as IFN-γ from splenocyte [11]. This cytokine in turn increases proliferation of lymphocytes such as NK cells, promoting Type 1 T helper activity expressing anti-tumour immunity and has anti-angiogenic effects [11]. TGF-β is an additional cytokine, involved in blocking T-cell production, that tumors produce in high concentrations to ensure they are unaffected by immune surveillance. Expression of this cytokine is hindered by L. acidophilus, as a result reduces tumour growth rate and increases lymphocyte proliferation.
Transfering Micrococcus luteus in in vivo models hinders 4TI tumor growth and this may be due to the microbiota upregulating M1-macrophage-related genes which include among others, IL-6IL,-8 and IL12 genes [12]. Potentially introducing Micrococcus luteus peritoumorally can improve patient outcome, however before reaching this stage further safety and effective evaluations are necessary. Sphingomonas yanoikuyae affect the immune response through the proliferation of invariant NKT cells involved in immunosurveillance as well as the expression of TLR2,5, and 9 [2]. Toll-like receptor 5 (TLR5) expression is also associated with microbial expression, e.g., Salmonella typhimurium flagellin, and activates the innate immune response in breast cancer patients, marking TLR5 as a therapeutic target [13]. Absence of inflammation in breast cancer patients is also instigated by the absence of Roseburia inulinivorans microbiota. A case common in post-menopausal breast cancer patients than premenopausal breast cancer patients [14].
Escherichia Coli produce antimicrobial peptides that are activated under cellular stress conditions and affect cell survival through cell cycle arrest and DNAse activity, [15,16]. Colicin A for example increases apoptosis in multiple breast cancer cell lines (MCF7, MDA-MD-231, MRC5 and Osteosarcoma cell line HOS) by 7–28%, while Colicin E1 increases apoptosis even more in MCF7 (58%) and in HS913T cells (14%) whilst Colicin A was ineffective [16]. Anti-cancer expression also involves membrane disintegration of breast cancer cells following expression by a defensin peptide expressed by Brevibacillus sp. [17].
Focus, evolves around Bifidobacterium, because of their anaerobic status is ideal for successfully delivering therapeutic genetic material to the necrotic center of tumours. A plasmid has been assembled carrying a DNA binding protein and E.coli as a source of cytosine deaminase required to convert the prodrug 5-Fluorocytosine to the tumor toxic 5-Fluorouracil [18]. Bifidobacterium sp. also tackle cancer indirectly, through metabolism of lapachol, synthesizing cytotoxic metabolites against breast cancer, as proven in SKBR-3 cell lines [19]. Bifidobacterium is also effective in synergy with Bacteroides. The mixture of the two-microbiota exibit anti-breast cancer properties as they suppress angiogenesis, proliferation and apoptosis against breast cancer cells [20]. The combination of these two-microbiota increased secretion of interferon γ, inducing tumor cell lysis.
Overall, modifying or eliminating certain communities of microbes can reverse the environmental conditions favouring cancer existence and triggering apoptosis. Complementary to traditional breast cancer therapies, restoring breast microbiota to their local natural concentrations does not only ensure a functional immune response but can also prevent host microenvironment interference to the cancer drugs administered or the metabolites they react.
2.1. Bacterial therapeutics for tumor treatment and immune modulation
Recent scientific discourse has highlighted the significance of microbiota in mitigating breast cancer via its anti-tumor activities [21]. Contemporary studies assert that specific intestinal bacteria can obstruct oncogenesis and promote tumor regression. Nonetheless, indiscriminate modulation of the gut microbiota can manifest unforeseen consequences. Precise targeting of tumor-associated bacteria is thus crucial for ensuring the safety and effectiveness of therapeutic modalities [22,23]. Current research underscores the imperative of regulating specific tumor-inducing bacteria through methodologies such as bacteria-mediated tumor therapy (BMTT), fecal bacterial and bacteriophage transplantation, prebiotic enhancement, and the utilization of bacterial toxins and enzymes [24].
However, a salient concern in harnessing bacterial agents for cancer therapeutics pertains to their potential cytotoxicity and pathogenic manifestations. To mitigate these risks, the scientific community has explored genetic engineering as a solution, allowing the excision of virulence-inducing genes while preserving therapeutic features [25]. Yet, the selection of bacteria is paramount: an ideal bacterial candidate should effectively permeate tumors, possess a minimal infectious propensity, and remain amenable to antibiotic-mediated elimination post-intervention. Numerous bacterial candidates await rigorous clinical evaluations, leaving certain aspects of their metabolic functions ambiguous. Challenges encompass inadvertent cytotoxic effects on healthy cells, incomplete tumor eradication, and unpredictable bacterial genome mutations. The transient existence of bacterial peptides in the human body further perplexes their therapeutic potential. As such, there is an emergent demand for exhaustive clinical trials to elucidate these bacteria-tumor cell dynamics and the potential ramifications of such interventions [26].
Conventional cancer treatments, primarily radiotherapy and chemotherapy, remain central to breast cancer intervention paradigms. Despite their dominance, the associated adverse effects and non-selective toxicity of these treatments necessitate the exploration of more targeted therapeutic options. In this context, genetically engineered bacteria, which exhibit a selective affinity for cancer cells while sparing healthy tissues, have emerged as promising agents in cancer research [27].
These bacteria inherently possess the ability to produce and excrete proteinaceous toxins that can inhibit specific cellular functions, a property that may be harnessed for therapeutic purposes. These agents can target cancer cells, thereby minimizing damage to surrounding healthy cells. Studies have suggested a potential symbiotic relationship between certain bacterial strains and tumor regression observed in animal models. Nevertheless, there is an unequivocal need for additional research to validate and understand these associations further [28].
While the exclusive use of bacterial agents might not secure complete tumor elimination, their combination with conventional drugs presents a promising avenue for cancer treatment. This integrated approach—incorporating drug-laden bacteria—appears especially propitious for overcoming the limitations of existing treatments, primarily their inability to effectively penetrate the tumor microenvironment [29,30].
The utilization of bacteria, particularly Listeria, Clostridium, and Salmonella, offers a solution to this challenge as they can navigate and infiltrate the tumor’s environment. For instance, Salmonella typhimurium has demonstrated remarkable anti-tumor properties, showing efficiency in invading and eliminating various cancer cells in in vivo studies. This strain has yielded significant results as a monotherapy against pancreatic, prostate, and breast cancers in animal models [31,32].
Furthermore, Clostridium perfringens has been acknowledged for producing an enterotoxin that, upon interaction with specific transmembrane proteins, can induce tumor regression. The FDA, recognizing the potential of BMTT, approved the use of Bacillus-Calmette-Guerin, a weakened strain of Mycobacterium bovis, for a specific bladder cancer treatment in the late 1970s, a testament to the enduring clinical relevance of this approach [33,34].
Despite these promising advances, bacterial-mediated therapies are not without challenges. Concerns like potential antibiotic resistance and infection risks associated with the use of live bacteria in treatments need careful consideration and resolution. Hence, while the paradigm of BMTT isn’t new in oncology, its widespread application and implementation remain subjects of ongoing debate and investigation. The preliminary findings, although promising, underscore the importance of continued and expanded research to fully comprehend and validate the potential symbiosis between bacterial strains and tumor regression.
2.2. Bacteriotherapy approaches
Bacteriotherapy, an evolving field in the domain of anticancer therapies, employs a diverse range of bacterial forms, inclusive of genetically modified organisms (GMOs), in both their living and attenuated states. These bacterial entities function via promoting apoptosis or disrupting cell membrane that primarily targets cancer cells through a range of anticancer agents, including bacteriocins, spores, and bacterial peptides [35].
Historical records indicate the rudimentary utilization of bacteriotherapy as far back as 1550 BC. A seminal advancement in cancer immunotherapy can be attributed to Dr. William Coley, an American orthopedic surgeon. His pioneering work involved the development of a heat-inactivated concoction of Streptococcus pyogenes and Serratia marcescens, subsequently referred to as “Coley’s toxin.” Administering this therapeutic concoction to patients with inoperable cutaneous carcinoma yielded substantial clinical outcomes, notably tumor regression and complete remission in a significant proportion of patients [36].
Various bacterial strains, encompassing the likes of Vibrio, Shigella, Salmonella, Listeria, and Bifidobacteria, have manifested profound efficacy in tumor invasion, colonization, and subsequent eradication. However, the relationship between bacteria and cancer remains multifaceted. Certain bacterial strains possess the potential to instigate carcinogenesis or induce malignancies. A case in point is Helicobacter pylori, which, through the secretion of specific cytokines and chemokines, can incite chronic inflammatory responses with detrimental cellular implications. Pertinently, the cytotoxin associated gene A (cagA) has emerged as a pivotal bacterial protein with profound implications in oncogenesis, primarily by compromising the function of the tumor suppressor protein, p53 [37,38].
Though historically malignancies were often associated with pathogenic bacterial infections, contemporary research is increasingly highlighting the anticarcinogenic potentials of certain bacteria. This paradigm shift can be epitomized by the nuanced understanding of how tumor cells proliferate by subverting host immunologic defenses. Recent studies accentuate the anticarcinogenic activities of Salmonella, which appear to operate through a multifaceted mechanism involving both adaptive and innate immune response activations.
Bacteriocins, intricate peptides or proteins synthesized ribosomally, first garnered attention in 1920, courtesy of the seminal work by the Belgian scientist André Gratia and the discovery of colicin (the first bacteriocin) from E. coli [39]. Today, their applications transcend in clinical domains, with their antimicrobial properties finding utility in food preservation as well. Based on their molecular weight, they are categorized into four classes. Significant bacteriocins include Bovicin HC5 from S. bovis and Nisin A from Lactococcus lactis. Some research indicates that the enterotoxin (TcdA) and cytotoxin (TcdB) produced by Clostridioides difficile could be pivotal in treating colorectal cancer [40].
Bacteria, in their myriad forms, are increasingly being recognized as potent immunotherapeutic agents. Their ability to modulate tumor antigenicity holds promise in augmenting immune responses. The novel insights into bacterial interactions, particularly infections with Clostridium novyi, underscore the profound potential in harnessing bacteria for therapeutic advancements in oncological properties. Therefore, bacteria are hailed as promising immunotherapeutic agents due to their ability to amplify the antigenicity of tumor cells, thus bolstering immune responses. By using bacterial cancer immunotherapy, tumor cells are identified as infected cells rather than mere cancer cells, elevating the chances of their elimination. For instance, infections with C. novyi can trigger the formation of heat shock proteins like Hsp70, promoting the maturation of professional dendritic and antigen-presenting cells, leading to potent antigen-specific immune responses [41,42].
2.3. Oncolytic virotherapy and phage-based immunotherapies in cancer treatment
Oncolytic virotherapy employs either naturally occurring or genetically engineered viruses with an affinity for tumor cells, leading to their selective targeting and replication. This process culminates in tumor regression attributed not only to direct cytotoxicity but also the stimulation of anti-tumor immune responses. Remarkably, this occurs without detriment to healthy cells and tissues [43].
In both in vitro and in vivo preclinical investigations, the third-generation oncolytic herpes simplex virus-1 (HSV-1) vector, G47Δ, exhibited amplified cytotoxic effects across multiple BC cell lines, inclusive of MCF-7, MDA-MB-468, and the tamoxifen-resistant variant MCF-7/TAM-R [44,45,46]. Notably, a synergistic cytotoxic effect was observed in breast cancer cells when G47Δ was combined with the chemotherapeutic agent, paclitaxel. This potentiated the anti-tumor efficacy of paclitaxel, resulting in a five-fold dosage reduction to achieve equivalent tumor reduction in vivo[46], a shift that could minimize chemotherapy-associated adversities.
In 2015, the U.S. Food and Drug Administration (FDA) granted approval for talimogene laherparepvec (T-VEC) as the inaugural oncolytic agent for melanoma treatment. Derived from the genetically modified HSV-1, its utility was further highlighted in a phase 2 clinical trial, which revealed that, in conjunction with neoadjuvant chemotherapy (NAC), it enhanced pathological complete response rates in triple-negative breast cancer (TNBC) patients, resulting in an 89% 2-year disease-free rate [47]. It warrants mention that observed adverse effects, though generally mild, ranged from injection site pain and headaches to low-grade fevers. This was notably in contrast to the more severe immune-mediated toxicities associated with TNBC treatment involving both chemotherapy and pembrolizumab [48] .
Likewise, phage therapy is emerging as a promising strategy in breast cancer immunotherapies. It utilizes the ability of phages to invoke anti-tumor immune responses. Specifically, in phage display immunotherapy, antigens (proteins or peptides) fused to phage coat-proteins, function as protective vaccines against cancer. Certain peptides, including E75, AE37, and GP2, have shown potential in breast cancer tests on BALB/c mice. Phages offer two main vaccine delivery techniques: (i) showcasing immunogenic peptides through modified phage coat proteins, and (ii) acting as delivery mediums for DNA vaccines by inserting a eukaryotic promoter-driven vaccine gene within their genome. These vaccines, presenting numerous antigen copies on immunogenic phage particles, incite strong immune reactions. They are also stable, cost-effective, and potent. Experiments have validated their effectiveness in mice and rabbits. Vaccines against Human Papilloma Viruses (HPV), such as Gardasil-9, demonstrate the applications beyond breast cancer. Another anti-breast cancer development is a phage-based anti-HER2 vaccine, designed to bypass immune tolerance. Other studies have developed vaccines for prostate cancer and utilized inovirus-associated vector vaccines for antibody production. Furthermore, a dual anthrax-plague vaccine and a lambda phage-based vaccine for hepatocellular carcinoma underscore the expansive potential of phage-based therapies.
Additionally, in a recent study by Catala and colleagues (2021), Protein-Lipid Particles (PLPs) have been innovatively crafted using bacteriophage lambda to display a fluorescent probe and the therapeutic antibody trastuzumab (Trz), leading to the formation of Trz-PLPs [49]. These are designed to target HER2-positive breast cancer cells. By increasing Trz’s density on PLPs, more prolonged inhibition of cell growth is achieved compared to using free Trz. Trz-PLPs have impacts on numerous cellular pathways, influencing amino acid metabolism, mitochondrial function, and more. They modulate the phosphorylation of key signaling proteins, such as Akt and mTOR, influencing the vital PI3K/Akt/mTOR signaling pathway in cancer cells [49]. Dong and colleagues (2022) have also spotlighted the potential of the M13 phage-based vaccines [50]. By combining M13 phage with a cationic polymer, PEI, a hybrid platform (M13@PEI) was designed capable of efficiently absorbing negatively charged antigens. This resulted in the MPO vaccine, combined with the Ovalbumin (OVA) antigen, which improved the maturation of antigen-presenting cells (APCs) and boosted antigen presentation. The M13 phage genome’s CpG regions and PEI’s role as a TLR5 agonist facilitated this. Enhanced antigen processing and uptake were further confirmed through in vitro assays, emphasizing the robust cytotoxic T lymphocyte (CTL) response. In vivo studies also confirmed the MPO vaccine’s ability to deliver antigens effectively and enhance antigen-specific T-cell mediated responses. When combined with α-PD1 treatment, the vaccine showcased powerful anti-tumor effects, improved survival rates, and enhanced immune memory responses, proving the significant potential of M13 phage-based vaccines in anti-tumor immunotherapy [50].
2.4. Probiotics and breast cancer pathogenesis
Emerging data suggests a nexus between microbial dysbiosis in breast and gut regions and the onset and progression of BC [51]. BC pathogenesis is frequently linked to sustained inflammation, incited by intestinal bacteria that activate NF-κB, subsequently releasing pro-inflammatory cytokines such as TNF-alpha [52,53,54]. Variations in the gut microbiome have been observed across different BC stages [55,56]. These alterations can influence therapeutic efficacy and potential toxicities. Interventions aimed at modifying the gut microbiome, employing probiotics and prebiotics, could be instrumental in attenuating systemic inflammation and alleviating treatment-associated toxicities. Specific strains such as Bifidobacterium and Lactobacillus have shown promise due to their immunomodulatory and antigenotoxic characteristics [57]. Recent research by Yazdi et al. (2012) revealed that the prophylactic administration of Lactobacillus plantarum augmented with selenium nanoparticles led to a significant reduction in tumor volume and elevated survival rates in a murine model of advanced human BC [58]. Furthermore, milk fermented with Lactobacillus casei CRL 431 administered to the same model demonstrated a marked decrease in tumor invasiveness and metastatic potential [59].
In addition, there have been a multitude of clinical trials in oncology field investigating the efficacy of probiotics alongside standard anti-cancer regimens. These studies predominantly indicate a beneficial reduction in gastrointestinal adversities that often arise from conventional cancer therapies [60,61,62,63,64,65]. A significant finding is seen in RCC patients who received the bacterial supplement, CBM588, alongside immunotherapy, reporting improved progression-free survival rates and response outcomes [66]. Probiotics play a crucial role in enhancing the immune system, significantly elevating immunoglobulin A (IgA) levels in the gut, which is vital for immune function. Specific probiotic bacteria, including L. casei and Sphingomonas yanoikuyae, have been noted to bolster the production of natural killer (NK) cells, playing a pivotal role in regulating cancer progression by actively participating in the body’s defence against cancer. Moreover, probiotics not only foster immune defenses but also produce compounds instrumental in protecting against DNA damage and breaking down carcinogens, thereby potentially preventing cancer. For example, L. casei strain Shirota (BLS) has been studied for its probable cancer-preventive properties, showing an inverse correlation between its consumption and the incidence of BC [67]. Furthermore, probiotics have shown promise in reducing chemotherapy-related cognitive impairment (CRCI) in BC patients and alleviating various other chemotherapy-induced side effects [68,69].
Species like L. casei Shirota and Bifidobacterium Bb12 have exhibited antigenotoxic activity, a crucial component in preventing genetic mutations that may lead to cancer, although the degree of this effect varies between bacterial species and is dependent on long-term exposure. Furthermore, the effectiveness of immune cells activation by probiotics varies and is based on dosage and bacterial strain.
However, despite the optimistic preliminary findings, it is essential to acknowledge that the effects of probiotics tend to diminish over time. This diminishing effect underscores the need for ongoing research to understand the long-term role and potential benefits of probiotics in both cancer prevention and treatment, and in mitigating side effects associated with cancer therapies. Given the initial promising results predominantly based on in vivo experiments, there is a palpable need for enhanced, comprehensive research, including in vitro exploration to overcome challenges observed in existing studies, such as inefficient mucosal adhesion and reduced gastrointestinal activity.
Current clinical trials are sparse, especially those focusing on probiotics specific to BC. Nevertheless, the initial findings are promising, and the results from ongoing clinical trials are anticipated to provide a clearer understanding of the role probiotics play in potentially enhancing BC treatment outcomes and in cancer prevention more broadly.
2.5. Microbial polysaccharides
Microbial polysaccharides (MPs) play roles in various cellular processes, including signal transduction and immune response modulation [70]. Glycans and Basidiomycetes-derived MPs, like krestin, schizophyllan, and lentinan, exhibit anticancer properties through immunostimulation, downregulation of NF-κB responses, and induction of tumor cell apoptosis [71,72].
Polysaccharide peptides from the mushroom YunZhi (Coriolus versicolor/Trametes versicolor) have been used in combination with chemotherapy to treat BC patients in Asian countries for decades. These peptides exhibit anti-proliferative properties, as they significantly reduce BC cell (MDA-MB-231) proliferation by upregulating the p21 gene expression [73]. Moreover, a meta-analysis by L.Y. Eliza et al. (2012) concluded that Coriolus versicolor can increase survival rates in cancer patients, including those with BC [74].
Additional beneficial MPs include Levan, which induces apoptotic cell death in MCF-7 BC cells, and the proteoglucan D-Fraction from Grifola frondosa, which reduces mammary tumor cell migration and lung metastases [75,76]. A clinical trial on D-Fraction in breast and lung cancer patients (stage II–IV) found that it hindered cancer progression, metastasis, and increased NK cell activity [77]. This suggests MPs’ potential in enhancing anti-tumor immunity in BC patients without significant toxicities. However, there’s a need for additional clinical trials to study D-fraction’s efficacy in combination with chemotherapeutics in a larger patient population.
3. Cancer Immunotherapy and Microbiome Immunomodulation
Immunotherapy represents a prominent emerging therapeutic approach for certain haematological and solid malignancies, including BC. Bacterial metabolites directly influence the activities of local immune cells. These effects include modulation of immunoglobulin secretion, the promotion of lymphocyte differentiation into regulatory T-lymphocytes and T-helper 17 cells, the generation of immunomodulatory cytokines, and even the epigenetic regulation of histone deacetylase enzymes.
Concerning the connection between the human microbiota and breast cancer, various metabolites have been identified as potential risk factors or modifiers. These include substances such as estrogens, active phytestrogens, short-chain fatty acids, lithocholic acid, and cadaverine. In particular, the gut microbiota’s production of estrogens, largely driven by the enzyme β-glucuronidase from specific intestinal bacteria, can result in the deconjugation of xenobiotics and sex hormones like estrogens. This process increases the reabsorption of estrogens into the systematic circulation, potentially elevating the risk of hormone-dependent breast cancer in women. Conversely, some metabolites such as phytestrogens, lithocholic acid, and cadaverine have been associated with a protective or risk-reducing influence on breast cancer development [78].
Gut bacteria can also promote breast cancer through the induction of chronic inflammation, which is closely linked to tumorigenesis. These bacteria, via pathogen-associated molecular patterns, can upregulate toll-like receptors and activate NF-κB, a critical regulator of inflammation and cancer. NF-κB activation leads to the release of several cytokines, including IL-6, IL-12, IL-17, IL-18, and tumor necrosis factor-alpha, contributing to persistent inflammation within the tumor microenvironment [79]. Secondary metabolites released by intestinal bacteria, along with pro-inflammatory molecules reaching the liver via the portal vein, may further promote carcinogenesis. For instance, butyrate, a microbial metabolite, can enhance the antitumor cytotoxic CD8 T cell response by modulating the ID2-dependent IL-12 signaling pathway [80].
The gut microbiome also plays a role in epigenetic deregulation, potentially affecting tumor development. Microorganisms produce bioactive substances with low molecular weight, such as folates, short-chain fatty acids, and biotin, which can participate in epigenetic processes by altering substrates used for methylation or influencing the activity of epigenetic enzymes [81].
Immune checkpoint inhibitors are a class of therapies that leverage the immune system to combat tumors by blocking inhibitory interactions between T-lymphocyte receptors and ligands on malignant cells. While BC is typically not considered highly immunogenic compared to other malignancies like lung cancer or melanoma, recent data have shown benefits of immunotherapy, particularly in the triple-negative subtype (ER/PR and HER2 negative).
Clinical trials have demonstrated varying response rates with different immunotherapies, alone or in combination with chemotherapy, in these patients. The KEYNOTE-012 trial investigated pembrolizumab monotherapy in previously treated triple-negative breast patients, showing an overall response rate (ORR) of 18.5% and a median time to response of 17.9 weeks [82]. The KEYNOTE-086 trial tested pembrolizumab as a first-line therapy for metastatic triple-negative breast, achieving an ORR of 23% [83]. Other trials, such as NCT01375842 and JAVELIN, assessed atezolizumab and avelumab, with observed ORRs of 10% and 5.2%, respectively [84,85].
Combinations of immunotherapy with chemotherapy were also explored, with atezolizumab combined with nab-paclitaxel yielding an ORR of 67% in first-line treatment [86]. The IMpassion 130 trial examined the combination of atezolizumab and nab-paclitaxel in untreated metastatic triple-negative breast patients, revealing a progression-free survival (PFS) benefit. The ENHANCE-1/KEYNOTE-150 trial evaluated eribulin combined with pembrolizumab, showing a higher ORR in PD-L1-positive breast cancer patients [87]. The KEYNOTE-355 trial combined pembrolizumab with chemotherapy, demonstrating a significant progression-free survival benefit in patients with high PD-L1 values [88]. These trials collectively indicate the potential of immune checkpoint inhibitors in improving outcomes for triple-negative breast patients, particularly in cases with high PD-L1 expression.
4. Treatment Outcomes and Therapy Resistance
The dynamic nature of the gut microbiome, combined with tumor heterogeneity, can lead to varied observations across scientific studies. Numerous reports highlight the alterations in both alpha and beta diversity of the gut microbiome across various cancers, including BC.
A study by Aarnoutse et al., (2022) identified a significant reduction in species richness (p = 0.042) within the gut microbiome of estrogen receptor-positive BC patients during treatment with (neo)adjuvant chemotherapy drugs [89]. A similar trend in decreased gut microbiome richness during chemotherapy was documented by Bilenduke et al., (2022), which later returned to baseline post-treatment [90]. Conversely, Horigome et al. (2019) found no notable shifts in gut microbiome composition between chemotherapy-treated BC patients and untreated counterparts [91]. This disparity was attributed to the fact that the patients had completed their chemotherapy two years before the study’s commencement, suggesting any chemotherapy-induced dysbiosis might have reverted. Various other studies, such as the CANTO trial, reported increased α diversity post-chemotherapy. Wu et al., (2022) made a similar observation for neoadjuvant chemotherapy patients [92].
A depleted microbial richness is linked to intensified depression symptoms, heightened fear of cancer recurrence, and the onset of diarrhea in BC patients undergoing chemotherapy [89,90,93]. Chemotherapy-associated cognitive impairment (CACI) is a widely documented side effect in BC patients, with deficits manifesting in memory retention, processing speed, and visuospatial ability [92,94,95,96]. An array of chemotherapeutic drugs has been implicated in inducing oxidative stress within the brain and promoting inflammation markers within the central nervous system.
Another possible mechanism underlying CACI involves the gut-brain interaction mediated by the peripheral immune system. The “Gut-Immune-Brain Axis” (GBA) theory proposes a communication triad between the intestinal microbiome, immune system, and the brain. Chemotherapy-induced dysbiosis might disrupt the intestinal barrier, leading to immune cell infiltration and the release of pro-inflammatory cytokines into the bloodstream [92]. These cytokines can penetrate the blood-brain barrier (BBB), inducing neuroinflammation, resulting in cognitive disturbances [97]. Several studies support this theory by identifying a correlation between elevated pro-inflammatory cytokines and CACI in BC patients [95,98].
On a positive note, certain bacteria, known for producing butyrate, demonstrate potential anti-inflammatory and anti-cancer properties. The abundance of such beneficial bacteria, including Coprococcus, Ruminococcus, and Faecalibacterium, was noted in BC patients devoid of neurotoxicity post-chemotherapy, pointing to their possible role in curbing neuroinflammation [92].
4.1. Radiotherapy
Radiation therapy, or radiotherapy, primarily functions by damaging the DNA of cancer cells to induce cell death. Typically administered locally, it can also be combined with other treatments. Notably, it’s a well-recorded observation that radiation therapy frequently causes gastrointestinal discomfort as a side effect, even if the gastrointestinal region isn’t directly treated. This indicates a possible involvement of gut microbes in this side effect [99].
Few studies have delved into the impacts of Radiotherapy (RT) or Chemoradiation (CRT) on cancer patients’ gut microbiomes. Specifically, for BC, clinical studies detailing RT’s influence on the gut microbiome are scant, even though RT is a standard treatment for a majority of BC patients. Gynecological cancer patients undergoing RT show a marked decline in gut microbiota richness, exemplified by the decrease in beneficial gut commensal Firmicutes and an increase in opportunistic pathogens like Fusobacterium [100]. Several studies corroborate this finding, revealing that reduced alpha diversity and gut microbiome dysbiosis post-RT and CRT often accompany gastrointestinal side effects or fatigue in various cancers.
In cases of RT, it’s noteworthy that higher alpha gut diversity has been associated with increased tumor infiltration of activated CD4+ T-cells, subsequently leading to better recurrence-free and overall survival rates in cancer patients. While animal studies are foundational in understanding the intricate interactions of radiation, bacteria, and fungi, only one study, as of now, has delved into these interactions in a BC mouse model [101]. This study accentuated the importance of both bacteria and fungi in modulating the outcomes of RT.
Furthermore, studies have shown a connection between gut bacteria and the effectiveness of radiation therapy in treating various cancers. Eradicating Gram-positive bacteria with antibiotics improved radiation’s antitumor effects in melanoma, lung, and cervical cancer mouse models. However, reintroducing the metabolite sodium butyrate, produced by these bacteria, negated this benefit [102]. Complete removal of gut bacteria reduced radiation effectiveness in some cancer models, while gut fungi depletion enhanced it [101]. Fungal sensor Dectin-1’s higher concentrations correlated with poorer breast cancer survival rates. Mice’s response to radiation varied based on their microbiota, with Enterococcaceae and Lachnospiraceae being prominent in responsive mice. Leukemia patients with these bacterial families experienced fewer gastrointestinal symptoms post-radiation. Metabolites from these bacteria were linked to radioprotection. Though research is limited, gut microbiota’s role in radiation response emphasizes the need for more studies on this relationship [103].
4.2. Chemotherapy
Chemotherapy primarily involves the use of medicinal drugs to chemically treat cancer by interfering with cell division (mitosis). The effectiveness of chemotherapy differs across various types of cancer. Its main objective is to harm or overwhelm cancer cells to induce programmed cell death (apoptosis), and some chemotherapy drugs can also stimulate immune reactions. Since chemotherapy is administered throughout the body, it may also impact normal cells that undergo rapid division. The gut microbiome was initially identified as a factor influencing the effectiveness of chemotherapy because systemic administration is likely to affect these microbial communities [104].
Administering antibiotics to neutralize the gut microbiome decreased the effectiveness of chemotherapy drugs like cisplatin and oxaliplatin in treating lymphoma and colon cancer in mice, attributed to reduced reactive oxygen species (ROS) production. This indicates the necessity of a functional commensal microbiome for the success of these platinum-based treatments. Antibiotics also lowered oxaliplatin’s effectiveness in a specific colon cancer mouse model. Subsequent experiments revealed associations between certain microbes and the drug’s effectiveness. For instance, the presence of Paraprevotella clara was linked with a lack of response to oxaliplatin, while B. fragilis correlated with a positive response. In terms of the chemotherapy drug cyclophosphamide, it resulted in the movement of particular gut microbes like Lactobacillus johnsonii and Enterococcus hirae to the spleen and lymph nodes, initiating an immune response in melanoma and sarcoma mouse models. Additional research showed that the existence of E. hirae and Barnesiella intestinihominis in the gut could enhance cyclophosphamide activity and rejuvenate its effectiveness post-antibiotic administration.
Additionally, the CANTO trial documented significant changes in the gut microbiome composition throughout chemotherapy [92]. Favourable commensals like Dorea formicigenerans and Methanobrevibacter smithii archaea, commonly found in healthy individuals, increased in abundance in BC patient’s post-chemotherapy. Coprococcus and members of the Ruminococcaceae and Eubacteriaceae families were positively linked with a good prognosis and the absence of axillary lymph node metastasis. Conversely, harmful bacterial species such as certain Klebsiella and Bacteroides species, along with members of the Lachnospiraceae and Clostridiaceae families, were associated with axillary lymph node invasion and advanced BC stages after chemotherapy. In particular, B. uniformis was prominently abundant in non-metastatic BC of advanced stage but reduced post-chemotherapy. High abundance of Bacteroides also correlated with non-responsiveness to trastuzumab treatment in HER2-positive BC patients [105].
A metabolite from the gut microbiome, indole-3-acetic acid, has been observed to enhance the effectiveness of FOLFIRINOX, a combination chemotherapy used in treating metastatic PDAC. This enhancement is due to the accumulation of ROS and a reduction in the cancer cells’ autophagy. However, influences on chemotherapy effectiveness are not limited to the gut microbiome alone; bacteria in and around the tumor also play a significant role. There’s a growing interest in exploring how these local bacteria impact the effectiveness of chemotherapy and can contribute to drug resistance in cancer patients.
Studies have shown that the commensal bacteria E. coli has the ability to modify the effectiveness of various chemotherapy drugs. It increased the toxicity of some drugs, while reducing the toxicity of others like gemcitabine, doxorubicin, and mitoxantrone. Further experiments involving E. coli and gemcitabine in a mouse model indicated a decrease in the drug’s ability to combat tumors. Likewise, both Mycoplasma hyorhinis and E. coli, have been found to metabolize gemcitabine into an inactive form, making cancer cells resistant. In another study, the commensal F. nucleatum, when combined with colon cancer cells, reduced the cells’ apoptosis when exposed to certain chemotherapy drugs, leading to increased drug resistance. It implies that the local microbes and those within the tumor microenvironment can substantially impact chemotherapy’s effectiveness. On the other hand, manipulating the lung microbiota using aerosolized antibiotics and specifically probiotics has shown improvement in the efficacy of a chemotherapeutic drug, dacarbazine. Such findings underscore the potential of further exploring microbial interactions in various body regions and within the tumor to enhance the outcomes of chemotherapy treatments.
5. Integrating One Health Approach in Cancer Ecology
The holistic approach to health promoted by the One Health principles mainly focuses on infectious diseases and zoonoses [106]. However, the mechanisms underlying oncogenesis and novel management strategies in oncology recognize the interrelations between human health, animal health and the environment, thus placing emphasis to the transdisciplinary and multisectoral relevance of One Health. The connection between environmental, animal and human health and oncogenesis be supported by several observations [107,108]. The transmissibility of specific malignancies between animals: although rare, transmission of cancer between individuals of the same or related species (such as the canine transmissible venereal sarcoma) has been documented [109]. In addition, the transmission mode in several cases remains undefined (e.g., in the case of a leukemia-like disease among marine bivalves, such as clams and mussels [109]. Similarly, from an evolutionary perspective, transmission of cancer between different species remains a possibility [109,110]. The evolutionary cancer suppression mechanisms detected in animal species: one such example is the development of immune-modulating resistance against devil facial tumour disease (DFTD) reported in Tasmanian devils (Sarcophilus harrisii), which probably contributed to survival of the species over this fatal type of transmissible cancer [111]. Another example is that of the myxoma virus (MYXV) and the European rabbit (Oryctolagus cuniculus), which co-evolved, selecting for viruses of attenuated virulence that caused extended (over fatal) disease and in parallel, for rabbits that developed resistance to myxomatosis [112] through genetic alterations that facilitated an enhanced immune response the disease [113]. Such recorded cases provide evidence that transmissible cancers can exert selection pressure on affected species, promoting the domination of organisms with genetic alterations and immune mechanisms that confer a survival benefit. The mutagenetic and oncogenetic ability of human activities to animals: the extent, intensity and nature of human activity have significant impact on the diversity of the environment and on the health and habits of different animal species, resulting in variable contact patterns between animals, arthropods and humans and facilitating the emergence and re-emergence of infectious diseases [114]. Simultaneously, it is well established that environmental alterations resulting from urbanization, air, soil noise, light, and water pollution, environmental degradation, and dietary interventions, precipitate ongogenic processes in humans, thus increasing the risk of cancer. Accumulating literature suggests that these conditions also affect the health of animals, including wildlife, through various pathways, including endocrine and immune dysregulation, chronic inflammation, nutritional disruptions, oncogenic infections, epigenetic changes, chromosomal alterations, and microbiome changes [115,116,117].
The shared environment between animals and humans, affecting microbiota composition and microbiome and unraveling possible causative relationships with cancer development. An organisms’ homeostasis and physiology are closely intercalated with its microbiome. Similarly, environmental perturbations, anthropogenic or not, can influence the microbiota of both humans and animals, with health implications across different organs and systems [118,119,120].
In animal studies, westernized diet-associated bacteria exerted transgenerational effects in utero resulting in chronic disease and cancer in mice [121]. Furthermore, antibiotic-affected microbial communities in bees led to significant alterations in gene expressions in the host, which could also be passed across generations [122]. Such findings signify the close relationship between microbiome, normal host functions, genetic changes and the ability to pass these changes across generations.
Taken together, these observations advocate for collaborative efforts to address the various mechanisms underlying cancer development by acknowledging similarities that exist among animals and humans in a shared ecosystem. Acknowledging that the environment and disease can cause disruptions and imbalances in the host microbiota composition, predisposing to physiologic and homeostatic changes, a One Health approach to cancer comprises of the following:
Surveillance: cancer surveillance across human medicine, veterinary medicine, and environmental science. Areas and species can serve as sentinel targets for early detection of carcinogens in the environment and for cancer patterns that might reflect eco-environmental exposures or cancer types that warrant further research.
Detection, including early warning systems: interdisciplinary collaboration to create diagnostic tools (e.g., biomarkers, screening protocols, geographic information systems). Employment of artificial intelligence and machine learning tools can help combine surveillance, diagnosis and warning systems to facilitate early diagnosis and prompt detection.
Elucidating and addressing common risk factors: several risk factors for cancer, such as exposure to carcinogens and lifestyle choices (e.g., nutrition, physical activity), affect both humans and animals [123]. Understanding the presence and pathophysiologic mechanisms of shared risk factors can help elucidate oncogenetic pathways or design and implement preventive strategies. Surveillance and prompt detection can facilitate the design of future studies in order to elucidate unidentified risks for cancer development in different species [124].
Environmental health: the association between environment and disease is bidirectional. The behavior of animal species can increase cancer risk, through the induction of cancer-associated environmental exposures. Spatial, temporal, ecological and population changes can have documented and unpredicted effects on the emergence, re-emergence and frequency of diseases [114,123]. These are not limited to communicable diseases, but can span across endocrine conditions, malignancies, cardiovascular disease, metabolic conditions, skin conditions, toxin exposures, etc [125]. Up to one fourth of global deaths and one-fifth of disability-adjusted life years (DALYs) in humans are associated to environmental exposures [125]. Conversely, environment-attributable diseases can affect the behavior of animals, leading to further environmental changes. For example, poor living conditions in rural areas drive urbanization, further increasing air and noise pollution in urban areas. In wildlife, diseased animals are more prone to fall to prey, thus increasing the transmission risk of communicable diseases, while representing an inferior nutritional source for preying [89] species. Animals and humans who are malnourished or suffer from chronic disease, have a higher risk of other diseases after exposure, either to environmental factors, pollutants or transmissible agents, increasing the incidence of environment-associated diseases and affecting the growth, behavior and health of animal populations [123]. Cumulatively, environmental degradation increases risk for disease and diseased populations can affect the environment, creating a vicious cycle of eco-environmental health [126].
Cancer prevention: prevention strategies span over different levels, including primordial, primary, secondary, tertiary and quaternary [126]. Health promotion, a principle focused on humans, concerns empowering people to increase control over their own health [127]. In the context of One Health, cancer prevention extends beyond individual and populational levels of health, to the broad geospatial and interspecies framework of ecosystems [123].
Antimicrobial stewardship: an important and emerging aspect of One Health, the ecological effects of antimicrobials at the population and environmental level are globally recognized. Exposure to antimicrobials facilitates the dominance of resistant bacterial populations in the environment, acquiring genes which have the potential to transmit in the environment, from organism to organisms, and between species [128]. Antimicrobial usage has been found to be directly related to the number of resistance genes in the gut microbiome of humans, even in the absence of direct antimicrobial administration [129]. Water and soil constitute significant environmental reservoirs of antimicrobial resistant genes [130]. Antimicrobial resistant genes can spread in the environment to such an extent that can even reach air masses in areas of high antimicrobial usage [131]. These observations raise the imminent need for application stringent efforts to control transmission of antimicrobial resistance in the community. The strongest risk for emergence of antimicrobial resistance in community settings is inappropriate use of antimicrobials in humans, animals and agriculture [130]. Indeed, the volume of inappropriate use of antimicrobials in animal husbandry, veterinary medicine and agriculture, significantly exceeds their use in human medicine, representing a key target for stewardship interventions[132]. Antibiotic-associated tissue dysbiosis has been associated with tumor development, further supporting the need for prudent use of antimicrobials in the animals [133]. In addition, disruption of normal immune responses to malignant cells, induced by antimicrobials, can have detrimental outcomes in organisms with cancer, by affecting their responses both to cancer and to therapeutic agents [134].
Ethical considerations: considerations related to ethical issues surrounding animal welfare, humane treatment, healthy livelihood, access to optimal healthcare and experimental studies, for all living organisms are integrated into the One Health approach, ensuring that research, medical interventions and environmental health are addressed under the same moral boundaries [127,135,136].
6. Conclusion
Employing agents involved in multiple human biological reactions such as immune response and metabolism may be key to developing tumour drugs with higher selectivity and specificity hence reducing adverse side effects for patients. These drugs could be prescribed in synergy with traditional cancer treatments which includes radiation, immunotherapy or chemotherapy. These forms of treatment can potentially diminish drug resistance. A microbiome screen test can pinpoint potential target that may help improve patient prognosis, as previously described, but may also avert drug resistance. Additionally, standardised protocols need to be composed to ensure clinical guidelines and protocols are available. Such a uniform system will also allow for collection of efficiency and safety evidence. Breast tumours can be accessed by adjusting the gut microbiota or by means of microbiota transport vectors introduced peritoumorally. Ultimately the relationship between gut and breast microbiome colonies needs to be scrutinised prior to designing new drugs that work in this way and evaluate what effects these microbiomes will have on the immune system.
6.1. Integration of microbial therapy within the one health approach
The concept of ONE Health describes a transdisciplinary approach considering the interaction between plants, animals, people, and the environment they share. The basis of this concept is to recognise and eliminate zoonotic diseases, water contaminants, antimicrobial-resistant germs, vector-borne diseases and more. Evidence so far supports that there is a strong link between microbiome and cancer development, cancer proliferation and apoptosis. Specific human microbiome also serve as biomarkers for certain cancers. Shifting the microbiome (expression) accordingly reverses these affects and it does so through a more holistic approach [137]. Manipulating the gut microbiota through such an approach makes for more personalised treatments with fewer side effects and potentially greater health outcomes. This could also improve patients mental position as they may appreciate the concept being offered a more holistic treatment option as to chemical drugs.
Interdisciplinary collaboration between human and animal researchers can advance microbial research by providing epidemiological insights of the disease. A partnership between veterinary medicine and human medicine can increase the amount of data available on cancer and behaviour of microbiota and provide valuable information that can help progress therapeutic methods. As comparative and spontaneous oncology emerge, more information becomes available as to how an animals’ immune system fights off neoplasms. Extrapolation of this information can increase understanding on the behaviour of a cancer and hence provide novel therapeutic targets and treatment schemes [138]. More importantly as ownership of both domestic and exotic pets is increasing, the need to survey ONE Health elements it crucial to monitor and potentially prevent outbreaks.
Transmissible cancers are emerging, accelerating the loss of biodiversity across ecosystems redirecting research as to why these cancers arise and how they unfold and prevent any potential pandemics [108]. An additional aspect that’s requires further evaluation is the degree to which malignancies in animals, such as breast cancer, are transmissible to humans.
Identifying the exact microbiome species through genetic analysis can uncover their environmental origin, this could be animal or other food products. Chemical agents also disturb microbiome. Fertilisers provide crops with nonspecific nutrients meaning that microbiota populations are also nourished hence proliferate, once produce is consumed by human the gut microbiota is subsequently increased [139]. Infant feeding, infections, medication and diet are among the environmental factors that need to be further assessed to further understand the impact they could have on breast cancer. An ongoing study (NCT03885648) is evaluating the hypothesis that risk of breast cancer for humans could be due to environmental contaminants affecting the mammary/gut microbiota.
6.2. Regulatory considerations and ethical implications
Despite emerging microbiome-based therapies presenting promising therapeutic results against BC therapy both regulatory and ethical considerations must be thoughtfully evaluated. This is to ensure patient safety and diagnosis. Prior to marketing a drug for microbiome-based therapy, clinical trials are essential to evaluate drug safety and efficacy followed by evaluation of the results from the Food and Drug Administration (FDA) and European Medicines Agency (EMA). Following the marketing of drugs, off-label use by clinicians may also be a factor to consider in terms of both regulatory and ethical concerns, ensuring the patient is constantly informed of the efficacy and limitations of the treatment they are receiving. It is critical to ensure that with informed consent patients are fully aware of the limited knowledge available on long-term effects of administering microbiome therapies [140]. Microbiome can express both tumour suppressor and promoter properties, therefore interventions causing dysbiosis must be investigated to identify how genes and lifestyle (e.g., diet) affect the natural biodiversity of microbiome. This information will also affect patient response to microbial therapy. Access to microbiome therapies is also of ethical concern, to ensure individuals from different populations and socioeconomical groups all have access to these drugs. Utilisation of big data and machine learning for precision medicine is already being discussed. This raises data privacy concerns as use of omics technologies will involve databases that store and constantly analyse sensitive data on the gut microbiome of multiple individuals. Setting up such data sets calls for adherence to ethical guidelines and approval from ethical committees and requires completion of informed consent forms [141].
6.3. Future directions
Suitable regulation of gastrointestinal microbiome by antibiotics or dietary regimes restrains estrogen effects and regulates bacterial activities which in turn can prevent breast cancer or breast cancer reoccurrence. As microbes translocate to mammary tissue from the gut they also affect how the inflammatory response is triggered [142]. Further gene analysis is required to map all commensal microorganisms and other wild-type microorganisms naturally expressed in the human body that contribute to additional genetic material necessary for human metabolism. The focal aspects that need to be determined to ensure a microbiome can be labelled as therapeutic agents are to first identify the species naturally present in the human body under ‘healthy’ conditions, determine the ‘healthy’ concentrations of these microbiota and finally the levels at which these microorganisms activate harmful responses.
Mapping out all microbes that inhabit a patient’s body can provide insights of the treatment plan that should be administrated. This is important as microbiota in both the gut and within the tumour environment can affect drug metabolism and disturb activity of chemotherapeutic agents [143]. By reversing dysbiosis the microbiome can be manipulated to reverse microbiota to ‘health’ levels avoiding tumour drug resistance and subsequently harmful effects instigated by these drugs. Dietary plans may also accompany drug administration for more efficient results, for instance in vivo results presented that hyperbaric oxygen therapy accompanied by a ketogenic diet express significant antitumor effect [144].
Proceeding with microbial therapies will also require supplementary investigation to understand how microbiome-to-microbiome interaction can affect patient prognosis. This is necessary as the same drug can shape up differently in every human individual due to the unique composition of their microbiome [145]. Additionally, the shift age exerts on microbiome concentrations is also vital as it may affect a patient’s diagnosis and prognosis. The stage of the cancer should also be considered as this affects the concentration of the microbiome but also other conditions such as whether the patient is in a pre- or postmenopausal state [146]. Evidently, Lachnospiraceae and Clostridium are abundant in stage II and III breast cancer patient gut microbiome as compared to stage 0 and stage I. The level of dysbiosis is also affected by the menopausal state of breast cancer. Post-menopausal breast cancer patients express a higher presence of pathogenic bacteria than pre-menopausal patients proposing a non-invasive approach as these concentrations can be rereferred to as markers for detection, prevention and potentially therapeutic targets [147].
Fucobacterium nucleatum is an anaerobe that suppresses T cell accumulation while promoting tumour growth and stepping up metastasis [148]. Additional evaluation as to whether eliminating the microbiota will have the opposite effect and allow for anticancer cells to react effectively. An additional pathway requiring further exploration is the servicing of E.coli secretome as biomarkers for breast cancer as they disrupt key metabolic pathways of MCF-7 cells [75]. As microbial settlements are volatile, continuous monitoring is essential to ensure suitable adjustments are made to increase treatment efficacy. Clinical trials are necessary to further evaluate the effectiveness and safety of these therapies both short and long-term. The latter is especially crucial given that some microbiota are key for normal biological functions thus altering their concentrations or introducing them in other parts of the human body than their normal environment can have unintentional consequences.
6.4. Promising avenues for further research and development
Bacteriotherapy has been a possibility since 1988 and involves implantation of gut flora from a healthy individual to a patient reversing gut dysbiosis to improve diagnosis [149]. Bacteria have been enlisted in the treatment regime of multiple cancers; Salmonella Typhimurium for colon breast carcinoma, Escherichia coli for nasopharyngeal carcinoma, Klebsiella pneumoniae for cervical cancer and pseudomonas aeruginosa in multiple cancers, C. botulinum in breast cancer [150,151]. Due to their anaerobic properties’ microbiota can enter a tumour microenvironment without being affected by its necrotic and hypoxic properties. One mode of action is utilising the microbiome as a vector to deliver chemotherapeutic drugs deep into the tumour so that they may exert their apoptotic tumour effects [152]. An alternative mechanism is one where microbiome expression is tuned to ‘healthy’ levels permitting chemotherapeutic drugs to perform their effects successfully within the human body without interference and with limited adverse side effects. Having determined the fluctuations that microbial community concentrations undergo due to the presence of a tumour, has marked novel biomarkers to be targeted for tumour diagnosis. Future diagnoses of cancer may involve completion of genomic profile screening on a patient biopsy to identify the dysregulated microbes that need to be targeted. These findings can then be utilised to establish an effective patient-specific cancer-microbial therapy plan with the least side effects.
Assuming microbial therapy where fecal microbiota transplantation is involved will require detailed screening of the donor to ensure the material that will be retrieved is free from alternate diseases or even infectious agents that could maltreat the patient. As materials are extracted from another human donor this may also lead to unexpected immunological side effects within the patient. Finally, similarly to other novel treatments, long-term safety is unknown.
In this review, we explore the influence of both gut and local microbiomes on various aspects of cancer care, encompassing its development, progression, diagnosis, and treatment. Recent advancements have seen groups harnessing synthetic biology to modify commensal microbes, triggering specific immune responses against diseases, including cancer. As research techniques and analytical models evolve, our understanding of the microbiomes within and around us will deepen. We foresee a marked rise in the discovery of microbes associated with cancer, going beyond just the easily cultured ones. These newly discovered microbes present promising avenues for refining diagnostic and therapeutic strategies, setting the stage for enhanced cancer prevention and more effective treatment outcomes. Additionally, interdisciplinary collaborations between human, animal, and environmental health researchers will provide invaluable insights into cancer-microbiome interplay. Comprehending the identities, distributions, and activities of human microbes in health and disease through a One Health approach is essential for strategically modulating the microbiome and harnessing its intricate ties with breast cancer for improved patient outcomes.
Figure 1.
One health Conceptual Diagram. One health includes Human Health and Well-being, Animal Health, Environment health as well as the Microbial and biochemical Ecology of Soils and Plants. The interconnectivity and dynamic interaction between all of them emphasizes the level of each can affect the other by stressing the need of their balance.
Figure 1.
One health Conceptual Diagram. One health includes Human Health and Well-being, Animal Health, Environment health as well as the Microbial and biochemical Ecology of Soils and Plants. The interconnectivity and dynamic interaction between all of them emphasizes the level of each can affect the other by stressing the need of their balance.

Figure 2.
Microbiome and Tumor Microenvironment interplay. Schematic illustration of the Microbiome –mediated modulation of the immune system, including Toll-Like Receptor activation, Cytokine release, anti-tumor responses, inhibition of tumor vasculature and immune cell activation.
Figure 2.
Microbiome and Tumor Microenvironment interplay. Schematic illustration of the Microbiome –mediated modulation of the immune system, including Toll-Like Receptor activation, Cytokine release, anti-tumor responses, inhibition of tumor vasculature and immune cell activation.

Author Contributions
Conceptualization, A.Y. and C.F.; data curation, A.Y, C.F, S.C.T., G.M., Y.P., M.F.; writing—original draft preparation, A.Y., C.F., S.C.T., C.A.K., Z.-D.P. and C.T.; writing—review and editing, A.Y., C.F., S.C.T., C.A.K, Z.-D.P. and E.O.J.All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Artusa, V. , Calabrone, L., Mortara, L., Peri, F., Bruno, A.: Microbiota-Derived Natural Products Targeting Cancer Stem Cells: Inside the Gut Pharma Factory. IJMS. 24, 4997 (2023). [CrossRef]
- Xuan, C. , Shamonki, J.M., Chung, A., DiNome, M.L., Chung, M., Sieling, P.A., Lee, D.J.: Microbial Dysbiosis Is Associated with Human Breast Cancer. PLoS ONE. 9, e83744 (2014). [CrossRef]
- Nejman, D. , Livyatan, I., Fuks, G., Gavert, N., Zwang, Y., Geller, L.T., Rotter-Maskowitz, A., Weiser, R., Mallel, G., Gigi, E., Meltser, A., Douglas, G.M., Kamer, I., Gopalakrishnan, V., Dadosh, T., Levin-Zaidman, S., Avnet, S., Atlan, T., Cooper, Z.A., Arora, R., Cogdill, A.P., Khan, M.A.W., Ologun, G., Bussi, Y., Weinberger, A., Lotan-Pompan, M., Golani, O., Perry, G., Rokah, M., Bahar-Shany, K., Rozeman, E.A., Blank, C.U., Ronai, A., Shaoul, R., Amit, A., Dorfman, T., Kremer, R., Cohen, Z.R., Harnof, S., Siegal, T., Yehuda-Shnaidman, E., Gal-Yam, E.N., Shapira, H., Baldini, N., Langille, M.G.I., Ben-Nun, A., Kaufman, B., Nissan, A., Golan, T., Dadiani, M., Levanon, K., Bar, J., Yust-Katz, S., Barshack, I., Peeper, D.S., Raz, D.J., Segal, E., Wargo, J.A., Sandbank, J., Shental, N., Straussman, R.: The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science. 368, 973–980 (2020). [CrossRef]
- Dohlman, A.B. , Klug, J., Mesko, M., Gao, I.H., Lipkin, S.M., Shen, X., Iliev, I.D.: A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell. 185, 3807-3822.e12 (2022). [CrossRef]
- Fu, A. , Yao, B., Dong, T., Chen, Y., Yao, J., Liu, Y., Li, H., Bai, H., Liu, X., Zhang, Y., Wang, C., Guo, Y., Li, N., Cai, S.: Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 185, 1356-1372.e26 (2022). [CrossRef]
- Lakritz, J.R. , Poutahidis, T., Mirabal, S., Varian, B.J., Levkovich, T., Ibrahim, Y.M., Ward, J.M., Teng, E.C., Fisher, B., Parry, N., Lesage, S., Alberg, N., Gourishetti, S., Fox, J.G., Ge, Z., Erdman, S.E.: Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. 6, 9387–9396 (2015). [CrossRef]
- Liu, M. , Jia, S., Dong, T., Zhao, F., Xu, T., Yang, Q., Gong, J., Fang, M.: Metabolomic and Transcriptomic Analysis of MCF-7 Cells Exposed to 23 Chemicals at Human-Relevant Levels: Estimation of Individual Chemical Contribution to Effects. Environ Health Perspect. 128, 127008 (2020). [CrossRef]
- Xu, J.-Y. , Liu, M.-T., Tao, T., Zhu, X., Fei, F.-Q.: The role of gut microbiota in tumorigenesis and treatment. Biomedicine & Pharmacotherapy. 138, 111444 (2021). [CrossRef]
- Roy, S. , Trinchieri, G.: Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 17, 271–285 (2017). [CrossRef]
- Tzeng, A. , Sangwan, N., Jia, M., Liu, C.-C., Keslar, K.S., Downs-Kelly, E., Fairchild, R.L., Al-Hilli, Z., Grobmyer, S.R., Eng, C.: Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 13, 60 (2021). [CrossRef]
- Maroof, H. , Hassan, Z.M., Mobarez, A.M., Mohamadabadi, M.A.: Lactobacillus acidophilus Could Modulate the Immune Response Against Breast Cancer in Murine Model. J Clin Immunol. 32, 1353–1359 (2012). [CrossRef]
- Bernardo, G. , Le Noci, V., Ottaviano, E., De Cecco, L., Camisaschi, C., Guglielmetti, S., Di Modica, M., Gargari, G., Bianchi, F., Indino, S., Sartori, P., Borghi, E., Sommariva, M., Tagliabue, E., Triulzi, T., Sfondrini, L.: Reduction of Staphylococcus epidermidis in the mammary tumor microbiota induces antitumor immunity and decreases breast cancer aggressiveness. Cancer Letters. 555, 216041 (2023). [CrossRef]
- Cai, Z. , Sanchez, A., Shi, Z., Zhang, T., Liu, M., Zhang, D.: Activation of Toll-like Receptor 5 on Breast Cancer Cells by Flagellin Suppresses Cell Proliferation and Tumor Growth. Cancer Research. 71, 2466–2475 (2011). [CrossRef]
- Zhu, J. , Liao, M., Yao, Z., Liang, W., Li, Q., Liu, J., Yang, H., Ji, Y., Wei, W., Tan, A., Liang, S., Chen, Y., Lin, H., Zhu, X., Huang, S., Tian, J., Tang, R., Wang, Q., Mo, Z.: Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 6, 136 (2018). [CrossRef]
- Lakey, J.H., Slatin, S.L.: Pore-Forming Colicins and Their Relatives. In: Van Der Goot, F.G. (ed.) Pore-Forming Toxins. pp. 131–161. Springer Berlin Heidelberg, Berlin, Heidelberg (2001). 2001. [CrossRef]
- Kaur, S. , Kaur, S.: Bacteriocins as Potential Anticancer Agents. Front. Pharmacol. 6, (2015). [CrossRef]
- Baindara, P. , Gautam, A., Raghava, G.P.S., Korpole, S.: Anticancer properties of a defensin like class IId bacteriocin Laterosporulin10. Sci Rep. 7, 46541 (2017). [CrossRef]
- Hidaka, A. , Hamaji, Y., Sasaki, T., Taniguchi, S., Fujimori, M.: Exogeneous Cytosine Deaminase Gene Expression in Bifidobacterium breve I-53-8w for Tumor-Targeting Enzyme/Prodrug Therapy. Bioscience, Biotechnology, and Biochemistry. 71, 2921–2926 (2007). [CrossRef]
- Silva, E.O. , De Carvalho, T.C., Parshikov, I.A., Dos Santos, R.A., Emery, F.S., Furtado, N.A.J.C.: Cytotoxicity of lapachol metabolites produced by probiotics. Lett Appl Microbiol. 59, 108–114 (2014). [CrossRef]
- Karami, P. , Goli, H.R., Abediankenari, S., Chandani, S.R., Jafari, N., Ghasemi, M., Ahanjan, M.: Anti-tumor effects of Bacteroides fragilis and Bifidobacterium bifidum culture supernatants on mouse breast cancer. Gene Reports. 33, 101815 (2023). [CrossRef]
- Bernardo, G. , Le Noci, V., Di Modica, M., Montanari, E., Triulzi, T., Pupa, S.M., Tagliabue, E., Sommariva, M., Sfondrini, L.: The Emerging Role of the Microbiota in Breast Cancer Progression. Cells. 12, 1945 (2023). [CrossRef]
- Zhu, R. , Lang, T., Yan, W., Zhu, X., Huang, X., Yin, Q., Li, Y.: Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy. Advanced Science. 8, (2021). [CrossRef]
- Mendes, I. , Vale, N.: How Can the Microbiome Induce Carcinogenesis and Modulate Drug Resistance in Cancer Therapy? International Journal of Molecular Sciences. 24, 11855 (2023). [CrossRef]
- Trivanović, D. , Pavelić, K., Peršurić, Ž.: Fighting Cancer with Bacteria and Their Toxins. Int J Mol Sci. 22, 12980 (2021). [CrossRef]
- Khoshnood, S. , Fathizadeh, H., Neamati, F., Negahdari, B., Baindara, P., Abdullah, M.A., Haddadi, M.H.: Bacteria-derived chimeric toxins as potential anticancer agents. Frontiers in Oncology. 2022; 12. [Google Scholar] [CrossRef]
- Browne, K. , Chakraborty, S., Chen, R., Willcox, M.D., Black, D.S., Walsh, W.R., Kumar, N.: A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int J Mol Sci. 21, 7047 (2020). [CrossRef]
- Patyar, S. , Joshi, R., Byrav, D.P., Prakash, A., Medhi, B., Das, B.: Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 17, 21 (2010). [CrossRef]
- Nandi, D. , Parida, S., Sharma, D.: The gut microbiota in breast cancer development and treatment: The good, the bad, and the useful! Gut Microbes. 15, 2221452. [CrossRef]
- Allemailem, K.S. : Innovative Approaches of Engineering Tumor-Targeting Bacteria with Different Therapeutic Payloads to Fight Cancer: A Smart Strategy of Disease Management. International Journal of Nanomedicine. 16, 8159 (2021). [CrossRef]
- Liang, S. , Wang, C., Shao, Y., Wang, Y., Xing, D., Geng, Z.: Recent advances in bacteria-mediated cancer therapy. Frontiers in Bioengineering and Biotechnology. 2022; 10. [Google Scholar] [CrossRef]
- Duong, M.T.-Q. , Qin, Y., You, S.-H., Min, J.-J.: Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 51, 152 (2019). [CrossRef]
- Wei, X. , Du, M., Chen, Z., Yuan, Z.: Recent Advances in Bacteria-Based Cancer Treatment. Cancers (Basel). 14, 4945 (2022). [CrossRef]
- Freedman, J.C. , Shrestha, A., McClane, B.A.: Clostridium perfringens Enterotoxin: Action, Genetics, and Translational Applications. Toxins (Basel). 8, 73 (2016). [CrossRef]
- Cardillo, F. , Bonfim, M., da Silva Vasconcelos Sousa, P., Mengel, J., Ribeiro Castello-Branco, L.R., Pinho, R.T.: Bacillus Calmette–Guérin Immunotherapy for Cancer. Vaccines (Basel). 9, 439 (2021). [CrossRef]
- Nallar, S.C. , Xu, D.-Q., Kalvakolanu, D.V.: Bacteria and genetically modified bacteria as cancer therapeutics: Current advances and challenges. Cytokine. 89, 160–172 (2017). [CrossRef]
- McCarthy, E.F. : The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop J. 2006; 26, 154–158. [Google Scholar]
- Forbes, N.S. : Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 10, 785–794 (2010). [CrossRef]
- Hatakeyama, M. : Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 15, 306–316 (2014). [CrossRef]
- Felgner, S. , Kocijancic, D., Frahm, M., Heise, U., Rohde, M., Zimmermann, K., Falk, C., Erhardt, M., Weiss, S.: Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology. 7, e1382791 (2018). [CrossRef]
- Field, D. , Cotter, P.D., Ross, R.P., Hill, C.: Bioengineering of the model lantibiotic nisin. Bioengineered. 6, 187–192 (2015). [CrossRef]
- Roberts, N.J. , Zhang, L., Janku, F., Collins, A., Bai, R.-Y., Staedtke, V., Rusk, A.W., Tung, D., Miller, M., Roix, J., Khanna, K.V., Murthy, R., Benjamin, R.S., Helgason, T., Szvalb, A.D., Bird, J.E., Roy-Chowdhuri, S., Zhang, H.H., Qiao, Y., Karim, B., McDaniel, J., Elpiner, A., Sahora, A., Lachowicz, J., Phillips, B., Turner, A., Klein, M.K., Post, G., Diaz, L.A., Riggins, G.J., Papadopoulos, N., Kinzler, K.W., Vogelstein, B., Bettegowda, C., Huso, D.L., Varterasian, M., Saha, S., Zhou, S.: Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 6, 249ra111 (2014). [CrossRef]
- Bhardwaj, N. , Gnjatic, S., Sawhney, N.B.: TLR AGONISTS: Are They Good Adjuvants? Cancer journal (Sudbury, Mass.). 16, 382 (2010). [CrossRef]
- Kaufman, H.L. , Kohlhapp, F.J., Zloza, A.: Erratum: Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 15, 660–660 (2016). [CrossRef]
- Fan, J. , Jiang, H., Cheng, L., Liu, R.: The oncolytic herpes simplex virus vector, G47Δ, effectively targets tamoxifen-resistant breast cancer cells. Oncology Reports. 35, 1741–1749 (2016). [CrossRef]
- Wang, L.-J. , Han, S.-X., Bai, E., Zhou, X., Li, M., Jing, G.-H., Zhao, J., Yang, A.-G., Zhu, Q.: Dose-dependent effect of tamoxifen in tamoxifen-resistant breast cancer cells via stimulation by the ERK1/2 and AKT signaling pathways. Oncology Reports. 29, 1563–1569 (2013). [CrossRef]
- Zeng, W.-G. , Li, J.-J., Hu, P., Lei, L., Wang, J.-N., Liu, R.-B.: An oncolytic herpes simplex virus vector, G47Δ, synergizes with paclitaxel in the treatment of breast cancer. Oncology Reports. 29, 2355–2361 (2013). [CrossRef]
- Soliman, H. , Hogue, D., Han, H., Mooney, B., Costa, R., Lee, M.C., Niell, B., Williams, A., Chau, A., Falcon, S., Soyano, A., Armaghani, A., Khakpour, N., Weinfurtner, R.J., Hoover, S., Kiluk, J., Laronga, C., Rosa, M., Khong, H., Czerniecki, B.: Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial. Nat Med. 29, 450–457 (2023). [CrossRef]
- Schmid, P. , Cortes, J., Dent, R., Pusztai, L., McArthur, H., Kümmel, S., Bergh, J., Denkert, C., Park, Y.H., Hui, R., Harbeck, N., Takahashi, M., Untch, M., Fasching, P.A., Cardoso, F., Andersen, J., Patt, D., Danso, M., Ferreira, M., Mouret-Reynier, M.-A., Im, S.-A., Ahn, J.-H., Gion, M., Baron-Hay, S., Boileau, J.-F., Ding, Y., Tryfonidis, K., Aktan, G., Karantza, V., O’Shaughnessy, J.: Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med. 386, 556–567 (2022). [CrossRef]
- Catala, A. , Dzieciatkowska, M., Wang, G., Gutierrez-Hartmann, A., Simberg, D., Hansen, K.C., D’Alessandro, A., Catalano, C.E.: Targeted Intracellular Delivery of Trastuzumab Using Designer Phage Lambda Nanoparticles Alters Cellular Programs in Human Breast Cancer Cells. ACS Nano. 15, 11789–11805 (2021). [CrossRef]
- Dong, X. , Pan, P., Ye, J.-J., Zhang, Q.-L., Zhang, X.-Z.: Hybrid M13 bacteriophage-based vaccine platform for personalized cancer immunotherapy. Biomaterials. 289, 121763 (2022). [CrossRef]
- Wu, H. , Ganguly, S., Tollefsbol, T.O.: Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment. Microorganisms. 10, 1727 (2022). [CrossRef]
- Rutkowski, M.R. , Stephen, T.L., Svoronos, N., Allegrezza, M.J., Tesone, A.J., Perales-Puchalt, A., Brencicova, E., Escovar-Fadul, X., Nguyen, J.M., Cadungog, M.G., Zhang, R., Salatino, M., Tchou, J., Rabinovich, G.A., Conejo-Garcia, J.R.: Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell. 27, 27–40 (2015). [CrossRef]
- Schwabe, R.F. , Jobin, C.: The microbiome and cancer. Nat Rev Cancer. 13, 800–812 (2013). [CrossRef]
- Garrett, W.S. : Cancer and the microbiota. Science. 348, 80–86 (2015). [CrossRef]
- Eslami-S, Z. , Majidzadeh-A, K., Halvaei, S., Babapirali, F., Esmaeili, R.: Microbiome and Breast Cancer: New Role for an Ancient Population. Front. Oncol. 10, 120 (2020). [CrossRef]
- Luu, T.H. , Michel, C., Bard, J.-M., Dravet, F., Nazih, H., Bobin-Dubigeon, C.: Intestinal Proportion of Blautia sp. is Associated with Clinical Stage and Histoprognostic Grade in Patients with Early-Stage Breast Cancer. Nutrition and Cancer. 69, 267–275 (2017). [CrossRef]
- Malik, S.S. , Saeed, A., Baig, M., Asif, N., Masood, N., Yasmin, A.: Anticarcinogenecity of microbiota and probiotics in breast cancer. International Journal of Food Properties. 21, 655–666 (2018). [CrossRef]
- Yazdi, M. , Mahdavi, M., Kheradmand, E., Shahverdi, A.: The Preventive Oral Supplementation of a Selenium Nanoparticle-enriched Probiotic Increases the Immune Response and Lifespan of 4T1 Breast Cancer Bearing Mice. Arzneimittelforschung. 62, 525–531 (2012). [CrossRef]
- Aragón, F. , Carino, S., Perdigón, G., De Moreno De LeBlanc, A.: Inhibition of Growth and Metastasis of Breast Cancer in Mice by Milk Fermented With Lactobacillus casei CRL 431. Journal of Immunotherapy. 38, 185–196 (2015). [CrossRef]
- Chitapanarux, I. , Chitapanarux, T., Traisathit, P., Kudumpee, S., Tharavichitkul, E., Lorvidhaya, V.: Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol. 5, 31 (2010). [CrossRef]
- Mego, M. , Chovanec, J., Vochyanova-Andrezalova, I., Konkolovsky, P., Mikulova, M., Reckova, M., Miskovska, V., Bystricky, B., Beniak, J., Medvecova, L., Lagin, A., Svetlovska, D., Spanik, S., Zajac, V., Mardiak, J., Drgona, L.: Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complementary Therapies in Medicine. 23, 356–362 (2015). [CrossRef]
- Hibberd, A.A. , Lyra, A., Ouwehand, A.C., Rolny, P., Lindegren, H., Cedgård, L., Wettergren, Y.: Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 4, e000145 (2017). [CrossRef]
- Theodoropoulos, G.E. , Memos, N.A., Peitsidou, K., Karantanos, T., Spyropoulos, B.G., Zografos, G.: Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann Gastroenterol. 2016; 29, 56–62. [Google Scholar]
- Demers, M. , Dagnault, A., Desjardins, J.: A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clinical Nutrition. 33, 761–767 (2014). [CrossRef]
- Österlund, P. , Ruotsalainen, T., Korpela, R., Saxelin, M., Ollus, A., Valta, P., Kouri, M., Elomaa, I., Joensuu, H.: Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 97, 1028–1034 (2007). [CrossRef]
- Dizman, N. , Meza, L., Bergerot, P., Alcantara, M., Dorff, T., Lyou, Y., Frankel, P., Cui, Y., Mira, V., Llamas, M., Hsu, J., Zengin, Z., Salgia, N., Salgia, S., Malhotra, J., Chawla, N., Chehrazi-Raffle, A., Muddasani, R., Gillece, J., Reining, L., Trent, J., Takahashi, M., Oka, K., Higashi, S., Kortylewski, M., Highlander, S.K., Pal, S.K.: Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 28, 704–712 (2022). [CrossRef]
- Toi, M. , Hirota, S., Tomotaki, A., Sato, N., Hozumi, Y., Anan, K., Nagashima, T., Tokuda, Y., Masuda, N., Ohsumi, S., Ohno, S., Takahashi, M., Hayashi, H., Yamamoto, S., Ohashi, Y.: Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-control Study. CNF. 9, 194–200 (2013). [CrossRef]
- Juan, Z. , Chen, J., Ding, B., Yongping, L., Liu, K., Wang, L., Le, Y., Liao, Q., Shi, J., Huang, J., Wu, Y., Ma, D., Ouyang, W., Tong, J.: Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomised, double-blind, and placebo-controlled trial. European Journal of Cancer. 161, 10–22 (2022). [CrossRef]
- Khazaei, Y. , Basi, A., Fernandez, M.L., Foudazi, H., Bagherzadeh, R., Shidfar, F.: The effects of synbiotics supplementation on reducing chemotherapy-induced side effects in women with breast cancer: a randomized placebo-controlled double-blind clinical trial. BMC Complement Med Ther. 23, 339 (2023). [CrossRef]
- Shanmugam, M. , Abirami, R.G.: Microbial Polysaccharides - Chemistry and Applications. Journal of Biologically Active Products from Nature. 9, 73–78 (2019). [CrossRef]
- Ullah, S. , Khalil, A.A., Shaukat, F., Song, Y.: Sources, Extraction and Biomedical Properties of Polysaccharides. Foods. 8, 304 (2019). [CrossRef]
- Lemieszek, M. , Rzeski, W.: Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. wo. 4, 285–289 (2012). [CrossRef]
- Chow, L.W.C. , Lo, C.S.Y., Loo, W.T.Y., Hu, X., Sham, J.S.T.: Polysaccharide Peptide Mediates Apoptosis by Up-regulating p21 Gene and Down-regulating Cyclin D 1 Gene. Am. J. Chin. Med. 31, 1–9 (2003). [CrossRef]
- L.Y. Eliza, W., K. Fai, C., P. Chung, L.: Efficacy of Yun Zhi (Coriolus versicolor) on Survival in Cancer Patients: Systematic Review and Meta-Analysis. IAD. 6, 78–87 (2012). [CrossRef]
- Queiroz, E.A.I.F. , Fortes, Z.B., Da Cunha, M.A.A., Sarilmiser, H.K., Barbosa Dekker, A.M., Öner, E.T., Dekker, R.F.H., Khaper, N.: Levan promotes antiproliferative and pro-apoptotic effects in MCF-7 breast cancer cells mediated by oxidative stress. International Journal of Biological Macromolecules. 102, 565–570 (2017). [CrossRef]
- Alonso, E.N. , Ferronato, M.J., Fermento, M.E., Gandini, N.A., Romero, A.L., Guevara, J.A., Facchinetti, M.M., Curino, A.C.: Antitumoral and antimetastatic activity of Maitake D-Fraction in triple-negative breast cancer cells. Oncotarget. 9, 23396–23412 (2018). [CrossRef]
- Kodama, N. , Komuta, K., Nanba, H.: Effect of Maitake ( Grifola frondosa ) D-Fraction on the Activation of NK Cells in Cancer Patients. Journal of Medicinal Food. 6, 371–377 (2003). [CrossRef]
- Alpuim Costa, D. , Nobre, J.G., Batista, M.V., Ribeiro, C., Calle, C., Cortes, A., Marhold, M., Negreiros, I., Borralho, P., Brito, M., Cortes, J., Braga, S.A., Costa, L.: Human Microbiota and Breast Cancer—Is There Any Relevant Link?—A Literature Review and New Horizons Toward Personalised Medicine. Front. Microbiol. 12, 584332 (2021). [CrossRef]
- Laborda-Illanes, A. , Sanchez-Alcoholado, L., Dominguez-Recio, M.E., Jimenez-Rodriguez, B., Lavado, R., Comino-Méndez, I., Alba, E., Queipo-Ortuño, M.I.: Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment. Cancers. 12, 2465 (2020). [CrossRef]
- Wu, M. , Bai, J., Ma, C., Wei, J., Du, X.: The Role of Gut Microbiota in Tumor Immunotherapy. Journal of Immunology Research. 2021, 1–12 (2021). [CrossRef]
- Haque, S. , Raina, R., Afroze, N., Hussain, A., Alsulimani, A., Singh, V., Mishra, B.N., Kaul, S., Kharwar, R.N.: Microbial dysbiosis and epigenetics modulation in cancer development – A chemopreventive approach. Seminars in Cancer Biology. 86, 666–681 (2022). [CrossRef]
- Nanda, R. , Chow, L.Q.M., Dees, E.C., Berger, R., Gupta, S., Geva, R., Pusztai, L., Pathiraja, K., Aktan, G., Cheng, J.D., Karantza, V., Buisseret, L.: Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. JCO. 34, 2460–2467 (2016). [CrossRef]
- Adams, S. , Schmid, P., Rugo, H.S., Winer, E.P., Loirat, D., Awada, A., Cescon, D.W., Iwata, H., Campone, M., Nanda, R., Hui, R., Curigliano, G., Toppmeyer, D., O’Shaughnessy, J., Loi, S., Paluch-Shimon, S., Tan, A.R., Card, D., Zhao, J., Karantza, V., Cortés, J.: Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Annals of Oncology. 30, 397–404 (2019). [CrossRef]
- Dirix, L.Y. , Takacs, I., Jerusalem, G., Nikolinakos, P., Arkenau, H.-T., Forero-Torres, A., Boccia, R., Lippman, M.E., Somer, R., Smakal, M., Emens, L.A., Hrinczenko, B., Edenfield, W., Gurtler, J., Von Heydebreck, A., Grote, H.J., Chin, K., Hamilton, E.P.: Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 167, 671–686 (2018). [CrossRef]
- AACR Annual Meeting 2017 Online Proceedings and Itinerary Planner | Presentation, https://www.abstractsonline.com/pp8/#!/4292/presentation/1296.
- Emens, L.A. , Adams, S., Loi, S., Schneeweiss, A., Rugo, H.S., Winer, E.P., Barrios, C.H., Dieras, V., de la Haba-Rodriguez, J., Gianni, L., Chui, S.Y., Schmid, P.: IMpassion130: a Phase III randomized trial of atezolizumab with nab-paclitaxel for first-line treatment of patients with metastatic triple-negative breast cancer (mTNBC). JCO. 34, TPS1104–TPS1104 (2016). [CrossRef]
- Tolaney, S.M. , Kalinsky, K., Kaklamani, V.G., D’Adamo, D.R., Aktan, G., Tsai, M.L., O’Regan, R., Kaufman, P.A., Wilks, S., Andreopoulou, E., Patt, D.A., Yuan, Y., Wang, G., Xing, D., Kleynerman, E., Karantza, V., Diab, S.: A phase Ib/II study of eribulin (ERI) plus pembrolizumab (PEMBRO) in metastatic triple-negative breast cancer (mTNBC) (ENHANCE 1). JCO. 38, 1015–1015 (2020). [CrossRef]
- Cortes, J. , Cescon, D.W., Rugo, H.S., Nowecki, Z., Im, S.-A., Yusof, M.M., Gallardo, C., Lipatov, O., Barrios, C.H., Holgado, E., Iwata, H., Masuda, N., Otero, M.T., Gokmen, E., Loi, S., Guo, Z., Zhao, J., Aktan, G., Karantza, V., Schmid, P., Luis, F., Gonzalo, G.A., Diego, K., Ruben, K., Matias, M., Mirta, V., Sally, B.-H., Stephen, B., Philip, C., Sherene, L., Dhanusha, S., Andrea, G., Donatienne, T., Carlos, B., Leandro, B., Fabiano, C., Ruffo, D.F.J., Roberto, H., Domicio Carvalho, L., Fernando Cezar Toniazzi, L., Roberto Odebrecht, R., Antonio Orlando, S.N., Felipe, S., David, C., Danielle, C., Cristiano, F., Xinni, S., Joanne, Y., Alejandro, A., Carlos, G., Claudio, S., Cesar, S., Eduardo, Y., Alvaro, G.D., Jesus, S., Petra, H., Zdenek, K., Bohuslav, M., Katarina, P., Jana, P., Vesna, G., Erik, J., Jeanette, J., Soren, L., Tamas, L., Herve, B., Isabelle, D., Anthony, G., Anne-Claire, H.-B., Luis, T., Jens-Uwe, B., Peter, F., Dirk, F., Nadia, H., Jens, H., Anna, K.F.D.S., Christian, K., Sibylle, L., Diana, L., Tjoung-Won, P.-S., Raquel Von, S., Pauline, W., Louis, C., Ava, K., Kai Cheong Roger, N., Peter, A., Tibor, C., Zsuzsanna, K., Laszlo, L., Karoly, M., Gabor, R., John, C., Catherine, K., Seamus, O., Saverio, C., Antonietta, Da., Enrico, R., Tomoyuki, A., Takaaki, F., Kenichi, I., Takashi, I., Yoshinori, I., Tsutomu, I., Hiroji, I., Yoshimasa, K., Koji, M., Yasuo, M., Hirofumi, M., Seigo, N., Naoki, N., Shoichiro, O., Akihiko, O., Yasuaki, S., Eiji, S., Masato, T., Yuko, T., Kenji, T., Koichiro, T., Junichiro, W., Naohito, Y., Yutaka, Y., Teruo, Y., Anita, B., Mastura, M.Y., Angel, G.V., Alejandro, J.R., Jorge, M.R., Flavia, M.-V., Jessica, R.C., Karin, B., Vivianne, T.-H., David, P., Ewa, C., Ewa, N.-Z., Zbigniew, N., Barbara, R., Joanna, S., Cezary, S., Rafal, T., Bogdan, Z., Alexander, A., Natalia, F., Oleg, L., Andrey, M., Vladimir, M., Guzel, M., Jin Hee, A., Seock-Ah, I., Keun Seok, L., Kwong Hwa, P., Yeon Hee, P., Begona, B.D.L.H., Javier, C., Josefina, C.J., Luis, D.L.C.M., Jose, G.S., Maria, G., Esther, H., Esther, Z.A., Chien-Ting, L., Mei-Ching, L., Chiun-Sheng, H., Chao-Jung, T., Ling-Ming, T., Cagatay, A., Gul, B., Irfan, C., Erhan, G., Seyda, G., Nil, M.M., Mustafa, O., Ozgur, O., Sinan, Y., Steve, C., Janine, G., Iain, M., Peter, S., Nicholas, T., Mark, T., Christopher, T., Duncan, W., Hryhoriy, A., Oleksandr, B., Igor, B., Oleksii, K., Olena, K., Hanna, K., Anna, K., Iurii, L., Alla, N., Natalya, O., Olga, P., Andrii, R., Sergii, S., Yaroslav, S., Dmytro, T., Grygorii, U., Ihor, V., Sibel, B., Madhu, C., Michael, C., Patrick, C., Scott, C., Jennifer, D., Keerthi, G., Jeffrey, H., Kent, H., William, I., Randa, L., Janice, L., Raul, M., Susan, M., Rita, N., Ira, O., Coral, O., Timothy, P., Amit, P., Brian, P., Hope, R., Irina, R., Michael, S., Robert, S., Michael, S., Laura, S., Bradley, S., Michaela, T., Frances, V.-A.: Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. The Lancet. 396, 1817–1828 (2020). [CrossRef]
- Aarnoutse, R. , Ziemons, J., Hillege, L.E., De Vos-Geelen, J., De Boer, M., Bisschop, S.M.P., Vriens, B.E.P.J., Vincent, J., Van De Wouw, A.J., Le, G.N., Venema, K., Rensen, S.S., Penders, J., Smidt, M.L.: Changes in intestinal microbiota in postmenopausal oestrogen receptor-positive breast cancer patients treated with (neo)adjuvant chemotherapy. npj Breast Cancer. 8, 89 (2022). [CrossRef]
- Bilenduke, E. , Sterrett, J.D., Ranby, K.W., Borges, V.F., Grigsby, J., Carr, A.L., Kilbourn, K., Lowry, C.A.: Impacts of breast cancer and chemotherapy on gut microbiome, cognitive functioning, and mood relative to healthy controls. Sci Rep. 12, 19547 (2022). [CrossRef]
- Horigome, A. , Okubo, R., Hamazaki, K., Kinoshita, T., Katsumata, N., Uezono, Y., Xiao, J.Z., Matsuoka, Y.J.: Association between blood omega-3 polyunsaturated fatty acids and the gut microbiota among breast cancer survivors. Beneficial Microbes. 10, 751–758 (2019). [CrossRef]
- Terrisse, S. , Derosa, L., Iebba, V., Ghiringhelli, F., Vaz-Luis, I., Kroemer, G., Fidelle, M., Christodoulidis, S., Segata, N., Thomas, A.M., Martin, A.-L., Sirven, A., Everhard, S., Aprahamian, F., Nirmalathasan, N., Aarnoutse, R., Smidt, M., Ziemons, J., Caldas, C., Loibl, S., Denkert, C., Durand, S., Iglesias, C., Pietrantonio, F., Routy, B., André, F., Pasolli, E., Delaloge, S., Zitvogel, L.: Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 28, 2778–2796 (2021). [CrossRef]
- Okubo, R. , Kinoshita, T., Katsumata, N., Uezono, Y., Xiao, J., Matsuoka, Y.J.: Impact of chemotherapy on the association between fear of cancer recurrence and the gut microbiota in breast cancer survivors. Brain, Behavior, and Immunity. 85, 186–191 (2020). [CrossRef]
- Rodríguez Martín, B. , Fernández Rodríguez, E.J., Rihuete Galve, M.I., Cruz Hernández, J.J.: Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer. IJERPH. 17, 8896 (2020). [CrossRef]
- Williams, L.J. , Fletcher, E., Douglas, A., Anderson, E.D.C., McCallum, A., Simpson, C.R., Smith, J., Moger, T.A., Peltola, M., Mihalicza, P., Sveréus, S., Zengarini, N., Campbell, H., Wild, S.H.: Retrospective cohort study of breast cancer incidence, health service use and outcomes in Europe: a study of feasibility. European Journal of Public Health. 28, 327–332 (2018). [CrossRef]
- Jim, H.S.L. , Phillips, K.M., Chait, S., Faul, L.A., Popa, M.A., Lee, Y.-H., Hussin, M.G., Jacobsen, P.B., Small, B.J.: Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated With Standard-Dose Chemotherapy. JCO. 30, 3578–3587 (2012). [CrossRef]
- Wu, D. , Chen, Q., Chen, X., Han, F., Chen, Z., Wang, Y.: The blood–brain barrier: structure, regulation, and drug delivery. Sig Transduct Target Ther. 8, 217 (2023). [CrossRef]
- Cheung, Y.T. , Ng, T., Shwe, M., Ho, H.K., Foo, K.M., Cham, M.T., Lee, J.A., Fan, G., Tan, Y.P., Yong, W.S., Madhukumar, P., Loo, S.K., Ang, S.F., Wong, M., Chay, W.Y., Ooi, W.S., Dent, R.A., Yap, Y.S., Ng, R., Chan, A.: Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Annals of Oncology. 26, 1446–1451 (2015). [CrossRef]
- Shadad, A.K. : Gastrointestinal radiation injury: Symptoms, risk factors and mechanisms. WJG. 19, 185 (2013). [CrossRef]
- Nam, Y.-D. , Kim, H.J., Seo, J.-G., Kang, S.W., Bae, J.-W.: Impact of Pelvic Radiotherapy on Gut Microbiota of Gynecological Cancer Patients Revealed by Massive Pyrosequencing. PLoS ONE. 8, e82659 (2013). [CrossRef]
- Shiao, S.L. , Kershaw, K.M., Limon, J.J., You, S., Yoon, J., Ko, E.Y., Guarnerio, J., Potdar, A.A., McGovern, D.P.B., Bose, S., Dar, T.B., Noe, P., Lee, J., Kubota, Y., Maymi, V.I., Davis, M.J., Henson, R.M., Choi, R.Y., Yang, W., Tang, J., Gargus, M., Prince, A.D., Zumsteg, Z.S., Underhill, D.M.: Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell. 39, 1202-1213.e6 (2021). [CrossRef]
- Uribe-Herranz, M. , Rafail, S., Beghi, S., Gil-de-Gómez, L., Verginadis, I., Bittinger, K., Pustylnikov, S., Pierini, S., Perales-Linares, R., Blair, I.A., Mesaros, C.A., Snyder, N.W., Bushman, F., Koumenis, C., Facciabene, A.: Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. Journal of Clinical Investigation. 130, 466–479 (2019). [CrossRef]
- Guo, H. , Chou, W.-C., Lai, Y., Liang, K., Tam, J.W., Brickey, W.J., Chen, L., Montgomery, N.D., Li, X., Bohannon, L.M., Sung, A.D., Chao, N.J., Peled, J.U., Gomes, A.L.C., Van Den Brink, M.R.M., French, M.J., Macintyre, A.N., Sempowski, G.D., Tan, X., Sartor, R.B., Lu, K., Ting, J.P.Y.: Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 370, eaay9097 (2020). [CrossRef]
- Alexander, J.L. , Wilson, I.D., Teare, J., Marchesi, J.R., Nicholson, J.K., Kinross, J.M.: Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 14, 356–365 (2017). [CrossRef]
- Di Modica, M. , Gargari, G., Regondi, V., Bonizzi, A., Arioli, S., Belmonte, B., De Cecco, L., Fasano, E., Bianchi, F., Bertolotti, A., Tripodo, C., Villani, L., Corsi, F., Guglielmetti, S., Balsari, A., Triulzi, T., Tagliabue, E.: Gut Microbiota Condition the Therapeutic Efficacy of Trastuzumab in HER2-Positive Breast Cancer. Cancer Research. 81, 2195–2206 (2021). [CrossRef]
- One Health, https://www.who.int/news-room/questions-and-answers/item/one-health.
- Kleber, K.T. , Iranpur, K.R., Perry, L.M., Cruz, S.M., Razmara, A.M., Culp, W.T.N., Kent, M.S., Eisen, J.A., Rebhun, R.B., Canter, R.J.: Using the canine microbiome to bridge translation of cancer immunotherapy from pre-clinical murine models to human clinical trials. Front. Immunol. 13, 983344 (2022). [CrossRef]
- M. Dujon, A., Brown, J.S., Destoumieux-Garzón, D., Vittecoq, M., Hamede, R., Tasiemski, A., Boutry, J., Tissot, S., Alix-Panabieres, C., Pujol, P., Renaud, F., Simard, F., Roche, B., Ujvari, B., Thomas, F.: On the need for integrating cancer into the One Health perspective. Evolutionary Applications. 14, 2571–2575 (2021). [CrossRef]
- Kattner, P. , Zeiler, K., Herbener, V.J., Ferla-Brühl, K.L., Kassubek, R., Grunert, M., Burster, T., Brühl, O., Weber, A.S., Strobel, H., Karpel-Massler, G., Ott, S., Hagedorn, A., Tews, D., Schulz, A., Prasad, V., Siegelin, M.D., Nonnenmacher, L., Fischer-Posovszky, P., Halatsch, M.-E., Debatin, K.-M., Westhoff, M.-A.: What Animal Cancers teach us about Human Biology. Theranostics. 11, 6682–6702 (2021). [CrossRef]
- Dujon, A.M. , Gatenby, R.A., Bramwell, G., MacDonald, N., Dohrmann, E., Raven, N., Schultz, A., Hamede, R., Gérard, A.-L., Giraudeau, M., Thomas, F., Ujvari, B.: Transmissible Cancers in an Evolutionary Perspective. iScience. 23, 101269 (2020). [CrossRef]
- Epstein, B. , Jones, M., Hamede, R., Hendricks, S., McCallum, H., Murchison, E.P., Schönfeld, B., Wiench, C., Hohenlohe, P., Storfer, A.: Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nat Commun. 7, 12684 (2016). [CrossRef]
- Kerr, P. , Liu, J., Cattadori, I., Ghedin, E., Read, A., Holmes, E.: Myxoma Virus and the Leporipoxviruses: An Evolutionary Paradigm. Viruses. 7, 1020–1061 (2015). [CrossRef]
- Di Giallonardo, F. , Holmes, E.C.: Viral biocontrol: grand experiments in disease emergence and evolution. Trends in Microbiology. 23, 83–90 (2015). [CrossRef]
- Spernovasilis, N. , Tsiodras, S., Poulakou, G.: Emerging and Re-Emerging Infectious Diseases: Humankind’s Companions and Competitors. Microorganisms. 10, 98 (2022). [CrossRef]
- Giraudeau, M. , Sepp, T., Ujvari, B., Ewald, P.W., Thomas, F.: Human activities might influence oncogenic processes in wild animal populations. Nat Ecol Evol. 2, 1065–1070 (2018). [CrossRef]
- Sepp, T. , Ujvari, B., Ewald, P.W., Thomas, F., Giraudeau, M.: Urban environment and cancer in wildlife: available evidence and future research avenues. Proc. R. Soc. B. 286, 20182434 (2019). [CrossRef]
- Pesavento, P.A. , Agnew, D., Keel, M.K., Woolard, K.D.: Cancer in wildlife: patterns of emergence. Nat Rev Cancer. 18, 646–661 (2018). [CrossRef]
- White, J. , Amato, K.R., Decaestecker, E., McKenzie, V.J.: Editorial: Impact of anthropogenic environmental changes on animal microbiomes. Front. Ecol. Evol. 11, 1204035 (2023). [CrossRef]
- Sonnenburg, E.D. , Sonnenburg, J.L.: The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol. 17, 383–390 (2019). [CrossRef]
- Moustafa, M.A.M. , Chel, H.M., Thu, M.J., Bawm, S., Htun, L.L., Win, M.M., Oo, Z.M., Ohsawa, N., Lahdenperä, M., Mohamed, W.M.A., Ito, K., Nonaka, N., Nakao, R., Katakura, K.: Anthropogenic interferences lead to gut microbiome dysbiosis in Asian elephants and may alter adaptation processes to surrounding environments. Sci Rep. 11, 741 (2021). [CrossRef]
- Poutahidis, T. , Varian, B.J., Levkovich, T., Lakritz, J.R., Mirabal, S., Kwok, C., Ibrahim, Y.M., Kearney, S.M., Chatzigiagkos, A., Alm, E.J., Erdman, S.E.: Dietary Microbes Modulate Transgenerational Cancer Risk. Cancer Research. 75, 1197–1204 (2015). [CrossRef]
- Kowallik, V. , Das, A., Mikheyev, A.S.: Experimental inheritance of antibiotic acquired dysbiosis affects host phenotypes across generations. Front. Microbiol. 13, 1030771 (2022). [CrossRef]
- Dujon, A.M. , Ujvari, B., Thomas, F.: Cancer risk landscapes: A framework to study cancer in ecosystems. Science of The Total Environment. 763, 142955 (2021). [CrossRef]
- Efird, J.T. , Davies, S.W., O’Neal, W.T., Anderson, E.J.: Animal Viruses, Bacteria, and Cancer: A Brief Commentary. Front. Public Health. 2, (2014). [CrossRef]
- Prüss-Üstün, A. , Wolf, J., Corvalán, C.F., Bos, R., Neira, M.P.: Preventing disease through healthy environments: a global assessment of the burden of disease from environmental risks. World Health Organization, Geneva (2016).
- AbdulRaheem, Y. : Unveiling the Significance and Challenges of Integrating Prevention Levels in Healthcare Practice. J Prim Care Community Health. 14, 21501319231186500 (2023). [CrossRef]
- About: Health promotion and disease prevention through population-based interventions, including action to address social determinants and health inequity, http://www.emro.who.int/about-who/public-health-functions/health-promotion-disease-prevention.html.
- Grenni, P. , Ancona, V., Barra Caracciolo, A.: Ecological effects of antibiotics on natural ecosystems: A review. Microchemical Journal. 136, 25–39 (2018). [CrossRef]
- Lee, K. , Raguideau, S., Sirén, K., Asnicar, F., Cumbo, F., Hildebrand, F., Segata, N., Cha, C.-J., Quince, C.: Population-level impacts of antibiotic usage on the human gut microbiome. Nat Commun. 14, 1191 (2023). [CrossRef]
- Wang, W. , Weng, Y., Luo, T., Wang, Q., Yang, G., Jin, Y.: Antimicrobial and the Resistances in the Environment: Ecological and Health Risks, Influencing Factors, and Mitigation Strategies. Toxics. 11, 185 (2023). [CrossRef]
- Rossi, F. , Péguilhan, R., Turgeon, N., Veillette, M., Baray, J.-L., Deguillaume, L., Amato, P., Duchaine, C.: Quantification of antibiotic resistance genes (ARGs) in clouds at a mountain site (puy de Dôme, central France). Science of The Total Environment. 865, 161264 (2023). [CrossRef]
- Lloyd, D.H. , Page, S.W.: Antimicrobial Stewardship in Veterinary Medicine. Microbiol Spectr. 6, 6.3.03 (2018). [CrossRef]
- Roderburg, C. , Loosen, S.H., Joerdens, M.S., Demir, M., Luedde, T., Kostev, K.: Antibiotic therapy is associated with an increased incidence of cancer. J Cancer Res Clin Oncol. 149, 1285–1293 (2023). [CrossRef]
- Ibragimova, S. , Ramachandran, R., Ali, F.R., Lipovich, L., Ho, S.B.: Dietary Patterns and Associated Microbiome Changes that Promote Oncogenesis. Front. Cell Dev. Biol. 9, 725821 (2021). [CrossRef]
- emhj: One Health: perspectives on ethical issues and evidence from animal experiments, http://www.emro.who.int/emhj-volume-18-2012/issue-11/article-15.html.
- Van Herten, J. , Bovenkerk, B., Verweij, M.: One Health as a moral dilemma: Towards a socially responsible zoonotic disease control. Zoonoses and Public Health. 66, 26–34 (2019). [CrossRef]
- Ursell, L.K. , Metcalf, J.L., Parfrey, L.W., Knight, R.: Defining the human microbiome. Nutrition Reviews. 70, S38–S44 (2012). [CrossRef]
- Baba, A.I. , Câtoi, C.: COMPARATIVE ONCOLOGY. In: Comparative Oncology. The Publishing House of the Romanian Academy (2007).
- Dincă, L.C. , Grenni, P., Onet, C., Onet, A.: Fertilization and Soil Microbial Community: A Review. Applied Sciences. 12, 1198 (2022). [CrossRef]
- Woodworth, M.H. , Sitchenko, K.L., Carpentieri, C., Friedman-Moraco, R.J., Wang, T., Kraft, C.S.: Ethical Considerations in Microbial Therapeutic Clinical Trials. The New Bioethics. 23, 210–218 (2017). [CrossRef]
- Cammarota, G. , Ianiro, G., Ahern, A., Carbone, C., Temko, A., Claesson, M.J., Gasbarrini, A., Tortora, G.: Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat Rev Gastroenterol Hepatol. 17, 635–648 (2020). [CrossRef]
- Yang, J. , Tan, Q., Fu, Q., Zhou, Y., Hu, Y., Tang, S., Zhou, Y., Zhang, J., Qiu, J., Lv, Q.: Gastrointestinal microbiome and breast cancer: correlations, mechanisms and potential clinical implications. Breast Cancer. 24, 220–228 (2017). [CrossRef]
- Yin, B. , Wang, X., Yuan, F., Li, Y., Lu, P.: Research progress on the effect of gut and tumor microbiota on antitumor efficacy and adverse effects of chemotherapy drugs. Front. Microbiol. 13, 899111 (2022). [CrossRef]
- Poff, A.M. , Ari, C., Seyfried, T.N., D’Agostino, D.P.: The Ketogenic Diet and Hyperbaric Oxygen Therapy Prolong Survival in Mice with Systemic Metastatic Cancer. PLoS ONE. 8, e65522 (2013). [CrossRef]
- Gilbert, J.A. , Blaser, M.J., Caporaso, J.G., Jansson, J.K., Lynch, S.V., Knight, R.: Current understanding of the human microbiome. Nat Med. 24, 392–400 (2018). [CrossRef]
- Soto-Pantoja, D.R. , Gaber, M., Arnone, A.A., Bronson, S.M., Cruz-Diaz, N., Wilson, A.S., Clear, K.Y.J., Ramirez, M.U., Kucera, G.L., Levine, E.A., Lelièvre, S.A., Chaboub, L., Chiba, A., Yadav, H., Vidi, P.-A., Cook, K.L.: Diet Alters Entero-Mammary Signaling to Regulate the Breast Microbiome and Tumorigenesis. Cancer Research. 81, 3890–3904 (2021). [CrossRef]
- Hou, M.-F. , Ou-Yang, F., Li, C.-L., Chen, F.-M., Chuang, C.-H., Kan, J.-Y., Wu, C.-C., Shih, S.-L., Shiau, J.-P., Kao, L.-C., Kao, C.-N., Lee, Y.-C., Moi, S.-H., Yeh, Y.-T., Cheng, C.-J., Chiang, C.-P.: Comprehensive profiles and diagnostic value of menopausal-specific gut microbiota in premenopausal breast cancer. Exp Mol Med. 53, 1636–1646 (2021). [CrossRef]
- Parhi, L. , Alon-Maimon, T., Sol, A., Nejman, D., Shhadeh, A., Fainsod-Levi, T., Yajuk, O., Isaacson, B., Abed, J., Maalouf, N., Nissan, A., Sandbank, J., Yehuda-Shnaidman, E., Ponath, F., Vogel, J., Mandelboim, O., Granot, Z., Straussman, R., Bachrach, G.: Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 11, 3259 (2020). [CrossRef]
- Bennet, JustinD., Brinkman, M.: TREATMENT OF ULCERATIVE COLITIS BY IMPLANTATION OF NORMAL COLONIC FLORA. The Lancet. 333, 164 (1989). [CrossRef]
- Mills, H. , Acquah, R., Tang, N., Cheung, L., Klenk, S., Glassen, R., Pirson, M., Albert, A., Hoang, D.T., Van, T.N.: The Use of Bacteria in Cancer Treatment: A Review from the Perspective of Cellular Microbiology. Emergency Medicine International. 2022, 1–6 (2022). [CrossRef]
- Pang, Z. , Gu, M.-D., Tang, T.: Pseudomonas aeruginosa in Cancer Therapy: Current Knowledge, Challenges and Future Perspectives. Front. Oncol. 12, 891187 (2022). [CrossRef]
- Kasinskas, R.W. , Forbes, N.S.: Salmonella typhimurium Lacking Ribose Chemoreceptors Localize in Tumor Quiescence and Induce Apoptosis. Cancer Research. 67, 3201–3209 (2007). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
The Bio-Diversity and the Role of Gut Microbiota in Postmenopausal Women with Luminal Breast Cancer Treated with Aromatase Inhibitors: An Observational Cohort Study
Angioletta Lasagna
et al.
Pathogens,
2022
Dietary Impacts on Changes in Diversity and Abundance of the Murine Microbiome during Progression and Treatment of Cancer
Holly Paden
et al.
Nutrients,
2023
Intestinal Microbiota in Postmenopausal Breast Cancer Patients and Controls
Romy Aarnoutse
et al.
Cancers,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






