Submitted:
10 November 2023
Posted:
13 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. CD147/Basigin
2.1. Discovery of CD147/Basigin
2.2. Discovery of related molecules
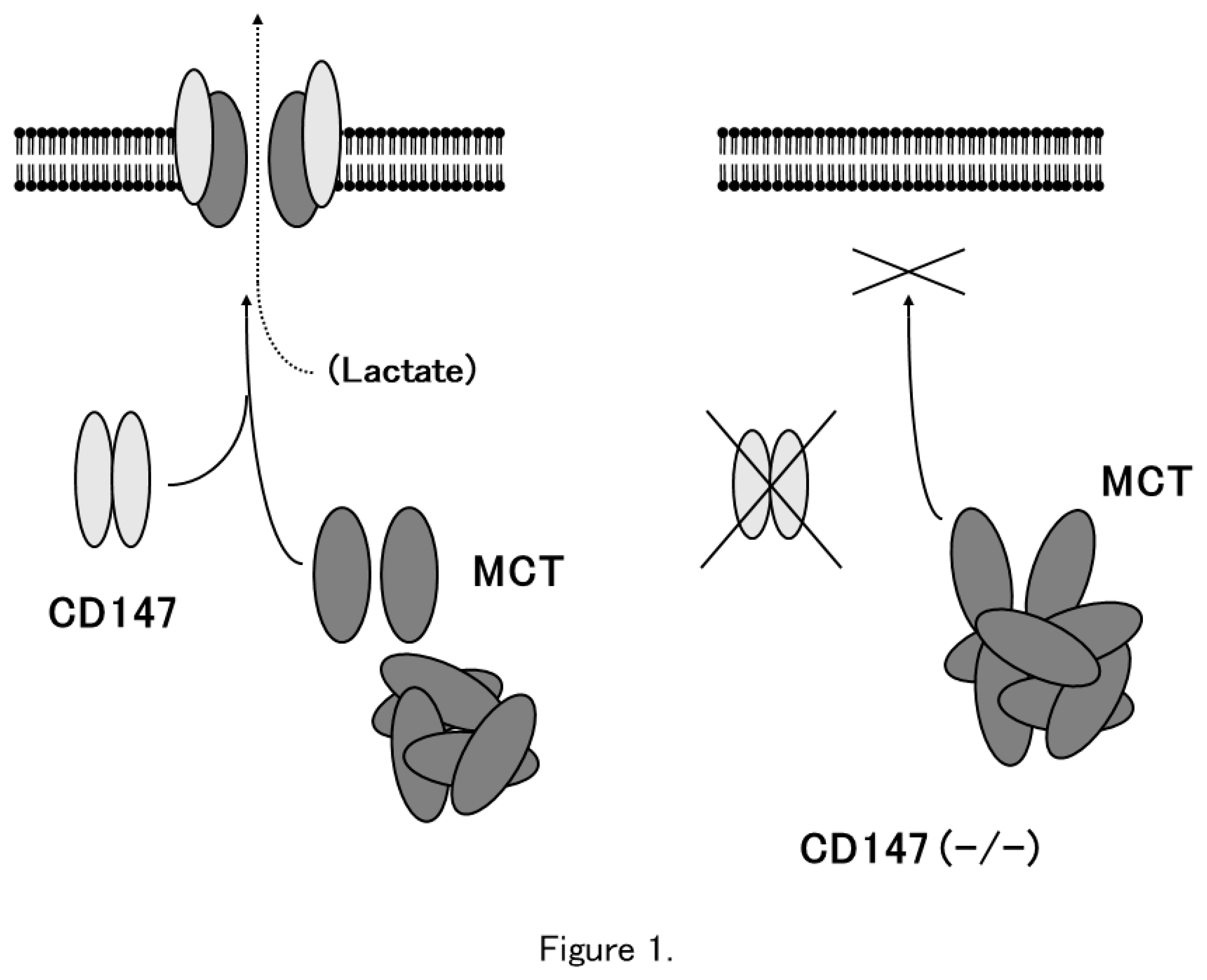
3. Chaperone-like function of CD147/Basigin
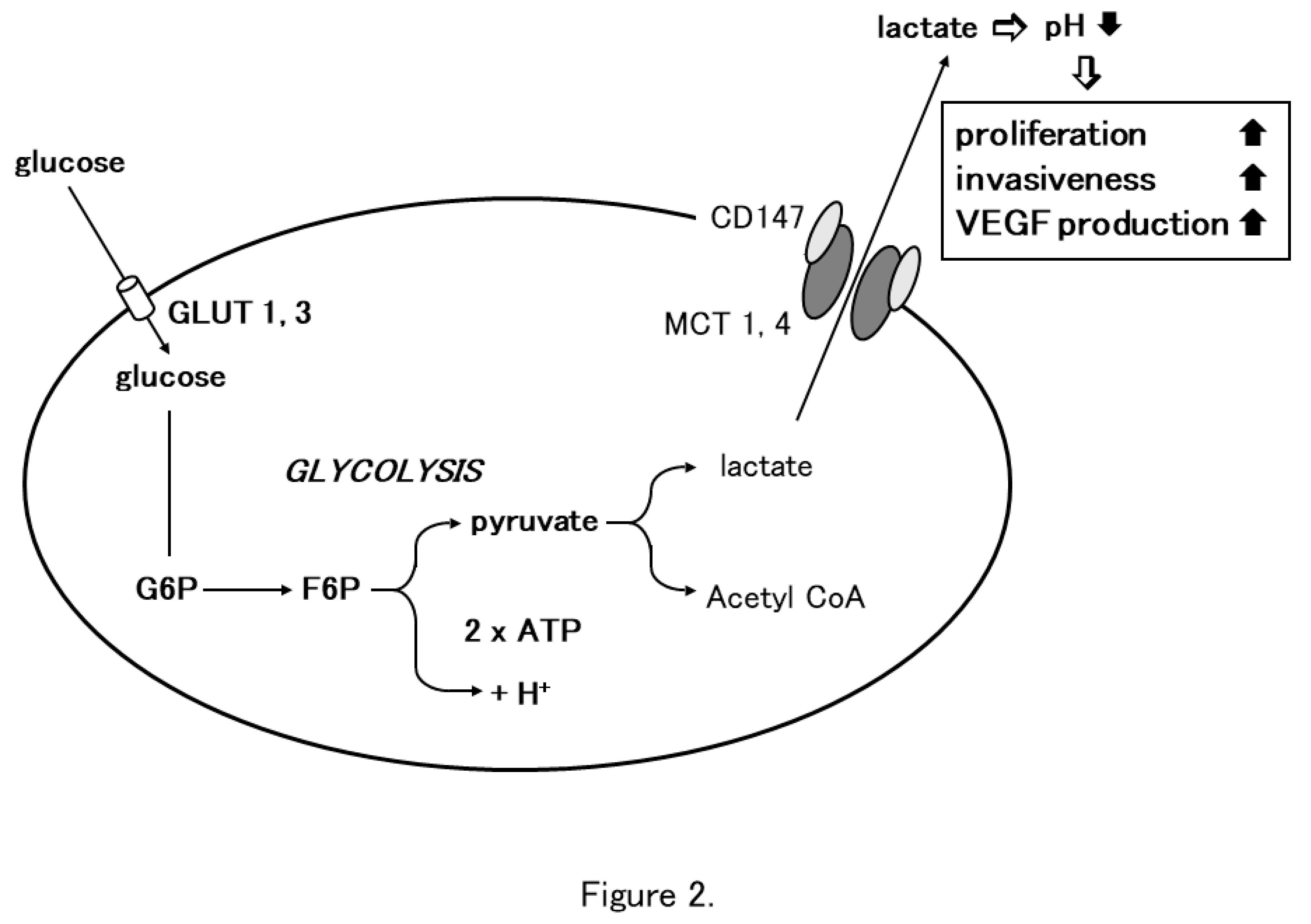
4. CD147 and glycolysis
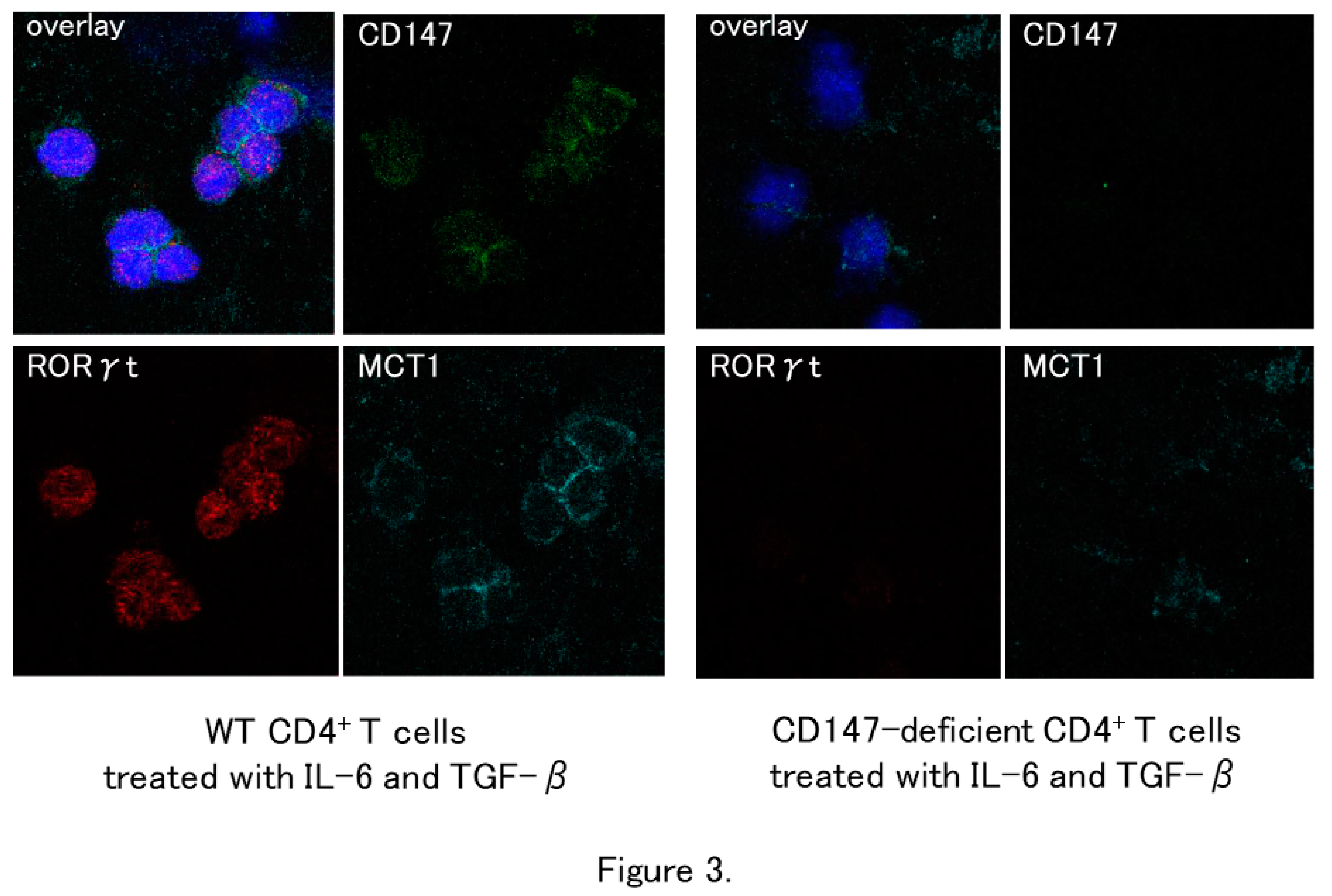
5. T cell differentiation/proliferation and glycolysis
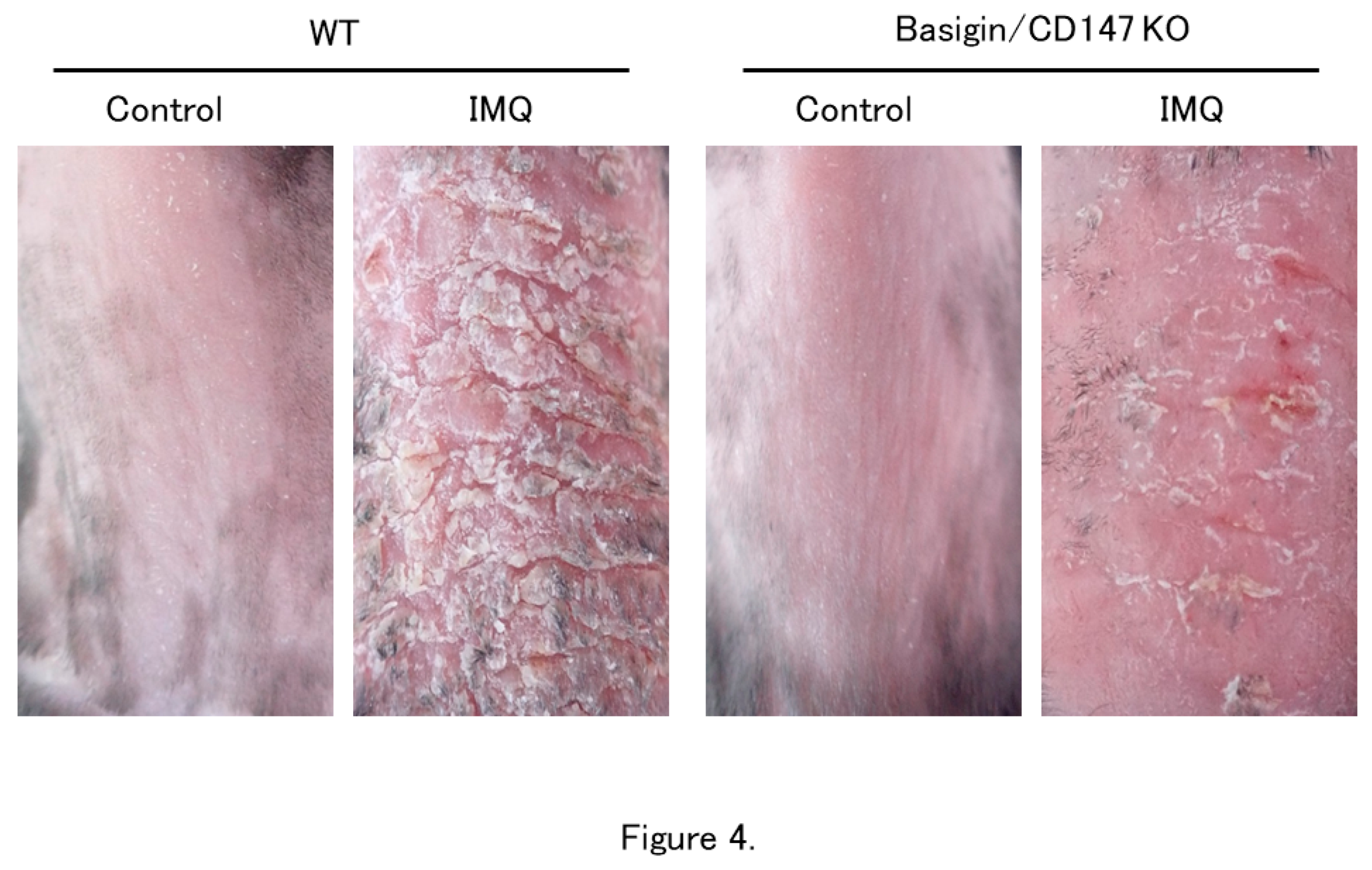
6. Psoriasis
7. CD147/Basigin and other immune disorders
8. Conclusion and future directions
References
- Miyauchi, T.; Kanekura, T.; Yamaoka, A.; Ozawa, M.; Miyazawa, S.; Muramatsu, T. Basigin, a new, broadly distributed member of the immunoglobulin superfamily, has strong homology with both the immunoglobulin V domain and the β-chain of major histocompatibility complex class II antigen. J. Biochem. 1990, 107, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Kanekura, T.; Miyauchi, T.; Tashiro, M.; Muramatsu, T. Basigin, a new member of the immunoglobulin super family: Genes in different mammalian species, glycosylation changes in the molecule from adult organs and possible variation in the N-terminal sequences. Cell Struct. Funct. 1991, 16, 23–30. [Google Scholar] [CrossRef]
- House, S.W.; Warburg, O.; Burk, D.; Schade, A.L. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Kirk, P.; Wilson, M.C.; Heddle, C.; Brown, M.H.; Barclay, A.N.; Halestrap, A.P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000, 19, 3896–3904. [Google Scholar] [CrossRef]
- Kanekura, T.; Chen, X. CD147/basigin promotes progression of malignant melanoma and other cancers. J. Dermatol. Sci. 2010, 57, 149–154. [Google Scholar] [CrossRef]
- Chen, X.; Lin, J.; Kanekura, T.; Su, J.; Lin, W.; Xie, H.; Wu, Y.; Li, J.; Chen, M.; Chang, J. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006, 66, 11323–11330. [Google Scholar] [CrossRef]
- Su, J.; Chen, X.; Kanekura, T. A CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis. Cancer Lett. 2009, 273, 140–147. [Google Scholar] [CrossRef]
- Donnelly, R.P.; Finlay, D.K. Glucose, glycolysis and lymphocyte responses. Mol. Immunol. 2015, 68, 513–519. [Google Scholar] [CrossRef]
- Perera, G.K.; Di Meglio, P.D.; Nestle, F.O. Psoriasis. Annu. Rev. Pathol. 2012, 7, 385–422. [Google Scholar] [CrossRef]
- Lynde, C.W.; Poulin, Y.; Vender, R.; Bourcier, M.; Khalil, S. Interleukin 17A: Toward a new understanding of psoriasis pathogenesis. J. Am. Acad. Dermatol. 2014, 71, 141–150. [Google Scholar] [CrossRef]
- Okubo, A.; Uchida, Y.; Higashi, Y.; Sato, T.; Ogawa, Y.; Ryuge, A.; Kadomatsu, K.; Kanekura, T. CD147 is essential for the development of psoriasis via the induction of Th17 cell differentiation. Int. J. Mol. Sci. 2021, 23, 177–191. [Google Scholar] [CrossRef]
- Muramatsu, T.; Miyauchi, T. Basigin (CD147): A multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol. Histopathol. 2003, 18, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Iacono, K.T.; Brown, A.L.; Greene, M.I.; Saouaf, S.J. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol. 2007, 83, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Kaname, T.; Miyauchi, T.; Kuwano, A.; Matsuda, Y.; Muramatsu, T.; Kajii, T. Mapping basigin (BSG), a member of the immunoglobulin superfamily, to 19p13.3. Cytogenet. Cell Genet. 1993, 64, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Kasinrerk, W.; Fiebiger, E.; Stefanová, I.; Baumruker, T.; Knapp, W.; Stockinger, H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J. Immunol. 1992, 149, 847–854. [Google Scholar] [CrossRef]
- Biswas, C.; Zhang, Y.; DeCastro, R.; Guo, H.; Nakamura, T.; Kataoka, H.; Nabeshima, K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995, 55, 434–439. [Google Scholar]
- Seulberger, H.; Lottspeich, F.; Risau, W. The inducible blood-brain barrier specific molecule HT7 is a novel immunoglobulin-like cell surface glycoprotein. EMBO J. 1990, 9, 2151–2158. [Google Scholar] [CrossRef]
- Schlosshauer, B.; Herzog, K.H. Neurothelin: An inducible cell surface glycoprotein of blood-brain barrier-specific endothelial cells and distinct neurons. J. Cell. Biol. 1990, 110, 1261–1274. [Google Scholar] [CrossRef]
- Fadool, J.M.; Linser, P.J. 5A11 antigen is a cell recognition molecule which is involved in neuronal-glial interactions in avian neural retina. Dev. Dyn. 1993, 196, 252–262. [Google Scholar] [CrossRef]
- Altruda, F.; Cervella, P.; Gaeta, M.L.; Daniele, A.; Giancotti, F.; Tarone, G.; Stefanuto, G.; Silengo, L. Cloning of cDNA for a novel mouse membrane glycoprotein (gp42): Shared identity to histocompatibility antigens, immunoglobulins and neural-cell adhesion molecules. Gene 1989, 85, 445–451. [Google Scholar] [CrossRef]
- Fossum, S.; Mallett, S.; Barclay, A.N. The MRC OX-47 antigen is a member of the immunoglobulin superfamily with an unusual transmembrane sequence. Eur. J. Immunol. 1991, 21, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Nehme, C.L.; Cesario, M.M.; Myles, D.G.; Koppel, D.E.; Bartles, J.R. Breaching the diffusion barrier that compartmentalizes the transmembrane glycoprotein CE9 to the posterior-tail plasma membrane domain of the rat spermatozoon. J. Cell. Biol. 1993, 120, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.; Troccoli, N.; Biswas, C. Coordinate increase in collagenase mRNA and enzyme levels in human fibroblasts treated with the tumor cell factor, TCSF. Biochem. Int. 1989, 19, 257–266. [Google Scholar] [PubMed]
- Bordador, L.C.; Li, X.; Toole, B.; Chen, B.; Regezi, J.; Zardi, L.; Hu, Y.; Ramos, D.M. Expression of EMMPRIN by oral squamous cell carcinoma. Int. J. Cancer. 2000, 85, 347–352. [Google Scholar] [CrossRef]
- Muraoka, K.; Nabeshima, K.; Murayama, T.; Biswas, C.; Koono, M. Enhanced expression of a tumor-cell-derived collagenase-stimulatory factor in urothelial carcinoma: Its usefulness as a tumor marker for bladder cancers. Int. J. Cancer. 1993, 55, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Polette, M.; Gilles, C.; Marchand, V.; Lorenzato, M.; Toole, B.; Tournier, J.M.; Zucker, S.; Birembaut, P. Tumor collagenase stimulatory factor (TCSF) expression and localization in human lung and breast cancers. J. Histochem. Cytochem. 1997, 45, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Sameshima, T.; Nabeshima, K.; Toole, B.P.; Yokogami, K.; Okada, Y.; Goya, T.; Koono, M.; Wakisaka, S. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in cocultures with brain-derived fibroblasts. Cancer Lett. 2000, 157, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kanekura, T.; Chen, X.; Kanzaki, T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int. J. Cancer 2002, 99, 520–528. [Google Scholar] [CrossRef]
- Kataoka, H.; DeCastro, R.; Zucker, S.; Biswas, C. Tumor cell-derived collagenase-stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72-kDa gelatinase. Cancer Res. 1993, 53, 3154–3158. [Google Scholar]
- Muramatsu, T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 2016, 159, 481–490. [Google Scholar] [CrossRef]
- Simon-Chazottes, D.; Matsubara, S.; Miyauchi, T.; Muramatsu, T.; Guénet, J.L. Chromosomal localization of two cell surface-associated molecules of potential importance in development: Midkine (Mdk) and basigin (Bsg). Mamm. Genome. 1992, 2, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.N.; Marison, M.H.; Law, S.K.A.; McKnight, A.J.; Tomlinson, M.G.; van der Merwe, P.A. edt. The leucocyte antigen facts book. London: Academic Press Harcourt Brace & Company Publishers, 1997, p. 408–409.
- Yurchenko, V.; Zybarth, G.; O’Connor, M.; Dai, W.W.; Franchin, G.; Hao, T.; Guo, H.; Huang, H.-C.; Toole, B.; Gallay, P.; Sherry, B.; Bukrinsky, M. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J. Biol. Chem. 2002, 277, 22959–22965. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Fox, D.A.; Horejsi, V.; Sagawa, K.; Skubitz, K.M.; Katz, D.R.; Chain, B. The functional interaction s between CD98, β1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood 2001, 98, 374–832. [Google Scholar] [CrossRef]
- Nishibaba, R.; Higashi, Y.; Su, J.; Furukawa, T.; Kawai, K.; Kanekura, T. CD147-targeting siRNA inhibits cell-matrix adhesion of human malignant melanoma cells by phosphorylating focal adhesion kinase. J. Dermatol. 2012, 39, 63–67. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, W.J.; Xu, J.D.; Cao, X.X.; Chen, Q.; Yang, J.M.; Xu, Z.D. Involvement of CD147 in regulation of multidrug resistance to P-gp substrate drugs and in vitro invasion in breast cancer cells. Cancer Sci. 2007, 98, 1064–1069. [Google Scholar] [CrossRef]
- Yoshida, S.; Shibata, M.; Yamamoto, S.; Hagihara, M.; Asai, N.; Takahashi, M.; Mizutani, S.; Muramatsu, T.; Kadomatsu, K. Homoligomer formation by basigin, an immunoglobulin superfamily member, via its N-terminal immunoglobulin domain. Eur. J. Biochem. 2000, 267, 4372–4380. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family – From monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004, 447, 619–628. [Google Scholar] [CrossRef]
- Köpnick, A.L.; Jansen, A.; Geistlinger, K.; Epalle, N.H.; Beitz, E. Basigin drives intracellular accumulation of L-lactate by harvesting protons and substrate anions. PLOS ONE. 2021, 16, e0249110. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jiang, X.; Zhang, S.; Zhu, A.; Yuan, Y.; Xu, H.; Lei, J.; Yan, C. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 2021, 184, 370–383.e13. [Google Scholar] [CrossRef]
- Manoharan, C.; Wilson, M.C.; Sessions, R.B.; Halestrap, A.P. The role of charged residues in the transmembrane helices of monocarboxylate transporter 1 and its ancillary protein basigin in determining plasma membrane expression and catalytic activity. Mol. Membr. Biol. 2006, 23, 486–498. [Google Scholar] [CrossRef]
- Philp, N.J.; Ochrietor, J.D.; Rudoy, C.; Muramatsu, T.; Linser, P.J. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Katayama, N.; Kachi, S.; Kondo, M.; Kadomatsu, K.; Usukura, J.; Muramatsu, T.; Mori, S.; Miyane, Y. Retinal dysfunction in basigin deficiency. Invest. Ophthalmol. Vis. Sci 2000, 41, 3128–3133. [Google Scholar]
- Weinhouse, S. Metabolism of respiratory fuels in cancer cells. Acta. Unio. Int. Contra. Cancrum. 1960, 16, 32–35. [Google Scholar] [PubMed]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Beckner, M.E.; Stracke, M.L.; Liotta, L.A.; Schiffmann, E. Glycolysis as primary energy source in tumor cell chemotaxis. J. Natl. Cancer Inst. 1990, 82, 1836–1840. [Google Scholar] [CrossRef] [PubMed]
- Rofstad, E.K.; Mathiesen, B.; Kindem, K.; Galappathi, K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006, 66, 6699–707. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Xu, L.; Chen, Y.; Gohongi, T.; Seed, B.; Jain, R.K. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001, 61, 6020–6024. [Google Scholar] [PubMed]
- Gallagher, S.M.; Castorino, J.J.; Wang, D.; Philp, N.J. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007, 67, 4182–4189. [Google Scholar] [CrossRef]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Wilson, M.C. The monocarboxylate transporter family - Role and regulation. IUBMB Life. 2012, 64, 109–119. [Google Scholar] [CrossRef]
- Murray, C.M.; Hutchinson, R.; Bantick, J.R.; Belfield, G.P.; Benjamin, A.D.; Brazma, D.; Bundick, R.V.; Cook, I.D.; Craggs, R.I.; Edwards, S.; Evans, L.R.; Harrison, R.; Holness, E.; Jackson, A.P.; Jackson, C.G.; Kingston, L.P.; Perry, M.W.D.; Ross, A.R.J.; Rugman, P.A.; Sidhu, S.S.; Sullivan, M.; Taylor-Fishwick, D.A.; Walker, P.C.; Whitehead, Y.M.; Wilkinson, D.J.; Wright, A.; Donald, D.K. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 2005, 1, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.N.; Kaushik, D.K.; Yong, V.W. The role of EMMPRIN in T cell biology and immunological diseases. J. Leuko.c Biol. 2015, 98, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, J.; Li, Y.; Yin, Z.J.; Lv, T.T.; Zhu, P.; Zhang, Y. CD147 modulates the differentiation of T-helper 17 cells in patients with rheumatoid arthritis. APMIS. 2017, 125, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Brembilla, N.C.; Boehncke, W.H. Revisiting the interleukin 17 family of cytokines in psoriasis: Pathogenesis and potential targets for innovative therapies. Front. Immunol. 2023, 14, 1186455. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Towne, J.E.; Kricorian, G.; Klekotka, P.; Gudjonsson, J.E.; Krueger, J.G.; Russell, C.B. The emerging role of IL-17 in the pathogenesis of psoriasis: Preclinical and clinical findings. J. Invest. Dermatol. 2013, 133, 17–26. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J. Th17 cell differentiation: The long and winding road. Immunity 2008, 28, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Marinoni, B.; Ceribelli, A.; Massarotti, M.S.; Selmi, C. The Th17 axis in psoriatic disease: Pathogenetic and therapeutic implications. Auto. Immun. Highlights 2014, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, D.; Xiong, H.; Shi, D. Transcriptional regulation of effector T cells in the pathogenesis of psoriasis. Eur. J. Med. Res. 2023, 28, 182–189. [Google Scholar] [CrossRef]
- Lu, H.; Kuang, Y.H.; Su, J.; Chang, J.; Wu, L.S.; Kanekura, T.; Li, D.; Chen, M.-L.; Chen, X. CD147 is highly expressed on peripheral blood neutrophils from patients with psoriasis and induces neutrophil chemotaxis. J. Dermatol. 2010, 37, 1053–1056. [Google Scholar] [CrossRef]
- Egawa, N.; Koshikawa, N.; Tomari, T.; Nabeshima, K.; Isobe, T.; Seiki, M. Membrane type 1 matrix metalloproteinase (MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J. Biol. Chem. 2006, 281, 37576–37585. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Hori, M.; Kosugi, T.; Kojima, H.; Sato, W.; Inaba, S.; Maeda, K.; Nagaya, H.; Sato, Y.; Ishimoto, T.; Ozali, T.; Tsuboi, N.; Muro, Y.; Yuzawa, Y.; Imai, E.; Johnson, R.J.; Matsuo, S.; Kadomatsu, K.; Maruyama, S. Plasma CD147 reflects histological features in patients with lupus nephritis. Lupus. 2014, 23, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liao, L.; Jia, X.; Kuang, Y.; Chen, X. Role of soluble CD147 in psoriatic patients: A preliminary study. J. Dermatol. 2018, 45, e266–267. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, S.; Lei, L.; Zhang, X.; Jia, X.; Luo, Z.; Huang, X.; Kuang, Y.; Zeng, W.; Su, J.; Chen, N. Epidermal CD147 expression plays a key role in IL-22-induced psoriatic dermatitis. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Wick, G.; Grundtman, C.; Mayerl, C.; Wimpissinger, T.F.; Feichtinger, J.; Zelger, B.; Sgonc, R.; Wolfram, D. The immunology of fibrosis. Annu. Rev. Immunol. 2013, 31, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.J.; Zhang, K.; Chen, L.N.; Miao, J.L.; Yao, M.; Ren, Y.; Fu, Z.; Chen, Z.; Zhu, P. Enhancement of CD147 on M1 macrophages induces differentiation of Th17 cells in the lung interstitial fibrosis. Biochim. Biophys. Acta. 2014, 1842, 1770–1782. [Google Scholar] [CrossRef]
- Kaushik, D.K.; Bhattacharya, A.; Mirzaei, R.; Rawji, K.S.; Ahn, Y.; Rho, J.M.; Yong, V.W. Enhanced glycolytic metabolism supports transmigration of brain-infiltrating macrophages in multiple sclerosis. J. Clin. Invest. 2019, 129, 3277–3292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




