Submitted:
10 November 2023
Posted:
13 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Strains, culture growth and co-cultures
2.2. Antifungal activity of the media
2.3. Medium solvent extraction and concentration
2.4. Extract antifungal activity on post-harvest cherry tomatoes
2.5. Statistical analysis
3. Results
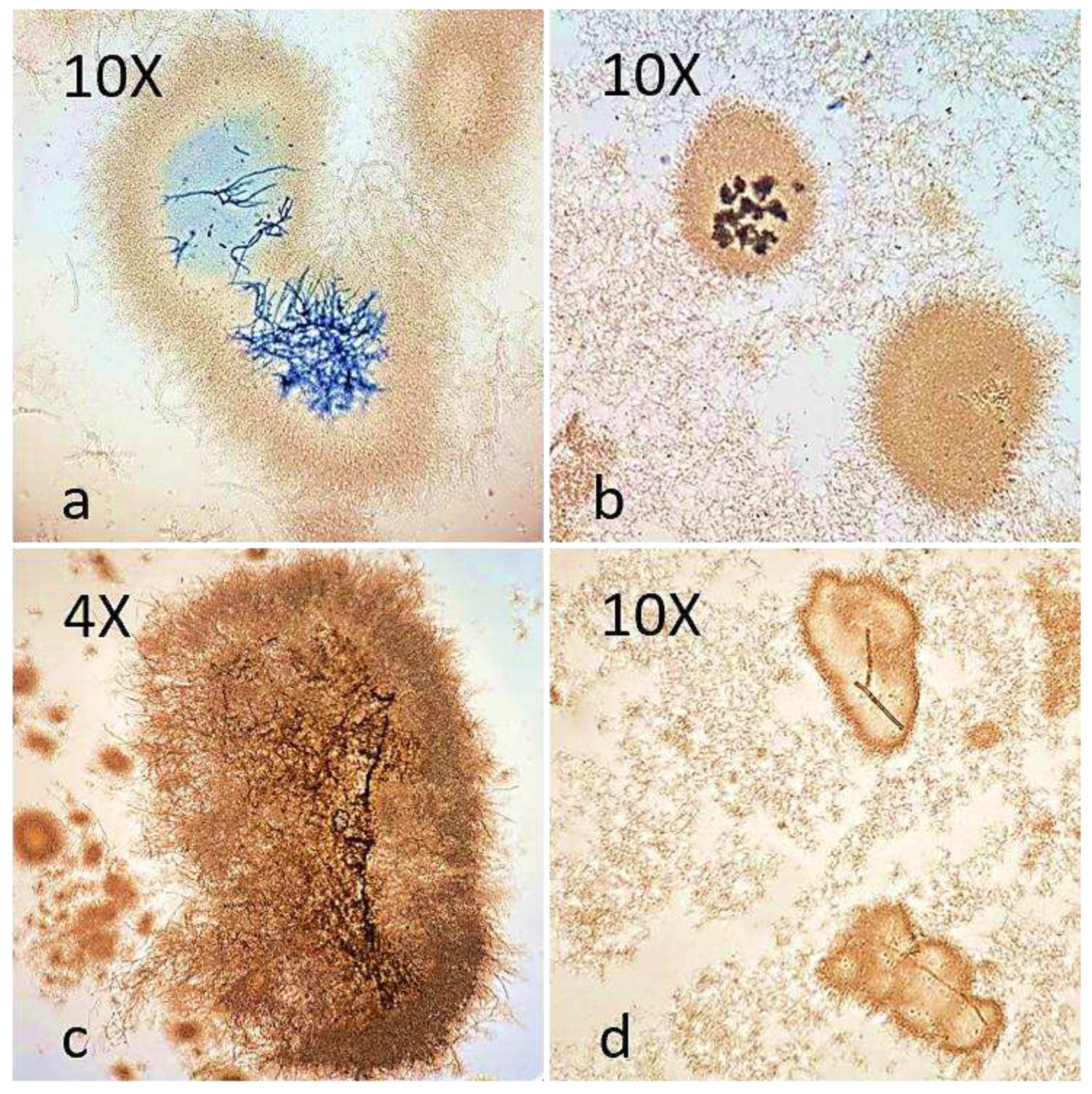
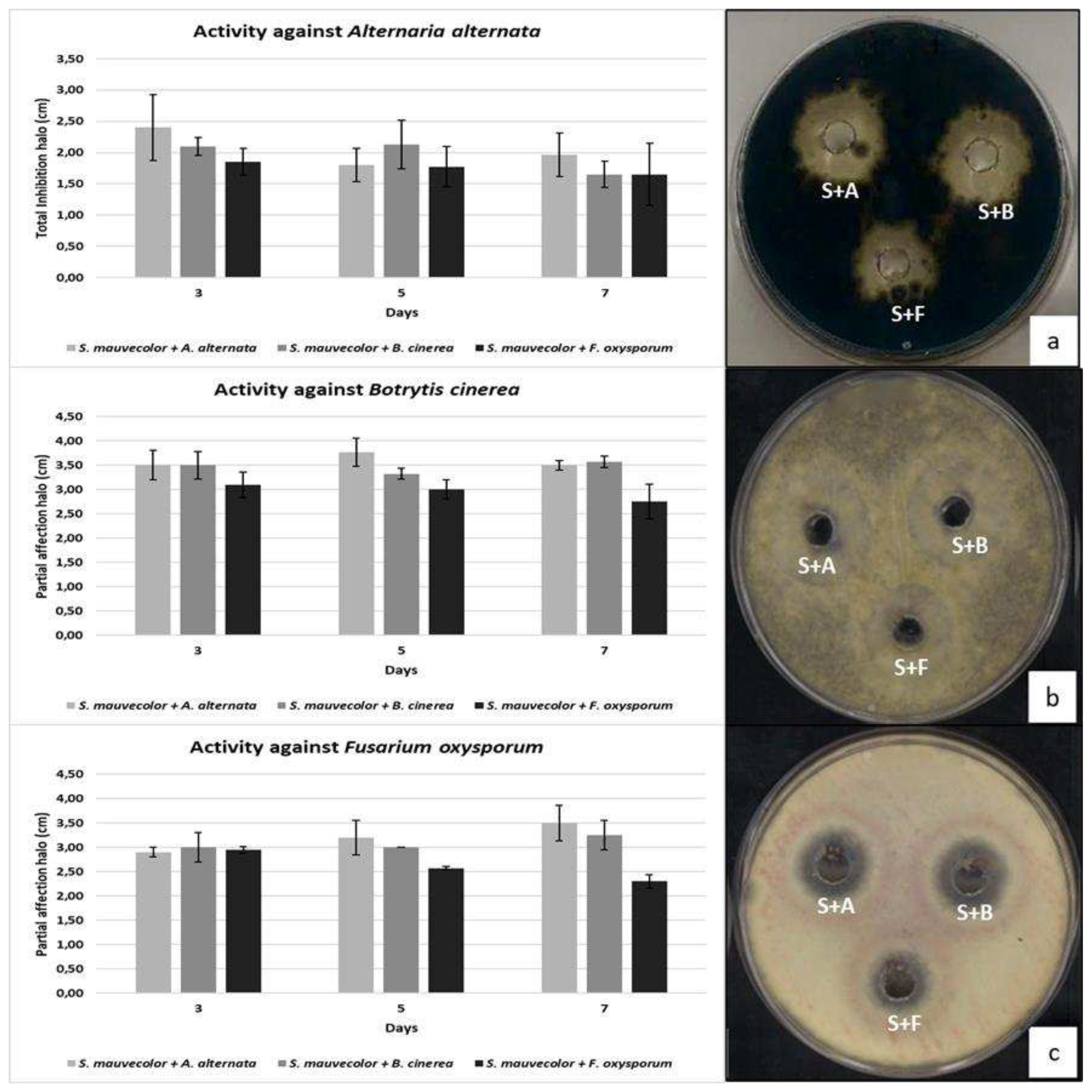
3.1. Actinomycetes co-culture growth inhibition and antifungal activity in vitro
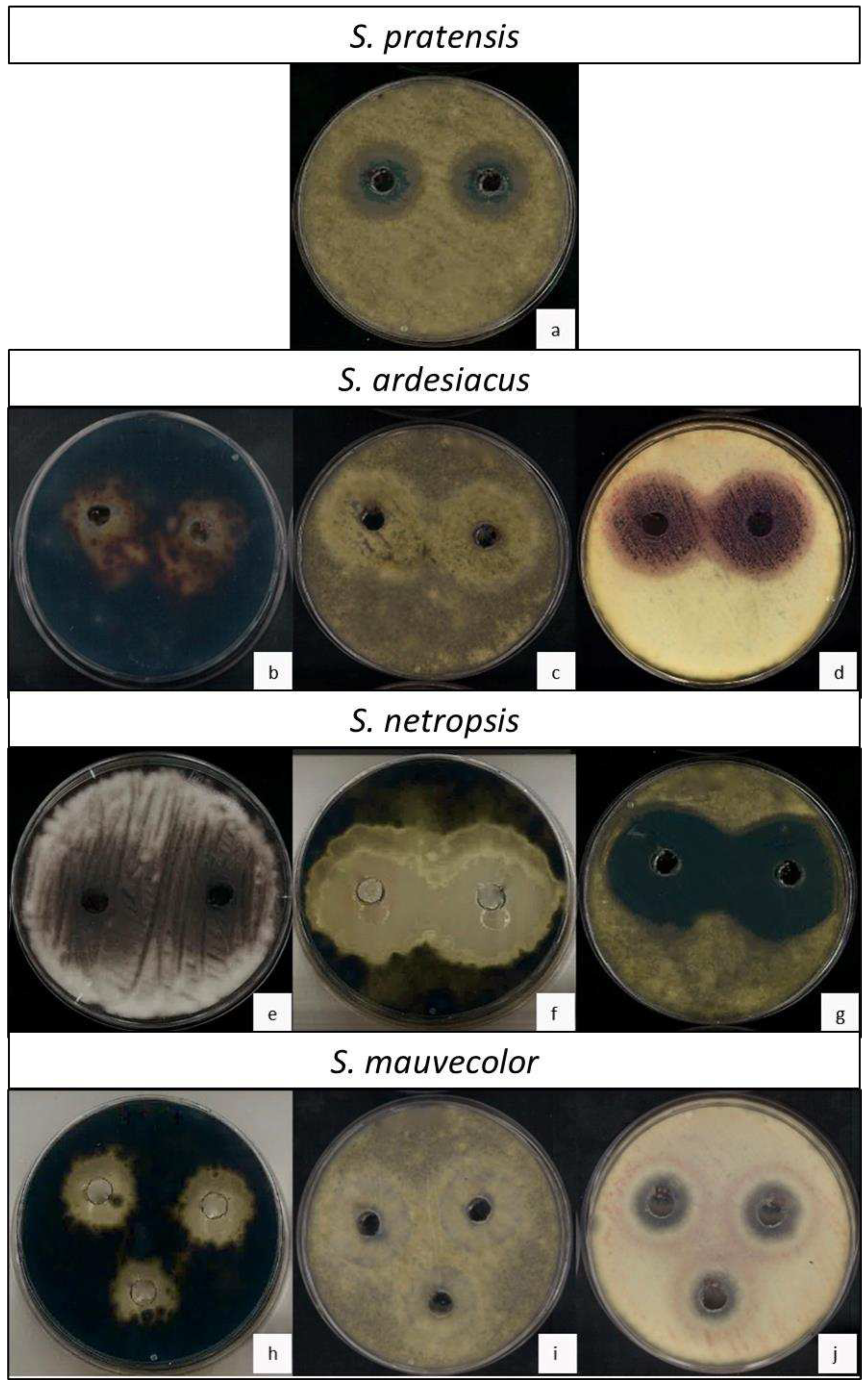
3.2. Actinomycetes species antifungal activity in vitro
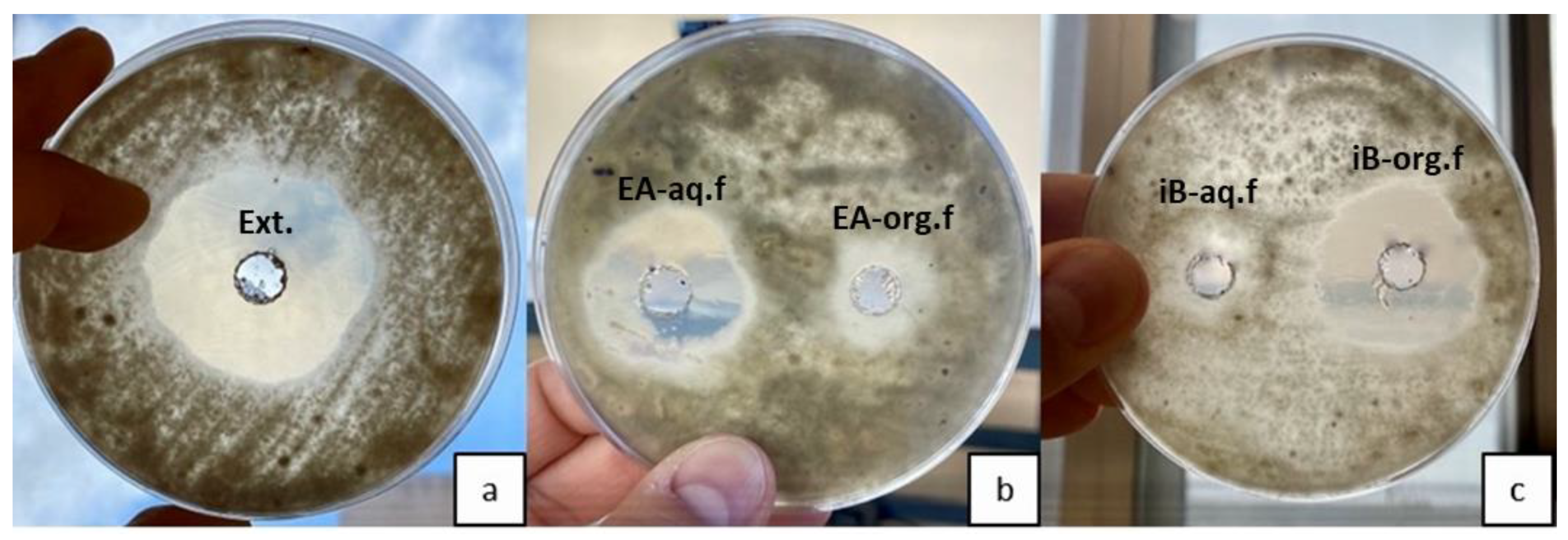
3.3. Organic solvent extraction and antifungal activity
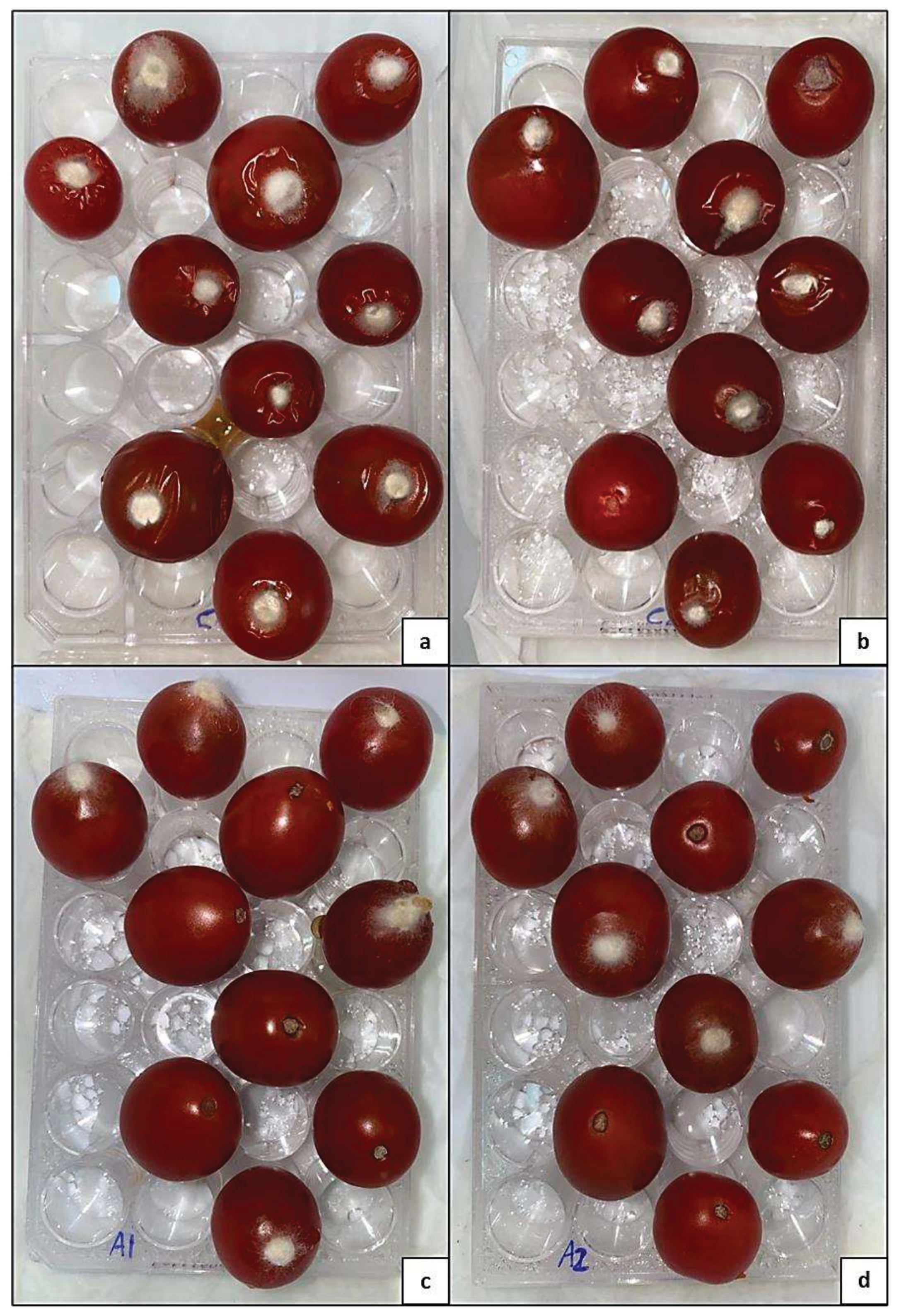
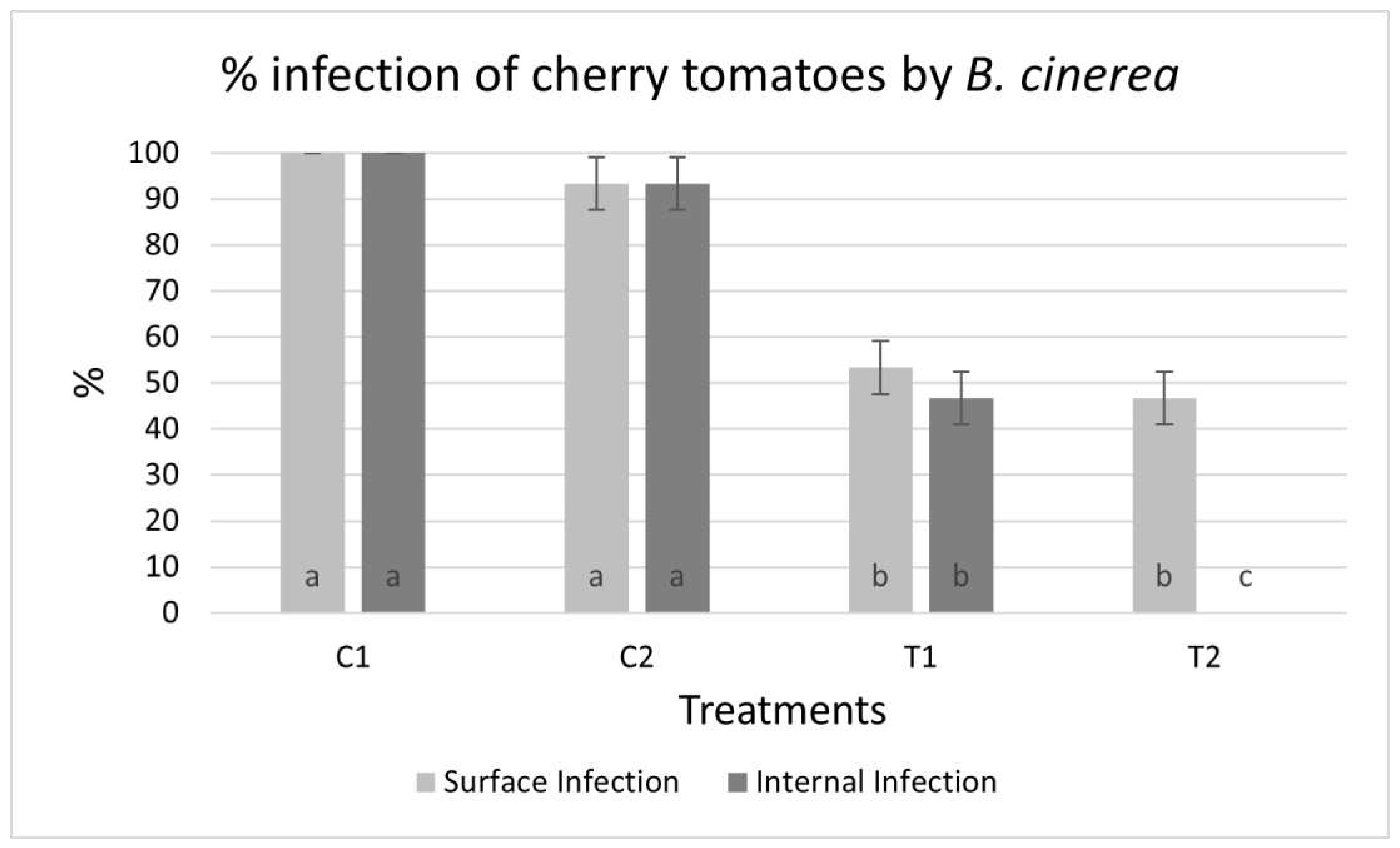
3.4. Botrytis growth inhibition on cherry tomatoes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahadur, I. The novel potassic bio-fertilizers: a promising approach for evergreen agriculture. Int. J. Microbiol. Res. 2015, ISSN, 0975-5276.
- Bahadur, I.; Maurya, R.; Roy, P.; Kumar, A. Potassium-solubilizing bacteria (KSB): a microbial tool for K-solubility, cycling, and availability to plants. In Plant Growth Promoting Rhizobacteria for Agricultural Sustainability: From Theory to Practices; Kumar, A., Meena, V., Eds.; Springer: Singapore; pp. 257–265. [CrossRef]
- Bandara, A.Y.; Weerasooriya, D.K.; Bradley, C.A.; Allen, T.W.; Esker, P.D. Dissecting the economic impact of soybean diseases in the United States over two decades. PLOS one. 2020, 15, e0231141. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-San Millan, A.; Larraya, L.; Farran, I.; Ancin, M.; Veramendi, J. Successful biocontrol of major postharvest and soil-borne plant pathogenic fungi by antagonistic yeasts. Biol. Control. 2021, 160, 104683. [Google Scholar] [CrossRef]
- Parra-Amin, J.E.; Cuca, L.E.; González-Coloma, A. Antifungal and phytotoxic activity of benzoic acid derivatives from inflorescences of Piper cumanense. Nat. Prod. Res. 2021, 35(16), 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Mederos-Torres, Y.; Bernabé-Galloway, P.; Ramírez-Arrebato, M.A. Películas basadas en polisacáridos como recubrimientos biodegradables y su empleo en la postcosecha de los frutos. Cultivos Tropicales. 2020, 41(3). [Google Scholar]
- Truchado, P.; Allende, A. La implicación de las frutas y hortalizas en las toxiinfecciones alimentarias y la relevancia del estado fisiológico de las bacterias. arbor. 2020, 196(795), a541–a541. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, V. Bioactive compounds of Streptomyces: biosynthesis to applications. Stud. Nat. Prod. Chem. 2020, 64, 467–491. [Google Scholar] [CrossRef]
- Hernández-Bolaños, E.; Montesdeoca-Flores, D.T; Abreu-Yanes, E.; Barrios, M.; Abreu-Acosta, N. Evaluating different methodologies for bioprospecting actinomycetes in Canary Islands soils. Curr. Microbiol. 2020, 77, 2510–2522. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, M.H.; Rasmey, A.H.M.; El-Sayed, E.S.A.; El-Kady, I.A.; Yassin, I.M. Biosynthesis of anti-inflammatory immunosuppressive metabolite by Streptomyces variabilis ASU319. Eur. J. Biol. Res. 2016, 6(3). [Google Scholar] [CrossRef]
- Bentley, S.D.; Chater, K.F.; Cerdeño-Tárraga, A.M.; Challis, G.L.; Thomson, N.R.; James, K.D.; ... Hopwood, D.A. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3 (2). Nature 2002, 417, 141–147. [CrossRef]
- Ōmura, S.; Ikeda, H.; Ishikawa, J.; Hanamoto, A.; Takahashi, C.; Shinose, M.; ... Hattori, M. Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 12215–12220. [CrossRef] [PubMed]
- Pérez, J.; Muñoz-Dorado, J.; Braña, A.F.; Shimkets, L.J.; Sevillano, L.; Santamaría, R.I. Myxococcus xanthus induces actinorhodin overproduction and aerial mycelium formation by Streptomyces coelicolor. Microb. Biotechnol. 2011, 4(2), 175–183. [Google Scholar] [CrossRef]
- Lee, N.; Kim, W.; Chung, J.; Lee, Y.; Cho, S.; Jang, K.S.; ... Cho, B.K. Iron competition triggers antibiotic biosynthesis in Streptomyces coelicolor during coculture with Myxococcus xanthus. ISME J. 2020, 14(5), 1111–1124. [CrossRef]
- Chen, H.; Xiao, X.; Wang, J.; Wu, L.; Zheng, Z.; Yu, Z. Antagonistic effects of volatiles generated by Bacillus subtilis on spore germination and hyphal growth of the plant pathogen, Botrytis cinerea. Biotechnol. Lett. 2008, 30, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Boorn, K.L.; Khor, Y.Y.; Sweetman, E.; Tan, F.; Heard, T.A.; Hammer, K.A. Antimicrobial activity of honey from the stingless bee Trigona carbonaria determined by agar diffusion, agar dilution, broth microdilution and time-kill methodology. J. Appl. Microbiol. 2010, 108(5), 1534–1543. [Google Scholar] [CrossRef]
- Esteban, A.; Abarca, M.L.; Cabañes, F.J. Comparison of disk diffusion method and broth microdilution method for antifungal susceptibility testing of dermatophytes. Med. Mycol. 2005, 43(1), 61–66. [Google Scholar] [CrossRef]
- Garmendia, G.; Vero, S. Métodos para la desinfección de frutas y hortalizas. Hortic. 2006, 197, 18–27. [Google Scholar]
- Abdelmoteleb, A.; González-Mendoza, D. A novel Streptomyces rhizobacteria from desert soil with diverse anti-fungal properties. Rhizosphere. 2020, 16, 100243. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, H.; Deng, Y.; Tian, W.; Fan, G.; Sun, X. Antifungal Activity of Streptomyces hygroscopicus JY-22 against Alternaria alternata and Its Potential Application as a Biopesticide to Control Tobacco Brown Spot. Agronomy 2023, 13(7), 1944. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Yu, Z. New Lactones Produced by Streptomyces sp. SN5431 and Their Antifungal Activity against Bipolaris maydis. Microorganisms. 2023, 11(3), 616. [Google Scholar] [CrossRef]
- Santamaría, R.I.; Martínez-Carrasco, A.; Tormo, J.R.; Martín, J.; Genilloud, O.; Reyes, F.; Díaz, M. Interactions of Different Streptomyces Species and Myxococcus xanthus Affect Myxococcus Development and Induce the Production of DK-Xanthenes. Int. J. Mol. Sci. 2023, 24(21), 15659. [Google Scholar] [CrossRef]
- Afroz-Toma, M.; Rahman, M.H.; Rahman, M.S.; Arif, M.; Nazir, K.N.H.; Dufossé, L. Fungal Pigments: Carotenoids, Riboflavin, and Polyketides with Diverse Applications. J. Fungi. 2023, 9(4), 454. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A. Metabolitos secundarios de actinomicetos. In Impacto de la biología molecular y las nuevas tecnologías en el conocimiento de la función celular y sus aplicaciones, 1st ed.; Fierro, F.F., Onofre, M.V. Universidad Autónoma Metropolitana, México, 2011; pp. 27-38.
- Evangelista-Martínez, Z.; Moreno-Enríquez, A. Metabolitos secundarios de importancia farmacéutica producidos por actinomicetos. Rev. Biotecnol. 2007, 11, 37–50. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; ... Foster, G.D. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13(4), 414–430. [CrossRef] [PubMed]
- Tsalidis, G.A. Human health and ecosystem quality benefits with life cycle assessment due to fungicides elimination in agriculture. Sustainability 2022, 14(2), 846. [Google Scholar] [CrossRef]
- Chacon-Lopez, A.; Guardado-Valdivia, L.; Banuelos-Gonzalez, M.; Lopez-Garcia, U.; Montalvo-González, E.; Arvizu-Gomez, J.; ... Aguilera, S. Effect of metabolites produced by Bacillus atrophaeus and Brevibacterium frigoritolerans strains on postharvest biocontrol of Alternaria alternata in tomato (Solanum lycopersicum L.). Biocontrol Sci. 2021, 26(2), 67–74. [CrossRef] [PubMed]
- Chaouachi, M.; Marzouk, T.; Jallouli, S.; Elkahoui, S.; Gentzbittel, L.; Ben, C.; Djébali, N. Activity assessment of tomato endophytic bacteria bioactive compounds for the postharvest biocontrol of Botrytis cinerea. Postharvest Biol. Technol. 2021, 172, 111389. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63(1), 34–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, J.H.; Liu, G.; Yao, X.F.; Li, P.F.; Yang, X.P. Characterization of the watermelon seedling infection process by Fusarium oxysporum f. sp. niveum. Plant Pathol. 2015, 64(5), 1076–1084. [Google Scholar] [CrossRef]
- Prins, T.W.; Tudzynski, P.; von Tiedemann, A.; Tudzynski, B.; Ten Have, A.; Hansen, M.E.; ... van Kan, J.A. Infection strategies of Botrytis cinerea and related necrotrophic pathogens. Fungal Pathol. 2000, 33–64. [CrossRef]
- Zhu, L.; Ni, W.; Liu, S.; Cai, B.; Xing, H.; Wang, S. Transcriptomics analysis of apple leaves in response to Alternaria alternata apple pathotype infection. Front. Plant Sci. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Carpena, R.; Ritter, A.; Socorro, A.R; Perez, N. Nitrogen evolution and fate in a Canary Islands (Spain) sprinkler fertigated banana plot. Agric. Water Manag. 2002, 52(2), 93–117. [Google Scholar] [CrossRef]
- de Cossío, Á.G. Nueva ayuda al sector de producción de plátanos de Canarias. Hacienda Canaria. 2008, (24), 129–152. [Google Scholar]
- Costa, J.M.; Heuvelink, E.P. The global tomato industry. In Tomatoes; Heuvelink, E.P., Ed.; Wallingford, United Kingdom, 2018; pp. 1–26. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




