1. Introduction
C-X-C motif chemokine receptor 3 (CXCR3, CD183) is a G-protein-coupled receptor expressed on T cells and NK cells [
1]. CXCR3 signaling contributes to the accumulation of these cells to inflammation sites. Interferons (IFN), cytokines secreted in inflammation sites, regulate CXCR3 signaling and expression. IFN-α, -β, and -γ elevate the expression of CXCL9, CXCL10, and CXCL11, which induce CXCR3-mediated chemotaxis in various types of cells including macrophages, dendritic cells, fibrocytes, and endothelial cells [
2,
3]. Additionally, IFN-γ is essential for CXCR3 expression induced by T cell receptor stimulation in T cells [
4]. CXCR3 activation leads to actin polymerization and migration of T cells to inflammation sites through G-protein and phospholipase C dependent pathways [
5].
CXCR3 is involved in inflammatory diseases [
6,
7]. Dextran Sulfate Sodium (DSS)-induced colitis is a commonly used mouse model for inflammatory bowel disease (IBD) [
8]. In CXCR3 deficient mice challenged with 2.5% DSS, body weight loss, a sign of the severity of colitis, was lower than that of wild type mice [
9]. The number of leukocytes in the colon did not increase after the challenge. Moreover, the elevations of pro-inflammatory cytokines, such as IL-6, TNF-α, and IFN-γ by DSS-challenge reduced in CXCR3 deficient mice [
9]. These facts indicate that CXCR3 plays a pivotal role in the development of IBD.
To understand pathogenesis and develop therapeutics for inflammatory diseases, various mouse models have been reported [
8,
10,
11,
12]. Although CXCR3 is an attractive target for anti-inflammatory therapies, few preclinical models using anti-mouse CXCR3 (mCXCR3) monoclonal antibodies (mAbs) have been developed. We have established mAbs to mouse CCR3 [
13] and CCR8 [
14] using the Cell-Based Immunization and Screening (CBIS) method. In this report, we developed novel anti-mCXCR3 mAbs using the CBIS method and evaluated its applications.
2. Materials and Methods
2.1. Antibodies, Cell Lines, and Plasmids
An anti-mCXCR3 mAb (clone CXCR3-173) was purchased from BioLegend (San Diego, CA). Alexa Fluor 488-conjugated anti-rat IgG (4416) and anti-Armenian hamster IgG (ab173003) were purchased from Cell Signaling Technology, Inc. (Danvers, MA) and Abcam (Cambridge, UK), respectively.
LN229, Chinese hamster ovary (CHO)-K1, and P3X63Ag8U.1 (P3U1) cells were obtained from the American Type Culture Collection (Manassas, VA).
The synthesized DNA (Eurofins Genomics KK) encoding mCXCR3 (Accession No.: NM_009910.3) was subcloned into a pCAGzeo_PAcH vector (PA tag [
15] added to the C-terminus of mCXCR3). The pCAGzeo_mCXCR3-PA was transfected into LN229 and CHO-K1 cells using a Neon transfection system (Thermo Fisher Scientific Inc., Waltham, MA). Clones stably expressing mCXCR3-PA were established using a cell sorter (SH800; Sony Corp., Tokyo, Japan) after culturing in a medium, containing 0.5 mg/mL of Zeocin (InvivoGen, San Diego, CA).
LN229 and mCXCR3-overexpressed LN229 (LN229/mCXCR3-PA) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Nacalai Tesque, Inc., Kyoto, Japan), supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc.), 100 µg/mL streptomycin, 100 U/mL of penicillin, and 0.25 µg/mL amphotericin B (Nacalai Tesque, Inc.). CHO-K1 and mCXCR3-PA-overexpressed CHO-K1 (CHO/mCXCR3-PA) cells were maintained in a Roswell Park Memorial Institute (RPMI)-1640 medium (Nacalai Tesque, Inc.), supplemented with 10% FBS, 100 μg/mL of streptomycin, 100 units/mL of penicillin, and 0.25 μg/mL of amphotericin B (Nacalai Tesque, Inc.). All cells were grown in a humidified incubator at 37oC, in an atmosphere of 5% CO2 and 95% air.
2.2. Production of Hybridomas
A five-week-old Sprague–Dawley rat was purchased from CLEA Japan (Tokyo, Japan). The animal was housed under specific pathogen-free conditions. All animal experiments were approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001).
To develop mAbs against mCXCR3, we intraperitoneally immunized one rat with LN229/mCXCR3-PA cells (1×109 cells) plus Alhydrogel adjuvant 2% (InvivoGen, San Diego, CA). After four additional injections every week (1×109 cells), the splenocytes were harvested. The splenocytes were fused with P3U1 cells using PEG1500 (Roche Diagnostics, Indianapolis, IN). Hybridomas were selected by cultivation in the RPMI-1640 medium with 10% FBS, 100 μg/mL of streptomycin, 100 units/mL of penicillin, 0.25 μg/mL of amphotericin B, 5 μg/mL of Plasmocin, 5% Briclone (NICB, Dublin, Ireland), and hypoxanthine/aminopterin/thymidine (HAT; Thermo Fisher Scientific, Inc.). The supernatants were screened using flow cytometry using CHO/mCXCR3-PA.
2.3. Flow Cytometry
CHO-K1 and CHO/mCXCR3-PA cells were harvested after a brief exposure to 1 mM ethylenediaminetetraacetic acid (EDTA, Nacalai Tesque, Inc.). The cells were washed with 0.1% bovine serum albumin (BSA) in PBS and treated with anti-mCXCR3 mAbs for 30 min at 4oC. After washing, the cells were treated with Alexa Fluor 488-conjugated secondary antibodies. Flow cytometric analysis was performed using the SA3800 Cell Analyzer (Sony Corporation, Tokyo, Japan).
To determine the dissociation constant (KD), anti-mCXCR3 mAbs were diluted serially from 100 μg/mL to 6 ng/mL. The geometric mean of fluorescence intensities of CHO/mCXCR3-PA at each concentration was calculated by FlowJo v10.8.1 (Becton, Dickinson & Company, Ashland, OR). The KD was estimated by fitting saturation binding curves to the built-in; one-site binding models in GraphPad PRISM 8 (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Development of Anti-mCXCR3 mAbs
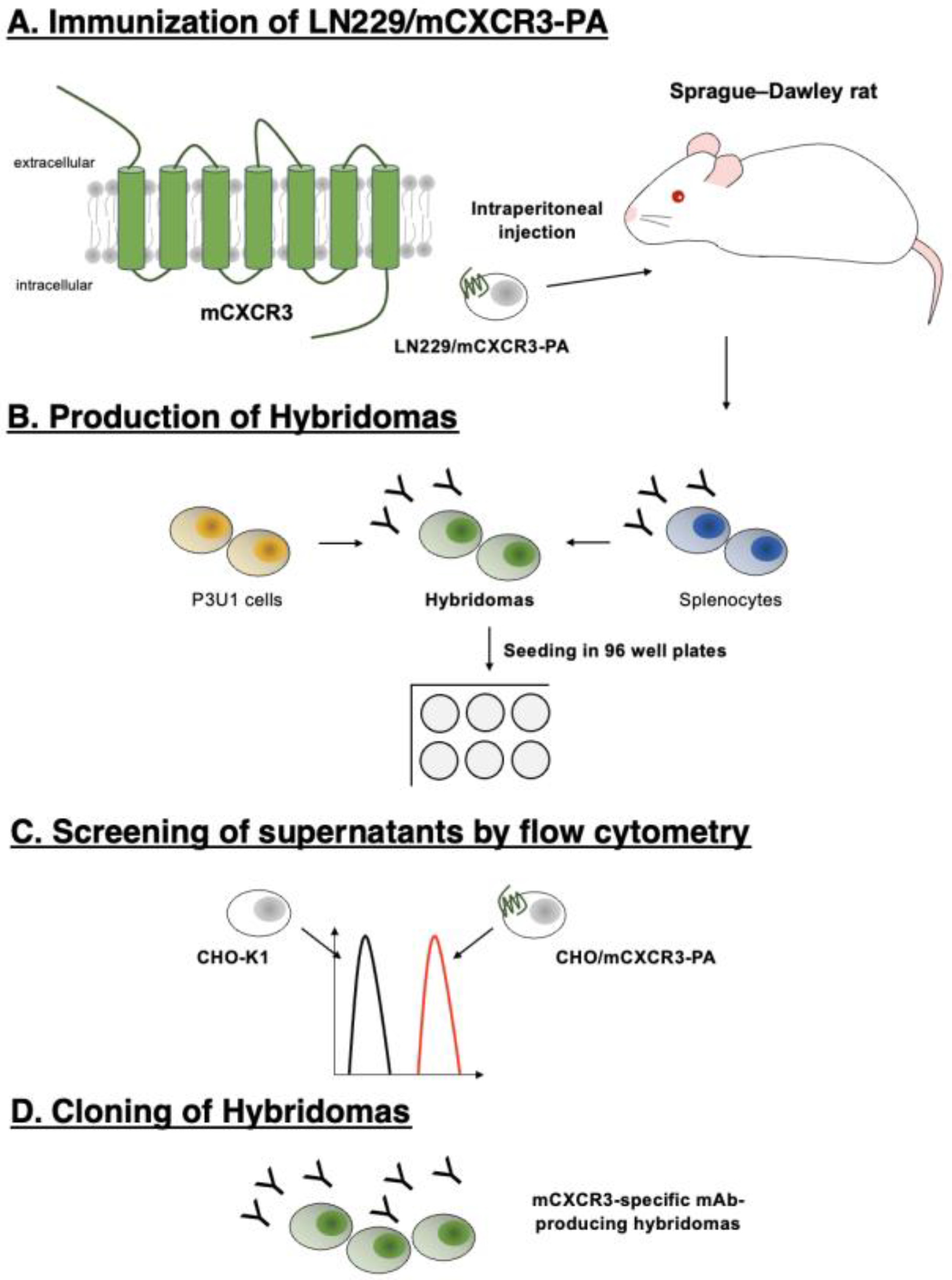
We developed anti-mCXCR3 mAbs using the CBIS method. The CBIS method is composed of four steps (
Figure 1). First, a rat was immunized with LN229/mCXCR3-PA cells (
Figure 1A). The splenocytes were collected and fused with P3U1 cells (
Figure 1B). The reactivities to CHO/mCXCR3-PA cells of each supernatant were observed by flow cytometry (
Figure 1C). Finally, Cx
3Mab-4 (rat IgG
1, kappa) was developed (
Figure 1D).
3.2. Flow Cytometry
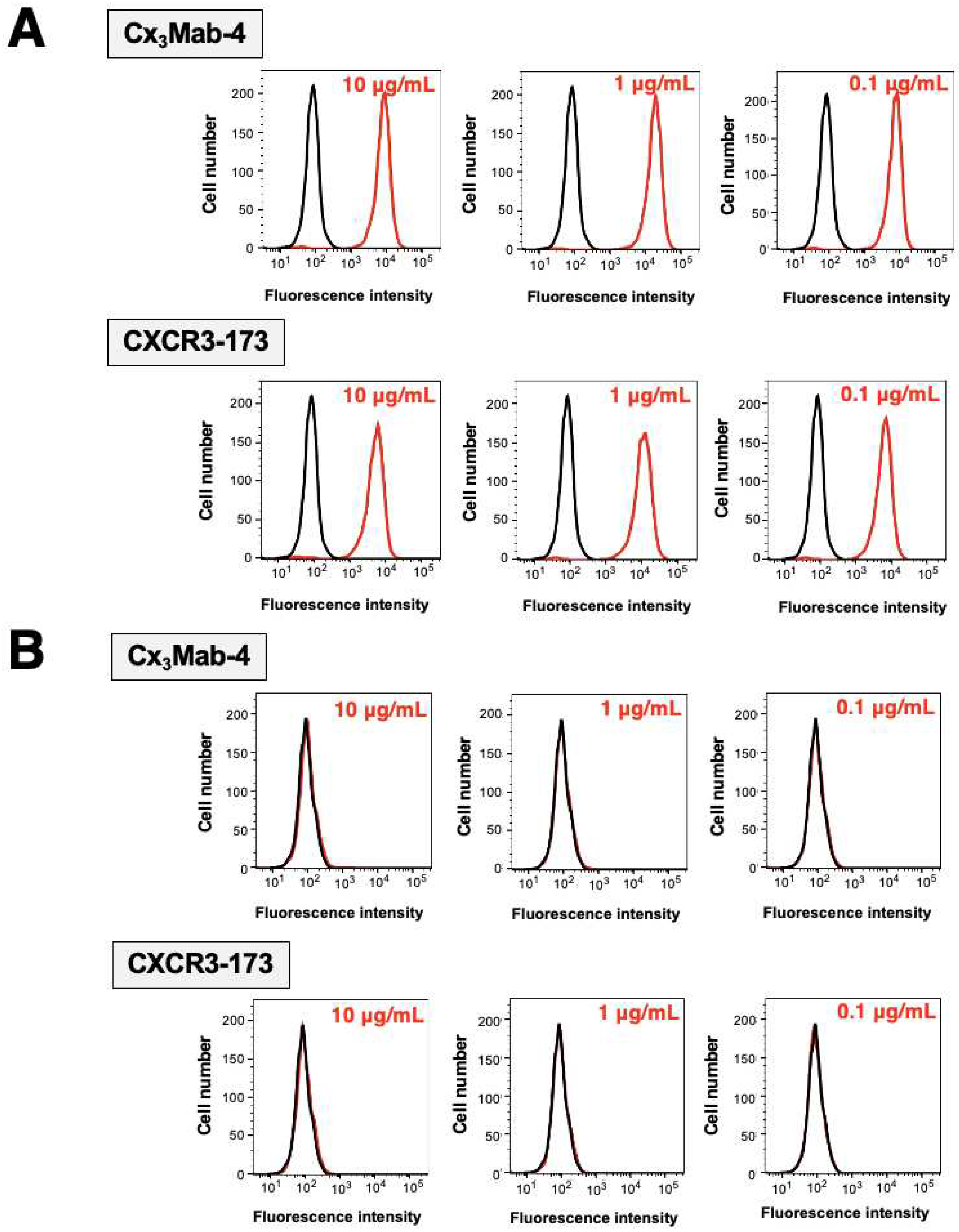
To check the specificity of Cx
3Mab-4 and CXCR3-173 (a commercially available anti-mCXCR3 mAb), we performed flow cytometry against CHO/mCXCR3-PA and CHO-K1. Both Cx
3Mab-4 and CXCR3-173 reacted to CHO/mCXCR3-PA cells in a dose-dependent manner (
Figure 2A). In contrast, Cx
3Mab-4 and CXCR3-173 did not bind to CHO-K1 cells even at 10 µg/mL (
Figure 2B). No difference was observed between two anti-mCXCR3 mAbs.
3.3. Determination of Dissociation Constant of Anti-mCXCR3 mAbs against CHO/mCXCR3-PA Cells
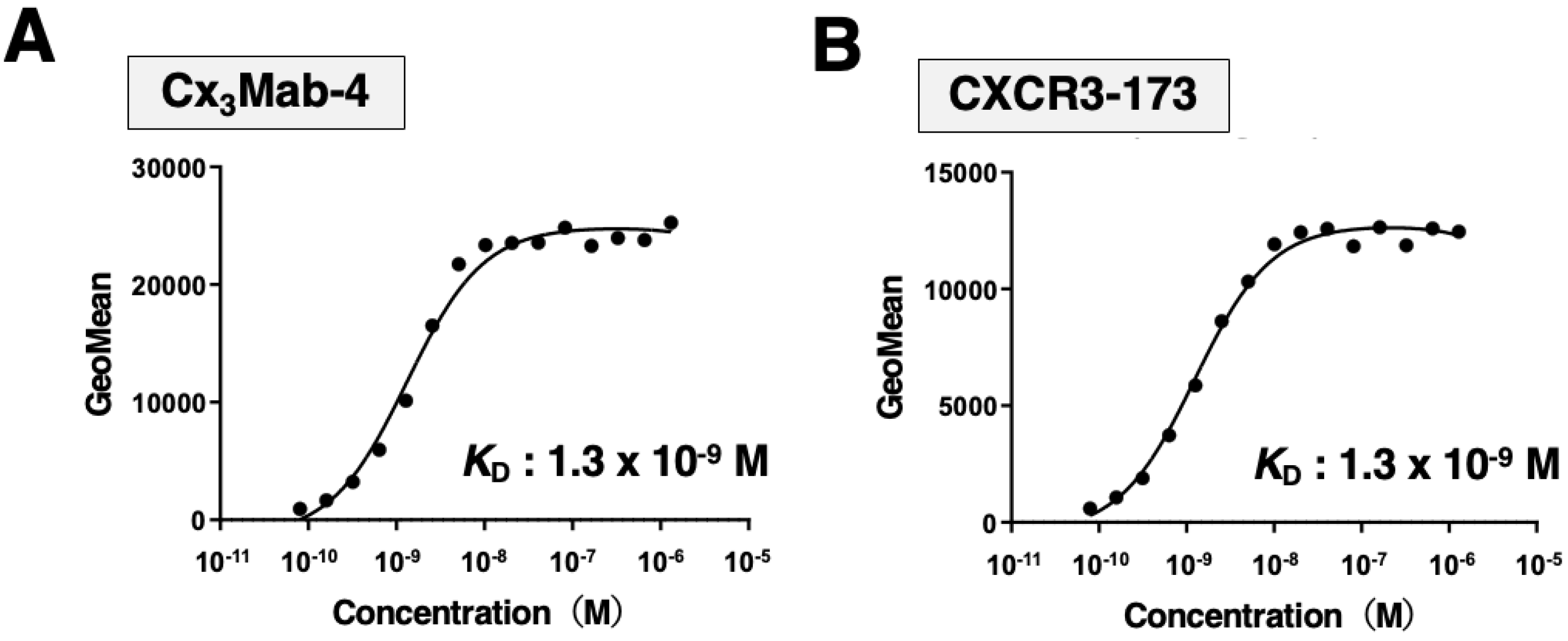
We determined the apparent dissociation constant (K
D) of Cx
3Mab-4 and CXCR3-173 against mCXCR3 by flow cytometry. The geometric mean of the fluorescence intensity of CHO/mCXCR3-PA at each concentration of Cx
3Mab-4 and CXCR3-173 was plotted. By fitting one-site binding models, both K
D values of Cx
3Mab-4 and CXCR3-173 for CHO/mCXCR3-PA were determined as 1.3×10
-9 M (
Figure 3), indicating that both Cx
3Mab-4 and CXCR3-173 possess high affinity for CHO/mCXCR3-PA cells.
4. Discussion
The CXCR3 ligands, CXCL9, CXCL10, and CXCL11, are abundantly expressed in the intestinal mucosa of IBD patients [
16,
17,
18]. IBD is characterized by chronic, uncontrolled inflammation in the intestinal mucosa [
19]. The chemokines induce cellular trafficking and enhance inflammation through binding to CXCR3 expressed on proinflammatory lymphocytes. The inhibition of infiltration has been shown to attenuate inflammation [
19]. Eldelumab (MDX-1100, BMS-936557) is a fully human IgG
1 mAb to CXCL10 and have been developed for IBD treatment. Unfortunately, in the phase II study, the eldelumab exposure-remission was not sufficient in patients with IBD [
20,
21]. Since not only CXCL10 but also CXCL9 and CXCL11 are elevated in IBD, anti-CXCR3 therapy is also expected for IBD treatment. In this study, we developed a novel anti-mCXCR3 mAb, Cx
3Mab-4 which is useful for flow cytometry (Figs. 2 and 3). To apply preclinical mouse models of IBD, we should determine the epitope of Cx
3Mab-4 and investigate the neutralizing effect against the ligands. We have already determined the epitopes of anti-mouse chemokine receptor mAbs against CCR2 (C
2Mab-6) [
22], CCR3 (C
3Mab-3, 4, 6, and 7) [
23,
24], CCR6 (C
6Mab-13) [
25], CCR9 (C
9Mab-24) [
26], and CXCR6 (Cx
6Mab-1) [
27,
28].
Infiltration of regulatory T (Treg) cells into solid tumors represents a barrier to cancer immunotherapy [
29]. Chemokine receptors including CXCR3 play a critical role in Treg cell recruitment into inflamed tumor tissue, and are important therapeutic targets [
30]. CXCR3
+ Treg cells increase in multiple tumor models, and are activated through interaction with dendritic cells (DCs) [
30]. CXCR3 ablation in Treg cells disrupted the DC-Treg cells interactions and increased DC-CD8
+ T cell interactions, which resulted in increased CD8
+ T cell priming and activation in tumors [
30]. These results show that CXCR3 is critical for Treg cell accumulation and immune suppression in tumors. Therefore, depletion of CXCR3
+ Treg cells in tumors is expected to potentiate antitumor immunity. In our previous studies, we changed the isotype of mAbs into mouse IgG
2a to retain antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) [
31]. Since the subclass of Cx
3Mab-4 is rat IgG
1, it does not possess ADCC and CDC. Therefore, in further studies, the subclass of Cx
3Mab-4 will be converted into mouse IgG
2a to evaluate the effect of depletion of mCXCR3
+ Treg cells in preclinical mouse models.
Author Contributions
T.O., Y.I., and T.T. performed the experiments. M.K.K. and Y.K. designed the experiments. H.S. and M.K.K. analyzed the data. T.O. and Y.K. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Numbers: JP23ama121008 (to Y.K.), JP23am0401013 (to Y.K.), 23bm1123027h0001 (to Y.K.), and JP23ck0106730 (to Y.K.), and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant nos. 23K19494 (to T.O.), 21K20789 (to T.T.), 22K06995 (to H.S.), 21K07168 (to M.K.K.), and 22K07224 (to Y.K.).
Conflicts of Interest
The authors have no conflict of interest.
References
- Groom, J.R.; Luster, A.D. CXCR3 in T cell function. Exp Cell Res 2011, 317, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Metzemaekers, M.; Vanheule, V.; Janssens, R.; Struyf, S.; Proost, P. Overview of the Mechanisms that May Contribute to the Non-Redundant Activities of Interferon-Inducible CXC Chemokine Receptor 3 Ligands. Front Immunol 2017, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, C.; Mukai, T.; Yamaguchi, N.; Morimoto, Y.; Park, W.R.; Iwasaki, M.; Gao, P.; Ono, S.; Fujiwara, H.; Hamaoka, T. Induction of the chemokine receptor CXCR3 on TCR-stimulated T cells: dependence on the release from persistent TCR-triggering and requirement for IFN-gamma stimulation. Eur J Immunol 2002, 32, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.J.; Verdijk, P.; van der Raaij-Helmer, E.M.; Navis, M.; Hensbergen, P.J.; Leurs, R.; Tensen, C.P. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood 2003, 102, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Lacotte, S.; Brun, S.; Muller, S.; Dumortier, H. CXCR3, inflammation, and autoimmune diseases. Ann N Y Acad Sci 2009, 1173, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Szentes, V.; Gazdag, M.; Szokodi, I.; Dezsi, C.A. The Role of CXCR3 and Associated Chemokines in the Development of Atherosclerosis and During Myocardial Infarction. Front Immunol 2018, 9, 1932. [Google Scholar] [CrossRef]
- Baydi, Z.; Limami, Y.; Khalki, L.; Zaid, N.; Naya, A.; Mtairag, E.M.; Oudghiri, M.; Zaid, Y. An Update of Research Animal Models of Inflammatory Bowel Disease. ScientificWorldJournal 2021, 2021, 7479540. [Google Scholar] [CrossRef] [PubMed]
- Chami, B.; Yeung, A.W.; van Vreden, C.; King, N.J.; Bao, S. The role of CXCR3 in DSS-induced colitis. PLoS One 2014, 9, e101622. [Google Scholar] [CrossRef]
- Gallage, S.; Avila, J.E.B.; Ramadori, P.; Focaccia, E.; Rahbari, M.; Ali, A.; Malek, N.P.; Anstee, Q.M.; Heikenwalder, M. A researcher’s guide to preclinical mouse NASH models. Nat Metab 2022, 4, 1632–1649. [Google Scholar] [CrossRef]
- Caplazi, P.; Baca, M.; Barck, K.; Carano, R.A.; DeVoss, J.; Lee, W.P.; Bolon, B.; Diehl, L. Mouse Models of Rheumatoid Arthritis. Vet Pathol 2015, 52, 819–826. [Google Scholar] [CrossRef]
- McGaha, T.L.; Madaio, M.P. Lupus Nephritis: Animal Modeling of a Complex Disease Syndrome Pathology. Drug Discov Today Dis Models 2014, 11, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Tanaka, T.; Sano, M.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 3 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 107–112. [Google Scholar] [CrossRef]
- Tanaka, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Sano, M.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 8 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kaneko, M.; Neyazaki, M.; Nogi, T.; Kato, Y.; Takagi, J. PA tag: a versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr Purif 2014, 95, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, S.; Garrido-Martin, E.M.; Harris, R.J.; Bateman, A.C.; Collins, J.E.; Cummings, J.R.F.; Sanchez-Elsner, T. Human Intestinal Macrophages Are Involved in the Pathology of Both Ulcerative Colitis and Crohn Disease. Inflamm Bowel Dis 2021, 27, 1641–1652. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Murphy, E.A.; Price, R.L.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine 2016, 77, 44–49. [Google Scholar] [CrossRef]
- Singh, U.P.; Venkataraman, C.; Singh, R.; Lillard, J.W., Jr. CXCR3 axis: role in inflammatory bowel disease and its therapeutic implication. Endocr Metab Immune Disord Drug Targets 2007, 7, 111–123. [Google Scholar] [CrossRef]
- Danese, S.; Vuitton, L.; Peyrin-Biroulet, L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol 2015, 12, 537–545. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Colombel, J.F.; Ghosh, S.; Sands, B.E.; Dryden, G.; Hebuterne, X.; Leong, R.W.; Bressler, B.; Ullman, T.; Lakatos, P.L.; et al. Eldelumab [Anti-IP-10] Induction Therapy for Ulcerative Colitis: A Randomised, Placebo-Controlled, Phase 2b Study. J Crohns Colitis 2016, 10, 418–428. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Rutgeerts, P.; Colombel, J.F.; Ghosh, S.; Petryka, R.; Sands, B.E.; Mitra, P.; Luo, A. Eldelumab [anti-interferon-gamma-inducible protein-10 antibody] Induction Therapy for Active Crohn’s Disease: a Randomised, Double-blind, Placebo-controlled Phase IIa Study. J Crohns Colitis 2017, 11, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Suzuki, H.; Asano, T.; Li, G.; Nanamiya, R.; Tateyama, N.; Isoda, Y.; Okada, Y.; Kobayashi, H.; Yoshikawa, T.; et al. Epitope Mapping of an Anti-Mouse CCR2 Monoclonal Antibody (C(2)Mab-6) Using Enzyme-Linked Immunosorbent Assay. Monoclon Antib Immunodiagn Immunother 2022, 41, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Tateyama, N.; Asano, T.; Suzuki, H.; Li, G.; Yoshikawa, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Epitope Mapping of Anti-Mouse CCR3 Monoclonal Antibodies Using Flow Cytometry. Antibodies (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Tateyama, N.; Asano, T.; Tanaka, T.; Isoda, Y.; Okada, Y.; Kobayashi, H.; Li, G.; Nanamiya, R.; Yoshikawa, T.; Kaneko, M.K.; et al. Epitope Mapping of Anti-Mouse CCR3 Monoclonal Antibodies (C(3)Mab-6 and C(3)Mab-7). Monoclon Antib Immunodiagn Immunother 2023, 42, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tawara, M.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Identification of the Binding Epitope of an Anti-Mouse CCR6 Monoclonal Antibody (C(6)Mab-13) Using 1× Alanine Scanning. Antibodies (Basel) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Asano, T.; Tanaka, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Determination of the Binding Epitope of an Anti-Mouse CCR9 Monoclonal Antibody (C(9)Mab-24) Using the 1× Alanine and 2× Alanine-Substitution Method. Antibodies (Basel) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Isoda, Y.; Tanaka, T.; Suzuki, H.; Asano, T.; Nakamura, T.; Yanaka, M.; Handa, S.; Komatsu, Y.; Okuno, S.; Takahashi, N.; et al. Epitope Mapping of an Anti-Mouse CXCR6 Monoclonal Antibody (Cx(6)Mab-1) Using the 2 × Alanine Scanning Method. Monoclon Antib Immunodiagn Immunother 2022, 41, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Isoda, Y.; Tanaka, T.; Suzuki, H.; Asano, T.; Yoshikawa, T.; Kitamura, K.; Kudo, Y.; Ejima, R.; Ozawa, K.; Kaneko, M.K.; et al. Epitope Mapping Using the Cell-Based 2 × Alanine Substitution Method About the Anti-mouse CXCR6 Monoclonal Antibody, Cx(6)Mab-1. Monoclon Antib Immunodiagn Immunother 2023, 42, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.; Tanaka, A.; Sakaguchi, S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell 2023, 41, 450–465. [Google Scholar] [CrossRef]
- Moreno Ayala, M.A.; Campbell, T.F.; Zhang, C.; Dahan, N.; Bockman, A.; Prakash, V.; Feng, L.; Sher, T.; DuPage, M. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8(+) T cell antitumor immunity. Immunity 2023, 56, 1613–1630.e1615. [Google Scholar] [CrossRef]
- Li, G.; Suzuki, H.; Ohishi, T.; Asano, T.; Tanaka, T.; Yanaka, M.; Nakamura, T.; Yoshikawa, T.; Kawada, M.; Kaneko, M.K.; et al. Antitumor activities of a defucosylated anti-EpCAM monoclonal antibody in colorectal carcinoma xenograft models. Int J Mol Med 2023, 51. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).