1. Introduction
The Renal arteries originate directly from the abdominal aorta and the exact level of origin may vary among individual dogs but is most often found between the second and third lumbar vertebrae [
1]. Further the renal artery branches and gives rise to 5 to 7 segmental arteries, with collateral arteries for delivering oxygenated blood to various parts of the renal tissue. Interlobar arteries pass along the wall of the calyx, and pyramids, these arteries serve to supply the renal cortex and medulla. It is characterized by a complex network of blood vessels that ensure proper blood supply to the nephrons, which are the functional units of the kidneys[
1,
2].
Chronic Kidney disease (CKD) is an irreversible structural or functional impairment of one or both kidneys, occurring over 3 months. [
3,
4]. Even though Renal failure is considered a geriatric disease, it occurs in all age groups of canines and felines. The reported incidence of CKD in dogs varies from 0.05% to 3.74% in the UK and from 0.05% to 1.15% in the US. [
3]; O'Neill DG,
et al., 2013). The characteristic structural lesions in CKD vary among individuals, ranging from mild changes in one kidney to substantial nephron loss in both kidneys, depending on the spectrum of the disease. [
3,
4]. In renal failure, more than 70% of nephrons were destroyed and they were replaced by fibrous tissue. As it results in vasculature and structural changes were observed in renal failure.[
3]
During the late 70’s, the utilization of visceral angiograms in veterinary medicine gained more attention for diagnosis similar to humn medicine. [
5,
6,
7] In recent years, the angiogram techniques in veterinary medicine for diagnosis were shifted towards renal rather than cardiac and greater blood vessels. [
7,
8]. Renal radiographs were evaluated through three methods such as abdominal radiograph, contrast urography and intravenous renal angiogram. The abdominal plain radiograph was useful for the evaluation of morphological alteration of the kidney. Whereas contrast radiograph is essential in excretory urography and intravenous pyelogram for evaluation of vasculature. [
5,
9,
10,
11,
12]
In human medicine, the renal angiogram was utilized as an important feature for the evaluation of dynamic renal vasculature as opposed to the inherent limitation of biopsy and laparoscopic visualization [
7,
8,
12,
13]. This method is more suitable for evaluating the nephrographic stage of renal diseases, while poor distinguish in cortico-medullary junction [
7].
Renal angiogram is an interventional procedure used to visualize the vascular pattern and parenchymal architecture in the kidney and to diagnose renal diseases ([
6,
8,
14]. The renal angiogram is categorized into three types such as non-selective, semi-selective and selective methods. Among these methods, the selective is more precise and avoids superimposition of other abdominal vasculature [
8,
15]). This angiogram consists of three phases arterial, venous and nephrographic phase. [
7,
16]. Using an angiogram, the pathological conditions involving the blood vessels (arterial, arteriolar) nephrosclerosis, stenosis, vasculitis, and hypertensive vascular diseases were diagnosed.[
17]
Renal artery stenosis (RAS) is the narrowing of the renal arteries that supply blood to the kidneys. Renal artery stenosis is caused by fibromuscular dysplasia and arteriosclerosis. This condition can lead to reduced blood flow to the kidneys and can cause hypertension. Renal angiogram is often used to confirm the diagnosis of RAS and to assess the severity of the stenosis. Treatment options may include angioplasty with or without stent placement ([
18]
In few cases of unexplained haematuria, a renal angiogram can be helpful in identifying vascular abnormalities or sources of bleeding within the kidneys [
19,
20,
21]. Thus the renal angiogram has many benefits in the diagnosis of renal diseases.[
22]
In chronic kidney diseases, there will be a structural and functional loss of nephrons in single or both kidneys.[
3] Halpern [
17] reported that the renal changes were observed at both microscopic and macroscopic changes along with vascular alterations. This study has been aimed to determine the location of origin of renal arteries, vascular alteration or changes in renal and standardization of renal angiogram procedure in chronic kidney disease.
2. Materials and Methods
This study involved six dogs diagnosed with chronic kidney disease which underwent renal angiography between May and October 2023. Among these dogs, two had stage III chronic kidney disease, while the remaining dogs had stage II which was classified based on their serum creatinine level. further sub-classified based on proteinuria and blood pressure. Among the six four had proteinuria, two had mild hypertension, one had moderate and one had severe hypertension. Renal angiograms were performed in the client-owned dogs. Before the procedure, comprehensive information was provided to the clients, including potential complications of the renal angiographic procedures and an informed consent was obtained from all clients.
Patient preparation
The patients underwent thorough examination, including physical assessments, haematological tests, serum biochemistry, and systolic blood pressure measurements. The dogs with CKD were selected randomly, who could fit for anaesthesia with minimal risk. The choice of anaesthesia protocol was based on each patient's health condition. Injectable anaesthetic agents, such as butorphanol, xylazine, diazepam, and propofol, were used for induction, while the inhalant anaesthetic agent isoflurane was administered for maintenance.
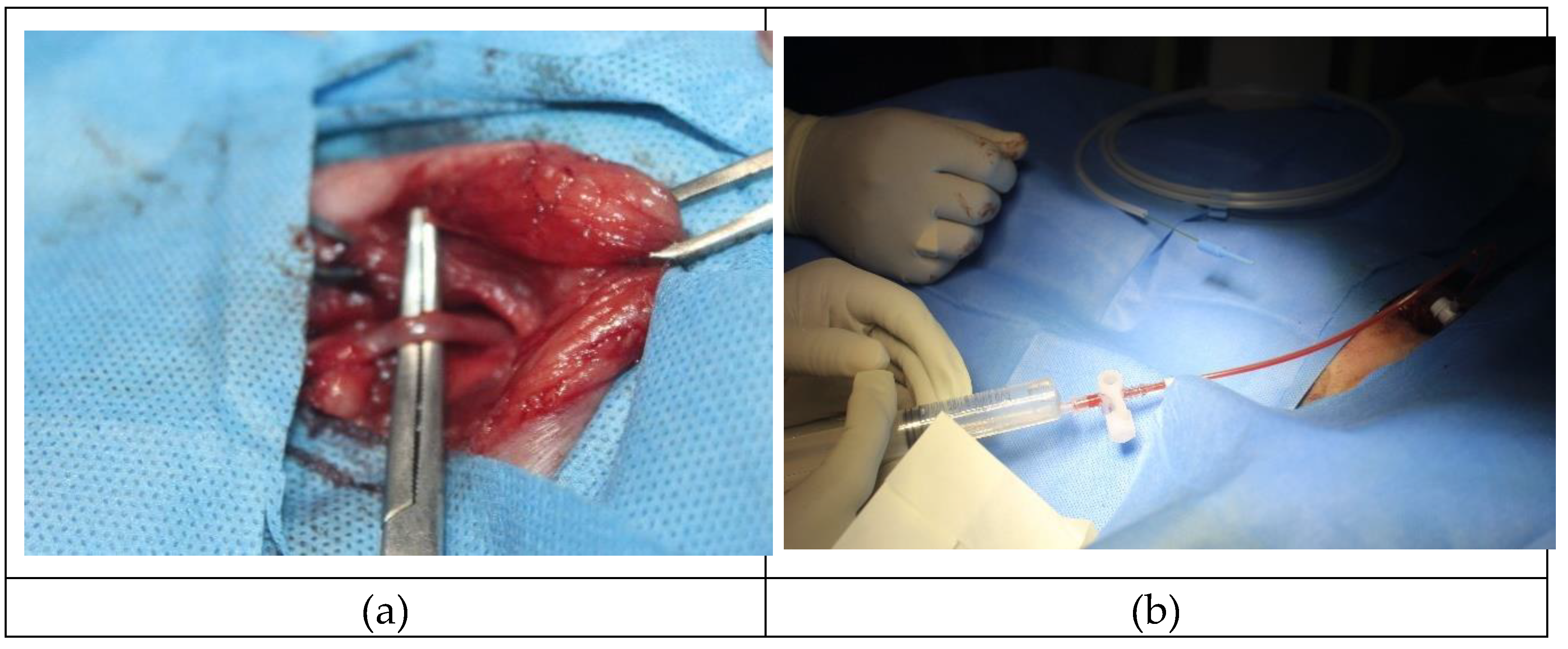
The selected animals were sedated, and the surgical site was meticulously prepared for femoral access. (
Figure 1a)During the procedure, the animals were positioned in a Dorso-ventral position to prevent superimposition of abdominal organs and enhance visualization of renal vasculature and its architectures, as recommended[
23] Roughly a 4 cm incision was made on the medial aspect of the inguinal region, and the fascia and muscles were separated using a blunt dissection technique. Subsequently, the artery, vein, and nerves were carefully isolated, and the femoral artery was identified by its pulsation. A 18G percutaneous needle was then inserted into the artery, followed by the introduction of a 5french guide wire and a femoral sheath (Cook Company). The femoral sheath was secured to the adjacent muscle using absorbable suture material with PGA 2-0 (
Figure 2b). With the assistance of a hydrophilic guide wire (0.035", 504- 605X, Cook Company USA.), cobra C2 catheter was threaded, and an ionic contrast agent (Iohexol saline in a 50:50 ratio) was infused for the evaluation of renal arteries.[
24,
25]
SURGICAL SITE CARE
After the procedure, the femoral artery was sutured with absorbable suture material (PGA 4-0). Muscles were sutured individually with the PGA 2-0 as the ford interlocking and subcutis pattern were placed. The skin was sutured with non-absorbable suture (polyamide 2-0) with cross mattress pattern.
INTENSIVE MONITORING.
After the procedure, the patients were monitored intensively for 4 hours and all the vital parameters were recorded. Ringers Lactate solution was administered to correct the dehydration, antibiotics and opioid analgesic (butorphanol) were administered. Once the patients stabilised, they were discharged and subsequently monitored. The animals were medicated with Beta-lactam group antibiotics (Ampicillin and cloxacillin) and anti-oxidants like N-acetylcysteine were administered intravenously for one day and followed by per-os for 7days.
STATISTICAL ANALYSIS
Changes in serum biochemical data over the period in the renal angiogram in dogs were evaluated by repeated measures of ANOVA, using SPSS (SPSS 20.1 IBM,). Values were considered statistically significant at 𝑃 < 0.0 5 if the confidence interval did not contain 1.0.
3. Results
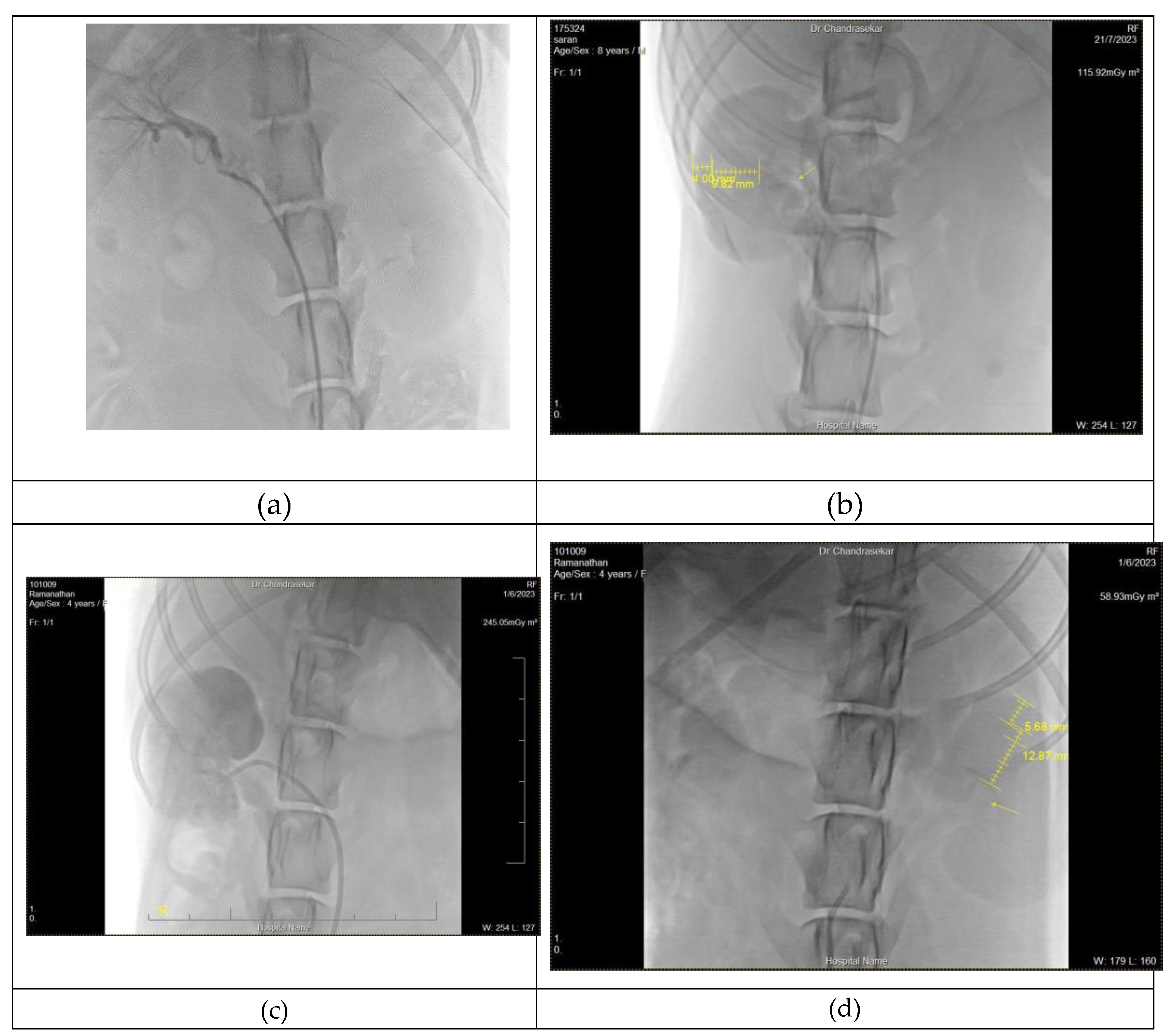
The primary objective of this study is to locate the renal arteries. Both Renal arteries arose from different positions such as the right originates between the lumbar vertebrae 1-3, and the left artery originates near to second and third lumbar vertebrae. During an angiogram, the right renal artery was found near the second lumbar vertebrae -intervertebral disc and the left renal artery originated between the second to third lumbar vertebrae. Among six cases, two cases with demarcation of renal borders and poor vascular access were observed. Thin attenuated renal arterial branches were visualized in both the cortex and medullary regions of three CKD dogs. In few cases shrunken arteries along with reduced renal perfusion were observed unilaterally, may indicate as single fibrosed kidney.
3.1. CASE MATERIAL
3.1.1. CORTEX-MEDULLARY RATIO(C:M ratio)
An eight-year-old boxer (case 1) and three-year-old mixed breed (case 2) on selective renal angiogram, the renal cortex medullary ratio (C:M ratio) as 0.40 with thinning of renal medulla. A C:M ratio of 0.45 was observed in six-year-old mixed breed (case4) was which was within the normal range. Under non selective Nephro-angiogram, four-year-old mixed breed (case3 ) had the cortex medullary ratio 0.42, The remaining cases on aortic–renal angiogram show the cortex medullary ratio as 0.38.
In (Case 5 ) and (case 6 ) the mean of cortex medullary ratio of the left kidney was observed as 0.42 (ranging from 0.37 to 0.44) and the right kidney C: M ratio as 0.39 (ranging between 0.36 to 0.45). Renal angiogram measurement of cortex and medullary ratio were tabulated form. (
Table 1.)
3.1.2. Vasculature and architectural changes
Selective renal angiogram of Boxer(case 1) and 3y mixed breed (case 2), revealed renal hypoplasia with attenuated thin renal arteries. In case 1 irregular architecture and lack of prominent renal arteries observed on the right kidney, whereas in case 2 renal hypoplasia with attenuated thin renal arteries found in both kidneys. (
Figure 2a) Based on findings, it could be either renal atrophy or renal hypoplasia . The angiographic findings of mixed 4y old dog (case 3), revealed reduction in the flow of segmental and interlobular arteries with a symmetrical reduction of parenchyma unilaterally.(
Figure 2b)
In selective angiogram of a six-year-old mixed breed(case 4), a stenosis of interlobular arteries and renal hypoplasia was observed on the cranial portion and atrophy(
Figure 2c) or hypoplasia of the caudal region of the left kidney.Also in the left kidney, the complete hypoplasia of renal parenchyma was observed. In remaining cases(case 5 & 6), there was a poor perfusion of renal arteries in both kidneys.In case 5 renal hypoplasia was observed in left kidney (fig 2d), while Renal hypoplasia with complete lack of associated interlobular arteries was observed in Case 6 From this study, the approach to the left kidney was easier compared to catheterization of the right kidney and in few cases vice-versa.
3.1.3. Serum Biochemical Changes
The serum creatinine and BUN were elevated and continuous medication with hydration therapy reduced the serum creatinine and BUN in the blood. After the renal angiogram, the BUN and Serum creatine levels showed an elevation within 48 hours as the Mean ± S.D. value was 110.61±3.39 and 6.94±0.65 with 95% confidence
. (Table 2)
3.1.4. Necropsy findings
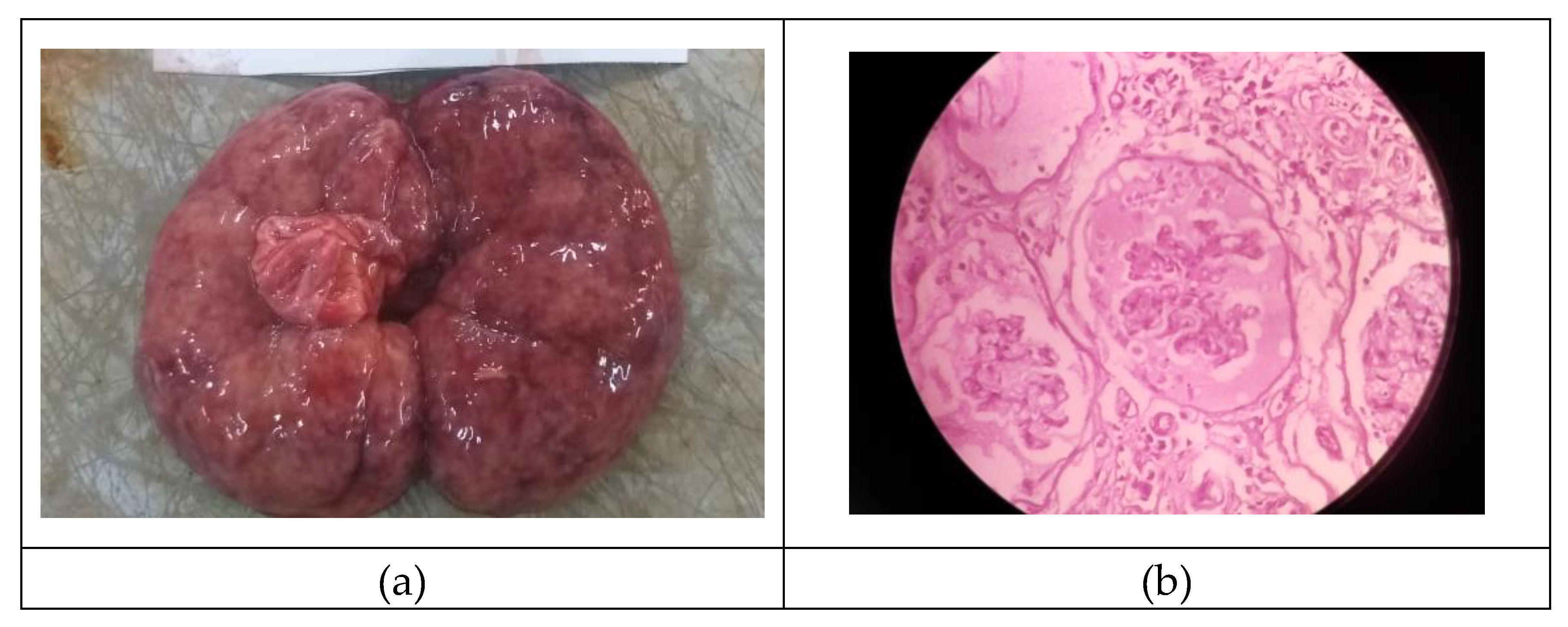
3y old Non-descript dog and terrier cross was died within 14 days of angiogram and they were necropsied. The necropsy revealed a small contracted kidney, pale grey in colour, shrunken cortex with grey and red mottling, and capsule peels with difficulty suggestive of non-suppurative nephritis possibly chronic interstitial nephritis. (
Figure 3a). Histopathology revealed severe tubular epithelial degeneration and necrosis replaced by diffuse proliferation of interstitial fibrous tissue along with mononuclear cell infiltration and some tubules showing cystic dilatation; distorted bowman’s capsule with eosinophilic exudate with loss of glomeruli tuft. (
Figure 3b).
Presence of exfoliated cells in bowman’s space with periglomerular fibrosis and lymphocytic infiltration. Some glomeruli showed atrophy with increased bowman’s space. Necrotic tubular epithelium with cast formation were also visualized.
3.1.5. Complications
Complications were observed in 3 of 6 renal angiogram procedures. In the present study, haematoma on the surgical site was the major complication of angiogram found in 3 animals. anaesthetic complications like hypothermia and muscle tremor were observed in two cases. Excessive salivation was observed in one-third of the study population. The most common complication of contrast agents as elevated serum creatinine levels were observed within 48hours of renal angiogram procedure in all dogs may indicate contrast-induced nephropathy
3.2. Figures, Tables and Schemes
All figures and tables should be cited in the main text as
Figure 1,
Table 1, etc.
4. Discussion
During renal angiogram, the following parameters were studied such as cortex and medullary ratio, appearance and filling of segmental and interlobular arteries, and renal parenchymal evaluation in renal failure.[
8,
17].
Fluoroscopy procedure.
Femoral artery catheterization was performed with a 5French catheter. 5 French Catheter and femoral sheath, which was found ideal for all breeds and size of the animal. Among the various renal Angio catheters available, this entire study was performed using the Cobra 2 catheter (Cook Company) stood out as an ideal and preferred choice for renal and cardiac angiograms.[
12,
19,
24]
Findings
The right renal artery typically originates near the second lumbar vertebra, while the left renal artery arises between the second and third lumbar intervertebral spaces. It is important to note that the exact origins of these arteries can vary among different breeds and sizes of animals were concurred with finding of Kinacid[
16]
Angiogram findings of case I revealed a slower filling with reduced number of interlobular arteries, it was similar to renal hypoplasia which may be a glomerular nephritis, correlated with Mena [
26]findings who observed renal changes in human medicine. Whereas, renal hypoplasia and atrophy were observed unilaterally in case 2 and 3. These dogs exhibits CKD at stage III clinical signs. In case 2, renal hypoplasia was restricted to cranial part of right kidney, while in case 3 renal atrophy was observed in left kidney. Khatami [
16]and Mena [
26] also correlated the cortical hypoplasia and thinning of cortical area in histopathological as typical lesion of CKD.
Interestingly, slender interlobular arteries with slow flow rate in left kidney was observed in case 4 and 5. similar to the findings of Davis et al and [
7].
During the angiogram, a terrier cross (case 6) exhibited unilateral(Left kidney) complete renal hypoplasia and a lack of associated interlobular arteries. Subsequent histopathological staining of the kidney revealed diffuse, dense fibrous tissue scarring. The histopathological diagnosis confirmed renal cortical atrophy and glomerular nephritis. ([
7]
In cases of chronic kidney disease, the mean cortex-to-medullary (C: M) ratio was determined to be 0.41, with a range between 0.39 and 0.41. This indicates that the C: M ratio is positioned towards the lower end of the range generally considered normal for kidneys. In Barber's study, the mean C: M ratio in healthy kidneys was reported as 0.53, with a range spanning from 0.40 to 0.69. It is noteworthy that both hypoplasia and atrophy conditions exhibited a symmetrical reduction in renal parenchyma. As a result, the cortex-medullary ratio remained within the normal range, as noted by Khademi.,1974.
Complications.
Enumerate of complications were reported in human medicine on angiogram procedures such as allergic reactions, contrast-induced nephropathy, hematoma, thrombosis, embolism and blood vessel injury [
27,
28,
29]. In the present study, haematoma on the surgical site was the major complication of angiogram found in 3 animals, which were medicated with anti-inflammatory and enzymatic medication for wound healing. As delaying in wound healing which may end in septic wounds. The adverse effects of contrast medium and anaesthetic risks were observed in half of the study population. As anaesthetic complication, hypothermia was observed in two cases, which had muscle tremor as a homeostatic mechanism.
Excessive salivation with no oral ulcerations were observed in one third of the study population. ptyalism may occurred to reduce the acidity condition, which was associated with contrast induced nephropathy [
30,
31].
Elevated serum creatinine and BUN was observed within 48 hours of angiogram in study population. The animal with well hydrated status shows least elevation of serum creatinine than the dehydrated and animal with normal hydration which agreement with Merten
et al. 2004 findings and loop diuretics like Frusemide were administered intra-venously to reduce the incidences. The exact mechanism of contrast induced nephropathy is unknown. Faucon [
25] stated that it may due to increased diuretic effect and sticking of contrast agents in nephrons lead to nephritis.
To restore hydration and reduce adverse effects, Normal saline [
32] was administered at 1ml/kg per hour and antioxidant N-acetylcysteine as a bolus and CRI, and frusemide[
32,
33,
34] as a bolus through intravenous for 6 hours after the procedure [
32,
34,
35]
Many other pharmacological agents including ascorbic acid, calcium channel blockers, furosemide, prostaglandin analogs, endothelin agonists, adenosine antagonists, L-arginine, hypertonic mannitol, dopamine, and theophylline[
33,
36,
37]. None of these treatments seem to be effective in the prevention of ICM-induced renal toxicity. As there is no specific treatment for the adverse effects and contrast induced nephropathy condition, to overcome these adverse effects, either restoration of hydration status and increased excretion or haemodialysis removes the contrast medium.
5. Conclusions
The angiogram was performed in dogs with chronic kidney diseases to study the renal vasculature and architectural changes in various kidney diseases. Both the renal artery and femoral artery vary in their size to each individual. In an angiogram, renal hypoplasia and reduction in interlobular artery were found more common in renal failure condition than renal atrophy. The patient with renal hypoplasia shows a slow filling in the interlobular artery than normal kidney. The renal atrophy was found unilaterally in patient with stage III renal diseases. Renal angiogram helps to differentiate the unsuspected case of renal failure from various renal diseases. In medicine, localized renal scarring is usually diagnosed at necropsy while other techniques like laparoscopic examinations and biopsies have their own limitations. It is difficult to ascertain the aetiology of renal scarring. The angiogram can be visualized better at selective magnification and the rate, volume of contrast agent infused into artery. The iodinated contrast agents such as Iohexol was used as common medium for angiogram. These ionic contrast agents produce more adverse effect such as contrast induced nephropathy, allergic reactions, thrombosis and hematoma. As these agents may remain in the renal tubules and also produces nephron damage. These adverse effects can be prevented by several techniques such as hydration of the patients, administration of antioxidants, and diuretics. iso-osmolarity contrast agents were least toxic than the low osmolarity contrast medium.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
In this article, the corresponding author, Kishorekumar Arumugam, conducted the complete clinical examination, angiogram procedure, and postoperative follow-ups. The co-corresponding authors, Chandrasekar M, Bharanidharan G R, and Giridharan S, assisted in the angiogram. Anesthesia was administered and monitored by Pushkin Raj, and the research was funded by Kavitha S.
Funding
This research was funded by TANUVAS, grant number S.C.No.1114.
Institutional Review Board Statement
There was IRBS approved from Directorate of clinics, MVC, TANUVAS.
Informed Consent Statement
The procedure was performed in client owned animals with chronic kidney diseases. A detailed information about the procedure, aim of this study and possible complication were informed to the owners. With their full consent the procedure was performed.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interest.”.
References
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of veterinary anatomy-E-Book; Elsevier Health Sciences, 2009. [Google Scholar]
- Evans, H.E.; De Lahunta, A. Guide to the Dissection of the Dog-E-Book; Elsevier Health Sciences, 2016. [Google Scholar]
- Polzin, D.J. Chronic kidney disease in small animals. Veterinary Clinics: Small Animal Practice 2011, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Bartges, J.W. Chronic kidney disease in dogs and cats. Veterinary Clinics: Small Animal Practice 2012, 42, 669–692. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.; Lawson, T.; Korobkin, M.; Moss, A. Selective Abdominal Angiography in the Dog 1. Veterinary Radiology 1973, 14, 72–80. [Google Scholar] [CrossRef]
- Culp, W.T.; Mayhew, P.D.; Pascoe, P.J.; Zwingenberger, A. Angiographic anatomy of the major abdominal arterial blood supply in the dog. Veterinary Radiology & Ultrasound 2015, 56, 474–485. [Google Scholar]
- BARBER, D.L. Renal Angiography in Veterinary Medicine 1. Veterinary Radiology 1975, 16, 187–204. [Google Scholar] [CrossRef]
- Abrams, H.L. Abrams' angiography: interventional radiology; Lippincott Williams & Wilkins, 2006. [Google Scholar]
- LORD, R.S.; HAYES, J.M. Renovascular reconstruction in hypertension. Medical Journal of Australia 1975, 1, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.A.; Low, D.G.; Finco, D.R. Canine and feline urology; 1972. [Google Scholar]
- Ranganath, L.; David, W. Non-selective renal angiography (aortography) in dogs. Indian Journal of Veterinary Surgery 2000, 21, 25–27. [Google Scholar]
- Rademacher, N. Diagnostic imaging of the urinary tract. Veterinary Clinics: Small Animal Practice 2019, 49, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Abrams, H.L. Quantitative derivates of renal radiologic studies: An overview. Investigative Radiology 1972, 7, 240–279. [Google Scholar] [CrossRef]
- FOSTER, R.S.; SHUFORD, W.H.; WEENS, H.S. Selective renal arteriography in medical diseases of the kidney. American Journal of Roentgenology 1965, 95, 291–308. [Google Scholar] [CrossRef]
- Obrez, I.; Abrams, H.L. Temporary occlusion of the renal artery: effects and significance. Radiology 1972, 104, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, O.W. Renal angiography. (No Title) 1966.
- HALPERN, M. Angiography in chronic renal disease and renal failure. Radiologic Clinics of North America 1972, 10, 467–494. [Google Scholar] [CrossRef] [PubMed]
- Willicombe, M.; Sandhu, B.; Brookes, P.; Gedroyc, W.; Hakim, N.; Hamady, M.; Hill, P.; McLean, A.; Moser, S.; Papalois, V. Postanastomotic transplant renal artery stenosis: association with de novo class II donor-specific antibodies. American Journal of Transplantation 2014, 14, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Berent, A. Interventional treatment of idiopathic renal hematuria. Veterinary Image-Guided Interventions 2015, 301–308. [Google Scholar]
- MISHINA, M.; WATANABE, T.; YUGETA, N.; MAEDA, H.; FUJII, K.; WAKAO, Y.; TAKAHASHI, M.; YAMAMURA, H. Idiopathic renal hematuria in a dog; the usefulness of a method of partial occlusion of the renal artery. Journal of veterinary medical science 1997, 59, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Misra, S. Endovascular treatment of renal artery stenosis. Renal Vascular Disease 2014, 317–323. [Google Scholar]
- Choi, J.; Yoon, J. Primary pyonephrosis in a young dog. Journal of Small Animal Practice 2012, 5, 304–304. [Google Scholar] [CrossRef] [PubMed]
- Lindell, S.; Olin, T. Catheterization of the renal arteries in dogs and cats. Acta Physiologica Scandinavica 1957, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Weisse, C. Intra-Arterial Chemotherapy Infusion. Veterinary Image-Guided Interventions 2015, 389–397. [Google Scholar]
- Faucon, A.-L.; Bobrie, G.; Clément, O. Nephrotoxicity of iodinated contrast media: From pathophysiology to prevention strategies. European Journal of Radiology 2019, 116, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Bookstein, J.J.; Gikas, P.W. Angiographic diagnosis of renal parenchymal disease: chronic glomerulonephritis, chronic pyelonephritis, and arteriolonephrosclerosis. Radiology 1973, 108, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; Patel, M.V.; Masrani, A.; Chong, B.; Osman, M.; Tasse, J.; Soni, J.; Turba, U.C.; Arslan, B. Assessing intra-arterial complications of planning and treatment angiograms for Y-90 radioembolization. Cardiovascular and interventional radiology 2017, 40, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Crudu, V.; Blankenship, J.; Berger, P.; Scott, T.; Skelding, K. Complications related to access site after percutaneous coronary interventions: are the adverse events underreported? Catheterization and Cardiovascular Interventions 2011, 77, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Yamaguchi, K.; Kozuka, T.; Takashima, T.; Seez, P.; Matsuura, K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990, 175, 621–628. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A. Contrast-induced acute kidney injury. Journal of the American College of Cardiology 2008, 51, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Khambatta, S.; Jazrawi, A. Minimizing the renal toxicity of iodinated contrast. Am Heart Assoc: 2011; Vol. 124, pp 1210-1211.
- Martin-Paredero, V.; Dixon, S.M.; Baker, J.D.; Takiff, H.; Gomes, A.S.; Busuttil, R.W.; Moore, W.S. Risk of renal failure after major angiography. Archives of Surgery 1983, 118, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Tao, A.; Bai, Y.; Deng, Y.; Chen, G. Effectiveness of N-acetylcysteine for the prevention of contrast-induced nephropathy: a systematic review and meta-analysis of randomized controlled trials. Journal of the American Heart Association 2016, 5, e003968. [Google Scholar] [CrossRef] [PubMed]
- Allaqaband, S.; Tumuluri, R.; Malik, A.M.; Gupta, A.; Volkert, P.; Shalev, Y.; Bajwa, T.K. Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheterization and Cardiovascular Interventions 2002, 57, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Merten, G.J.; Burgess, W.P.; Gray, L.V.; Holleman, J.H.; Roush, T.S.; Kowalchuk, G.J.; Bersin, R.M.; Van Moore, A.; Simonton III, C.A.; Rittase, R.A. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. Jama 2004, 291, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.R.; Kjellstrand, C.M.; Tymchak, W.J.; Hervas-Malo, M.; Taylor, D.A.; Teo, K.K. Forced euvolemic diuresis with mannitol and furosemide for prevention of contrast-induced nephropathy in patients with CKD undergoing coronary angiography: a randomized controlled trial. American Journal of Kidney Diseases 2009, 54, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.; Gordon, P.; Manoukian, S.V.; Abbott, J.D.; Kereiakes, D.J.; Jeremias, A.; Kim, M.; Dauerman, H.L.; Investigators, B.T. Randomized trial of bicarbonate or saline study for the prevention of contrast-induced nephropathy in patients with CKD. Clinical journal of the American Society of Nephrology: CJASN 2015, 10, 1519. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).