1. Introduction
Intravitreal injections are among the most performed procedures in medicine. Their positive impact on preserving vision in retinal diseases such as neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME) and retinal vein occlusions (RVO) has been extensively demonstrated [
1,
2,
3,
4]. The widespread use of these injections has contributed to a reduction in blindness and low vision [
5,
6]. However, individual perspectives on treatment may differ from a population health standpoint, as they require continuous motivation over many years [
7,
8]. Consequently, non-persistence and non-adherence remain significant challenges that threaten the long-term maintenance of visual function [
9]. It is essential to understand the patients´ attitudes and commitment to treatment in order to explain and enhance treatment adherence [
10]. Previous publications have revealed substantial disparities in treatment practices between clinical studies and real-life scenarios. Notably, the injection frequency and treatment adherence are often much lower in the real-world-settings compared to clinical trials [
11,
12]. As a result, the patients’ perspective has garnered increased attention in recent years to gain a deeper understanding of their concerns, relevant barriers, and behavioral aspects.
Retinal diseases and comorbidities have a profound impact on the quality of life (QoL) [
13]. Since a significant portion of treated patients are elderly, this target group often suffers from other health problems and experiences daily limitations even before regular treatment visits and ocular discomfort introduce additional burdens for both patients and potential caregivers [
14]. Questionnaires that focus solely on QoL dimensions do not adequately capture the personal situations and treatment commitment of patients. Therefore, we have developed an exploratory tool aimed at comprehensively elucidating patients’ social, physical, and psychological conditions. This tool should aid in understanding the utilization of care by patients and in potentially identifying internal or external barriers and limitations to therapy.
2. Materials and Methods:
The exploratory questionnaire was implemented within the multi-centered ALBATROS data collection to monitor the situation of anti-VEGF-patients when receiving treatment for neovascular age-related macular degeneration (nAMD), diabetic macula edema (DME) or retinal vein occlusion (RVO) in Germany. The study design and clinical outcomes have been recently reported [
15]. One hundred and two study centers participated in the project throughout Germany. Patients were invited to join the data collection before commencing their therapy and were followed-up over the first twelve months of treatment. No specifications regarding anti-VEGF treatment or treatment frequency were given. Only patients without prior anti-VEGF treatment, aged ≥18 years and a written informed consent for participation were included in the study.
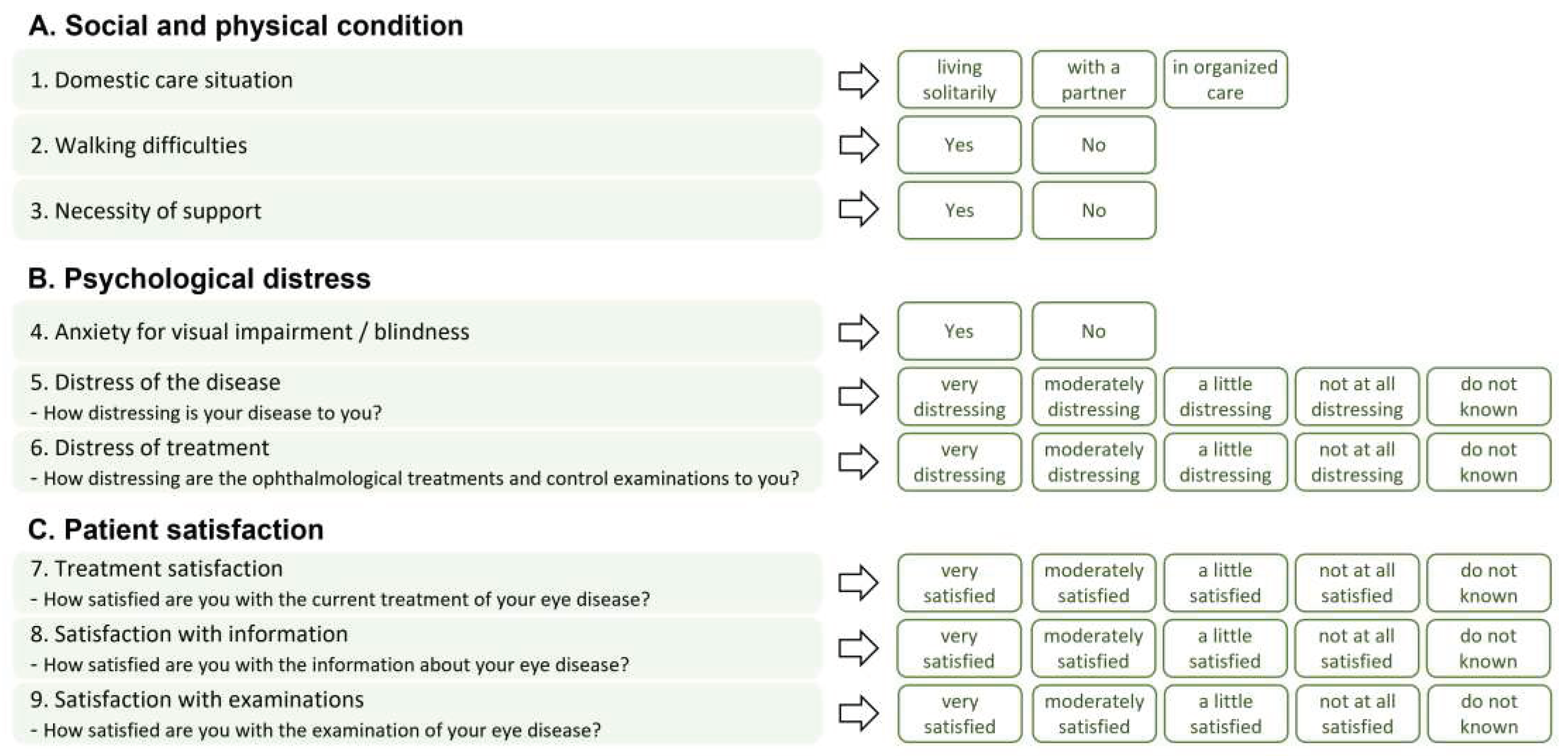
The questionnaire about the patients’ situation of Basic care and Patient satisfaction (in German: “
Fragebogen zur Basisversorgung und Patientenzufriedenheit”, BPZ-9) comprised nine questions in three different domains about social, physical, and psychological conditions. Patients’ distress and satisfaction with treatment were assessed using a five-point Likert-scale. The answers were collected at baseline and the final study visit (after twelve months). An overview of the questions and answer options is provided in
Figure 1.
For this analysis data from patients who completed the full 12-month observation period were included. Statistical analyses were descriptive with absolute and relative frequencies, tabulated using SAS Version 9.4 (SAS Institute Inc., Cary, North Carolina, United States of America).
3. Results:
In total 1,478 patients with a baseline and a 12-month visit were included in the completed analysis set (CAS) of the ALBATROS data collection. During the 12-month observation period, patients received on average (mean±SD) 6.1±3.2 anti-VEGF injections.
The mean(±SD) age of the study population was 74.5±10.9 years, with 54.9% female patients. Irrespective of sex, older patients experienced worse visual acuity (
Figure S1). Nearly two thirds of the study population (65.2%) were treated for nAMD, with 32.8 % receiving bilateral treatment (DME: 9.9%). Demographic parameters varied across different indications, e.g., patients diagnosed with nAMD were generally older and more likely to be female, while patients diagnosed with DME were younger and more likely to be male (
Table 1).
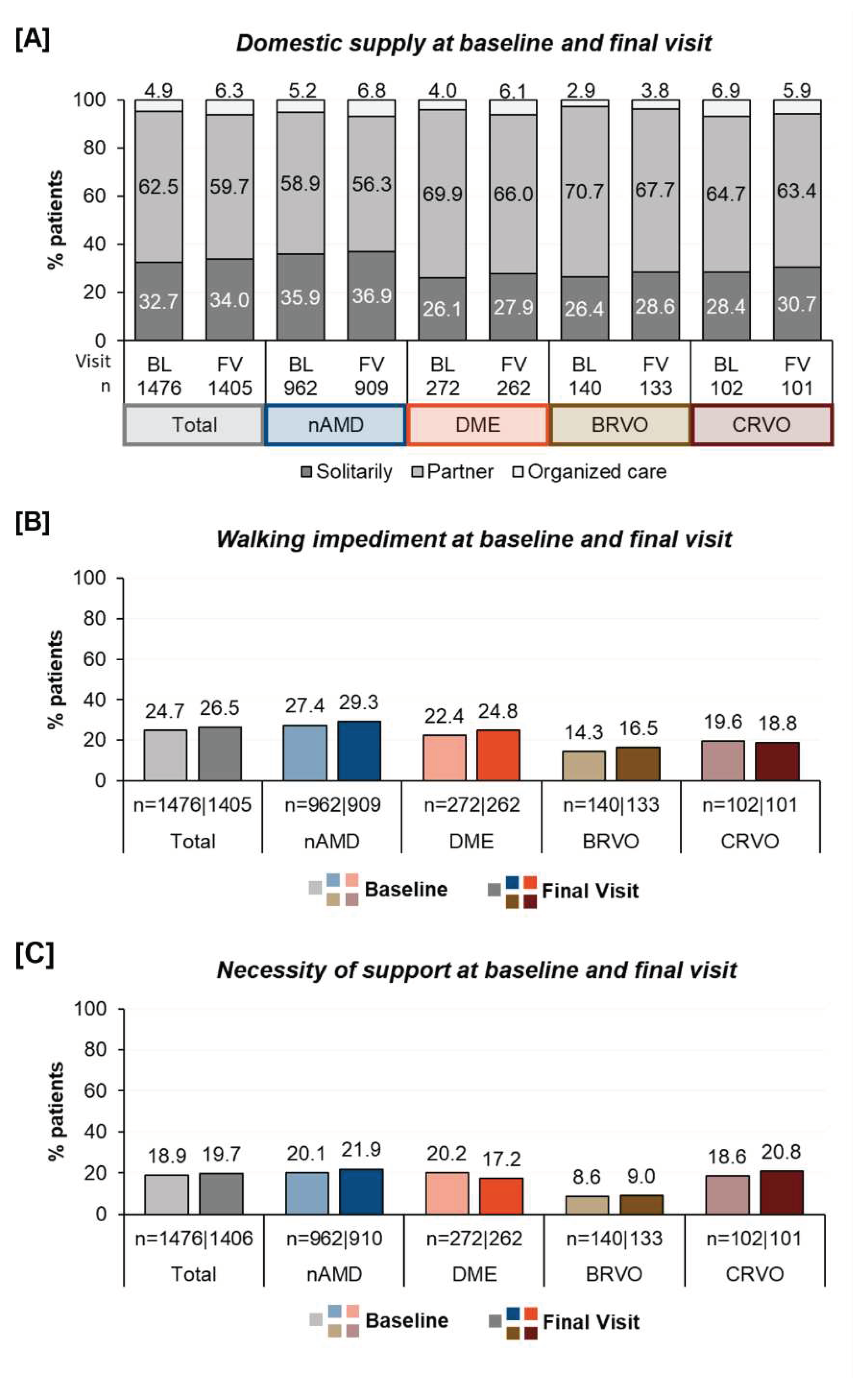
Social and physical condition: The living situation of patients varied significantly. At baseline, 4.9% of the patients lived in organized care, 62.5% lived with a partner and approximately one third (32.7%) lived alone. At baseline, 24.7% of the patients reported walking impairments and 18.9% reported dependency on an accompanying person. The percentages varied by indication as demonstrated in
Figure 2 and showed that nAMD patients more often suffered from walking difficulties and required more assistance. In contrast BRVO-patients were less often living in an organized care and reported a better walking situation and less need for accompany.
After twelve months of treatment, the proportion of individuals in a single household increased from 32.7% to 34.0% and the percentage of those in organized care rose from 4.9% to 6.3%. Walking difficulties slightly increased from 24.7% to 26.5% and the need for assistance during anti-VEGF-treatment from 18.9% to 19.7%. Fewer patients with DME were dependent of support than in the beginning of treatment (20.2% vs. 17.2%).
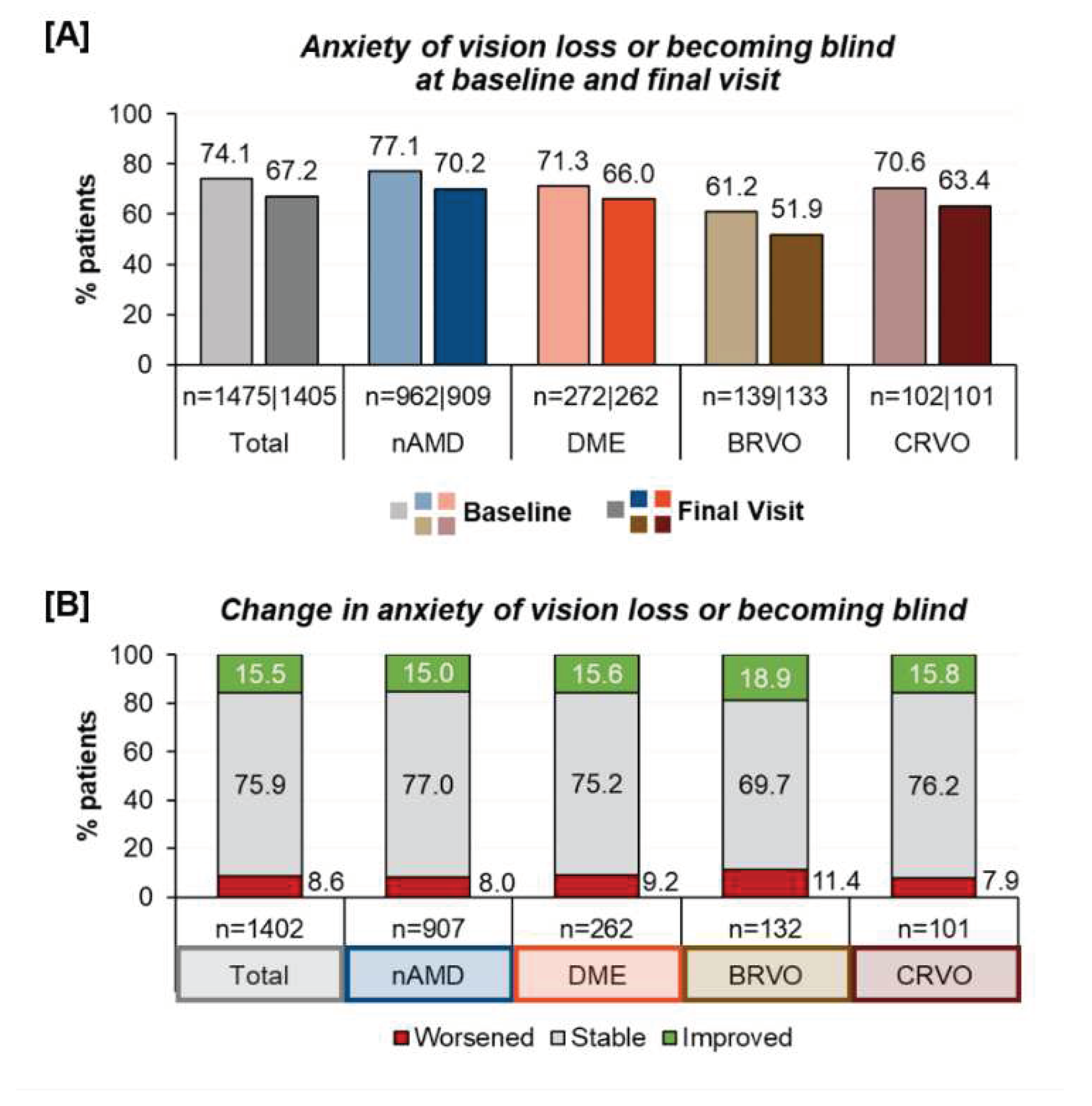
Psychological distress | Anxiety of vision loss: Prior to the first anti-VEGF therapy, most patients (74.1%) expressed anxiety about developing visual impairment and blindness. This concern remained present in 67.2% of the patients after twelve months. When examining different underlying indications, the fear for vision loss was less prominent in branch retinal vein occlusions (BRVO) and more prominent in nAMD. Over the course of one year of treatment, a reduced prevalence of anxiety was observed across all indications (
Figure 3). However, while 15.5% had less anxiety about vision loss, a small group (8.6 % of patients) reported being more anxious than prior to the anti-VEGF therapy.
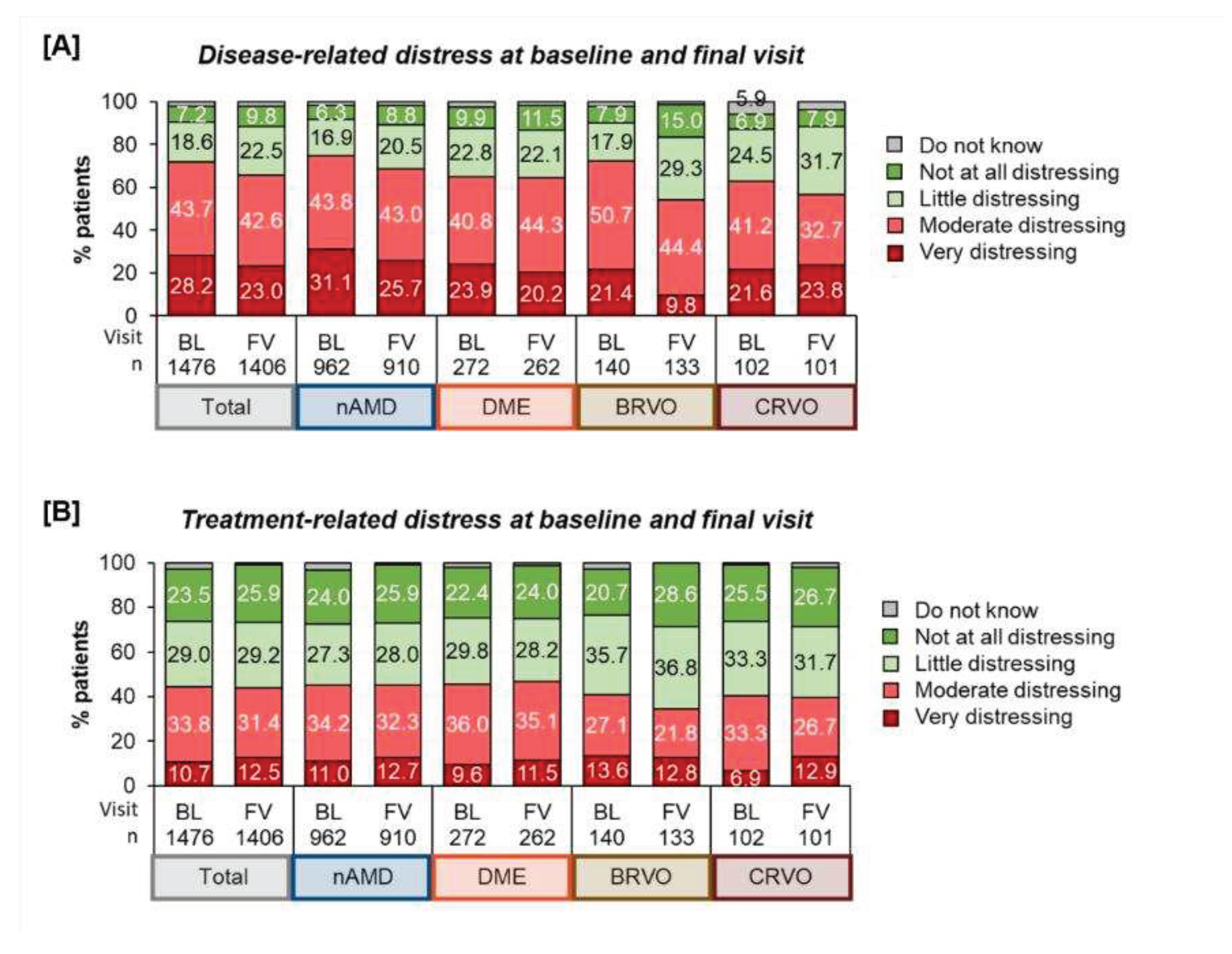
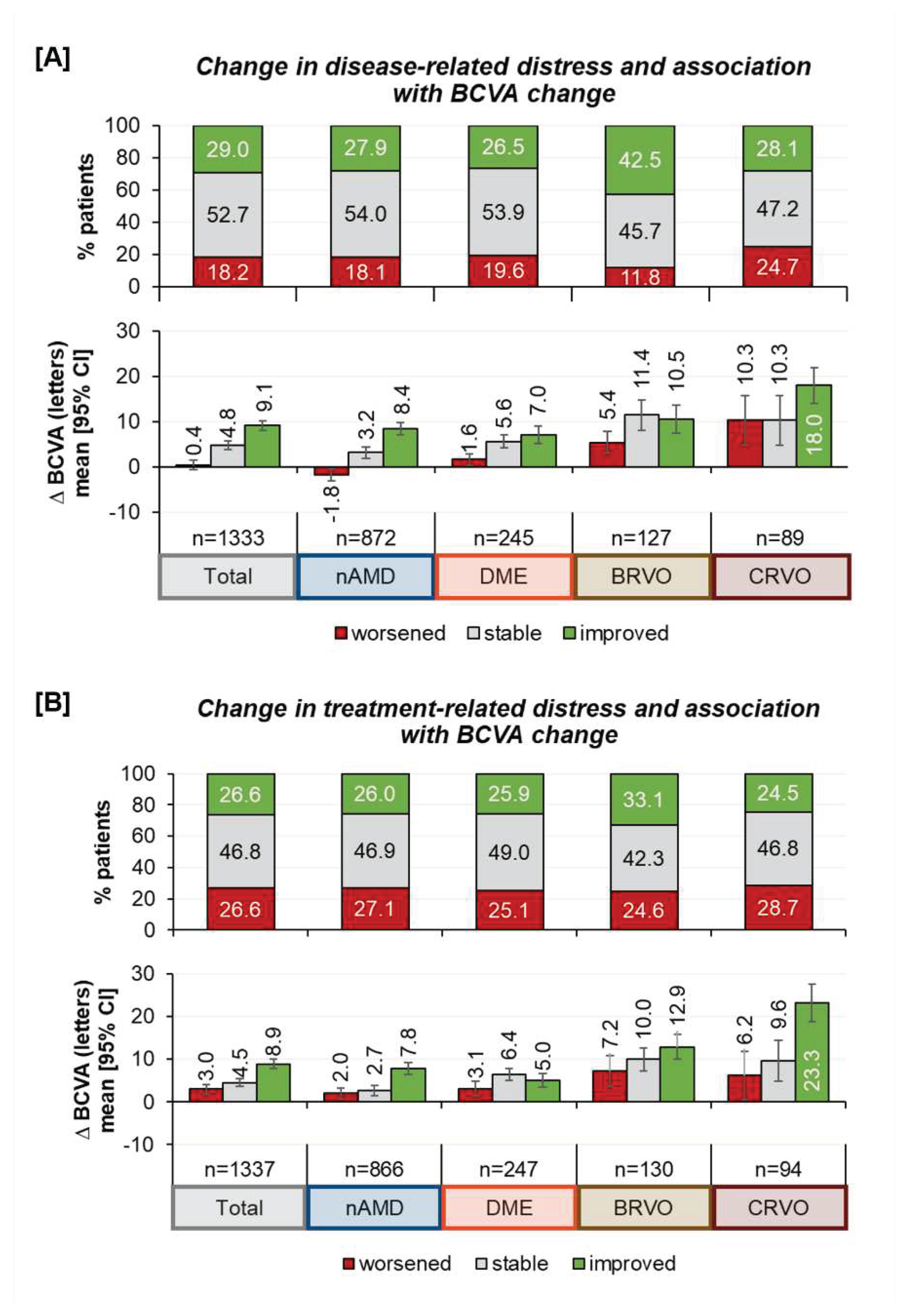
Psychological distress | Disease and treatment-related distress: At baseline, 71.9 % of patients perceived their disease as moderately to very distressing, while 25.8 % perceived it as little or not at all distressing. At the final visit, fewer patients reported disease-related distress, but it was still prevalent in nearly two thirds of the patients (65.6%). Regarding treatment distress, we found that a majority (52.5%) experienced it as little or not at all distressing as compared to 44.5% who valued treatment as moderately to very distressing. After twelve months the ratio had not changed substantially (55.1% - “little/not at all distressing” vs. 43.9% - “moderately/ very distressing”).
Comparing disease- and treatment-related distress, patients more frequently struggled with the disease itself than with treatment (
Figure 4).
Regarding differences in disease- and treatment-related distress at the beginning and after twelve months of treatment, we observe that about half of the patients reported similar emotional views. At least, more patients experienced a reduction of disease related distress than an increase in distress. The results differed by indication, showing that more BRVO patients experienced improvement than patients of other indications. However, 18.2 % of all patients reported more distress in dealing with the retinal disease, which was also less pronounced among those with BRVO.
Concerning treatment-related distress, about three out of four patients (73.4%) did not change their perception of treatment distress or even found it improved, with little variation among the different indications (
Figure 5, upper part of the figures).
Improvements in distress estimation were correlated with changes in the best corrected visual acuity (BCVA), as shown in
Figure 5 (lower part of the figures). A heat map analyzing the correlation of patients` disease and treatment distress perception, showed no clear correlation of disease and treatment distress, highlighting the value of separately assessing both disease and treatment distress (
Figure S2).
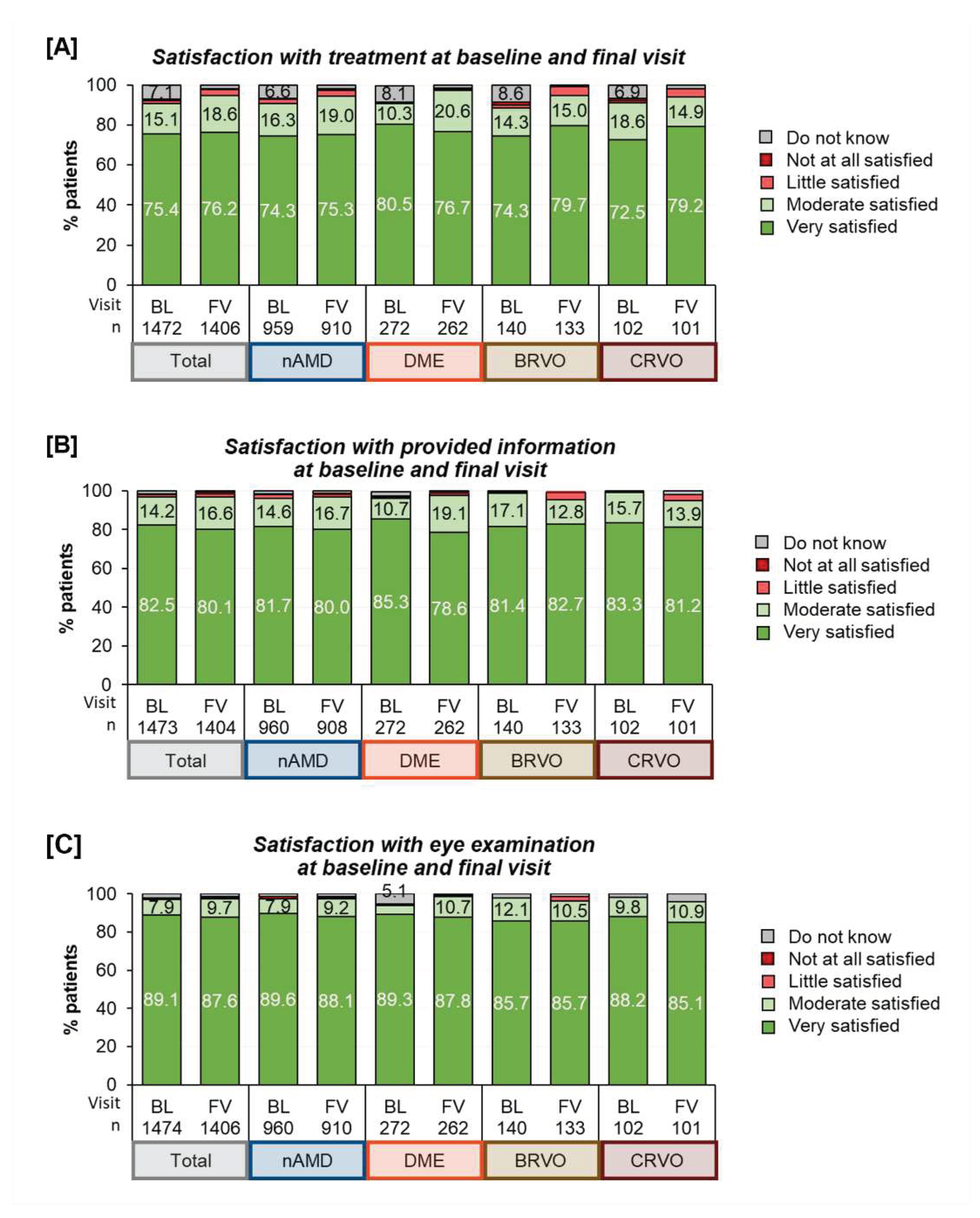
Patient Satisfaction: Most patients were generally highly satisfied regardless of the underlying indication. The satisfaction varied across different areas with the highest satisfaction seen for ‘control examinations’ (seven out of eight patients being “very satisfied”), followed by ‘information about the disease’ (four out of five being “very satisfied”) and ‘satisfaction with treatment’ (three of four being “very satisfied”), see
Figure 6. Over the course of twelve months, satisfaction remained generally stable for the patients under study observation.
4. Discussion
The findings from the ALBATROS study provide intriguing insights into the patients’ social, physical, and psychological conditions, when confronted with a retinal disease and intravitreal anti-VEGF treatment. The extensive size of this study cohort with data collected from more than one hundred study centers throughout Germany helped to capture a realistic picture. The broad categories hinted at the variety of practical issues and concerns patients face when receiving injection therapy.
The social backgrounds of anti-VEGF patients are naturally diverse, as reflected in the ALBATROS data, which represented the affected population [
16]. Although the vast majority was found to live in their own homes, attention should be given to difficulties or mobility restrictions. Roughly six percent of patients in our cohort resided in a domestic care situation. More than one-fifth of patients needed to be accompanied and even every forth patient experienced walking difficulties. These facts reveal that logistical issues and limitations may frequently hinder the daily practice of anti-VEGF treatment.
Previous studies have already demonstrated a high need of support. For instance, a survey among nAMD patients reported that even 82.1% of patients received assistance from a caregiver [
17]. In contrast to support in daily activities (57.8%), other studies described a higher rate (77%) of patients, attending medical appointments without assistance [
18]. This corroborates our findings that 18.9% of anti-VEGF-patients required an accompanying person. The accessibility of care should be closely monitored. Previous surveys suggest that means of transportation does not influence the dependency of affected individuals [
19]. Even in cities with a good and efficient public transport, visually impaired might not manage the injection visit without caregivers.
However, even more important than the issues of transport and mobility, the doctor-patient relationship proved to have a significant impact on patients [
20]. Accompanying persons were able to enhance adherence by overcoming barriers and increasing self-efficacy [
21].
The fact that patients experienced more distress due to their disease than the actual treatment underscores the high psychological burden of having a retinal disease. A review on the psychological impacts of neovascular macular degeneration described that up to 42% of patients showed signs of depression [
22]. Other studies reported a prevalence of depression among anti-VEGF-patients ranging between 20% and 26% [
23] and also among caregivers to AMD-patients, with 24.4% affected [
17]. Our findings emphasize the specific impact of anxiety for vision loss as a key psychological element, which was present in as much as 74.1% of patients at the beginning of their treatment. This high prevalence of anxiety for vision loss confirmed anxiety to be a psychological dimension with a lower threshold than depression, as described in the literature. Assessing anxiety can identify that there is also a fear of the injection itself during treatment and that efficacy of the treatment is the most important outcome for patients as previous studies pointed out [
24,
25]. Patients therefore declared their willingness to take certain risks or accept discomfort and inconveniences [
26,
27]. Although aversions and unconscious preferences cannot be ruled out, the acceptance of intravitreal therapy is generally very high [
28], supported by the very positive findings for treatment satisfaction observed in our study.
Our analysis has highlighted psychological and logistical dimensions of treatment. Literature has described treatment barriers for patients that can be categorized into four groups [
20]: tolerability, clinical factors, logistical parameters and human factors, which can all contribute to a low confidence in their therapy and its effects resulting in the decision to discontinue treatment. The experience of side effects was identified as the most important factor for non-adherence [
20]. Previous investigations demonstrated that 28.8% of anti-VEGF-patients were lost to follow up during treatment, defined as a discontinuation of therapy for at least six months [
29]. Other studies described that 32% of patients considered to interrupt their anti-VEGF therapy [
30] and that up to 50% of patients stopped treatment within 24 months [
31]. Possible reasons may be disbelief in the benefit of treatment or fear of the injection [
32]. The follow-up of this study might have been too short to access the full impact of longer treatment durations [
33].
Non-adherence is therefore a major issue in retinal care, found to be higher in DME than in nAMD and other indications [
32]. A key role in maintaining adherence was seen in the quality of the relationship between physician and patient [
32]. Practical recommendations include better monitoring of the patients’ treatment course to identify interruptions or non-adherence [
32] and actively assessing the patients' expectations regularly along the course of treatment [
32]. Even though our analysis focused primarily on those patients who adhered to their therapy, we do observe that psychological and logistical problems are very common already among these patients. We can assume that such issues are even more important for those, who have difficulties to commit to a continuous therapy [
34]. Future “real-world”- research should therefore focus even more on those patient groups who do not continue treatment and further investigate behavioral factors.
Our findings were limited because patients may have responded in an overly positive way and avoided being critical (social desirability bias). In real-world care practice satisfaction values as demonstrated here may be too high and practical barriers or comorbidities may be more prevalent among those who interrupted or even stopped treatment within the first year. Furthermore, our research questions were exploratory, and we cannot supply final information about the validity and reliability of our questionnaire at this point. Further methodological validation is needed to measure accuracy and consistency of our research questions. The introduction of validated questionnaires can complement established instruments of life-quality and patient-related outcome measures. Future research may also introduce possible means to measure commitment and adherence to ease the delivery of retinal care. As anti-VEGF-treatment remains one of the most performed medical procedures, even minor improvements can have a greater impact for the needs in the population.
5. Conclusions
In summary, having a retinal disease and undergoing a continuous anti-VEGF treatment was shown to be challenging for patients both logistically as well as psychologically. On the other hand, patients demonstrated high levels of satisfaction with their treatment. Understanding patients` needs and fears are prerequisites to help patients overcoming logistical and psychological barriers and may result in enhanced and maintained commitment, adherence, and treatment satisfaction. Our exploratory study questions may help to widen the understanding of the patients’ background, attitudes and behavior and may serve as a useful complement to other instruments of patient-related outcome measures.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Box-Whisker-Plot of worst eye visual acuity vs age group and sex at baseline. Note: Distribution of worst eye visual acuity (VA): Study eye=69.6%, Partner eye=30.4%. Horizontal lines show median, boxes indicate the interquartile range (IQR) with whiskers providing 1.5xIQR and black dots showing outlier values. Figure S2: Heat maps showing correlation of disease- and treatment distress at baseline [A], final visit [B] and change from baseline to final visit [C] for the overall population. Numbers indicate patients with a distinct combination of distress perception. Shadings show frequencies. The darker the color, the higher the frequency.
Author Contributions
Conceptualization, A.K.S., B.S., C.W., M.R., N.P.; Methodology, A.K.S, B.S., C.W., F.Z., M.R., N.P.; Software, n/a.; Validation, A.K.S, B.S., C.W., F.Z., M.R.; Formal Analysis, n/a; Investigation, A.K., A.K.S, C.W., T.H., F.Z. and N.P.; Resources, A.K., A.K.S, B.S., C.W., F.Z., M.R., T.H., N.P.; Data Curation, B.S., M.R.; Writing – Original Draft Preparation, C.W., A.K.S, F.Z.; Writing – Review & Editing, A.K.S, C.W., T.H., A.K., F.Z., N.P.; Visualization, A.K.S, B.S., F.Z.; Supervision, M.R., B.S., N.P.; Project Administration, B.S., M.R.; Funding Acquisition, M.R.
Funding
This research was funded by Novartis Pharma GmbH, Nuremberg, Germany.
Institutional Review Board Statement
ALBATROS: The study was conducted according to the guidelines of the Declaration of Helsinki. According to national law, the ALBATROS data collection did not require formal vote in favor by an independent ethics committee (IEC) but was reviewed by the IEC according to medical professional code of conduct of state Rhineland-Palatinate (EC State Medical Association of Rhineland-Palatinate) for the corresponding investigator.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to intellectual property reasons.
Acknowledgments
The authors would like to thank Markos Schulte (CROLLL GmbH) for medical writing support.
Conflicts of Interest
C.W. received research grants from Bayer Healthcare, Novartis Pharma GmbH and Santen | N.P. received financial support and grants from Allergan/Abbvie GmbH, Bayer AG, Ivantis (Alcon GmbH), Nicox S.A., Novartis GmbH and Santen GmbH | T.H. received research grants from Novartis Pharma GmbH | A.K. received grants from Bayer Healthcare and Novartis Pharma GmbH | B.S. and M.R. are employees of Novartis Pharma GmbH | F.Z. received financial support and grants from Acelyrin, Allergan/Abbvie GmbH, Alimera Sciences GmbH, Bayer Healthcare AG, Clearside Biomedical, BDI Pharma Inc., Boehringer-Ingelheim Pharma GmbH & Co. KG, CME Health, DFG (Deutsche Forschungsgemeinschaft e.V.), Genentech GmbH, Gerling GmbH, Ionis, Janssen, Kodiak GmbH, Novartis Pharma GmbH, Novo Nordisk Pharma GmbH, MSD GmbH, ODOS Pharma S.A., Ophtea Pharma, Oxurion NV, Regeneron Pharmaceuticals, Inc., Roche AG, Sandoz and Stada; | A.K.S. received financial support and grants from Novartis, Abbvie, Apples, Bayer Healthcare, Heidelberg Engineering, and Santen;.
References
- Brown, D.M.; Campochiaro, P.A.; Singh, R.P.; Li, Z.; Gray, S.; Saroj, N.; Rundle, A.C.; Rubio, R.G.; Murahashi, W.Y.; CRUISE Investigators. Ranibizumab for Macular Edema Following Central Retinal Vein Occlusion: Six-Month Primary End Point Results of a Phase III Study. Ophthalmology 2010, 117, 1124–1133.e1. [Google Scholar] [CrossRef]
- Mitchell, P.; Bandello, F.; Schmidt-Erfurth, U.; Lang, G.E.; Massin, P.; Schlingemann, R.O.; Sutter, F.; Simader, C.; Burian, G.; Gerstner, O.; et al. The RESTORE Study: Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy for Diabetic Macular Edema. Ophthalmology 2011, 118, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; Le Goff, M.; Von Hanno, T.; Mirshahi, A.; Khawaja, A.P.; Verhoeven, V.J.M.; Hogg, R.E.; Anastosopoulos, E.; Cachulo, M.L.; Höhn, R.; et al. The Decreasing Prevalence of Nonrefractive Visual Impairment in Older Europeans. Ophthalmology 2018, 125, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.P.; Bertram, B.; Wolfram, C.; Holz, F.G. Blindness and Visual Impairment in Germany. Deutsches Ärzteblatt international 2012. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Okada, A.; Matsui, H.; Yasunaga, H.; Aihara, M.; Obata, R. Recent Trends in Anti-Vascular Endothelial Growth Factor Intravitreal Injections: A Large Claims Database Study in Japan. Jpn J Ophthalmol 2023, 67, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Reitan, G.; Kjellevold Haugen, I.B.; Andersen, K.; Bragadottir, R.; Bindesbøll, C. Through the Eyes of Patients: Understanding Treatment Burden of Intravitreal Anti-VEGF Injections for nAMD Patients in Norway. Clin Ophthalmol 2023, 17, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Ehlken, C.; Ziemssen, F.; Eter, N.; Lanzl, I.; Kaymak, H.; Lommatzsch, A.; Schuster, A.K. Systematic Review: Non-Adherence and Non-Persistence in Intravitreal Treatment. Graefes Arch Clin Exp Ophthalmol 2020, 258, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Junker, S.; Wilke, T.; Lommatzsch, A.; Schuster, A.K.; Kaymak, H.; Ehlken, C.; Ziemssen, F. Questionnaire for the Assessment of Adherence Barriers of Intravitreal Therapy: The ABQ-IVT. Int J Retina Vitreous 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, F.; Wachtlin, J.; Kuehlewein, L.; Gamulescu, M.-A.; Bertelmann, T.; Feucht, N.; Voegeler, J.; Koch, M.; Liakopoulos, S.; Schmitz-Valckenberg, S.; et al. Intravitreal Ranibizumab Therapy for Diabetic Macular Edema in Routine Practice: Two-Year Real-Life Data from a Non-Interventional, Multicenter Study in Germany. Diabetes Ther 2018, 9, 2271–2289. [Google Scholar] [CrossRef] [PubMed]
- Wecker, T.; Ehlken, C.; Bühler, A.; Lange, C.; Agostini, H.; Böhringer, D.; Stahl, A. Five-Year Visual Acuity Outcomes and Injection Patterns in Patients with pro-Re-Nata Treatments for AMD, DME, RVO and Myopic CNV. Br J Ophthalmol 2017, 101, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Pesudovs, K.; Gothwal, V.K.; Wright, T.; Lamoureux, E.L. Remediating Serious Flaws in the National Eye Institute Visual Function Questionnaire. Journal of Cataract and Refractive Surgery 2010, 36, 718–732. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Bucher, F.; Liczenczias, E.; Maslanka Figueroa, S.; Müller, B.; Agostini, H. nAMD: Optimization of Patient Care and Patient-Oriented Information with the Help of an Internet-Based Survey. Graefes Arch Clin Exp Ophthalmol 2022, 260, 3241–3253. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.K.; Wolfram, C.; Hudde, T.; Klatt, A.; Schnegelsberg, B.; Midani-Oezkan, H.; Ross, M.; Ziemssen, F.; Pfeiffer, N. Impact of Routinely Performed Optical Coherence Tomography Examinations on Quality of Life in Patients with Retinal Diseases-Results from the ALBATROS Data Collection. J Clin Med 2023, 12, 3881. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, F.; Feltgen, N.; Holz, Fg.; Guthoff, R.; Ringwald, A.; Bertelmann, T.; Wiedon, A.; Korb, C. Demographics of Patients Receiving Intravitreal Anti-VEGF Treatment in Real-World Practice: Healthcare Research Data versus Randomized Controlled Trials. BMC Ophthalmol 2017, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Varano, M.; Eter, N.; Winyard, S.; Wittrup-Jensen, K.U.; Navarro, R.; Heraghty, J. The Emotional and Physical Impact of Wet Age-Related Macular Degeneration: Findings from the wAMD Patient and Caregiver Survey. Clin Ophthalmol 2016, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Ehlken, C.; Bauer-Steinhusen, U.; Lechtenfeld, W.; Hasanbasic, Z.; Agostini, H.; Wilke, T. Treatment of Age-Related Neovascular Macular Degeneration: The Patient’s Perspective. Graefes Arch Clin Exp Ophthalmol 2017, 255, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Bucher, F.; Liczenczias, E.; Maslanka Figueroa, S.; Müller, B.; Agostini, H. nAMD: Optimization of Patient Care and Patient-Oriented Information with the Help of an Internet-Based Survey. Graefes Arch Clin Exp Ophthalmol 2022, 260, 3241–3253. [Google Scholar] [CrossRef]
- Giocanti-Aurégan, A.; García-Layana, A.; Peto, T.; Gentile, B.; Chi, G.C.; Mirt, M.; Kosmas, C.E.; Lambert, J.; Lanar, S.; Lewis, H.B.; et al. Drivers of and Barriers to Adherence to Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema Treatment Management Plans: A Multi-National Qualitative Study. Patient Prefer Adherence 2022, 16, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Stokes, J.; Priestman, L.; Holmes, C.; Said, P. Impact of a Patient Support Program on Patient Beliefs About Neovascular Age-Related Macular Degeneration and Persistence to Anti-Vascular Endothelial Growth Factor Therapy. Patient Prefer Adherence 2021, 15, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.P.; Finger, R.P.; Eldem, B.; Aslam, T.; Barratt, J.; Daien, V.; Kodjikian, L.; Loewenstein, A.; Okada, M.; Wong, T.Y.; et al. The Management of Neovascular Age-Related Macular Degeneration: A Systematic Literature Review of Patient-Reported Outcomes, Patient Mental Health and Caregiver Burden. Acta Ophthalmol 2022. [Google Scholar] [CrossRef] [PubMed]
- Senra, H.; Ali, Z.; Balaskas, K.; Aslam, T. Psychological Impact of Anti-VEGF Treatments for Wet Macular Degeneration-a Review. Graefes Arch Clin Exp Ophthalmol 2016, 254, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, S.; Finkelstein, E.; Lee, J.J.; Too, I.H.K.; Teo, K.Y.C.; Tan, A.C.S.; Wong, T.Y.; Cheung, G.C.M. Understanding Patient Preferences in Anti-VEGF Treatment Options for Age-Related Macular Degeneration. PLoS ONE 2022, 17, e0272301. [Google Scholar] [CrossRef] [PubMed]
- McClard, C.K.; Wang, R.; Windham, V.; Munoz, J.; Gomez, S.; Fried, S.; Saroj, N.; Regillo, C.; Wykoff, C.C.; Strutt, A.M. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII): Development of a Patient-Reported Measure to Assess Treatment Burden of Repeat Intravitreal Injections. BMJ Open Ophth 2021, 6, e000669. [Google Scholar] [CrossRef] [PubMed]
- Skelly, A.; Taylor, N.; Fasser, C.; Malkowski, J.-P.; Goswami, P.; Downey, L. Patient Preferences in the Management of Wet Age-Related Macular Degeneration: A Conjoint Analysis. Adv Ther 2022, 39, 4808–4820. [Google Scholar] [CrossRef] [PubMed]
- Skelly, A.; Taylor, N.; Fasser, C.; Malkowski, J.-P.; Goswami, P.; Downey, L. Correction to: Patient Preferences in the Management of Wet Age-Related Macular Degeneration: A Conjoint Analysis. Adv Ther 2023, 40, 720. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Varano, M.; Pilotto, E.; Staurenghi, G.; Camparini, M.; Pece, A.; Battaglia Parodi, M.; Vadalà, M.; Donati, S.; Frizziero, L.; et al. Real-Life Patient Journey in Neovascular Age-Related Macular Degeneration: A Narrative Medicine Analysis in the Italian Setting. Eye (Lond) 2022, 36, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Angermann, R.; Rauchegger, T.; Nowosielski, Y.; Casazza, M.; Bilgeri, A.; Ulmer, H.; Zehetner, C. Treatment Compliance and Adherence among Patients with Diabetic Retinopathy and Age-Related Macular Degeneration Treated by Anti-Vascular Endothelial Growth Factor under Universal Health Coverage. Graefes Arch Clin Exp Ophthalmol 2019, 257, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Sii, S.; Aspinall, P.; Borooah, S.; Dhillon, B. Exploring Factors Predicting Changes in Patients’ Expectations and Psychosocial Issues during the Course of Treatment with Intravitreal Injections for Wet Age-Related Macular Degeneration. Eye (Lond) 2018, 32, 673–678. [Google Scholar] [CrossRef]
- Okada, M.; Mitchell, P.; Finger, R.P.; Eldem, B.; Talks, S.J.; Hirst, C.; Paladini, L.; Barratt, J.; Wong, T.Y.; Loewenstein, A. Nonadherence or Nonpersistence to Intravitreal Injection Therapy for Neovascular Age-Related Macular Degeneration: A Mixed-Methods Systematic Review. Ophthalmology 2021, 128, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Polat, O.; İnan, S.; Özcan, S.; Doğan, M.; Küsbeci, T.; Yavaş, G.F.; İnan, Ü.Ü. Factors Affecting Compliance to Intravitreal Anti-Vascular Endothelial Growth Factor Therapy in Patients with Age-Related Macular Degeneration. Turk J Ophthalmol 2017, 47, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, B.; Sabsabi, M.; Ziemssen, F. Importance of Treatment Duration: Unmasking Barriers and Discovering the Reasons for Undertreatment of Anti-VEGF Agents in Neovascular Age-Related Macular Degeneration. Clin Ophthalmol 2021, 15, 4317–4326. [Google Scholar] [CrossRef] [PubMed]
- Angermann, R.; Franchi, A.; Frede, K.; Stöckl, V.; Palme, C.; Kralinger, M.; Zehetner, C. Long-Term Persistence with Aflibercept Therapy among Treatment-Naïve Patients with Exudative Age-Related Macular Degeneration in a Universal Health Care System: A Retrospective Study. BMC Ophthalmol 2022, 22, 372. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).