Submitted:
11 November 2023
Posted:
14 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Material Preparation:
2.2. Multi-Material Bottom-Up Stereolithography:
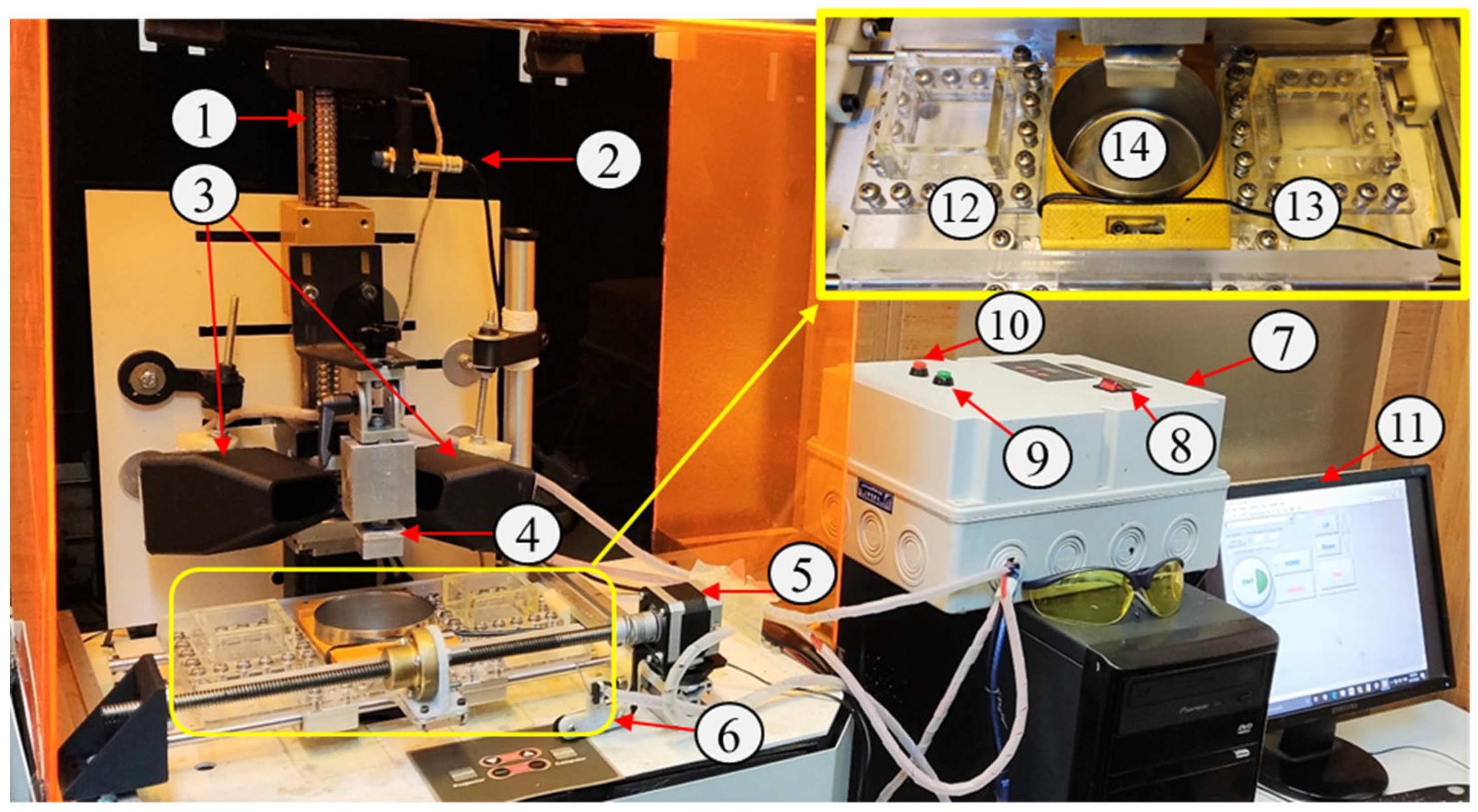
2.2.1. Multi-Material Setup:
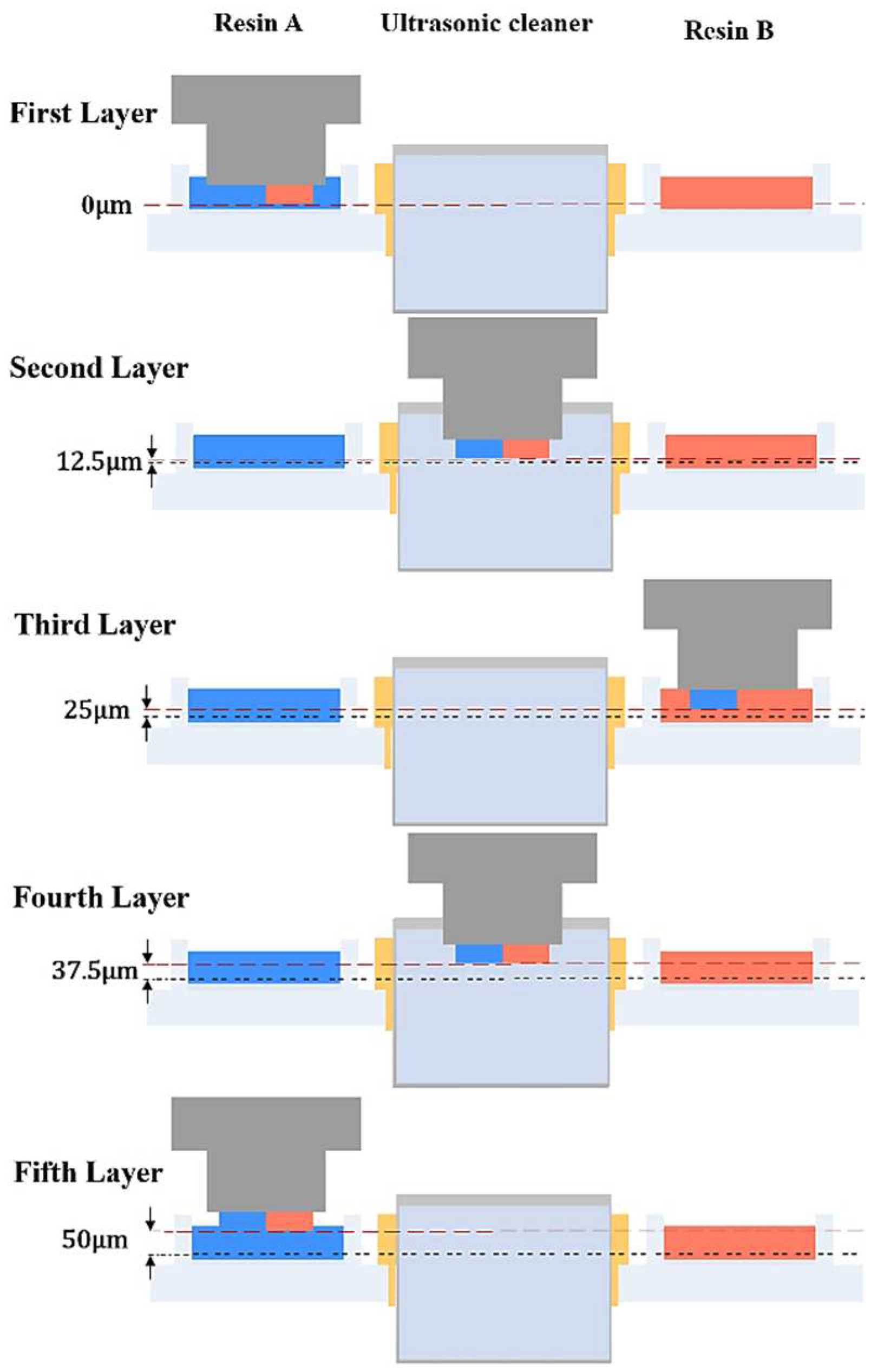
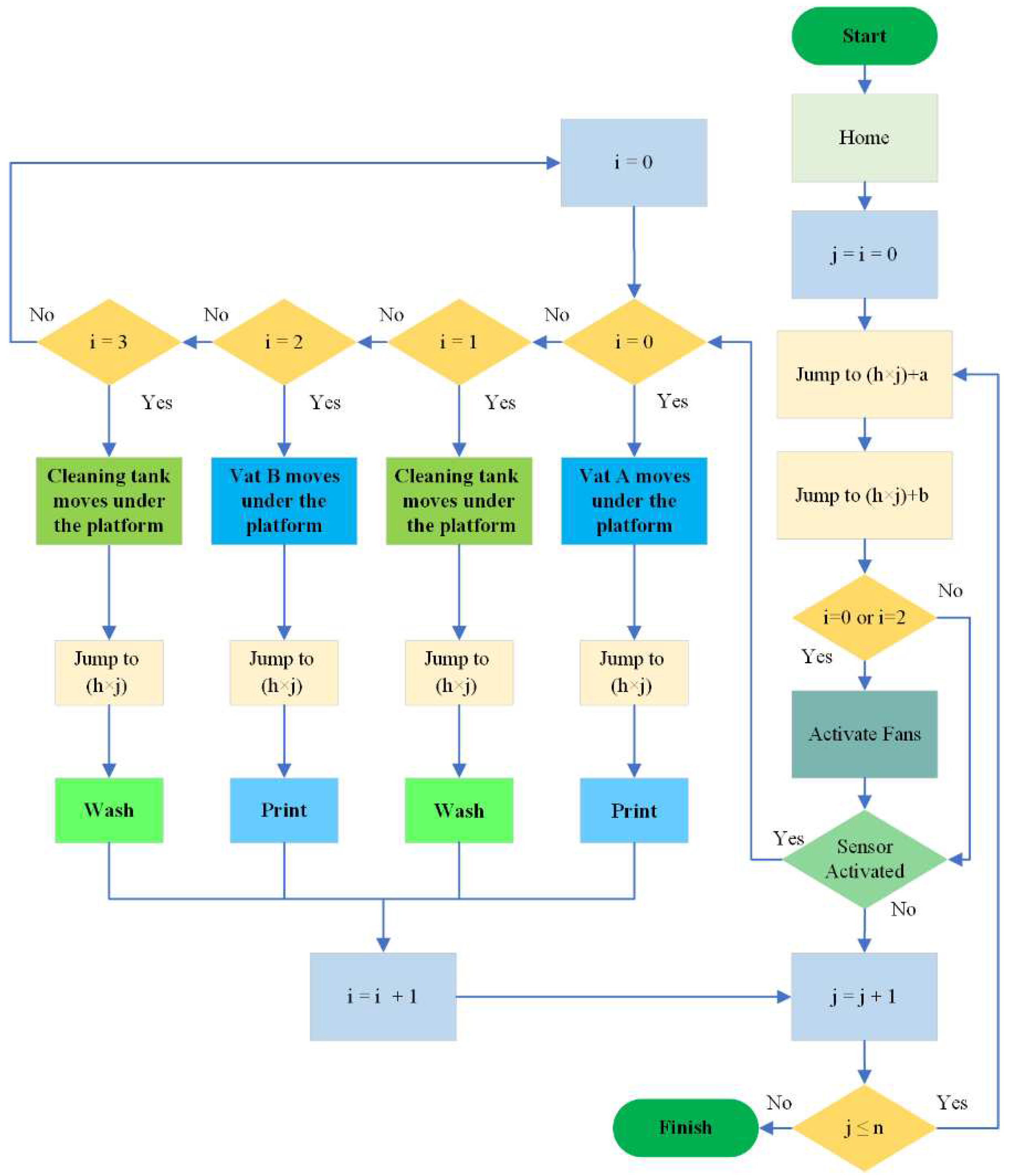
2.2.2. Multi-Material Printing Algorithm:
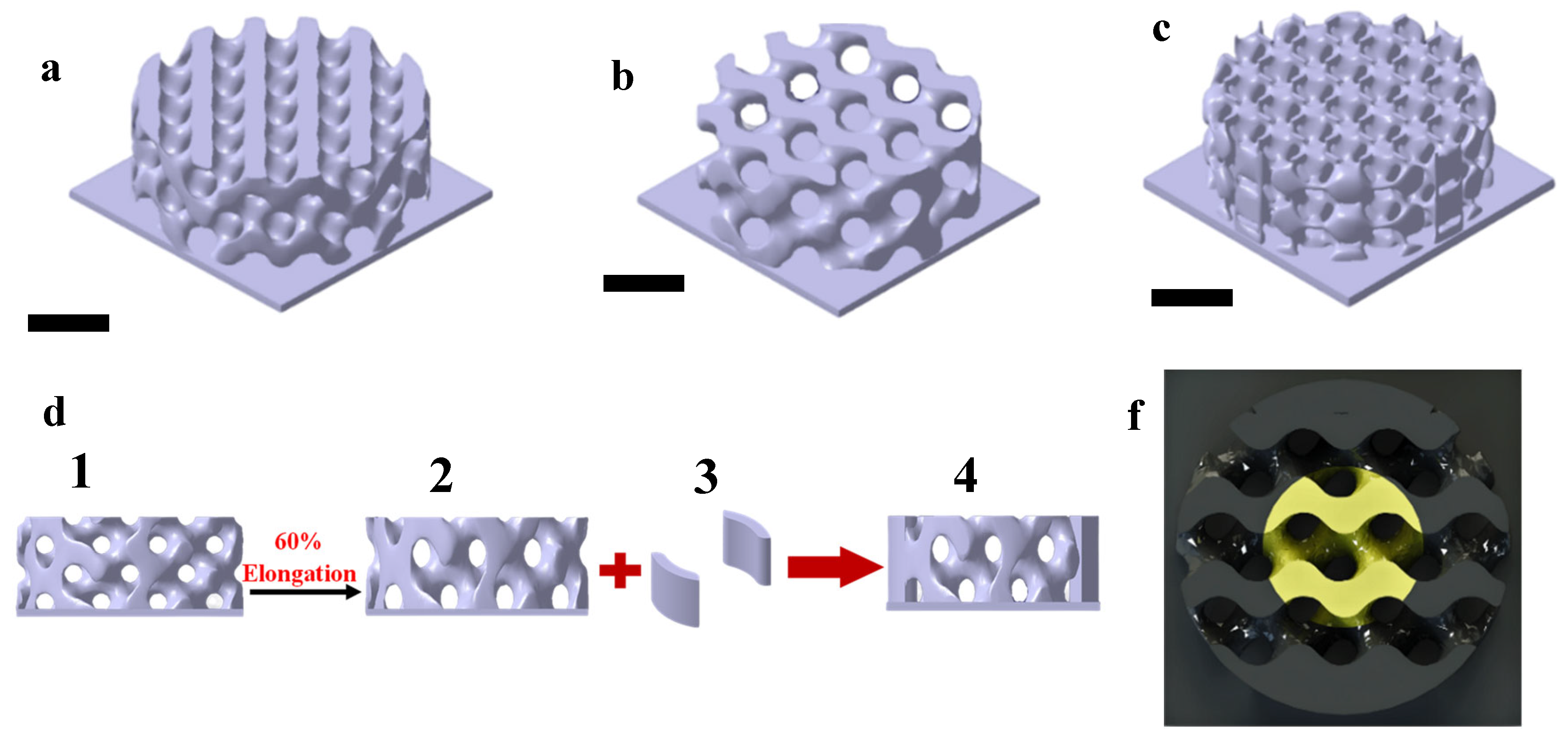
2.3. D Printing Samples:
2.3.1. Parts:
2.3.2. Scaffold:
2.4. Scaffold Characterization:
2.4.1. Mechanical Properties:
| samples | Size (length×width×height) in mm | |
| 1 | Commercial resin cylinders | 2×2×4 |
| 2 | Multi-material cubics | 4×4×4 |
| 3 | Bio resin samples (PLLA) | 6×6×5 |
| 4 | Bio resin cylinders (PLLA/GO) | 6×6×5 |
2.4.2. Morphology:
2.4.3. Cell Seeding
2.4.4. Cell Viability
2.4.5. Cell Morphology:
3. Result and Discussion
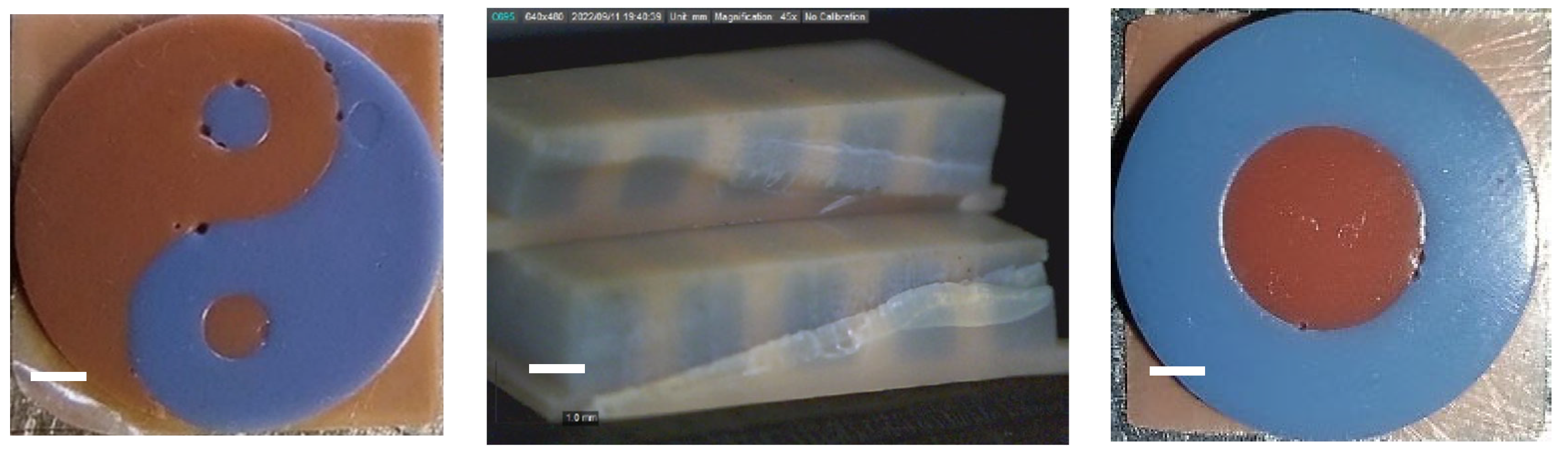
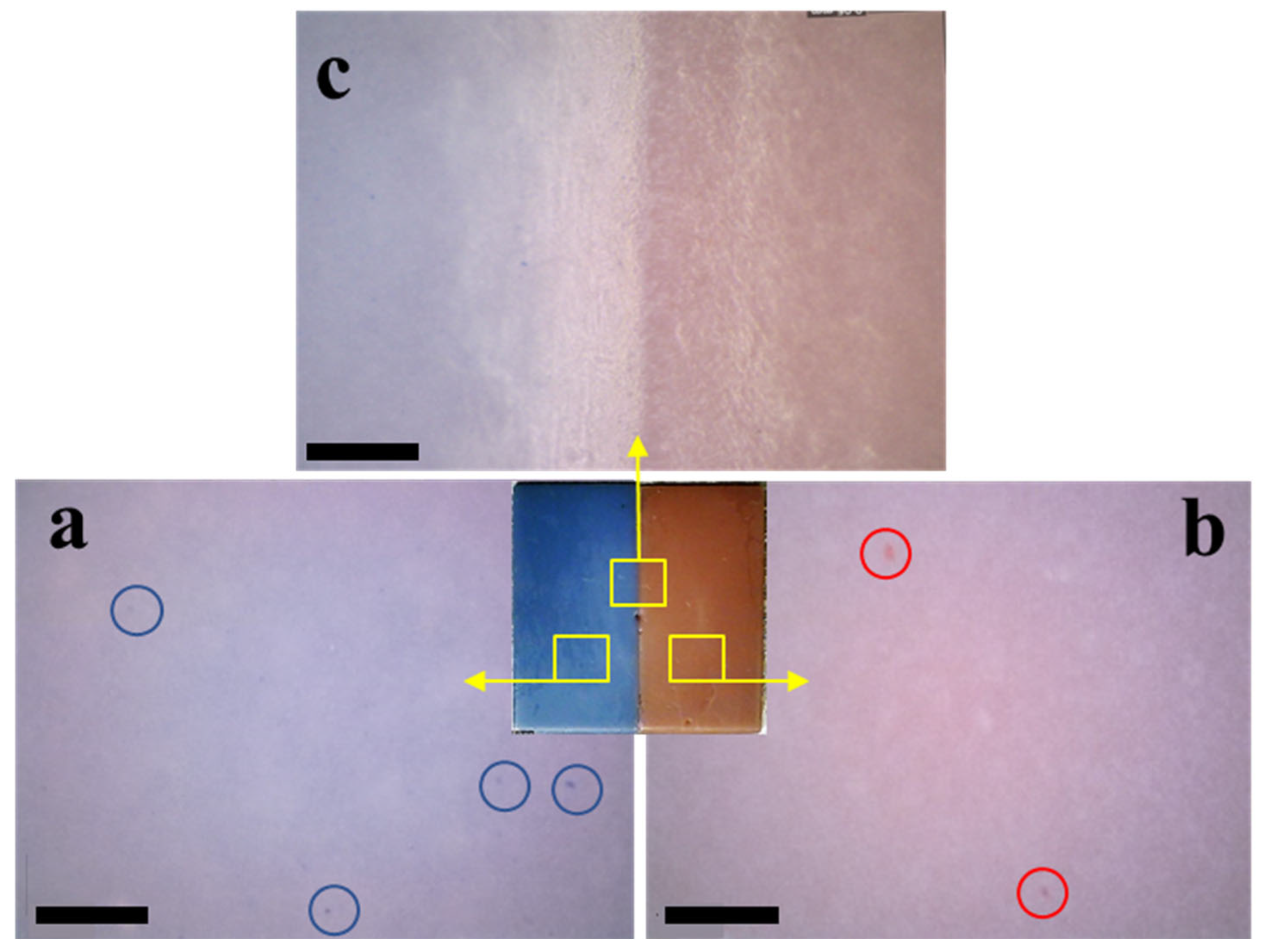
3.1. Multi-Material Bottom-Up Stereolithography
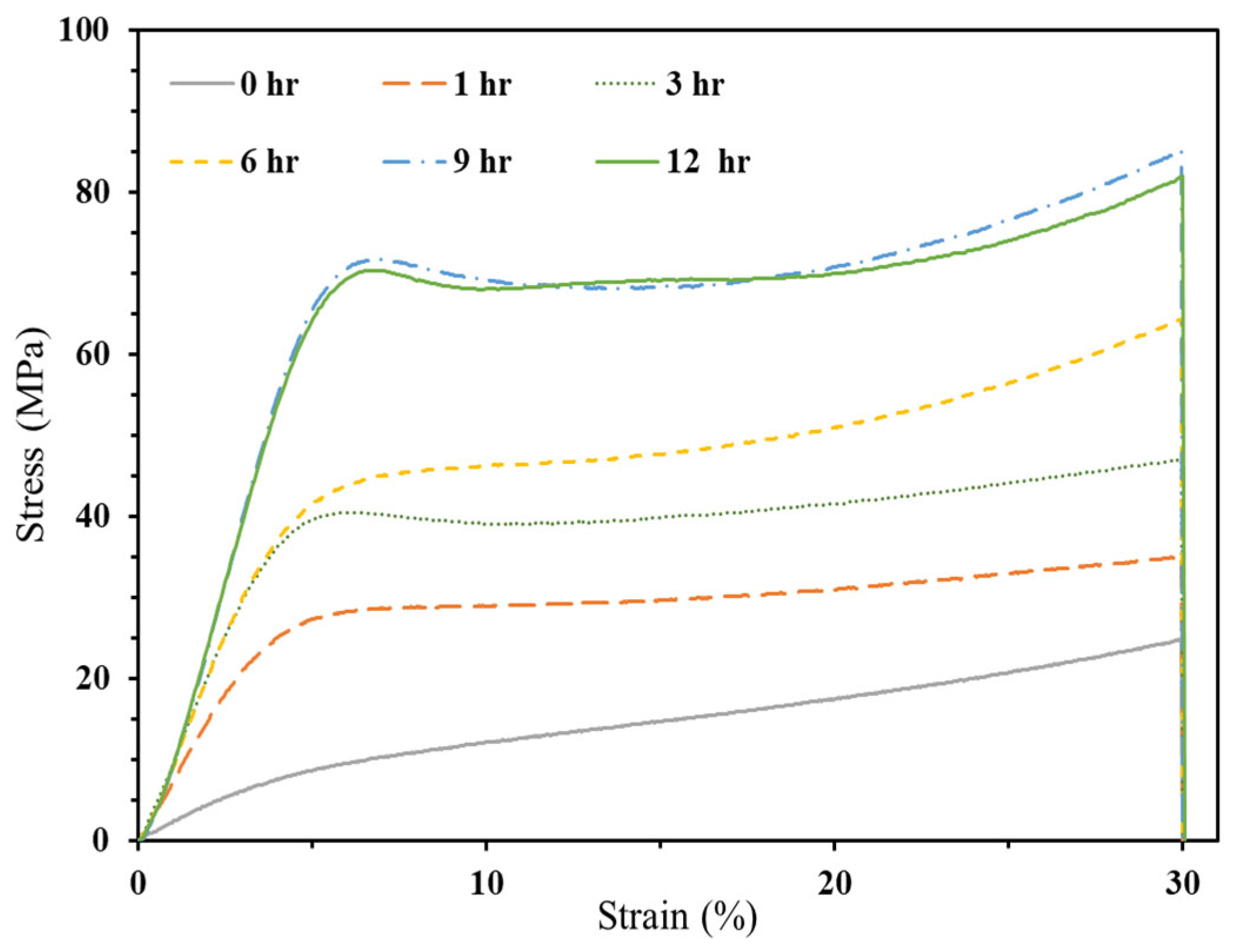
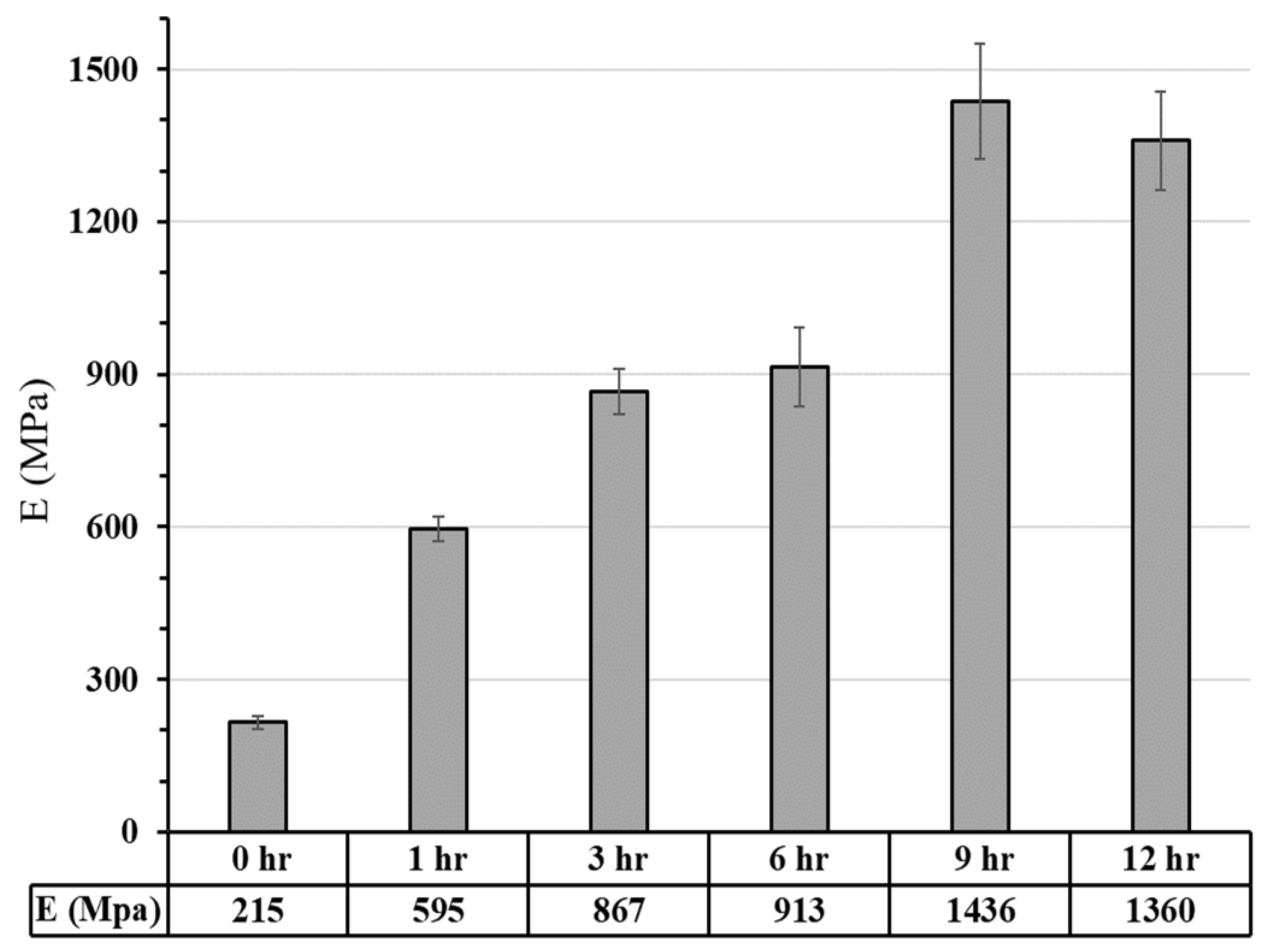
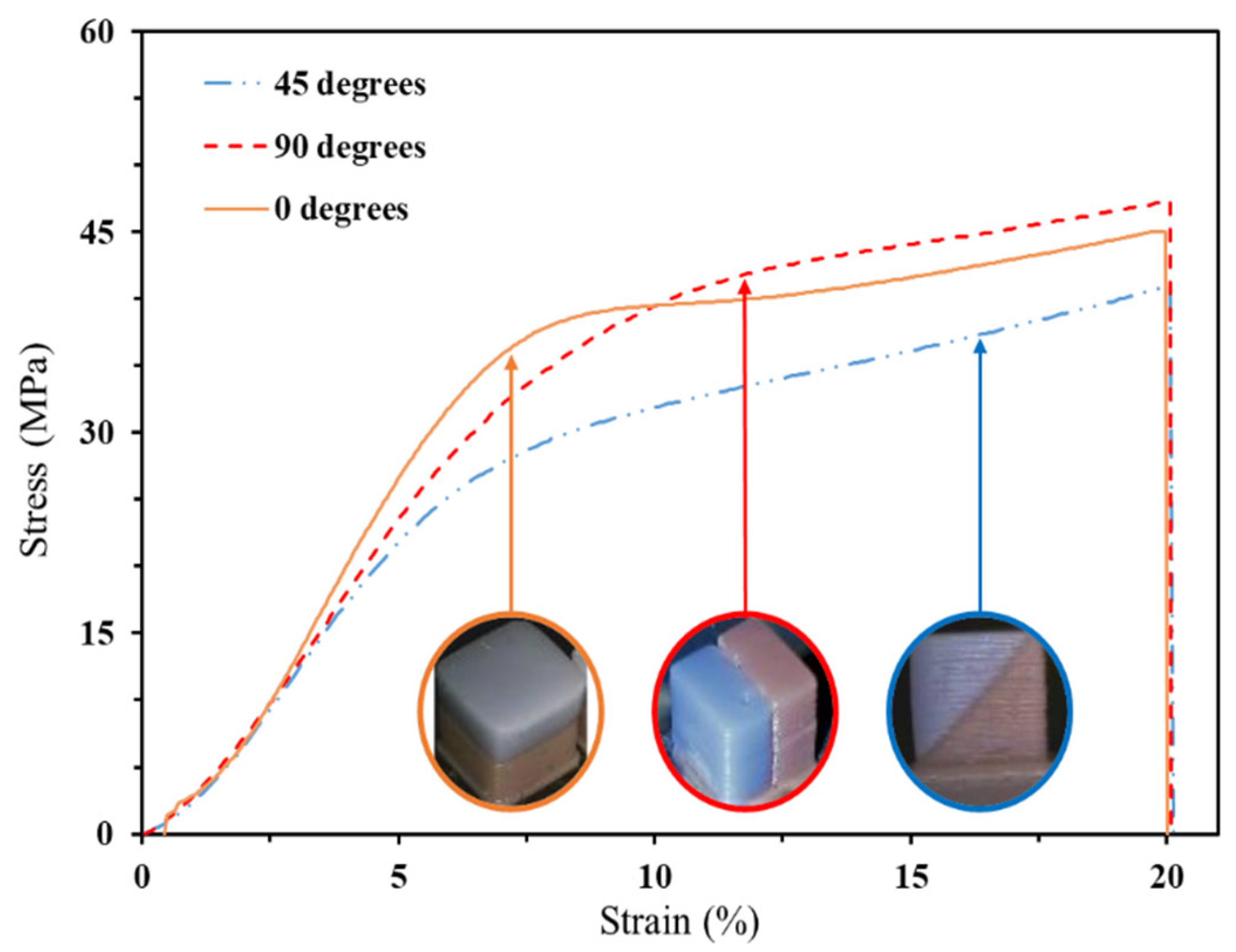
3.2.1. Commercial Resin Samples:
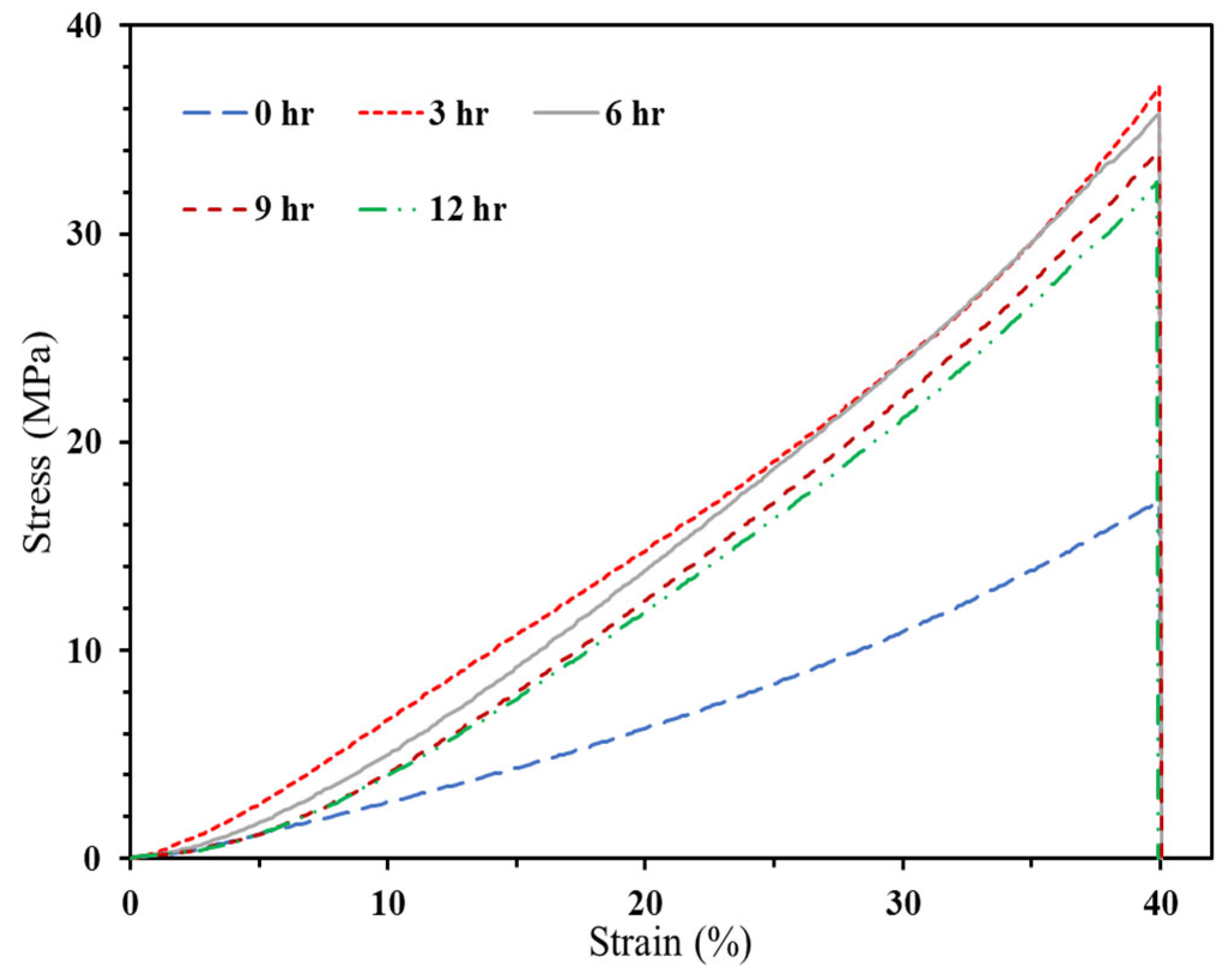
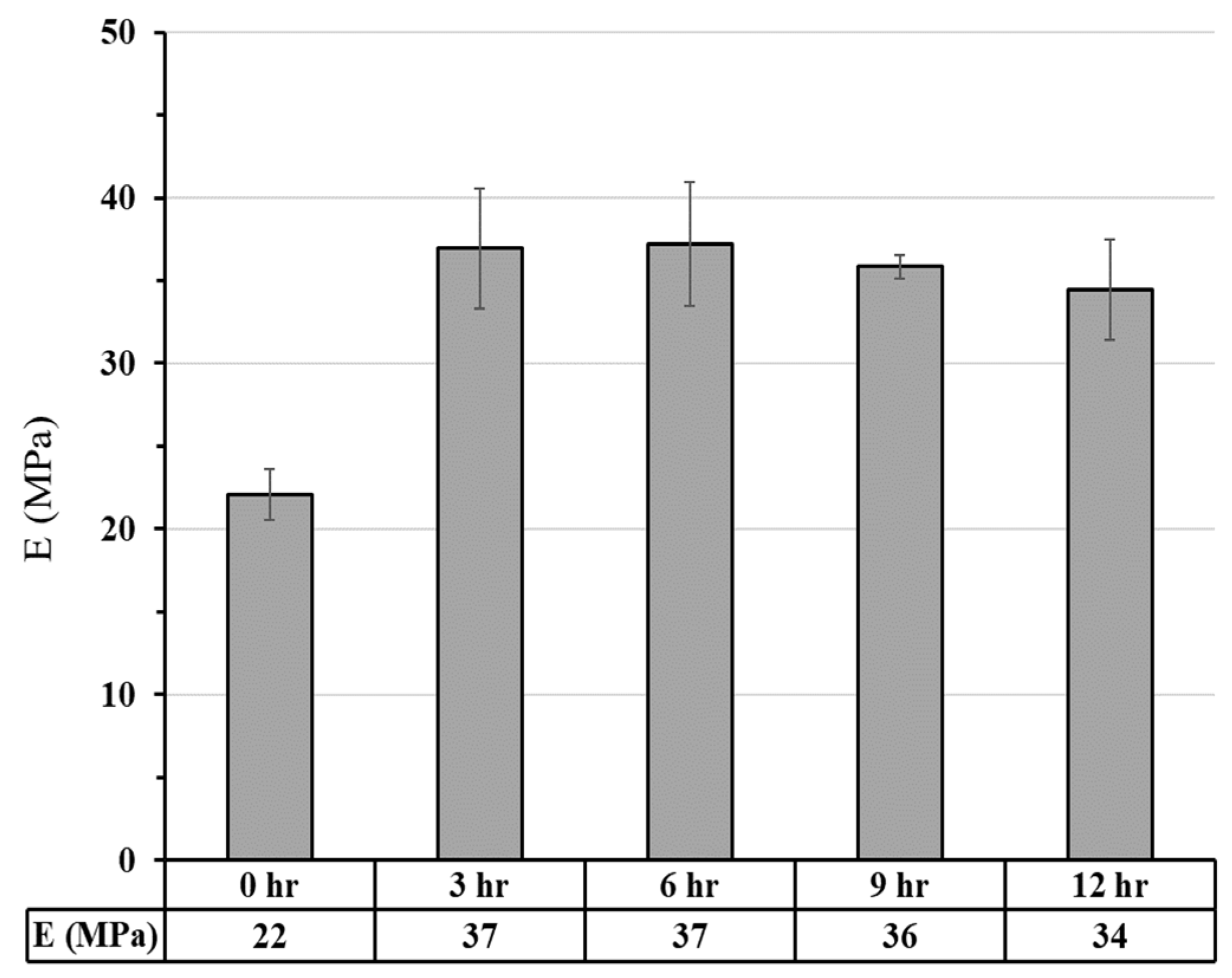
3.2.2. Bio-Resin Samples
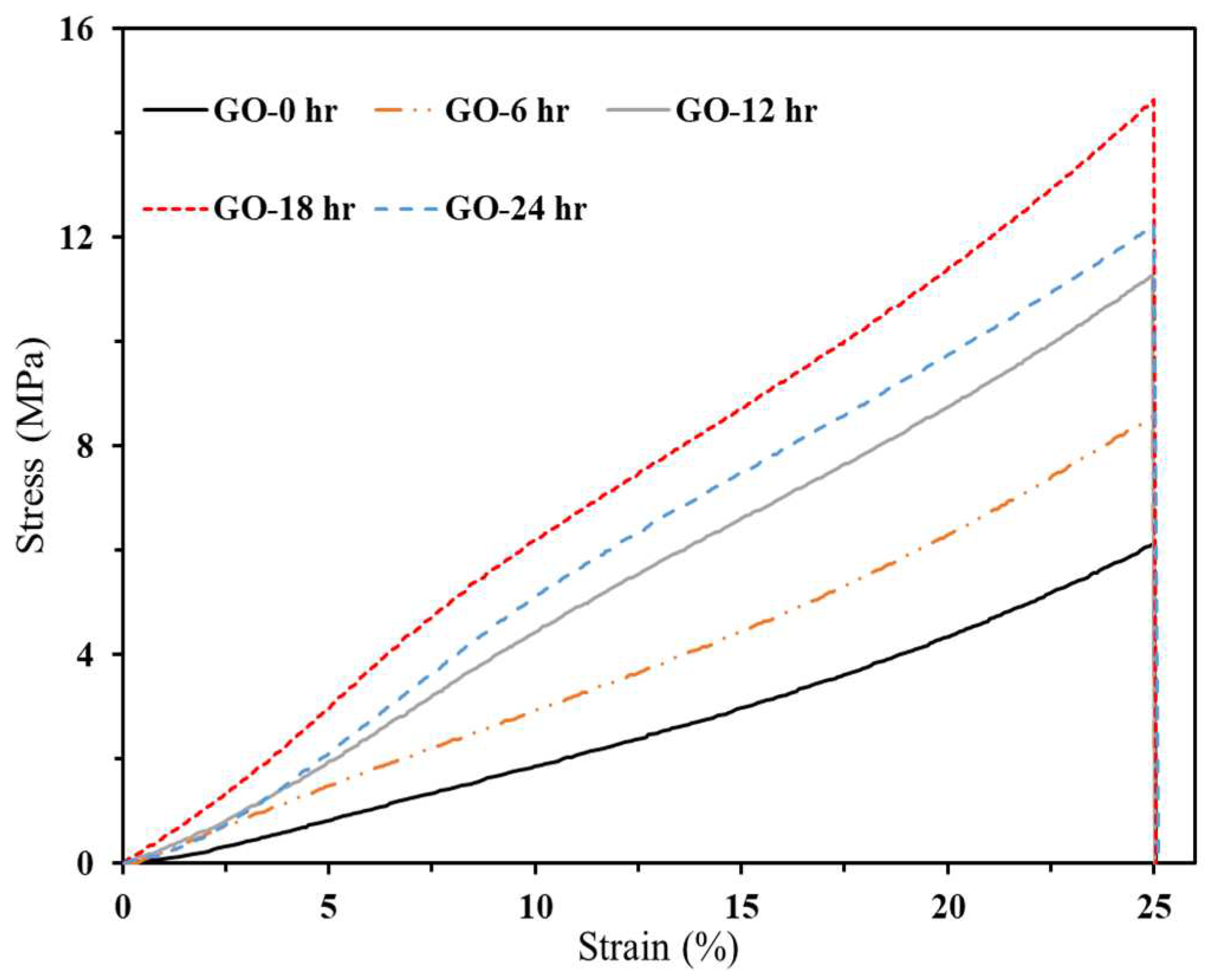
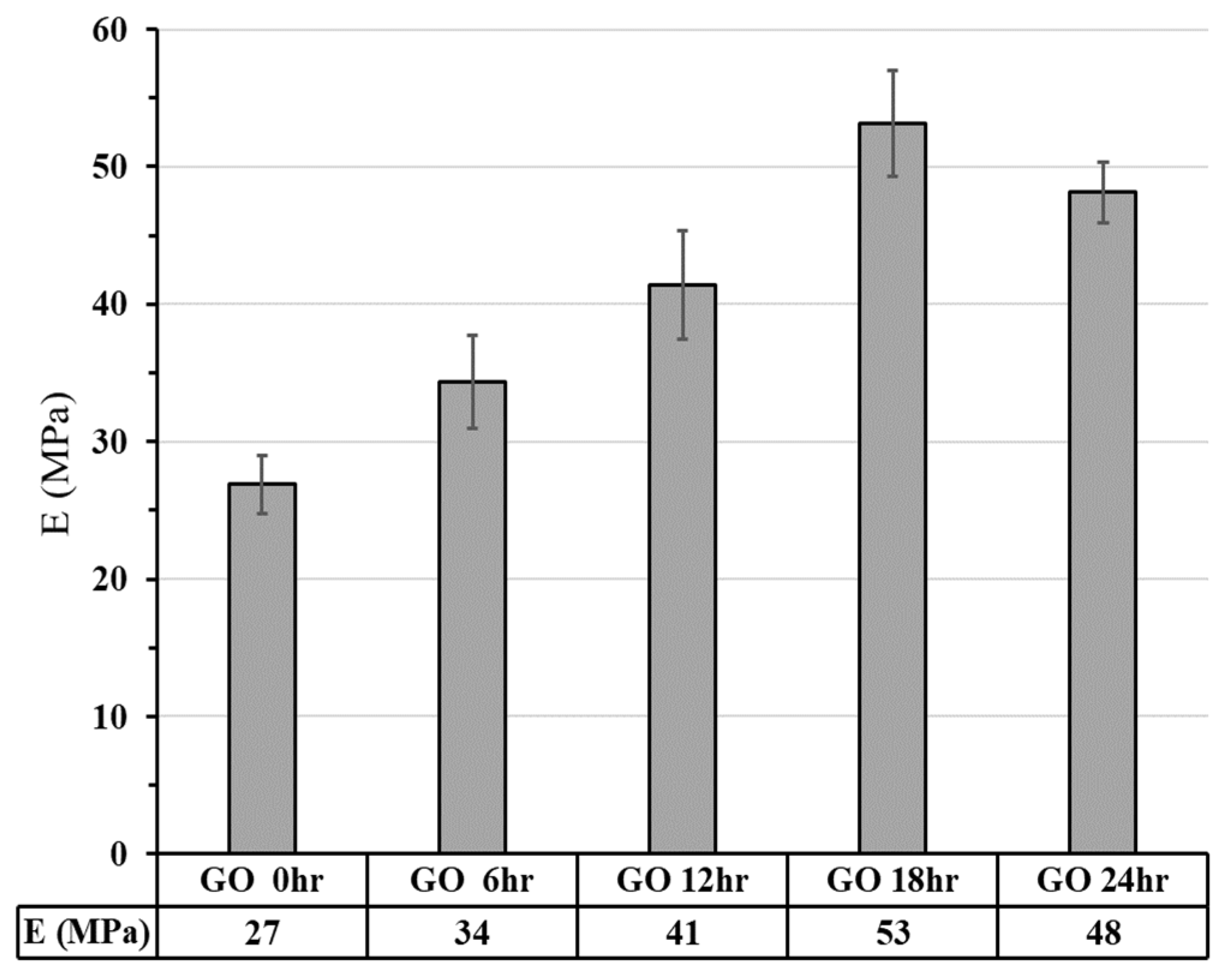
3.2.2.1. Compressive Strength
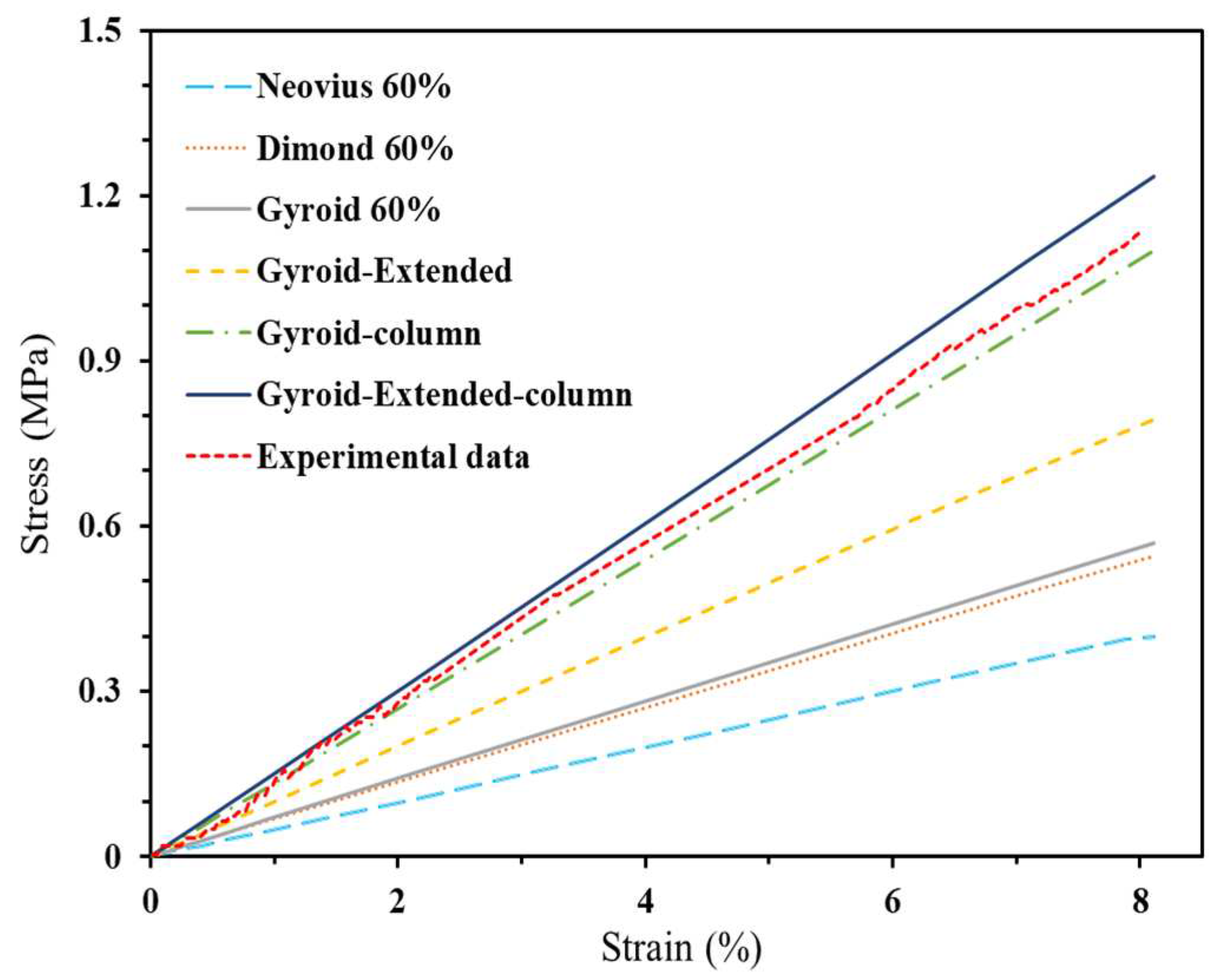
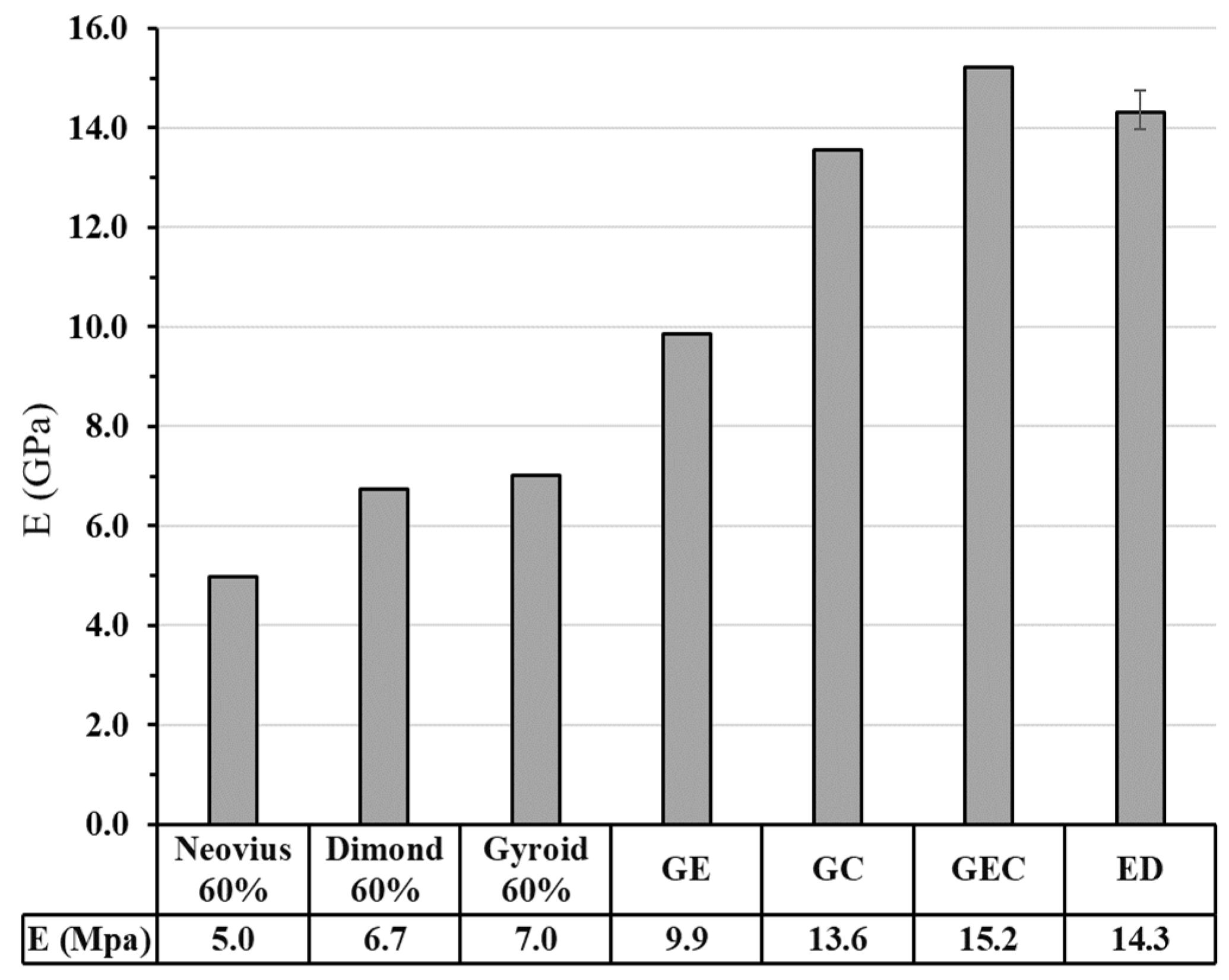
3.2.2.2. Finite Element Analysis
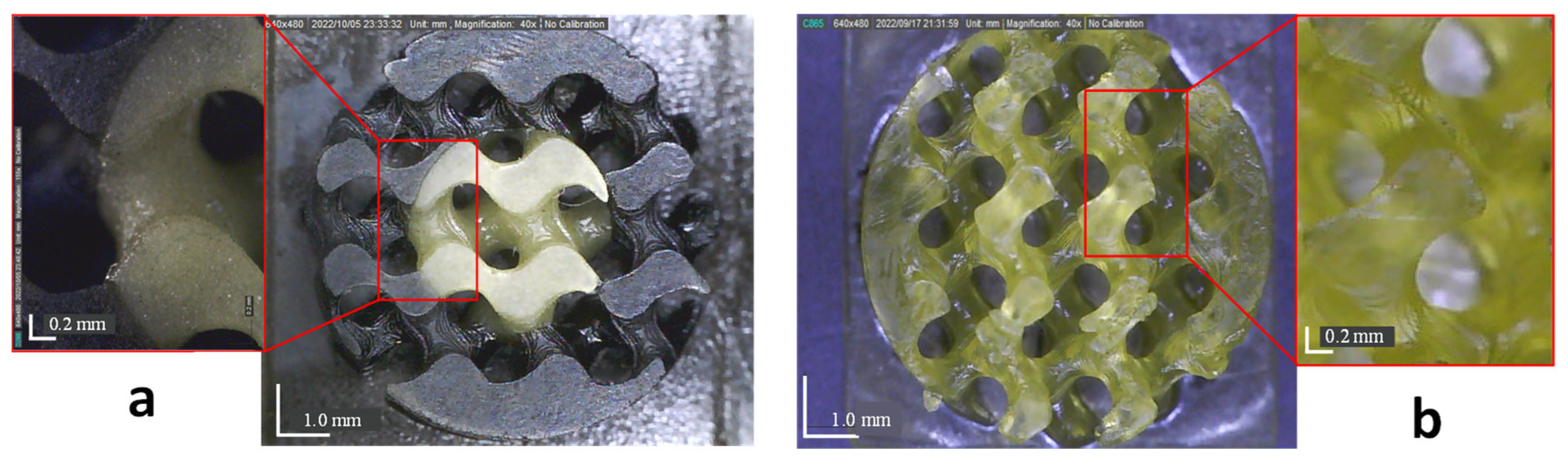
3.3. Scaffolds Morphology
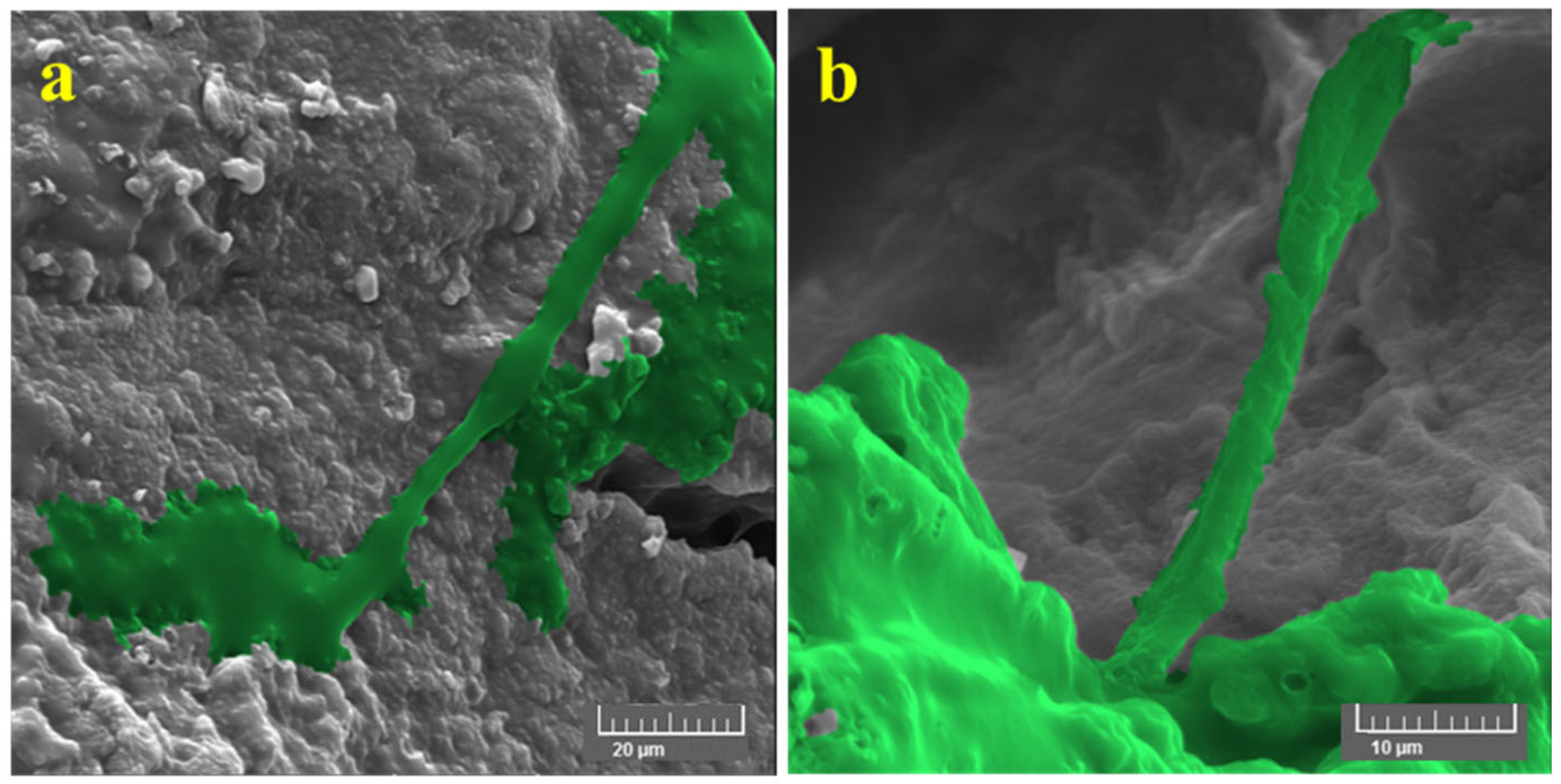
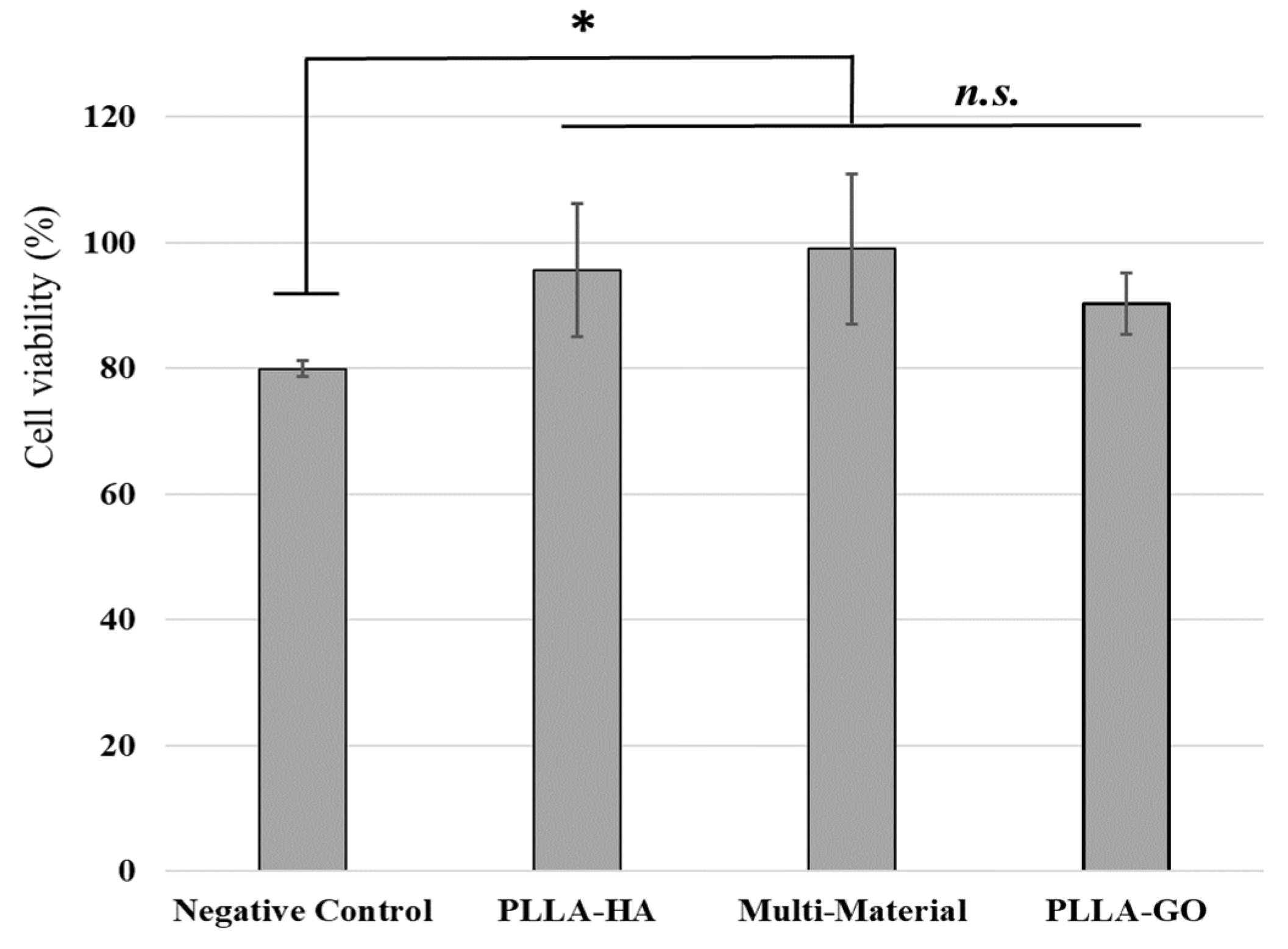
3.4. In Vitro Studies
4. Conclusion
References
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Miszuk, J.M.; Zhao, Y.; Sun, H.; Fong, H. Electrospun Polycaprolactone 3D Nanofibrous Scaffold with Interconnected and Hierarchically Structured Pores for Bone Tissue Engineering. Adv. Heal. Mater. 2015, 4, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, Z.; Baradaran-Rafii, A.; Bakhshaiesh, N.L.; Ai, J.; Ebrahimi-Barough, S.; Malekabadi, H.E.; Nazeri, N.; Vaez, A.; Salehi, M.; Sefat, F.; et al. Design and characterization of biodegradable multi layered electrospun nanofibers for corneal tissue engineering applications. J. Biomed. Mater. Res. Part A 2019, 107, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Özarslan, A.C.; Yücel, S. Fabrication and characterization of strontium incorporated 3-D bioactive glass scaffolds for bone tissue from biosilica. Mater. Sci. Eng. C 2016, 68, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Primo, F.A.; Kumar, S.A.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2020, 17, 100248. [Google Scholar] [CrossRef]
- Hunziker, E.; Lippuner, K.; Keel, M.; Shintani, N. An educational review of cartilage repair: precepts & practice – myths & misconceptions – progress & prospects. Osteoarthr. Cartil. 2015, 23, 334–350. [Google Scholar] [CrossRef]
- Saed, A.B.; Behravesh, A.H.; Hasannia, S.; Akhoundi, B.; Hedayati, S.K.; Gashtasbi, F. An in vitro study on the key features of Poly L-lactic acid/biphasic calcium phosphate scaffolds fabricated via DLP 3D printing for bone grafting. Eur. Polym. J. 2020, 141, 110057. [Google Scholar] [CrossRef]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Gholivand, K.; Ardebili, S.A.A.; Mohammadpour, M.; Malekshah, R.E.; Hasannia, S.; Onagh, B. Preparation and examination of a scaffold based on hydroxylated polyphosphazene for tissue engineering: In vitro and in vivo studies. J. Appl. Polym. Sci. 2022, 139, 52179. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Liu, C. Biomaterials Act as Enhancers of Growth Factors in Bone Regeneration. Adv. Funct. Mater. 2016, 26, 8810–8823. [Google Scholar] [CrossRef]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Yagihara, K.; Okabe, S.; Ishii, J.; Amagasa, T.; Yamashiro, M.; Yamaguchi, S.; Yokoya, S.; Yamazaki, T.; Kinoshita, Y. Mandibular reconstruction using a poly(l-lactide) mesh combined with autogenous particulate cancellous bone and marrow: a prospective clinical study. Int. J. Oral Maxillofac. Surg. 2013, 42, 962–969. [Google Scholar] [CrossRef]
- Al-Wafi, R.; Mansour, S.; Ahmed, M. Mechanical, microstructural properties and cell adhesion of Sr/Se-hydroxyapatite/graphene/polycaprolactone nanofibers. J. Thermoplast. Compos. Mater. 2020, 34, 536–556. [Google Scholar] [CrossRef]
- Grant, J.J.; Pillai, S.C.; Hehir, S.; McAfee, M.; Breen, A. Biomedical Applications of Electrospun Graphene Oxide. ACS Biomater. Sci. Eng. 2021, 7, 1278–1301. [Google Scholar] [CrossRef] [PubMed]
- Moyano, J.; Mosa, J.; Aparicio, M.; Pérez-Coll, D.; Belmonte, M.; Miranzo, P.; Osendi, M. Corrigendum to “Strong and light cellular silicon carbonitride – Reduced graphene oxide material with enhanced electrical conductivity and capacitive response’’ [Addit. Manuf. 30 (2019) 100849]. Addit. Manuf. 2020, 32. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B: Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Barradas, A.W.C.; van Blitterswijk, C.A.; de Boer, J.; Feijen, J.; Grijpma, D.W. Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 2010, 6, 4208–4217. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, Y.-H.; Lee, H.; Koh, Y.-H.; Kim, H.-E. Improving mechanical properties of porous calcium phosphate scaffolds by constructing elongated gyroid structures using digital light processing. Ceram. Int. 2020, 47, 3252–3258. [Google Scholar] [CrossRef]
- Jin, Y.; Zou, S.; Pan, B.; Li, G.; Shao, L.; Du, J. Biomechanical properties of cylindrical and twisted triply periodic minimal surface scaffolds fabricated by laser powder bed fusion. Addit. Manuf. 2022, 56, 102899. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Yan, X.-C.; Fang, G.; Liu, M. Biomechanical influence of structural variation strategies on functionally graded scaffolds constructed with triply periodic minimal surface. Addit. Manuf. 2020, 32, 101015. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Barradas, A.W.C.; van Blitterswijk, C.A.; de Boer, J.; Feijen, J.; Grijpma, D.W. Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 2010, 6, 4208–4217. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Fu, J.; Yao, X.; He, Y. Triply periodic minimal surface (TPMS) porous structures: from multi-scale design, precise additive manufacturing to multidisciplinary applications. Int. J. Extreme Manuf. 2022, 4, 022001. [Google Scholar] [CrossRef]
- THE JOURNAL OF Prosthetic Dentistry. J. Prosthet. Dent. 2000, 84, 8A. [CrossRef]
- Xing, Z.; Zhou, H.; Liu, W.; Nie, J.; Chen, Y.; Li, W. Efficient cleaning of ceramic green bodies with complex architectures fabricated by stereolithography-based additive manufacturing via high viscoelastic paste. Addit. Manuf. 2022, 55, 102809. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, P.; Li, J.; Lu, Z. High-resolution multiceramic additive manufacturing based on digital light processing. Addit. Manuf. 2022, 54, 102732. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Y.; Yang, Z.; Khoshnevis, B. Digital material fabrication using mask-image-projection-based stereolithography. Rapid Prototyp. J. 2013, 19, 153–165. [Google Scholar] [CrossRef]
- Fuchs, F.J. Ultrasonic cleaning and washing of surfaces. In Power Ultrasonics; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 577–609. [Google Scholar]
- Saed, A.B.; Behravesh, A.H.; Hasannia, S.; Ardebili, S.A.A.; Akhoundi, B.; Pourghayoumi, M. Functionalized poly L-lactic acid synthesis and optimization of process parameters for 3D printing of porous scaffolds via digital light processing (DLP) method. J. Manuf. Process. 2020, 56, 550–561. [Google Scholar] [CrossRef]
- Wu, X.; Lian, Q.; Li, D.; Jin, Z. Biphasic osteochondral scaffold fabrication using multi-material mask projection stereolithography. Rapid Prototyp. J. 2019, 25, 277–288. [Google Scholar] [CrossRef]
- Choi, J.-W.; MacDonald, E.; Wicker, R. Multi-material microstereolithography. Int. J. Adv. Manuf. Technol. 2009, 49, 543–551. [Google Scholar] [CrossRef]
- Matte, C.-D.; Pearson, M.; Trottier-Cournoyer, F.; Dafoe, A.; Kwok, T.-H. Multi-material digital light processing printer with material tower and spray cleaning. ASME 2018 13th Int. Manuf. Sci. Eng. Conf. MSEC 2018, vol. 4, pp. 1–11, 2018. [CrossRef]
- Choi, J.-W.; Kim, H.-C.; Wicker, R. Multi-material stereolithography. J. Am. Acad. Dermatol. 2011, 211, 318–328. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, X.; Yang, Y.; Yang, X.; Yi, Y. Mechanical behavior of TPMS-based scaffolds: a comparison between minimal surfaces and their lattice structures. SN Appl. Sci. 2019, 1, 1145. [Google Scholar] [CrossRef]
- Fantini, M.; Curto, M.; De Crescenzio, F. TPMS for interactive modelling of trabecular scaffolds for bone tissue engineering. Lect. Notes Mech. Eng. 2017, 425–435. [Google Scholar] [CrossRef]
- Schoen, A.H. Reflections concerning triply-periodic minimal surfaces. Interface Focus 2012, 2, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Blanquer, S.B.G.; Werner, M.; Hannula, M.; Sharifi, S.; Lajoinie, G.P.R.; Eglin, D.; Hyttinen, J.; A Poot, A.; Grijpma, D.W. Surface curvature in triply-periodic minimal surface architectures as a distinct design parameter in preparing advanced tissue engineering scaffolds. Biofabrication 2017, 9, 025001. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, M.; Yufa, N.; Hoffman, J.; Thomas, E.L. Triply Periodic Bicontinuous Cubic Microdomain Morphologies by Symmetries. Macromolecules 2001, 34, 6083–6089. [Google Scholar] [CrossRef]
- Hedayati, S.K.; Behravesh, A.H.; Hasannia, S.; Kordi, O.; Pourghaumi, M.; Saed, A.B.; Gashtasbi, F. Additive manufacture of PCL/nHA scaffolds reinforced with biodegradable continuous Fibers: Mechanical Properties, in-vitro degradation Profile, and cell study. Eur. Polym. J. 2022, 162, 110876. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Mangadlao, J.D.; Tiu, B.D.B.; Tritchler, G.C.; Advincula, R.C. High-Strength Stereolithographic 3D Printed Nanocomposites: Graphene Oxide Metastability. ACS Appl. Mater. Interfaces 2017, 9, 10085–10093. [Google Scholar] [CrossRef] [PubMed]
- Diez-Escudero, A.; Harlin, H.; Isaksson, P.; Persson, C. Porous polylactic acid scaffolds for bone regeneration: A study of additively manufactured triply periodic minimal surfaces and their osteogenic potential. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, F.; Krüger, M.; de Torre, I.G.; Sierra, L.Q.; Rodrigo, M.A.; Kock, L.; Rodriguez-Cabello, J.C. Cartilage Regeneration in Preannealed Silk Elastin-Like Co-Recombinamers Injectable Hydrogel Embedded with Mature Chondrocytes in an Ex Vivo Culture Platform. Biomacromolecules 2018, 19, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, M.-B.; Li, Y.; Ma, Y.; Li, J.-S.; Dai, F.-Y. Recent Progress in Tissue Engineering and Regenerative Medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Monterubbianesi, R.; Bencun, M.; Pagella, P.; Woloszyk, A.; Orsini, G.; Mitsiadis, T.A. A comparative in vitro study of the osteogenic and adipogenic potential of human dental pulp stem cells, gingival fibroblasts and foreskin fibroblasts. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]

| Function name | Mathematical expression |
| Schwarz G (Gyroid) | |
| Neovius | |
| Schwarz D (Dimond) |
| Nominal Porosity (%) | Nominal Pore size (µm) | Calculated porosity (%) | Error Porosity (%) | Calculated pore size (µm) | Error Pore size (%) | |
| Multi-material PLLA-PLLA scaffold | 50 | 580 | 47 | 6 | 547 ± 80 | 5.7 |
| Multi-material PLLA/GO-HA scaffold | 50 | 580 | 43 | 14 | 454 ± 80 | 21.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





