1. Introduction
Vanadium chalcogenides have remarkable properties in terms of magnetism and catalytic activity and are being used in important industrial processes. Exemplarily, the contact-process for the synthesis of sulfuric acid uses vanadium(V) oxide (V
2O
5) as a highly active catalyst in the oxidation step from SO
2 to SO
3 [
1]. Vanadium selenides and tellurides exhibit promising electronic properties coupled to their structure and physical behavior. VSe
2 (
Pm1) shows good mechanical properties such as strength and durability together with electrical and optical qualities and is therefore applied in solar cells [
2]. As anode material, VSe
2 is being tested for lithium- and sodium-ion batteries [
3].
The bulk structures of vanadium chalcogenides were first explored in the middle of the 20
th century [
4,
5,
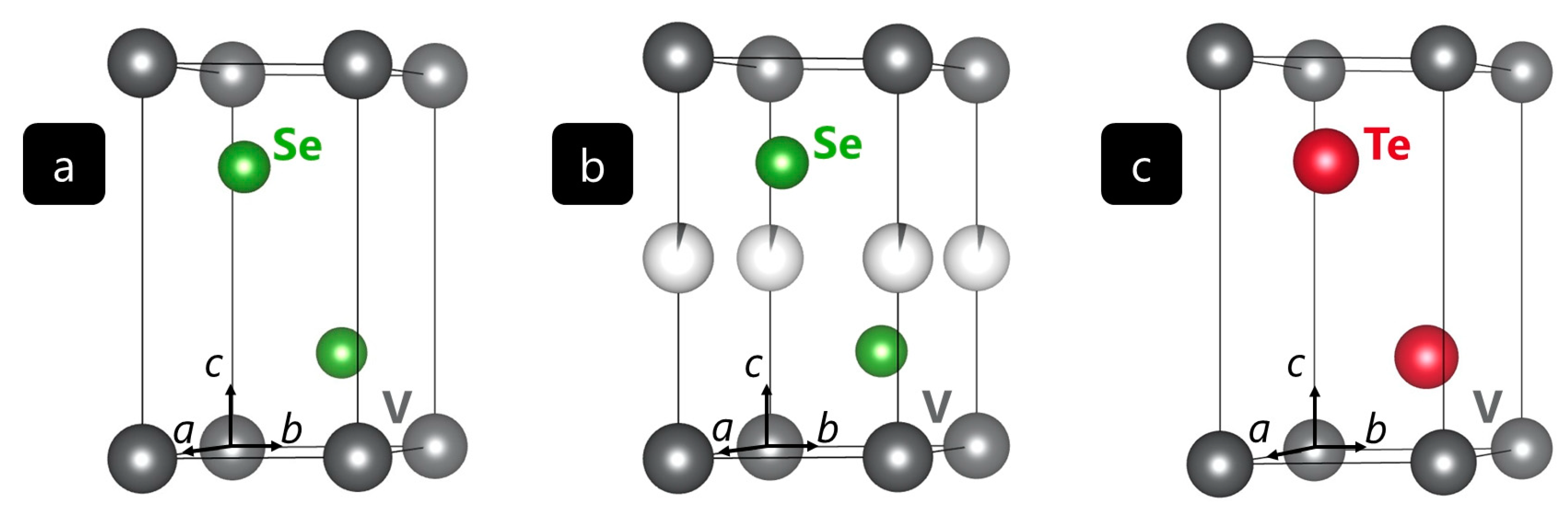
6]. Later, additional compounds belonging to these systems were found. This article elaborates on new members of the V–Se–Te system. The crystal structures of the previously reported materials VSe
2, V
1.04Se
2 and VTe
2 are shown in
Figure 1.
As depicted in
Figure 1, the crystal structures of the known vanadium(IV) selenides and tellurides adopt the trigonal space group
Pm1 (
Z = 1). The structures (a) and (c) are representatives of the CdI
2-type in which vanadium atoms are located on Wyckoff site 1
a. In structure (b), vanadium atoms additionally occupy Wyckoff site 1
b, but only by 4%. The chalcogenide atoms are located on Wyckoff position 2
d with
z = 0.257 (a,b) and
z = 0.250 (c) [
7,
8,
9]. The crystal-chemical motif alludes to layers of vanadium cations that are octahedrally coordinated by the respective chalcogenide anions.
The different chalcogenide anions in the compounds VSe
2 and VTe
2 give rise to different properties, for example in terms of magnetism: VSe
2 is known as a paramagnetic compound and from a Curie-type contribution to its magnetic susceptibility originating from the vanadium atoms [
10,
11]. In contrast, VTe
2 evidences antiferromagnetic spin ordering below a Néel temperature of 418 °C. Furthermore, a transition of the polymorphic charge density wave at 753 °C can influence the magnetic behavior even at elevated temperatures [
12,
13].
The synthesis of new ternary vanadium dichalcogenides is not only auspicious because of versatile magnetic properties that could qualify mixed vanadium dichalcogenides for use in various applications. In contrast to the systems Cr–Se–Te [
14,
15,
16,
17] and Ti–Se–Te [
5,
18,
19], where several ternary phases have been known since the middle of the 20
th century, the system V–Se–Te is widely unexplored to date. Hence, we have decided to fill this gap, and we have synthesized a series of V
wSe
yTe
2–y compounds supposedly belonging to a solid solution.
2. Results and Discussion
To validate both structure and composition, two single crystals and their structures were determined by X-ray diffraction. The stoichiometry found was then compared with energy dispersive X-ray spectroscopy data. The structural model was furthermore tested against Rietveld-derived results from powder data. According to Vegard’s law, increasing lattice parameters were found with higher tellurium content. Also, the range of thermal decomposition was determined by simultaneous differential scanning calorimetry/thermogravimetric analysis. In addition, the magnetic behavior was studied.
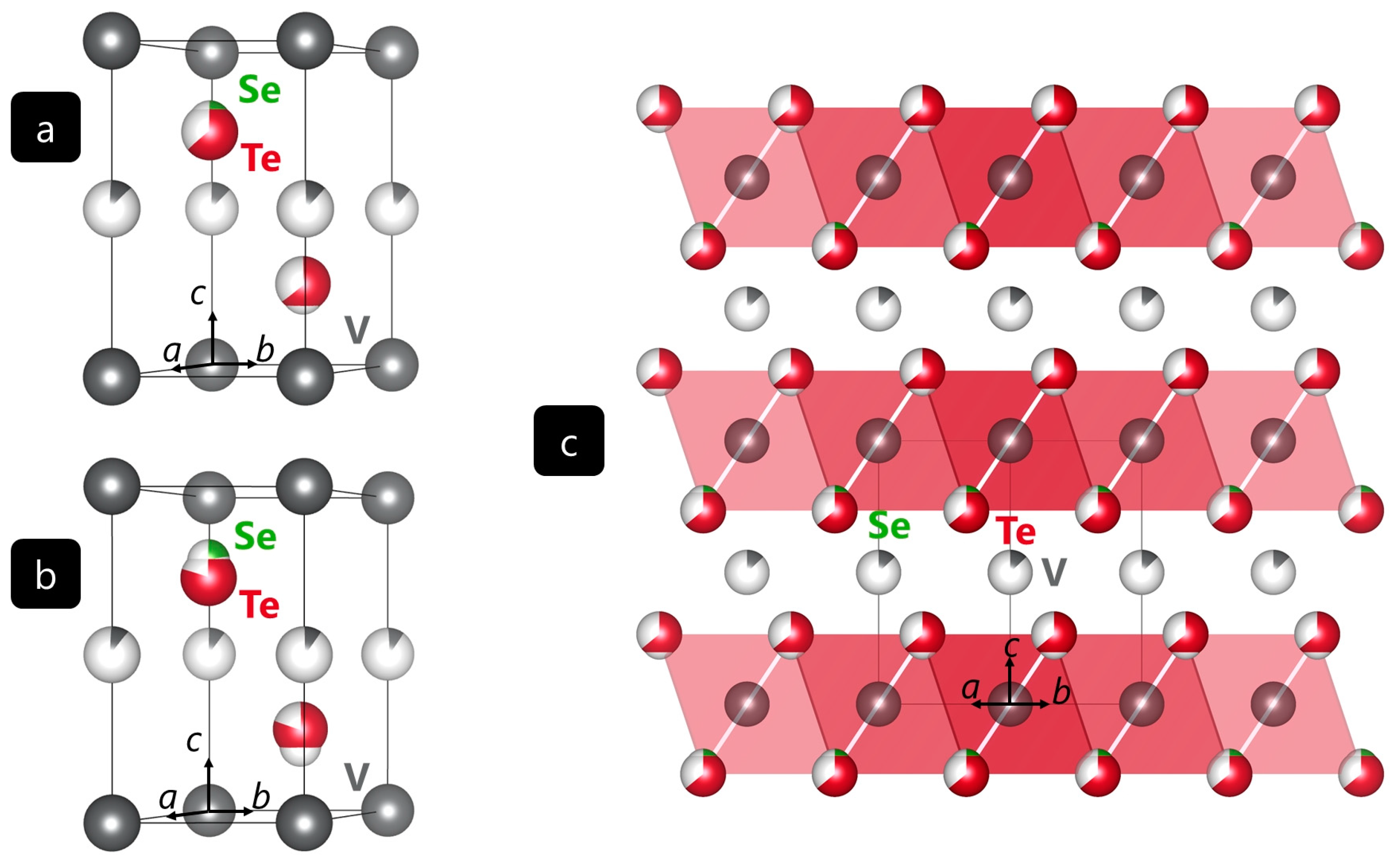
2.1. Crystal structure description of VwSeyTe2–y
The V
wSe
yTe
2–y compounds were obtained as gray, crystalline powders from ceramic syntheses as described in 3.1. V
1.13Se
0.72Te
1.28 and V
1.10Se
0.42Te
1.58 crystallize with one structural formula per unit cell in the CdI
2-type belonging to the trigonal space group
Pm1 (no. 164) [
7]. As alluded to already, CdI
2-type structures are known for their layered appearance consisting of layers of octahedrally coordinated metal ions. These layers are held together by van-der-Waals interactions.
Figure 2 shows the unit cells as derived from single-crystal structure determination, as well as the layered structures.
In both structures, vanadium ions that are octahedrally coordinated by selenium or tellurium ions, respectively, reside on Wyckoff site 1
a (V1, 0 0 0). Additionally, Wyckoff site 1
b (V2, 0 ½ 0) is occupied with vanadium ions by 13% in the case of V
1.13Se
0.72Te
1.28 (a) and 10% in the case of V
1.10Se
0.42Te
1.58 (b), the Se atom found a little closer to the fully occupied layer formed by V1 than Te. In more detail, the selenium and tellurium ions coordinating the vanadium ions are located on Wyckoff site 2
d (⅓ ⅔
z) with
z = 0.235(3) for selenium and
z = 0.265(1) for tellurium in V
1.13Se
0.72Te
1.28. In V
1.10Se
0.42Te
1.58, the selenium and tellurium
z positions are 0.214(3) and 0.266(1), respectively. The results of the single-crystal refinements are summarized in
Table 1, and the refined Wyckoff sites are given in Table S1.
It is worth mentioning that VS
4 is a promising material for magnesium ion batteries with an interchain spacing of 5.83 Å [
20]. As the distances between the fully occupied vanadium layers are 6.29 Å and 6.36 Å for V
1.13Se
0.72Te
1.28 and V
1.10Se
0.42Te
1.58, respectively, it may well be the case that the aforementioned V-Se-Te phases may serve well in such magnesium ion batteries, since these distances appears to be large enough for Mg intercalation and deintercalation.
Table 1.
Refinement details of the single-crystal studies of V1.13Se0.72Te1.28 and V1.10Se0.42Te1.58.
Table 1.
Refinement details of the single-crystal studies of V1.13Se0.72Te1.28 and V1.10Se0.42Te1.58.
| Formula |
V1.13Se0.72Te1.28
|
V1.10Se0.42Te1.58
|
| form wt. (g mol-1) |
277.74 |
290.81 |
| space group |
m1 |
m1 |
|
a (Å) |
3.626(2) |
3.633(7) |
|
c (Å) |
6.290(2) |
6.365(12) |
| volume (Å3) |
71.6(1) |
72.7(3) |
| Z |
1 |
1 |
| calc. density (g cm−3) |
6.441 |
6.639 |
|
µ (mm−1) |
25.541 |
24.140 |
|
F(000) |
117 |
122 |
|
θ range (°) |
3.24–30.40 |
3.20–31.00 |
| index range |
−5 ≤ h ≤ 4 −5 ≤ k ≤ 5−8 ≤ l ≤ 8 |
−5 ≤ h ≤ 4 −4 ≤ k ≤ 5−9 ≤ l ≤ 7 |
| reflections collected |
1049 |
585 |
| independent reflections |
108 |
111 |
| refinement method |
Full matrix least squares on F2
|
| data/restraints/parameters |
108/0/11 |
111/0/14 |
| goodness-of-fit on F2
|
1.208 |
0.987 |
| final R indices [I > 2σ(I)] |
R1 = 0.021; wR2 = 0.049 |
R1 = 0.020; wR2 = 0.042 |
|
R indices (all data) |
R1 = 0.023; wR2 = 0.050 |
R1 = 0.021; wR2 = 0.042 |
|
Rint
|
0.025 |
0.029 |
| largest diff. peak and hole (e–Å−3) |
1.454 and –1.198 |
0.807 and –0.904 |
2.2. Elemental analysis by means of energy dispersive X-ray diffraction
The elemental compositions were analyzed by energy dispersive X-ray spectroscopy (EDX) measurements of the isolated single crystals in a scanning electron microscope (SEM). The SEM images of both crystals are shown in
Figure 3. The averaged elemental composition of the V
1.13Se
0.72Te
1.28 specimen analyzed from four single-crystal locations (white rectangles) was 30.7(4) at% vanadium, 27.0(4)% selenium, and 42.3(3)% tellurium. The vanadium content, according to the EDX data, is lower compared to findings from single-crystal XRD analysis. Seemingly, this is due to partial overlap of the vanadium
Lα1 and
Lβ1 signals with the oxygen
Kα1 peak, which was found as a surface artefact [
21]. The Se/Te ratio determined from the single-crystal XRD measurement is 0.56, close to the EDX value which arrives at 0.64. The EDX analysis thus confirms the composition of the newly synthesized phase, at least semi-quantitatively.
For the crystal with the stoichiometry V
1.10Se
0.42Te
1.58, the EDX measurement also supports the finding of a lower selenium content. Again, the four-spot single-crystal composition was found as 34.7(2) at% vanadium, 9.4(3)% selenium, and 55.9(3)% tellurium. Clearly, the selenium content determined is lower compared to the single-crystal X-ray diffraction value, and we suspect the presence of tellurium-richer regions closer to the crystal surface. As EDX probes in a region of about 2 micrometers in depth, this might influence the result of this measurement [
21]. Carbon artefacts were found in all EDX analyses. This contribution is a known effect for measurements on a carbon adhesive tape and was therefore neglected in data evaluation.
2.3. Powder X-Ray diffraction analysis of compounds in the V–Se–Te system.
Compounds with the refined compositions VSe
0.45Te
1.55, VSe
0.53Te
1.47, VSe
0.64Te
1.36, VSe
0.72Te
1.28, VSe
0.77Te
1.23, VSe
0.82Te
1.18, VSe
0.94Te
1.06 were synthesized as described in 3.1. All obtained products were gray and polycrystalline. Powder X-ray diffraction was carried out as described in 3.3, and the data were Rietveld-refined using the FullProf suite [
22,
23]. As a starting model, the solution of the single-crystal structure of V
1.13Se
0.72Te
1.28 was used. The chemical composition of tellurium and selenium was refined individually for each stoichiometry with the coefficient sum fixed to 2.
Interestingly, preferred orientation of the crystal to the (001) plane was observed, so the
G1 parameter was refined to values between 0.27 and 0.40 indicating platy texture of the crystallites. This matches the shape of the hexagonal single-crystals and, reflecting the layered structure of the material, is also known for related compounds such as VTe
2 [
12].
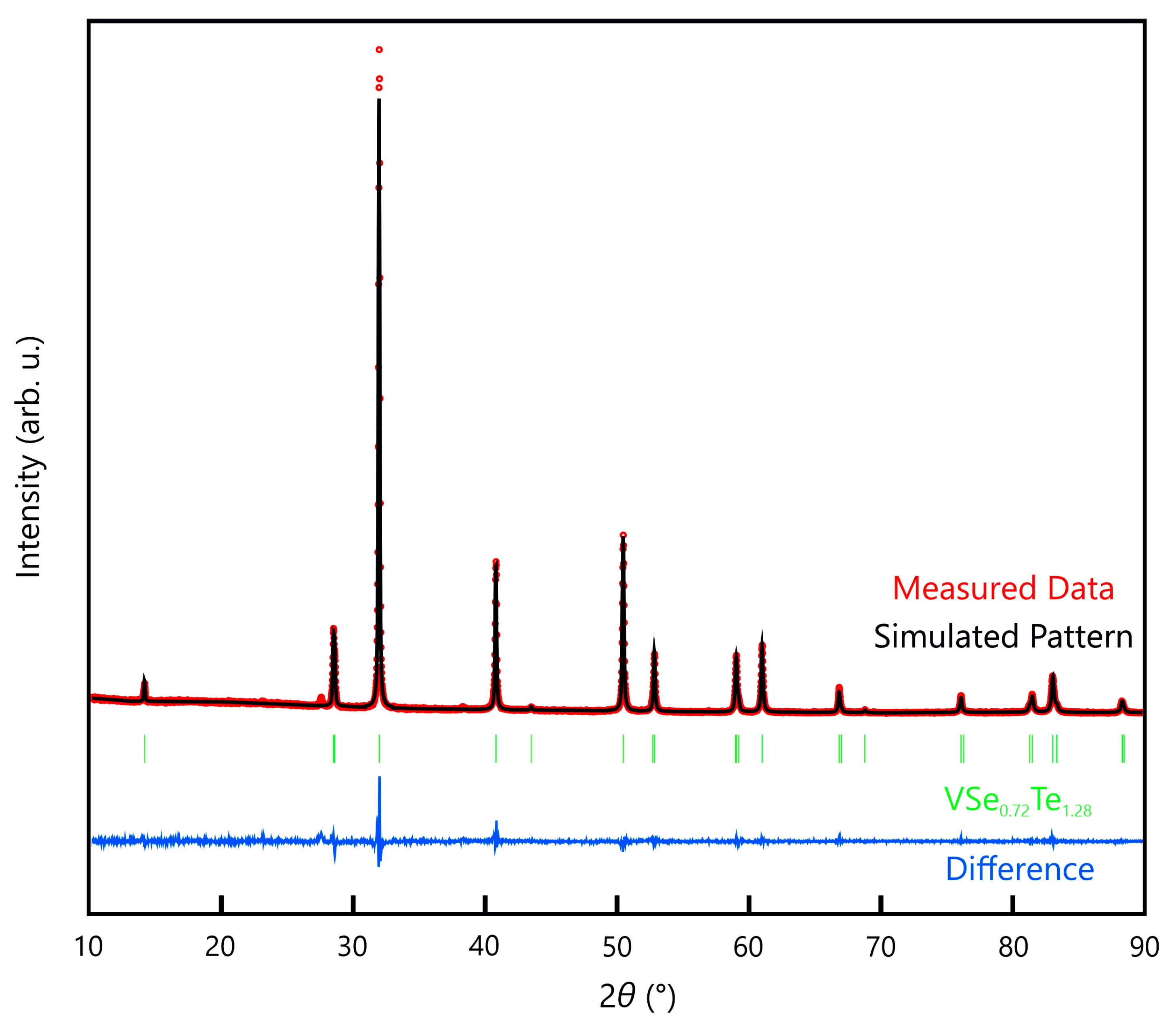
Figure 4 shows the powder pattern of VSe
0.72Te
1.28 with the corresponding Rietveld refinement.
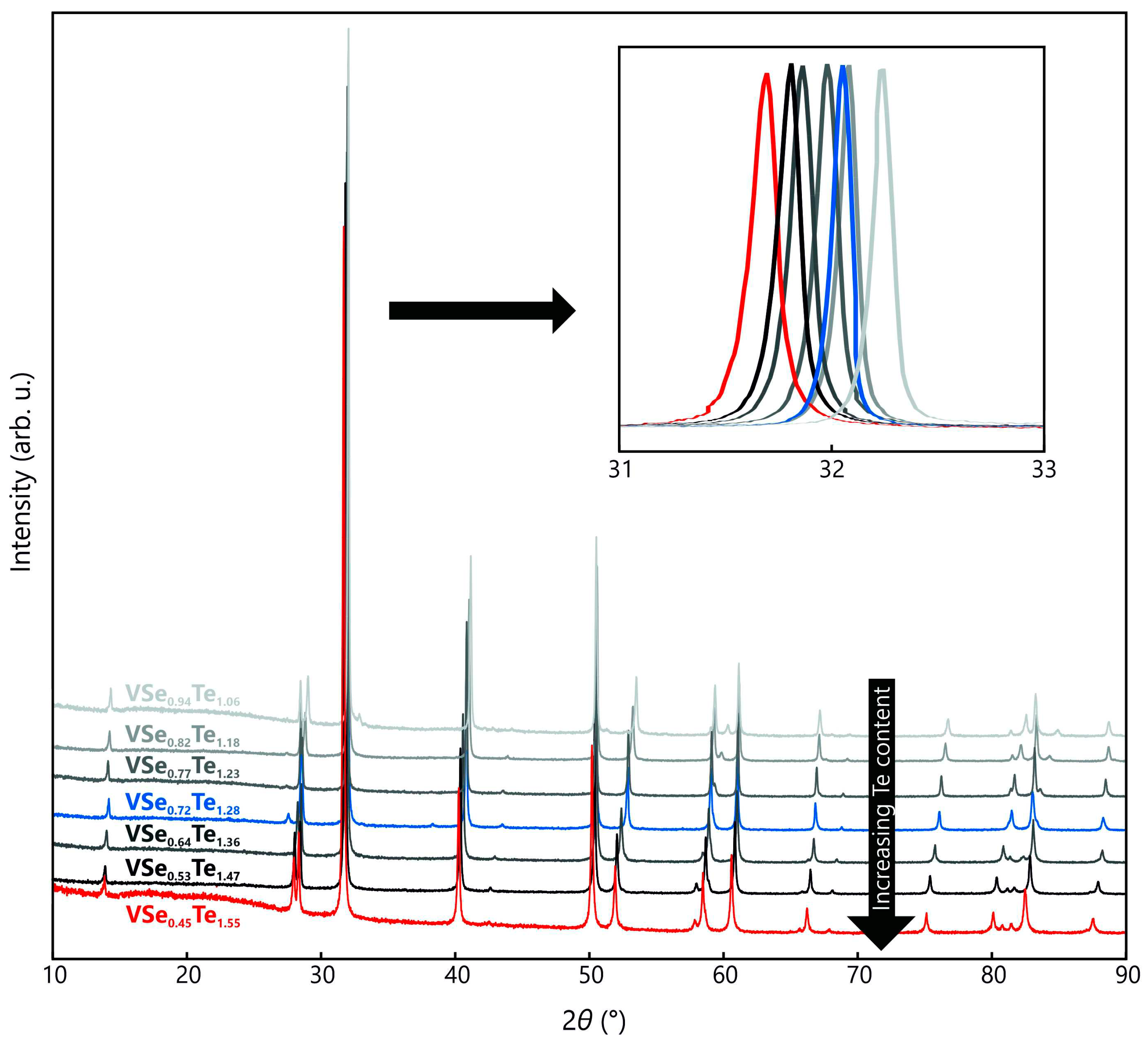
Figure 5 depicts powder-XRD patterns of VSe
yTe
2–y samples of different stoichiometries, with all reflections attributable to the respective Miller indices. With increasing tellurium content, we observe a shift towards lower 2
θ values due to the lattice expansion satisfying the spatial requirement of the tellurium anion.
For describing the lattice parameters and the resulting unit-cell volume of similar mixed-ion compounds of the same space group, Vegard’s law can be employed [
24,
25], relating the lattice parameters of mixed crystals to the lattice parameters of the corresponding pure solids weighted by the molar fraction of the respective constituent. For the ternary vanadium chalcogenides, Vegard’s law is applicable upon comparing VSe
2 with 1
T-VTe
2 [
7,
9].
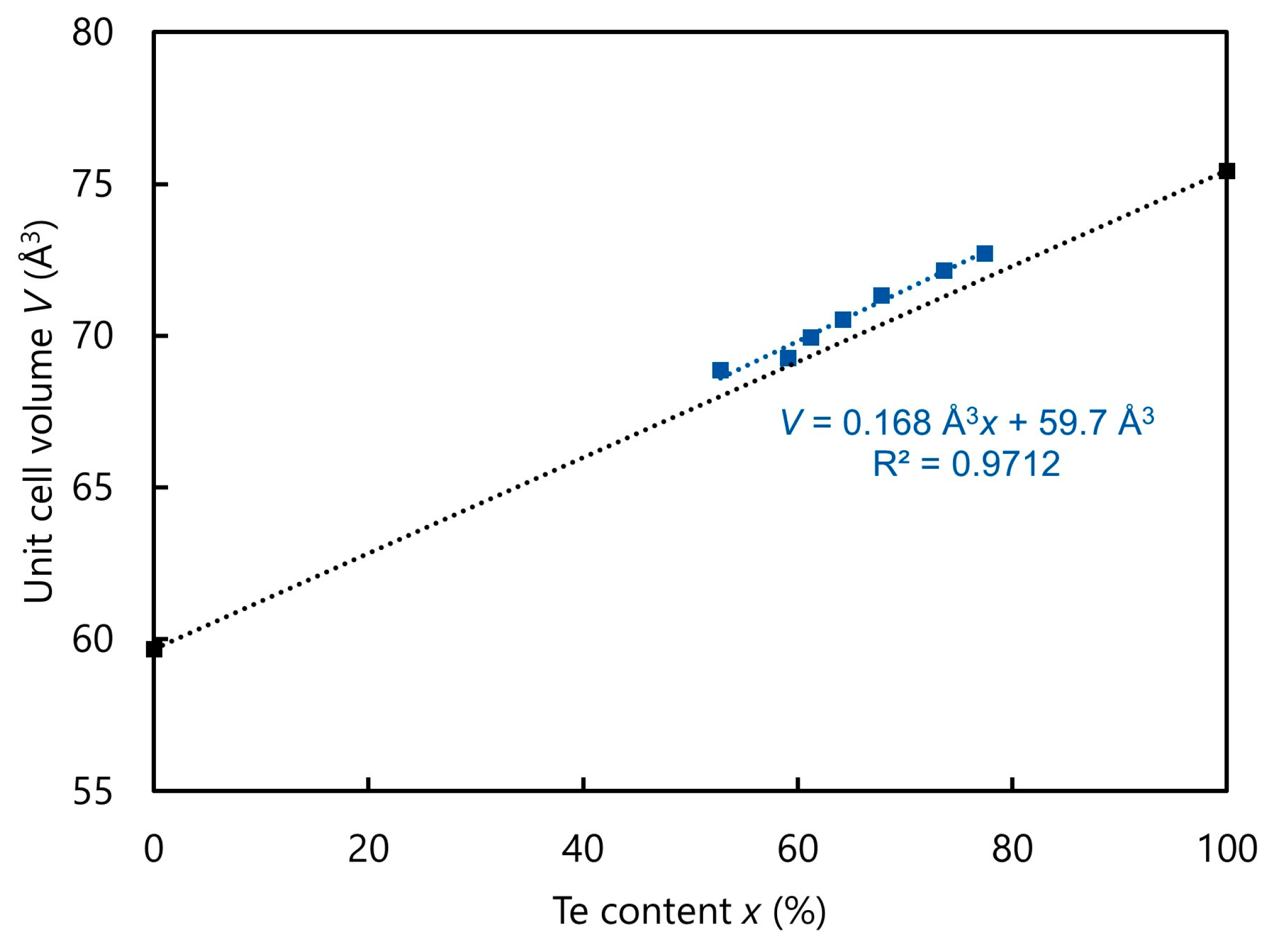
Figure 6 shows the correlation of the unit cell volume with the tellurium content of the powder samples.
As expected, the unit-cell volume increases with increasing tellurium content, explainable by the larger ionic radius of tellurium (2.21 Å for coordination number = 6) compared to selenium (1.98 Å for CN = 6) and thus an increase in spatial requirement [
26]. The linear fit describes the experimental volumes well, so Vegard’s law applies to this system. Furthermore, the predicted unit cell volume of 59.7 ų for pure VSe
2 corresponds to experimental findings by Wiegers (59.67 ų) [
7].
Simultaneous DSC/TGA measurements in nitrogen atmosphere revealed an endothermic decomposition to VN at temperatures above approximately 850 °C. Mass loss and heat flow are shown in
Figure S1 and the powder pattern in
Figure S2. Initial investigations at low temperature show no obvious structural changes in VSe
0.72Te
1.28 down to 30K. The collected powder pattern is shown in
Figure S3.
2.4. Magnetic measurements of VSe0.72Te1.28
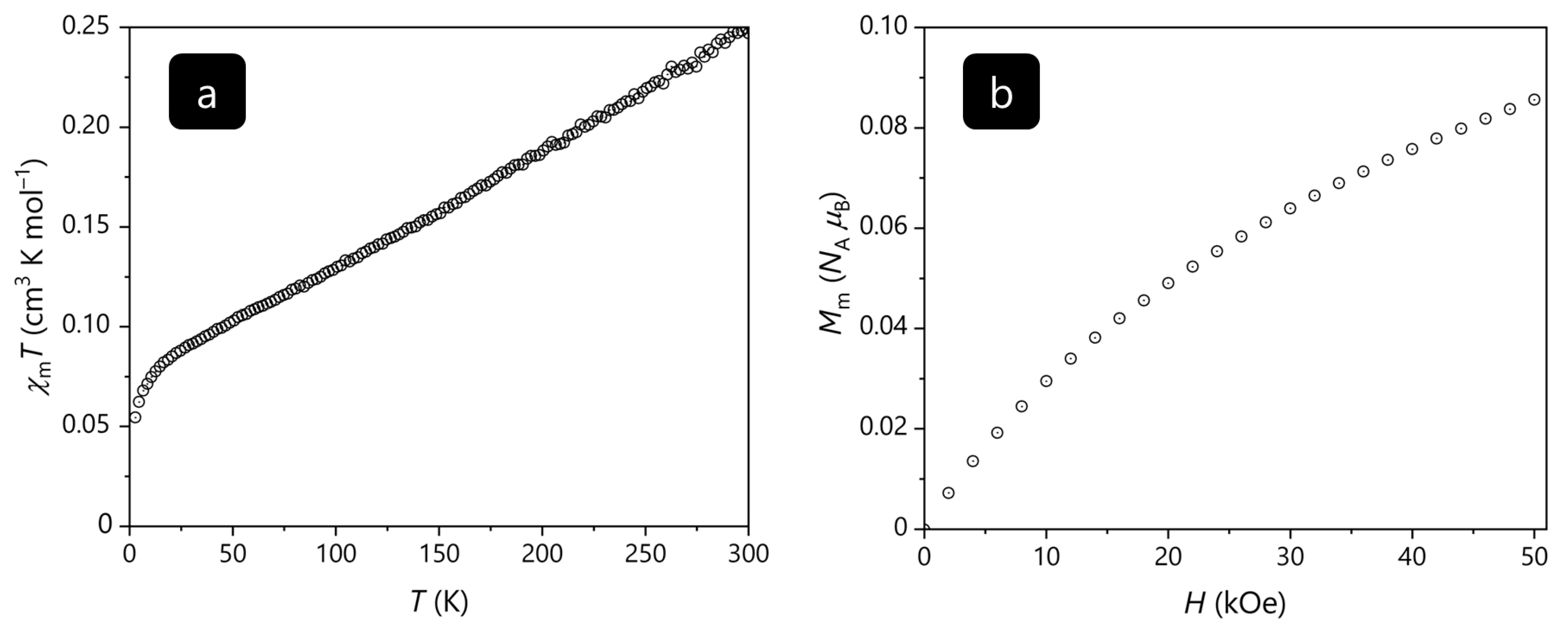
The magnetic data of VSe
0.72Te
1.28 normalized to one formula unit are shown in
Figure 7 as a
χmT vs.
T plot at 0.1 T and as a
Mm vs.
H plot at 2.0 K. At 300 K,
χmT is 0.25 cm
3 K mol
–1, which is well below the range 0.34–0.38 cm
3 K mol
–1 expected [
27] from an isolated vanadium(IV) center. Upon cooling the compound,
χmT almost linearly decreases to 0.083 cm
3 K mol
–1 at 17 K, and subsequently drops to 0.054 cm
3 K mol
–1 at 2.8 K. At 2.0 K, the molar magnetization continuously increases by increasing the applied magnetic field. At 50 kOe, the
Mm vs.
H curve is characterized by a distinct slope and the value
Mm = 0.09
NA μB. Both observations show that the magnetization is far from being saturated under these conditions. E.g., the saturation value is
Mm,sat =
gS NA μB ≈ 1.0
NA μB for a vanadium(IV) center with the valence electron configuration 3
d1, an effective
g value of
g ≈ 2.0 and spin
S = ½.
Even though a slight deviation from the spin-only value of χmT = 0.375 cm3 K mol–1 (S = ½) is expected for a 3d1 center even in a perfect octahedral ligand field, since contributions from the orbital momentum are not fully quenched, the found value at 300 K is beyond such an explanation. However, the value of 0.25 cm3 K mol–1 as well as the distinct slope of the χmT vs. T curve over the entire measured temperature range (instead of being almost constant) indicate predominantly strong antiferromagnetic exchange interactions between the vanadium centers. This conclusion is also supported by the magnetization data at 2.0 K, since strong antiferromagnetic interactions result in the observed and above discussed features of the Mm vs. H curve.
3. Materials and Methods
3.1. Syntheses
Ceramic syntheses were carried out starting from pure vanadium (99.5%, Alfa Aesar), selenium (99.999%, MaTeck), and tellurium (≥ 99.7%, Fluka AG) in stoichiometric quantities (≈ 300 mg per experiment) in evacuated quartz glass ampoules (length 9 cm, diameter 0.7 cm) in a horizontal tube furnace. The applied temperature program involved heating to 800 °C with a rate of 80 °C h‒1, holding this temperature for 120 h, and subsequently quenching in icy water for all phases. The reaction products were gray, metallically lustering powders containing small crystals that were isolated and analyzed with single-crystal X-ray diffraction experiments. So far, the powders appear as being stable in air for at least one year.
3.2. Single-crystal X-Ray diffraction experiments and structure resolution
Single-crystal X-ray diffraction experiments were carried out on a Bruker APEX II diffractometer (Bruker Corporation, Billerica, MA, USA) with Mo-
Kα (λ = 0.71073 Å) radiation using a CCD detector. Integration and absorption correction of the experimental data was carried out with APEX2 [
28]. The structures were solved and refined using direct methods in WinGX 2023.1 [
29] and SHELXL 2018/1 [30-32]. The well-resolved data allowed to refine the fully occupied V1 (1
a) and sub-occupied V2 (1
b) positions with independent anisotropic displacement parameters (ADPs). Because the Se and Te essentially occupy the same (2
b) site, with only a small displacement difference of about 0.33 Å (V
1.10Se
0.42Te
1.58) and 0.19 Å (V
1.13Se
0.72Te
1.28), their site-occupation factors were coupled to full occupancy for the 2
b site. The larger displacement difference in V
1.10Se
0.42Te
1.58 allowed to refine Se and Te with individual ADPs while in V
1.13Se
0.72Te
1.28 a common ADP set for Se and Te atoms was used. Full details concerning the structure determinations are available in CIF format and have been deposited as CCDC 2306778 and 2306779. These data can be obtained free of charge via
www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
3.3. Powder X-Ray diffraction experiments and refinement
To obtain powder X-ray diffraction data, the gray reaction products were mechanically ground and placed between Mylar sheets in a sample holder. Powder X-ray diffraction analysis was carried out on a STADI P powder diffractometer (STOE & CIE GmbH, Darmstadt, Germany) equipped with a DECTRIS® MYTHEN detector and Ge single-crystal monochromator with Cu-
Kα1 radiation (λ = 1.54059 Å). Powder X-Ray diffraction measurements were controlled with the aid of the WinXPow software [
33].
Data refinement was carried out using the Rietveld method with the FullProf suite [
22], starting with a model derived from the single-crystal structure refinement of V
1.13Se
0.72Te
1.28 .
Low temperature data were collected on a Huber G670 powder diffractometer (HUBER Diffraktionstechnik GmbH & Co. KG, Rimsting, Germany) equipped with a copper X-ray tube in combination with a Ge Huber 616.2 monochromator.
3.4. Scanning Electron Microscopy (SEM) with Energy dispersive X-ray spectroscopy measurements (EDX)
Energy dispersive X-ray spectroscopy measurements (EDX) were carried out on a Leo Supra 35 VP scanning electron microscope (SEM) from Carl Zeiss AG (Oberkochen, Germany). By means of an acceleration voltages of 10 kV, the measurements were performed using an INCA Energy 200 spectroscope with a SiLi crystal (133 eV, 10 mm2) from Oxford Instruments (Abingdon, UK). For the measurement, the sample was deposited on a carbon sticky tape pasted on an aluminium holder.
3.5. Measurements of the magnetic properties
A Quantum Design DynaCool physical property measurement system (PPMS) (Quantum Design International, Inc., San Diego, CA, USA) was used in the vibrating sample magnetometry (VSM) option. A polycrystalline sample of VSe0.77Te1.23 was immobilized using a polypropylene sample capsule and a brass holder. The data were measured as a function of temperature (2–300 K at 0.1 T) and magnetic field (0.1−5 T at 2 K), then corrected for the diamagnetic contributions of the sample holder and the compound (χm,dia = –1.37×10‒4 cm3 mol‒1).
4. Conclusions
The new ternary compounds exhibit similar structures to the already known V
1.04Se
2 phase and a high-temperature modification of VTe
2 [
8,
9]. The selenium and tellurium atoms, respectively, occupy the Wyckoff site 2
d, and the lattice parameters were found to lie between the ones of the structures of the binaries. Powder diffractograms of seven compounds of different stoichiometries within the V
wSe
yTe
2–y system evidence the expected trend, namely that a larger tellurium content leads to a shift of the reflection positions to lower 2 values.
Simultaneous DSC/TGA measurements in nitrogen atmosphere revealed an endothermic decomposition to VN at temperatures above approximately 850 °C. The magnetic measurements evidences predominantly strong antiferromagnetic coupling.
Supplementary Materials
Single-crystal data in CIF format can be downloaded at: Preprints.org, Table S1: Spatial parameters in V1.13Se0.72Te1.28 and V1.10Se0.42Te1.58; Figure S1: Thermogravimetric analysis and differential scanning calorimetry of VSe0.72Te1.28; Figure S2: Comparison of VSe0.72Te1.28 powder X-ray data after synthesis, after DSC/TGA, and Bragg positions of VSe and VN; Figure S3: Comparison of VSe0.72Te1.28 powder X-ray data at room temperature and at 30 K.
Author Contributions
Conceptualization S.K.; methodology S.K., A.F., B.V.H. and J.v.L.; validation J.v.L. and R.D.; formal analysis F.K., S.K., A.F. and J.v.L.; investigation F.K., S.K., A.F. and B.V.H.; resources B.V.H., A.F., K.F. and R.D.; data curation S.K.; writing—original draft preparation F.K. and S.K.; writing—review and editing S.K., F.K., A.F., B.V.H., J.v.L., K.F. and R.D.; visualization F.K., S.K. and J.v.L.; supervision K.F. and R.D.; project administration, S.K., K.F. and R.D.; funding acquisition K.F. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Research Foundation (DFG, Bonn, Germany; SFB 917 “Nanoswitches”; S.K., B.V.H., K.F. and R.D.).
Data Availability Statement
The crystallographic data of V1.10Se0.42Te1.58 and V1.13Se0.72Te1.28 are provided in separate CIF files.
Acknowledgments
The authors thank Tobias Storp for the collection of powder and single-crystal X-ray diffraction data, Birgit Hahn for EDX measurements and Dr. Shibabrata Nandi and Hend Shahed for their assistance with the PPMS measurement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dunn, J.P.; Stenger, H.G.; Wachs, I.E. Oxidation of SO2 over Supported Metal Oxide Catalysts. J. Catal. 1999, 181, 233–243. [Google Scholar] [CrossRef]
- Sanap, P.P.; Gupta, S.P.; Kahandal, S.S.; Gunjakar, J.L.; Lokhande, C.D.; Sankapal, B.R.; Said, Z.; Bulakhe, R.N.; Man Kim, J.; Bhalerao, A.B. Exploring vanadium-chalcogenides toward solar cell application: A review. J. Ind. Eng. Chem. 2023. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z. Carbon-coated vanadium selenide as anode for lithium-ion batteries and sodium-ion batteries with enhanced electrochemical performance. Mater. Lett. 2017, 189, 152–155. [Google Scholar] [CrossRef]
- Hoschek, E.; Klemm, W. Vanadinselenide. Z. Anorg. Allg. Chem. 1939, 242, 49–62. [Google Scholar] [CrossRef]
- Ehrlich, P. Über die binären Systeme des Titans mit den Elementen Stickstoff, Kohlenstoff, Bor und Beryllium. Z. Anorg. Chem. 1949, 259, 1–41. [Google Scholar] [CrossRef]
- Biltz, W.; Köcher, A. Beiträge zur systematischen Verwandtschaftslehre. 88. Über das System Vanadium/Schwefel. Z. Anorg. Allg. Chem. 1939, 241, 324–337. [Google Scholar] [CrossRef]
- Wiegers, G.A. Physical properties of first-row transition metal dichalcogenides and their intercalates. Physica B+C 1980, 99, 151–165. [Google Scholar] [CrossRef]
- Nakahira, M.; Hayashi, K. Characterization of the layered transition-metal dichalcogenides with octahedral coordination. Mat. Res. Bull. 1978, 13, 1403–1408. [Google Scholar] [CrossRef]
- Bronsema, K.D.; Bus, G.W.; Wiegers, G.A. The crystal structure of vanadium ditelluride, V1+xTe2. J. Solid State Chem. 1984, 53, 415–421. [Google Scholar] [CrossRef]
- van Bruggen, C.F.; Haas, C. Magnetic susceptibility and electrical properties of VSe2 single crystals. Solid State Commun. 1976, 20, 251–254. [Google Scholar] [CrossRef]
- DiSalvo, F.J.; Waszczak, J.V. Magnetic studies of VSe2. Phys. Rev. B 1981, 23, 457–461. [Google Scholar] [CrossRef]
- Won, D.; Kiem, D.H.; Cho, H.; Kim, D.; Kim, Y.; Jeong, M.Y.; Seo, C.; Kim, J.; Park, J.-G.; Han, M.J.; et al. Polymorphic Spin, Charge, and Lattice Waves in Vanadium Ditelluride. Adv. Mat. 2020, 32, 1906578. [Google Scholar] [CrossRef] [PubMed]
- Duvjir, G.; Jung, J.-A.; Ly, T.T.; Lam, N.H.; Chang, Y.J.; Lee, S.; Kim, H.; Kim, J. Fine structure of the charge density wave in bulk VTe2. APL Mater. 2022, 10. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Bensch, W.; Benea, D.; Ebert, H. Anion substitution effects on structure and magnetism in the chromium chalcogenide Cr5Te8—Part I: Cluster glass behavior in trigonal Cr(1+x)Q2 with basic cell (Q=Te, Se; Te:Se=7:1). J. Solid State Chem. 2004, 177, 3245–3253. [Google Scholar] [CrossRef]
- Lotgering, F.K.; Gorter, E.W. Solid solutions between ferromagnetic and antiferromagnetic compounds with NiAs structure. J. Phys. Chem. Solids 1957, 3, 238–249. [Google Scholar] [CrossRef]
- Tsubokawa, I. The Magnetic Properties of Chromium-Tellurium-Selenium System. J. Phys. Soc. Jpn. 1956, 11, 662–665. [Google Scholar] [CrossRef]
- Wontcheu, J.; Bensch, W.; Mankovsky, S.; Polesya, S.; Ebert, H.; Kremer, R.K.; Brücher, E. Anion substitution effects on the structure and magnetism of the chromium chalcogenide Cr5Te8—Part III: Structures and magnetism of the high-temperature modification Cr(1+x)Q2 and the low-temperature modification Cr(5+x)Q8 (Q=Te, Se; Te:Se=5:3). J. Solid State Chem. 2008, 181, 1492–1505. [Google Scholar] [CrossRef]
- Arnaud, Y.; Chevreton, M. Etude comparative des composés TiX2 (X = S, Se, Te). Structures de TiTe2 et TiSeTe. J. Solid State Chem. 1981, 39, 230–239. [Google Scholar] [CrossRef]
- Rimmington, H.P.B.; Balchin, A.A. The growth by iodine vapour transport techniques and the crystal structures of layer compounds in the series TiSxSe2−x, TiSxTe2−x, TiSexTe2−x. J. Crystal Growth 1974, 21, 171–181. [Google Scholar] [CrossRef]
- Dey, S.; Lee, J.; Britto, S.; Stratford, J.M.; Keyzer, E.N.; Dunstan, M.T.; Cibin, G.; Cassidy, S.J.; Elgaml, M.; Grey, C.P. Exploring Cation–Anion Redox Processes in One-Dimensional Linear Chain Vanadium Tetrasulfide Rechargeable Magnesium Ion Cathodes. J. Am. Chem. Soc. 2020, 142, 19588–19601. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4 ed.; Springer New York, NY: New York, NY, USA, 2017. [Google Scholar]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B: Condens. Matter. 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent developments of the program FULLPROF. Commission on Powder Diffraction (IUCr) Newsletter 2001, 26, 12. [Google Scholar]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Lueken, H. Magnetochemie; Teubner Verlag: Stuttgart, Germany, 1999. [Google Scholar]
- APEX2, v2014. 11-0, Bruker AXS: Madison, WI, USA, 2015.
- Farrugia, L. WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- WinXPOW Powder Diffraction Software, 3. 7.0.0; STOE & Cie GmbH: Hilpertstr. 10, 64295 Darmstadt, Germany, 2021. [Google Scholar]
- Sakuma, T.; Xianglian; Siagian, S. ; Basar, K.; Takahashi, H.; Igawa, N.; Kamishima, O. Correlation effects among thermal displacements of atoms in VSe by diffuse neutron scattering measurement. J. Therm. Anal. Calorim. 2010, 99, 173–176. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Ripp, G.S.; Yakovlev, G.A.; Seryotkin, Y.V.; Karmanov, N.S.; Izbrodin, I.A.; Grokhovsky, V.I.; Khromova, E.A. Uakitite, VN, a New Mononitride Mineral from Uakit Iron Meteorite (IIAB). Minerals 2020, 10, 150. [Google Scholar] [CrossRef]
- Lengauer, W.; Ettmayer, P. Lattice parameters and thermal expansion of δ-VN1−x from 298–1000 K. Monatsh. Chem. 1986, 117, 713–719. [Google Scholar] [CrossRef]
- Ettmayer, P.; Schebesta, W.; Vendl, A.; Kieffer, R. Beitrag zur Kenntnis des Systems Vanadin—Chrom—Stickstoff. Monatsh. Chem. 1978, 109, 929–941. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).