1. Introduction
An atrophied posterior mandible, because of tooth loss, periodontal pathologies, tumor removal, trauma, or congenital diseases, represents a challenge for implant-supported rehabilitation because of the reduction in bone volume between the residual alveolar ridge and the mandibular canal [
1,
2,
3]. In this scenario, bone augmentation has become an integral part of implant dentistry [
4,
5,
6].
The inlay grafts showed optimal incorporation to the recipient sites, presenting small resorption over time and high implant survival rates [
7,
8,
9]. Although good results have been reported for posterior mandibular bone augmentation using this technique [
10,
11,
12,
13], it demands considerable surgical experience and requires sufficient bone height above the mandibular canal [
11,
12].
Onlay bone grafts had been successfully applied in mandibular bone augmentation. However, high rates of bone resorption were reported with this technique at implant installation [
2,
14]. An important concern in the restoration is the lack of keratinized tissue that may contribute to wound dehiscence and possible infection and subsequent necrosis of the onlay graft [
15].
Autogenous bone collected from extra- or intraoral donor sites is considered the gold standard graft for alveolar ridge augmentation due to its osteoconductive, osteoinductive, and osteogenic properties [
16,
17,
18,
19]. Nevertheless, autografts have several disadvantages such as wound complications and infection at the donor site, paresthesia, increased timing for surgery and recovery, and enhanced postoperative pain. Moreover, the availability of bone might limit the volume of the graft [
20,
21,
22,
23,
24,
25].
Xenogenous might be considered an alternative to overcome the disadvantages of autogenous grafting [
26,
27,
28]. A recent study compared graft healing of autogenous graft and xenogeneic equine bone graft applied as onlay for bone augmentation in rabbits [
29]. Similar proportions on new bone were observed after 60 days. More recently, collagenated bone grafts from equine were used as inlay or onlay on the lateral wall of the mandible of rabbits. It has been observed that the proportion of newly formed bone increased faster and was higher in the inlay group [
30]. It was observed that the proportion of newly formed bone increased more rapidly and was greater in the inlay group.
Installing implants simultaneously with a bone graft can reduce the total treatment time by eliminating a second surgery. Several clinical [
31,
32,
33,
34,
35,
36] and experimental [
37,
38,
39,
40,
41] studies reported data on implant installed with ring grafts secured by implant for vertical bone augmentation.
To investigate the efficacy of this approach, a randomized clinical trial evaluated marginal bone loss of autogenous bone grafts collected from the chin used for either inlay or onlay grafting with concurrent implant placement [
42]. Bone gain was significantly higher for the inlay group. However, the comparison between xenogeneic bone blocks used for inlay and onlay grafts with implants has not been investigated yet.
Thus, the aim of the experiment was to study bone dynamics at inlay and onlay xenografts used for bone augmentation applying a ring technique.
2. Materials and Methods
2.1. Ethical Statements
The Ethical Committee for Animals use of the Dental Faculty of Ribeirão Preto, USP, Brazil approved the experiment on October 16, 2019, as reported in protocol 2019.1.694.58.4. The rules reported in the rules for animal experimentation in Brazil were strictly followed. The ARRIVE Checklist was followed.
2.2. Study Design
This was paired, prospective, controlled, randomized study. Xenograft blocks were applied to the lateral wall of the mandible using the onlay or inlay techniques. Both blocks were fixed to the recipient sites using an implant. The healing was evaluated after 10 weeks.
2.3. Experimental Model and Sample Size Calculation
The results from a similar experiment in rabbits were used to determine the sample size [
43]. Considering the maximum standard deviation found to be 7.4% and 10% of the difference between the two groups as histologically relevant, an n=8 was obtained applying α = 0.05 and a power of 0.9 (PS, Dupont and Walton D. Plummer). The sample size was increased to 12 to account for possible loss of animals or experimental sites.
Hence, 12 adult male New Zealand White rabbits, 3.5–4.0 kg of wight, and 5-6 months of age, were comprised incorporated in the study.
2.4. Randomization and Allocation Concealment
An author not involved in animal selection, surgery and histological evaluation performed electronically the randomization (S.P.X.). The allocation treatment was concealed in coded envelopes which were opaque and sealed. The envelopes opened after recipient site preparation just before the placement of the first xenogeneic block. The histological slides were examined by an assessor (E.F.D.R., see acknowledgements) who was not informed about the allocation treatment. However, the treatment inlay/onlay was easily identified on histological slides.
2.5. Biomaterials
SpBlock is a xenograft block of exclusively collagenated cancellous equine bone (Tecnoss, Giaveno, Italy). This process prevents ceramization of hydroxyapatite crystals, aiming to accelerate resorption. Cylindrical blocks were prepared with diameters of 7 mm and thicknesses of 3 mm. A hole, in the dimensions of the implant, was prepared in the center of the blocks (
Figure 1).
Bio-Gide (Geistlich Biomaterials, Wolhusen, LU, Switzerland) is a porcine-derived resorbable membrane composed of types I and III collagen. The bilayer structure contains an outer smooth layer that prevents the invasion of soft tissues and an inner layer that favors the growth of vessels and cells. [
44].
2.6. Anesthetic Procedures
Acepromazine I.M. (1.0 mg/kg; Acepran, Vetnil, Louveira, São Paulo), and xylazine (3.0 mg/Kg; Laboratórios Calier S/A, Barcelona, Spain) in conjunction with ketamine I.M. (50.0 mg/Kg; União Química Farmacêutica Nacional S/A, Embuguaçú, São Paulo, Brazil) were administered. Oxytetracycline was administered once the animals were sedated. (0.2 ml/Kg; Biovet, Vargem Grande Paulista, São Paulo, Brazil). After shaving and disinfecting the experimental region, local anesthesia was injected.
2.7. Surgical Procedure
One qualified expert surgeon performed all surgeries (V.F.B.; see acknowledgments).
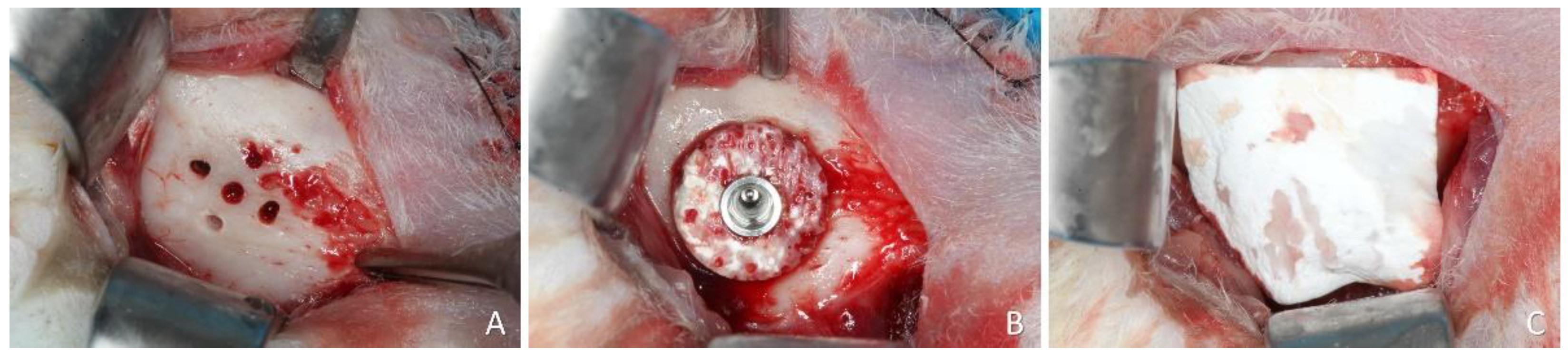
The lower edge of the mandible was incised and the bone on the lateral wall of the mandible was exposed. At the onlay sites, the cortical bone was perforated up to the marrow compartment with a 1.0 mm truncated-conical drill (
Figure 2A).
The central perforation was enlarged for implant placement. The xenogeneic block was secured on the recipient region using an implant 8.5 mm long and 3.25 mm in diameter (Leader Medica, Padua, Italy) (
Figure 2B). The shoulder of the implant was placed at about the level of the graft. A healing cap was placed on the implant and the experimental site was covered with a resorbable membrane (BioGuide®; Geistlich Pharma AG, Wolhusen, Switzerland) (
Figure 2C).
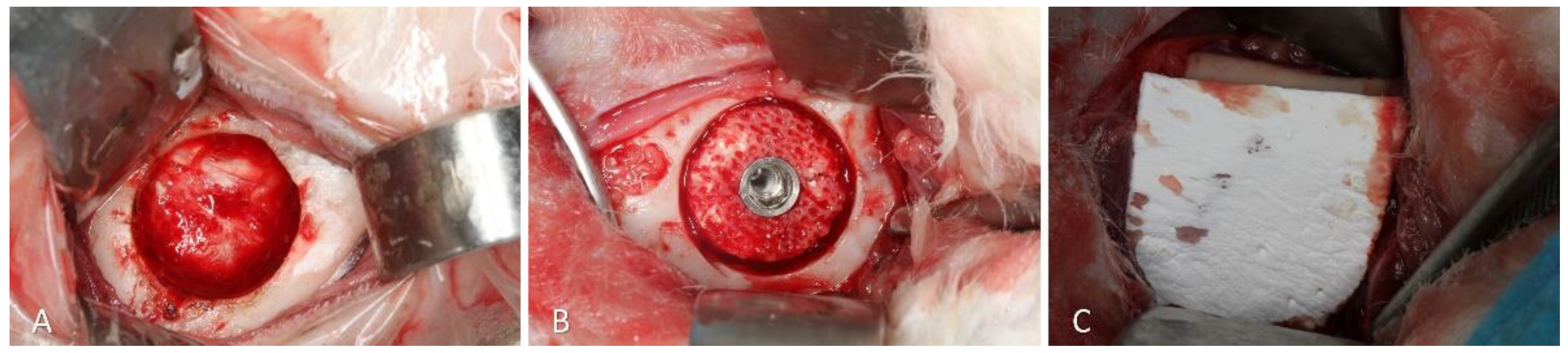
Trephines and drills were used on the opposite side to obtain a defect about 7 mm in diameter and about 3 mm deep (
Figure 3A). The xenogeneic graft was adapted within the defect and fixed with an implant 8.5 mm long and 3.25 mm in diameter (Leader Medica, Padua, Italy) (
Figure 3B). After placement of the cover screw, the region was protected with a resorbable membrane (BioGuide®; Geistlich Pharma AG, Wolhusen, Switzerland) ((
Figure 3C). The wounds were closed with double sutures, internal with Vicryl 4-0, and external with Nylon 4-0.
2.8. Animal Maintenance
After surgery and during the following 3 days, the animals received ketoprofen (3.0 mg/kg, 12/12h, i.m., 10% Ketofen, Merial, Campinas, São Paulo, Brazil) and 2% tramadol hydrochloride (1.0 mg/kg 12/12h, subcutaneous; Cronidor, Agener União Saúde Animal, Apucarana, Paraná, Brazil).
The rabbits were housed in individual metal cages (1 animal/4500 cm²) in an acclimatized room with split air conditioning, an exhaust fan (27 to 34 air changes/h), and automatic lighting control (12-hour light-dark cycle) at the Animal Facility of the Faculty of Dentistry of Ribeirão Preto, University of São Paulo. The animals received dedicated food and had ad libitum access to water. Every day, a careful check of the basic biological functions was performed.
2.9. Euthanasia
Euthanasia was performed with an overdose of thiopental I.V. (Thiopentax; Cristália, Itapira, São Paulo, Brazil) and the biopsies of the experimental regions were harvested.
2.10. Histological Processing
After fixation in paraformaldehyde, the biopsies were dehydrated in alcohol and then, they were included in resin (LR WhiteTM HardGrid, London Resin Co Ltd, Berkshire, United Kingdom) and polymerized. Subsequently, the histological slides were prepared using a cutting/grinding equipment (Exakt, Apparatebau, Norderstedt, Germany. Stevenel’s Blue and Alizarin Red or Toluidine Blue were applied for staining.
2.11. Histomorphometric Evaluation
An expert assessor (E.F.D.R., see acknowledgements) performed all histological measurements, after a calibration with another expert (D.B.). The inter-rater agreement has to reach a coefficient k > 0.90.
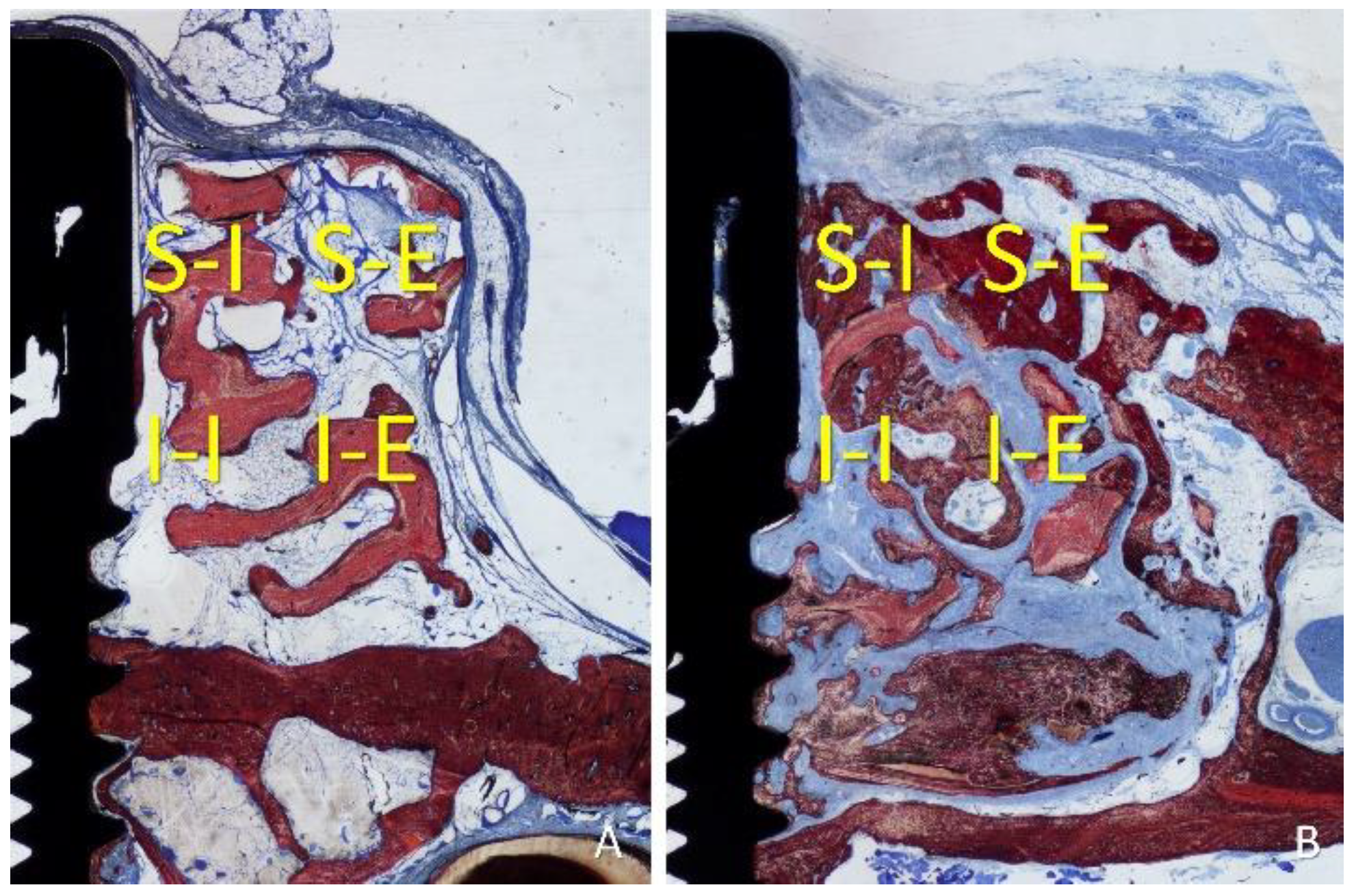
An Eclipse Ci microscope (Nikon Corporation, Tokyo, Japan) connected to a computer was used for histological assessments applying a x10 lens. Histological measurements were performed with the NIS Elements D software (v 5.0, Laboratory Imaging, Nikon Corporation, Tokyo, Japan). As linear evaluation, the distance between the implant margin (M) and the most coronal contact of the bone with the implant surface (B) was measured. The gain of osseointegration was evaluated as the difference between the depth of the original defect (3 mm) and the distance M-B. For morphometric measurements, a lattice was superposed on the image applying a point counting methods. Four regions were evaluated within the grafted region, both sides lateral to the implant: inferior/internal (I-I), inferior/external (I-E), superior/internal (S-I), and superior/external (S-E). The following tissues were assessed: new bone, xenograft, soft tissues (marrow spaces, provisional matrix, dense and loose tissues, and connective tissue), and inflammatory infiltrate (
Figure 4A,B). A correlation between the bone percentage in the S-I region and the gain of osseointegration was carried out.
2.12. Experimental Outcomes and Statistical Methods
The primary variables were mineralized new bone percentage within the grafts and osseointegration gain while the other tissues were considered secondary variables. Differences between onlay and inlay were evaluated using a paired t-test or Wilcoxon matched-pairs signed-rank test. The selection of the test was based on the results of normality assessed by applying the Shapiro-Wilk test. GraphPad Prism (version 10.0.2 for Windows, GraphPad Software, Boston, Massachusetts, USA) was used for statistical analysis. The level of significance was 5%.
3. Results
3.1. Clinical Outcomes
The post-surgical period of the animals was uneventful. No biopsies were lost, and the histological analysis could be performed in all sites, maintaining the original sample of n=12.
3.2. Descriptive Histological Evaluation
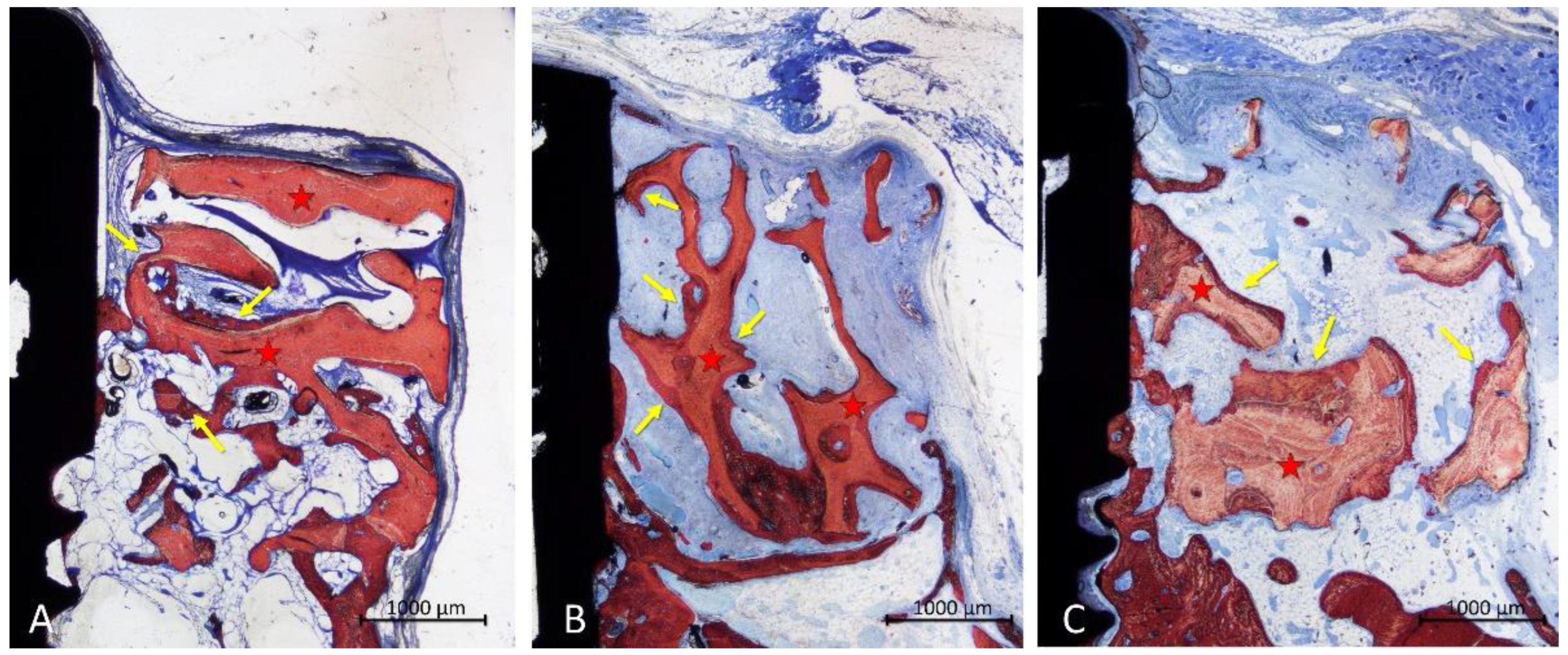
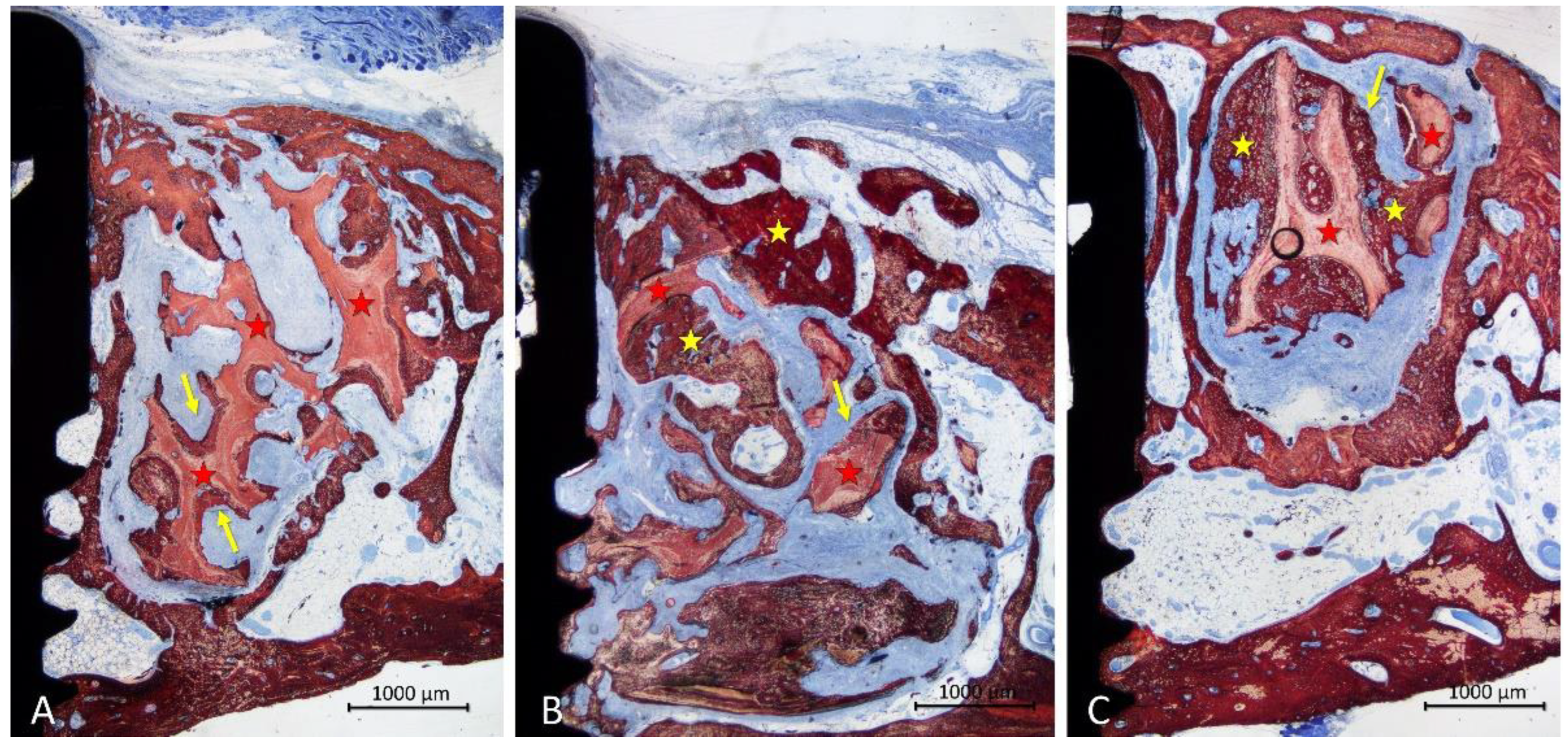
In the onlay group, new bone was mainly laying on the xenograft trabeculae (
Figure 5A,B), reaching in some cases the most superficial region (
Figure 5 C). The areas within the trabeculae were filled in a few cases with bone marrow (
Figure 5A,C), or more often by dense soft tissue (
Figure 5B).
The configuration of the trabeculae of the graft seemed to have been maintained in several grafts, whereas in other cases, the trabeculae appeared to have lost the original conformation. An inflammatory infiltrate was observed in only one specimen. Most of the implants showed new bone on the surface of the implant within the defect, in some cases up to a distance from the implant margin <1 mm (
Figure 5C).
In the inlay group, new bone was distributed on the trabeculae but also occupying the spaces among them (
Figure 6A-C). A dense soft tissue was occupying the remaining spaces among the trabeculae. The coronal entrance of the defect was often found closed by bone produced from the edges of the defect, interconnected to the bone formed within the graft (
Figure 6A-C). The trabeculae of the graft were still present in the region, however, in different proportions in the various specimens. A small inflammatory infiltrate was observed only in one specimen. Most implants showed vast areas of osteointegration within the defect region, in some case formed up to <1 mm from the implant margin (
Figure 6A-C).
3.3. Histomorphometric Assessments
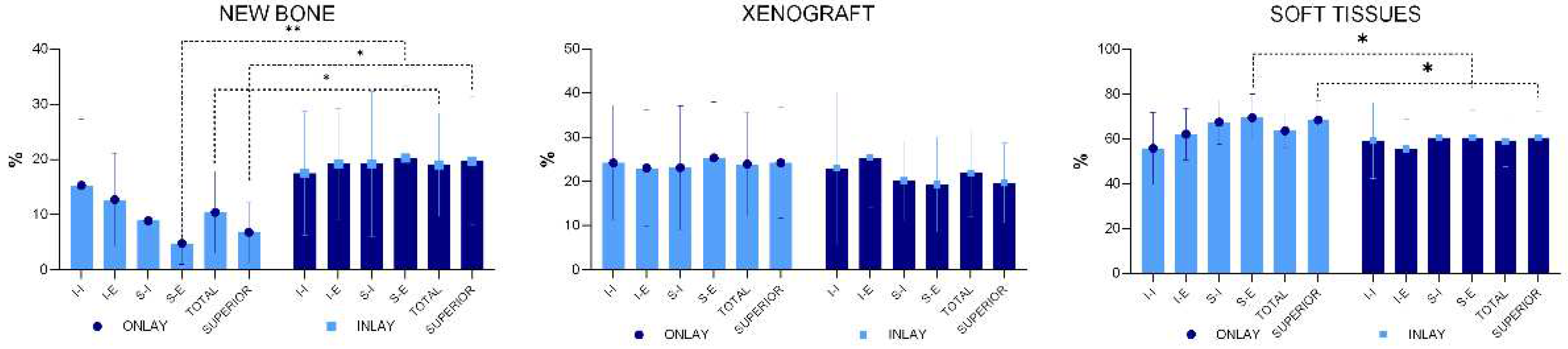
Statistically significant higher percentage of newly formed bone was present in the inlay compared to the onlay grafts, being 19.0 ±9.3% and 10.4 ±7.4% respectively (p=0.031) (
Figure 7). The percentage of new bone was higher in inlay grafts compared to onlay grafts in all four regions examined, the difference being statistically significant only for the S-E region. When the bone percentages of the two superior regions were merged (mean S-E and S-I), a statistically significant difference was found between the two groups (p=0.010).
The mean percentage of the xenograft did not yield statistically significant differences in any of the regions evaluated. An inflammatory infiltrate was observed only in one specimen of the onlay graft.
Even though in some cases the most coronal level of osteointegration on the implant was positioned <1 mm from the implant margin, other sites presented any or very little bone gain on the implant surface above the 3 mm deep original defect. The mean gains were 0.95 ±1.05% at the onlay group and 0.78 ±0.71% at the inlay group (p=0.603). The r value correlation between new bone percentage in the superior interior region S-I and level of osseointegration was 0.7 (strong positive linear relationship).
4. Discussion
The results from the experiment showed a higher new bone mean percentage in the inlay graft (19.0 ±9.3%) compared to the onlay graft (10.4 ±7.4%; p=0.031). The highest differences in bone formation between the two groups were observed in the region closer to the top of the grafts, i.e., the furthest regions from the walls/base of the defects.
The reasons for these differences in the proportion of new bone are to be referred to the different characteristics of the recipient regions. Indeed, inlay grafts were inserted into self-contained defects presenting walls at the base and around the graft. This means that the grafts could rely on bone production from various sources located around them. Instead, the onlay grafts could only rely on bone formed from the cortex at the base of the graft. From that recipient site, the new bone had to grow toward the top and the lateral sides of the graft.
Aiming to favor bone formation, several perforations of the cortical layer were made reaching the marrow spaces. This procedure has been shown to improve the healing within the graft. In a study in rabbits, the lateral aspect of the mandible was prepared with perforations only at one side while the opposite side was left intact [
45]. The iliac crest was used as donor site and the block grafts were secured on the mandibular recipient sites. Revascularization, volume/density maintenance and occurrence of bone remodeling proteins were evaluated at different periods of healing. Greater volume loss was observed in the intact site graft, while greater bone density was measured in the perforated sites. The VEGF labeling was present already after 3 days in the perforated grafts while, in the intact sites, the labeling appeared after 5 days. The immuno-labeling of osteoblastic lineage showed an accelerated bone remodeling process in the perforated sites. The effect of these perforations was illustrated in detail in an experiment in which grafts collected from the calvaria were applied on the mandibular body prepared with perforations [
46].
The onlay graft can only count on the osteoconductivity of the biomaterial that is of foremost importance. The lack of osteoconductivy of the biomaterial might lead to failures. In a dog study, block grafts composed of deproteinized bovine bone material (DBBM) were placed as onlay on the lateral wall of the mandible. A minimal incorporation of the xenograft was observed, close to the cortical bone of the mandible [
47]. In another dog experiment [
48], similar DBBM blocks were placed as inlay grafts in defects prepared in the alveolar bone crest in the mandible. In the opposite side of the mandible, a block of autogenous bone was used as graft. While the autogenous bone block presented a perfect incorporation to the recipient site, the xenograft failed to be incorporated, showing in most cases connective tissue between the graft and the recipient sites. Instead, collagenated blocks used as graft showed new bone within the graft, laying onto the trabeculae of the xenograft, reaching the furthest regions from the recipient site [
43].
The self-contained conformation of the defect used for the inlay graft offers multiple sources for bone formation. Depending on the dimension, self-contained defects have the potential to heal spontaneously, similarly to the extraction sockets. The spontaneous healing of critical-sized defects was evaluated in rabbits in both calvaria and mandible [
49]. In each animal, through-and-through circumferential defects were prepared with 10 mm of diameter in the calvaria, and 11 mm of diameter in the mandible. The defects in one side were filled with autologous bone or biphasic calcium phosphate granules while the opposite defects were left heal spontaneously. Both microCT and histological analysis revealed a failure in the closure of the empty defects. The defect applied in the present study presented more favorable conditions compared to the study mentioned above, i.e., smaller dimensions and a box conformation that allowed bone formation also from the base of the defect.
In another experimental study in rabbit [
50], 2 to 3 mm deep box defects of different dimensions (4, 5, 6, 8, and 10-mm) were prepared in the mandible. After 12 weeks of healing, the smallest defects were closed with newly formed bone. However, the 8 mm and 10 mm wide defects persisted underfilled. The defects applied in the present study had a box conformation and a diameter of 7 mm. Under such conditions, it is reasonable to assume that a certain degree of spontaneous healing should be expected. However, an implant was placed in the center of the defect to fix the graft. This implant affected the total volume of the defect and eventually transformed a 7 mm critical-sized defect into a circumferential peri-implant marginal defect with a gap <2 mm. This allows us to suppose that the placement of an implant might accelerate the healing. On the contrary, it has been already demonstrated that the implant retards the healing at marginal defects [
51]. In fact, in a marginal defect around an implant, new bone is formed from the lateral walls towards the implant surface, and it stops at about 0.4 mm from the surface, leaving a residual narrow defect around the implant surface, occupied by connective tissues. This defect is closed over time by newly formed bone only if the surface has osteoconductive properties [
52]. If the implant surface is not osteoconductive, the residual narrow defect will be not filled by new bone (Botticelli rough/turned; Akimoto) [
52,
53]. In the present study, an incomplete mean gain of implant osseointegration within the graft region was observed both in the inlay (0.78 mm) and in the onlay (0.95 mm) groups. In several cases the osseointegration reached a distance <1 mm from the implant margin in both groups, showing good osteoconductive properties of the implant surface. The low mean gain of osseointegration is due to a large variability of the results the reasons of which should be imputed to an insufficient new bone content in the internal regions of some grafts, especially in the superior-internal region. In the absence of bone close to the implant surface, the gain in osseointegration is hampered. This assumption is substantiated by the strong positive linear correlation between osseointegration gain and percentage of new bone in the superior-internal region.
A rabbit experiment showed bone formation features like those observed in the present study. [
30]. In that study, the onlay and inlay xenografts were of the same nature as those used in the present study. In that experiment, the block grafts were secured on the recipient sites with a fixation screw. Two healing periods were analyzed, i.e., 2 and 10 weeks. The results showed a higher proportion of new bone at the inlay compared to the onlay grafts. The percentage of the xenograft decreased of about 1/3 between the two periods of healing. In the present study, the evaluation was performed only after 10 weeks of healing. However, being the percentage of the residual grafts after 10 weeks similar between the two studies, it might be supposed that a similar percentage of graft resorption occurred in both studies.
A closure of the coronal entrance of the defects was observed in several specimens of the inlay blocks of the present study. This agrees with similar observations reported in the study mentioned above [
30].
The present study adopted a “ring” technique with immediate implant installation. This technique was used for vertical augmentation, the results of which have been reported in systematic reviews on both clinical [
54] and animal [
55] studies.
As every animal study, caution should be applied in the interpretation of data. However, it should be considered that these experiments provide outcomes that should be considered when similar procedures are applied in humans, and eventually confirmed or refuted by clinical studies. Further biomaterials and longer periods of healing should be analyzed.
5. Conclusions
The inlay grafts exhibited a higher new bone percentage than the onlay grafts. The reason might be credited to the defect conformation at the recipient site of the inlay grafts that presents more sources for bone formation compared to the onlay sites. The trabecular conformation and the composition of the grafts made possible the expression of the osteoconductive properties of the material used. This resulted, in several specimens, in growth of bone toward the most superior regions in both grafts, and in the closure of the coronal entrance of the defects in the inlay group.
Author Contributions
Conceptualization, N.K., S.P.X., K.M., and D.B.; methodology, N.K., S.P.X., and D.B.; validation, K.M., Y.N., S.B.; formal analysis, N.K., E.R.S., and D.B.; investigation, S.P.X. and E.R.S.; resources, S.P.X., D.B. and Y.N.; data curation, N.K. and D.B.; writing—original draft preparation, N.K., E.R.S., and D.B; writing—review and editing, N.K., S.P.X., D.B. and S.B.; visualization, K.M. and Y.N.; supervision, S.P.X., D.B., and S.B.; project administration, S.P.X., D.B. and Y.N.; funding acquisition, D.B., and Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
The experiment was financially supported by ARDEC Academy, Rimini, Italy.
Institutional Review Board Statement
This research project was approved by the Committee on Ethics in the Use of Animals of the Faculty of Dentistry of Ribeirão Preto, University of São Paulo, Brazil, on October 16, 2019, protocol #2019.1.694.58.4.
Data Availability Statement
The data is available following a reasonable request.
Acknowledgments
We thank Dr. Vitor Ferreira Balan for the surgical procedures and Mr Sebastiao Blanco (University of São Paulo, Faculty of Dentistry of Ribeirão Preto) for processing the histological slides. The scientific contribution in the histological assessment by Dr Ermenegildo Federico De Rossi (ARDEC Academy, Italy) is greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Felice P, Marchetti C, Piattelli A, Pellegrino G, Checchi V, Worthington H, Esposito M. Vertical ridge augmentation of the atrophic posterior mandible with interpositional block grafts: bone from the iliac crest versus bovine anorganic bone. Eur J Oral Implantol. 2008 Autumn;1(3):183-98. PMID: 20467621.
- Cordaro L, Amadé DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res. 2002 Feb;13(1):103-11. PMID: 12005140. [CrossRef]
- Chiapasco M, Zaniboni M, Rimondini L. Autogenous onlay bone grafts vs. alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: a 2-4-year prospective study on humans. Clin Oral Implants Res. 2007 Aug;18(4):432-40. Epub 2007 May 14. PMID: 17501979. [CrossRef]
- Marx RE. Bone and bone graft healing. Oral Maxillofac Surg Clin North Am. 2007 Nov;19(4):455-66, v. [CrossRef]
- Habal MB. Bone grafting in craniofacial surgery. Clin Plast Surg. 1994 Jul;21(3):349-63. [CrossRef]
- Louis PJ, Sittitavornwong S. Managing Bone Grafts for the Mandible. Oral Maxillofac Surg Clin North Am. 2019 May;31(2):317-330. Epub 2019 Mar 7. PMID: 30852175. [CrossRef]
- Stellingsma C, Raghoebar GM, Meijer HJ, Batenburg RH. Reconstruction of the extremely resorbed mandible with interposed bone grafts and placement of endosseous implants. A preliminary report on outcome of treatment and patients' satisfaction. Br J Oral Maxillofac Surg. 1998 Aug;36(4):290-5. PMID: 9762457. [CrossRef]
- Choi BH, Lee SH, Huh JY, Han SG. Use of the sandwich osteotomy plus an interpositional allograft for vertical augmentation of the alveolar ridge. J Craniomaxillofac Surg. 2004 Feb;32(1):51-4. PMID: 14729051. [CrossRef]
- Stoelinga PJ, Blijdorp PA, Ross RR, De Koomen HA, Huybers TJ. Augmentation of the atrophic mandible with interposed bone grafts and particulate hydroxylapatite. J Oral Maxillofac Surg. 1986 May;44(5):353-60. PMID: 3009757. [CrossRef]
- Yeung R. Surgical management of the partially edentulous atrophic mandibular ridge using a modified sandwich osteotomy: a case report. Int J Oral Maxillofac Implants. 2005 Sep-Oct;20(5):799-803. PMID: 16274157.
- Jensen OT. Alveolar segmental "sandwich" osteotomies for posterior edentulous mandibular sites for dental implants. J Oral Maxillofac Surg. 2006 Mar;64(3):471-5. PMID: 16487811. [CrossRef]
- Marchetti C, Trasarti S, Corinaldesi G, Felice P. Interpositional bone grafts in the posterior mandibular region: a report on six patients. Int J Periodontics Restorative Dent. 2007 Dec;27(6):547-55. PMID: 18092449.
- Bianchi A, Felice P, Lizio G, Marchetti C. Alveolar distraction osteogenesis versus inlay bone grafting in posterior mandibular atrophy: a prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Mar;105(3):282-92. PMID: 18280960. [CrossRef]
- Schwartz-Arad D, Levin L, Sigal L. Surgical success of intraoral autogenous block onlay bone grafting for alveolar ridge augmentation. Implant Dent. 2005 Jun;14(2):131-8. PMID: 15968184. [CrossRef]
- Felice P, Pistilli R, Lizio G, Pellegrino G, Nisii A, Marchetti C. Inlay versus onlay iliac bone grafting in atrophic posterior mandible: a prospective controlled clinical trial for the comparison of two techniques. Clin Implant Dent Relat Res. 2009 Oct;11 Suppl 1:e69-82. Epub 2009 Aug 3. PMID: 19681938. [CrossRef]
- Donovan MG, Dickerson NC, Mitchell JC. Calvarial bone harvest and grafting techniques for maxillary and mandibular implant surgery. Atlas Oral Maxillofac Surg Clin North Am. 1994 Sep;2(2):109-22. PMID: 11905359. [CrossRef]
- von Arx T, Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D. Lateral ridge augmentation and implant placement: an experimental study evaluating implant osseointegration in different augmentation materials in the canine mandible. Int J Oral Maxillofac Implants. 2001 May-Jun;16(3):343-54. PMID: 11432654.
- Chiriac G, Herten M, Schwarz F, Rothamel D, Becker J. Autogenous bone chips: influence of a new piezoelectric device (Piezosurgery) on chip morphology, cell viability and differentiation. J Clin Periodontol. 2005 Sep;32(9):994-9. PMID: 16104964. [CrossRef]
- Nowzari H, Aalam AA. Mandibular cortical bone graft part 2: surgical technique, applications, and morbidity. Compend Contin Educ Dent. 2007 May;28(5):274-80; quiz 281-2. PMID: 17607892.
- Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003;5(3):154-60. PMID: 14575631. [CrossRef]
- Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006 Oct;17 Suppl 2:136-59. PMID: 16968389. [CrossRef]
- Nkenke E, Schultze-Mosgau S, Radespiel-Tröger M, Kloss F, Neukam FW. Morbidity of harvesting of chin grafts: a prospective study. Clin Oral Implants Res. 2001 Oct;12(5):495-502. PMID: 11564110. [CrossRef]
- Nkenke E, Weisbach V, Winckler E, Kessler P, Schultze-Mosgau S, Wiltfang J, Neukam FW. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: a prospective study. Int J Oral Maxillofac Surg. 2004 Mar;33(2):157-63. PMID: 15050072. [CrossRef]
- von Arx T, Häfliger J, Chappuis V. Neurosensory disturbances following bone harvesting in the symphysis: a prospective clinical study. Clin Oral Implants Res. 2005 Aug;16(4):432-9. PMID: 16117767. [CrossRef]
- McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007 Mar;78(3):377-96. PMID: 17335361. [CrossRef]
- Pistilli R, Felice P, Piatelli M, Nisii A, Barausse C, Esposito M. Blocks of autogenous bone versus xenografts for the rehabilitation of atrophic jaws with dental implants: preliminary data from a pilot randomized controlled trial. Eur J Oral Implantol. 2014 Summer;7(2):153-71. PMID: 24977250.
- Al Ruhaimi KA. Bone graft substitutes: a comparative qualitative histologic review of current osteoconductive grafting materials. Int J Oral Maxillofac Implants. 2001 Jan-Feb;16(1):105-14. PMID: 11280355.
- Troeltzsch M, Troeltzsch M, Kauffmann P, Gruber R, Brockmeyer P, Moser N, Rau A, Schliephake H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J Craniomaxillofac Surg. 2016 Oct;44(10):1618-1629. Epub 2016 Aug 18. PMID: 27622971. [CrossRef]
- Silva ER, Balan VF, Botticelli D, Soldini C, Okamoto R, Xavier SP. Histomorphometric, Immunohistochemical and Microtomographic Comparison between Autogenous and Xenogenous Bone Blocks for Mandibular Lateral Augmentation in Rabbits. Materials (Basel). 2021 Oct 13;14(20):6049. PMID: 34683641; PMCID: PMC8540416. [CrossRef]
- Sakaguchi, R.; Xavier, S.P.; Morinaga, K.; Botticelli, D.; Silva, E.R.; Nakajima, Y.; Baba, S. Healing of Collagenated Cancellous Equine Bone Blocks Used as Inlay or Onlay for Lateral Bone Augmentation in Rabbits. Preprints 2023, 2023090756. [CrossRef]
- Peñarrocha-Diago M, Gómez-Adrián MD, García-Mira B, Ivorra-Sais M. Bone grafting simultaneous to implant placement. Presentation of a case. Med Oral Patol Oral Cir Bucal. 2005 Nov-Dec;10(5):444-7. English, Spanish. PMID: 16264379.
- Omara M, Abdelwahed N, Ahmed M, Hindy M. Simultaneous implant placement with ridge augmentation using an autogenous bone ring transplant. Int J Oral Maxillofac Surg. 2016 Apr;45(4):535-44. Epub 2015 Nov 28. PMID: 26644216. [CrossRef]
- Yuce MO, Adali E, Turk G, Isik G, Gunbay T. Three-dimensional bone grafting in dental implantology using autogenous bone ring transplant: Clinical outcomes of a one-stage technique. Niger J Clin Pract. 2019 Jul;22(7):977-981. PMID: 31293264. [CrossRef]
- Chandra RV, Shivateja K, Reddy AA. Autogenous Bone Ring Transplant vs Autologous Growth Factor-Enriched Bone Graft Matrix In Extraction Sockets With Deficient Buccal Bone: A Comparative Clinical Study. Int J Oral Maxillofac Implants. 2019 November/December;34(6):1424–1433. Epub 2019 Sep 18. PMID: 31532824. [CrossRef]
- Giraddi GB, Saifi AM. Bone Ring Augmentation Around Immediate Implants: A Clinical and Radiographic Study. Ann Maxillofac Surg. 2017 Jan-Jun;7(1):92-97. PMID: 28713743; PMCID: PMC5502523. [CrossRef]
- Wychowanski P, Woliński J, Morawiec T, Kownacki P, Starzynska A, Kosieradzki M, Fiedor P. Preliminary Clinical Data and the Comparison of the Safety and Efficacy of Autogenous Bone Grafts Versus Xenograft Implantations in Vertical Bone Deficiencies Before Dental Implant Installation. Transplant Proc. 2020 Sep;52(7):2248-2251. Epub 2020 Apr 3. PMID: 32252999. [CrossRef]
- Benlidayi ME, Tatli U, Salimov F, Tükel HC, Yuksel O. Comparison of autogenous and allograft bone rings in surgically created vertical bone defects around implants in a sheep model. Clin Oral Implants Res 2018;29:1155-62. [CrossRef]
- Jinno Y, Jimbo R, Lindstrom M, Sawase T, Lilin T, Becktor JP. Vertical bone augmentation using ring technique with three different materials in the sheep mandible bone. Int J Oral Maxillofac Implants 2018;33:1057-63. [CrossRef]
- Haga-Tsujimura M, Nakahara K, Kobayashi E, Igarashi K, Schaller B, Saulacic N. Single-staged implant placement using bone ring technique with and without membrane placement: an experimental study in the beagle dog. Clin Oral Implants Res 2018;29:263-76. [CrossRef]
- Nakahara K, Haga-Tsujimura M, Sawada K, Kobayashi E, Mottini M, Schaller B, et al. Single-staged vs. two-staged implant placement using bone ring technique in vertically deficient alveolar ridges - part 1: histomorphometric and micro-CT analysis. Clin Oral Implants Res 2016;27:1384-91.
- Nakahara K, Haga-Tsujimura M, Sawada K, Kobayashi E, Schaller B, Saulacic N. Single-staged vs. two-staged implant placement in vertically deficient alveolar ridges using bone ring technique - part 2: implant osseointegration. Clin Oral Implants Res 2017;28:e31-8.
- El Zahwy M, Taha SAAK, Mounir R, Mounir M. Assessment of vertical ridge augmentation and marginal bone loss using autogenous onlay vs inlay grafting techniques with simultaneous implant placement in the anterior maxillary esthetic zone: A randomized clinical trial. Clin Implant Dent Relat Res. 2019 Dec;21(6):1140-1147. Epub 2019 Nov 19. PMID: 31743566. [CrossRef]
- Kanayama M, Botticelli D, Apaza Alccayhuaman KA, Yonezawa D, Silva ER, Xavier SP. The Impact on the Healing of Bioactivation with Argon Plasma of a Xenogeneic Graft with Adequate Fixation but Poor Adaptation to the Recipient Site: An Experimental Study in Rabbits. Int J Oral Maxillofac Implants. 2021 Jul- Aug;36(4):703-714. [CrossRef]
- Schwarz F, Sager M, Ferrari D, Mihatovic I, Becker J. Influence of recombinant human platelet-derived growth factor on lateral ridge augmentation using biphasic calcium phosphate and guided bone regeneration: a histomorphometric study in dogs. J Periodontol. 2009 Aug;80(8):1315-23. [CrossRef]
- Faria PE, Okamoto R, Bonilha-Neto RM, Xavier SP, Santos AC, Salata LA. Immunohistochemical, tomographic and his-tological study on onlay iliac grafts remodeling. Clin Oral Implants Res. 2008 Apr;19(4):393-401. [CrossRef]
- Caneva M, Botticelli D, Carneiro Martins EN, Caneva M, Lang NP, Xavier SP. Healing at the interface between recipient sites and autologous block bone grafts affixed by either position or lag screw methods: a histomorphometric study in rabbits. Clin Oral Implants Res. 2017 Dec;28(12):1484-1491. Epub 2017 Apr 5. [CrossRef]
- Araújo MG, Sonohara M, Hayacibara R, Cardaropoli G, Lindhe J. Lateral ridge augmentation by the use of grafts comprised of autologous bone or a biomaterial. An experiment in the dog. J Clin Periodontol. 2002 Dec;29(12):1122-31. [CrossRef]
- De Santis E, Lang NP, Favero G, Beolchini M, Morelli F, Botticelli D. Healing at mandibular block-grafted sites. An experimental study in dogs. Clin Oral Implants Res. 2015 May;26(5):516-22. Epub 2014 Jun 12. [CrossRef]
- Kotagudda Ranganath S, Schlund M, Delattre J, Ferri J, Chai F. Bilateral double site (calvarial and mandibular) critical-size bone defect model in rabbits for evaluation of a craniofacial tissue engineering constructs. Mater Today Bio. 2022 Apr 20;14:100267. [CrossRef]
- Wang Y, Zhang X, Mei S, Li Y, Khan AA, Guan S, Li X. Determination of critical-sized defect of mandible in a rabbit model: Micro-computed tomography, and histological evaluation. Heliyon. 2023 Jul 17;9(7):e18047. [CrossRef]
- Botticelli D, Berglundh T, Buser D, Lindhe J. Appositional bone formation in marginal defects at implants. Clin Oral Implants Res. 2003 Feb;14(1):1-9. PMID: 1256235. [CrossRef]
- Botticelli D, Berglundh T, Lindhe J. Resolution of bone defects of varying dimension and configuration in the marginal portion of the peri-implant bone. An experimental study in the dog. J Clin Periodontol. 2004 Apr;31(4):309-17. PMID: 15016260. [CrossRef]
- Akimoto K, Becker W, Persson R, Baker DA, Rohrer MD, O'Neal RB. Evaluation of titanium implants placed into simulated extraction sockets: a study in dogs. Int J Oral Maxillofac Implants. 1999 May-Jun;14(3):351-60. PMID: 10379108.
- Sáez-Alcaide LM, Brinkmann JC, Sánchez-Labrador L, Pérez-González F, Molinero-Mourelle P, López-Quiles J. Effectiveness of the bone ring technique and simultaneous implant placement for vertical ridge augmentation: a systematic review. Int J Implant Dent. 2020 Dec 12;6(1):82. PMID: 33313968; PMCID: PMC7732905. [CrossRef]
- Gaikwad AM, Joshi AA, Padhye AM, Nadgere JB. Autogenous bone ring for vertical bone augmentation procedure with simultaneous implant placement: A systematic review of histologic and histomorphometric outcomes in animal studies. J Prosthet Dent. 2021 Nov;126(5):626-635. Epub 2020 Oct 7. PMID: 33039188. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).