Preprint
Review
The Role of Sleep in Multiple Sclerosis
Altmetrics
Downloads
179
Views
75
Comments
0
This version is not peer-reviewed
Submitted:
13 November 2023
Posted:
14 November 2023
You are already at the latest version
Alerts
Abstract
Multiple Sclerosis (MS) is a neurodegenerative disease that mainly affects young adults. The high prevalence of the disease and its impact on patients' quality of life led to priority being given to the etiopathogenesis of the disease to identify possible modifiable factors and consequent effective intervention strategies. Recent hypotheses suggest that the etiopathogenesis of MS is multifactorial and includes factors related to the immune system, neuroinflammation and neuro-degeneration. In this scenario, sleep has a close - although indirect - relationship with MS, through its relationship with each of those factors. In particular, the role of cytokines in immune system impairment and neuroinflammation, mitochondrial dysfunction and oxidative stress in neurodegeneration all are associated with sleep in different forms. Furthermore, melatonin in relation with vitamin D has a potential therapeutic effect in MS sleep-related problems. Given the growing interest of research in the mechanisms underlying MS and therapies able to alleviate MS-related symptoms at all stages of the disease, a more in-depth study of the role of sleep disturbances and the factors intrinsically related to sleep and MS could be useful both to investigate the etiopathogenetic factors of MS and to develop potential non-invasive intervention strategies for MS treatment.
Keywords:
Subject: Biology and Life Sciences - Cell and Developmental Biology
1. Introduction
Multiple Sclerosis (MS) is one of the most debilitating neurodegenerative diseases affecting about 2.4 million people in the world's population [1]. MS mainly affects young adults between the second and fourth decade of life. Its high prevalence, early onset and the effects of the disease on the quality of life have led to a significant increase in research on MS in recent decades. It is possible to assume that most patients, in the early years of the disease, present the clinical form called "relapsing-remitting MS" (RRMS), which presents with relapses followed by recovery. Over the years, the corresponding clinical forms, progressive relapsing-remitting MS (PRMS) and secondary progressive MS (SPMS) [2], lead to a worsening of the quality of life and clinical conditions, in terms of persistent symptoms and lack of recovery after relapses.
Several factors make the course of the disease difficult to predict, which is highly subjective. In a first phase, visual disturbances may occur (such as optic neuritis), changes in gait and balance, fatigue, episodes of paralysis, sensory deficits [3].
The effects of MS on the brain are known, with the appearance of focal areas of inflammation and demyelination, neuronal and oligodendrocyte loss and reactive astrogliosis [4]. Brain tissue and spinal cord damage is initiated by T-cell mediated inflammation and demyelination and neurodegeneration are driven by heterogeneous mechanisms, including innate and adaptive immune systems [5]. With regard to the etiopathogenesis of the disease, little is yet known about the causes leading to demyelination and neuronal loss and the great clinical variability in terms of different symptomatology, different prognosis and different progression create confusion in achieving a unified hypothesis on the MS pathogenic mechanisms.
Within the different hypotheses, the factors that are commonly recognized as being directly or indirectly part of the pathogenesis in MS are some cytokines, which would contribute to the process of neuroinflammation and immune system dysregulation; and mitochondrial dysfunction and oxidative stress closely involved in the neurodegenerative process [3]. Furthermore, the role of melatonin as a possible “future vitamin D” is growing in interest in the field of MS research.
Although little is known about the etiopathogenesis of MS, some pharmacological treatments are effective - although not resolutive - in slowing the progression of the disease. In the last decade, research on the etiopathogenesis of the disease has focused attention on the possible multifactorial nature of MS, assuming that many factors act and interact each other, triggering to onset of the disease.
Within this scenario, sleep seems to have a more or less direct relationship with each of the factors related to the etiopathogenesis of MS, confirming the presence of marked sleep disorders throughout the course of the disease. In particular, patients with MS complain of insomnia, sleep breathing disorders (SBD), restless leg syndrome (RLS), disorders of the sleep-wake rhythm [6]. In addition, there is growing evidence of the bidirectional relationship between sleep and the immune system [7], whose malfunctioning in MS is at the root of the origin and progression of the disease.
This review discusses empirical evidence that assumes the possible multifactorial nature of the etiopathogenesis of MS. In the light of recent advances and evidence on the implications of many factors to which sleep is linked, we examine in depth the relationships between sleep, disturbed sleep and sleep loss with each of the factors considered, with particular reference to immune system, neuroinflammation and neurodegeneration. Furthermore, considerable space will be given to new evidence concerning the cellular and molecular mechanisms of MS, with particular reference to cytokines. These molecules, in fact, are produced by T lymphocytes and monocytes and play a fundamental role in both innate and acquired immune responses. Of specific interest for the relationship between sleep and MS is the role of specific cytokines, such as some types of pro-inflammatory interleukins (IL-1ß and IL-6) and tumour necrosis factor (TNF).
On the basis of the state of the art of the empirical evidence on the mechanisms linking sleep and MS, possible intervention strategies for the prevention and treatment of disturbed sleep are described. The role of melatonin, in particular, is currently considered a candidate of preference for therapeutic interventions in sleep-related disorders in MS. Other therapeutic hypotheses assume that acting on sleep in terms of: (i) improving its quality, (ii) regulating normal circadian rhythms and (iii) resolving sleep disturbances can have a beneficial effect on the quality of life of MS patients.
2. Etiopathogenesis of MS: A New Multifactorial Approach
Although little is yet known about the causes of MS, the literature is consistent in stating that genetic, environmental and immune factors are all implicated in the etiopathogenesis of the disease [8].
Considering the etiopathogenesis of MS as multifactorial means taking into account all the factors that could contribute to the onset of the disease. Research on the causes of MS is hampered by the great heterogeneity of the disease, from different points of view: radiological and histopathological changes, the appearance of very different symptoms, the inability to define the course of the disease and the different responses of patients to pharmacological treatments [9].
Although there is no definite knowledge on the explanation of the pathophysiology in MS, two different hypotheses have been formulated regarding the possible peripheral or central origins of the disease. In recent years, both hypotheses have been supported by some empirical evidence, suggesting that the combination of both paradigms may be the most comprehensive model for revealing the complex origins of MS. The theory that postulates a peripheral origin of the initiation of processes that triggers MS is called “outside-in” [10]. According to this theory, the autoimmune attack against myelin would originate from the peripheral activation of reactive T cells migrating into the central nervous system (CNS). At the molecular level the development of the autoimmune response, typical of MS, would be due to the cross-reactivity between self and non-self antigens [11]. Underlying the formation of lesions in MS is the recruitment of pathogenic T cells called CD4+ 'helper' and CD8+ 'killer' cells, that migrate from the periphery to the CNS. Antigen presenting cells (APCs), such as monocytes, macrophages, microglia, dendritic cells and B cells activate naïve CD4+ T cells and encourage differentiation of CD4+ Th17 and Th1 cells through inflammatory cytokines, such as IL-1ß, IL-6, IL-23 and IL-12 [12]. The recruitment of these cells would be responsible for the mobilisation of phagocytes that are capable of producing harmful inflammatory mediators and, with the presence of infiltrated T cells, of degrading the myelin sheath [13].
The 'inside-out' theory, on the other hand, recognises MS as a primary neurodegenerative disease and points to cytodegeneration, mainly directed at the oligodendrocyte-myelin complex, leading to the release of strongly antigenic constituents, as the initial event that triggers the inflammatory and autoimmune response in predisposed individuals [11]. The basis for this theory is axonal damage, which has been found to occur at an early stage of the disease. This suggests the possibility of an initial event in the CNS where apoptosis of oligodendrocytes and activation of microglia would take place, which would be at the origin of the early lesions in MS. The death of oligodendrocytes would alter the myelin sheath, allowing microglia to scavenge and accumulate at the site of injury, as evidenced by the fact that macrophages are the predominant cell type in both chronic and active plaques [13]. In this case, the recruitment of immune cells would be a secondary response to the events initiated in the CNS [13]. Even this theory, however, recognises the immune cell reaction to antigenic components as a highly characterising event in MS, although it identifies the origin of the triggering events in the CNS.
Lesion types can be divided on the basis of the major constituents of inflammatory infiltrates and CSF characteristics: lesions of pattern I display infiltration of T cells and macrophages; in the pattern II there is a contribution of humoral mechanisms to disease pathology, with the add of antibodies and complement deposition. The main features of pattern III are the distal oligodendrogliopathy with the dysregulation of mielin protein expression and apoptosis of olygodendrocytes, evident in an inflammatory background [14,15].
Yet, currently knowledge indicates and classifies MS as an autoimmune disease. The key mechanisms underlying MS mainly concern the immune system, neuroinflammation and neurodegeneration.
Beyond the peripheral or central origin of MS, the disease is characterized by two phases: at the beginning of a new lesion there is predominance of acute inflammation and then a state of chronic inflammation followed by neurodegeneration [16]. During the first phase there is the penetration of blood brain barrier (BBB) by the immune cells activated against the myelin sheath. The inflammation in MS is partly due to the components of the innate and adaptive immune systems [17,18]. In summary, there is proliferation and dysregulation of pro-inflammatory T lymphocytes (Th1 and Th17) and activation of B cells and secretion of inflammatory cytokines [19].
Inflammatory pathognomic events in MS also activate neurodegenerative processes that lead to the destruction of oligodendrocytes, axons and ultimately neurons [17,18]. The brain, spinal cord and retinal atrophy are the result of the presence of neurodegeneration even in the early stages of the disease, which means that both acute inflammation and neurodegeneration processes coexist from the very first symptoms of the disease in the grey matter and the white matter [20].
Thus, neuroinflammation - defined as the response of the reactive elements of the central nervous system (CNS) to an altered homeostasis from inside or outside the CNS - and neurodegeneration are closely related and seem to occur also in parallel, and an important role could be played by cytokines, which determine synaptic dysfunction. Cytokines, in fact, together with microglial cells, myeloid infiltrating cells, astrocytes and oligodendrocytes are the main reactive components of the CNS [21,22].
Hence, starting from the deficit of the immune system, the processes of neuroinflammation and neurodegeneration and all the factors underlying these systems contribute in different ways to the development, course and different clinical manifestations of the disease.
Bearing in mind the involvement of these systems, recent literature has indicated which factors, closely related to the immune system and the processes of neuroinflammation and neurodegeneration play a role in MS. It should be noted that there are many factors that appear to contribute directly or indirectly to the etiopathogenesis, development and progression of the disease, and this review will consider the factors that could be primarily implicated in MS and related to sleep in various forms. According to Tobore's multifactorial approach [3], mitochondrial dysfunction (MtD), oxidative stress and vitamin D all seem to play an important role in MS and also have a modulatory effect on many other factors involved in the disease [3]. Each of these factors has a relationship with sleep, which in turn seems to have a close indirect relationship with MS.
3. Disturbed Sleep in MS
Although recent research suggests that sleep disorders are common in MS and have the potential to contribute to chronic symptoms, sleep assessment is not routinely incorporated into MS clinical care [23]. It has been found that the presence of sleep disturbances in MS patients is about 50% [24] and they appear 4 times more frequently than in the general population [25].
Many studies in the literature agree that the most common sleep disorders in MS are insomnia, sleep-disordered breathing (SDB; with particular reference to Obstructive Sleep Apnoea Syndrome; OSA) and motor disorders, such as Restless Legs Sydrome (RLS) and Periodic Limb Movement of Sleep (PLMS). Moreover, although there is no definite association with disturbed sleep yet, many patients complain of a sort of feeling of tiredness during the day. This symptom is often associated with fatigue, a very common condition in MS. Fatigue is defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities” and, more recently, as reversible motor and cognitive impairment with reduced motivation and desire to rest [26,27]. It is important to emphasize that the conditions of fatigue and sleepiness are to be considered distinct, sleepiness being defined as an abnormal probability of falling asleep during normal waking hours. This distinction, when based self-report measures, may not be so clear as compared to objective measures (e.g., via the Multiple Sleep Latency Test, MSLT, for evaluating daytime sleepiness) [28].
Beyond the association between sleep disorders and MS, it is interesting to discuss the results of the numerous works that have investigated MS and disturbed sleep in terms of sleep fragmentation, a common feature of insomnia, OSA, RLS and PLMS. In other words, in the light of recent evidence linking sleep and MS also at the etiopathogenetic level, it is necessary to consider sleep structure itself - and not only primary sleep disorders - as a modifiable factor linked to MS at various levels. Indeed, sleep fragmentation, poor sleep efficiency or sleep deprivation (SD), whatever the cause, are factors closely linked to a number of typical MS disturbances. In particular, sleep fragmentation, could be a cause or concomitant cause of the cognitive disturbances found in many MS patients.
For this reason, a number of studies have investigated the relationship between disturbed sleep, MS and cognition. A recent study aimed to determine direct and indirect longitudinal associations between sleep disturbances and self-perceived cognitive dysfunction in women with MS compared with a control group (n=524) [29]. Specifically, the aim was to define whether the perceived dysfunction in MS was mediated and/or moderated by sleep disorders. The working hypothesis was that the higher prevalence of suspected or diagnosed OSA, sleepiness and insomnia in MS patients should predict cognitive difficulties after a 4-years period. The results of the study showed that OSA, insomnia and sleepiness could differentially moderate or mediate the effect of MS on perceived cognition in women. The cognitive functions under consideration were multiple, including working memory, comprehension and visuospatial functions. Different sleep disorders appeared to moderate or mediate cognitive dysfunction differentially: OSA was strongly associated with verbal comprehension, whereas insomnia and sleepiness showed the strongest associations with working memory and, to a lesser extent, with verbal comprehension and attention.
Again, with reference to cognition, objective sleep measures assessed by polysomnography (PSG) were also analysed in relation to cognitive and physical fatigue measures in MS patients [30]. The results of this cross-sectional work pointed out that sleep impairment was strongly correlated with cognitive but not with physical fatigue. Specifically, patients who had lower sleep efficiency, increased sleep onset latency and Wakefulness After Sleep Onset (WASO) had more cognitive fatigue. This could imply that the lack of good sleep quality would make these patients more easily fatigued in cognitive tasks that could interfere with their profession. Indeed, the finding that PSG measures do not show significant correlations with age, disease duration or degree of severity suggests that sleep impairment could be present at any stage of MS, beyond disease severity and even in professionally active young patients. Furthermore, this study also emphasises the increase in N1 and concomitant decrease in N3 (Slow Wave Sleep, SWS) and REM in patients with increased cognitive fatigue. This could imply that the lack of good sleep quality would make these patients more easily fatigued in cognitive tasks that could interfere with their profession. Indeed, the finding that PSG measures do not show significant correlations with age, disease duration or degree of severity suggests that sleep impairment could be present at any stage of MS, beyond disease severity and even in professionally active young patients. Furthermore, this study also emphasises the increase in N1 and concomitant decrease in N3 (Slow Wave Sleep, SWS) and REM in patients with increased cognitive fatigue. Learning and synaptic homeostasis in the brain are considered to be largely dependent on SWS. Lack of N3 could make the patient less receptive to new information and result in worsening learning ability. Preserved memory, the ability to learn new information and the ability to form new memories all depend on N3 which, when affected, results in worsening in all these domains. The worsening or absence of REM can also worsen memory and learning. Interestingly, only 38% of patients complained of sleep disturbances but at PSG level 88% of patients had at least one alteration among the sleep markers considered: worse sleep efficiency, increased latency, reduced or absent N3 and REM, increased WASO, increased respiratory event index (REI) and increased PLMS. This suggests a possible discrepancy between subjective and objective measures of sleep quality [30]. In this regard, a study evaluated the discrepancies between self-reported and device-measured sleep parameters [31] to determine the agreement between subjective and objective measures of sleep quality. Patients completed a sleep diary for 7 days and wore an actigraph as an objective measure of sleep quality for 7 nights. There was considered acceptable agreement between the two methods concerning total bedtime and total sleep time, but not for sleep onset latency, number of nocturnal awakenings and sleep efficiency. This suggests that it would be important to use both measures to capture all aspects of sleep quality in MS patients.
In general, it can be stated that many of the studies that have examined the main sleep disorders in MS have focused on subjective measures of sleep quality and sleep disturbance and on assessing the association between sleep disorders and quality of life (QoL) in MS patients, suggesting a relation between sleep disorders and MS, given the high percentage of MS patients who suffer from sleep disorders and the consequent negative impact on patients' QoL.
The recent literature evidence, however, especially with reference to the last decade, also includes several studies that have investigated sleep with objective recording methods that are considered more reliable.
Among the studies that have investigated sleep by PSG recordings, Braley and co-workers analysed for the first time the relationship between PSG data and a key group of self-reported symptoms (fatigue, tiredness, lack of energy and drowsiness) in MS patients compared to a control group [32]. The findings on sleepiness were similar between the two groups, whereas fatigue, tiredness and lack of energy were reported more in the MS patient group. Among these patients, fatigue correlated with sleepiness, and decreased sleep efficiency correlated with fatigue, tiredness and lack of energy. Thus, fatigue and related symptoms could be dependent on MS per se or in relation to reduced sleep efficiency, which suggests the need of measuring and possibly intervening on sleep impairment as a potentially modifiable factor which worsens MS symptoms in the form of fatigue and further related symptoms.
These results point to the possibility of acting early and, above all, on modifiable elements which impact on the QoL of MS patients: treating sleep disorders would mean being able to intervene more or less directly on MS-related cognitive dysfunctions as well.
Concerning the assessment of sleep-disorder-related to QoL in MS, a recent cross-sectional study was conducted on 152 MS patients with different levels of physical disability, who were administered questionnaires with socio-demographic and clinical information and questionnaires on sleep, sleepiness and QoL [25]. The results showed that 66% of the patients suffered from insomnia and that this was significantly more frequent in patients suffering from sphincter-related disorders, fatigue, sexual disorders and mood disorders. Excessive Daytime Sleepiness (EDS), on the other hand, was present in 41% of the patients and was unrelated to mood disorders. The presence of sleep disorders, however, significantly worsened the patients' QoL, particularly in the area of their family life. Even more recently, QoL in relation to SD was evaluated in a cross-sectional study that analysed data from a longitudinal study of 1717 MS patients [33]. In particular, this study assessed whether (i) sleep difficulties are prevalent and common in MS compared to the general population; (ii) how sleep is related to some common symptoms in MS; and (iii) whether sleep quality is associated with QoL. The results of this work also confirmed that poor sleep quality (assessed by questionnaires) was strongly associated with a deterioration in QoL. In particular, clusters related to fatigue, cognition, anxiety and depression, pain and sensitivity were independently associated with poor sleep quality. Sleep quality, daytime sleepiness and RLS symptoms were associated with reduced self-reported QoL independently of MS-related symptoms.
The presence of sleep disturbances in a high percentage of MS patients, the deleterious effects of disturbed sleep on symptoms and QoL, and the fact that disturbed sleep is present from the earliest stages of the disease - when the first brain damage evidenced by Magnetic Resonance Imaging (MRI) occurs - have led in recent years to investigate the involvement of specific brain regions related to sleep disturbances and MS. In this regard, a review analysed the relationship between the presence of sleep disorders (SBD, RLS, PLMD and insomnia) and MRI images of MS patients to identify possible alterations of specific neuroanatomical patterns [34]. While insomnia is considered secondary to other MS-related symptoms such as nocturia and pain, SBD and RLS could be a consequence of damage to specific areas of the CNS. With regard to SBD, multiple lesions at the level of the pontine tegmentum and medulla have been found in MS patients with pronounced sleep apnoea detected with PSG [35]. In the case of RLS, the neuropathological background in MS patients could involve the descending dopaminergic fibres controlling motor and sensory pathways with particular reference to the hypothalamus, which represents the main source of dopamine for the spinal cord via the diencephalon-spinal pathway, as suggested by Koblinger and co-workers [36]. In the case of high numbers of PLMS, the review also suggests a possible relationship with demyelination of axons involved in motor movements in the frontal lobe and right insular white matter [37]. Ferini-Strambi and colleagues [38], on the other hand, found that major MRI lesions in MS patients with PLMS were identified in the infratentorial regions, particularly in the brainstem and cerebellum, but not in other regions.
When referring to sleep and wakefulness, but also to phenomena such as sleepiness, alertness, learning, memory and stress response, one of the most involved brain regions is the locus coeruleus (LC). This brain region is located on the lateral face of the fourth ventricle in the upper dorsolateral pontine tegmentum and is considered the primary source of noradrenaline (NA) [39], a neurotransmitter with powerful anti-inflammatory and neuroprotective effects [40]. The interest of NA processes in relation with MS is evident due to the fact that NA can modulate synaptic transmission, membrane potential and excitability of of neurons and, in blood vessels, contribute to the regulation of blood flow and BBB permeability [40]. Furthermore, LC has been studied with respect to regulation of neuroinflammation [41]. What happens in the LC/NA system in case of MS? There is evidence of NA changes in MS and its animal models, and treatments that increase them are beneficial. Alterations in peripheral NA and adrenoceptor levels in MS patients have been reported by some studies, as well as changes in NA in the CSF [42]. Significant increases in glial activation in and around LC were observed in MS brains, as well as reductions in NA-LC levels. This confirms that LC damage occurs in humans and animals, although the loss of LC neurons has not yet been directly observed.
In conclusion, these works suggest that sleep alterations are implicated in the clinical expression of MS. Considering the role of sleep in myelin regeneration and, consequently, on disease outcome and the possibility that sleep alterations may be implicated in the promotion of fatigue, cognitive dysfunction, mood alterations and other clinical features of MS [43], further studies on this issue would be important.
4. MS and Sleep: A Strong Indirect Relationship
4.1. Immune System and Sleep: Basic Principles Related to MS
Communication between the peripheral immune system and the CNS occurs through various mechanisms, such as direct neural innervation, humoral mediator activities and active transport systems that move substances through the BBB [44,45]. The CNS, on its side, actively modulates the information received from the peripheral immune system by creating their bidirectional communication [46]. Sleep-related changes during an immune challenge are active processes: the interactions between sleep and the immune system are also bidirectional, with immune activation altering sleep and disrupted sleep affecting immune function [44,47].
Direct communication between the immune system and the CNS occurs through the action of cytokines and other molecular patterns on the vagus nerve, which innervates peripheral organs, and vagal afferents project to different parts of the brain involved in sleep regulation, such as the supraoptic and paraventricular nucleus of the hypothalamus, the nucleus of the solitary tract, the ventrolateral medulla and amygdala [44]. In the context of the relationship between sleep and the immune system with regard to MS, the mechanisms of active transport of substances from the peripheral immune system to the CNS via the BBB are crucial. Indeed, substance transport mechanisms are influenced by sleep, circadian processes and disturbed sleep [48,49]. In particular, the active transport of cytokines such as IL-1, IL-6 and TNF is a key contributor to the sleep changes that occur during the course of chronic, inflammatory diseases, such as MS.
Neuroimmunological research has accumulated much evidence on the relationship between the brain and the cytokine-immune-endocrine system and sleep is associated with changes at peripheral cytokines, cellular immune functions end endocrine levels [50]. It has been suggested that certain cytokines and neuroendocrine substances impact on sleep and sleep-wake rhythms and such peptides also influence the immune system [50]. Within this context, it is necessary to define this relationship, as MS is considered an autoimmune disease, showing a malfunctioning of the immune system leading to a state of acute (at the beginning of a new lesion) and/or chronic inflammation (later in the course of the disease) in which cellular and molecular mechanisms also involved in sleep regulation play fundamental roles.
Several neuroimmune pathways promote framework by which sleep can impact host defences [50]. Sleep plays an important role in the regulation of both adaptive and innate immune responses; it appears that sleep disturbances can lead to downregulation (worse response to infectious challenge) or up-regulation (increased genomic markers of inflammation) of adaptive immunity [51]. Sleep impacts the two principal effector system: the hypothalamus-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) which, in turn, regulate both adaptive and innate immune responses [51]. The HPA axis facilitates the interaction between the brain and the immune system by the action of adrenocorticotropin hormone (ACTH) and cortisol on immune cells [52]. In humans, the nocturnal sleep shows a strong down-regulation of the hypothalamus-pituitary-adrenal axis (HPA) and the sympathetic nervous system (SNS) that express the concomitant drop in cortisol, epinephrine and norepinephrine blood levels [52]. On the other hand, some hormones considered to be serving mediators for cell growth, differentiation and restoration show increased levels in the blood during sleep: these are pituitary growth hormone (GH), melatonin, prolactin and leptin [53,54]. These hormones, although having different cellular sources, have an action that can be considered synergistic on the immune system.
The evidence of the sleep contribution in the regulation of innate immunity is also manifest with the progressive increase of natural killer (NK) cell activity that reaches its minimum during the first part of the night and shows the peak in the late morning hours [55], with an attenuation in case of disturbed sleep [56,57]. With regard to the influence of sleep on adaptive-type immunity, it is observed that the number of leucocytes and monocytes and subsets of lymphocytes including T-helper cells (CD4+), cytotoxic T-cells (CD8+), activated T-cells and B-cells reach their maximum in the evening and decrease throughout the night with the minimum in the morning hours [53].
There is large evidence that focused on SD effects on immune and inflammatory functions [58]. Total SD impact several immune components involved in the early host responses to infection: it has been observed progressive increase in neutrophil and monocyte levels and NK activity during 64 hours of total SD [59]; in vitro-stimulated lymphocytes showed enhanced capacity to produce interferon (IFN) and neutrophils had reduced ability to phagocytose [60]. The increases in leukocyte counts, in particular neutrophil and monocyte were observed also in chronic partial sleep deprivation protocols (4 h of sleep for three consecutive nights) and in acute partial sleep deprivation (2 h of sleep for one night) [61,62].
A partial SD protocol (4 hours of sleep for 5 consecutive nights) measured the immunological effects of sleep loss in healthy young adults by assessing peripheral blood mononuclear cells (PBMC) [63]. The results showed an increase in lymphocyte activation and production of pro-inflammatory cytokines such as IL1-ß, IL-6 and IL-17 that remained elevated even after two nights of recovery sleep, suggesting possible persistent changes in the immune system. Some evidence points that when sleep fragmentation is limited to one or two nights, the levels of inflammatory markers did not change [64,65], as well as in case of daytime naps [61,66]. It interesting to note that nap, in this sense, could be considered protective: in healthy adults the increase in IL-6 levels after sleep deprivation was reversed after two-hours nap [67].
4.2. Possible Cellular and Molecular Links between Sleep and MS in Neuroinflammation
As mentioned above, although the pathogenesis of MS is unclear, some underlying dynamics are known: there is an autoimmune response directed against CNS antigens due to myelin-reactive CD4+ T cells and B cells, and underlying the autoimmune response would be a violation of tolerance to myelin and other CNS antigens, leading to obstinate peripheral activation of self-reactive T lymphocytes [68]. Once T-lymphocytes are activated peripherally, they can cross the BBB via interaction with receptors on the surface of T-lymphocytes (VLA-4) and via receptors found on the capillaries of epithelial cells (VCAM-1). This type of process is facilitated by other factors consisting of adhesion molecules, chemokines and matrix metalloproteinases (MMPs) [69]. Once in the CNS, T lymphocytes undergo reactivation due to encountering autoantigenic peptides in the brain parenchyma that bind with MHC II-type molecules expressed by cells residing in the CNS, i.e. dendritic cells, macrophages and B cells [70]. The result of this process is an inflammatory response that leads to the release of cytokines and chemokines and the recruitment of additional immune cells to the site of inflammation with further activation of myelin (in particular, macrophages present in the CNS), which causes persistent inflammation and damage to myelin [71,72,73].
Data from recent literature investigating the development of neuroinflammation in neurodegenerative diseases emphasize the importance of external factors, including sleep quality, in the development of neuroinflammation, with particular reference to the role of microglia and astrocytes [74,75,76]. It has been shown that astrocytes contribute in homeostatic and circadian regulation of sleep and could be considered as possible therapeutic candidate for sleep disorders in neurodegenerative diseases [77]. Although these studies were not directly referred to MS, it is reasonable to suggest a possible implication of microglia and astrocytes also in the processes of neurodegeneration and neuroinflammation in relation with MS.
Currently, it is well known that certain biochemical factors play a role in sleep regulation, although many mechanisms still need to be investigated [78]. Since the 1990s, many studies have provided evidence for such substances termed “sleep regulatory substances” (SRS) [79,80,81,82,47]. To be defined as SRS a substance must have certain necessary characteristics, such as (i) undergoing changes with the propensity to sleep (the substance or its receptor), (ii) the administration must have a somnogenic effect or inhibit sleep, (iii) the blockage of its action or the inhibition in its production must have effects on sleep and (iv) the changes in the substance must vary during a disease-state associated with altered sleep and (v) the substance must act on known sleep-regulatory circuits [47,78,82]
In this scenario, the cytokines IL-1, TNF-α and IL-6 are certainly recognised as SRS: many molecular, genetic, electrophysiological and biochemical studies have shown specific effects on sleep-related behaviour or the sleep-wake cycle [78]. Other neurochemicals are important in sleep regulation, such as adenosine, reactive oxygen species (ROS), prostaglandin D2 and growth hormone-releasing hormone (GHRH) [83].
SD stimulates the increased expression of immune-signalling cytokines that promote inflammatory processes associated with a range of disease states, including autoimmune disorders. Cytokines, in turn, affect sleep and this suggests a pathway through which high levels of cytokines involved in inflammatory processes can dysregulate sleep [84].
Certain cytokines, such as IL-1, TNF and IL-6 are implicated in the regulation of the sleep-wake cycle and are the same cytokines that stimulate inflammatory responses [85]. For instance, high circulating levels of these cytokines promote the development of a cascade of pro-inflammatory processes that include the activation of immune cells and stimulate the recruitment and infiltration of these cells at sclerotic plaque sites [86]. Thus, some proinflammatory cytokines that play a role in exacerbating neuroinflammation in MS are cytokines that also play a role in sleep regulation (IL-1, TNF-α and IL-6). Although the mechanisms underlying the relationship between sleep and MS are unclear, there are many 'common grounds' that would lead to investigate this relationship.
The immune system shows dynamic variation during a normal sleep-wake cycle due to a combination of circadian and sleep-related factors [84]. Immune cells show their peak early in the night and progressively decrease to their lowest value in the morning, probably due to increased adhesion to blood vessel walls and/or migration into lymphoid tissue [57]. IL-1, IL-6 and TNF reach their peak during the night [87,88]. These changes are similar to those of growth hormone and opposite to cortisol, whose levels are at their nadir before sleep and peak between 7:00 and 9:00 AM [84].
In order to summarise the possible molecular mechanisms linking sleep and MS, it is necessary to consider the main works in the literature that have investigated, even indirectly, the role of IL-1, IL-6 and TNF in relation to sleep, immune system and inflammation mechanisms.
IL-1
The IL-1 cytokine family has several functions in the immune response, inflammation and sleep [89,90]. IL-1 are dysregulated in several diseases in which patients also have sleep disorders, such as vascular disorders, cancer and brain damage [91,92,93,94]. The sleep-altering actions of IL-1 family members appear to depend, at least in part, on the downstream activity of the receptors on which they act [89]. In animals, low doses of IL-1 increase NREM sleep, whereas with increasing dose, NREM sleep is inhibited in a circadian-rhythm dependent manner [95]. In a study in which IL-1 was administered to rabbits at the onset of darkness, it was observed that the animals slept approximately 3 hours longer than usual in the 12-hour period following the administration [96]. In the case of mice, injection of IL-1 into the intracerebroventricular space in the brain was associated with increased NREM and reduced REM sleep [97]. It was also found that IL-1ß applied in the brainstem, at the level of the raphe nuclei and LC, increased NREM in rats [98,99]. Interestingly, high dosages of IL-1ß could lead to increased wakefulness [79], possibly due to the increased expression of anti-inflammatory cytokines (IL-4, IL-10 or IL-13) that could attenuate the increased sleep following sleep-promoting stimuli and lead to increased induction of compensatory wakefulness molecules [89,100]. Spontaneous sleep and sleep responses following deprivation were attenuated after administration of anti-IL-1α and anti-IL-1ß antibodies in rabbits [101].
In healthy adults, administration of anakinra, a recombinant IL-1 receptor antagonist drug, does not alter total sleep duration or the relative length of the different sleep stages, although EEG spectral power analysis shows an increase in Slow Wave Activity (SWA; 0.5-4 Hz) during NREM [103].
TNF-α
TNF-α is produced by most nucleated cells such as glia and neurons [104]. It is involved in immune functioning, inflammation, cell survival, proliferation and differentiation, cognitive functions, mood and fatigue [105]. Alteration in TNFα expressions are related with pathological conditions that have sleep dysregulation [105] showing its role in sleep regulation and circadian biology [106]. Levels of mRNA encoding IL-1 and TNF-α are at their highest in the brain during sleep, just as TNF-α protein levels in the brain and IL-1 levels in the CSF have a sleep-related diurnal rhythm [105]. IL-1 e TNF-α have somnogenic effects that express through the activation of the nuclear factor kappa B (NFkB), that increases pro-inflammatory cytokines and adhesion molecules as a result of overexpression in macrophages at sites of inflammation. Activated microglia in lesions presents large amounts of activated NFκB that may contribute at the low rate of oligodendrocyte death in MS [107]. Thus, systemic injection of IL-1 and TNF- α has been shown to cause increased delta electroencephalographic (EEG) activity, typical of slow wave sleep, and increase the duration of NREM sleep [96]. Both of these cytokines are associated with clinical conditions such as fatigue, rheumatoid arthritis, chronic insomnia and influenza, with TNF-α also associated with MS [80,108].
Production of both TNF-α and IL-1 is enhanced following sleep deprivation and correlates with greater amount of recovery sleep; the blockade of TNF-α or IL-1 blocks recovery sleep and mice lacking type I IL-1 receptor and/or type I TNF-α receptor sleep less than controls [47]. These data concerning TNF roles suggest that TNF drive the propensity to sleep [109]. In human, total sleep deprivation provoked significant increases in the monocyte production of TNF-α in the successive morning and increased in the transcription of TNF-α mRNA [110].
IL-6
IL-6 is classified as one of the pro-inflammatory cytokines that stimulate B-cell function [111] and also plays an important role in non-immunological tissues: it is secreted by a variety of non-immunological cells, such as adipocytes and endothelial cells [112,113]. Together with C-reactive protein (CRP), it is one of the most important biomarkers of chronic or low-grade inflammation [114]. Many studies have noted the crucial role of IL-6 for the interaction between the immune system and the CNS in inflammatory diseases, with particular reference to diseases whose symptoms are fatigue, sleep disturbances and drowsiness [115].
IL-6 levels have a circadian profile peaking at 19:00 and 05:00 and night sleep appears to be crucial for the nocturnal increase in IL-6 and TNF production by Toll-like receptor 4 (TLR4)-stimulated monocytes [116,118]. Experimental sleep curtailment led to decreased IL-6 secretion during the night [116], delaying the nocturnal increase of IL-6 and attenuating TNF production by monocytes during the night. This would, therefore, lead to the overexpression of IL-6 during the day [88,116,118]. Indeed, among those with sleep disorders with shorter SWS duration and longer REM duration, IL-6 production is reduced at night and increases during the day [119]. Nocturnal IL-6 levels appear to differ between sleep stages, but the evidences are still conflicting. Some data show the IL-6 peak early in the night, coinciding with SWS [116], while others show the highest IL-6 levels later, coinciding with REM sleep [88]. A sleep deprivation study showed that a night of total SD led to an increase in IL-6 during the day and a decrease in IL-6 during the following night [116]. Furthermore, these changes were correlated with increased sleepiness and fatigue during the day and deeper sleep during the night following deprivation. Partial SD appeared to have similar effects: healthy adult who slept 6 hours per night for one week showed higher rates of IL-6 secretion over 24 hours and deeper sleep at night with higher levels of self-reported daytime sleepiness [120]. Thus, changes in IL-6 levels appear to have an effect on both sleep and self-reported sleepiness [121]. IL-6 levels also underwent changes in patients with insomnia (particularly difficulty falling asleep), who showed increased IL-6 levels at night compared to the control group [122]. The levels changed in proportion to the amount of sleep and showed a negative correlation with the amount of SWS, emphasising the importance of both quality and quantity of sleep. As sleep or lack of sleep seems to have an effect on IL-6 levels, inhibition of recombinant human IL-6 also influenced sleep and daytime symptoms, in terms of increased fatigue, changes in sleep architecture, decreased ability to concentrate compared to placebo [123].
4.3. Neurodegeneration: Mytochondria Dysfunction and Oxidative Stress
The processes of acute inflammation and neurodegeneration in MS co-exist since the early stages of the disease as confirmed by the presence of brain, spinal cord and retinal atrophy (affecting both white and gray matter) that could represent the first symptoms in MS presentation [20]. Among the different form of MS, RRMS has a highly neuro-inflammatory phenotype, while the SPMS and PPMS forms are mainly characterized by neurodegeneration [16,20].
Mitochondria are energy-producing organelles found within the cell and are present in many neurodegenerative diseases, although it remains unclear whether they play a role in triggering neurodegeneration or, instead, represent innocent bystanders during neuroinflammation [124]. The main functions of mitochondria in the cell are the production of ATP, the contribution to many vital metabolic components, the role in the generation of reactive oxygen species (ROS) that are important signalling molecules and the initiation of the internal apoptosis system and the antiviral immune response [70]. In the case of MS, the development of the dysfunction at mitochondrial level is related to insufficient ATP generation reflected in a decrease in the functional activity of neurons, leading to neurodegeneration [70]. With the chronic inflammation and myelin destruction there is a redistribution of the ion channels with increased number of Na+/K+-ATPase that leads to increased ATP consumption. In this scenario, mitochondria try to make compensatory changes (e.g., increasing in size and number, modyifing the morphology and the localization in the neurons), in order to balance the ratio and demand for energy. Neurons are characterized by high requirements of energy for the implementation of high-energy processes underlying the conduction of a nerve impulse and they are highly dependent on the functioning of mitochondria and highly vulnerable to their dysfunction [70]. Mitochondria abnormalities in MS include mutations in mitochondrial DNA, disturbances in the balance of mitochondrial dynamics and changes in the content of energy metabolism in the cell and deterioration in the expression of mitochondrial genes [125,126].
The oxidative damage to mitochondrial DNA and the disruption of the activity of mitochondrial enzyme complexes in MS lesions lead to the destruction of oxidative phosphorylation process and increased reactive oxygen species (ROS) production by mitochondria [127]. ROS are fundamental cell signalling molecules and, in normal conditions, ensures cellular homeostasis [128]. ROS levels that are either too low or too high have negative impacts on cells. In particular, too low levels prevent to ensure working functioning of cell in regulating a cascade of biochemical reactions, while too high levels lead to a change in their function, which leads to oxidative stress. In case of oxidative stress, the role of ROS shifts from signalling role to destroyer of macromolecules such as proteins, nucleic acids or lipids and contributes to the development of inflammation and cell death [129]. Furthermore, ROS directly damage the myelin sheath facilitating the release of new autoantigenic particles that cause an increase in autoimmune inflammation and consequent damage to neuronal structures [130,131].
In MS, inflammation and oxidative stress are in a self-perpetuating vicious cycle: inflammation promotes oxidative stress and levels of ROS overcomes the antioxidant defences within lesions resulting in damage in cell mithocondria, proteins, nucleic acids and cell death [132,133].
How does sleep fit into the contest related to mitochondrial damage and oxidative stress in MS?
Sleep in association with MS fits into the framework concerning oxidative stress as one of the functions of sleep is to promote anti-oxidant mechanisms by removing free radicals accumulating during wakefulness [134,135,136]. This was the view expressed by Reimund in its “The free radical flux theory of sleep” to explain the relationship between sleep and ROS at brain level, which hypothesised that bioenergetics, redox and temperature regulation promote a more protective and restorative waking phase at the level of the nucleus ('nucleorestorative'), while sleep would be more protective and restorative at the mitochondrial level ('mitorestorative') [137].
A recent review summarizes the studies that tested the hypothesis of the role of sleep as a sort of anti-oxidant [138]. These studies measure changes in antioxidant enzyme activity and markers of damage or oxidative stress in SD conditions. Considering the methodological heterogeneity, the results were controversial: some studies showed indirect signs of oxidative stress in SD conditions, but did not detect oxidative damage and others reported no damage [139,140,141]. Recent studies, however, emphasised the fact that sleep deprivation had an effect on other organs: it seems that acute and chronic sleep deprivation lead to ROS accumulation and oxidative stress in the gut [142,143]. It is, therefore, conceivable that sleep deprivation acts on a group of cells specialised in homeostatic sleep control and not on brain-wide changes in oxidation [138].
Several studies suggested that the functions of mitochondria, in addition to the impact on cellular and physiological processes, may also influence other dynamics, including sleep, linking mitochondrial energetics – specifically cellular redox state – to some behavioural states [138,144,145]. Some evidence supports the existence of a particular link among sleep, circadian rhythms and metabolism with an important role of mitochondria [146]. Some studies coming from animal studies in mice, rats and drosophila found an implication of mitochondria in different aspects of the sleep-wake cycle [142,147,148], highlighting changes in expression/morphology at the gene level suggesting the possibility that mitochondria adapt to the new energy demand/supply during the sleep-wake cycle [146].
Enzymatic antioxidant and non-enzymatic antioxidants are important in counteracting oxidative stress. Among the non-enzymatic antioxidants, melatonin, together with glutathione (GSH), vitamin C and vitamin E, seems to have an important role. The non-enzymatic antioxidants peak during the dark phase, in contrast with enzymatic antioxidants that peak during light phase [149,150,151].
Some animal models suggested that lack of sleep or disturbed sleep could contribute to oxidative stress showing decreases in the activity of antioxidant enzymes in the brainstem and hippocampus following sleep deprivation in rats [152], but the results are controversial. In general, it is possible that short periods of SD increase the antioxidant response in the brain and other organs, whereas prolonged periods of SD reduce the antioxidant response, suggesting a possible role of prolonged period of wakefulness in chronic oxidative stress that would lead to a kind of failure in antioxidant mechanisms useful for sustaining the accumulation of pro-oxidants [153].
Thus, there is much evidence to suggest that sleep and MS may share a variety of mechanisms underlying the processes of autoimmune response, neuroinflammation and neurodegeneration (Figure 1).
5. New Potential Evaluative and Therapeutic Scenario
5.1. Melatonin and Vitamin D
Melatonin is synthetized from the amino-acid tryptophan via serotonin (5-HT) and its normal release is timed by input from the suprachiasmatic nuclei (SCN) in the hypothalamus [154,155]. Once synthesised, melatonin is released into the circulation and, due to its high solubility in water and lipids, can penetrate the cell membrane and reach various body fluids such as saliva, urine and CSF via the BBB [156,157]. In humans, melatonin expresses its biological effects via two specific receptors: MT1 and MT2 [158,159]. The membrane receptor for melatonin MT1 is expressed in various areas of the brain including the SCN [160], hippocampus, cerebellum, amygdala and hypothalamus [161], and also in peripheral tissues such as the pancreas cells, retina and immune system [162,163,164]. The MT2 receptor, on the other hand, is mainly expressed in peripheral tissues and, secondarily, in the brain [165,166,167]. Its release by pineal gland occurs with the arrival of darkness and is also associated with a fall in body temperature [168]. Specifically, melatonin levels in plasma have regular ups and downs within the 24 hours period [155] with night concentrations 3-10 times higher than the daytime with its secretion beginning at 21:00-22:00 h and the peak reached between 03:00 and 04:00 h. The decrease in melatonin plasma levels starts between 07:00 and 09:00 in the morning [155,169,170,171].
Research over the last decade on MS led to greater awareness of melatonin for its ability in acting as a strong antioxidant, immune active agent and mitochondria regulator [172,173]. Melatonin levels are associated with neuroimmunological diseases and are inversely correlated with MS severity and relapses [174,175,176,177,178]. In MS patients it has been observed low levels of melatonin in urine and blood related to fatigue and disability [179,180,181].
Recent works suggest that melatonin supplementation could have beneficial effects in MS due to its role in anti-inflammatory mechanisms and oxidative stress reduction [172], as observed in some clinical data showing that the melatonin administration at low doses was associated with better quality of life [182] and reduced oxidative stress and inflammatory markers in MS patients [183,184]. Furthermore, melatonin supplementation reduced inflammatory compounds, such as TNFα and IL-6 with a more significant effect at a dosage of 10 mg/day (or more) for three months (or more) [185]. It is known that IL-6 is associated with MS and increases Th17 levels contributing to processes related to neuro-inflammation [186]. At epigenetic level, melatonin is able to down-regulate IL-6 inhibiting some crucial immune-inflammatory processes in MS and also inhibits the permeability of the BBB and the deleterious peripheral and central immune and inflammatory processes [187,188,189]. Melatonin seems to reduce pathogenic inflammation also decreasing Th17 cells and IL-17 cytokines increasing anti-inflammatory cytokines, such as IL-10 and T regulatory cells [190]. It is possible that melatonin could provide a more protective microenvironment for cytokines [191].
A recent review synthetized literature evidence on the evaluation of melatonin action mechanisms on inflammation and oxidative stress due to MS and the interaction among melatonin and several crucial factors that could influence MS pathophysiology [192]. It emerges that biomarkers of oxidative stress were reduced in all studies considered indendently from the dosage (3-25 mg) and duration of administration (1 month-1 year) [183,193,194,195]. With regard to inflammation-related parameters, the results differed with regard to the effects of melatonin on proinflammatory cytokine levels: with the dose of 25 mg/day for 6 months there was a reduction in TNFα, IL-1 and IL-6 levels [183], but there were no changes in TNFα in another study after melatonin treatment of 3 mg/day for 6 months, although a reduction in IL-1ß levels was observed [184]. Furthermore, the dose of 3 mg/day did not show changes on clinical outcome, functional disability and development of brain lesions [196], suggesting that it should be necessary to administer high doses of melatonin, in order to reduce clinical activity of MS.
Thus, the effects of melatonin on oxidative stress have been noted in some studies. In MS, the increase in inflammatory cytokines following the infiltration of macrophages and lymphocytes into the CNS increases ROS generation, which further promotes inflammation leading to oxidative stress [197]. Among the many consequences of oxidative stress are increased production of lipid peroxidation (LPO), damage to nuclear and mitochondrial DNA and subsequent disturbances associated with its own mechanisms of replication and cellular tissue damage and walls and increased protein carbonylation (CO) [198,199,200,201]. Given these mechanisms, melatonin could act as an antioxidant by reducing macromolecular damage in all organs and the main biomarkers associated with oxidative stress [202,203], such as CP, LPO, nitric oxide (NO) [195,204,205,206].
Melatonin also appears to be able to counteract the damage linked to mitochondrial dysfunction in MS. Oxidative stress could lead to mutations and deletions in mtDNA due to the damage caused by ROS affecting energy metabolism and ATP production [207,208,209]. Melatonin is said to be able to stabilise the inner mitochondrial membrane by improving electron transport chain activity and reducing oxygen consumption [203,210]. In addition, melatonin would implement its beneficial effect by acting as a protective factor, by increasing oxidative phosphorylation required for activation of the melatonergic pattern of mitochondria [211] and by increasing the activity of enzymes involved in oxidative phosphorylation and optimising mitochondrial function, such as NADH-coenzyme Q redictase (Complex I) and cytochrome C oxidase (Complex IV) [204,212,213,214,215].
A recent study investigated the effects of melatonin intake on sleep quality and sleep disturbances in MS patients [216]. Thirty MS patients underwent to melatonin supplementation or placebo for a two-week period and vice versa in the following two weeks. During the second and fourth week they wore an actigraph to measure total sleep time and sleep efficiency and self-reported clinical outcomes were acquired. The results of the study showed that melatonin supplementation between 0.5 and 3 mg/day significantly improved total sleep time and there was also a trend for improved sleep efficiency in those taking melatonin compared to placebo. There were no statistically significant differences in patient-reported outcomes, although there was a trend for a decrease in insomnia severity in those taking melatonin.
Another aspect to be discussed is the relationship between sleep and vitamin D with regard to MS. Until a few years ago, the effects of vitamin D were attributed exclusively to phosphocalcic metabolism [217], but it is now known that vitamin D deficiency is a risk factor for MS and is also correlated with its severity [218,219,220].
There are two forms of vitamin D: vitamin D2 (ergocalciferol) produced by some plants in response to UV radiation and vitamin D3 (cholecalciferol) synthesised in the skin of humans and animals via UV irradiation of 7-dehydrcholesterol to provitamin D3, the biologically more active form [221,222].
Vitamin D undergoes two steps of hydroxylation: first in the liver to 25(OH)D, the most abundant 'pre-hormone' in serum and measured in serum tests, and then in the kidney, to a potent metabolite 1,25-dihydroxyvitamin D (1,25(OH)2D), which also plays a role in inflammatory and immunological function [223]. Locally produced 1,25(OH)2D in immune type cells has effects on the innate, adaptive and humoral immune systems by promoting the innate immune system response, inhibiting humoral and cell-mediated immunity with anti-proliferative and anti-inflammatory effects [223,224]. The way these effects are mediated is via vitamin D receptors and vitamin D-responsive elements found in the promoter regions of many genes [225]. There is evidence that 1,25(OH)2D in vitro inhibits the production of pro-inflammatory Th1 cytokines and stimulates Treg activity [226]. Although the biological basis of the relationship between vitamin D and MS is not yet known, studies are mainly investigating two fronts: the possible association between vitamin D and the risk of inflammation and the possible role of vitamin D in myelinisation and remyelination processes [227,228].
Vitamin D metabolism is present in the CNS, is involved in myelinisation and may be influenced by many external factors such as exposure to sunlight, diet and vitamin D supplementation [228].
The mechanisms by which vitamin D could influence MS are numerous. Immunological effects are mainly implicated in the inflammatory period of MS, but other types of mechanisms, in parallel, also have neurological and central effects [229]. The role of vitamin D associated with the immune system is immunomodulatory and includes different categories of T lymphocytes, B cells and certain cytokines [230,231,232,233]. Vitamin D supplementation could be immunologically beneficial as it decreases pro-inflammatory lymphocytes Th17 and pro-inflammatory cytokines IL-17 and is able to attenuate B-cell immunoreactivity and, conversely, stimulates Treg and a type of anti-inflammatory cytokine IL-10 (anti-inflammatory cytokine) [234,235,236,237,238].
In addition to immunomodulatory properties, effects concerning the CNS have also been found: vitamin D enters many CNS cell types, such as microglia, neurons, oligodendrocytes and astrocytes that have receptors for vitamin D. Vitamin D is said to play a role in neuroprotection, remyelination and axonal degeneration in MS patients [239,240]. Evidence on vitamin D administration in MS suggests that a moderate dose taken orally would be recommended for all types of MS (between 2000 and 4000 IU/d) [241,242].
Some lines of research, although evidence is rather scarce, are considering a possible relationship between sleep and vitamin D associated with autoimmune diseases. The rationale is that these diseases are mediated by alterations in immunomodulation, increased susceptibility to infection, and increased levels of inflammatory substances, including those related to sleep regulation, such as TNFα and prostaglandin2 (PD2) [243]. Furthermore, inadequate vitamin D levels could have an effect in the development of daytime symptoms commonly associated with sleep disorders [243]. In relation to sleep, intranuclear vitamin D receptors and retinoid X receptors (VDR-RXR) heterodimers downregulate the transcription of RelB, a gene encoding the RelB protein, belonging to the family of transcription factors that collectively refer to NFkB [244], which plays a pivotal pro-inflammatory role both in terms of the production of sleep-regulating substances, such as IL-1 and TNF-alpha [245] and in activating inflammatory patterns known to appear in the setting of intermittent hypoxia, as observed in OSA [246].
At the clinical level, there is little evidence that has directly evaluated the role of vitamin D metabolism in the presentation of sleep-related daytime symptoms or sleep disorders. One study showed that a woman with hypersomnia reported a complete resolution of daytime hypersomnolence following treatment for vitamin D insufficiency [247], suggesting that an alteration in systemic metabolism associated with low vitamin D levels may have promoted sleepiness via a central signalling mechanism. Another finding linking the symptom of drowsiness with vitamin D is the significant association between drowsiness and 25(OH)D in patients who complained of chronic musculoskeletal pain [248].
Other more recent evidence suggests the role of vitamin D in sleep regulation with the finding of an association between vitamin D deficiency and increased risk of sleep disturbances, general sleep difficulties, shorter sleep duration and nocturnal awakenings in children and adults [249,250,251].
A recent meta-analysis has suggested that vitamin D supplementation is useful for improving sleep quality, while further investigation is needed to assess its effects on sleep quantity and sleep disorders [252]. The same evidence also highlights which mechanisms might be involved in direct and indirect vitamin D association to sleep. One potential mechanism could be the extensive presence of vitamin D receptors also present in areas implicated in sleep regulation [253]. Furthermore, in areas implicated in sleep regulation, such as the substantia nigra and the supraoptic and paraventricular nuclei of the hypothalamus, expression enzymes implicated in vitamin D activation and degradation (25OHD and 1-hydroxylase and 24-CYP24A1) would be present [253,254]. Another possible mechanism is related to vitamin D as an immunomodulatory molecule playing a role in the downregulation of inflammatory markers implicated in sleep regulation, such as TNFα, cytokines, PD2 and, in the case of vitamin D deficiency, some inflammatory markers could increase with deleterious effects on sleep [255].
Furthermore, the relationship could also be due to sunlight influencing both vitamin D and sleep-wake rhythm [256,257,258,259]. Melatonin production is regulated by vitamin D, so abnormal levels of vitamin D may decrease melatonin levels leading to sleep disturbances [154,260]. Recent evidence has suggested that vitamin D and melatonin would be two sides of the same coin having a major impact on many aspects of life and health [261].
The synthesis pattern of melatonin and vitamin D is said to be opposite: vitamin D begins to be produced by the skin during exposure to light and sunlight, while melatonin synthesis by the pineal gland begins in the absence of light [217]. Although their biosynthesis systems are opposites, vitamin D and melatonin share the ability to act as powerful modulators of the immune system with anti-inflammatory properties as they are both light-dependent mediators [262]. It has been suggested that MS could represent a kind of cornerstone between them that could establish their mutual connection [217].
It is precisely the link between vitamin D and melatonin that could play a role in MS, as suggested by Golan and co-workers [262] which hypothesised that vitamin D and melatonin might have related influences in MS patients, in particular basing on the assumption that they are both light-dependent mediators and have shared immunomodulatory properties. The study was conducted in MS patients treated with IFN-ß and the experimental protocol involved a subgroup of patients taking a low dose of vitamin D3 daily (800 IU) and another subgroup receiving a considered high dose daily (4,370 IU) for one year. At baseline, after 3 months and after 12 months, 25(OH)D levels in serum and melatonin metabolites (6-SMT) in urine were measured. After 3 months, 25(OH)D levels increased and nocturnal melatonin secretion decreased significantly in MS patients taking high-dose vitamin D3, but not in those taking low-dose vitamin D3. After one year there was a decrease in 25(OH)D levels (probably due to seasonal climatic changes) accompanied by an increase in 6-SMT in the urine at night in the group taking high-dose vitamin D3. The change in 25(OH)D percentages was significantly negatively correlated with the change in 6-SMT percentages after 3 months and in the period between 3 months and 12 months. Thus, melatonin should be considered as a potential mediator of the neuromodulatory effects of vitamin D in MS patients treated with IFN-ß. What has been hypothesised by the authors is that 25(OH)D and the pineal gland of the CNS would communicate by influencing each other, with 25(OH)D carrying a kind of “light message” to the pineal gland resulting in a decrease in melatonin synthesis.
What emerges, however, is that supplements of melatonin and vitamin D could have beneficial effects in MS, but it is crucial to check their long-term balance, which is fundamental for the fine-tuning of immune cells. In clinical practice, a regular check of their fluctuations in MS patients would be necessary as the effects are considered variable between individuals. The best efficiency could be achieved by balancing both factors [261].
5.2. Sleep Management and Care in MS Clinical Routine
Given the high incidence of sleep disorders in the MS patient population and given the possible neuroimmunological, neuroinflammatory and neurodegenerative mechanisms that may be shared by sleep and MS, it would be conceivable to suggest that, in many cases, treating sleep-related disorders could contribute to improve some of the MS-related symptoms and, consequently, the quality of life of this patient population.
This section discusses some evidence that suggests possible intervention strategies in case of disturbed sleep in MS, but it is necessary to clarify that the first step for clinical practice would be to introduce sleep assessment and monitoring into the clinical-diagnostic routine of the disease, in order to address patients to a specific treatment in case of symptoms attributable to sleep-related problems.
Cognitive Behavioural Therapy for Insomnia (CBT-I) promotes healthy habits on sleep-related behaviours and suggests strategies to improve psychological processes and cognitive distortions that may contribute to persistent insomnia. There is well established evidence that has shown the effectiveness of CBT-I in cases of insomnia [263,264,265,266,267]. Recent data suggest that this approach is also useful in MS patients who also experience psychological comorbidities, such as, for example, depressive states [268]. Recent work investigated the efficacy of CBT-I in MS by assessing sleep through the use actigraphy and sleep log (a sort of sleep habits recording) [269]. Following intervention with CBT-I there was an increase in sleep efficiency, a decrease in time spent in bed and variability in sleep efficiency from sleep log data. The objective data recorded by the actigraphy showed, as in the sleep log, a decrease in total bedtime and also a decrease in total sleep time. There would be, therefore, an improvement following CBT-I, but the results are still scarce and not conclusive for the MS patient population.
Recently, the efficacy of tele-health-delivered CBT-I (tele-CBTi) was also evaluated given the possible limitations of patients' access to CBT-i due to possible symptoms such as motor difficulties, fatigue or living in areas that do not allow in-person access to such therapies [270]. Tele-CBT-i and classical CBT-I showed overlapping outcomes, both being beneficial in terms of lower sleep latency, improved efficiency, lower severity of insomnia level, lower fatigue and lower level of depressive state, all measured subjectively.
In the management of insomnia in MS, it is necessary to consider not only the aspects of the disease that can cause insomnia, but also the pharmacological treatments that patients assume to treat MS. For instance, patients treated with IFNß (immunotherapy) complain of symptoms such as drowsiness, tiredness, reduced sleep efficiency and flu-like side effects that can be linked to the treatment [271,272]. In this regard, recent work compared self-reported sleep quality and sleep patterns measured with actigraphy in MS patients treated with IFNß for more than 6 months (also comparing drug-free nights and nights following drug intake) and control subjects [273]. The results showed that MS patients had lower self-perceived sleep efficiency, longer sleep latency, higher objective sleep efficiency and more frequent intra-night awakenings than controls. When compared, the drug-free night and the night following treatment with IFNß, the sole significant difference was the total bedtime, which was greater on the night following drug treatment. These results suggest that IFNß may also contribute to sleep disturbances and, therefore, screening with objective measures in case of drug assumption would be useful. Such a case, for instance, it would be possible to minimise some effects by shifting the time of administration from the evening to the morning hours [271,274].
In case of SDB, the issue is often complicated, as breathing disorders are generally under-diagnosed and, in the case of MS, it is even more difficult to establish whether it is a primary sleep disorder or whether it may depend on another set of comorbidities that may or not be related to MS. In this case, the diagnostic phase of identifying the primary apnoea subtype, the severity of apnoea events and other specific MS-related symptoms is crucial [274]. Thus, at the diagnostic stage, the patient should undergo PSG to assess sleep apnoea levels and MSLT to assess excessive daytime sleepiness and the possibility of narcolepsy [275]. The therapy of choice for OSA is continuous positive air pressure (CPAP), which uses a mechanical device to keep the upper airway pervious during sleep. It has been found to improve oxygen saturation during the night also having beneficial effects on fatigue in MS patients [276].
No specific treatment guidelines exist for RLS in MS patients, but treatments should be based on the patient's symptoms and any comorbidities [274]. As for any other patients, dopaminergic (first-line) agents, anticonvulsants, opioids, benzodiazepines and treatment of iron deficiency are generally indicated, but any treatment choice must consider not only MS-related symptoms but also the patient's ongoing MS therapy [277].
In presence of circadian rhythm disorders, there are no specific treatments for MS patients, but the same strategies of scheduled daytime sleep, bright light exposure and supplementation of melatonin are suggested as for other types of patients.
A study investigated for the first time the relationship between physical activity and sleep characteristics in MS patients [278], assessing sleep by using actigraphy and physical activity levels with a mobile accelerometer. The results showed a positive relationship between physical activity and sleep parameters. Specifically, total sleep time correlated positively with light and moderate levels of physical activity. moderate physical activity (physical activity or exercise for 30 minutes a day) was also correlated with better sleep quality, while light physical activity was correlated with a higher number of intra-night awakenings. More recently, another work investigated the effects of moderate-intensity aerobic exercise and home-based exercise (control group) on sleep characteristics by measuring sleep-related biomarkers, such as serotonin, melatonin and cortisol in MS patients [6]. Those who performed moderate-intensity aerobic exercise had a significant improvement in self-reported sleep quality, in the degree of insomnia severity and in some objective parameters measured by actigraphy. Among the biomarkers, only serotonin increased, with higher levels in the patients who engaged in aerobic exercise than in the control group, and the change in serotonin levels correlated with changes in sleep quality and insomnia severity index only in the group of patients who engaged in moderate-intensity aerobic exercise.
Beyond specific sleep disorders, sleep quality in MS patients was also assessed by investigating patients' sleep hygiene habits in a descriptive correlational study [279]. The correlations examined the relationship of fatigue and other MS-related symptoms with sleep quality and sleep hygiene behaviours (patients were asked to state how often they engaged in sleep hygiene behaviours (watching TV, eating, drinking, smoking, etc.) in the period immediately before sleep. The results showed that MS-related symptoms were correlated with poor sleep hygiene behaviour, confirming previous works [272,280].
A schematic representation of possible sleep-based interventions in MS is shown in Figure 2.
5. Conclusions
The perspective that emerges from the contributions available in the literature on the relationship between sleep and MS, points to a complex framework of reciprocal interactions at different levels. Assuming that more than 50% of MS patients suffer from sleep disorders and considering the importance given in recent years to investigating modifiable risk and protective factors relating to the development and course of MS, an in-depth study of the mechanisms linking sleep and MS seems necessary.
In the complexity of the processes underlying MS, some mechanisms are still unclear, but there is general consensus of the involvement of mechanisms including impairment of the immune response, neuroinflammation and neurodegeneration, with the widely accepted belief that these processes are present in parallel from the onset of the disease (possibly even earlier). Sleep fits into the complex scenario of the autoimmune, neuroinflammatory and neurodegenerative reactions of the disease as it appears to be involved in many molecular and cellular dynamics underlying the abnormal peripheral and central responses typical of all stages of MS. A recent strand of literature on sleep has emphasised the importance of cytokines as sleep regulating substances, and certain types of pro-inflammatory cytokines are implicated in the autoimmune and inflammatory processes of MS and contribute to the malfunctioning of the various autoimmune processes that lead to the signature attack on the myelin sheath and the typical clinical manifestations of MS.
MS affects mainly young individuals, with the onset of the disease largely occurring between the second and fourth decade of life. One of the challenges of both research and clinic is to ensure the greatest attainable quality of life for patients. It is therefore necessary to address all the variables that may have an impact on patients' daily lives. It is well known that chronic sleep fragmentation, whatever the underlying cause, has an impact on the daytime life of individuals, causing sleepiness, cognitive difficulties and a general deterioration in performance. In the case of MS, the distress caused by disturbed sleep adds to an already compromised general situation and, if we consider that sleep and MS share mechanisms at an autoimmune, neuroinflammatory and neurodegenerative level, such that they affect each other, it is clear that sleep management is fundamental from the beginning of the disease, even at the diagnostic stage. Currently, the diagnostic routine of MS does not include sleep monitoring, despite the fact that more than half of MS patients have at least one sleep disorder. Detecting the presence of sleep problems at the diagnostic stage would mean being able to act on a modifiable factor related to MS and, consequently, treating disturbed sleep would mean acting positively on the daytime consequences of this disorder and alleviating the discomfort resulting from sleep impairment. Furthermore, one of the most common symptoms complained by MS patients is fatigue, which is closely related to sleep and often also confused with sleepiness due to non-restorative sleep (as in the case of continuous awakenings due to pain or nocturia).
Thus, for all the reasons expressed, the assessment, monitoring and potential intervention on disturbed sleep, as well as further studies directly investigating the relationship between sleep and MS, would be necessary to advance the most important challenge in relation to the current state of knowledge on MS: the improvement of MS patients' quality of life.
Author Contributions
Conceptualization, S.C. and L.D.G..; resources, S.C. and V.A.; writing—original draft preparation, S.C.; writing—review and editing, S.C, V.A. and L.D.G..; supervision, L.D.G. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wallin M.T., Culpepper W.J., Campbell J.D., Nelson L.M., Langer-Gould A., Marrie R.A., Cutter G.R., Kaye W.E., Wagner L., Tremlett H., Buka S.L., Dilokthornsakul P., Topol B., Chen L.H., LaRocca N.G.; US. Multiple Sclerosis Prevalence Workgroup. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurol. 2019, 92, e1029-e1040. [CrossRef]
- Loma I., Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol. 2011, 9, 409-16. [CrossRef]
- Tobore T.O. Towards a comprehensive etiopathogenetic and pathophysiological theory of multiple sclerosis. Int J Neurosci. 2020, 130, 279-300. [CrossRef]
- Mohammed E.M. Environmental Influencers, MicroRNA, and Multiple Sclerosis. J. of Cent. Nerv. System Dis. 2020;12. [CrossRef]
- Lassmann H., Brück W., Lucchinetti C.F. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007, 17, 210-8. [CrossRef]
- Al-Sharman A., Khalil H., El-Salem K., Aldughmi M., Aburub A. The effects of aerobic exercise on sleep quality measures and sleep-related biomarkers in individuals with Multiple Sclerosis: A pilot randomised controlled trial. NeuroRehab. 2019;45:107-115. [CrossRef]
- Lashley F.R. A review of sleep in selected immune and autoimmune disorders. Holist Nurs Pract. 2003, 17, 65-80. [CrossRef]
- Lazibat I., Rubinić Majdak M., Županić S. Multiple Sclerosis: New Aspects of Immunopathogenesis. Acta Clin Croat. 2018, 57, 352-361. [CrossRef]
- Filippi M., Rocca M.A., Barkhof F., Brück W., Chen J.T., Comi G., DeLuca G., De Stefano N., Erickson B.J., Evangelou N., Fazekas F., Geurts J.J., Lucchinetti C., Miller D.H., Pelletier D., Popescu B.F., Lassmann H.; Attendees of the Correlation between Pathological MRI findings in MS workshop. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012, 11, 349-60. [CrossRef]
- Lucchinetti C.F., Popescu B.F., Bunyan R.F., Moll N.M., Roemer S.F.., Lassmann H, Brück W., Parisi J.E., Scheithauer B.W., Giannini C., Weigand S.D., Mandrekar J., Ransohoff R.M. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011, 365, 2188-97. [CrossRef]
- das Neves S.P., Sousa J.C., Sousa N., Cerqueira J.J., Marques F. Altered astrocytic function in experimental neuroinflammation and multiple sclerosis. Glia. 2021, 69, 1341-1368. [CrossRef]
- Titus H.E., Chen Y., Podojil J.R., Robinson A.P., Balabanov R., Popko B., Miller S.D. Pre-clinical and Clinical Implications of "Inside-Out" vs. "Outside-In" Paradigms in Multiple Sclerosis Etiopathogenesis. Front Cell Neurosci. 2020 Oct 27;14:599717. [CrossRef]
- Yong H., Chartier G., Quandt J. Modulating inflammation and neuroprotection in multiple sclerosis. J Neurosci Res. 2018 Jun;96:927-950. [CrossRef]
- Papiri G., D'Andreamatteo G., Cacchiò G., Alia S., Silvestrini M., Paci C., Luzzi S., Vignini A. Multiple Sclerosis: Inflammatory and Neuroglial Aspects. Curr Issues Mol Biol. 2023, 45, 1443-1470. [CrossRef]
- Jarius S., König F., Metz I., Ruprecht K., Paul F., Brück W., Wildemann B. Pattern II and pattern III MS are entities distinct from pattern I MS: evidence from cerebrospinal fluid analysis. J Neuroinflamm. 2017; 14, 171. [CrossRef]
- de Barcelos I.P., Troxell R.M., Graves J.S. Mitochondrial Dysfunction and Multiple Sclerosis. Biology (Basel). 2019, 8, 37. [CrossRef]
- Steinman R.M. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med. 2001, 68, 160-6. , Zipp F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol. 2014 Dec;262 Pt A:8-17. [CrossRef]
- Sospedra M., Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005 ;23:683-747. [CrossRef]
- Friese M.A., Schattling B., Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol. 2014, 10, 225-38. [CrossRef]
- Romee R., Schneider S.E., Leong J.W., Chase J.M., Keppel C.R., Sullivan R.P., Cooper M.A., Fehniger T.A. Cytokine activation induces human memory-like NK cells. Blood. 2012, 120, 4751-60. [CrossRef]
- Peferoen L., Kipp M., van der Valk P., van Noort J.M., Amor S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology. 2014, 141, 302-13. [CrossRef]
- Braley T.J. Overview: A Framework for the Discussion of Sleep in Multiple Sclerosis. Curr Sleep Med Rep. 2017, 3, 263-271.
- Hughes A.J., Dunn K.M., Chaffee T. Sleep Disturbance and Cognitive Dysfunction in Multiple Sclerosis: a Systematic Review. Curr Neurol Neurosci Rep. 2018, 18, 2. [CrossRef]
- Kołtuniuk A., Chojdak-Łukasiewicz J. Adherence to Therapy in Patients with Multiple Sclerosis-Review. Int J Environ Res Public Health. 2022, 19, 2203. [CrossRef]
- Sater R., Gudesblatt M., Kresa-Reahl K., Brandes D., Sater P. The relationship between objective parameters of sleep and measures of fatigue, depression, and cognition in multiple sclerosis. Multiple Sclerosis J. - Experimental, Translational and Clinical. 2015;1. [CrossRef]
- Bakalidou D., Giannopapas V., Giannopoulos S. Thoughts on Fatigue in Multiple Sclerosis Patients. Cureus. 2023, 15, e42146. [CrossRef]
- Arand D.L., Bonnet M.H. The multiple sleep latency test. Handb Clin Neurol. 2019;160:393-403. [CrossRef]
- Braley T.J., Shieu M.M., Zaheed A.B., Dunietz G.L. Pathways between multiple sclerosis, sleep disorders, and cognitive function: Longitudinal findings from The Nurses' Health Study. Mult Scler. 2023, 29, 436-446. [CrossRef]
- Chinnadurai S.A., Gandhirajan D., Pamidimukala V.., Kesavamurthy B, Venkatesan S.A. Analysing the relationship between polysomnographic measures of sleep with measures of physical and cognitive fatigue in people with multiple sclerosis. Mult Scler Relat Disord. 2018 Aug;24:32-37. [CrossRef]
- Cederberg K.L.J., Mathison B.G., Schuetz M.L., Motl R.W. Discrepancies between self-reported and device-measured sleep parameters in adults with multiple sclerosis. J Clin Sleep Med. 2022 Feb 1;18:415-421. https://doi.org/10.5664/jcsm.9586. J., Chervin R.D., Segal B.M. Fatigue, tiredness, lack of energy, and sleepiness in multiple sclerosis patients referred for clinical polysomnography. Mult Scler Int. 2012;2012:673936. doi:10.1155/2012/673936.
- Laslett L.L., Honan C., Turner J.A., Dagnew B., Campbell J..A, Gill T.K., Appleton S., Blizzard L., Taylor B.V., van der Mei I. Poor sleep and multiple sclerosis: associations with symptoms of multiple sclerosis and quality of life. J Neurol Neurosurg Psychiatry. 2022, jnnp-2022-329227. [CrossRef]
- Foschi M., Rizzo G., Liguori R., Avoni P., Mancinelli L., Lugaresi A., Ferini-Strambi L. Sleep-related disorders and their relationship with MRI findings in multiple sclerosis. Sleep Med. 2019, 56, 90-97. https://doi.org/10.1016/j.sleep.2019.01.010. , Howard R.S., Hirsch N.P., Miller D.H., Moseley I.F., Fish D. Sleep problems in multiple sclerosis. Eur Neurol. 1994;34:320-3. doi:10.1159/000117070.
- Koblinger K., Füzesi T., Ejdrygiewicz J., Krajacic A., Bains J.S., Whelan P.J. Characterization of A11 neurons projecting to the spinal cord of mice. PLoS One. 2014, 9, e109636. [CrossRef]
- Clark C.M., Fleming J.A., Li D., Oger J., Klonoff H., Paty D. Sleep Disturbance, Depression, and Lesion Site in Patients With Multiple Sclerosis. Arch Neurol. 1992;49:641–643. [CrossRef]
- Ferini-Strambi L., Filippi M., Martinelli V., Oldani A., Rovaris M., Zucconi M., Comi G., Smirne S. Nocturnal sleep study in multiple sclerosis: correlations with clinical and brain magnetic resonance imaging findings. J Neurol Sci. 1994, 125, 194-7. [CrossRef]
- Benarroch E.E. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology. 2009, 73, 1699-704. [CrossRef]
- Polak P.E., Kalinin S., Feinstein D.L. Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2011 Mar;134(Pt 3):665-77. [CrossRef]
- Marien M.R., Colpaert F.C., Rosenquist A.C. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004, 45, 38-78. [CrossRef]
- Biernacki T., Kokas Z., Sandi D., Füvesi J., Fricska-Nagy Z., Faragó P., Kincses T.Z., Klivényi P, Bencsik K., Vécsei L. Emerging Biomarkers of Multiple Sclerosis in the Blood and the CSF: A Focus on Neurofilaments and Therapeutic Considerations. Int. J. of Mol. Sci. 2022; 23:3383. [CrossRef]
- Whibley D., Goldstein C., Kratz A.L., Braley T.J. A multidimensional approach to sleep health in multiple sclerosis. Mult Scler Relat Disord. 2021, 56, 103271. [CrossRef]
- Irwin M.R., Opp M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology. 2017, 42, 129-155. [CrossRef]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008, 9, 46-56. [CrossRef]
- Olofsson P.S., Rosas-Ballina M., Levine Y.A., Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012, 248, 188-204. [CrossRef]
- Imeri L., Opp M.R. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009, 10, 199-210. [CrossRef]
- Pan W., Kastin A.J. The Blood-Brain Barrier: Regulatory Roles in Wakefulness and Sleep. Neuroscientist. 2017, 23, 124-136. [CrossRef]
- He J., Hsuchou H., He Y., Kastin A.J., Wang Y., Pan W. Sleep restriction impairs blood-brain barrier function. J Neurosci. 2014, 34, 14697-706. [CrossRef]
- Dickstein J.B., Moldoksky. Sleep, cytokines and immune function. Sleep Med Rev. 1999. Vol.3, No.3, pp 219-228.
- Irwin M.R. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015, 66, 143-72. [CrossRef]
- Besedovsky L., Lange T., Born J. Sleep and immune function. Pflugers Arch. 2012, 463, 121-37. [CrossRef]
- Born W., Cady C., Jones-Carson J., Mukasa A., Lahn M., O'Brien R. Immunoregulatory functions of gamma delta T cells. Adv Immunol. 1999;71:77-144.
- Haus E.L., Smolensky M.H. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013, 17, 273-84. [CrossRef]
- Kronfol Z., Nair M., Zhang Q., Hill E.E., Brown M.B. Circadian immune measures in healthy volunteers: relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom Med. 1997, 59, 42-50. [CrossRef]
- Irwin M., McClintick J., Costlow C., Fortner M., White J., Gillin J.C. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996, 10, 643-53. [CrossRef]
- Redwine L., Dang J., Irwin M. Cellular adhesion molecule expression, nocturnal sleep, and partial night sleep deprivation. Brain Behav Immun. 2004, 18, 333-40. [CrossRef]
- Faraut B., Boudjeltia K.Z., Vanhamme L., Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012, 16, 137-49. [CrossRef]
- Dinges D.F., Douglas S.D., Hamarman S., Zaugg L., Kapoor S. Sleep deprivation and human immune function. Adv Neuroimmunol. 1995;5:97-110. [CrossRef]
- Palmblad J., Petrini B., Wasserman J., Akerstedt T. Lymphocyte and granulocyte reactions during sleep deprivation. Psychosom Med. 1979, 41, 273-8. [CrossRef]
- Faraut B., Boudjeltia K.Z., Dyzma M., Rousseau A., David E., Stenuit P., Franck T., Van Antwerpen P., Vanhaeverbeek M., Kerkhofs M. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011, 25, 16-24. [CrossRef]
- Boudjeltia K.Z., Faraut B., Stenuit P., Esposito M.J., Dyzma M., Brohée D., Ducobu J., Vanhaeverbeek M., Kerkhofs M. Sleep restriction increases white blood cells, mainly neutrophil count, in young healthy men: a pilot study. Vasc Health Risk Manag. 2008;4:1467-70. [CrossRef]
- van Leeuwen W.M., Lehto M., Karisola P., Lindholm H., Luukkonen R., Sallinen M., Härmä M., Porkka-Heiskanen T., Alenius H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. [CrossRef]
- Schmid S.M., Hallschmid M., Jauch-Chara K., Wilms B., Lehnert H., Born J., Schultes B. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011, 34, 371-7. [CrossRef]
- Stamatakis K.A., Punjabi N.M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010, 137, 95-101. [CrossRef]
- Shearer W.T., Reuben J.M.., Mullington J.M., Price N.J., Lee B.N., Smith E.O., Szuba M.P., Van Dongen H.P., Dinges D.F. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001, 107, 165-70. [CrossRef]
- Vgontzas A.N., Pejovic S., Zoumakis E., Lin H.M., Bixler E.O., Basta M., Fang J., Sarrigiannidis A., Chrousos G.P. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007, 292, E253-61. [CrossRef]
- Selter R.C., Hemmer B. Update on immunopathogenesis and immunotherapy in multiple sclerosis. Immunotargets Ther. 2013, 2, 21-30. [CrossRef]
- Gold R., Wolinsky J.S. Pathophysiology of multiple sclerosis and the place of teriflunomide. Acta Neurol Scand. 2011, 124, 75-84. [CrossRef]
- Blagov A.V., Grechko A.V., Nikiforov N.G., Borisov E.E., Sadykhov N.K., Orekhov A.N. Role of Impaired Mitochondrial Dynamics Processes in the Pathogenesis of Alzheimer's Disease. Int J Mol Sci. 2022, 23, 6954. [CrossRef]
- Koriem M. "Corrigendum to ‘Multiple sclerosis: New insights and trends’" Asian Pacific Journal of Tropical Biomedicine, 2017 vol. 7, no. 5,. [CrossRef]
- Bjartmar C., Wujek J.R., Trapp B.D. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003, 206, 165-71. [CrossRef]
- Brück W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005 Nov;252 Suppl 5:v3-9. [CrossRef]
- Gudkov S.V., Burmistrov D.E., Kondakova E.V., Sarimov R.M., Yarkov R.S., Franceschi C., Vedunova M.V. An emerging role of astrocytes in aging/neuroinflammation and gut-brain axis with consequences on sleep and sleep disorders. Ageing Res Rev. 2023, 83, 101775. [CrossRef]
- Morais L.H., Schreiber H.L. 4th, Mazmanian S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021, 19, 241-255. [CrossRef]
- Pak V.M., Onen S.H., Bliwise D.L., Kutner N.G., Russell K.L., Onen F. Sleep Disturbances in MCI and AD: Neuroinflammation as a Possible Mediating Pathway. Front Aging Neurosci. 2020, 12, 69. [CrossRef]
- Vaidyanathan T.V., Collard M., Yokoyama S., Reitman M.E., Poskanzer K.E. Cortical astrocytes independently regulate sleep depth and duration via separate GPCR pathways. Elife. 2021, 10, e63329. [CrossRef]
- Jewett K.A., Krueger J.M. Humoral sleep regulation; interleukin-1 and tumor necrosis factor. Vitam Horm. 2012;89:241-57. [CrossRef]
- Krueger J.M., Rector D.M., Roy S., Van Dongen H.P., Belenky G., Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008, 9, 910-9. [CrossRef]
- Obal F. Jr, Krueger J.M. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003, 8, d520-50. [CrossRef]
- Roky R., Obál F. Jr, Valatx J.L., Bredow S., Fang J., Pagano L.P., Krueger J.M. Prolactin and rapid eye movement sleep regulation. Sleep. 1995, 18, 536-42.
- Kilduff T.S., Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000, 23, 359-65. [CrossRef]
- Barun B. Pathophysiological background and clinical characteristics of sleep disorders in multiple sclerosis. Clin Neurol Neurosurg. 2013 Dec;115 Suppl 1:S82-5. [CrossRef]
- Motivala, S.J., & Irwin, M.R. Sleep and Immunity: Cytokine Pathways Linking Sleep and Health Outcomes. Current Directions in Psychological Science, 2007, 16(1), 21-25. [CrossRef]
- Opp M.R. Cytokines and sleep. Sleep Med Rev. 2005, 9, 355-64. [CrossRef]
- Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002, 105, 1135-43. [CrossRef]
- Born J., Lange T., Hansen K., Mölle M., Fehm H.L. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997, 158, 4454-64.
- Redwine L., Hauger R..L, Gillin J.C., Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000, 85, 3597-603. [CrossRef]
- Zielinski M.R., McKenna J.T., McCarley R.W. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016;3:67-104. [CrossRef]
- Dinarello C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018, 281, 8-27. [CrossRef]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [CrossRef]
- Viola-Saltzman M., Watson N.F. Traumatic brain injury and sleep disorders. Neurol Clin. 2012, 30, 1299-312. [CrossRef]
- Boutin H., LeFeuvre R.A., Horai R.., Asano M, Iwakura Y., Rothwell N.J. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001, 21, 5528-34. Erratum in: J Neurosci 2001, 21, 1a. [CrossRef]
- Berger A.M., Parker K.P., Young-McCaughan S., Mallory G.A., Barsevick A.M., Beck S.L., Carpenter J.S., Carter P.A., Farr L.A., Hinds P.S., Lee K.A., Miaskowski C., Mock V., Payne J.K., Hall M. Sleep wake disturbances in people with cancer and their caregivers: state of the science. Oncol Nurs Forum. 2005, 32, E98-126. [CrossRef]
- Opp M.R., Obal F. Jr, Krueger J.M. Interleukin 1 alters rat sleep: temporal and dose-related effects. Am J Physiol. 1991 Jan;260(1 Pt 2):R52-8. [CrossRef]
- Krueger J.M., Obál F.J., Fang J., Kubota T., Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001, 933, 211-21. [CrossRef]
- Imeri L., Ceccarelli P., Mariotti M., Manfridi A., Opp M.R., Mancia M. Sleep, but not febrile responses of Fisher 344 rats to immune challenge are affected by aging. Brain Behav Immun. 2004, 18, 399-404. [CrossRef]
- De Sarro G., Gareri P., Sinopoli V.A., David E., Rotiroti D. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci. 1997;60:555-64. [CrossRef]
- Manfridi A., Brambilla D., Bianchi S., Mariotti M., Opp M.R., Imeri L. Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci. 2003, 18, 1041-9. [CrossRef]
- Zielinski M.R., Krueger J.M. Sleep and innate immunity. Front Biosci (Schol Ed). 2011, 3, 632-42. [CrossRef]
- Obal F. Jr, Opp M., Cady A.B., Johannsen L., Postlethwaite A.E., Poppleton H.M., Seyer J.M., Krueger J.M. Interleukin 1 alpha and an interleukin 1 beta fragment are somnogenic. Am J Physiol. 1990 Sep;259(3 Pt 2):R439-46. [CrossRef]
- Zielinski M.R., Krueger J.M. Sleep and innate immunity. Front Biosci (Schol Ed). 2011 Jan 1;3:632-42. [CrossRef]
- Schmidt E.M., Linz B., Diekelmann S., Besedovsky L., Lange T., Born J. Effects of an interleukin-1 receptor antagonist on human sleep, sleep-associated memory consolidation, and blood monocytes. Brain Behav Immun. 2015, 47, 178-85. [CrossRef]
- Probert L. TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience. 2015, 302, 2-22. [CrossRef]
- Zielinski M.R., Systrom D.M., Rose N.R. Fatigue, Sleep, and Autoimmune and Related Disorders. Front Immunol. 2019, 10, 1827. [CrossRef]
- Zielinski M.R., Gibbons A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front Cell Infect Microbiol. 2022, 12, 853096. [CrossRef]
- Leibowitz S.M, Yan J. NF-κB Pathways in the Pathogenesis of Multiple Sclerosis and the Therapeutic Implications. Front Mol Neurosci. 2016, 9, 84. [CrossRef]
- Opp M.R., Toth L.A. Neural-immune interactions in the regulation of sleep. Front Biosci. 2003, 8, d768-79. [CrossRef]
- Irwin M.R. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019, 19, 702-715. [CrossRef]
- Irwin M.R., Wang M., Campomayor C.O., Collado-Hidalgo A., Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006, 166, 1756-62. [CrossRef]
- Nishimoto N. and Kishimoto T. Interleukin 6: From Bench to Bedside. Nature Clinical Practice Rheumatology 2006, 2, 619-626. [CrossRef]
- Mohamed-Ali V., Flower L., Sethi .J, Hotamisligil G., Gray R., Humphries S.E., York D.A., Pinkney J. beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J Clin Endocrinol Metab. 2001, 86, 5864-9. [CrossRef]
- Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989, 142, 549-53.
- Bruunsgaard H., Andersen-Ranberg K., Hjelmborg J.v., Pedersen B.K., Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003, 115, 278-83. [CrossRef]
- Rohleder N., Aringer M., Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012, 1261, 88-96. [CrossRef]
- Vgontzas A.N., Papanicolaou D.A., Bixler E.O., Lotsikas A., Zachman K., Kales A., Prolo P., Wong M.L., Licinio J., Gold P.W., Hermida R.C., Mastorakos G., Chrousos G.P. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999, 84, 2603-7. [CrossRef]
- Lange T., Dimitrov S., Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010, 1193, 48-59. [CrossRef]
- Dimitrov S., Besedovsky L., Born J., Lange T. Differential acute effects of sleep on spontaneous and stimulated production of tumor necrosis factor in men. Brain Behav Immun. 2015, 47, 201-10. [CrossRef]
- Redwine L., Dang J., Hall M., Irwin M. Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom Med. 2003, 65, 75-85. [CrossRef]
- Vgontzas A.N., Zoumakis E., Bixler E.O., Lin H.M., Follett H., Kales A., Chrousos G.P. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004, 89, 2119-26. [CrossRef]
- Simpson N., Dinges D.F. Sleep and inflammation. Nutr Rev. 2007 Dec;65(12 Pt 2):S244-52. [CrossRef]
- Burgos I., Richter L., Klein T., Fiebich B., Feige B., Lieb K., Voderholzer U., Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006, 20, 246-53. [CrossRef]
- Späth-Schwalbe E., Hansen K., Schmidt F., Schrezenmeier H., Marshall L., Burger K., Fehm H.L., Born J. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998, 83, 1573-9. [CrossRef]
- Bargiela D., Chinnery P.F. Mitochondria in neuroinflammation - Multiple sclerosis (MS), leber hereditary optic neuropathy (LHON) and LHON-MS. Neurosci Lett. 2019, 710, 132932. [CrossRef]
- Patergnani S., Morciano G., Carinci M., Leo S., Pinton P., Rimessi A. The "mitochondrial stress responses": the "Dr. Jekyll and Mr. Hyde" of neuronal disorders. Neural Regen Res. 2022, 17, 2563-2575. [CrossRef]
- Mao, P. and Reddy, P.H. Is multiple sclerosis a mitochondrial disease? Biochimica et Biophysica Acta, 2010 1802, 66-79.
- Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014 May 19;24:R453-62. [CrossRef]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979, 59, 527-605. [CrossRef]
- Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014, 94, 909-50. [CrossRef]
- Michaličková D., Hrnčíř T., Canová N.K., Slanař O. Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur J Pharmacol. 2020, 873, 172973. [CrossRef]
- van Horssen J., Schreibelt G., Drexhage J., Hazes T., Dijkstra C.D., van der Valk P., de Vries H.E. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med. 2008, 45, 1729-37. [CrossRef]
- Ortiz G.G., Pacheco-Moisés F.P., Bitzer-Quintero O.K., Ramírez-Anguiano A.C., Flores-Alvarado L.J., Ramírez-Ramírez V., Macias-Islas M.A., Torres-Sánchez E.D. Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol. 2013;2013:708659. [CrossRef]
- Park J.S., Chyun J.H., Kim Y.K., Line L.L., Chew B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond). 2010, 7, 18. [CrossRef]
- Reimund E. The free radical flux theory of sleep. Med Hypotheses. 1994, 43, 231-3. [CrossRef]
- Teixeira, N.B., Picolo, G., Giardini, A.C. Alterations of peripheral nerve excitability in an experimental autoimmune encephalomyelitis mouse model for multiple sclerosis. J Neuroinflammation 2020; 17, 266. [CrossRef]
- Villafuerte G., Miguel-Puga A., Rodríguez E.M., Machado S., Manjarrez E., Arias-Carrión O. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev. 2015;2015:234952. [CrossRef]
- Richardson R.B., Mailloux R.J. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants (Basel). 2023, 12, 674. [CrossRef]
- Hartmann C., Kempf A. Mitochondrial control of sleep. Curr Opin Neurobiol. 2023, 81, 102733. [CrossRef]
- Hill V.M., O'Connor R.M., Sissoko G.B., Irobunda I.S., Leong S., Canman J.C., Stavropoulos N., Shirasu-Hiza M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018, 16, e2005206. [CrossRef]
- Gulec M., Ozkol H., Selvi Y., Tuluce Y., Aydin A., Besiroglu L., Ozdemir P.G. Oxidative stress in patients with primary insomnia. Prog Neuropsychopharmacol Biol Psychiatry. 2012, 37, 247-51. [CrossRef]
- Silva R.H., Abílio V.C., Takatsu A.L., Kameda S.R., Grassl C., Chehin A.B., Medrano W.A., Calzavara M.B., Registro S., Andersen M.L., Machado R.B.., Carvalho R.C., Ribeiro Rde A., Tufik S., Frussa-Filho R. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004, 46, 895-903. [CrossRef]
- Vaccaro A., Kaplan Dor Y., Nambara K., Pollina E.A., Lin C., Greenberg M.E., Rogulja D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell. 2020, 181, 1307-1328.e15. [CrossRef]
- Everson C.A., Henchen C.J., Szabo A., Hogg N. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep. 2014, 37, 1929-40. [CrossRef]
- Kempf A., Song S.M., Talbot C.B., Miesenböck G. A potassium channel β-subunit couples mitochondrial electron transport to sleep. Nature. 2019, 568, 230-234. [CrossRef]
- Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015, 163, 560-9. [CrossRef]
- Melhuish Beaupre L.M., Brown G.M., Braganza N.A., Kennedy J.L., Gonçalves V.F. Mitochondria's role in sleep: Novel insights from sleep deprivation and restriction studies. World J Biol Psychiatry. 2022, 23, 1-13. [CrossRef]
- Rodriguez A.M., Nakhle J., Griessinger E., Vignais M.L. Intercellular mitochondria trafficking highlighting the dual role of mesenchymal stem cells as both sensors and rescuers of tissue injury. Cell Cycle. 2018;17:712-721. [CrossRef]
- Somarajan B.I., Khanday M.A., Mallick B.N. Rapid Eye Movement Sleep Deprivation Induces Neuronal Apoptosis by Noradrenaline Acting on Alpha1 Adrenoceptor and by Triggering Mitochondrial Intrinsic Pathway. Front Neurol. 2016, 7, 25. [CrossRef]
- Singh M., Jadhav H.R. Melatonin: functions and ligands. Drug Discov Today. 2014, 19, 1410-8. [CrossRef]
- Blanco R.A., Ziegler T.R., Carlson B.A., Cheng P.Y., Park Y., Cotsonis G.A., Accardi C.J., Jones D.P. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007, 86, 1016-23. [CrossRef]
- Wilking M., Ndiaye M., Mukhtar H., Ahmad N. Circadian rhythm connections to oxidative stress: implications for human health. Antioxid Redox Signal. 2013, 19, 192-208. [CrossRef]
- Ramanathan L., Gulyani S., Nienhuis R., Siegel J.M. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002, 13, 1387-90. [CrossRef]
- Atrooz F., Salim S. Sleep deprivation, oxidative stress and inflammation. Adv Protein Chem Struct Biol. 2020;119:309-336. [CrossRef]
- Zhao D., Yu Y., Shen Y., Liu Q., Zhao Z., Sharma R., Reiter R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front Endocrinol (Lausanne). 2019, 10, 249. [CrossRef]
- Gunata M., Parlakpinar H., Acet H.A. Melatonin: A review of its potential functions and effects on neurological diseases. Rev Neurol (Paris). 2020, 176, 148-165. [CrossRef]
- Claustrat B., Leston J. Melatonin: Physiological effects in humans. Neurochirurgie. 2015 Apr-Jun;61(2-3):77-84. [CrossRef]
- Pardridge W.M., Mietus L.J. Transport of albumin-bound melatonin through the blood-brain barrier. J Neurochem. 1980, 34, 1761-3. [CrossRef]
- Dubocovich M.L., Delagrange P., Krause D.N., Sugden D., Cardinali D.P., Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010, 62, 343-80. [CrossRef]
- Cecon E., Oishi A., Jockers R. Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br J Pharmacol. 2018, 175, 3263-3280. [CrossRef]
- Weaver D.R., Liu C., Reppert S.M. Nature's knockout: the Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol Endocrinol. 1996, 10, 1478-87. [CrossRef]
- Mazzucchelli C., Pannacci M., Nonno R., Lucini V., Fraschini F., Stankov B.M. The melatonin receptor in the human brain: cloning experiments and distribution studies. Brain Res Mol Brain Res. 1996 Jul;39(1-2):117-26. [CrossRef]
- Savaskan E., Wirz-Justice A., Olivieri G., Pache M., Kräuchi K., Brydon L., Jockers R., Müller-Spahn F., Meyer P. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem. 2002, 50, 519-26. [CrossRef]
- Blodgett T.J., Blodgett N.P. Melatonin and melatonin-receptor agonists to prevent delirium in hospitalized older adults: An umbrella review. Geriatr Nurs. 2021, 42, 1562-1568. [CrossRef]
- Ren W., Liu G., Chen S., Yin J., Wang J., Tan B., Wu G., Bazer F.W., Peng Y., Li T., Reiter R.J., Yin Y. Melatonin signaling in T cells: Functions and applications. J Pineal Res. 2017 Apr;62(3). [CrossRef]
- Reppert S.M., Godson C., Mahle C.D., Weaver D.R., Slaugenhaupt S.A., Gusella J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995, 92, 8734-8. [CrossRef]
- Al-Ghoul W.M., Herman M.D., Dubocovich M.L. Melatonin receptor subtype expression in human cerebellum. Neuroreport. 1998, 9, 4063-8. [CrossRef]
- Wu Y.H., Ursinus J., Zhou J.N., Scheer F.A., Ai-Min B., Jockers R., van Heerikhuize J., Swaab D.F. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. 2013 Jun;148(2-3):357-67. [CrossRef]
- Esposito S., Laino D., D'Alonzo R., Mencarelli A., Di Genova L., Fattorusso A., Argentiero A., Mencaroni E. Pediatric sleep disturbances and treatment with melatonin. J Transl Med. 2019, 17, 77. [CrossRef]
- Sack R.L., Lewy A.J., Hughes R.J. Use of melatonin for sleep and circadian rhythm disorders. Ann Med. 1998, 30, 115-21. [CrossRef]
- Liebmann P.M., Wölfler A., Felsner P., Hofer D., Schauenstein K. Melatonin and the immune system. Int Arch Allergy Immunol. 1997, 112, 203-11. [CrossRef]
- Reiter R.J. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991 Aug;79(1-3):C153-8. [CrossRef]
- Minich D.M., Henning M., Darley C., Fahoum M., Schuler C.B., Frame J. Is Melatonin the "Next Vitamin D"?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients. 2022 Sep 22;14:3934. [CrossRef]
- Hardeland R., Cardinali D.P., Srinivasan V., Spence D.W., Brown G.M., Pandi-Perumal S.R. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011, 93, 350-84. [CrossRef]
- Akpinar Z., Tokgöz S., Gökbel H., Okudan N., Uğuz F., Yilmaz G. The association of nocturnal serum melatonin levels with major depression in patients with acute multiple sclerosis. Psychiatry Res. 2008, 161, 253-7. [CrossRef]
- Ghorbani A., Salari M., Shaygannejad V., Norouzi R. The role of melatonin in the pathogenesis of multiple sclerosis: a case-control study. Int J Prev Med. 2013 May;4(Suppl 2):S180-4.
- Melamud L., Golan D., Luboshitzky R., Lavi I., Miller A. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci. 2012 Mar 15;314(1-2):37-40. [CrossRef]
- Sandyk R., Awerbuch G.I. Nocturnal melatonin secretion in multiple sclerosis patients with affective disorders. Int J Neurosci. 1993 Feb;68(3-4):227-40. [CrossRef]
- López-González A., Álvarez-Sánchez N., Lardone P.J., Cruz-Chamorro I., Martínez-López A., Guerrero J.M., Reiter R.J., Carrillo-Vico A. Melatonin treatment improves primary progressive multiple sclerosis: a case report. J Pineal Res. 2015, 58, 173-7. [CrossRef]
- Ghareghani M., Scavo L., Jand Y., Farhadi N., Sadeghi H., Ghanbari A., Mondello S., Arnoult D., Gharaghani S., Zibara K. Melatonin Therapy Modulates Cerebral Metabolism and Enhances Remyelination by Increasing PDK4 in a Mouse Model of Multiple Sclerosis. Front Pharmacol. 2019, 10, 147. [CrossRef]
- Damasceno A., Moraes A.S., Farias A., Damasceno B.P., dos Santos L.M., Cendes F. Disruption of melatonin circadian rhythm production is related to multiple sclerosis severity: A preliminary study. J Neurol Sci. 2015;353(1-2):166-8. [CrossRef]
- Gholipour T., Ghazizadeh T., Babapour S., Mansouri B., Ghafarpour M., Siroos B., Harirchian M.H. Decreased urinary level of melatonin as a marker of disease severity in patients with multiple sclerosis. Iran J Allergy Asthma Immunol. 2015, 14, 91-7.
- Adamczyk-Sowa M., Pierzchala K., Sowa P., Mucha S, Sadowska-Bartosz I., Adamczyk J., Hartel M. Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem Res. 2014, 39, 1585-93. [CrossRef]
- Sánchez-López A.L., Ortiz G.G., Pacheco-Moises F.P., Mireles-Ramírez M.A., Bitzer-Quintero O.K., Delgado-Lara D.L.C., Ramírez-Jirano L.J., Velázquez-Brizuela I.E. Efficacy of Melatonin on Serum Pro-inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis. Arch Med Res. 2018, 49, 391-398. [CrossRef]
- Yosefifard M., Vaezi G., Malekirad A.A., Faraji F., Hojati V. A Randomized Control Trial Study to Determine the Effect of Melatonin on Serum Levels of IL-1β and TNF-α in Patients with Multiple Sclerosis. Iran J Allergy Asthma Immunol. 2019, 18, 649-654. [CrossRef]
- Zarezadeh M., Khorshidi M., Emami M., Janmohammadi P., Kord-Varkaneh H., Mousavi S.M., Mohammed S.H., Saedisomeolia A., Alizadeh S. Melatonin supplementation and pro-inflammatory mediators: a systematic review and meta-analysis of clinical trials. Eur J Nutr. 2020, 59, 1803-1813. [CrossRef]
- Serada S., Fujimoto M., Mihara M., Koike N., Ohsugi Y., Nomura S., Yoshida H., Nishikawa T., Terabe F., Ohkawara T., Takahashi T., Ripley B., Kimura A., Kishimoto T., Naka T. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008, 105, 9041-6. [CrossRef]
- Michaud M., Balardy L., Moulis G., Gaudin C., Peyrot C., Vellas B., Cesari M., Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013, 14, 877-82. [CrossRef]
- Chen M., Wang Y.H., Wang Y., Huang L., Sandoval H., Liu Y.J., Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. lFeb 24;311:1160-4. [CrossRef]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013, 39, 1-10. [CrossRef]
- 187. Farez MF, Mascanfroni ID, Méndez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, Balbuena Aguirre ME, Patel B, Ysrraelit MC, Zhu C, Kuchroo VK, Rabinovich GA, Quintana FJ, Correale J. Melatonin Contributes to the Seasonality of Multiple Sclerosis Relapses. Cell. 2015, 162, 1338-52. [CrossRef]
- Álvarez-Sánchez N, Cruz-Chamorro I, Díaz-Sánchez M, Sarmiento-Soto H, Medrano-Campillo P, Martínez-López A, Lardone PJ, Guerrero JM, Carrillo-Vico A. Melatonin reduces inflammatory response in peripheral T helper lymphocytes from relapsing-remitting multiple sclerosis patients. J Pineal Res. 2017 Nov;63(4). [CrossRef]
- Muñoz-Jurado A, Escribano BM, Caballero-Villarraso J, Galván A, Agüera E, Santamaría A, Túnez I. Melatonin and multiple sclerosis: antioxidant, anti-inflammatory and immunomodulator mechanism of action. Inflammopharmacology. 2022 Oct;30:1569-1596. [CrossRef]
- Adamczyk-Sowa M, Sowa P, Adamczyk J, Niedziela N, Misiolek H, Owczarek M, Zwirska-Korczala K. Effect of melatonin supplementation on plasma lipid hydroperoxides, homocysteine concentration and chronic fatigue syndrome in multiple sclerosis patients treated with interferons-beta and mitoxantrone. J Physiol Pharmacol. 2016, 67, 235–42.
- Yosefifard, Masoomeh & Vaezi, Gholamhassan & Maleki-Rad, Ali & Faraji, Fardin & Hojati, Vida. (2020). Effect of Melatonin on Serum Levels of INF-1β and Vitamin B12 in Patients With Multiple Sclerosis: A Randomized Controlled Trial. Iranian Journal of Toxicology. 14. 19-24. [CrossRef]
- Miller E, Walczak A, Majsterek I, Kędziora J. Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J Neuroimmunol. 2013 Apr 15;257(1-2):97-101. [CrossRef]
- Roostaei T, Sahraian MA, Hajeaghaee S, Gholipour T, Togha M, Siroos B, Mansouri S, Mohammadshirazi Z, Aghazadeh Alasti M, Harirchian MH. Impact of Melatonin on Motor, Cognitive and Neuroimaging Indices in Patients with Multiple Sclerosis. Iran J Allergy Asthma Immunol. 2015 Dec;14:589-95.
- Padureanu R, Albu CV, Mititelu RR, Bacanoiu MV, Docea AO, Calina D, Padureanu V, Olaru G, Sandu RE, Malin RD, Buga AM. Oxidative Stress and Inflammation Interdependence in Multiple Sclerosis. J Clin Med. 2019, 8, 1815. [CrossRef]
- Senthil Kumaran V, Arulmathi K, Srividhya R, Kalaiselvi P. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp Gerontol. 2008, 43, 176-83. [CrossRef]
- Maes M, Landucci Bonifacio K, Morelli NR, Vargas HO, Barbosa DS, Carvalho AF, Nunes SOV. Major Differences in Neurooxidative and Neuronitrosative Stress Pathways Between Major Depressive Disorder and Types I and II Bipolar Disorder. Mol Neurobiol. 2019, 56, 141-156. [CrossRef]
- Holton KF, Kirkland AE. Moving past antioxidant supplementation for the dietary treatment of multiple sclerosis. Mult Scler. 2020, 26, 1012-1023. [CrossRef]
- Morris JL, Gillet G, Prudent J, Popgeorgiev N. Bcl-2 Family of Proteins in the Control of Mitochondrial Calcium Signalling: An Old Chap with New Roles. Int J Mol Sci. 2021, 22, 3730. [CrossRef]
- Maldonado MD, Mora-Santos M, Naji L, Carrascosa-Salmoral MP, Naranjo MC, Calvo JR. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol Res. 2010, 62, 282-7. [CrossRef]
- Escribano BM, Colín-González AL, Santamaría A, Túnez I. The role of melatonin in multiple sclerosis, Huntington's disease and cerebral ischemia. CNS Neurol Disord Drug Targets. 2014;13:1096-119. [CrossRef]
- Túnez I, Montilla P, Del Carmen Muñoz M, Feijóo M, Salcedo M. Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington's disease. J Pineal Res. 2004, 37, 252-6. [CrossRef]
- Bahamonde C, Conde C, Agüera E, Lillo R, Luque E, Gascón F, Feijóo M, Cruz AH, Sánchez-López F, Túnez I. Elevated melatonin levels in natalizumab-treated female patients with relapsing-remitting multiple sclerosis: relationship to oxidative stress. Eur J Pharmacol. 2014, 730, 26-30. [CrossRef]
- Alghamdi BS, AboTaleb HA. Melatonin improves memory defects in a mouse model of multiple sclerosis by up-regulating cAMP-response element-binding protein and synapse-associated proteins in the prefrontal cortex. J Integr Neurosci. 2020, 19, 229-237. [CrossRef]
- Campbell A, Sharman E, Bondy SC. Age-related differences in the response of the brain to dietary melatonin. Age (Dordr). 2014, 36, 49-55. [CrossRef]
- Lassmann H, van Horssen J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim Biophys Acta. 2016, 1862, 506-10. [CrossRef]
- Michaličková D, Šíma M, Slanař O. New insights in the mechanisms of impaired redox signaling and its interplay with inflammation and immunity in multiple sclerosis. Physiol Res. 2020, 69, 1-19. [CrossRef]
- Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res. 2010, 48, 297-310. [CrossRef]
- Anderson, G. 2019. Mitochondria and the Gut as crucial hubs for the interactions of melatonin with sirtuins, inflammation, butyrate, tryptophan metabolites, and alpha 7 nicotinic receptor across a host of medical conditions. Melatonin Research. 2, 2 (June 2019), 70-85. [CrossRef]
- Leon J, Acuña-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ. Melatonin and mitochondrial function. Life Sci. 2004, 75, 765-90. [CrossRef]
- Martín M, Macías M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuña-Castroviejo D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res. 2000, 28, 242-8. [CrossRef]
- Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002, 19, 695-707. [CrossRef]
- Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014, 71, 2997-3025. [CrossRef]
- Hsu WY, Anderson A, Rowles W, Peters KE, Li V, Stone KL, Ashbrook LH, Gelfand AA, Bove RM. Effects of melatonin on sleep disturbances in multiple sclerosis: A randomized, controlled pilot study. Mult Scler J Exp Transl Clin. 2021, 7, 20552173211048756. [CrossRef]
- Mocayar Marón FJ, Ferder L, Reiter RJ, Manucha W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J Steroid Biochem Mol Biol. 2020, 199, 105595. [CrossRef]
- El-Salem K, Khalil H, Al-Sharman A, Al-Mistarehi AH, Yassin A, Alhayk KA, Qawasmeh MA, Bashayreh SY, Kofahi RM, Obeidat AZ. Serum vitamin d inversely correlates with depression scores in people with multiple sclerosis. Mult Scler Relat Disord. 2021, 48, 102732. [CrossRef]
- Munger KL, Zhang SM, O'Reilly E, Hernán MA, Olek MJ, Willett WC, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004, 62, 60-5. [CrossRef]
- Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, Freedman MS, Hartung HP, Miller DH, Montalbán X, Edan G, Barkhof F, Pleimes D, Radü EW, Sandbrink R, Kappos L, Pohl C. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014, 71, 306-14. [CrossRef]
- Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010, 9, 941-55. [CrossRef]
- Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016, 4, 16041. [CrossRef]
- Hewer S, Lucas R, van der Mei I, Taylor BV. Vitamin D and multiple sclerosis. J Clin Neurosci. 2013, 20, 634-41. [CrossRef]
- McLaughlin L, Clarke L, Khalilidehkordi E, Butzkueven H, Taylor B, Broadley SA. Vitamin D for the treatment of multiple sclerosis: a meta-analysis. J Neurol. 2018, 265, 2893-2905. [CrossRef]
- Toell A, Polly P, Carlberg C. All natural DR3-type vitamin D response elements show a similar functionality in vitro. Biochem J. 2000 Dec 1;352 Pt 2(Pt 2):301-9.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004 Dec;3:377-93. [CrossRef]
- Montava M, Garcia S, Mancini J, Jammes Y, Courageot J, Lavieille JP, Feron F. Vitamin D3 potentiates myelination and recovery after facial nerve injury. Eur Arch Otorhinolaryngol. 2015, 272, 2815-23. [CrossRef]
- Matías-Guíu J, Oreja-Guevara C, Matias-Guiu JA, Gomez-Pinedo U. Vitamin D and remyelination in multiple sclerosis. Neurologia (Engl Ed). 2018, 33, 177-186. English, Spanish. [CrossRef]
- Pierrot-Deseilligny C, Souberbielle JC. Vitamin D and multiple sclerosis: An update. Mult Scler Relat Disord. 2017, 14, 35-45. [CrossRef]
- von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010, 11, 344-9. [CrossRef]
- Danner OK, Matthews LR, Francis S, Rao VN, Harvey CP, Tobin RP, Wilson KL, Alema-Mensah E, Newell Rogers MK, Childs EW. Vitamin D3 Suppresses Class II Invariant Chain Peptide Expression on Activated B-Lymphocytes: A Plausible Mechanism for Downregulation of Acute Inflammatory Conditions. J Nutr Metab. 2016;2016:4280876. [CrossRef]
- Fawaz L, Mrad MF, Kazan JM, Sayegh S, Akika R, Khoury SJ. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol. 2016 May;166-167:59-71. https://doi.org/10.1016/j.clim.2016.02.011. Vitamin D effects on B cell function in autoimmunity. Ann N Y Acad Sci. 2014 May;1317:84-91. doi:10.1111/nyas.12440.
- da Costa DS, Hygino J, Ferreira TB, Kasahara TM, Barros PO, Monteiro C, Oliveira A, Tavares F, Vasconcelos CC, Alvarenga R, Bento CA. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J Neuroimmunol. 2016, 299, 8-18. [CrossRef]
- Sotirchos ES, Bhargava P, Eckstein C, Van Haren K, Baynes M, Ntranos A, Gocke A, Steinman L, Mowry EM, Calabresi PA. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016, 86, 382-90. [CrossRef]
- Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation. 2015;22:400-4. [CrossRef]
- Mosayebi G, Ghazavi A, Ghasami K, Jand Y, Kokhaei P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Invest. 2011;40:627-39. [CrossRef]
- Haas MJ, Jafri M, Wehmeier KR, Onstead-Haas LM, Mooradian AD. Inhibition of endoplasmic reticulum stress and oxidative stress by vitamin D in endothelial cells. Free Radic Biol Med. 2016, 99, 1-10. [CrossRef]
- Smolders J, Hupperts R, Barkhof F, Grimaldi LM, Holmoy T, Killestein J, Rieckmann P, Schluep M, Vieth R, Hostalek U, Ghazi-Visser L, Beelke M; SOLAR study group. Efficacy of vitamin D3 as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon β-1a: a Phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci. 2011 Dec 15;311(1-2):44-9. [CrossRef]
- 236. Sandberg L, Biström M, Salzer J, Vågberg M, Svenningsson A, Sundström P. Vitamin D and axonal injury in multiple sclerosis. Mult Scler. 2016 Jul;22:1027-31. [CrossRef]
- Wagner CL, Hollis BW, Kotsa K, Fakhoury H, Karras SN. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev Endocr Metab Disord. 2017, 18, 307-322. [CrossRef]
- Pierrot-Deseilligny C, Rivaud-Péchoux S, Clerson P, de Paz R, Souberbielle JC. Relationship between 25-OH-D serum level and relapse rate in multiple sclerosis patients before and after vitamin D supplementation. Ther Adv Neurol Disord. 2012, 5, 187-98. [CrossRef]
- McCarty DE, Chesson AL Jr, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. 2014, 18, 311-9. [CrossRef]
- Dong X, Craig T, Xing N, Bachman LA, Paya CV, Weih F, McKean DJ, Kumar R, Griffin MD. Direct transcriptional regulation of RelB by 1alpha,25-dihydroxyvitamin D3 and its analogs: physiologic and therapeutic implications for dendritic cell function. J Biol Chem. 2003, 278, 49378-85. [CrossRef]
- Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005, 116, 1188-98. [CrossRef]
- Ryan S, Nolan GM, Hannigan E, Cunningham S, Taylor C, McNicholas WT. Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax. 2007, 62, 509-14. [CrossRef]
- McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin d deficiency. J Clin Sleep Med. 2010, 6, 605-8.
- McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012, 8, 693-7. [CrossRef]
- Muscogiuri G, Barrea L, Savastano S, Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. 2020, 74, 850-851. [CrossRef]
- Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients. 2018, 10, 1395. [CrossRef]
- Al-Shawwa B, Ehsan Z, Ingram DG. Vitamin D and sleep in children. J Clin Sleep Med. 2020, 16, 1119-1123. [CrossRef]
- Abboud M. Vitamin D Supplementation and Sleep: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients. 2022, 14, 1076. [CrossRef]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005, 29, 21-30. [CrossRef]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002, 13, 100-5. [CrossRef]
- Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011, 57, 63-9. [CrossRef]
- Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health. 2001;25:85-93.
- Lucock M, Jones P, Martin C, Beckett E, Yates Z, Furst J, Veysey M. Vitamin D: Beyond Metabolism. J Evid Based Complementary Altern Med. 2015, 20, 310-22. [CrossRef]
- Jamilian H, Amirani E, Milajerdi A, Kolahdooz F, Mirzaei H, Zaroudi M, Ghaderi A, Asemi Z. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog Neuropsychopharmacol Biol Psychiatry. 2019, 94, 109651. [CrossRef]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517-49. [CrossRef]
- Kaneko I, Sabir MS, Dussik CM, Whitfield GK, Karrys A, Hsieh JC, Haussler MR, Meyer MB, Pike JW, Jurutka PW. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: implication for behavioral influences of vitamin D. FASEB J. 2015, 29, 4023-35. [CrossRef]
- Ghareghani M, Zibara K, Rivest S. Melatonin and vitamin D, two sides of the same coin, better to land on its edge to improve multiple sclerosis. Proc Natl Acad Sci U S A. 2023, 120, e2219334120. [CrossRef]
- Golan D, Staun-Ram E, Glass-Marmor L, Lavi I, Rozenberg O, Dishon S, Barak M, Ish-Shalom S, Miller A. The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav Immun. 2013, 32, 180-5. [CrossRef]
- Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, Nielsen GH, Nordhus IH. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006, 295, 2851-8. [CrossRef]
- Morin CM, Vallières A, Guay B, Ivers H, Savard J, Mérette C, Bastien C, Baillargeon L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009, 301, 2005-15. [CrossRef]
- Morin CM, Benca R. Chronic insomnia. Lancet. 2012, 379, 1129-41. Erratum in: Lancet. 2012, 379, 1488. [CrossRef]
- Edinger JD, Means MK. Cognitive-behavioral therapy for primary insomnia. Clin Psychol Rev. 2005, 25, 539-58. [CrossRef]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008, 4, 487-504.
- Baron KG, Corden M, Jin L, Mohr DC. Impact of psychotherapy on insomnia symptoms in patients with depression and multiple sclerosis. J Behav Med. 2011, 34, 92-101. [CrossRef]
- Williams-Cooke C, LeSuer L, Drerup M, Siengsukon C. The Impact of Cognitive Behavioral Therapy for Insomnia on Sleep Log and Actigraphy Outcomes in People with Multiple Sclerosis: A Secondary Analysis. Nat Sci Sleep. 2021, 13, 1865-1874. [CrossRef]
- Turkowitch D, Ludwig R, Nelson E, Drerup M, Siengsukon CF. Telehealth-Delivered Cognitive Behavioral Therapy for Insomnia in Individuals with Multiple Sclerosis: A Pilot Study. Mult Scler Int. 2022, 2022, 7110582. [CrossRef]
- Nadjar Y, Coutelas E, Prouteau P, Panzer F, Paquet D, Saint-Val C, Créange A. Injection of interferon-beta in the morning decreases flu-like syndrome in many patients with multiple sclerosis. Clin Neurol Neurosurg. 2011, 113, 316-22. [CrossRef]
- Bøe Lunde HM, Aae TF, Indrevåg W, Aarseth J, Bjorvatn B, Myhr KM, Bø L. Poor sleep in patients with multiple sclerosis. PLoS One. 2012;7:e49996. [CrossRef]
- Rocchi C, Pulcini A, Vesprini C, Totaro V, Viticchi G, Falsetti L, Danni MC, Bartolini M, Silvestrini M, Buratti L. Sleep in multiple sclerosis patients treated with interferon beta: an actigraphic study. Neurol Res. 2020, 42, 744-748. [CrossRef]
- Braley TJ, Chervin RD. A practical approach to the diagnosis and management of sleep disorders in patients with multiple sclerosis. Ther Adv Neurol Disord. 2015, 8, 294-310. [CrossRef]
- Morrison I, Riha RL. Excessive daytime sleepiness and narcolepsy--an approach to investigation and management. Eur J Intern Med. 2012, 23, 110-7. [CrossRef]
- Côté I, Trojan DA, Kaminska M, Cardoso M, Benedetti A, Weiss D, Robinson A, Bar-Or A, Lapierre Y, Kimoff RJ. Impact of sleep disorder treatment on fatigue in multiple sclerosis. Mult Scler. 2013, 19, 480-9. [CrossRef]
- Sivam S, Yee BJ. Role of gabapentin enacarbil XR in restless legs syndrome. Ther Clin Risk Manag. 2012;8:201-8. [CrossRef]
- Aburub A, Khalil H, Al-Sharman A, Alomari M, Khabour O. The association between physical activity and sleep characteristics in people with multiple sclerosis. Mult Scler Relat Disord. 2017, 12, 29-33. [CrossRef]
- Newland P, Lorenz RA, Smith JM, Dean E, Newland J, Cavazos P. The Relationship Among Multiple Sclerosis-Related Symptoms, Sleep Quality, and Sleep Hygiene Behaviors. J Neurosci Nurs. 2019, 51, 37-42. [CrossRef]
- Amtmann D, Askew RL, Kim J, Chung H, Ehde DM, Bombardier CH, Kraft GH, Jones SM, Johnson KL. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015, 60, 81-90. [CrossRef]
Figure 1.
The indirect relationship between sleep and MS. Schematic representation of the bidirectional relationship between multiple sclerosis (MS) and sleep. IL: Interleukin;TNF: tumour necrosis factor; HPA: hypothalamus-pituitary-adrenal; SNS: sympathetic nervous system; NK: natural killer; ROS: reactive oxygen species.
Figure 1.
The indirect relationship between sleep and MS. Schematic representation of the bidirectional relationship between multiple sclerosis (MS) and sleep. IL: Interleukin;TNF: tumour necrosis factor; HPA: hypothalamus-pituitary-adrenal; SNS: sympathetic nervous system; NK: natural killer; ROS: reactive oxygen species.
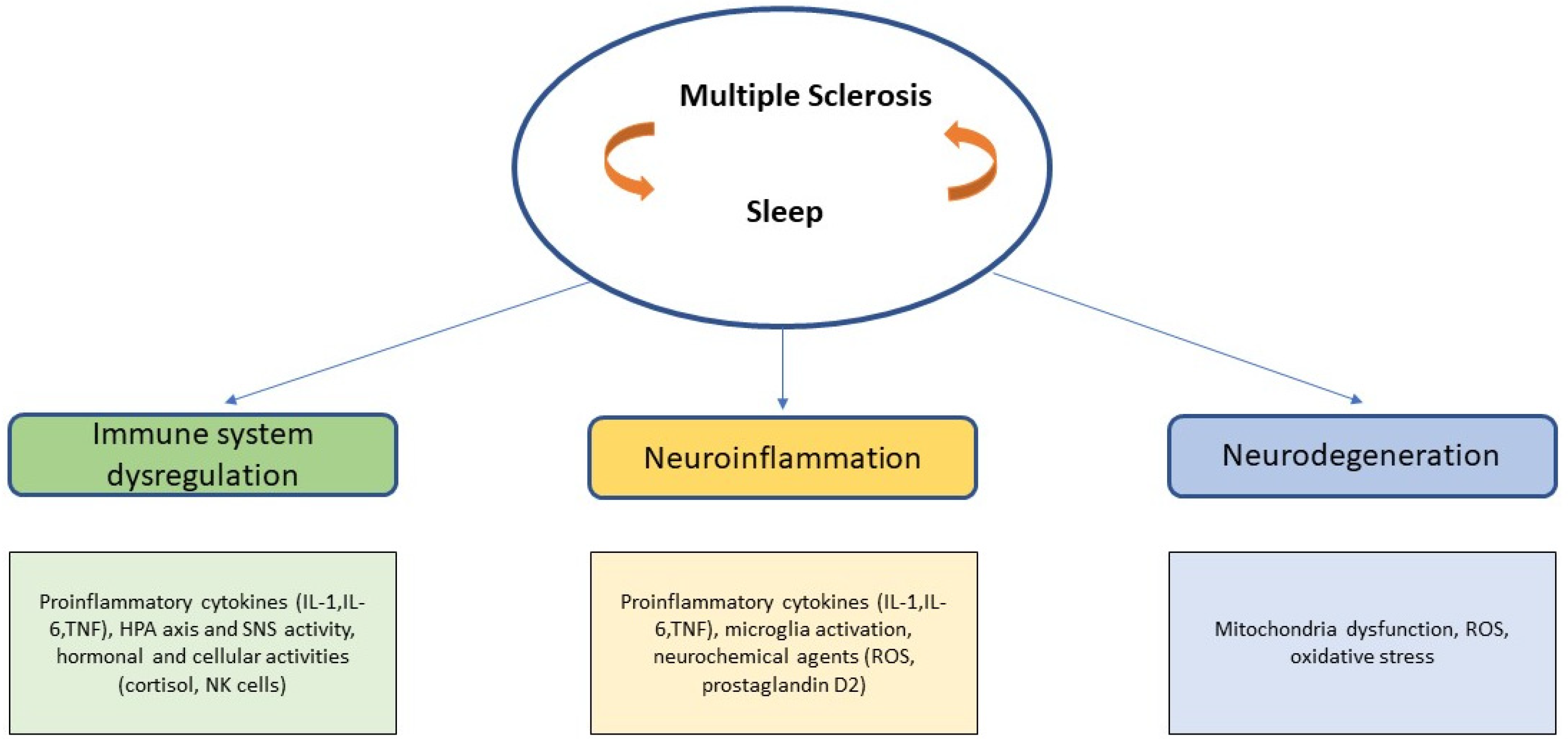
Figure 2.
New potential therapeutical scenario. Schematic representation of the sleep-based potential therapeutic interventions in MS. MS: Multiple Sclerosis; CBT-I: Cognitive Behavioral Therapy for Insomnia.
Figure 2.
New potential therapeutical scenario. Schematic representation of the sleep-based potential therapeutic interventions in MS. MS: Multiple Sclerosis; CBT-I: Cognitive Behavioral Therapy for Insomnia.
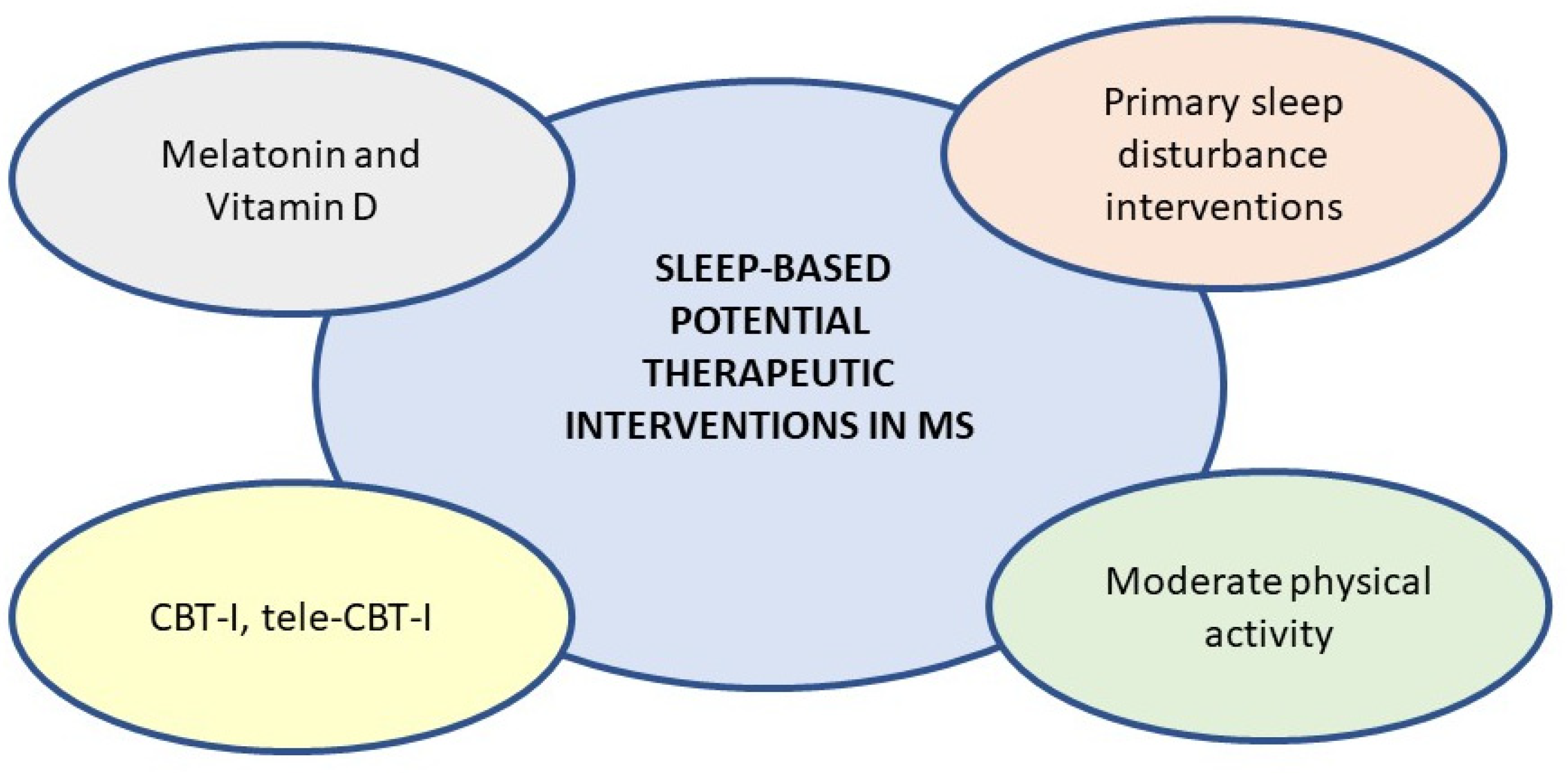
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







