Submitted:
14 November 2023
Posted:
15 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
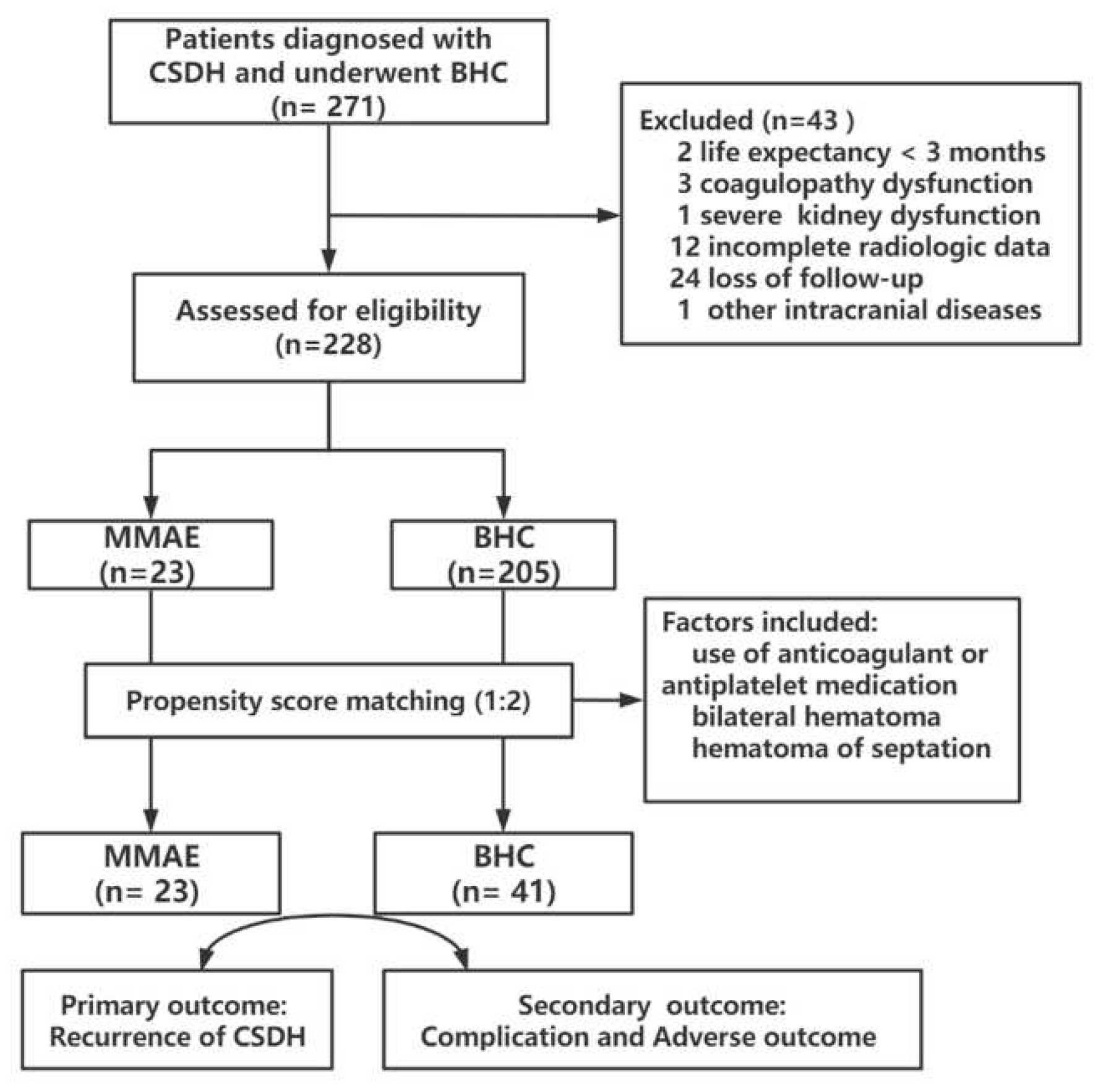
2.1. Study design
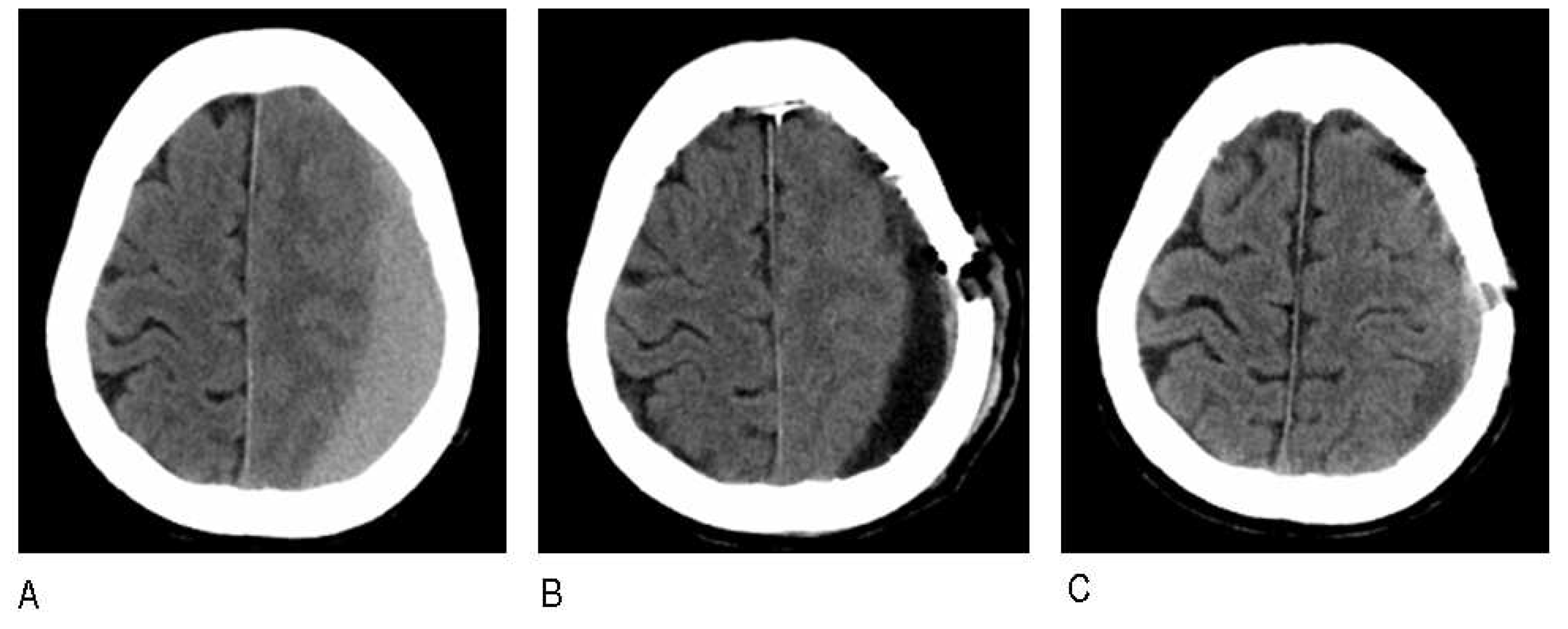
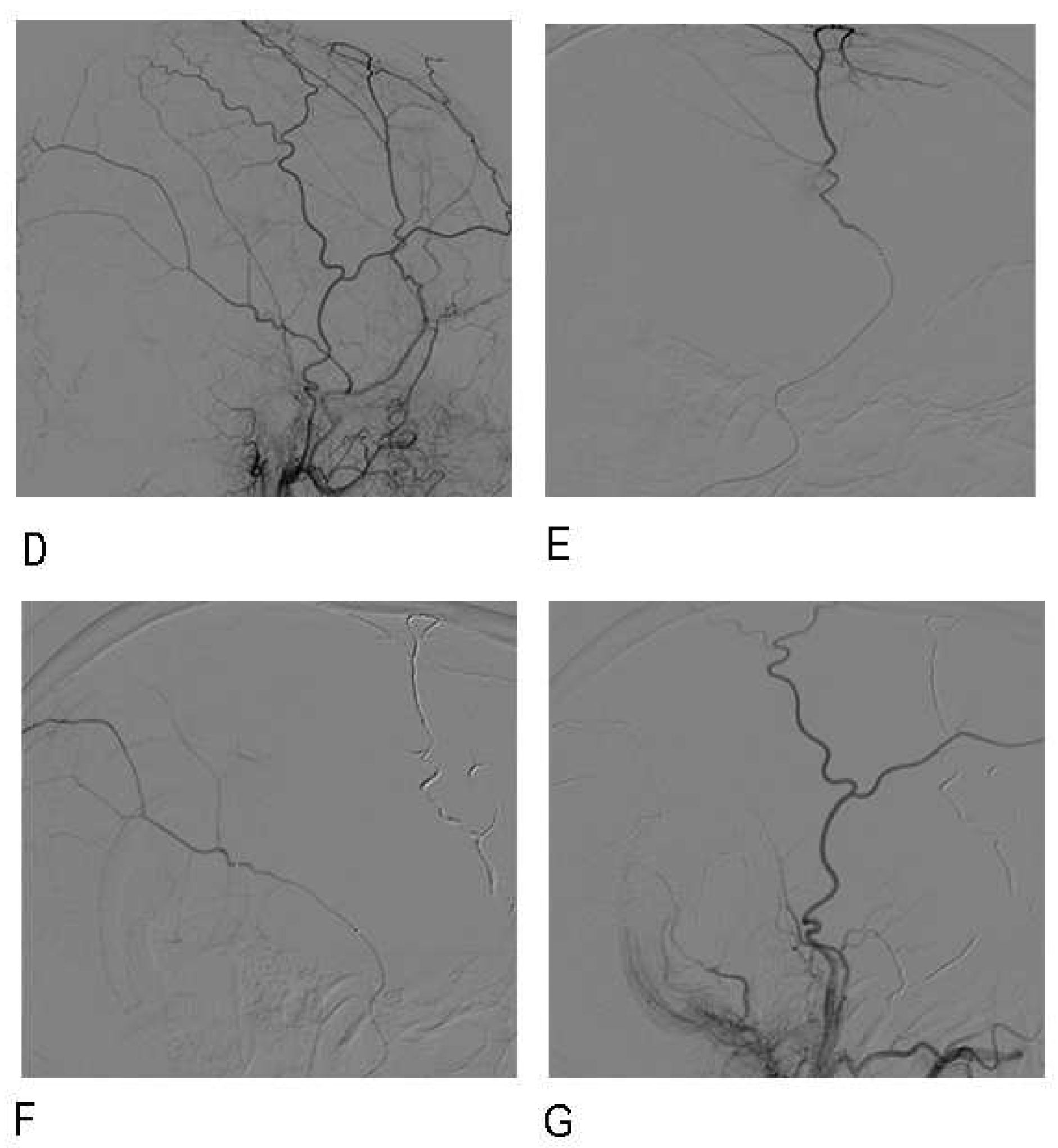
2.2. Embolization procedure
2.3. Follow-up and Data Collection
2.4. Study outcomes
2.5. Statistical analysis
3. Results
3.1. Baseline characteristics
| Factors | MMAE N=23 |
BHC N=205 |
P value |
|---|---|---|---|
| Age (y) | 71.7 ± 8.9 | 67.6 ± 11.2 | 0.064 |
| Male sex (%) | 15 (65.2) | 162 (79.0) | 0.132 |
| Trauma history (%) | 9 (39.1) | 82 (40.0) | 0.944 |
| Use of anticoagulant or antiplatelet medication (%) | 11 (47.8) | 46 (22.4) | 0.008 |
| Atorvastatin (%) | 20 (87.0) | 186 (90.7) | 0.564 |
| Hypertension (%) | 12 (52.2) | 86 (42.0) | 0.352 |
| Diabetes mellitus (%) | 3 (13.0) | 46 (22.4) | 0.305 |
| Smoking (%) | 2 (8.7) | 36 (17.6) | 0.277 |
| Chronic alcoholism (%) | 3 (13.0) | 18 (8.4) | 0.452 |
| Brain atrophy (%) | 4 (17.4) | 23 (11.2) | 0.384 |
| Hematoma width (mm) | 22.4 ± 4.6 | 19.8 ± 12.9 | 0.895 |
| Bilateral hematoma (%) | 9 (39.1) | 40 (19.5) | 0.043 |
| Midline shift >10 (%) | 7 (30.4) | 51 (24.9) | 0.566 |
| Hematoma density (%) | 0.087 | ||
| Hypodense/ isodense | 9 (39.1) | 119 (58.0) | |
| Hyperdense/ mixed density | 14 (60.9) | 86 (42.0) | |
| Hematoma of septation (%) | 11 (47.8) | 44 (21.5) | 0.005 |
3.2. Multivariate logistic regression
| Factors | All patients N=228 |
aOR# | 95% CI | P value |
|---|---|---|---|---|
| Age | 68.1 ± 10.9 | 1.014 | 0.995~1.023 | 0.197 |
| Use of antiplatelet or anticoagulants medication | 57 (25.3) | 6.721 | 1.416~13.549 | 0.024 |
| Bilateral hematoma | 49 (21.5) | 1.357 | 0.758~2.237 | 0.234 |
| Hematoma of hyperdense or mixed density | 100 (48.9) | 2.432 | 0.942~1.455 | 0.062 |
| Hematoma of septation | 55 (24.1) | 2.947 | 1.291~3.328 | 0.035 |
3.3. propensity score matching
| Factors | MMAE N=23 |
BHC after PSM N=205 |
P value |
|---|---|---|---|
| Age (y) | 71.7 ± 8.9 | 69±7.9 | 0.741 |
| Male sex (%) | 15 (65.2) | 28 (68.2%) | 0.804 |
| Trauma history (%) | 9 (39.1) | 16 (39.0%) | 0.995 |
| Use of anticoagulant or antiplatelet medication (%) | 11 (47.8) | 12 (29.3%) | 0.143 |
| Atorvastatin (%) | 20 (87.0) | 38 (92.7%) | 0.215 |
| Hypertension (%) | 12 (52.2) | 16 (39.0%) | 0.316 |
| Diabetes mellitus (%) | 3 (13.0) | 6 (14.6%) | 0.918 |
| Smoking (%) | 2 (8.7) | 6 (14.6%) | 0.493 |
| Chronic alcoholism (%) | 3 (13.0) | 3 (7.3%) | 0.456 |
| Brain atrophy (%) | 4 (17.4) | 5 (12.2%) | 0.572 |
| Hematoma width (mm) | 22.4 ± 4.6 | 21.3 ± 7.3 | 0.657 |
| Bilateral hematoma (%) | 9 (39.1) | 9 (22.0%) | 0.277 |
| Midline shift >10 (%) | 7 (30.4) | 11 (26.8%) | 0.763 |
| Hematoma density (%) | 0.578 | ||
| Hypodense/ isodense | 9 (39.1) | 19 (46.3) | |
| Hyperdense/ mixed density | 14 (60.9) | 22 (53.7%%) | |
| Hematoma of septation (%) | 11 (47.8) | 15 (36.6%) | 0.381 |
3.4. Comparison of Treatment Outcomes
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutchinson, P.J., E. Edlmann, D. Bulters, A. Zolnourian, P. Holton, N. Suttner, K. Agyemang, S. Thomson, I.A. Anderson, Y.Z. Al-Tamimi, D. Henderson, P.C. Whitfield, M. Gherle, P.M. Brennan, A. Allison, E.P. Thelin, S. Tarantino, B. Pantaleo, K. Caldwell, C. Davis-Wilkie, H. Mee, E.A. Warburton, G. Barton, A. Chari, H.J. Marcus, A.T. King, A. Belli, P.K. Myint, I. Wilkinson, T. Santarius, C. Turner, S. Bond, and A.G. Kolias, Trial of Dexamethasone for Chronic Subdural Hematoma. N Engl J Med, 2020. 383(27): p. 2616-2627. [CrossRef]
- Feghali, J., W. Yang, and J. Huang, Updates in Chronic Subdural Hematoma: Epidemiology, Etiology, Pathogenesis, Treatment, and Outcome. World Neurosurg, 2020. 141: p. 339-345. [CrossRef]
- Miranda, L.B., E. Braxton, J. Hobbs, and M.R. Quigley, Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg, 2011. 114(1): p. 72-6. [CrossRef]
- Kolias, A.G., A. Chari, T. Santarius, and P.J. Hutchinson, Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol, 2014. 10(10): p. 570-8. [CrossRef]
- Edlmann, E., P.C. Whitfield, A. Kolias, and P.J. Hutchinson, Pathogenesis of Chronic Subdural Hematoma: A Cohort Evidencing De Novo and Transformational Origins. J Neurotrauma, 2021. 38(18): p. 2580-2589. [CrossRef]
- Mehta, V., S.C. Harward, E.W. Sankey, G. Nayar, and P.J. Codd, Evidence based diagnosis and management of chronic subdural hematoma: A review of the literature. J Clin Neurosci, 2018. 50: p. 7-15. [CrossRef]
- Rauhala, M., P. Helén, H. Huhtala, P. Heikkilä, G.L. Iverson, T. Niskakangas, J. Öhman, and T.M. Luoto, Chronic subdural hematoma-incidence, complications, and financial impact. Acta Neurochir (Wien), 2020. 162(9): p. 2033-2043.
- Cecchini, G., Chronic subdural hematoma pathophysiology: a unifying theory for a dynamic process. J Neurosurg Sci, 2017. 61(5): p. 536-543. [CrossRef]
- Bounajem, M., R. Campbell, F. Denorme, and R. Grandhi, Paradigms in chronic subdural hematoma pathophysiology: Current treatments and new directions. The journal of trauma and acute care surgery, 2021. 91(6): p. e134-e141. [CrossRef]
- Smith, M., L. Kishikova, and J. Norris, Surgical management of chronic subdural haematoma: one hole or two? International journal of surgery (London, England), 2012. 10(9): p. 450-2.
- Tiwari, A., A.A. Dmytriw, R. Bo, N. Farkas, P. Ye, D.S. Gordon, K.M. Arcot, D. Turkel-Parrella, and J. Farkas, Recurrence and Coniglobus Volumetric Resolution of Subacute and Chronic Subdural Hematoma Post-Middle Meningeal Artery Embolization. Diagnostics (Basel), 2021. 11(2).
- Duerinck, J., J. Van Der Veken, S. Schuind, F. Van Calenbergh, J. van Loon, S. Du Four, S. Debacker, E. Costa, C. Raftopoulos, O. De Witte, W. Cools, R. Buyl, V. Van Velthoven, J. D'Haens, and M. Bruneau, Randomized Trial Comparing Burr Hole Craniostomy, Minicraniotomy, and Twist Drill Craniostomy for Treatment of Chronic Subdural Hematoma. Neurosurgery, 2022. [CrossRef]
- Berghauser Pont, L., R. Dammers, J. Schouten, H. Lingsma, and C. Dirven, Clinical factors associated with outcome in chronic subdural hematoma: a retrospective cohort study of patients on preoperative corticosteroid therapy. Neurosurgery, 2012. 70(4): p. 873-80; discussion 880.
- Quan, W., Z. Zhang, Q. Tian, X. Wen, P. Yu, D. Wang, W. Cui, L. Zhou, E. Park, A.J. Baker, J. Zhang, and R. Jiang, A rat model of chronic subdural hematoma: Insight into mechanisms of revascularization and inflammation. Brain Res, 2015. 1625: p. 84-96. [CrossRef]
- Edlmann, E., S. Giorgi-Coll, P.C. Whitfield, K.L.H. Carpenter, and P.J. Hutchinson, Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation, 2017. 14(1): p. 108. [CrossRef]
- Holl, D.C., V. Volovici, C.M.F. Dirven, W.C. Peul, F. van Kooten, K. Jellema, N.A. van der Gaag, I.P. Miah, K.H. Kho, H.M. den Hertog, H.F. Lingsma, and R. Dammers, Pathophysiology and Nonsurgical Treatment of Chronic Subdural Hematoma: From Past to Present to Future. World Neurosurg, 2018. 116: p. 402-411.e2. [CrossRef]
- Hashimoto, T., T. Ohashi, D. Watanabe, S. Koyama, H. Namatame, H. Izawa, R. Haraoka, H. Okada, N. Ichimasu, J. Akimoto, and J. Haraoka, Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int, 2013. 4: p. 104. [CrossRef]
- Srivatsan, A., A. Mohanty, F.A. Nascimento, M.U. Hafeez, V.M. Srinivasan, A. Thomas, S.R. Chen, J.N. Johnson, and P. Kan, Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: Meta-Analysis and Systematic Review. World Neurosurg, 2019. 122: p. 613-619. [CrossRef]
- Haldrup, M., B. Ketharanathan, B. Debrabant, O.S. Schwartz, R. Mikkelsen, K. Fugleholm, F.R. Poulsen, T.S.R. Jensen, L.V. Thaarup, and B. Bergholt, Embolization of the middle meningeal artery in patients with chronic subdural hematoma-a systematic review and meta-analysis. Acta Neurochir (Wien), 2020. 162(4): p. 777-784. [CrossRef]
- Fiorella, D. and A. Arthur, Middle meningeal artery embolization for the management of chronic subdural hematoma. Journal of neurointerventional surgery, 2019. 11(9): p. 912-915. [CrossRef]
- Manickam, A., L.A. Marshman, and R. Johnston, Long-term survival after chronic subdural haematoma. J Clin Neurosci, 2016. 34: p. 100-104. [CrossRef]
- Santarius, T., P.J. Kirkpatrick, D. Ganesan, H.L. Chia, I. Jalloh, P. Smielewski, H.K. Richards, H. Marcus, R.A. Parker, S.J. Price, R.W. Kirollos, J.D. Pickard, and P.J. Hutchinson, Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet, 2009. 374(9695): p. 1067-73. [CrossRef]
- Ducruet, A.F., B.T. Grobelny, B.E. Zacharia, Z.L. Hickman, P.L. DeRosa, K.N. Andersen, E. Sussman, A. Carpenter, and E.S. Connolly, Jr., The surgical management of chronic subdural hematoma. Neurosurg Rev, 2012. 35(2): p. 155-69; discussion 169. [CrossRef]
- Shen, J., L. Yuan, R. Ge, Q. Wang, W. Zhou, X.C. Jiang, and X. Shao, Clinical and radiological factors predicting recurrence of chronic subdural hematoma: A retrospective cohort study. Injury, 2019. 50(10): p. 1634-1640. [CrossRef]
- Molnár, A., G.L. Nádasy, G. Dörnyei, B.B. Patai, J. Delfavero, G. Fülöp, A.C. Kirkpatrick, Z. Ungvári, and B. Merkely, The aging venous system: from varicosities to vascular cognitive impairment. Geroscience, 2021. 43(6): p. 2761-2784. [CrossRef]
- Zhang, X., D. Wang, Y. Tian, H. Wei, X. Liu, T. Xiang, Y. Fan, C. Gao, J. Huang, Z. Sha, W. Quan, J. Zhang, and R. Jiang, Risk Factors for Atorvastatin as a Monotherapy for Chronic Subdural Hematoma: A Retrospective Multifactor Analysis. Front Aging Neurosci, 2021. 13: p. 726592. [CrossRef]
- Chihara, H., H. Imamura, T. Ogura, H. Adachi, Y. Imai, and N. Sakai, Recurrence of a Refractory Chronic Subdural Hematoma after Middle Meningeal Artery Embolization That Required Craniotomy. NMC Case Rep J, 2014. 1(1): p. 1-5.
- Kwon, S., C. Jin, and K.H. Cho, Oreongsan, an herbal medicine prescription developed as a new alternative treatment in patients with chronic subdural hematoma: a narrative review. Integr Med Res, 2019. 8(1): p. 26-30.
- Wang, H., M. Zhang, H. Zheng, X. Xia, K. Luo, F. Guo, and C. Qian, The effects of antithrombotic drugs on the recurrence and mortality in patients with chronic subdural hematoma: A meta-analysis. Medicine (Baltimore), 2019. 98(1): p. e13972.
- Gong, Z., D. Zhan, M. Nie, X. Li, C. Gao, X. Liu, T. Xiang, J. Yuan, W. Jiang, J. Huang, W. Quan, D. Wang, Y. Tian, H. Yuan, J. Zhang, and R. Jiang, Dexamethasone enhances the efficacy of atorvastatin in inhibiting excessively inflammation-induced abnormal angiogenesis by regulating macrophages. J Neuroinflammation, 2021. 18(1): p. 203.
- Catapano, J.S., C.L. Nguyen, A.A. Wakim, F.C. Albuquerque, and A.F. Ducruet, Middle Meningeal Artery Embolization for Chronic Subdural Hematoma. Front Neurol, 2020. 11: p. 557233.
- Scerrati, A., A. Mangiola, F. Rigoni, S. Olei, M. Santantonio, G. Trevisi, C. Anile, M.A. Cavallo, and D.E.B. P, Do antiplatelet and anticoagulant drugs modify outcome of patients treated for chronic subdural hematoma? Still a controversial issue. J Neurosurg Sci, 2021. 65(6): p. 626-633. [CrossRef]
- Ironside, N., C. Nguyen, Q. Do, B. Ugiliweneza, C.J. Chen, E.P. Sieg, R.F. James, and D. Ding, Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis. J Neurointerv Surg, 2021. 13(10): p. 951-957. [CrossRef]
- Ng, S., I. Derraz, J. Boetto, C. Dargazanli, G. Poulen, G. Gascou, P.H. Lefevre, N. Molinari, N. Lonjon, and V. Costalat, Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: a pilot study assessing hematoma volume resorption. J Neurointerv Surg, 2020. 12(7): p. 695-699. [CrossRef]
- Ban, S.P., G. Hwang, H.S. Byoun, T. Kim, S.U. Lee, J.S. Bang, J.H. Han, C.Y. Kim, O.K. Kwon, and C.W. Oh, Middle Meningeal Artery Embolization for Chronic Subdural Hematoma. Radiology, 2018. 286(3): p. 992-999.
| Outcome | MMAE N=23 |
BHC after PSM N=41 |
OR (95%CI) | P value |
|---|---|---|---|---|
| Recurrence | 1 (4.3) | 5 (12.1) | 0.072 (0.322~0.746) | 0.028 |
| Complications | 0 (0) | 2 (4.8) | NA | NA |
| Adverse prognosis | 4 (17.8) | 11 (26.8) | 0.065 (0.533~4.786) | 0.562 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




