Submitted:
14 November 2023
Posted:
14 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
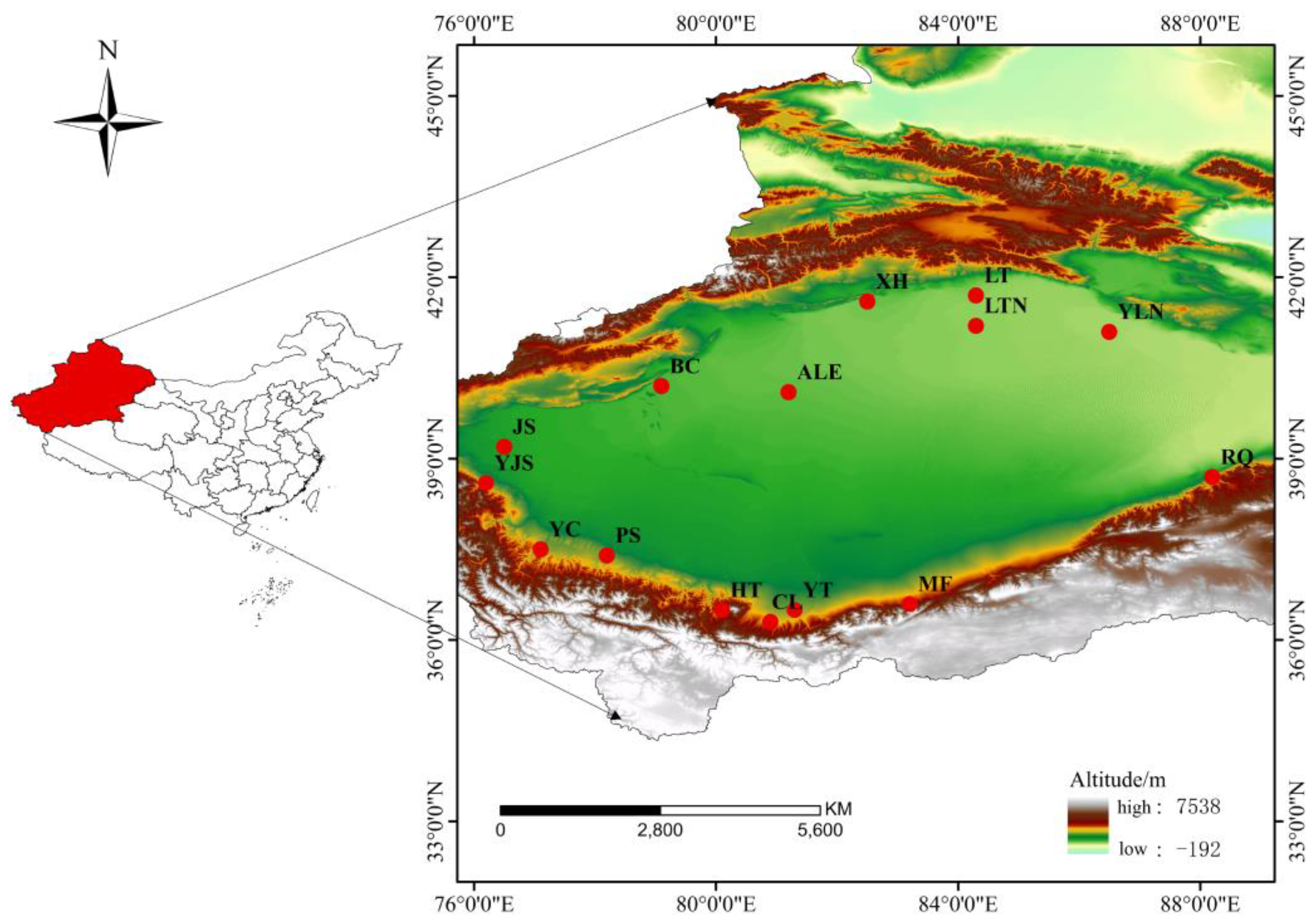
2.1. Study area and sample collection
2.2. DNA extraction and PCR amplification
2.3. Genetics data Processing and Analysis
2.3.1. Genetic diversity
2.3.2. Genetic differentiation and population structure
2.3.3. Spatial scales of genetic variation
2.3.4. Correlation of climatic factors with genetic structure
3. Results
3.1. Genetic diversity
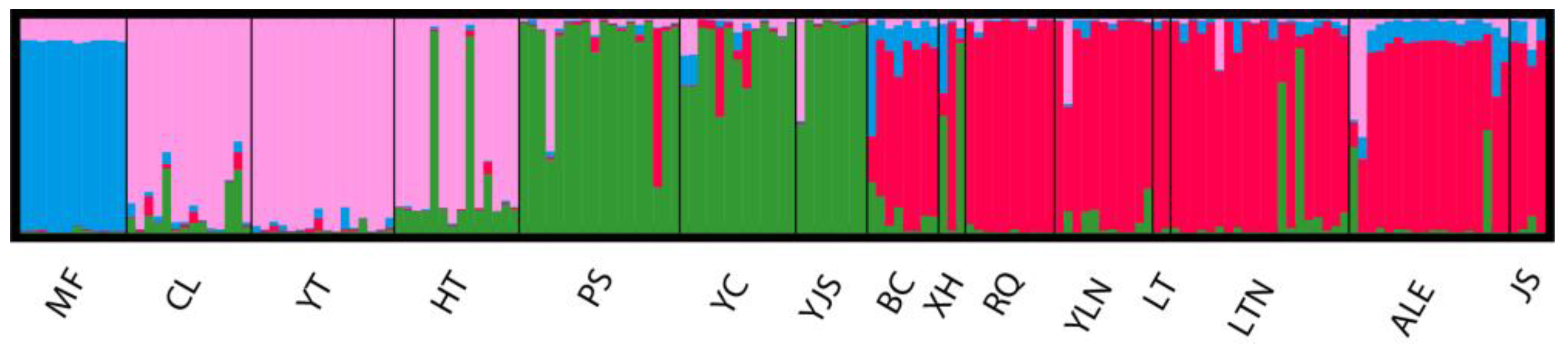
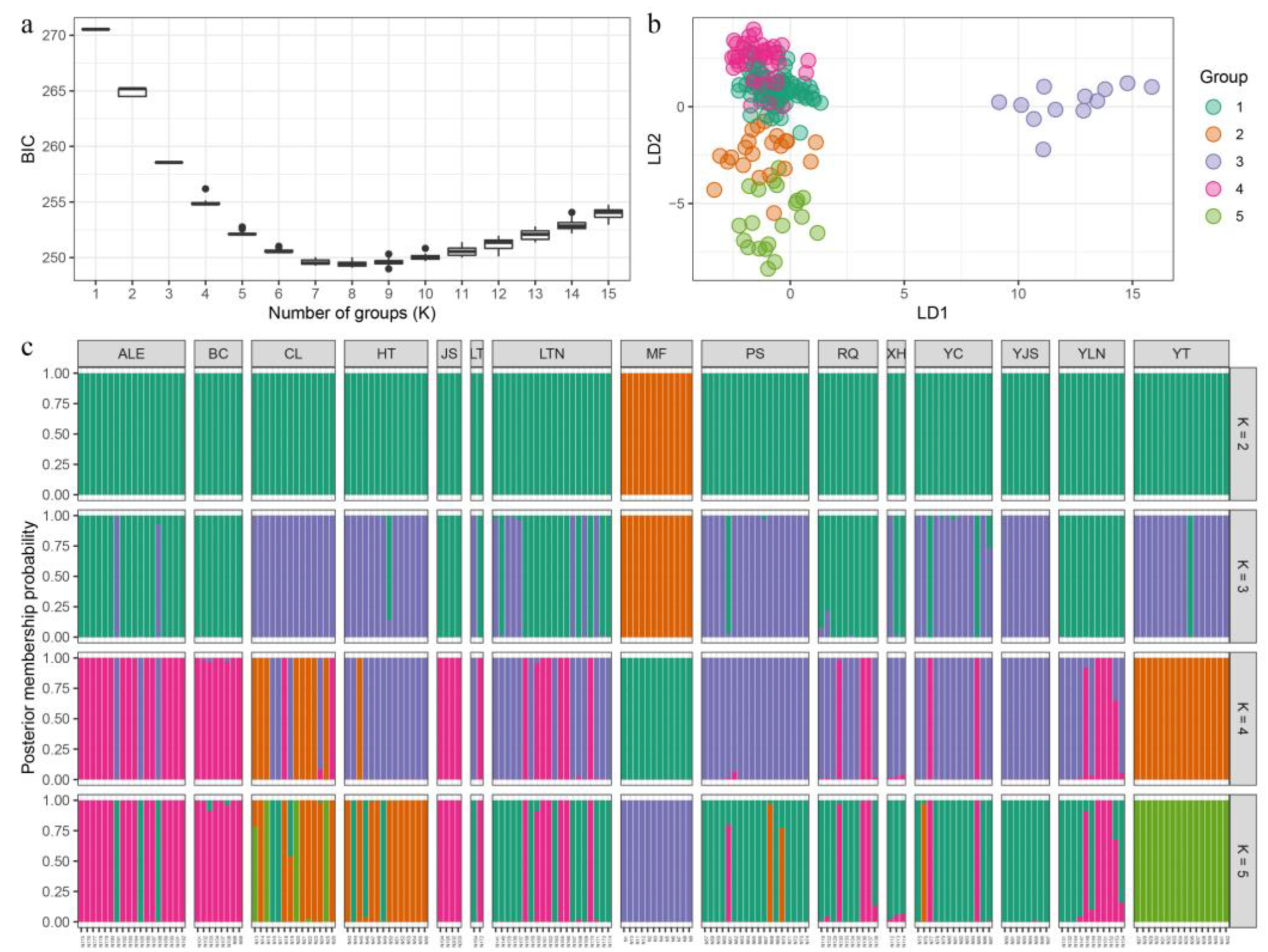
3.2. Genetic differentiation and population structure
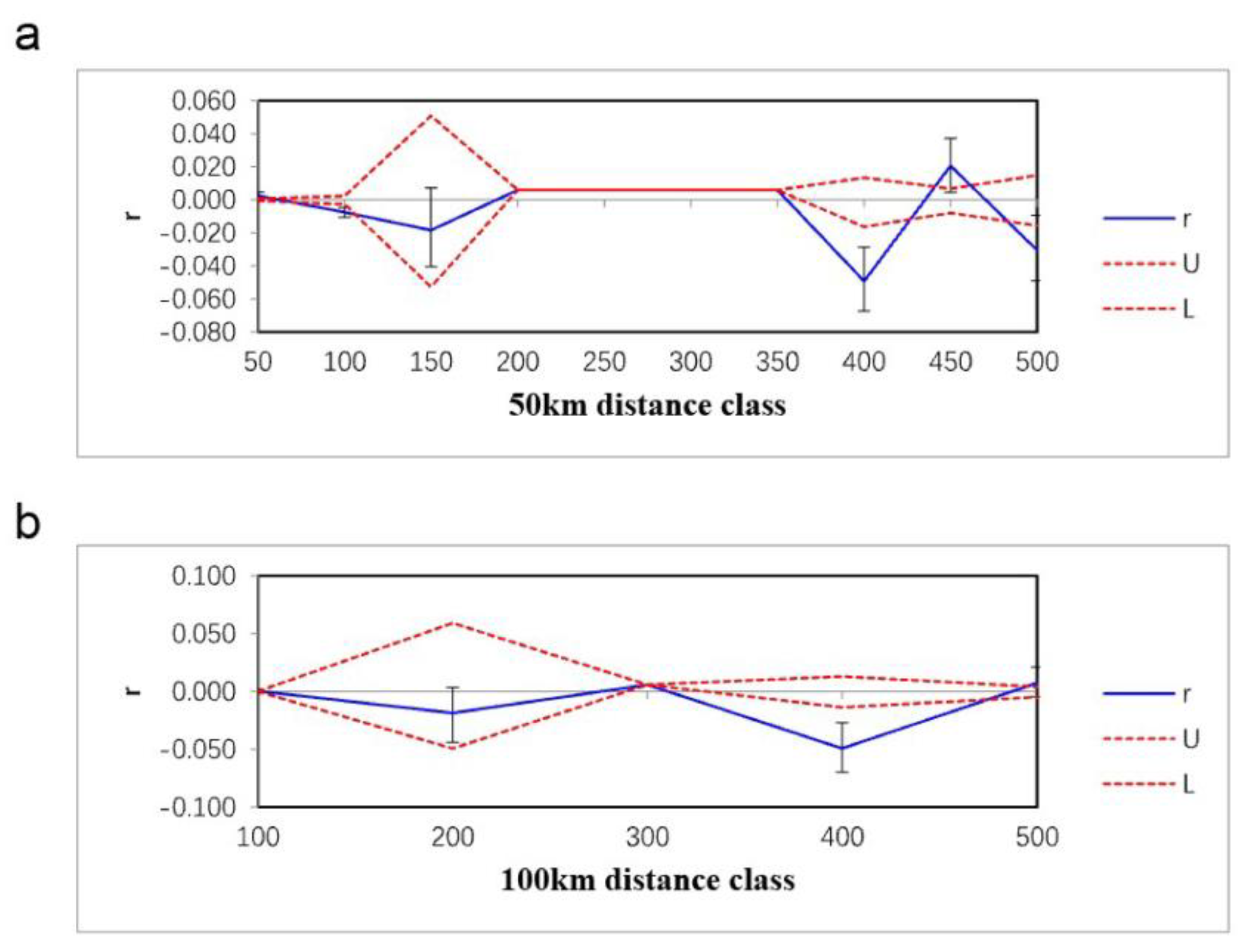
3.3. Spatial scales of genetic variation
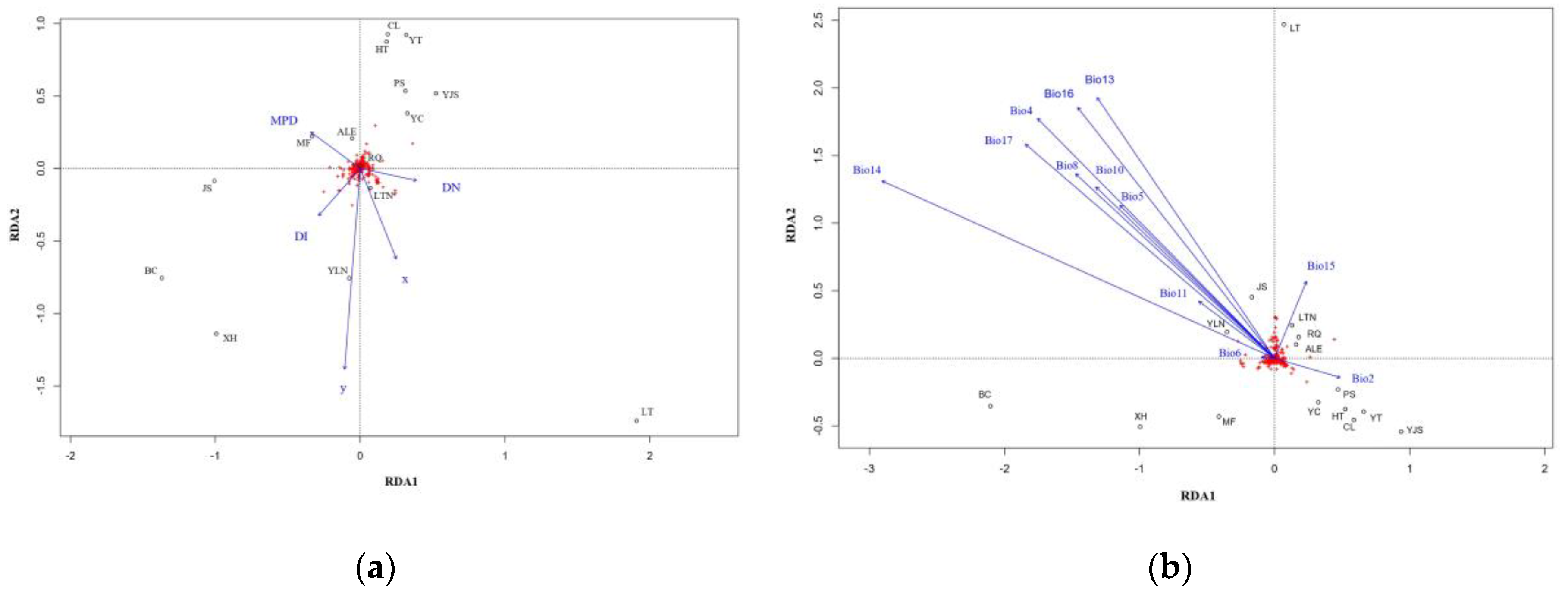
3.4. Correlation of climatic factors with genetic structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| code | climatic environmental factor |
|---|---|
| BIO2 | Mean Diurnal Range (Mean of monthly (max temp - min temp)) |
| BIO4 | Temperature Seasonality (standard deviation ×100) |
| BIO5 | Max Temperature of Warmest Month (°C) |
| BIO6 | Min Temperature of Coldest Month (°C) |
| BIO8 | Mean Temperature of Wettest Quarter (°C) |
| BIO10 | Mean Temperature of Warmest Quarter (°C) |
| BIO11 | Mean Temperature of Coldest Quarter (°C) |
| BIO13 | Precipitation of Wettest Month (mm) |
| BIO14 | Precipitation of Driest Month (mm) |
| BIO15 | Precipitation Seasonality (Coefficient of Variation) |
| BIO16 | Precipitation of Wettest Quarter (mm) |
| BIO17 | Precipitation of Driest Quarter (mm) |
References
- Wang, X.M.; Geng, X.; Liu, B.; Cai, D.W.; Li, D.F.; Xiao, F.Y.; Zhu, B.Q.; Hua, T.; Lu, R.J.; Liu, F. Desert Ecosystems in China: Past, Present, and Future. Earth-Sci. Rev. 2022, 234, 104206. [Google Scholar] [CrossRef]
- Bachelet, D.; Ferschweiler, K.; Sheehan, T.; Strittholt, J. Climate Change Effects on Southern California Deserts. J. Arid. Environ. 2016, 127, 17–29. [Google Scholar] [CrossRef]
- Iknayan, K.J.; Beissinger, S.R. Collapse of a Desert Bird Community over the Past Century Driven by Climate Change. PNAS. 2018, 115, 8597–8602. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.; Ojima, D.S.; Buchmann, B.; Canadell, J.G. ; Coomes; O.; Graumlich, L.; Jackson, R.; Jaramillo, V.; Lavorel, S.; Leadley, P. Global Land Project: Science Plan and Implementation Strategy. Environmental Policy Collection 2005. [Google Scholar]
- Huang, J.; Li, Y.; Fu, C.; Chen, F.; Fu, Q.; Dai, A.; Shinoda, M.; Ma, Z.; Guo, W.; Li, Z.; Zhang, L.; Liu, Y.; Yu, H.; He, Y.; Xie, Y.; Guan, X.; Ji, M.; Lin, L.; Wang, S.; Yan, H.; Wang, G. Dryland Climate Change: Recent Progress and Challenges. Rev. Geophys. 2017, 55, 719–778. [Google Scholar] [CrossRef]
- Feng, Q.; Ma, H.; Jiang, X.M.; Wang, X. Cao, S.X. What Has Caused Desertification in China? Sci. Rep. 2015, 5, 15998. [Google Scholar] [CrossRef]
- Huang, J.P.; Zhang, G.L.; Zhang, Y.T.; Guan, X.D.; Wei, Y.; Guo, R.X. Global Desertification Vulnerability to Climate Change and Human Activities. Land. Degrad. Dev. 2020, 31, 1380–1391. [Google Scholar] [CrossRef]
- Chen, F.H.; Chen, J.H.; Huang, W.; Chen, S.Q.; Huang, X.Z.; Jin, L.Y.; Jia, J.; Zhang, X.J.; An, C.B.; Zhang, J.W.; Zhao, Y.; Yu, Z.C.; Zhang, R.H.; Liu, J.B.; Zhou, A.F.; Feng, S. Westerlies Asia and Monsoonal Asia: Spatiotemporal Differences in Climate Change and Possible Mechanisms on Decadal to Sub-Orbital Timescales. Earth-Sci. Rev. 2019, 192, 337–354. [Google Scholar] [CrossRef]
- Whitford, W.G. Desertification and Animal Biodiversity in the Desert Grasslands of North America. J. Arid. Environ. 1997, 37, 709–720. [Google Scholar] [CrossRef]
- Honda, M.; Shimizu, H. Geochemical, Mineralogical and Sedimentological Studies on the Taklimakan Desert Sands. Sedimentology. 1998, 45, 1125–1143. [Google Scholar] [CrossRef]
- Sun, J.M.; Liu, T.S. The Age of the Taklimakan Desert. Science. 2006, 312, 1621. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.M.; Li, W.H. Oasis Cold Island Effect and Its Influence on Air Temperature: A Case Study of Tarim Basin, Northwest China. J. Arid. Land. 2016, 8, 172–183. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, L.; He, J.B.; Wu, Y.H.; Fu, J.Z.; Yang, Q.S. Comparison of Phylogeographic Structure and Population History of Two Phrynocephalus Species in the Tarim Basin and Adjacent Areas. Mol. Phylogenet. Evol. 2010, 57, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.J.; Jiang, L.; Li, Y. Genetic consequences of postglacial colonization by the endemic Yarkand hare (Lepus yarkandensis) of the arid Tarim Basin. Sci. Bull. 2011, 56, 1370–1382. [Google Scholar] [CrossRef]
- Borokini, I.T.; Klingler, K.B.; Peacock, M.M. Life in the Desert: The Impact of Geographic and Environmental Gradients on Genetic Diversity and Population Structure of Ivesia Webberi. Ecol. Evol. 2021, 11, 17537–17556. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.S.; Zhai, J.T.; Chen, X.X.; Jiao, P.P.; Zhang, S.H.; Sun, J.H.; Qin, R.; Liu, H.; Wu, Z.H.; Li, Z.J. Phylogeography Reveals Geographic and Environmental Factors Driving Genetic Differentiation of Populus Sect. Turanga in Northwest China. Front. Plant Sci. 2021, 12, 705083. [Google Scholar] [CrossRef]
- Yisilam, G.; Wang, C.X.; Xia, M.Q.; Comes, H.P.; Li, P.; Li, J.; Tian, X.M. Phylogeography and Population Genetics Analyses Reveal Evolutionary History of the Desert Resource Plant Lycium Ruthenicum (Solanaceae). Front. Plant Sci. 2022, 13, 915526. [Google Scholar] [CrossRef]
- Kumar, B.; Cheng, J.L.; Ge, D.E.; Xia, L.; Yang, Q.S. Phylogeography and Ecological Niche Modeling Unravel the Evolutionary History of the Yarkand Hare, Lepus Yarkandensis (Mammalia: Leporidae), through the Quaternary. BMC Evol. Biol. 2019, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Shirazinejad, M.P.; Aliabadian, M.; Mirshamsi, O. The Evolutionary History of the White Wagtail Species Complex, (Passeriformes: Motacillidae: Motacilla Alba). Contrib. Zool. 2019, 88, 257–276. [Google Scholar] [CrossRef]
- Zhang, C.L.; Liu, C.J.; Zhang, J.H.; Zheng, L.M.; Chang, Q.Q.; Cui, Z.L.; Liu, S.D. Analysis on the Desert Adaptability of Indigenous Sheep in the Southern Edge of Taklimakan Desert. Sci. Rep. 2022, 12, 12264. [Google Scholar] [CrossRef]
- Ababaikeri, B.; Liqun, N.; Eli, S.; Ismayil, Z.; Halik, M. Microsatellite Analyses of Genetic Diversity and Population Structure of Goitered Gazelle Gazella Subgutturosa (Guldenstadt, 1780) (Artiodactyla: Bovidae) in Xinjiang, China. Acta. Zool. Bulg. 2019, 71, 407–416. [Google Scholar]
- Pie, M.R. Campos, L.L.F.; Meyer, A.L.S.; Duran, A. The evolution of climatic niches in squamate reptiles. Proc. R. Soc. B. 2017, 284, 20170268. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, S.D. Ecophysiology of Australian Arid-Zone Reptiles. In On the Ecology of Australia’s Arid Zone; Springer International Press: Berlin, 2018; pp. 133–148. [Google Scholar]
- Qi, Y.; Zhao, W.; Li, Y.; Zhao, Y.Y. Environmental and Geological Changes in the Tarim Basin Promoted the Phylogeographic Formation of Phrynocephalus Forsythii (Squamata: Agamidae). Gene. 2021, 768, 145264. [Google Scholar] [CrossRef] [PubMed]
- Humphries, C. J. , Williams, P. H., Vane-Wright, R. I. Measuring Biodiversity Value for Conservation. Annu. Rev. Ecol. Evol. Syst. 1995, 26, 93–111. [Google Scholar] [CrossRef]
- Sinervo, B.; Miles, D.B.; Wu, Y.Y.; Méndez-De La Cruz, F.R.; Kirchhof, S.; Qi, Y. Climate Change, Thermal Niches, Extinction Risk and Maternal-Effect Rescue of Toad-Headed Lizards, Phrynocephalus, in Thermal Extremes of the Arabian Peninsula to the Qinghai-Tibetan Plateau. Integr. Zool. 2018, 13, 450–470. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.F.; Wang, Y.Z.; Zhong, Y.; Hoelzel, R.; Papenfuss, T.J.; Zeng, X.M.; Ananjeva, N.B.; Zhang, Y.P. A Phylogeny of Chinese Species in the Genus Phrynocephalus (Agamidae) Inferred from Mitochondrial DNA Sequences. Mol. Phylogenet. Evol. 2003, 27, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Melville, J, Hale, J. ; Mantziou, G.; Ananjeva, N.B.; Milto, K.; Clemann, N. Historical Biogeography, Phylogenetic Relationships and Intraspecific Diversity of Agamid Lizards in the Central Asian Deserts of Kazakhstan and Uzbekistan. Mol. Phylogenet. Evol. 2009, 53, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Zhan, A.B.; Fu, J.Z. Microsatellite DNA Markers for Three Toad-Headed Lizard Species (Phrynocephalus Vlangalii, P. Przewalskii and P. Guttatus): PERMANENT GENETIC RESOURCES NOTE. In Mol. Ecol. Resour.; 2009; Volume 9, pp. 535–538. [Google Scholar]
- Urquhart, J.; Bi, K.; Gozdzik, A.; Fu, J.Z. Isolation and Characterization of Microsatellite DNA Loci in the Toad-Headed Lizards, Phrynocephalus Przewalskii Complex. Mol. Ecol. Notes. 2005, 5, 928–930. [Google Scholar] [CrossRef]
- Hayden, M.J.; Nguyen, T.M.; Waterman, A.; McMichael, G.L.; Chalmers, K.J. Application of Multiplex-Ready PCR for Fluorescence-Based SSR Genotyping in Barley and Wheat. Mol. Breed. 2008, 21, 271–281. [Google Scholar] [CrossRef]
- Holland, M.M.; Parson, W. GeneMarker® HID: A Reliable Software Tool for the Analysis of Forensic STR Data: GENEMARKER® HID. J. Forensic Sci. 2011, 56, 29–35. [Google Scholar] [CrossRef]
- Oosterhout, C.V.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. Micro-Checker: Software for Identifying and Correcting Genotyping Errors in Microsatellite Data. Mol. Ecol. Notes. 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Rousset, F. GENEPOP ’ 007: A Complete Re-Implementation of the GENEPOP Software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.R. Analyzing Tables of Statistical Tests. Evolution. 1989, 43, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research-an Update. Bioinformatics. 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.; McGinnity, P.; Cross, T.F.; Crozier, W.W.; Prodoehl, P.A. DiveRsity: An R Package for the Estimation and Exploration of Population Genetics Parameters and Their Associated Errors. Methods. Ecol. Evol. 2013, 4, 782–788. [Google Scholar] [CrossRef]
- Amos, W.; Wilmer, J.W.; Fullard, K.; Burg, T.M.; Croxall, J.P.; Bloch, D.; Coulson, T. The Influence of Parental Relatedness on Reproductive Success. Proceedings. 2001, 268, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Alho, J.S.; Valimaki, K.; Merila, J. Rhh: An R Extension for Estimating Multilocus Heterozygosity and Heterozygosity-Heterozygosity Correlation. Mol. Ecol. Resour. 2010, 10, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Jost, L. GST and Its Relatives Do Not Measure Differentiation. Mol. Ecol. 2008, 17, 4015–4026. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Jueterbock, A.; Kraemer, P.; Deppermann, J.; Harmand, P. Calculations of Population Differentiation Based on GST and D: Forget GST but Not All of Statistics!: NEWS AND VIEWS: COMMENT. Mol. Ecol. 2010, 19, 3845–3852. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; VonHoldt, B.M. STRUCTURE HARVESTER: A Website and Program for Visualizing STRUCTURE Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software STRUCTURE: A Simulation Study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A Cluster Matching and Permutation Program for Dealing with Label Switching and Multimodality in Analysis of Population Structure. Bioinformatics. 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.A. DISTRUCT: A Program for the Graphical Display of Population Structure. Mol. Ecol. Notes. 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant Analysis of Principal Components: A New Method for the Analysis of Genetically Structured Populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T. Adegenet: A R Package for the Multivariate Analysis of Genetic Markers. Bioinformatics. 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Legendre, P.; Fortin, M.J. Comparison of the Mantel Test and Alternative Approaches for Detecting Complex Multivariate Relationships in the Spatial Analysis of Genetic Data. Mol. Ecol. Resour. 2010, 10, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Makanjuola, B.O.; Maltecca, C.; Miglior, F.; Schenkel, F.S.; Baes, C.F. Effect of Recent and Ancient Inbreeding on Production and Fertility Traits in Canadian Holsteins. BMC Genomics. 2020, 21, 605. [Google Scholar] [CrossRef]
- Driscoll, D. Ecological Genetics: Design, Analysis, and Application. Austral Ecol. 2005, 30, 815–816. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Brucker, L.; Tessier, N.G.; Liu, H.W.; Penedo, M.C.T.; Hughes, S.; Oberbauer, A.; Sacks, B. The Effect of Genetic Bottlenecks and Inbreeding on the Incidence of Two Major Autoimmune Diseases in Standard Poodles, Sebaceous Adenitis and Addison’s Disease. Canine. Genet. Epidemiol. 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Geiser, C.; Ray, N.; Lehmann, A.; Ursenbacher, S. Unravelling Landscape Variables with Multiple Approaches to Overcome Scarce Species Knowledge: A Landscape Genetic Study of the Slow Worm. Conserv. Genet. 2013, 14, 783–794. [Google Scholar] [CrossRef]
- Li, Y.; Lancaster, M.L.; Cooper, S.J.B.; Taylor, A.C.; Carthew, S.M. Population Structure and Gene Flow in the Endangered Southern Brown Bandicoot (Isoodon Obesulus Obesulus) across a Fragmented Landscape. Conserv. Genet. 2015, 16, 331–345. [Google Scholar] [CrossRef]
- Xu, Y.; Mai, J.W.; Yu, B.J.; Hu, H.X.; Yuan, L.; Jashenko, R.; Ji, R. Study on the Genetic Differentiation of Geographic Populations of Calliptamus Italicus (Orthoptera: Acrididae) in Sino-Kazakh Border Areas Based on Mitochondrial COI and COII Genes. J. Econ. Entomol. 2019, 112, 1912–1919. [Google Scholar] [CrossRef]
- Kodandaramaiah, U. Vagility: The Neglected Component in Historical Biogeography. Evol. Biol. 2009, 36, 327–335. [Google Scholar] [CrossRef]
- Abbas, A.; Jin, L.L.; He, Q.; Lu, B.; Yao, J.Q.; Li, Z.J.; Salam, A. Temporal and Spatial Variations of the Air Temperature in the Taklamakan Desert and Surrounding Areas. Theor. Appl. Climatol. 2021, 144, 873–884. [Google Scholar] [CrossRef]
- DeNardo, D.F.; Zubal. T.E.; Hoffman, T.C.M. Cloacal Evaporative Cooling: A Previously Undescribed Means of Increasing Evaporative Water Loss at Higher Temperatures in a Desert Ectotherm, the Gila Monster Heloderma Suspectum. J. Exp. Biol. 2004, 207, 945–953. [CrossRef] [PubMed]
- Lenormand, T. Gene Flow and the Limits to Natural Selection. Trends. Ecol. Evol. 2002, 17, 183–189. [Google Scholar] [CrossRef]
- Stockwell, C.A.; Hendry, A.P.; Kinnison, M.T. Contemporary Evolution Meets Conservation Biology. Trends. Ecol. Evol. 2003, 18, 94–101. [Google Scholar] [CrossRef]
- Pincheira, D.D.; Tregenza, T.; Witt, M.J.; Hodgson, D.J. The Evolution of Viviparity Opens Opportunities for Lizard Radiation but Drives It into a Climatic Cul-de-Sac: Viviparity and Climate Change. Glob. Ecol. Biogeogr. 2013, 22, 857–867. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.R.; Zeng, Z.G.; Liang, L.; Du, W.G. Maternal Food Availability Affects Offspring Performance and Survival in a Viviparous Lizard. Funct. Ecol. 2017, 31, 1950–1956. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, W.; Huang, Y.J.; Wang, X.N.; Zhao, Y.Y. Correlation between Climatic Factors and Genetic Diversity of Phrynocephalus forsythii. Asian. Herpetol. Res. 2019, 10, 270–275. [Google Scholar]
| Site abbreviation |
Sampling site | N | Ne | Ho | He | Fis | A | AR | IR |
|---|---|---|---|---|---|---|---|---|---|
| MF | Minfeng | 12 | 5.946 | 0.594 | 0.765 | 0.224 | 184 | 2.540 | 0.344 |
| CL | Cele | 14 | 6.631 | 0.515 | 0.751 | 0.314 | 205 | 2.420 | 0.394 |
| YT | Yutian | 16 | 4.360 | 0.394 | 0.672 | 0.414 | 165 | 2.040 | 0.505 |
| HT | Hetian | 14 | 4.860 | 0.486 | 0.728 | 0.332 | 166 | 2.190 | 0.428 |
| PS | Pishan | 18 | 8.915 | 0.494 | 0.829 | 0.404 | 274 | 2.440 | 0.429 |
| YC | Yecheng | 13 | 6.092 | 0.418 | 0.759 | 0.449 | 178 | 2.010 | 0.496 |
| YJS | Yingjisha | 8 | 3.663 | 0.466 | 0.613 | 0.240 | 101 | 1.850 | 0.474 |
| BC | Bachu | 8 | 2.790 | 0.207 | 0.427 | 0.603 | 75 | 1.160 | 0.713 |
| XH | Xinhe | 3 | 1.341 | 0.135 | 0.268 | 0.819 | 30 | 0.810 | 0.740 |
| RQ | Ruoqiang | 10 | 4.217 | 0.371 | 0.638 | 0.419 | 127 | 1.990 | 0.505 |
| YLN | Yulinan | 11 | 3.381 | 0.277 | 0.558 | 0.543 | 102 | 1.350 | 0.655 |
| LT | Luntai | 2 | 2.108 | 0.381 | 0.435 | 0.210 | 46 | 1.730 | 0.914 |
| LTN | Luntainan | 20 | 7.590 | 0.356 | 0.780 | 0.544 | 248 | 2.100 | 0.543 |
| ALE | Alaer | 18 | 6.003 | 0.370 | 0.752 | 0.508 | 210 | 2.120 | 0.535 |
| JS | Jjiashi | 4 | 2.680 | 0.381 | 0.489 | 0.290 | 68 | 1.830 | 0.468 |
| population | MF | CL | YT | HT | PS | YC | YJS | BC | XH | RQ | YLN | LT | LTN | ALE | JS |
| MF | —— | 0.662 | 0.712 | 0.700 | 0.697 | 0.747 | 0.788 | -0.077 | -7.747 | 0.707 | 0.370 | 0.327 | 0.671 | 0.672 | 0.360 |
| CL | 0.139 | —— | 0.163 | 0.176 | 0.217 | 0.309 | 0.410 | -0.075 | -6.069 | 0.420 | 0.149 | 0.161 | 0.322 | 0.284 | 0.129 |
| YT | 0.183 | 0.050 | —— | 0.297 | 0.299 | 0.398 | 0.532 | 0.276 | -3.870 | 0.380 | 0.161 | 0.278 | 0.414 | 0.388 | 0.280 |
| HT | 0.155 | 0.046 | 0.092 | —— | 0.270 | 0.383 | 0.468 | -0.013 | -6.140 | 0.477 | 0.154 | 0.164 | 0.308 | 0.305 | 0.210 |
| PS | 0.115 | 0.041 | 0.072 | 0.054 | —— | 0.158 | 0.366 | -0.107 | -10.079 | 0.353 | -0.035 | -0.292 | 0.252 | 0.315 | 0.110 |
| YC | 0.142 | 0.067 | 0.109 | 0.087 | 0.028 | —— | 0.470 | -0.053 | -9.274 | 0.399 | 0.033 | -0.281 | 0.414 | 0.392 | 0.178 |
| YJS | 0.197 | 0.116 | 0.179 | 0.139 | 0.081 | 0.127 | —— | 0.300 | -3.446 | 0.586 | 0.337 | 0.378 | 0.471 | 0.470 | 0.476 |
| BC | 0.181 | 0.090 | 0.138 | 0.113 | 0.093 | 0.095 | 0.165 | —— | 0.000 | 0.316 | 0.241 | -0.546 | -0.189 | -0.227 | -0.329 |
| XH | 0.137 | 0.063 | 0.128 | 0.095 | 0.032 | 0.005 | 0.157 | 0.008 | —— | -6.004 | 0.000 | 0.000 | -7.640 | -6.413 | 0.000 |
| RQ | 0.177 | 0.116 | 0.138 | 0.128 | 0.081 | 0.101 | 0.180 | 0.080 | 0.056 | —— | 0.038 | 0.021 | 0.269 | 0.321 | 0.001 |
| YLN | 0.181 | 0.129 | 0.131 | 0.110 | 0.083 | 0.102 | 0.173 | 0.067 | 0.073 | 0.104 | —— | 0.168 | -0.034 | -0.055 | 0.205 |
| LT | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | —— | -0.470 | -0.260 | 0.393 |
| LTN | 0.131 | 0.070 | 0.110 | 0.071 | 0.044 | 0.085 | 0.117 | 0.052 | 0.024 | 0.058 | 0.064 | 0.000 | —— | 0.129 | -0.126 |
| ALE | 0.142 | 0.068 | 0.112 | 0.077 | 0.060 | 0.081 | 0.128 | 0.055 | 0.051 | 0.090 | 0.092 | 0.000 | 0.030 | —— | -0.199 |
| JS | 0.194 | 0.127 | 0.210 | 0.153 | 0.129 | 0.168 | 0.193 | 0.055 | 0.104 | 0.126 | 0.110 | 0.000 | 0.065 | 0.059 | —— |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





