1. Introduction
The treatment of critically ill patients remains an evolving and controversial issue. Technological progress has made various mechanical circulatory assist devices (MCADs) available for the treatment of this group of patients. Some of these devices provide haemodynamic support to a dysfunctional left ventricle through a minimally invasive implantation approach using percutaneous techniques [
1,
2,
3,
4,
5,
6,
7]. This technological evolution allows the use of MCADs in patients with haemodynamic instability in which the speed and safety of the implant is critical and in more stable patients undergoing cardiovascular interventions, potentially associated with the risk of destabilisation. The choice of the appropriate percutaneous ventricular assist device (PVAD) system, the timing of the implant, the duration of support and the prevention of any complications are the key points in the management of patients requiring the insertion of MCADs. Nevertheless, the scientific evidence remains controversial and based on the experience of each centre leading to significant variability in indications, recommendations and implantation techniques [
8,
9]. Despite this variability, PVAD implant in the cath lab has been taking place with promising implications for the future.
There are many studies in the current literature on the use of combined peripheral venoarterial extracorporeal membrane oxygenation (VA-ECMO) and Impella 2.5 or VA-ECMO and intra-aortic balloon pump (IABP) for the management of patients in cardiogenic shock (CS) [
2,
4,
5,
8]. More recent experimental and clinical studies have focused their attention on the combined use of Impella 2.5 and IABP in CS patients [
1,
10,
11,
12,
13,
14]. Although the results presented are controversial [
15,
16], it is worth exploring this aspect further and determine its true potential as a prelude to a more definitive application on a routine basis.
In the study of the interaction between the cardiovascular and respiratory systems and different types of mechanical circulatory and respiratory assist devices, numerical and hybrid simulators have proved to be an excellent tool for the analysis and understanding of the effects induced by different modes of support on haemodynamic and energetic variables [
4,
5,
17,
18,
19,
20,
21,
22,
23].
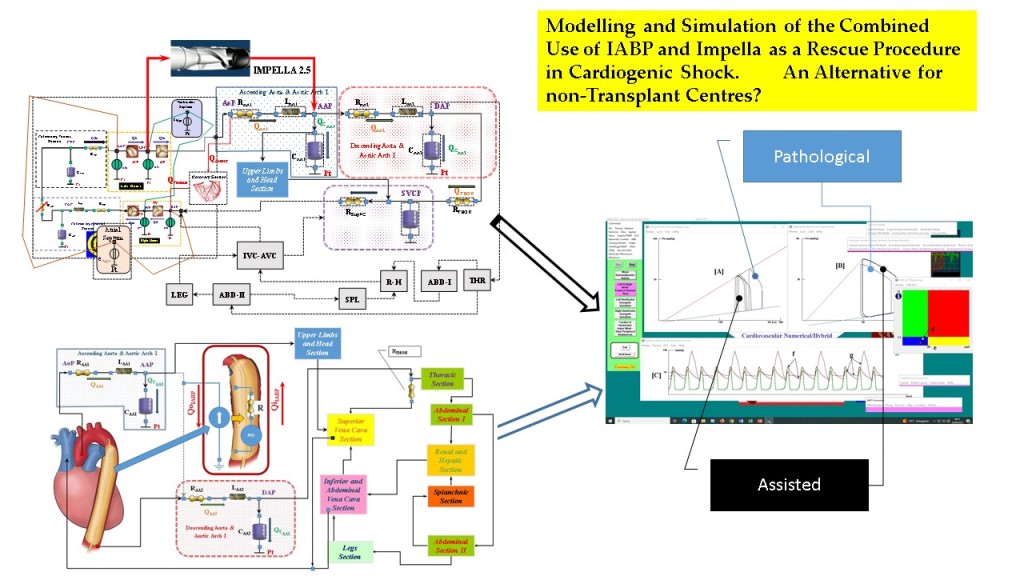
The purpose of this study was the use of CARDIOSIM
© software platform [
17,
24] to simulate the effects induced by the simultaneous administration of drug, activation of the IABP and the left ventricular assist device (LVAD) Impella 2.5 (Abiomed Europe GmbH, Aachen, Germany) on cardiovascular haemodynamic and energetic variables [
4]. CARDIOSIM
© features allow IABP support to be driven from full (1:1) to partial (1:2, 1:3 and 1:4) assistance. The study consisted of a virtual CS patient [
4,
25,
26] with its baseline status followed by the simultaneous administration of Milrinone to obtain a decrease in pulmonary vascular resistances of about 10% and activation of IABP. In addition, simultaneous activation of IABP (without drug administration) and Impella 2.5 was addressed. The full range of IABP setting (1:1, 1:2, 1:3 and 1:4) was considered during drug administration. Here we further develop aspects initially addressed in a previous study by our group [
27].
2. Materials and Methods
This study was designed and based on the use of CARDIOSIM
©, which is a numerical simulator of the cardiovascular system developed in the Cardiovascular Numerical/Hybrid Modelling Lab (Rome) of the Institute of Clinical Physiology (IFC-CNR) in collaboration with the Department of Human Movement and Sport Sciences, “Foro Italico” 4th University of Rome, the Department of Biomedical Engineering, University of Strathclyde (Glasgow), and the Department of Clinical, Internal Anesthesiology and Cardiovascular Sciences, “Sapienza” University of Rome and the Faculty of Medicine of Teaching University Geomedi [
4,
5,
17,
19,
24,
28]. The software simulator consists of lumped parameter models of the circulatory network and the time varying elastance concept to reproduce the native ventricular, atrial and septal behavior [
19,
28].
The cardiovascular network between the left ventricular output and the right atrial input, developed with 0-D models, described in [
5,
29], consists of: ascending and descending aorta, aortic arch, thoracic, abdominal, upper limbs and head, renal, hepatic, splanchnic, superior, inferior and abdominal vena cava and legs sections, respectively. The circulatory network between the right ventricular output and the left atrial input, described in [
30,
31] has been developed using the main and small arterial, pulmonary arteriole and capillary and pulmonary venous compartments.
The 0-D model described in [
32,
33] simulates the coronary circulation.
The numerical models of the IABP and the Impella 2.5 device implemented within CARDIOSIM
© platform have been modified in order to be activated simultaneously. The IABP was operated by adjusting the vacuum pressure to –10 mmHg and drive pressure to 260 mmHg, as described in [
4]. For the purposes of this study, the progressive timing reduction from full (1:1) to partial (1:2, 1:3 and 1:4) assistance was implemented for the IABP [
4,
27].
The Impella 2.5 (Abiomed Europe GmbH, Aachen, Germany) pump was implemented in the software platform as described in [
4].
The first part of the study reproduced a virtual CS patient with its baseline status according to literature data [
4,
34,
35,
36] as follows: systolic aortic pressure (SAP <90 mmHg), cardiac index (CI lower than 2.2 L/min/m
2), pulmonary capillary wedge pressure higher than 15 mmHg, and elevated left ventricular end-diastolic pressure (LVEDP >18 mmHg).
The second part of the study focused on the simulation settings with IABP support in full mode and simultaneous drug administration (Milrinone) to reduce the pulmonary vascular resistance by 10%. Subsequently, the weaning of the virtual patient was simulated by activating the IABP in 1:2, 1:3 and 1:4 mode. When the IABP operates in 1:2, or 1:3 or 1:4 mode, the relevant values have been calculated as average of twenty-four cardiac cycles, respectively. In the last part of the study, IABP and Impella 2.5 pump were applied simultaneously with different rotational speed at 35000, 40000 and 50000 rpm for the Impella device and full (1:1) to partial (1:2, 1:3 and 1:4) assistance for the IABP. Drug administration was not considered.
The hemodynamic parameters under investigation were: mean aortic pressure (AoP), systolic and diastolic aortic pressure (SAP and DAP), mean pulmonary arterial pressure (PAP), mean left atrial pressure (LAP), mean right atrial pressure (RAP), cardiac output (CO), cardiac index (CI), left and right ventricular end-systolic volume (LVESV and RVESV), left ventricular end-diastolic volume (LVEDV) and mean coronary blood flow (CBF). The energetic variables considered in the study were: left and right ventricular external work (LVEW and RVEW) and left and right atrial pressure-volume area (LAPVA and RAPVA) [
5].
3. Results
A virtual CS patient was developed according to literature data [
4,
34,
35,
36] (
Figure 1) using CARDIOSIM
© software.
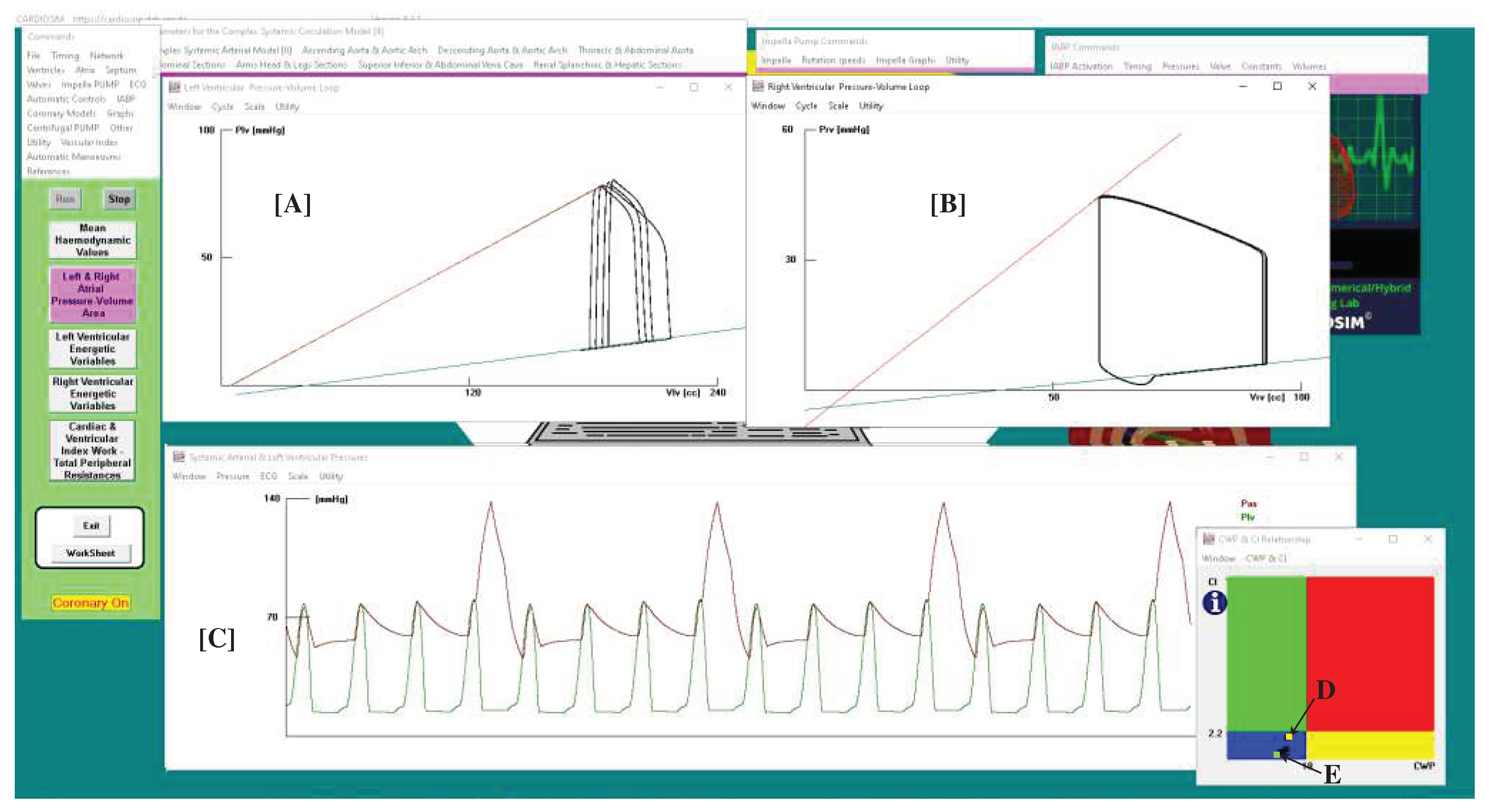
Figure 1 shows that systolic aortic pressure is lower than 90 mmHg, cardiac index is lower than 2.2 L/min/m
2 (panel d), pulmonary capillary wedge pressure is 23.7 mmHg, and left ventricular end-diastolic pressure is higher than 18 mmHg. The cardiac index (CI) and capillary wedge pressure (CWP) relationship graph (panel [f]), shows the four different regions describing:
the normal haemodynamic condition (green rectangle);
cardiorespiratory disease induced by insufficient CO (red rectangle);
hypoperfusion state caused by fluid volume depletion (blue rectangle);
low flow rate (yellow rectangle) requiring either drug therapy or IABP device, or ventricular assist device (VAD) support.
The simulated CS patient conditions show that the CI-CWP relationship presents a yellow point in the blue region (panel [f]), i.e. hypoperfusion state caused by fluid volume depletion.
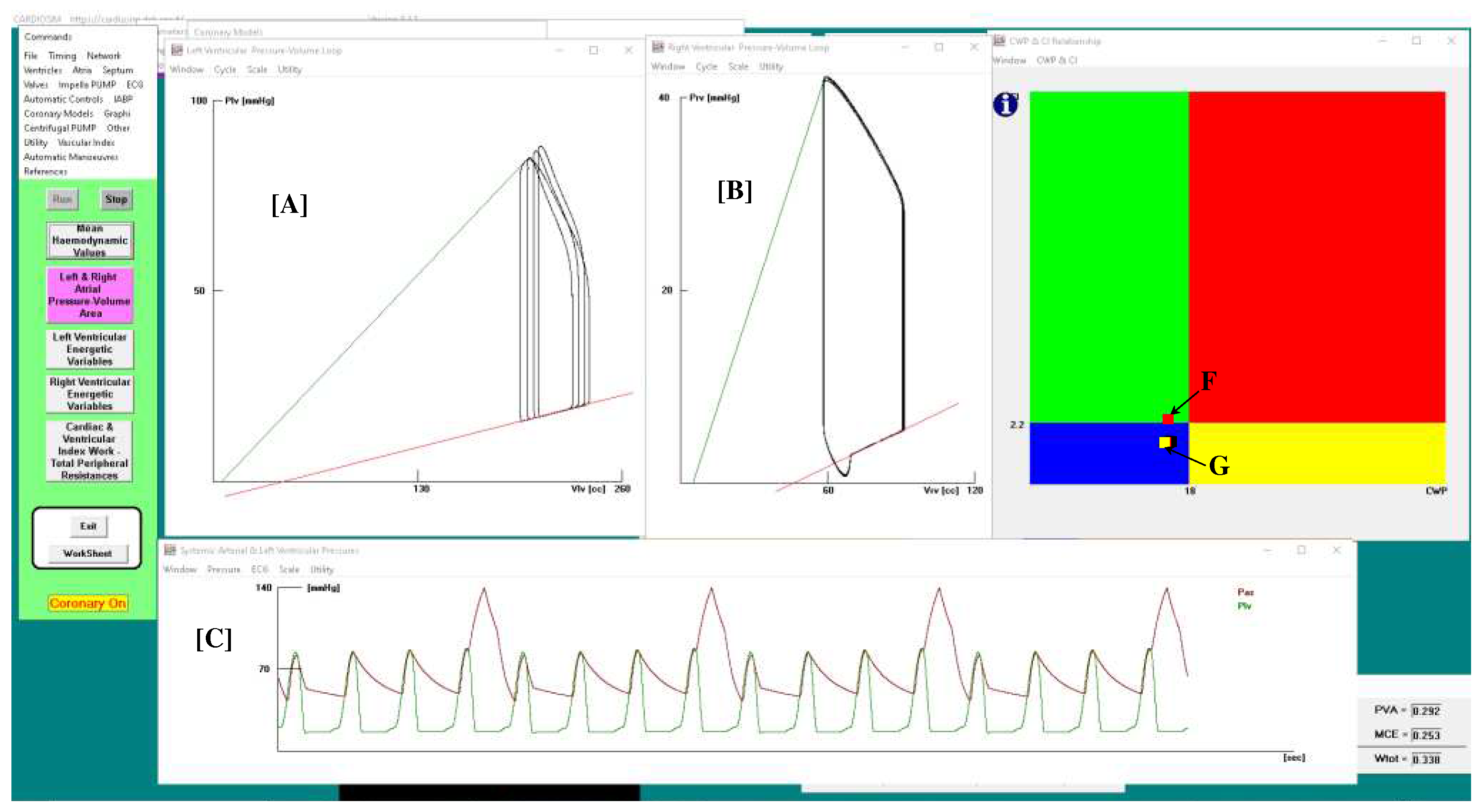
Figure 2 shows the results obtained when drug administration and IABP support were applied simultaneously. In this case, IABP was driven in 1:4 mode. In the left (A) and right (B) ventricular pressure-volume planes are plotted for four cardiac cycles: three cycles are without assistance (IABP off) and one cycle is with assistance (IABP on).
Panel C shows sixteen aortic and left ventricular pressures waveforms. After three unassisted waveforms, one assisted waveform follows. Finally, the point representing the relationship between the two parameters moves from position G (in the blue rectangle) to position F (in the green rectangle) in the CI-CWP graph.
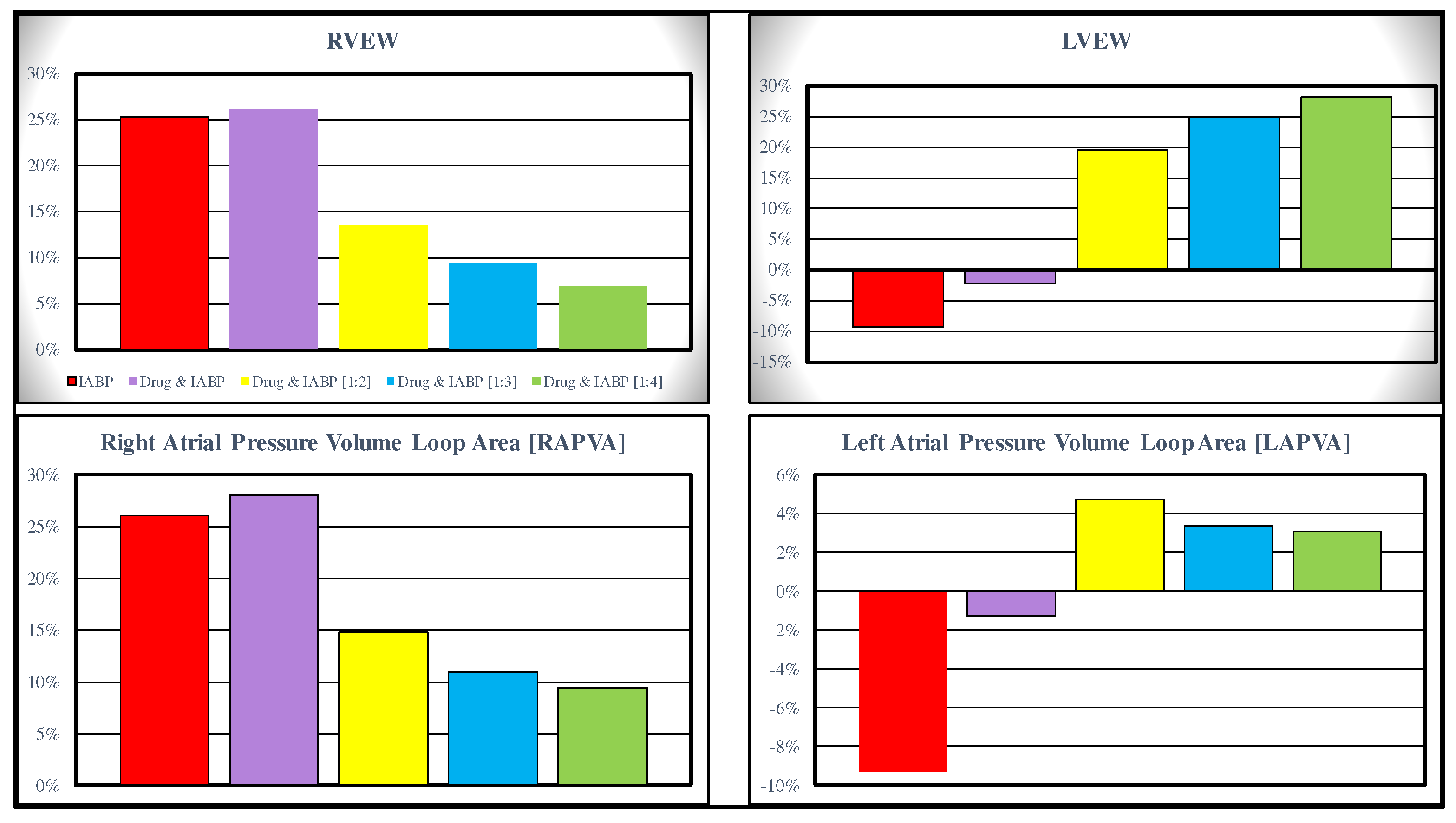
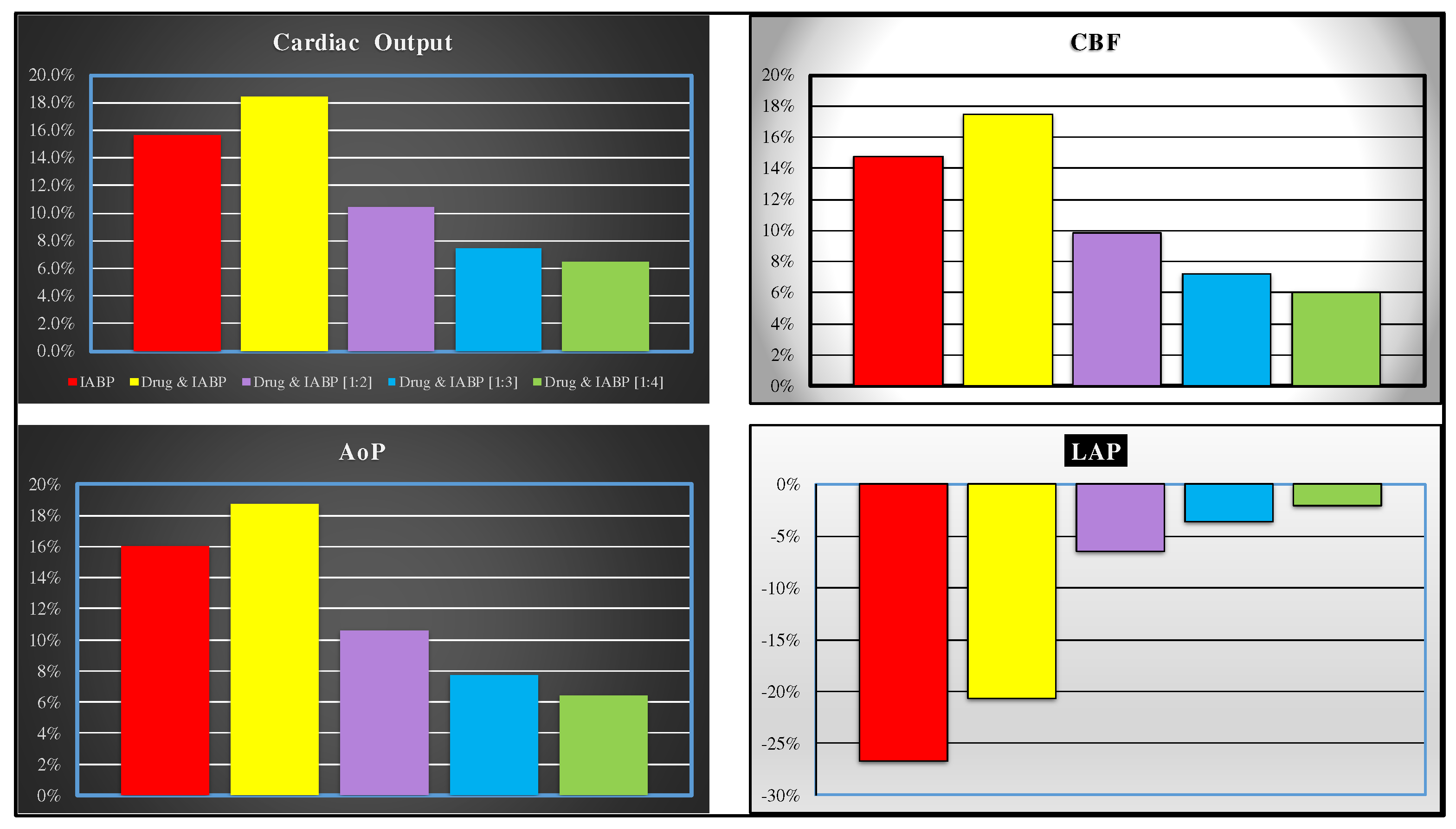
Starting from baseline conditions, the effects induced on RVEW, LVEW, RAPVA and LAPVA by IABP activation in full and partial mode are represented in
Figure 3. The top left panel shows that IABP (driven in full mode) without and with drug administration increases the RVEW by 25% compared to baseline conditions. The simultaneous simulation of drug administration and IABP driven in partial (1:4) mode gives a 6% increase. IABP support (full mode) decreases LVEW regardless of drug administration. Intra-aortic balloon pump increases LVEW when driven in partial mode (right top panel).
IABP support increases the RAPVA regardless of drug administration (bottom left panel), while different effects are induced in LAPVA (bottom right panel). The haemodynamic effects induced by different IABP settings and drug administration are reported in
Figure 4.
Simulation of simultaneous drug administration and IABP driven in full and partial (1:2) mode show more than 15% (16%) increase in CO (AoP) according to literature data (top and bottom left panels). More than 20% reduction is recorded for left atrial pressure (bottom right panel). Finally, an increase in coronary blood flow between 6% and 17% is obtained for all IABP actuation modes (top left panel).
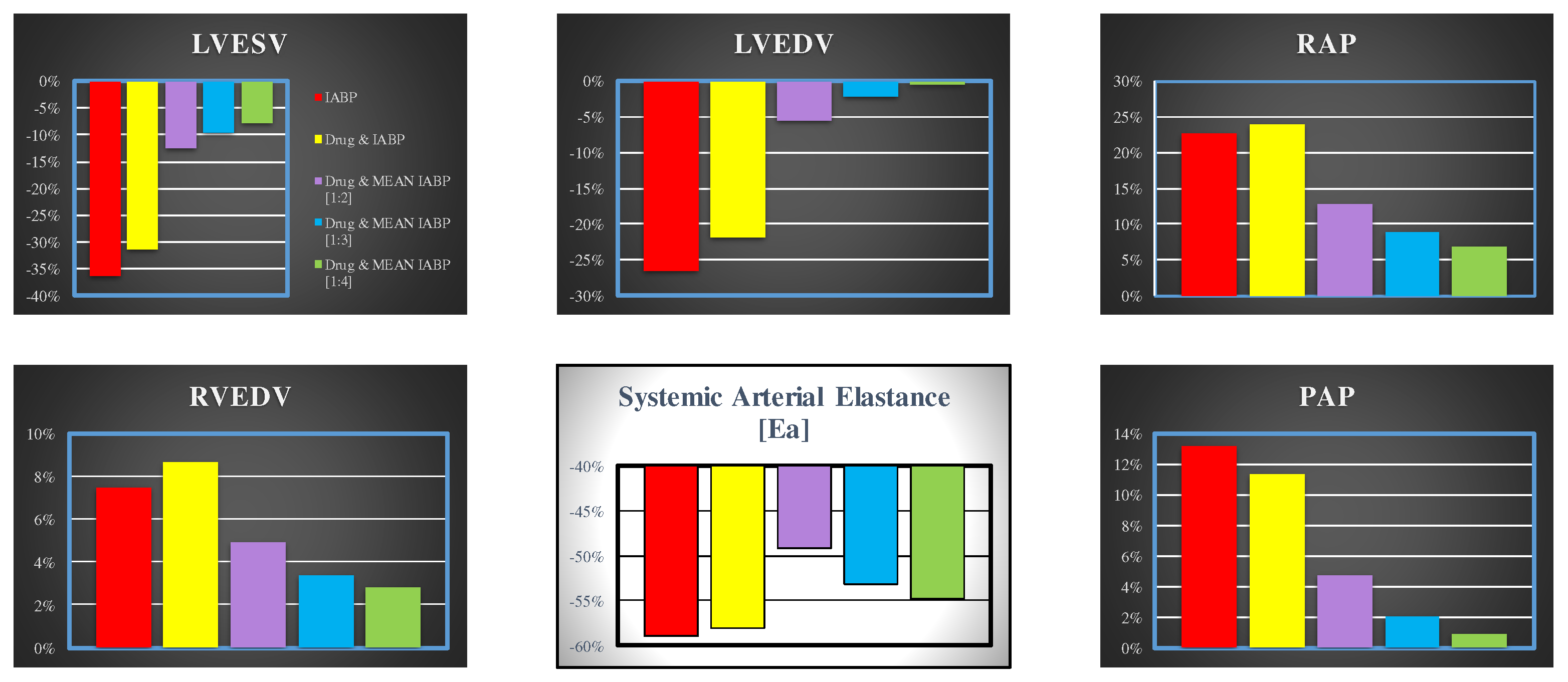
Figure 5 shows the effects induced on left ventricular end-systolic volume (top left panel), left ventricular end-diastolic volume (middle top panel), mean right atrial pressure (top right panel), mean pulmonary arterial pressure (bottom right panel), systemic arterial elastance (middle bottom panel) and on right ventricular end-diastolic volume (bottom left panel).
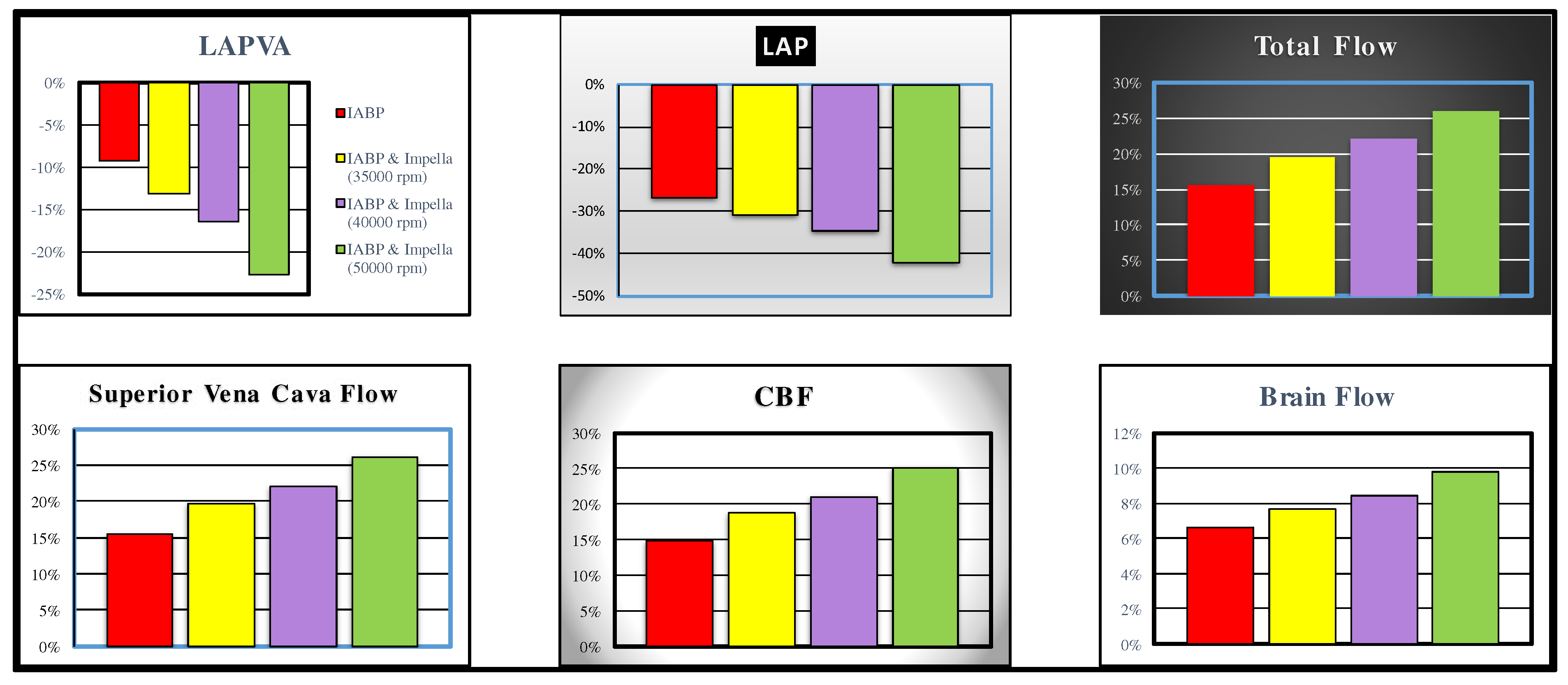
The following are the effects induced on the haemodynamic and energetic variables by the simultaneous activation of the IABP and the Impella 2.5. Drug administration was not simulated to avoid interference with the simultaneous activation of two support systems. The effects induced by simultaneous activation of IABP in full mode (1:1) and Impella 2.5 pump on left atrial pressure, left atrial pressure volume area (LAPVA), total flow, superior vena cava flow, coronary blood flow and cerebral flow are plotted in
Figure 6. LVAD was activated with different rotational speed (i.e. 35000, 40000 and 50000 rpm). The simultaneous activation of the two devices induces an approximately 22% reduction in LAPVA, a 25% increase in total flow and an approximately 25% increase in CBF when the rotational pump speed was set to 50000 rpm. Increases and reductions are calculated with respect to values measured in pathological conditions. Superior vena cava and brain blood flow show an increase of approximately 15% and 6%, respectively when IABP alone is driven in full mode.
Figure 7 shows the results obtained when both IABP driven in 1:4 mode and Impella 2.5 with 40000 rpm rotational speed were applied simultaneously. Four cardiac cycles are plotted in the left (A) and right (B) ventricular pressure-volume planes: three cycles without assistance (IABP off) and one cycle with assistance (IABP on).
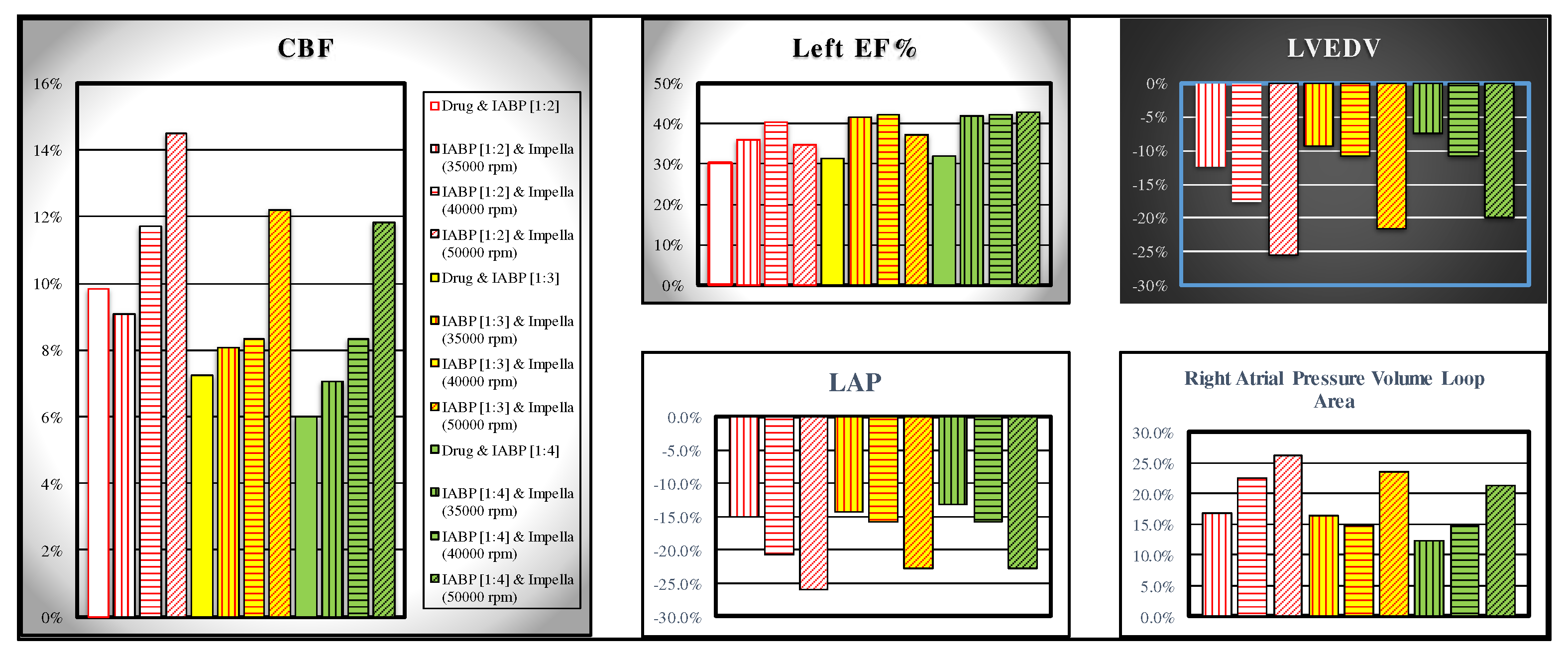
The comparison between the effects induced on some haemodynamic variables by the simultaneous activation of the IABP (driven in full and partial mode) and the Impella 2.5 pump (activated with different rotation speeds, i.e 35000, 40000 and 50000 rpm) is shown in
Figure 8. The simulations performed in this study have highlighted that for coronary blood flow the assistance provided with IABP driven in full and partial mode and drug administration produces similar effects on coronary blood flow compared to those induced by the simultaneous activation of IABP (in full and partial mode) and Impella 2.5 with 35000 rpm rotational speed (white bar and bar with vertical red line in the left panel of
Figure 8).
In contrast, different effects are observed on left ventricular ejection fraction (white bar and bar with vertical red line in the top middle panel) where the simultaneous application of IABP and Impella 2.5 generate higher percentage variations compared to the application of IABP (driven in full and partial mode) and drug administration (
Figure 8). The top right panel (bottom middle panel) shows that the combination of IABP (driven in partial mode) and LVAD (with 50000 rpm rotational speed) induces a reduction between 25.6% and 19.88% (25.95% and 22.84%) in left ventricular end-diastolic volume (left atrial pressure). Finally, the same combination of devices (driven in the same conditions) produces an increase between 26.2% and 21.35% in right atrial pressure volume loop area (right bottom panel of
Figure 8).
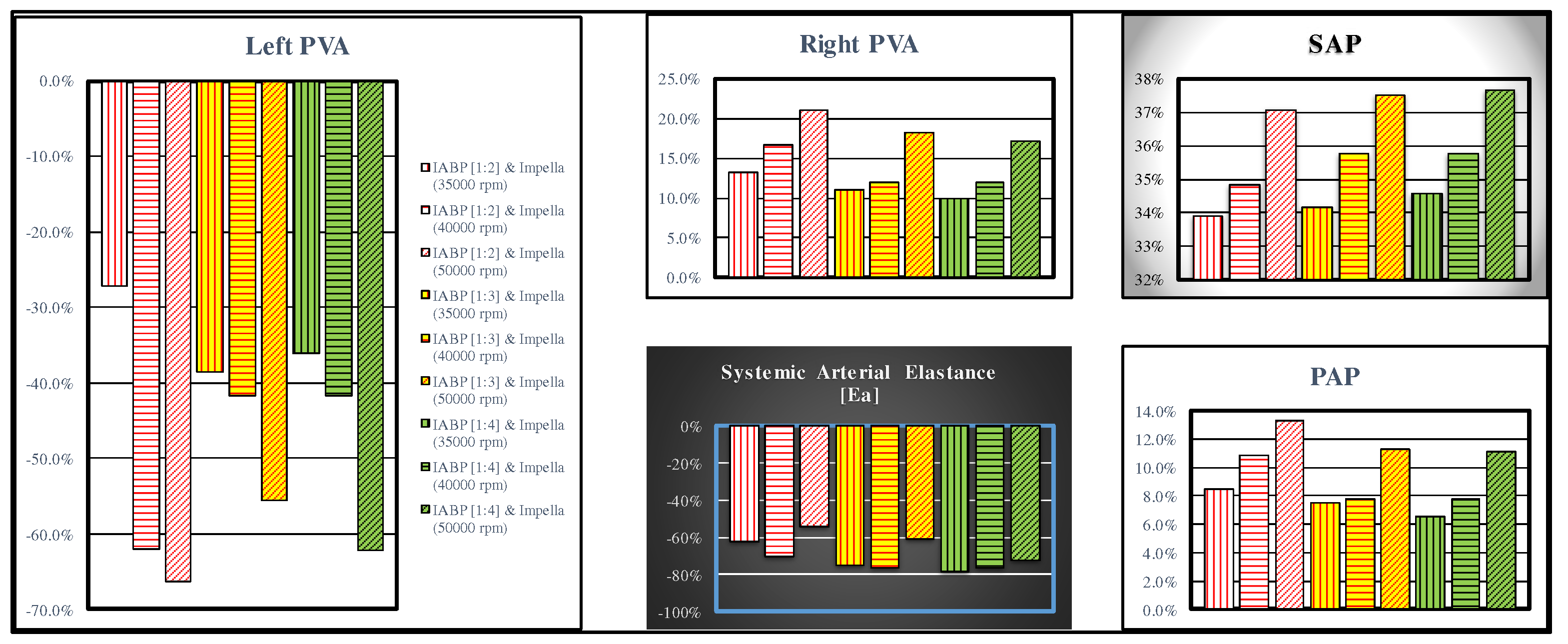
The combined action of the two devices produces opposite effects on left ventricular pressure volume area (left panel in
Figure 9) and right PVA (top middle panel). In both cases, the highest percentage variations occur when the LVAD is set to 50000 rpm. The effects induced on systemic arterial pressure and systemic arterial elastance are opposite, but in both cases, they are higher when IABP is activated in 1:2 mode (top right and bottom middle panel of
Figure 9, respectively).
3. Discussion
Acute myocardial infarction, acute decompensated heart failure and post cardiotomy failure are the common scenarios leading to cardiogenic shock. The aim is timing of intervention to stabilise the condition and buy time for the next step. The IABP and Impella 2.5 are commonly available in an acute setting, either in theatre or in the cath lab, mainly used as standalone devices. Their combined use has been previously proposed based on experimental evidence [
37]. Our simulations show that the highest left ventricular unloading is achieved with a combined used of Impella device at 50000 rpm and IABP activated in 1:2 mode (
Figure 9). The Impella device seems to play the major role. The same setting achieves significant increase in coronary blood flow and left ventricular ejection fraction (
Figure 8). When the IABP is fully activated (1:1), the highest effect is again achieved with Impella device at 50000 rpm (
Figure 6 and
Figure 7). The outcome of our simulations confirms that the combined use of the two devices seems to achieve a synergistic effect in terms of left ventricular unloading, organ perfusion with particular reference to coronary and cerebral blood flow, and oxygen demand/supply. This seems to match the experimental setting [
37]. These findings would suggest that a combined use of IABP and Impella device may play a key role in the acute setting in terms of stabilisation of cardiogenic shock with a view to upgrade to a more advanced device should escalation of treatment be required. This would give enough time for transfer to a transplant centre to carry out the procedure. Although the 1:4 mode for IABP is not used in clinical practice, it has been included for comparison purposes. The degree of assistance may be tailored according to the group of patients and the scenarios being faced. A simulation approach may be of value in terms of clinical decision-making. Although little is known about long-term outcomes beyond the first 30 days, this should not be a deterrent but a motivation to feed the audacity of pushing the boundaries and find solutions to address the problem. Undoubtedly, there remains an ongoing elevated mortality risk even in surviving patients who will require long-term follow up [
38]. Additionally, morbidity in terms of increased need for home support or transition to long-term care may generate discussions about patients’ willingness to undergo risky interventions, which may improve survival but increase dependence. Teams caring for patients with CS need to recognise this sustained ongoing risk and further review their approach to ensure sustained ongoing care. High-intensity follow-up, aggressive medical treatment and device-based intervention for heart failure may help modulate risk when failure occurs in the acute CS setting.
4. Conclusions
Mortality and morbidity in cardiogenic shock remains high despite efforts aimed at improving in-hospital or 30-day survival through revascularisation, the use of mechanical circulatory support and medical treatment optimisation. A focus on early intervention to prevent further deterioration may have a role to play. This may be achievable if designated non-transplant centres are available to offer the necessary expertise. Our simulations have shown that a combination of IABP and Impella device achieves adequate support to address the acute phase and allow transfer to a transplant centre should escalation to more advanced support be required. Close co-operation between clinician and engineering scientists may help with treatment optimisation and outcome prediction for different groups of patients.
Author Contributions
Conceptualization, B.D.L. and C.D.L.; methodology, B.D.L., C.D.L. and R.B.; software, B.D.L. and C.D.L.; formal analysis, M.C. and R.B.; investigation, B.D.L., and M.C.; resources, R.B.; data curation, B.D.L., M.C., R.B., and C.D.L.; writing—original draft preparation, B.D.L., and M.C.; writing—review and editing M.C. and R.B.; supervision, C.D.L., B.D.L. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
REMOTE project (n. F/310117/01/X56) financed by Italian Ministry of Economic Development.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhuiyan, R.; T. Bimal T.; Fishbein, J.;et al. Percutaneous coronary intervention with Impella support with and without intra-aortic balloon in cardiogenic shock patients. Cardiovascular Revascularization Medicine, 2023. [CrossRef]
- Wong, A.S.K.; Sin, S.W.C. Short-term mechanical circulatory support (intra-aortic balloon pump, Impella, extracorporeal membrane oxygenation, TandemHeart): a review. Ann Transl Med., 2020 Jul;8(13):829. [CrossRef] [PubMed] [PubMed Central]
- Merdji, H.; Levy, B.; Jung, C.; Ince, C.; Siegemund, M.; Meziani, F. Microcirculatory dysfunction in cardiogenic shock. Ann. Intensive Care, 2023,13(1), 38. [CrossRef]
- De Lazzari, B.; Capoccia, M.; Badagliacca, R.; Bozkurt, S.; De Lazzari, C. IABP versus Impella Support in Cardiogenic Shock: “In Silico” Study. J. Cardiovasc. Dev. Dis., 2023, 10, 140. [CrossRef]
- De Lazzari, B.; Iacovoni, A.; Mottaghy, K.; Capoccia, M.; Badagliacca, R.; Vizza, C. D.; De Lazzari, C. ECMO Assistance during Mechanical Ventilation: Effects Induced on Energetic and Haemodynamic Variables. Computer Methods and Programs in Biomedicine, 2021, 202, 106003. [CrossRef]
- Saito, S.; Shibasaki, I.; Matsuoka, T.; Niitsuma, K.; Hirota, S.; Kanno, Y.; Kanazawa, Y.; Tezuka, M.; Takei, Y.; Tsuchiya, G.; Konishi, T.; Ogata, K.; Fukuda, H. Impella support as a bridge to heart surgery in patients with cardiogenic shock. Interactive Cardiovascular and Thoracic Surgery, 2022, 35(2). [CrossRef]
- Meani, P.; Lorusso, R.; Pappalardo, F. ECPella: Concept, Physiology and Clinical Applications. Journal of Cardiothoracic and Vascular Anesthesia, 2022, 36(2), 557-566. [CrossRef]
- Russo, G., Burzotta, F., Aurigemma, C., Pedicino, D., Romagnoli, E., Trani, C. Can we have a rationalized selection of intra-aortic balloon pump, Impella, and extracorporeal membrane oxygenation in the catheterization laboratory? Cardiol J., 2022, 29(1):115-132. [CrossRef]
- Cavayas, Y.A.; Noly, P-E.; Singh, G.; Lamarche, Y. Controversies in extracorporeal membrane oxygenation: Immediate versus watchful waiting for venoarterial extracorporeal membrane oxygenation venting. JTCVS Open, 2021, 8, 70-76. [CrossRef]
- Pavlidis, A.N.; Redwood, S.R.; Clapp, B.R. Combined hemodynamic support with the Impella 2.5 device and intra-aortic balloon pump for management of refractory cardiogenic shock. J Invasive Cardiol., 2014, 26(5), E50-1. [PubMed]
- Gupta, A.; Allaqaband, S.; Bajwa, T. Combined use of Impella device and intra-aortic balloon pump to improve survival in a patient in profound cardiogenic shock post cardiac arrest. Catheter Cardiovasc Interv., 2009, 15, 74(6), 975-6. [CrossRef]
- Wiktor, D.M.; Sawlani, N.; Kanthi, Y.; Sipahi, I.; Fang, J.C.; Blitz, A. Successful combined use of Impella Recover 2.5 device and intra-aortic balloon pump support in cardiogenic shock from acute myocardial infarction. ASAIO J., 2010, 56(6), 519-21. [CrossRef]
- Cubeddu, R.J.; Lago, R.; Horvath, S.A.; Vignola, P.A.; O'Neill, W.; Palacios, I.F. Use of the Impella 2.5 system alone, after and in combination with an intra-aortic balloon pump in patients with cardiogenic shock: case description and review of the literature. EuroIntervention, 2012, 7(12), 1453-60. [CrossRef]
- Møller-Helgestad, O.K.; Poulsen, C.B.; Christiansen, E.H.; Lassen, J.F.; Ravn, H.B. Support with intra-aortic balloon pump vs. Impella2.5® and blood flow to the heart, brain and kidneys - an experimental porcine model of ischaemic heart failure. Int. J. Cardiol., 2015, 15, 178, 153-8. [CrossRef]
- Meani, P.; Lorusso, R.; Pappalardo, F. ECPella: Concept, Physiology and Clinical Applications. J. Cardiothorac. Vasc. Anesth., 2022, 36(2), 557-566. [CrossRef]
- Weber, D.M.; Raess, D.H.; Henriques, J.; Siess, T. Principles of Impella Cardiac Support. Cardiac Interventions Today, 2009, August/September 3-16.
- De Lazzari, B.; Badagliacca, R.; Filomena, D.; Papa S. et al. CARDIOSIM©: The First Italian Software Platform for Simulation of the Cardiovascular System and Mechanical Circulatory and Ventilatory Support. Bioengineering, 2022, 9(8), 383. [CrossRef]
- Kaye, D.M.; Wolsk, E.; Nanayakkara, S.; Mariani, J.; Hassager, C.; Gustafsson, F.; Moller, J.E.; Sunagawa, K.; Burkhoff, D. Comprehensive Physiological Modeling Provides Novel Insights Into Heart Failure With Preserved Ejection Fraction Physiology. J. Am. Heart Assoc., 2021, 10(19), e021584. [CrossRef]
- De Lazzari, B.; Iacovoni, A.; Capoccia, M.; Papa, S. et al. Ventricular and Atrial Pressure-Volume Loops: Analysis of the Effects Induced by Right Centrifugal Pump Assistance. Bioengineering, 2022, 9(5), 181. [CrossRef]
- Manzi, G.; Miotti, C.; Mariani, M.V.; Papa, S.; Luongo, F. et al. Computational Simulator Models and Invasive Hemodynamic Monitoring as Tools for Precision Medicine in Pulmonary Arterial Hypertension. J. Clin. Med., 2021, 11(1):82. [CrossRef]
- Ferrari, G.; Kozarski, M.; et al. Development of a hybrid (numerical-hydraulic) circulatory model: prototype testing and its response to IABP assistance. Int. J. Artif. Organs, 2005, 28(7), 750-9. [CrossRef]
- Kung, E.; Farahmand, M.; Gupta, A. A Hybrid Experimental-Computational Modeling Framework for Cardiovascular Device Testing. J. Biomech. Eng., 2019, 141(5), 051012. [CrossRef]
- Bozkurt, S.; Paracha, W.; Bakaya, K.; Schievano, S. Patient-Specific Modelling and Parameter Optimisation to Simulate Dilated Cardiomyopathy in Children. Cardiovasc. Eng. Technol., 2022, 13(5), 712-724. [CrossRef]
- De Lazzari, C.; Stalteri, D. 2011–2019, CARDIOSIM© Website. Available online: https://cardiosim.dsb.cnr.it/Esperimenti/Patient2 (accessed on 29 May 2022).
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc., 2019, 16, 8(8), e011991. [CrossRef]
- Ng, P.Y.; Ma T.S.K.; Ip, A.; Fang, S.; et al. Effects of varying blood flow rate during peripheral veno-arterial extracorporeal membrane oxygen (V-A ECMO) on left ventricular function measured by two-dimensional strain. Front Cardiovasc Med., 2023, 12; 10, 1147783. [CrossRef]
- Alkan, R.; De Lazzari, B.; Capoccia, M.; De Lazzari, C.; Bozkurt, S. Computational Evaluation of IABP, Impella 2.5, TandemHeart and Combined IABP and Impella 2.5 Support in Cardiogenic Shock. Mathematics, 2023, 11(16):3606, 1-17.
- De Lazzari, C. Interaction between the septum and the left (right) ventricular free wall in order to evaluate the effects on coronary blood flow: Numerical simulation. Comput. Methods Biomech. Biomed. Eng., 2012, 15, 1359–1368. [CrossRef] [PubMed]
- De Lazzari, C.; Stalteri, D. 2011–2019, CARDIOSIM© Website. Available online: https://cardiosim.dsb.cnr.it/CirculationModels/sci6 (accessed on 29 May 2022).
- Capoccia, M.; Marconi, S. and De Lazzari, C. Decision-making in advanced heart failure patients requiring LVAD insertion: Can preoperative simulation become the way forward? A case study. Journal of Biomedical Engineering and Informatics, 2018, 4 (2), 8-20. [CrossRef]
- De Lazzari, C.; Saltarocchi, S.; Genuini, I.; Capoccia, M. Clinical Applications. In: Concept, Mathematical Modelling and Applications in Heart Failure. Capoccia. M. and De Lazzari C. Eds., 2019, 337-366, Published by Nova Science Publishers, Inc. New York. ISBN 978-1-53614-771-1.
- De Lazzari, C.; Stalteri, D. 2011–2019, CARDIOSIM© Website. Available online: https://cardiosim.dsb.cnr.it/CirculationModels/ncm2 (accessed on 29 May 2022).
- De Lazzari, C. A pumping heart coupled to lumped description of the cardiovascular components. In: Modelling Cardiovascular System and Mechanical Circulatory Support. De Lazzari C. Ed., 2007, 57-76, Published by Consiglio Nazionale delle Ricerche. ISBN 978-88-8080-081-1.
- Schurtz, G.; Rousse, N.; Saura, O.; Balmette, V.; Vincent, F.; Lamblin, N.; Porouchani, S.; Verdier, B.; Puymirat, E.; Robin, E.; et al. IMPELLA® or Extracorporeal Membrane Oxygenation for Left Ventricular Dominant Refractory Cardiogenic Shock. J. Clin. Med., 2021, 10, 759. [CrossRef]
- Kosaraju, A.; Pendela, V.S.; Hai, O. Cardiogenic Shock. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482255/.
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D. et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation, 2017, 136, e232–e268. [CrossRef]
- Sauren, L.D.C.; Accord, R.E.; Hamzeh, K.; de Jong, M.; van der Nagel, T.; van der Veen, F.H.; Maessen, J.G. Combined Impella and Intra-aortic Balloon Pump Support to Improve Both Ventricular Unloading and Coronary Blood Flow for Myocardial Recovery: An Experimental Study. Artif Organs, 2007, 31(11), 839-842. [CrossRef]
- Sterling, L.H.; Fernando, S.M.; Talarico, R.; Qureshi, D.; van Diepen, S.; Herridge, M.S.; Price, S.; Brodie, D.; Fan, E.; Di Santo, P.; Jung, R.G.; Parlow, S.; Basir, M.B.; Scales, D.C.; Combes, A.; Mathew, R.; Thiele, H.; Tanuseputro, P.; Hibbert, B. Long-Term Outcomes of Cardiogenic Shock Complicating Myocardial Infarction. J Am Coll Cardiol, 2023, 82, 985-995.
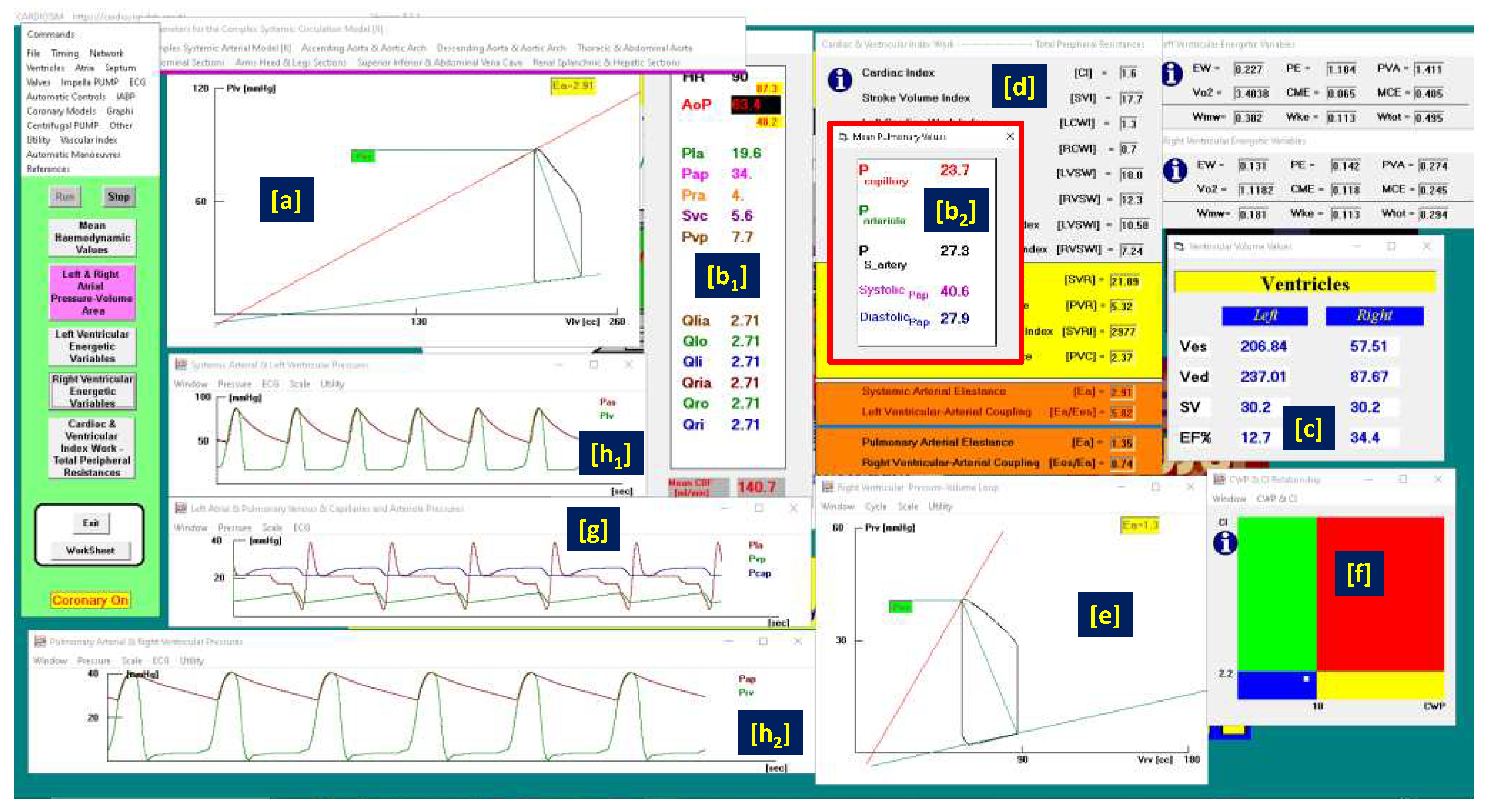
Figure 1.
Virtual CS patient developed according to literature data. Panels [a] and [e] show the left and right ventricular loops respectively; panels [h1], [h2] and [g] show the instantaneous waveforms of aortic (Pas) and left ventricular (Plv) pressures, pulmonary capillary, (Pcap) pulmonary venous (Pvp) and left atrial pressures (Pla) respectively, pulmonary arterial (Pap) and right ventricular pressures (Pra). Panels [b1] and [b2] show the mean values of pressures and flows (AoP≡Pas is the mean aortic pressure, 87.3 is the systolic aortic pressure (SAP) and 48.2 is the diastolic aortic pressure (DAP); CBF is the mean coronary blood flow). Panel [c] shows the left and right ventricular end systolic and end diastolic volume, the stroke volume and the ejection fraction. Finally, panel [f] reproduces the cardiac index (CI) and capillary wedge pressure (CWP) relationship.
Figure 1.
Virtual CS patient developed according to literature data. Panels [a] and [e] show the left and right ventricular loops respectively; panels [h1], [h2] and [g] show the instantaneous waveforms of aortic (Pas) and left ventricular (Plv) pressures, pulmonary capillary, (Pcap) pulmonary venous (Pvp) and left atrial pressures (Pla) respectively, pulmonary arterial (Pap) and right ventricular pressures (Pra). Panels [b1] and [b2] show the mean values of pressures and flows (AoP≡Pas is the mean aortic pressure, 87.3 is the systolic aortic pressure (SAP) and 48.2 is the diastolic aortic pressure (DAP); CBF is the mean coronary blood flow). Panel [c] shows the left and right ventricular end systolic and end diastolic volume, the stroke volume and the ejection fraction. Finally, panel [f] reproduces the cardiac index (CI) and capillary wedge pressure (CWP) relationship.
Figure 2.
Panel A (B) shows the left (right) ventricular pressure-volume loop. Plv≡LVP; Vlv≡LVV (left ventricular volume); Prv≡RVP; Vrv≡RVV (right ventricular volume); Pas≡AoP. Sixteen aortic and left ventricular pressures waveforms are plotted in panel C. In this case, the IABP was driven in 1:4 mode and drug administration was simulated reducing the pulmonary vascular resistances by 10%. In CI-CWP graph the rectangle point moves from G position (IABP off) to F position (IABP on).
Figure 2.
Panel A (B) shows the left (right) ventricular pressure-volume loop. Plv≡LVP; Vlv≡LVV (left ventricular volume); Prv≡RVP; Vrv≡RVV (right ventricular volume); Pas≡AoP. Sixteen aortic and left ventricular pressures waveforms are plotted in panel C. In this case, the IABP was driven in 1:4 mode and drug administration was simulated reducing the pulmonary vascular resistances by 10%. In CI-CWP graph the rectangle point moves from G position (IABP off) to F position (IABP on).
Figure 3.
Data representing the percent changes in relation to virtual pathological (CS) condition. The red columns represent the percent changes when IABP was activated in full mode (1:1). The purple, yellow, light blue and green columns show the percent changes when the effects of the drug administration and IABP (driven in partial mode) are simultaneously simulated. When the IABP operates in 1:2, or 1:3 or 1:4 mode, the values presented in this paper are calculated as average values of twenty-four cardiac cycles, respectively.
Figure 3.
Data representing the percent changes in relation to virtual pathological (CS) condition. The red columns represent the percent changes when IABP was activated in full mode (1:1). The purple, yellow, light blue and green columns show the percent changes when the effects of the drug administration and IABP (driven in partial mode) are simultaneously simulated. When the IABP operates in 1:2, or 1:3 or 1:4 mode, the values presented in this paper are calculated as average values of twenty-four cardiac cycles, respectively.
Figure 4.
The data represent the percent changes in relation to virtual pathological (CS) condition. The red columns represent the percent changes when IABP was activated in full mode (1:1). The purple, yellow, light blue and green columns show the percent changes when the effects of the drug administration and IABP (driven in partial mode) are simultaneously simulated. The percent changes of CO (coronary blood flow) are reported in the left (right) top panel. The bottom left (right) panel shows the mean aortic pressure (left atrial pressure).
Figure 4.
The data represent the percent changes in relation to virtual pathological (CS) condition. The red columns represent the percent changes when IABP was activated in full mode (1:1). The purple, yellow, light blue and green columns show the percent changes when the effects of the drug administration and IABP (driven in partial mode) are simultaneously simulated. The percent changes of CO (coronary blood flow) are reported in the left (right) top panel. The bottom left (right) panel shows the mean aortic pressure (left atrial pressure).
Figure 5.
Data represent the percent changes in relation to virtual pathological (CS) condition. The red columns represent the percent changes when IABP is driven in full mode (1:1). The purple, yellow, light blue and green columns show the percentage changes when the effects of drug administration and IABP support (driven in 1:2, 1:3 and 1:4 mode) are simultaneously simulated. When the IABP operates in 1:2, or 1:3 or 1:4 mode, the values are calculated as average values of twenty-four cardiac cycles, respectively.
Figure 5.
Data represent the percent changes in relation to virtual pathological (CS) condition. The red columns represent the percent changes when IABP is driven in full mode (1:1). The purple, yellow, light blue and green columns show the percentage changes when the effects of drug administration and IABP support (driven in 1:2, 1:3 and 1:4 mode) are simultaneously simulated. When the IABP operates in 1:2, or 1:3 or 1:4 mode, the values are calculated as average values of twenty-four cardiac cycles, respectively.
Figure 6.
Effects induced by simultaneous application of IABP driven in full mode (1:1) and Impella 2.5 (driven with rotational speed set to 35000, 40000 and 50000 rpm) on left atrial pressure volume loop area (top left panel), left atrial pressure (middle top panel), superior vena cava flow (bottom left panel) coronary blood flow (middle bottom panel) and brain flow (bottom right panel). The effects on total flow (i.e. cardiac natural flow pus Impella flow) are represented in the top right panel.
Figure 6.
Effects induced by simultaneous application of IABP driven in full mode (1:1) and Impella 2.5 (driven with rotational speed set to 35000, 40000 and 50000 rpm) on left atrial pressure volume loop area (top left panel), left atrial pressure (middle top panel), superior vena cava flow (bottom left panel) coronary blood flow (middle bottom panel) and brain flow (bottom right panel). The effects on total flow (i.e. cardiac natural flow pus Impella flow) are represented in the top right panel.
Figure 7.
Panel A (B) shows the left (right) ventricular pressure-volume loop. Plv≡LVP; Vlv≡LVV (left ventricular volume); Prv≡RVP; Vrv≡RVV (right ventricular volume); Pas≡AoP. Sixteen aortic and left ventricular pressures waveforms are plotted in panel C. In this case, both IABP driven in 1:4 mode and Impella 2.5 with 40000 rpm rotational speed were applied simultaneously. The rectangle point moves from E position (IABP off) to D position (IABP on) in the CI-CWP graph.
Figure 7.
Panel A (B) shows the left (right) ventricular pressure-volume loop. Plv≡LVP; Vlv≡LVV (left ventricular volume); Prv≡RVP; Vrv≡RVV (right ventricular volume); Pas≡AoP. Sixteen aortic and left ventricular pressures waveforms are plotted in panel C. In this case, both IABP driven in 1:4 mode and Impella 2.5 with 40000 rpm rotational speed were applied simultaneously. The rectangle point moves from E position (IABP off) to D position (IABP on) in the CI-CWP graph.
Figure 8.
White, yellow and green bars representing the effect induced by IABP driven in 1:2, 1:3 and 1:4, respectively, when drug administration has been simulated is available in the left and middle top panel graphs. Data represent the percent changes in relation to virtual pathological (CS) condition. The bars with vertical (horizontal) lines indicate the percentage change of a variable when the LVAD rotational speed was set to 35000 (40000) rpm, while the bars with oblique lines were obtained for a rotational speed of 50000 rpm. The simulations were performed setting the IABP in 1:2 (white bars with red lines), 1:3 (yellow bars with red lines) and 1:4 (green bars with black lines) mode. .
Figure 8.
White, yellow and green bars representing the effect induced by IABP driven in 1:2, 1:3 and 1:4, respectively, when drug administration has been simulated is available in the left and middle top panel graphs. Data represent the percent changes in relation to virtual pathological (CS) condition. The bars with vertical (horizontal) lines indicate the percentage change of a variable when the LVAD rotational speed was set to 35000 (40000) rpm, while the bars with oblique lines were obtained for a rotational speed of 50000 rpm. The simulations were performed setting the IABP in 1:2 (white bars with red lines), 1:3 (yellow bars with red lines) and 1:4 (green bars with black lines) mode. .
Figure 9.
The left (top middle) panel shows left (right) pressure volume area; systolic aortic pressure, systemic arterial elastance and pulmonary arterial pressure are available in the top left, bottom middle and bottom right panel, respectively. The bars with vertical (horizontal) lines indicate the percentage change of a variable when the LVAD rotational speed was set to 35000 (40000) rpm, while the bars with oblique lines were obtained for a rotational speed of 50000 rpm. The simulation was performed setting the IABP in 1:2 (white bars with red lines), 1:3 (yellow bars with red lines) and 1:4 (green bars with black lines) mode.
Figure 9.
The left (top middle) panel shows left (right) pressure volume area; systolic aortic pressure, systemic arterial elastance and pulmonary arterial pressure are available in the top left, bottom middle and bottom right panel, respectively. The bars with vertical (horizontal) lines indicate the percentage change of a variable when the LVAD rotational speed was set to 35000 (40000) rpm, while the bars with oblique lines were obtained for a rotational speed of 50000 rpm. The simulation was performed setting the IABP in 1:2 (white bars with red lines), 1:3 (yellow bars with red lines) and 1:4 (green bars with black lines) mode.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).