1. Introduction
The high-energy pulsed impact of explosive decomposition products of energetic materials (EM) upon different environmental objects is the basis of many modern industrial technologies. In this regard, creating new technologies related to the controlled impact of pulsed action on materials is an important promising direction in mining, metallurgical engineering, oil-extracting industries, construction, and mechanical engineering. These methods can be used in the explosive cutting of reinforced concrete structures, crushing and loosening of rocks, special processing of slabs and non-metallic materials, cleaning surfaces, containers, and holes from ice and metal, and compacting hard-to-press powders [
1].
It should be taken into consideration that in actual practice, the safe use of energetic materials (EMs) can only be partially guaranteed after some time. Thus, it is impossible to eliminate the risk of unpredictable explosive decomposition of EMs due to the influence of random external factors [
2]. The transition from electrical means of initiation to laser ones is a promising solution to these problems. So, the use of laser detonators eliminates the necessity to connect electrical wires to the initiation means, making the laser detonators (LDs) immune to such threats as electrostatic discharge, stray currents, or lightning. The use of fiber optic communication lines between the laser source and the LDs also increases the safety of blasting compared in comparison with traditional electrical blasting methods [
3,
4]. Another significant advantage of LDs is the possibility of replacing toxic, highly sensitive to mechanical impacts primary explosives in the blasting means with less toxic and less sensitive EMs, for example, photosensitive pyrotechnic compositions [
5]. Nowadays, it is clear that the best performance parameters are shown by LDs based not just on light-sensitive pyrotechnic compositions but also on their composites with nanocarbons [
6]. At the same time, graphene is increasingly used as a nanocarbon component of the EMs [for example, work [
7] and references therein]. The choice of graphene as a charge component is based on its inherent high electrical and thermal conductivity as a low-defect crystal. Currently, the choice of graphene is also associated with its ability to form a flow of electrons under the influence of an external photon stream [
8]. Accordingly, the use of graphene as a component of the laser devices can provide a synergistic effect of the influence of the thermal energy flow and the laser photon stream of the EMs [
9,
10].
So, due to its unique properties, graphene has become the most studied allotropic form of nanocarbon in the last decade. However, it should be noted that although the first work publication devoted to the production of graphene 2D structures goes back to 1958 [
11] - that is, long before the discovery of other allotropic forms of nanocarbon - graphene did not attract any significant attention to researchers—only the pioneer work of A. Geim and K. Novoselov awarded the Nobel Prize for the isolation and study of the properties of graphene1 obtained by mechanical exfoliation of graphite, which gave rise to numerous studies devoted to both the development of new synthetic methods and the search for areas of application of graphene [
12,
13,
14,
15,
16,
17,
18,
19]. The almost exponential annual growth of publications devoted to the search for new applications of graphene is mainly because, nowadays, the technique of mechanical splitting of graphite has been significantly improved [
20]. Note that, according to the IUPAC definition, the term graphene refers to a single sheet of graphite one atom thick. Two or more layers of graphene are referred to as multilayer graphene (few-layer graphene, graphene nanoplatelets)
To expand laboratory experiments to practical investigation in blasting technologies, the possibility of its large-scale production should be found. Methods of liquid exfoliation [
21], ion intercalation and exfoliation [
22], chemical vapor deposition (CVD) [
23], and water-chemical synthesis [
24] of graphene have been developed. They are used in practice, making it possible to produce graphene in amounts sufficient for interlaboratory research. However, the performance of these methods is insufficient to meet the needs of real materials science that, for example, prevents the possibility of using graphene in such a promising area of its application as photovoltaics [
25,
26].
It should be noted that modern stringent environmental requirements are an important factor determining the prospects for scaling up both known and newly developed methods of graphene synthesis.
Choice of synthesis technique. When developing graphene synthesis methods, researchers have to choose between techniques producing classical graphene that are impossible to provide the required level of performance and more productive techniques that lead to graphene line-organized 2D structures, minding the requirements of a specific task. In particular, as in this work, the task of laser detonator creation.
Therefore, many research groups are constantly looking for new possibilities for the synthesis of graphene-like structures, based both on different precursors and on new synthesis techniques.
Previously, we proposed the method for the synthesis of 2D graphene structures by carbonization of biopolymers under the conditions of the process of self-propagating high-temperature synthesis (SHS) that meets the given above requirements. The term SHS refers to moving a spin wave of a strong exothermic reaction through a mixture of reagents (oxidizing agent and reducing agent), where the heat release is located in the thin layer and is transferred from layer to layer by heat transfer. In the SHS process, branched-chain ignition, in contrast to thermal ignition, is caused by an avalanche multiplication of active intermediate products - free atoms, radicals, and excited particles - in their rapid reactions with the initial reagents and among themselves [
18].
While choosing a carbonization technique, we took into consideration the fact that the advantage in comparison with the processes of pyrolysis and hydrothermal carbonization traditionally used for the carbonization of biopolymers [
27] is the simplicity of the instrumentation of the SHS method. Other advantages of SHS methods are high synthesis rates, the possibility of carrying out the process without a constant supply of energy from external power sources, the possibility of synthesizing in any atmosphere or vacuum, and the absence of fundamental scale restrictions [
28,
29]. It should be noted that the synthesis of 2D nanocarbons under the conditions of the SHS process proceeds according to the “bottom-up” mechanism. The stages of the “bottom-up” mechanism include successive processes of thermolysis of the biopolymer to some primitives and the processes of their self-organization into 2D structures. However, the 2D nano carbons (G
svs) obtained in the SHS process do not correspond in their morphometric parameters to the graphene samples obtained using known methods, and so does not allow us to recommend this method as an alternative in EM charges.
T The purpose of this work was to develop a new method of complete 2D graphene structure synthesis and demonstrate the possibility of its application as a component of initiating energetic compositions with low laser ignition thresholds.
2. Experimental Part
2.1. Materials
Precursor. Lignin, a complex, irregularly structured, resistant to decomposition, insoluble in water and organic solvents, high molecular weight polymer with branched macromolecules of a cyclic structure, was used as a precursor [
30]. Lignin used in the experiments was obtained from long-term storage dumps under atmospheric conditions from the Arkhangelsk hydrolysis plant. The choice of lignin as a precursor of graphene-like material is explained by its reserves accumulated at storage sites in the amount of millions of tons and pose a severe environmental threat [
31]. In this regard, the choice of lignin as a precursor to graphene structures was also due to the desire to develop a method for its utilization to have some benefit.
2.2. Synthesis of Few-Layer Graphene (FLG)
The synthesis of 2D graphene structures was carried out by carbonization of lignin under the process of self-propagating high-temperature synthesis (SHS) [
11]. The method of obtaining FLG was carried out using a laboratory reactor, which is a quartz vessel (capacity 1 l) with a heating element in the lower part, which provides initial heating of the reaction zone to the temperature required to initiate the process (220 0C). The temperature in the reaction zone was controlled using a thermocouple. The preparation of the synthesis included a number of successive stages. The pre-dried lignin was transferred to a ball mill and grounded for 15 minutes. Next, a mechanical mixture of an oxidizing agent (ammonium nitrate NH4NO3, chemically pure, Sigma-Aldrich, St. Louis, USA) and a precursor (lignin) was prepared in a 1:1 weight ratio. The prepared mechanical mixture of oxidizer and precursor was placed in a “drunk barrel” type mixer and stirred for 15 minutes. Then, the resulting crushed mixture was transferred to a reactor preheated and purged with a current of dry argon. The start and end of the reaction were recorded by the start and end of the release of gaseous reaction products. The duration of the process was equal to 5-8 minutes. The yield of the carbonization product (based on the precursor) is 30-35% wt. The rest of the reaction mass passes into the gas phase and is captured in a trap cooled with liquid nitrogen.
2.3. Methods Used for Studying the Resulting Carbonized Structures
XRD diffraction. The phase composition of synthesized samples was studied using diffraction patterns recorded on an XRD-7000 diffractometer (CuKα radiation, λ = 0.154051 nm) (Shimadzu, Japan).
Raman spectroscopy. The quality of synthesized samples was estimated using Raman spectra recorded on a Horiba Jobin Yvon LabRam HR 800 spectrometer (532 nm laser; 1800 g/mm diffraction grating; micro-Raman system, microscopic objective, optical magnification ×20).
SEM and TEM. The synthesized images were obtained by scanning electron microscopy on a TESCAN Mira-3M microscope with an Oxford Instruments X-max EDX accessory and by transmission electron microscopy on 828 a 50 kV FEI Tecnai G2 30 S-TWIN microscope. In the TEM study, the powder samples were placed in ethanol, sonicated for 5 min, and mounted on a carbon grid. The obtained ring XRD patterns were identified using the software implemented in the Rigaku Ultima-IV diffractometer. XRD diffraction. The phase composition of synthesized samples was studied using diffraction patterns recorded on an XRD-7000 diffractometer (CuKα radiation, λ = 0.154051 nm) (Shimadzu, Japan).
2.4. Specific Surface Area Determination
The specific surface area of synthesized samples was determined by polymolecular adsorption (BET method) using an ASAP 2020 Accelerated Surface Area and Porosimeter analyzer (United States). Nitrogen was used as an adsorbate gas. Prior to measurement, heating in a vacuum at a temperature of 300°C for 3 h was used as a standard sample preparation method. The measurement error was no more than 3%.
The true density of FLG powder particles was determined by helium pycnometry using an Ultrapycnometer 1000 device (Quantachrome instruments, USA).
2.5. Production of Pyrotechnic Compositions
A promising composition based on porous silicon was used as the base pyrotechnic composition [
32], according to the method described in the article [
33]. Nanoporous silicon (por-Si) with a porosity of 70–80% and an average pore size of 12–15 nm was obtained. In this work, we used nanoporous silicon powder with a particle size in the range of 30 – 40 μm.
2.6. Samples of Charge of Pyrotechnic Composition (PC) Based on por-Si Powder and Calcium Perchlorate Ca(ClO4)2
Basic composition. 18 mg of por-Si powder was pressed into a metal cap of 4 mm high with an internal diameter of 4 mm and a hole in the bottom 3 mm in diameter under a pressure of 80 MPa. The pressed por-Si powder was impregnated with a solution of Ca(ClO4)2 in methanol so that after evaporation of methanol, the mass ratio between Ca(ClO4)2 and por-Si powder was equal to 1:1. The height of the charge in the cap was equal to 3 mm. Then, the caps were placed in a thermostat under the temperature of 80 0C and dried for 15 minutes until the complete evaporation of the remaining solvent. The process was checked by weighing control of the caps (their mass became constant).
Basic composition modified with graphene particles. Powders of por-Si and multilayer graphene sheets were mixed in a ball mill in a ratio (por-Si:FLG) of 1:0.5 or 1:1 for 1 hour until a total homogeneous mixture was obtained. T Then, from the resulting mixture, PC samples were prepared: 18 mg of the mixture was pressed into 4x4 mm metal caps under a pressure of 80 MPa. The pressed powder of the mixture was impregnated with a solution of Ca(ClO4)2 so that after the evaporation of methanol, the mass ratio between Ca(ClO4)2 and por-Si powder was at the level of 1:1. Thus, the following PC was produced. We obtained two PC with the resulting ratios between the components [por-Si: Ca(ClO4)2:FLG] 1:1:1 (PC-1) or 1:1:0.5 (PC-2).
Testing of pyrotechnic compositions. The susceptibility of PC to laser radiation was studied with the help of a semiconductor laser diode of the Focuslight ECSE01-08-976 type (USA) with a wavelength of 976 nm (infrared radiation) and an output power of up to 8 W. Under test conditions, charges of the PC were exposed to laser radiation with constant flux q ≈ 15 MW/m2. A Tektronix TDS 2014 oscilloscope was used to record the burning time of a pressed PC placed in metal caps. The first signal was detected by the oscilloscope at the moment the pulse was applied to the Focuslight FL-FCSE01-8-976 laser diode, and the second signal was fixed by the oscilloscope from the FD-256 photosensor at the moment the combustion front reached the free surface of the composition. The main camera of the Honor 10 smartphone (16 MP, aperture f/1.8), frame rate 720 fps (or 480 fps) recorded the process of PC burning after laser ignition.
3. Results and Their Discussion
The lignin carbonization product turned out to be a highly dispersed black powder. To confirm the stated model ideas for the carbonized product to have a graphene-like structure, a set of complementary research methods such as - electron microscopy, Raman spectroscopy, and X-ray diffraction was used.
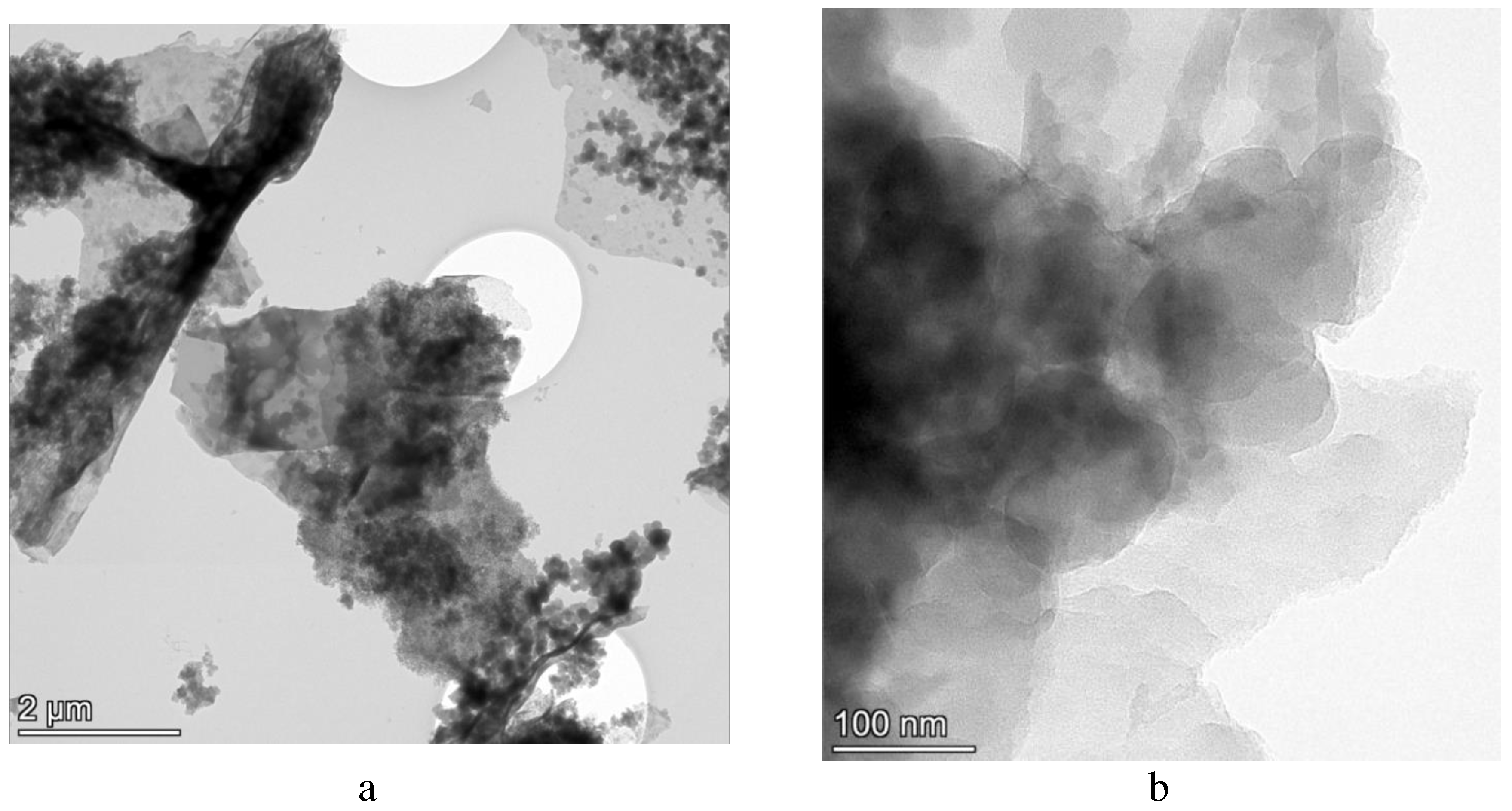
Electron microscopy. Electron micrographs of carbonized lignin are presented in
Figure 1. From
Figure 1, it can be concluded that the resulting particles have a volumetric-planar “scaly” form. These particles are typical for 2D carbon structures see, for example, [
34]. Attention should be paid to the electron microscopy method as the most visual way to determine the morphology of the resulting carbonized product.
Spectroscopic studies should confirm the fine structure of the product. To clarify the nature of the obtained product, spectroscopic studies were carried out.
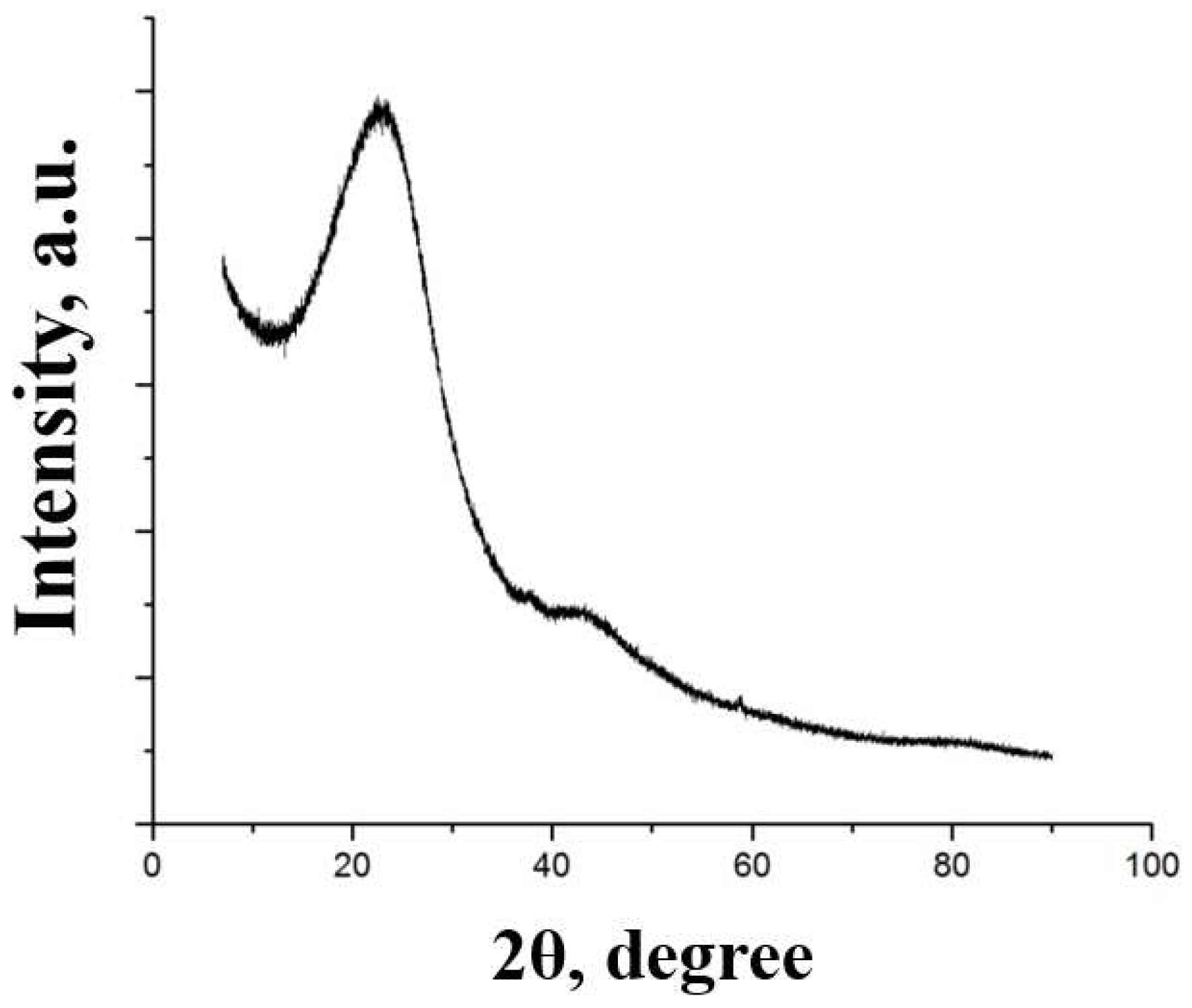
X-ray. The diffraction pattern of the carbonized product is shown in
Figure 2. A narrow, intense peak is observed in the diffraction patterns of graphite at 26.50. A weak, diffuse peak is typical for 2D carbon structures.
The blurring of the XRD peak indicates that the particles are formed by a stack of several graphene sheets [
35].
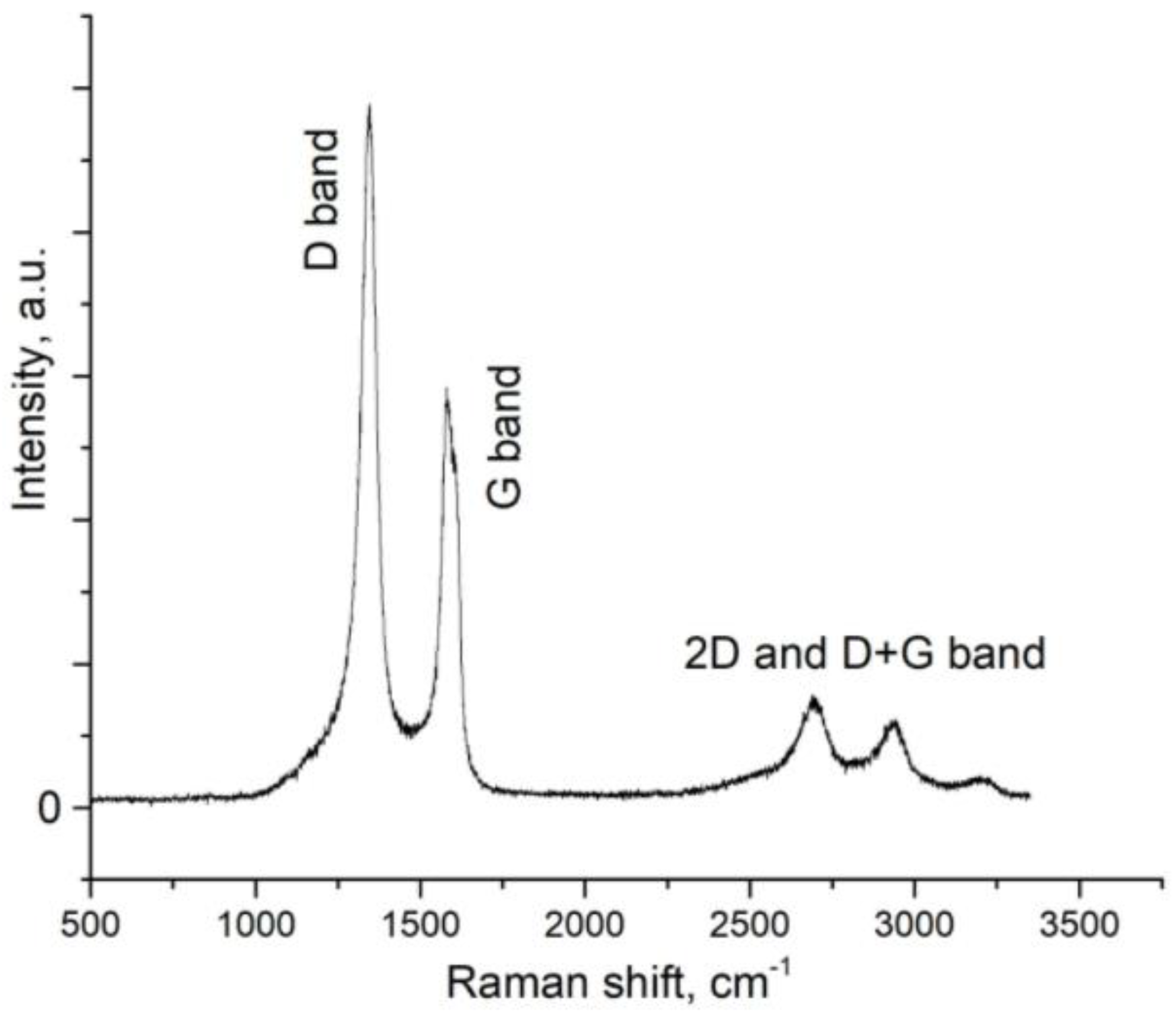
Raman spectroscopy. Raman spectroscopy is currently an effective way to determine the number of layers of 2D graphene structures without destroying their crystal lattice. In addition, the shape and position of the 2D peak allovers to clearly distinguish single-layer, bilayer, and multilayer graphene. Single-layer graphene usually exhibits a single and sharp 2D peak below 2700 cm-1, while bilayer graphene exhibits a broader and elevated 2D peak at 2700 cm-1. In the case of graphene sheets with more than five layers, we see broad 2D peaks that are shifted to positions 2700 + (20 ÷ 40) cm-1 and are similar to the 2D peaks of bulk graphite.
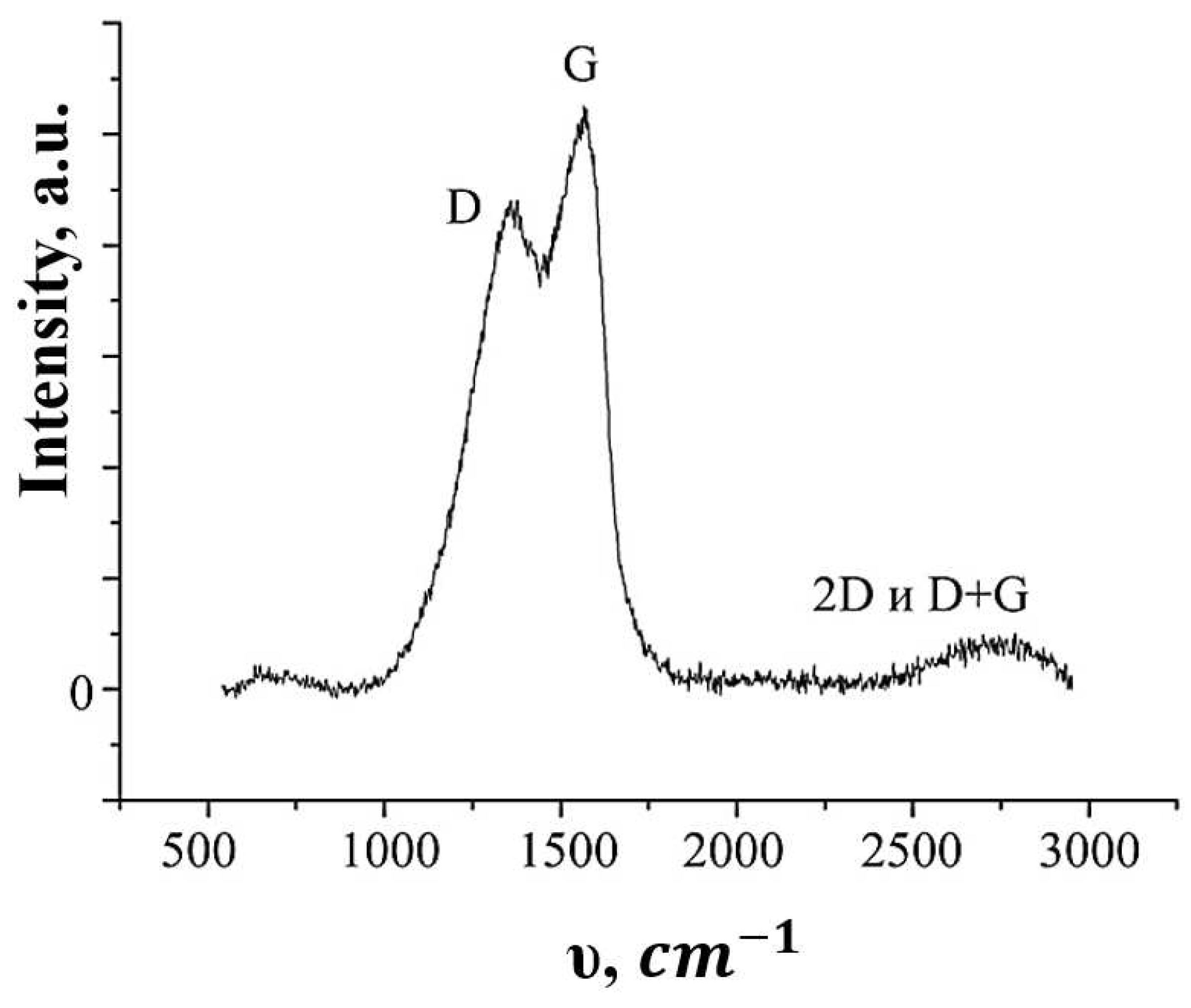
Raman spectroscopy data are shown in
Figure 3. The Raman spectrum has two distinct peaks - at 1300 cm
-1 and 1590 cm
-1. The first peak reflects defects in the sp
2 lattice (peak D), and the other - corresponds to hybridized carbon sp
2 (peak G). The relationship between D and G is the characteristics of each specific sample of graphene structures, determined both by the nature of the precursor and the method of the synthesis.
The true particle density of the carbonated product. Additionally, such an important characteristic of the carbonated product as its true density was determined. The measured value of the true density of powder particles turned out to be equal to ρ=1.98±0.02 g/cm
3 and satisfactorily coincided with the literature data for the true density of low-defect particles of few-layered graphene (ρ=2.21 g/cm
3) [
36].
Summarizing the data of complementary electron microscopy methods and spectrometric analysis methods, namely: the presence of a wide diffuse maximum around the value of 2θ = 26.5° in the diffraction pattern and the presence of three characteristic lines (peaks) in the Raman scattering spectrum of carbonized lignin samples, as well as data for the true density allows us to compare the obtained carbonized samples with samples few-layer graphene described in literature [
37,
38,
39]. Taking into account of the method, we denote it as FLG
svs.
4. Application of 2D Graphene Structures to Reduce the Ignition Threshold of Pyrotechnic Compositions
A pyrotechnic composition based on porous silicon and calcium perchlorate can be a likely replacement for traditional primary explosives (for example, lead azide) [
40]. The advantage of this composition over traditional primary EMs is the absence of heavy and toxic metals in the composition. Previously, similar pyrotechnic compositions showed fairly high sensitivity to various electrophysical pulses (such as a high-current electron beam of nanosecond duration, a high-voltage discharge, an electrical explosion of a semiconductor bridge) [
41,
42], and thermal effects [
43]. However, their susceptibility to coherent radiation remained unexplored.
We started the work by studying the fundamental possibility of using PS in laser means of ignition and initiation. At first, the susceptibility to radiation of a laser diode of a two-component pyrotechnic composition based on nanopore-Si powder and calcium perchlorate Ca(ClO
4)
2 was studied. During the experiment, five tests were carried out. In all cases, no ignition of charges was observed. It is known that graphene and its derivatives sensitize EM of various natures to coherent radiation. For example, when multilayer graphene was added, the threshold for ignition of a high-energy cobalt salt by a laser diode beam sharply decreased [
44]. The work [
45] proposed the use of graphene as a promising alloying component of solid rocket powders and primary and secondary explosives. The use of multilayer graphene as a promising alloying component of photosensitive PC based on fluorine rubber was demonstrated in [
46]. Based on the presented data, we started to study the sensitivity of the PC por-Si+Ca(ClO4)2 sensitized with graphene FLGsvs to infrared radiation of laser diode.
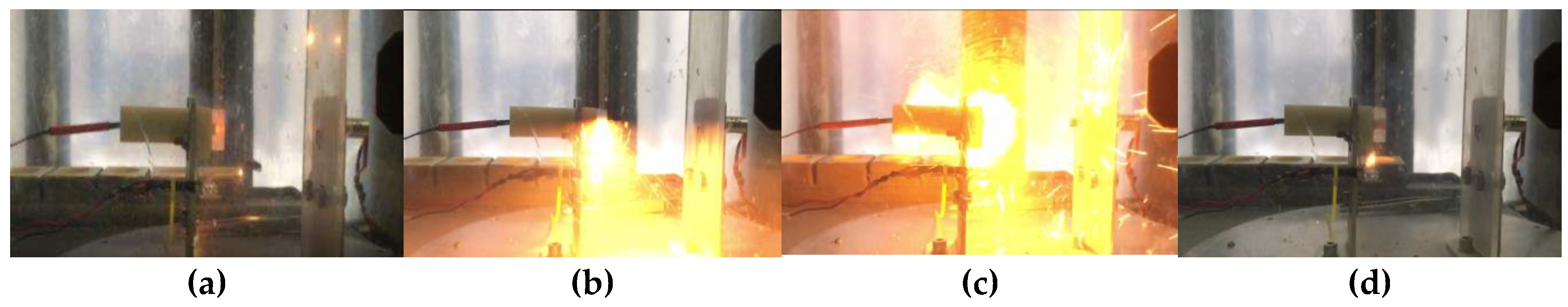
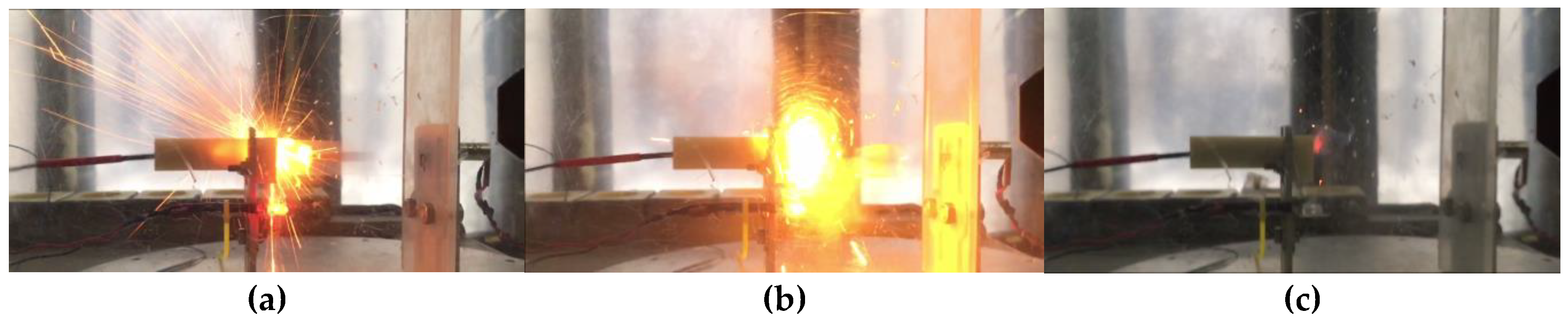
Two types of charges were prepared (see experimental part). Three charges of each type were examined while testing the samples; in all six cases, an explosive transformation (ignition with a strong sound effect) was observed. This effect can be interpreted as explosive combustion. The sequence of processes of explosive transformation of charges PC-1 and PC-2 are shown in
Figure 4 and
Figure 5.
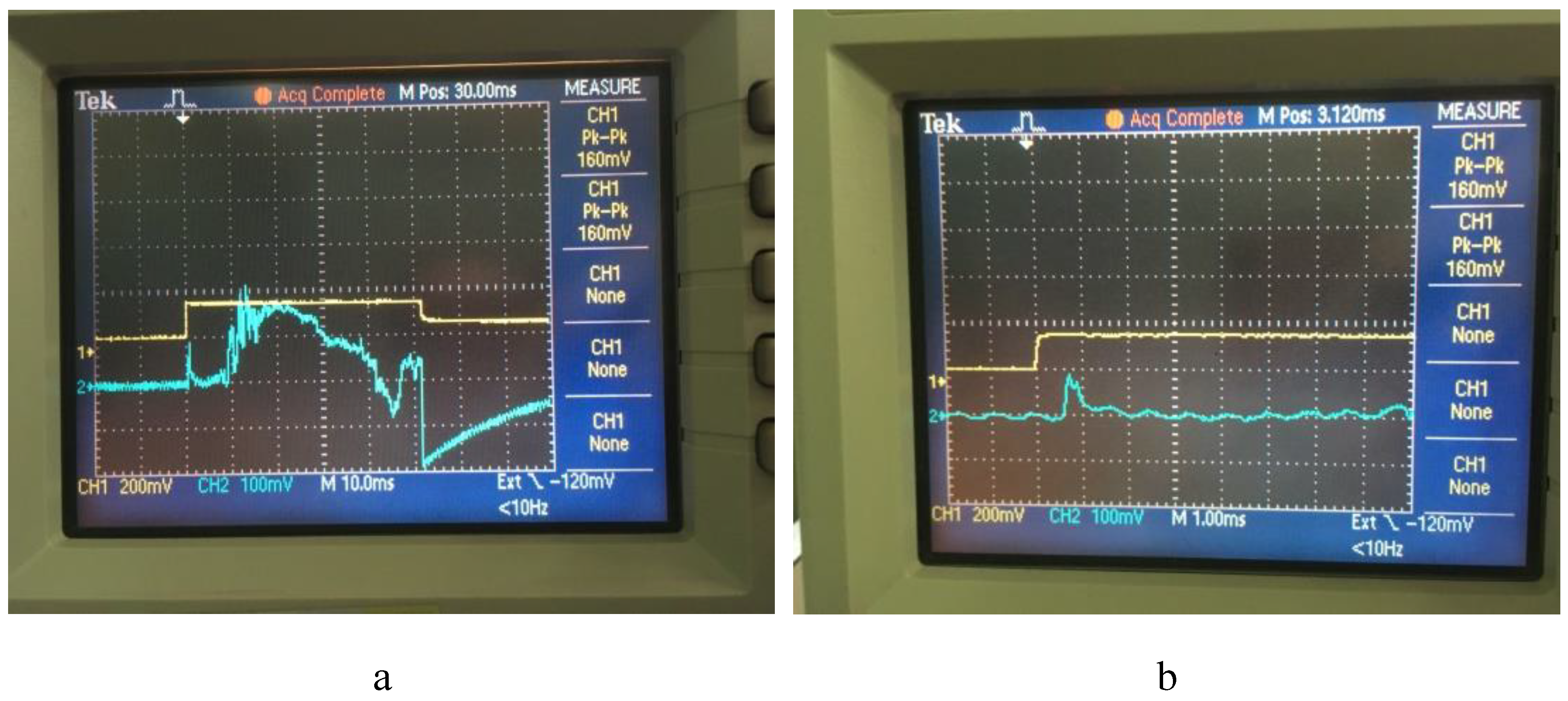
Oscillograms of the deflagration of compositions PC-1 and PC-2 showed that their combustion rates differ. So, under experimental conditions, the PC-1 composition burns out in ~ 10 ms (
Figure 6a), and the PC-2 composition burns faster in ~ 750 μs (
Figure 6b).
Thus, the addition of graphene-like material powder to pressed samples of PС-1 and PС-2 increases the likelihood of their ignition by laser diode (infrared) radiation in comparison with the original PС. Consequently, the addition of graphene-like powder FLGsvs increases the ignition ability of PС based on nanoporous silicon to laser diode (infrared) radiation. Moreover, if in the case of classical explosive compositions initiated with a laser beam, the share of the absorbing additive does not exceed (3 – 5)% (mass) (the amount of the sensitizing additive over 5% (mass) leads to failures during laser excitation), in the case of PC the share of the sensitizing additive of FLGsvs can be (20 – 33)% (mass.).
5. Model Representations of the Mechanism of Formation of 2D Graphene Structures under the Conditions of the SHS Process
Analyzing the experimental results, we tried to provide some considerations on the mechanism of carbonization of organic compounds with the formation of all nootropic forms of nanocarbon. As comes from the analysis of the literature, the first real example of obtaining an allotropic form of nanocarbon by carbonization of organic molecules is the synthesis of 3D nanocarbon (detonation nanodiamonds, DND) as the result of the explosive decomposition of the mixture of two organic molecules – trinitrotoluene and cyclotrimethylenetrinitramine (RDX) [
47]. The simplest idea of the mechanism of carbonization of organic compounds in the process of detonation synthesis is the idea of the mechanism of their carbonization through breaking the covalent bonds of the atoms of the original molecules up to their complete atomization [
48]. However, the activation energy of such reactions is very high and cannot be provided under the conditions of the detonation synthesis process [
49]. Consequently, even under conditions of high pressure and temperature during the explosive decomposition of trinitrotoluene and cyclotrimethylenetrinitramine, the destruction of their molecules goes up only some primitives - carbon structures, which in subsequent self-organization processes form 3D nanocarbon particles (DND). Several studies have shown that the specific morphometric parameters of DND particles depend not only on the conditions of detonation synthesis but also on the nature of the precursor (original explosive) [
50]. It should be noted that, despite the large number of works, the reliable structure of the resulting primitives is unknown and, until now, has been based only on phenomenological models.
Creating a model for the formation of allotropic forms of nanocarbon within the framework of the carbonization mechanism of organic molecules, we should proceed from the model developed by N.N. Semenov [
51]. According to this theory, the probable mechanism of destruction of materials is the mechanism associated with the formation of chemical radicals that, in turn, include multiple successive shear movements of atoms. Thus, covalent bond breaking is not a result but a consequence of the synergistic effect of elementary acts with lower activation energy. As a result, the energy of covalent bonds breaking is reduced, leading to their breaking. Minding such a model of material destruction, it can be assumed that the amount of energy supplied to the system plays a key role in determining the structure of final nanocarbons during the carbonization of organic substances.
To test the proposed model, we carried out detonation synthesis under conditions corresponding to the production of DND [
52] but excluding hexogen from the charge composition, using only trinitrotoluene as a nanocarbon precursor. The exclusion of hexogen from the composition of the charge results in a reduction in the energy of its explosive decomposition [
53]. To characterize the carbonized product synthesized under conditions of energy-deficient detonation synthesis, we used a set of complementary research methods already used in the work.
A comparison of the data in
Figure 1 and
Figure 3 with the data in
Figure 7 and
Figure 8 allows us to conclude that energy-deficient detonation synthesis does not lead to the formation of 3D nanocarbon but results in the formation of 2D graphene structures. It should be noted that although both SHS synthesis and energy-deficient detonation synthesis lead to the formation of 2D graphene structures, the particles of synthesized nanocarbons differ in their morphometric parameters. A rigorous comparison of morphometric parameters of synthesized particles with the help of different methods from various precursors of 2D graphene structures lies beyond the scope of this work and will be analyzed in future publications.
But based on our experimental results, we are able to make some following conclusions. Mechanisms of detonation and SHS syntheses have much in common, and the formation of nanoparticles, in the general case, is the combination of a high rate formation of initial primitives with their low rate of growth. The formation of nanocarbon particles is determined by the rate of diffusion of quantum primitives to the embryo of nanoparticles through their movement over a considerably t large distance. The size of a growing particle is proportional to the square root of the particle growth time [
11]. The growth of particles is limited by interfacial turbulence, being the result of unequal media density, a gradient of concentrations, and temperatures.
The difference in the mechanisms of detonation synthesis of 3D nanocarbons (DND) and SHS synthesis of 2D graphene structures, in the most general case, is the result of different amounts of supplied energy.
6. Conclusions
Using the lignin carbonation method under the conditions of a self-propagating high-temperature synthesis process, 2D nanocarbon corresponding to FLG in its morphometric parameters was obtained.
The resulting FLG was used as a component of a pyrotechnic composition based on porous silicon.
The addition of FLG powder to pressed samples of PС-1 [por-Si: Ca(ClO4)2:FLG) 1:1:1] and PС-2 [por-Si: Ca(ClO4)2:FLG) 1:1:0.5] increases the likelihood of their ignition with laser diode (infrared) radiation compared to the original PС [por-Si + Ca(ClO4)2].
The addition of 20–33% (mass.) graphene-like powder material FLGsvs to the PС leads to a sharp increase in sensitivity to infrared laser radiation and the excitation of explosive transformations in the PС.
A phenomenological model of organic compound carbonization with the formation of allotropic forms of carbon under the conditions of comparable carbonization mechanisms (SHS and detonation synthesis) is proposed.
Author Contributions
Conceptualization, A.P.V. and M.A.I.; methodology, A.P.V., A.A.V. and M.A.I.; validation, I.V.S. and G.G.S.; investigation, A.P.V., G.G.S. and A.A.V.; resources, A.P.V. and A.A.V.; data curation, A.P.V.; writing—original draft preparation, A.A.V., A.P.V., G.G.S. and M.A.I.; writing—review and editing, A.A.V., A.P.V., I.V.S. and M.A.I.; visualization, A.A.V.; supervision, M.A.I.; project administration, M.A.I.; funding acquisition, A.A.V. and M.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
The work of Vozniakovskii A.A. was supported by RFBR and BRFBR, project number 20-53-04026.
Conflicts of Interest
The authors declare that there are no conflicts of interest requiring disclosure in this article.
References
- Selivanov V.V., Kobylkin I. F., Novikov S. A. Explosive technologies: textbook for universities / 2nd ed., revised and additional. Moscow: Publishing house of MSTU im. N. E. Bauman. 2014. (In Russian).
- Kotomin A.A., Ilyushin M.A., Dushenok S.A. Methods for calculating of characteristics of energetic substances of various chemical structure. Mauritius: LAP LAMBERT Academic Pablishing. 2020.
- Ahmad S.R., Cartwright M. Laser Ignition of Energetic Materials. Chichester (GB): John Wiley & Sons, 2015.
- Ilyushin M.A., Shugalei I.V. Eco-friendly Energetic Substances for Initiation Devices. doi.org/10.1007/978-3-319-68255-6_46/ In “Handbook of Ecomaterials”/ Eds. L. M. T. Martínez, O.V. Kharissova, B.I. Kharisov. Cham (Switzerland): Springer Nature Switzerland AG. 2019. P. 3433 – 3449. doi.org/10.1007978-3-319-68255-6.
-
Ecotoxicology of Explosives / Eds. G.I. Sunahara, G. Lotufo, R.G. Kuperman, J. Hawari. London / New York: Taylor and Francis Group. 2009.
- Xiaolong Fu, Yonghu Zhu, Jizhen Li, Liping Jiang, Xitong Zhao, Xuezhong Fan. Preparation, Characterization and Application of Nano-Graphene-Based Energetic Materials. Nanomaterials. 2021. 11(9). 2374. [CrossRef]
- Yang Liu, Li-Shuang Hu, Shida Gong, Chunyu Guang, Lianqiang Li, Shuangqi Hu, Peng Deng. Study of Ammonium Perchlorate-based Molecula Perovskite (H2DABCO) [NH4(ClO4)3]/Graphene Energetic Composite with Insensitive Performance. Central European Journal of Energetic Materials. 2020. 17(3). 451-469. [CrossRef]
- Savenkov G.G., Ilyushin M.A. Initiation of explosive transitions in energy–saturated cobalt salt and nanosized carbonic additives compounds by means high-current electron beam // Proceedings of the 19-th Seminar “New trends in research of energetic materials” Pardubice. Czech Republic. April 20-22. 2016. Part 1I. 891-895.
- Savenkov G.G., Morozov V.A., Ilyushin M.A., Oskin I.A., Bragin V.A., Kozlov A.S. Influence of Nanosized Carbon Forms on the Properties and Susceptibility of Energy-Saturated Cobalt Salt to a Pulsed Electron Beam. Technical Physics. 2017. 62(11). 1703-1708. [CrossRef]
- Savenkov G.G., Morozov V. A., Ilyushin M. A., Kats V. M. Graphene As a Sensitizing Additive to High-Energy Cobalt Salt for Enhanced Initiation by a High-Current Electron Beam. Technical Physics Letters. 2018. 44(6). 522-524. [CrossRef]
- Voznyakovskii A. P., Neverovskaya A. Yu., Otvalko Ja. A., Gorelova E. V., Zabelina A. N. Facile synthesis of 2D carbon structures as a filler for polymer composites. Nanosystems: Physics, Chemistry, Mathematics. 2018. 9(1). 125–128. [CrossRef]
- Kuzmenko A. V., Tverjanovich A. S., Ilyushin M. A., Tveryanovich Yu. S. The effect of the concentration of high-absorbing inclusions on the laser initiation threshold of energetic materials: model and experiment. Journal of Energetic Materials. 2019. 37(4). 420-432. [CrossRef]
- Ilyushin M. A., Shugalei I. V. Light Sensitive Energetic Materials and Their Laser Initiation. In Nano and Micro-Scale Energetic Materials: Propellants and Explosives (Editors: W. Pang, L. T. DeLuca). Vol. 2. Part VI: Primary and Secondary Explosives. Chapter 18. Weinheim (FRG): Wiley-VCH. 2023. 541-566. [CrossRef]
- Wilson M. Electrons in Atomically Thin Carbon Sheets Behave Like Massless Particles. Physics Today. 2006. 59(1). 21-23. [CrossRef]
- Katsnelson M. I., Novoselov K. S. Graphene: New bridge between condensed matter physics and quantum electrodynamics. Solid State Communications. 2007. 143(1-2). 3–13. [CrossRef]
- Eletsky A.V., Zitserman V.Yu., Kobzev G.A. Graphene in solar energy. Russian nanotechnologies. 2015. 10(3– 4). 16-25 (In Russian).
- Zhang, X.; Hikal, W.; Zhang, Y.; Bhattcacharia, S.; Li, L.; Wang, S.R.; Weeks, B.L. Direct laser initiation and improved thermal stability of nitrocellulose/graphene oxide nanocomposites. Applied Physics Letters, 2013, 102(14):5428. [CrossRef]
- Liu, C.J.; Li, X.D.; Li, R.; Yang, Q.; Zhang, H.P.; Yang, B.; Yang, G.C.. Laser ignited combustion of graphene oxide/nitrocellulose membrane for solid propellant micro thruster and solar water distillation. Carbon, 2020, 166(1). 138-147. [CrossRef]
- Li, X.D.; Huang, B.; Li, R.; Zhang, H.P.; Qin, W.Z.; Qiao Z.Q.; Liu, Y.S.; Yang, G.C.. Laser-Ignited Relay-Domino-Like Reactions in Graphene Oxide/CL-20 Films for High - Temperature Pulse Preparation of Bi- Layered Photothermal Membranes. Small. 2019. 15(20):1900338. [CrossRef]
- Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.; Grigorieva, I. V.; Firsov, A. A. Electric Field Effect in Atomically Thin Carbon Films, Science. 2004. 306(5696). 666–669.
- Paton, K. R.; Varrla, E.; Backes, C.; Smith, R. J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O. M.; King, P.; et al. Scalable Production of Large Quantities of Defect-Free Few-Layer Graphene by Shear Exfoliation in Liquids. Nature Materials. 2014. 13(6). 624–630. [CrossRef]
- Parvez, K.; Wu, Z.-S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Müllen, K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014. 136(16). 6083–6091. [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H. R. Roll-to-Roll Production of 30-Inch Graphene Films for Transparent Electrodes. Nature Nanotechnology. 2010, 5(8). 574–578. [CrossRef]
- Choucair, M.; Thordarson, P.; Stride, J. A. Gram-Scale Production of Graphene Based on Solvothermal Synthesis and Sonication. Nature Nanotechnology.2009. 4. 30–33. [CrossRef]
- Chang D. W., Choi H.-J., Filer A., Baek J.-B. Graphene in photovoltaic applications: organic photovoltaic cells (OPVs) and dye-sensitized solar cells (DSSCs) Journal of Materials Chemistry A. 2014,2(31), 12136-12149. [CrossRef]
- Czerniak-Reczulska M., Niedzielska A., Jędrzejczak A. Graphene as a Material for Solar Cells Applications. Advances in Materials Science. 2015. 15(4)/(46). 67-81. [CrossRef]
- Adel M., El-Maghra A. Synthesis of few-layer graphene-like nanosheets from glucose: New facile approach for graphene-like nanosheets large-scale production. Journal of Materials Research. 2016. 31(4). 455-467. [CrossRef]
- Sytschev A. E., Merzhanov A. G. Self-propagating high-temperature synthesis of nanomaterials. Russian Chemical Reviews. 2004. 73(2). 147-159. [CrossRef]
- Rogachev A. S., Mukasin A. S. Combustion for the synthesis of materials: an introduction to structural macrokinetics. Moscow: PHYSMATLIT. 2012.
-
Lignins (Structure, properties and reactions) / Ed. K. V. Sarkanen and K. H. Ludwig; Translation from English by A.V. Obolenskaya. Moscow: Timber industry. 1975. (In Russian).
- Krutov S. M., Voznyakovskii A. P., Gordin A. A., Savkin D. I., Shugalei L V. Environmental Problems of Wood Biomass Processing. Waste Processing Lignin // Russian Journal of General Chemistry. 2015. 85(13). 2898-2907. [CrossRef]
- Hardt A.P. Pyrotechnics. Post Falls. Jdacho (USA): Pyrotechnica Publications. 2001.
- Zegrya G.G., Savenkov G.G., Zegrya A.G., Bragin V.A., Os’kin I.A., Poberezhnaya U.M. Laser Initiation of Energy-Saturated Composites Based on Nanoporous Silicon. Technical Physics. 2020. 65(10). 1636-1642. [CrossRef]
- Zhuangjun Fan, Kai Wang, Tong Wei, Jun Yan, Liping Song, Bo Shao. An environmentally friendly and efficient route for the reduction of graphene oxide by aluminum powder. Carbon. 2010. 48(5). 1670–1692. [CrossRef]
- Cheng I. F., Yuqun Xie, Gonzales R. A, Brejna P. R., Sundararajan J. P., Kengne B. A. F., Aston D. E., McIlroy D. N., Foutch J. D., Griffiths P. R. Synthesis of graphene paper from pyrolyzed asphalt. Carbon. 2011. 49. 2852–2861. [CrossRef]
- Wang, X., Liu, Q.C., Wu, S.Y., Xu, B.X., Xu, H.X. Multilayer polypyrrole nanosheets with self-organized surface structures for flexible and efficient solar-thermal energy conversion. Advanced Materials. 2019. 31(19): e1807716. [CrossRef]
- Katsnelson M. I. Graphene: Carbon in Two Dimensions. Cambridge (GB): Cambridge University Press. 2nd. Ed. 2020.
- Ilkiv B., Petrovska S., Sergiienko R., Tomai T., Shibata E.,Nakamura T., Honma I., Zaulychny Y. X-Ray Emission Spectra of Graphene Nanosheets. Journal of Nanoscience and Nanotechnology. 2012. 12(12). 8913–8919. [CrossRef]
- Johra F. T., Lee J.-W., Jung W.-G. Facile and safe graphene preparation on solution-based platform. Journal of Industrial and Engineering Chemistry. 2014. 20(5). 2883–2887. [CrossRef]
- Bezuidenhout H.C., Mukhopadhyay S. Nanoporous Silicon Based Energetic Formulations for Use in Explosives Initiating System. International Journal of Applied Engineering Research. 2016. 11(21). 10465 – 10471.
- Zegrya G. G., Savenkov G. G., Morozov V. A., Zegrya A. G., Ulin N. V., Ulin V. P., Lukin A. A., Bragin V. A., Oskin I. A., Mikhailov Yu. M. Sensitivity of energy-packed compounds based on superfine and nanoporous silicon to pulsed electrical treatments. Semiconductors. 2017. 51(4). 477–482. [CrossRef]
- Savenkov G. G., Kardo-Sysoev A.F., Zegrya A. G., Oskin I. A., Bragin V. A., Zegrya G. G. Initiation of explosive conversions in energy-saturated nanoporous silicon-based compounds with fast semiconductor switches and energy-releasing elements. Technical Physics Letters. 2017. 43(10). 896–898. [CrossRef]
- Savenkov G.G., Zegrya A.G., Zegrya G.G., Rumyantsev B.V., Sinani A.B., Mikhailov Yu.M. The Possibilities of Energy-Saturated Nanoporous Silicon-Based Composites (Review and New Results). Technical Physics. 2019. 64(3). 361-367. [CrossRef]
- Ilyushin M. A.,Voznyakovskii A. P., Shugalei I. V., Tverjanovich A. S. Laser initiation of modified complex cobalt (III) perchlorate. Zeitschrift für anorganische und allgemeine Chemie. 2021. 647(12). 1254-1260. [CrossRef]
- Qi-Long Yan, Gozin M., Feng-Qi Zhao, Cohen A., Si-Ping Pang. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale . 2016. 8. 4799 - 4851. [CrossRef]
- Poberezhnaya U.M., Freiman V.M., Ilyushin M.A., Zegrya G.G., Fadeev D.V., Os’kin I.A., Morozov V.A., Grigor’ev A.Yu., Savenkov G.G. Optical and electron-beam initiation of porous silicon films with different contents of oxidizer and graphene. Technical Physics. 2022. 67(11). 1469-1474. [CrossRef]
- Volkov K.V., Danilenko V.V., Elin V.I. Synthesis of diamond from the carbon in the detonation products of explosives. Combustion, Explosion and Shock Waves. 1990. 26(3). 366–368. [CrossRef]
- Danilenko V.V. Synthesis and sintering of diamond by explosion. Moscow: Energoatomizdat. 2003. (In Russian).
- Titov V.M., Anisichkin V.F., Mal’kov I.Yu. Synthesis of ultradispersed diamond in detonation waves. Combustion, Explosion and Shock Waves. 1989. 25(3). 372 – 379. [CrossRef]
- Pershin S.V., Petrov E.A., Tsaplin D.I. Influence of the molecular structure of explosives on the rate of formation, yield, and properties of ultradisperse diamond. Combustion, Explosion and Shock Waves. 1994. 30(2). 235–238. [CrossRef]
- Semenov N.N. On some problems of chemical kinetics and reactivity. Moscow: Publishing House of the USSR Academy of Sciences. 1958. (In Russian).
- Voznyakovskii A.P., Dolmatov V.Yu., Shumilov F.A. The Influence of Detonation Synthesis Conditions on Surface Properties of Detonation Nanodiamonds. Journal of Superhard Materials. 2014. 36(3). 165–170. [CrossRef]
- Vereshchagin A. L. Detonation nanodiamonds. Barnaul: ASTU Publishing House. 2001. (In Russian).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).