1. Introduction
Plant biostimulants refer to substances or microorganisms that can stimulate plant nutrient processes independently of the nutrient content of the products. They have the capacity to improve one or more characteristics of plants themselves or the root environment (Rouphael & Colla, 2020). Currently, organic substances constitute a large category of plant biostimulants, and many organic compounds or organic extracts have been reported for having biological stimulating activity, such as humic acid (HA). However, these organic substances are mainly complex mixtures, which poses a challenge for quality control in subsequent production and application processes. Therefore, screening structurally clear compounds that have plant stimulation activity is of great significance for the development, application, and mechanistic study of plant biostimulants.
HA is a type of plant biostimulant that is applied always to soil or plant roots. According to the currently proposed soil humus formation and cycling models, such as "humification", "selective preservation", "progressive decomposition", and "soil continuum model" (Johannes & Markus, 2015), the precursors and decomposition products of HA coexist in soil and may also contribute to HA’s plant biostimulant activities. However, due to the low content of HA and the complexity of the substances in the soil, it is not easy to separate or accurately identify these precursors and decomposition products from soil.
It has been suggested that the soil fauna plays an important role in litter decomposition and humification, and the soil-dwelling insect Protaetia brevitarsis larva (PBL) has been reported to feed on plant litter and form frass with a high HA content and whose 13C NMR spectrum is identical to that of soil HA. Furthermore, the PBL can consume humus substances without soil; thus, using this species can avoid the noise in soil substances from impeding the identification of HA-related compounds, and the PBL may serve as a model to investigate HA formation and cycling. With the aim of screening compounds that can be used as plant biostimulants, in this investigation, we fed PBL fermented corn straw, analysed the HA changes and identified metabolites from the digestive tract contents. Then, the aromatic compounds were selected for further assessment, and the plant biostimulant activity was assessed according to seed germination and growth-promoting effects. This research will not only provide insights into the insect-mediated transformation of HA, but the discovery of structurally clear biostimulant compounds also offers valuable guidance for industrial-scale synthesis of plant biostimulant production.
2. Materials and Methods
2. Preparation of fermented corn straw
The corn straw used in the experiment was collected from Fangshan, Beijing, and crushed to approximately 2 cm. Then, a compost pile with a square bottom of 4 m2 and a height of 70 cm was established to ferment the corn straw with 60% moisture content. Approximately two months later, the temperature of the compost pile dropped, and the fermented corn stalks were air-dried for later use.
2.1. Insects and feeding conditions
The Protaetia brevitarsis (PB) population used in the experiment originated from a wild population in Gongzhuling, Jiling. The 3rd instar larvae were used for the test, the body weight of PBL was between 1.8 g and 2.0 g, and the body length was approximately 3.0 cm. The PBLs were fed in a plastic box and kept in incubators with a relative humidity of 70%, a temperature of 25°C, and a day/night ratio of 16 h:8 h.
2.2. Preparation of samples
Healthy grown 3rd instar larvae were selected and immobilized on ice before dissection. After surface sterilization with sterile water - 70% ethanol - sterile water sequentially, the midgut and hindgut of the larvae were dissected to obtain gut contents for further analysis. To collect the frass, healthy grown PBLs were washed with sterile water and stored in an empty box at 25°C for 2 days, and the frass was collected for further analysis (Xuan et al., 2022).
2.3. Isolation and structural characterization of HA in samples
The improved HA extraction method was used to separate HA components from samples. During the experiment, each sample was suspended in 0.1 M NaOH with a weight-to-volume ratio of 1:10 and oscillated at 220 rpm for 24 h at 37°C. Centrifugation at 8000 rpm separated suspensions of different solubilities. The process was repeated twice, and the supernatant was mixed together. The extracted supernatant was acidified with 3 M HCl to pH 1.0 and stratified at room temperature for 24 h. After centrifugation at 8000 r/min, the precipitate obtained was the HA component. The organic carbon structure of HA is represented by 13C NMR chemical shifts. It was characterized by 13C dipolar, dephasing magic angle spinning nuclear magnetic resonance (13C DD–MAS NMR) analysis. The analysis was carried out by a 600 MHz NMR spectrometer (JNM-ECZ600R, JEOL RESONANCE Inc.) equipped with a 3.2 mm HXMAS probe at 12 kHz MAS. For each sample, the analysis ran for 11 hours, and the relaxation lifetime was 10 seconds. The obtained 13C CP-MAS NMR spectra were then baseline-corrected and integrated into the following chemical shift regions: 0-44 ppm for alkyl carbon; 44-64 ppm for methoxycarbon; 64-93 ppm for alkoxy carbon; 93-142 ppm for aromatic carbon; 142-162 ppm for phenolic carbon; and 162-188 ppm for carboxyl carbon.
2.4. Identification of organic compounds in digestive tract contents
Organic compound extraction: Approximately 30 mg of midgut or hindgut contents was suspended in 600 μl of methyl tertiary butyl ether (MTBE)/methanol/water (v/v/v=5:16:4) solvent containing 3 μg/mL L-norvaline as an internal standard and homogenized in an ice bath by a TissueLyser (JX-24, Jingxin, Shanghai) with zirconia beads at 40 Hz for 3 min. Following centrifugation at 16000 g and 4°C for 15 min, a total of 480 μl of supernatant was collected. Another 360 μl methanol was added to the residue, the extraction process was repeated, and the supernatants from the two extractions were combined (Tian et al., 2020).
Derivatization: One hundred microlitres of combined supernatants and 10 μl of 50 μg/ml L-norleucine were mixed and evaporated to dryness under a nitrogen stream. The residue was reconstituted in 30 μl of 20 mg/mL methoxyamine hydrochloride in pyridine (containing 5 μg/ml of n-alkane standards), and the resulting mixture was incubated at 37°C for 90 min. Then, 30 μl of BSTFA (with 1% TMCS) was added to the mixture and incubated at 70°C for 60 min for further derivatization (Tian et al., 2020).
Gas chromatography‒mass spectrometry (GC‒MS) analysis: Instrumental analysis was performed on an Agilent 7890A/5975C GC‒MS system (Agilent Technologies Inc., CA, USA). An OPTIMA® 5 MS Accent fused-silica capillary column (30 m × 0.25 mm × 0.25 μm; MACHEREY-NAGEL, Düren, GERMAN) was utilized to separate the derivatives (Tian et al., 2020).
2.5. Determination of the seed germination index of intestinal compounds
Based on the possible compounds generated during lignin degradation and their related KEGG metabolic pathways, seven aromatic compounds were selected from the detection results. These compounds are vanillic acid, p-coumaric acid, piperic acid, catechol, hydroquinone, benzoic acid and p-hydroxybenzoic acid.
To evaluate the effect of these compounds on plant growth, we first determined the seed germination index (GI) using
Brassica campestris L. Seed GI determination was conducted using a concentration of 40 μmol/L for the selected compounds based on their reported effective concentrations (Parvin et al., 2020). In 9 cm Petri dishes, three layers of sterilized filter paper were added, followed by the addition of 4 mL of the aforementioned culture solution. Each Petri dish contained 15 seeds of
B. campestris L. For the control group, distilled water was used for treatment. Each treatment was replicated three times. After incubation in the dark at 26°C for 4 days, the number of germinated seeds and the length of seedling roots were recorded. The formula for calculating the seed GI is shown below:
where ANT represents the average number of germinated seeds in the experimental group, ALT represents the average root length of seeds in the experimental group, ANC represents the average number of germinated seeds in the control group, and ALC represents the average root length in the control group.
2.6. Screening the concentration of plant growth-promoting intestinal compounds
We then screened the concentration of plant growth-promoting intestinal compounds. B. campestris L. seeds were germinated in 15 cm Petri dishes. Two layers of sterilized filter paper were placed in the Petri dish, and 8 mL of distilled water was added to completely wet the filter paper. Healthy B. campestris L. seeds were evenly spread on filter paper, and the dish was covered to maintain moisture. The Petri dishes were placed in a growth chamber for germination, with a temperature of 25°C, humidity of 70%, and a photoperiod of 16 hours of light and 8 hours of darkness, for approximately 48 hours until the hypocotyls emerged from the seed coat by approximately 2 to 4 mm. B. campestris L. seedlings with consistent growth were selected for the next experiment.
The nutrient solution used in the experiment was Arabidopsis nutrient solution, and the total volume was 40 mL. The prepared culture medium was added to 200 g of river sand and mixed thoroughly. The concentration gradient of each compound in the culture medium was 0 μmol/L, 20 μmol/L, 40 μmol/L, and 60 μmol/L. Healthy B. campestris L. seedlings were carefully selected and transplanted into the culture substrate with tweezers, with 3 replicates for each treatment and 5 seedlings per replicate. Then, the plants were cultured in a growth chamber with a temperature of 25°C, humidity of 70%, and a photoperiod of 16 hours of light and 8 hours of darkness. After 36 hours of cultivation, the B. campestris L. seedlings were carefully removed from the culture substrate and scanned to obtain images. Then, ImageJ was used to measure stem length and root length. The aboveground and belowground parts of the seedlings were separated, and their fresh weights were measured using a balance (CAV214C). The separated seedlings were then oven-dried at 80°C, and the dry weights of the aboveground and root parts were measured.
2.7. Validation of the plant growth-promoting activity of intestinal compounds
To further verify the role of intestinal compounds in promoting plant growth, the peanut variety HY22 was used for pot experiments. Full peanut seeds, each 1.0 to 1.3 g, were selected and potted individually. Vermiculite was used as a cultivation substrate, 1/2 MS solution (pH natural) was used to supply nutrients, and 50 μmol/L intestinal compounds were used as stimulants. The pots were placed in the greenhouse and watered when needed, and the daily temperature was also recorded. After 15 days of culture, peanut seedlings were removed from the culture substrate and carefully cleaned. The leaf chlorophyll relative content was measured, and the aboveground dry weight and root dry weight were measured after oven drying at 80°C.
2.8. Data analysis
Data analysis was performed using one-way ANOVA with a significance level of p<0.Multiple comparisons were conducted using the LSD test when homogeneity of variance was met, and Dunnett's T3 test was used for multiple comparisons when heterogeneity of variance was present.
Results
3.1. Analysis of HA structure in the gut of PBL
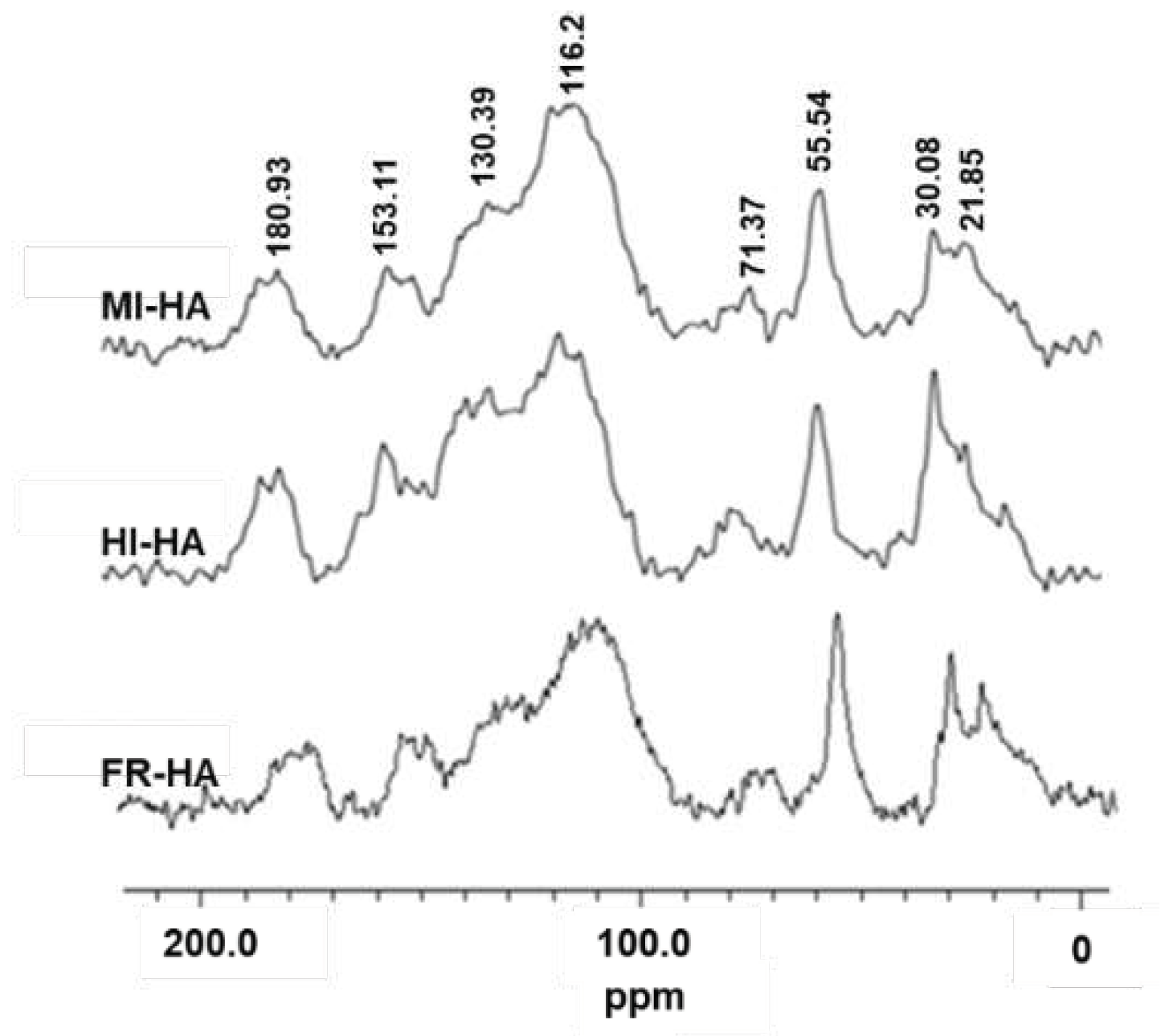
To analyse the structural changes of HA in the gut of PBL feeding on fermented corn stalks, we used solid
13C DD-MAS NMR to characterize the HA from the gut inclusions and frass. From the obtained carbon spectrum (
Figure 1), distinct structural differences between samples can be observed, and further integral analysis of the carbon spectrum shows the relative abundance changes of each functional group carbon. As shown in
Table 1, during the transition from the midgut to the hindgut, the relative abundance of methyl carbon and aromatic carbon in HA decreased, while the relative abundance of alkyl carbon, phenolic carbon, and carboxyl carbon increased; when transitioning from the hindgut to the frass, the relative abundance of alkyl carbon, methyl carbon, and aromatic carbon in HA increased, while alkoxy carbon, phenolic carbon, and carboxyl carbon decreased. These results indicate that HA undergoes changes within the PBL gut and that HA precursors or decomposition products may coexist in the gut.
3.2. Organic compounds in gut contents
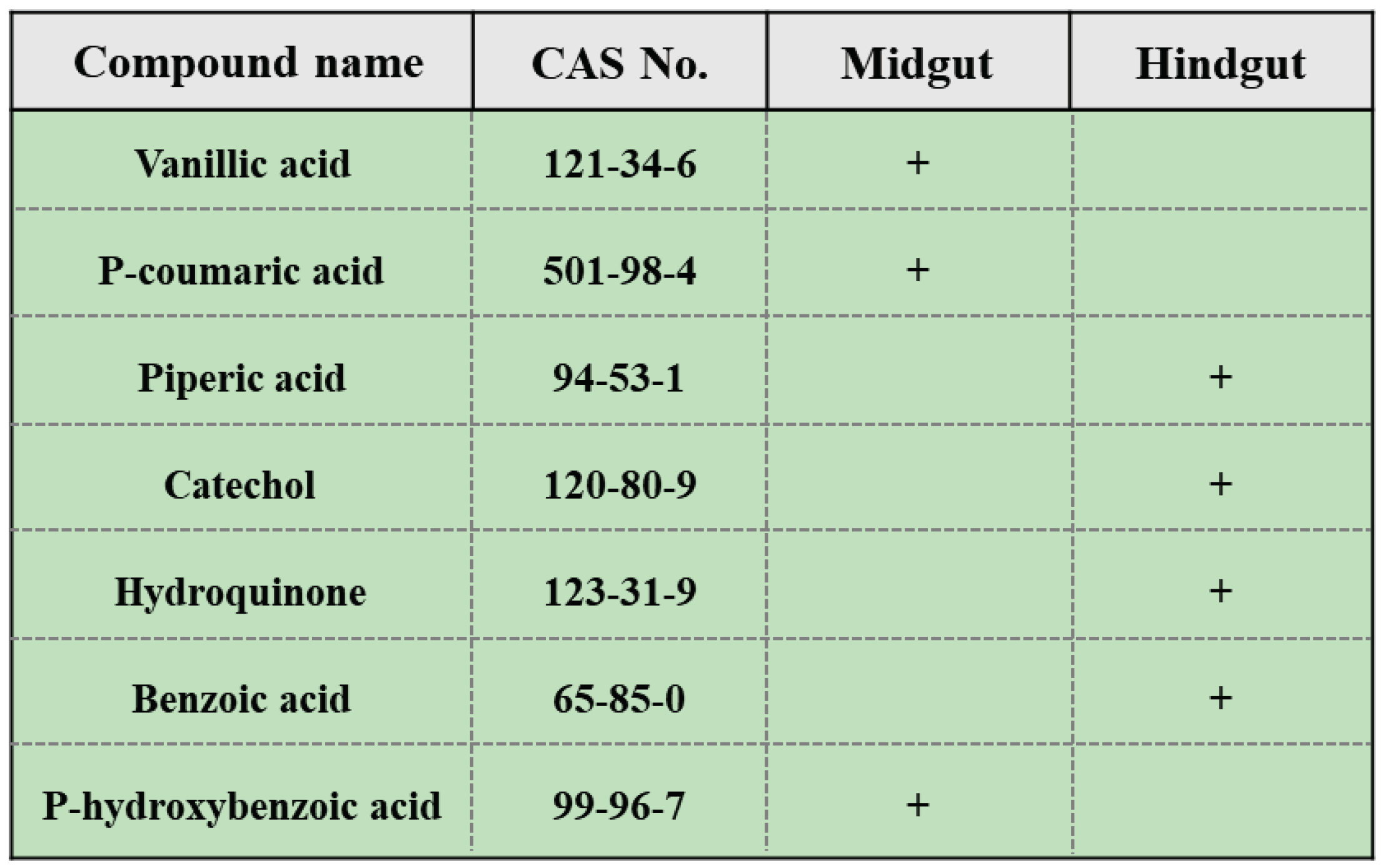
To identify potential HA precursors in the gut of PBL, an experimental detection and data analysis were conducted using a nontargeted metabolomics platform based on GC‒MS. A total of 146 gut compounds were detected, primarily including alcohols, acids, sugars, esters, and aromatic compounds, distributed in both the midgut and hindgut. Among them, 93 compounds were more prevalent in the midgut (Supplementary File 1), and 53 compounds were more prevalent in the hindgut (Supplementary File 1). Based on the possible types of compounds and relevant metabolic pathways in lignin degradation, seven benzene-ring-containing compounds were selected from the detected compounds: catechol, p-coumaric acid, vanillic acid, piperic acid, benzoic acid, p-hydroxybenzoic acid, and hydroquinone (
Figure 2). The stimulating effects of vanillic acid, p-coumaric acid, catechol, hydroquinone and p-hydroxybenzoic acid on plant growth have been reported by researchers, but the promoting effects of piperic acid on plant growth have not been reported.
3.3. Determination of the seed GI of the selected compounds
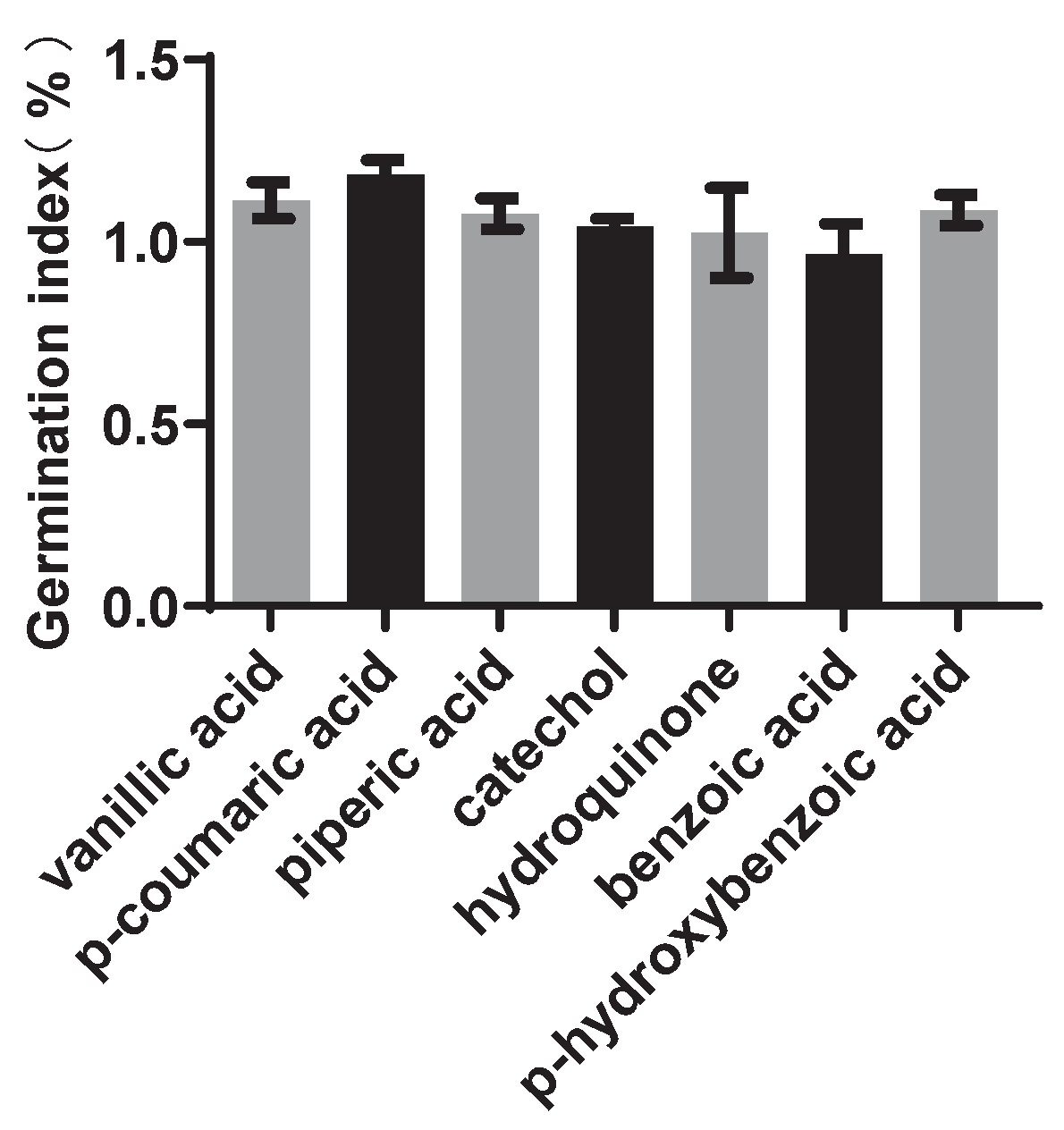
To assess the effects of these compounds on plant germination, a GI test was initially conducted using rapeseed (canola). The results indicated that except for benzoic acid, the other six compounds showed the ability to promote seed germination at a concentration of 40 μmol/L (
Figure 3). Thus, vanillic acid, p-coumaric acid, cinnamic acid, catechol, pyrocatechol, and p-hydroxybenzoic acid were used for further testing.
3.4. Screening of the plant growth-promoting concentration of these compounds
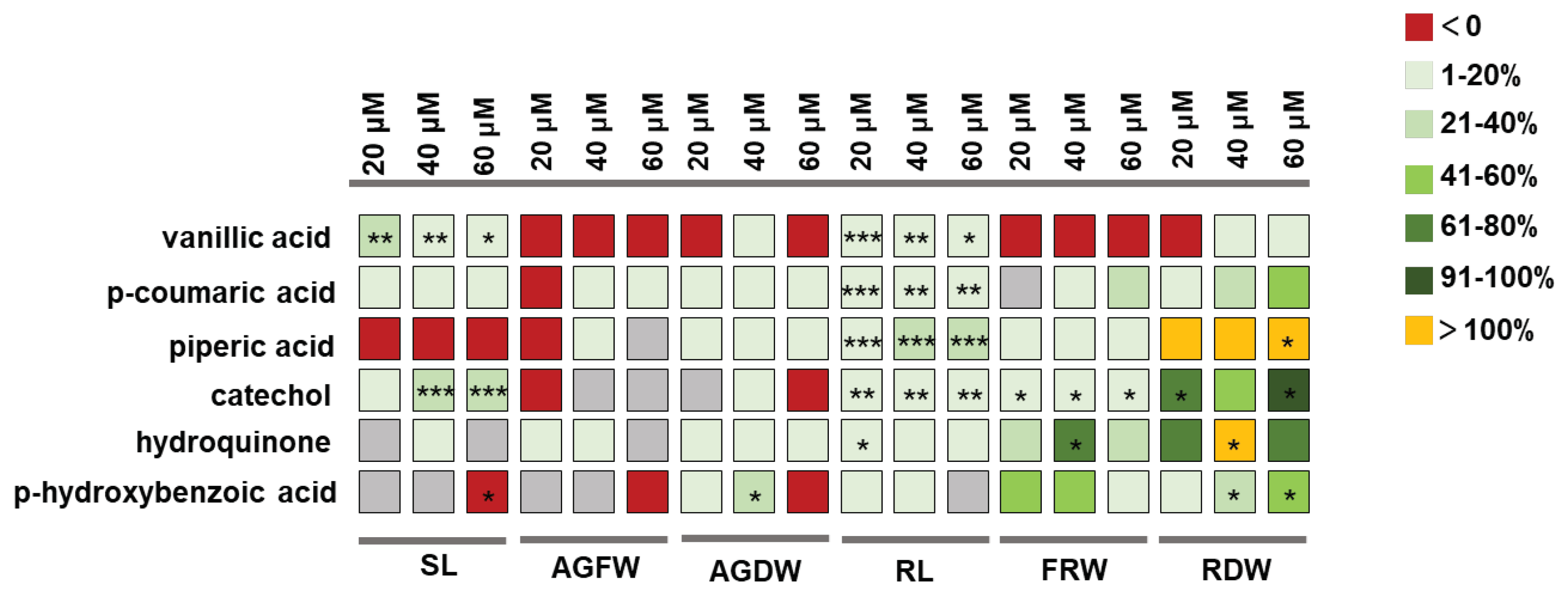
Further screening was conducted using sand culture experiments to determine the effective concentrations of the six gut compounds to promote plant growth. The results revealed that all six compounds showed growth-promoting effects on plants. Vanillic acid, catechol and p-hydroxybenzoic acid can promote the aboveground part and root of the plant. On the other hand, piperic acid, hydroquinone, and p-coumaric acid have specific growth-promoting effects on plant roots (
Figure 4), and these growth-promoting effects are somewhat related to the concentration of the compounds. Vanillic acid showed a significant growth-promoting effect at 20~60 μmol/L, and the effect was more obvious at 20 μmol/L, and coumaric acid at the same concentration also showed a significant growth-promoting effect. In the concentration range of 20~60 μmol/L, piperic acid can significantly promote plant growth. In the range of 20 to 60 μmol/L, catechol had a significant effect on growth, and the effect was more obvious at 40 μmol/L and 60 μmol/L. Hydroquinone can significantly promote plant growth in the concentration range of 20-40 μmol/L. P-hydroxybenzoic acid also has a significant growth-promoting effect at 40-60 μmol/L, of which the effect is more pronounced at 40 μmol/L (Supplementary File 2: Figure S1-S6).
3.5. Validation of the plant growth-promoting activity
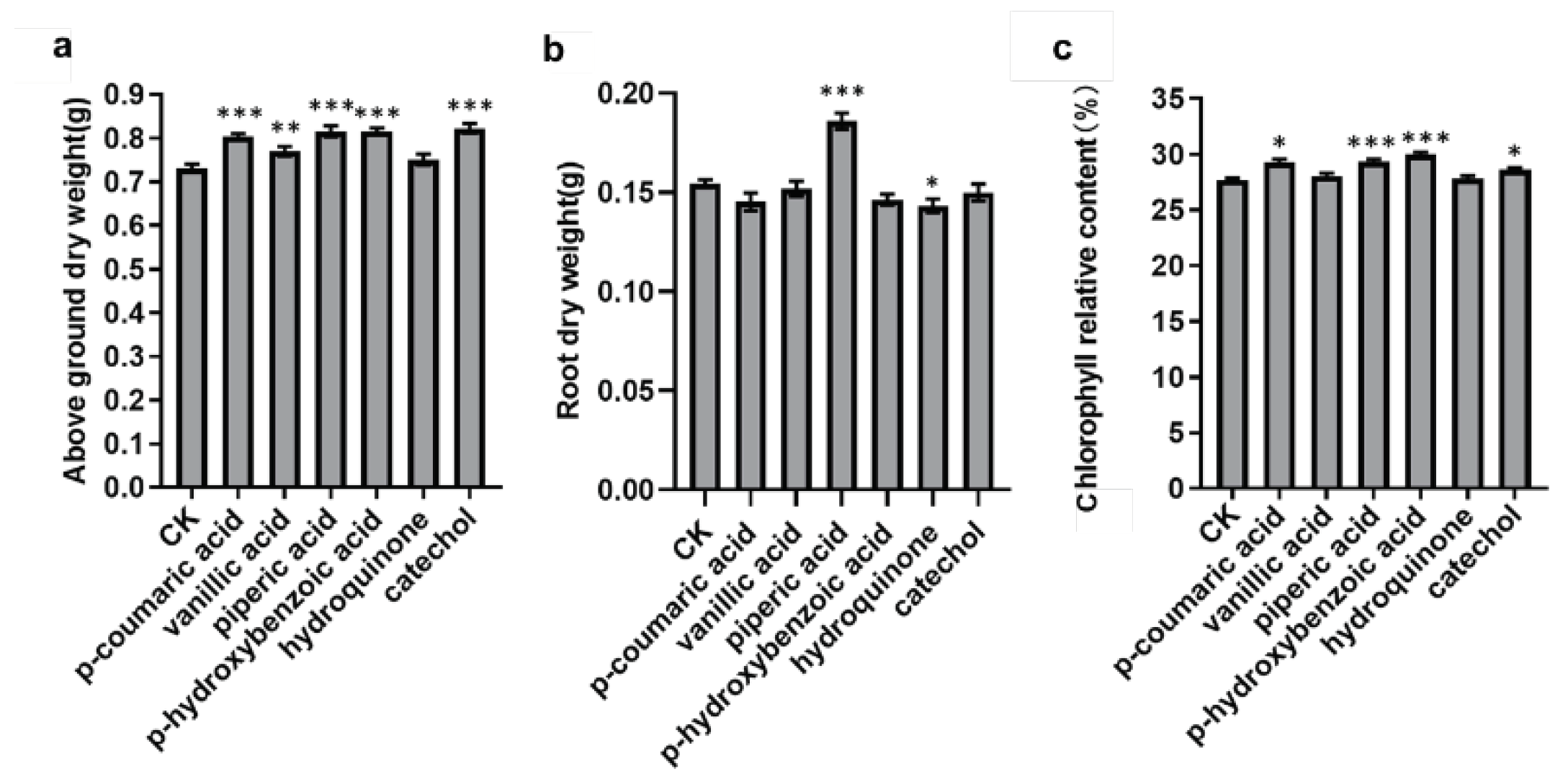
To validate the plant growth-promoting effects of these compounds, based on the data results from section 3.4, we selected a compound concentration of 50 μmol/L and conducted a pot experiment using peanut. Growth-related traits including above-ground dry weight, root dry weight and chlorophyll relative content were measured. Statistical analysis of variance and multiple comparisons were employed to confirm the growth-promoting capabilities of these compounds. As shows in
Figure 5, the results indicated that all six selected compounds have the ability to increase the above-ground dry weight of peanut seedlings and that p-coumaric acid, vanillic acid, piperic acid, p-hydroxybenzoic acid, and catechol exhibit a significant promoting effect. However, the root dry weight data shows that only piperic acid has a significant promoting effect, while the other five compounds exhibit an inhibitory effect.
Furthermore, the study also found that all six compounds can elevate the relative chlorophyll content of peanut seedlings. Among them, p-coumaric acid, piperic acid, p-hydroxybenzoic acid, and catechol significantly increase the relative chlorophyll content. We observed a positive correlation between the increase in chlorophyll content and the increase in above-ground dry weight, possibly because the higher chlorophyll content is conducive to photosynthesis, thereby increasing the above-ground dry weight.
Figure 5.
Functional verification results of 6 compounds promoting plant growth. (a, b, c) Represent the effects of 6 compounds on above-ground dry weight, root dry weight and the relative chlorophyll content, respectively. *** P < 0.001, ** P < 0.01, * P < 0.05.
Figure 5.
Functional verification results of 6 compounds promoting plant growth. (a, b, c) Represent the effects of 6 compounds on above-ground dry weight, root dry weight and the relative chlorophyll content, respectively. *** P < 0.001, ** P < 0.01, * P < 0.05.
4. Discussion
Soil-dwelling insects play a crucial role in the carbon cycle of terrestrial ecosystems because they assist in the breakdown and decomposition of cellulose and lignocellulosic components within biomass (Frouz, 2018). Research has shown that in natural environments, soil-dwelling insects can rapidly degrade and convert plant residues with high lignin content into humic substances within a matter of hours. In contrast, microorganisms typically require several months or even years to transform plant residues into humic substances. Soil-dwelling insects can degrade efficiently by participating in a series of processes, including fragmentation, microbial consumption, inducing changes in pH and redox conditions, removing easily degradable polysaccharides, increasing lignin proportions, and reducing levels of soluble polyphenols and carbon-to-nitrogen ratios. Through these biotic disruption processes, soil-dwelling insects can convert plant residues into nutrient-rich mull-type HA, while humic substances produced solely through microbial action are referred to as more-type HA (Frouz, 2018; Joly et al., 2018; Morriën & Prescott, 2018). Consequently, soil-dwelling insects play a pivotal role in the transformation of plant residues into mull-type HA. In China, soil-dwelling insects, such as PBL, are used as a resource and have been successfully employed to convert plant residues into humic acid-rich frass products (Li et al., 2019)(Lai et al., 2019). Based on these data, the innovative strategy in this study uses PBL that feeds on fermented maize straw as a model to screen compounds to use as plant biostimulants.
PBL is an easily reared soil-dwelling insect that feeds on decaying plant litter. Its digestive system is divided into three main parts: the foregut, located at the front end of the digestive tract, primarily serves as a food storage compartment; the midgut occupies a significant portion of the larva's body cavity and has an elongated tubular structure. The midgut fluid exhibits strong alkaline properties with a high pH value of approximately 10 to 11, creating a favourable environment for the dissolution and decomposition of organic matter. The hindgut is cylindrical in shape and maintains a neutral pH of around eight. Within the hindgut is a rich and active microbial community that plays a crucial role in the further fermentation of organic materials (Li et al., 2019; Wang et al., 2019; Wei et al., 2020; Xuan et al., 2022). Recent studies have found that due to its unique physicochemical properties in the gut and synergistic interactions with gut microbiota, PBL can effectively degrade corn straw and produce frass containing HA substances (Kui et al., 2022; Li et al., 2019). Previous research has suggested the crucial role of lignin and its degradation products in the formation of HA (Guo et al., 2019; Zhang et al., 2018), and in this study, we have discovered that the digestive contents of PBL show a high concentration of compounds containing benzene rings after a diet of fermented corn stalks (Supplementary Material 1) and the HA structure changes as it moves through the PBL intestinal tract (
Figure 1,
Table 1 and Table 2). These data indicated that, under the action of microbiota, HA in the PBL gut is continuously forming and decomposing, similar to the process described in the "soil continuum model" (Johannes & Markus, 2015). As such, screening compounds related to HA derived from PBL gut contents is a logical strategy for developing biostimulants.
Currently, many compounds with benzene rings have been reported to have plant biostimulation activity. Reports have shown that catechol can induce primary root growth by altering gene expression in the reactive oxygen species (ROS) pathway and regulating the distribution of H2O2 in the root elongation zone of plants (Ming et al., 2017). Furthermore, catechol can also enhance a plant's antifungal capabilities and boost its disease resistance by increasing the activity of peroxidases and polyphenol oxidases within the plant (Nira et al., 1974). Exogenous p-coumaric acid has the ability to promote plant growth and enhance its growth performance by activating the active oxygen signalling pathway involving O2·− (Nkomo et al., 2019). Additionally, exogenous vanillic acid can strengthen a plant's salt tolerance and growth performance by participating in the plant's antioxidant defence mechanisms and the aldehyde dehydrogenase system (B et al., 2020). Low concentrations of hydroquinone can stimulate seed germination and the growth of seedlings, while concurrently inhibiting the growth of fungal pathogens on seeds. The mechanism of action may be associated with redox reactions between quinones and phenols (Li et al., 2010)(Elwakil, 2003; Muhammad et al., 2017). Similarly, p-hydroxybenzoic acid can promote seed germination and the growth of seedlings at low concentrations, and it can also affect the activity of the plant's antioxidant enzymes (Gao et al., 2020; Shen et al., 2020). The promoting effect of these compounds on plants may be the reason why HA can serve as a biostimulant. Furthermore, it is worth noting that this study first reported the growth-stimulating effect of piperic acid on plants, even though it is known to play important roles as an antioxidant, antimicrobial and anti-inflammatory agent in the food and pharmaceutical fields (Araujo et al., 2018; Singh et al., 2022; Zarai et al., 2013). In this report, we assume that it also acts as a plant antioxidant, however, the details of the specific mechanisms of action still require further comprehensive research.
5. Conclusions
In summary, this study not only provides insights into the insect-mediated transformation of HA but also illustrates a new strategy for discovering structurally clear plant biostimulant compounds. We found six structurally clear compounds with growth-stimulating effects, and reported for the first time, the biostimulant activity of piperic acid. Thus, this work will contribute to advancing industrial synthetic biostimulant production.
Author Contributions
Conceptualization, C.S.; Data curation, P.G., K.W.; Formal analysis, P.G., K.W. and C.S.; Investigation, C.Q., Y.Z. and K.C.; Resources, J.Z.; Supervision, J.Z. and C.S.; Validation P.G.; Writing—original draft, P.G.; Writing—review and editing, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Autonomous region Key R&D Program of Xinjiang,China (Grant No. 2022B02046), the National Natural Science Foundation of China (No. 32070511).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Geng Ting and Langfang Scientific Research Trial Station (Chinese Academy of Agricultural Sciences, Langfang, China) for their help and for providing greenhouses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Araujo, O.P.D., Brito, D.A.T., Guerra, D.O.R., Mastrangelo, G.A.G., Mattos, D.O.J., Benedito, A.D.S.B., Roberto, L.M., Silva, C.R.W.D., Nascimento, F.A.T.D., Almeida, V.M.L.A.D. Evaluation of the antinociceptive and anti-inflammatory activities of piperic acid: Involvement of the cholinergic and vanilloid systems. European journal of pharmacology, 834, 54-64.
- B, K.P.A., C, K.N., D, M.H., F, M.H.M.B.B., E, S.M.M.A., A, M.F. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiology and Biochemistry, 150, 109-120.
- Chen, Y.A., Zhou, Y., Qin, Y., Liu, D., Zhao, X. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and lignin structure. Bioresour Technol, 269, 329-338.
- Elwakil, M.A. Use of Antioxidant Hydroquinone in the Control of Seed-borne Fungi of Peanut with Special Reference to the Production of Good Quality Seed. Plant Pathology Journal, 2(2), 75-79.
- Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma, 332.
- Guo, X.X., Liu, H.T., Wu, S.B. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci Total Environ, 662, 501-510.
- Johannes, L., Markus, K. The contentious nature of soil organic matter. Nature, 528(7580).
- Joly, F.X., Coq, S., Coulis, M., Nahmani, J., Hättenschwiler, S. Litter conversion into detritivore faeces reshuffles the quality control over C and N dynamics during decomposition. Functional Ecology, 32(11).
- Kui, W., Peiwen, G., Lili, G., Chunqin, L., Jie, Z., Changlong, S. Lignocellulose degradation in Protaetia brevitarsis larvae digestive tract: refining on a tightly designed microbial fermentation production line. Microbiome, 10(1).
- Li, Y., Fu, T., Geng, L., Shi, Y., Chu, H., Liu, F., Liu, C., Song, F., Zhang, J., Shu, C. Protaetia brevitarsis larvae can efficiently convert herbaceous and ligneous plant residues to humic acids. Waste Management, 83.
- Ming, W., Matthias, S., Shuqing, X., Christian, P., Julia, W., T, B.I., Karin, G. Catechol, a major component of smoke, influences primary root growth and root hair elongation through reactive oxygen species-mediated redox signaling. The New phytologist, 213(4).
- Morriën, E., Prescott, C.E. Pellets or particles? How can we predict the effect of soil macro-arthropods on litter decomposition? Functional Ecology, 32(11).
- Muhammad, K., Khan, A.L., Liaqat, A., Javid, H., Muhammad, W., Ahmed, A.H., Imran, Q.M., Yoon-Ha, K., Sang-Mo, K., Byung-Wook, Y. Hydroquinone; A Novel Bioactive Compound from Plant-Derived Smoke Can Cue Seed Germination of Lettuce. Frontiers in Chemistry, 5.
- Nira, Retig, and, I., Chet. Catechol-induced resistance of tomato plants to Fusarium wilt. Physiological Plant Pathology.
- Nkomo, M., Gokul, A., Keyster, M., Klein, A. Exogenous p-Coumaric Acid Improves Salvia hispanica L. Seedling Shoot Growth. Plants, 8(12).
- Parvin, K., Nahar, K., Hasanuzzaman, M., Bhuyan, M.H.M.B., Mohsin, S.M., Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiology and Biochemistry, 150(prepublish).
- Rouphael, Y., Colla, G. Editorial: Biostimulants in Agriculture. Front Plant Sci, 11, 40.
- Singh, G., Wani, N.A., Rahim, J.U., Shankar, S., Rai, R., Katoch, M. Synergistic antimicrobial and antibiofilm activities of piperic acid and 4-ethylpiperic acid amides in combination with ciprofloxacin. The Journal of Antibiotics, 75(4), 236-242.
- Wang, K., Li, P., Gao, Y., Liu, C., Wang, Q., Yin, J., Zhang, J., Geng, L., Shu, C. De novo genome assembly of the white-spotted flower chafer (Protaetia brevitarsis). Gigascience, 8(4).
- Wei, P., Li, Y., Lai, D., Geng, L., Liu, C., Zhang, J., Shu, C., Liu, R. Protaetia brevitarsis larvae can feed on and convert spent mushroom substrate from Auricularia auricula and Lentinula edodes cultivation. Waste Management, 114.
- Xuan, H., Gao, P., Du, B., Geng, L., Wang, K., Huang, K., Zhang, J., Huang, T., Shu, C. Characterization of Microorganisms from Protaetia brevitarsis Larva Frass. Microorganisms, 10(2).
- Zarai, Z., Boujelbene, E., Salem, N.B., Gargouri, Y., Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT- Food Science and Technology, 50(2), 634-641.
- Zhang, Z., Zhao, Y., Wang, R., Lu, Q., Wu, J., Zhang, D., Nie, Z., Wei, Z. Effect of the addition of exogenous precursors on humic substance formation during composting. Waste Manag, 79, 462-471.
- Lai, D.; Wang, Q.; Wu, Y.; Shu, C.; Zhang, Y.; Liu, C. Effect of Protaetia brevitarsis (Lewis) larvae larvae dung on development of pepper seedling stage under low temperature. North. Hortic. 2019, 8, 63–66. [Google Scholar]
- Li, M.; Li, W.; Du, W.; Li, J. Dormancy Mechanism and Bioactivity of Hydroquinone Extracted from Seed of Podophyllum hexandrum Royle. Bulletin of Botanical Research. 2010, 30(2), 215–220. [Google Scholar]
- Gao, B.; Jin, X.; Chen, Y. Effect of P-hydroxybenzoic Acid on Seedling Growth of Isatis indigotica. Bulletin of Botanical Research. 2010, 30( 2), 215–220. [Google Scholar]
- Shen, F.; Xu, Y.; Huang, X.; Yang, M. Effects of p-Hydroxybenzoic Acid and Cinnamic Acid on the Growth and the Physiological Indexes of Seedlings of Eucalyptus grandis×E. urophylla No.Journal of Southwest Forestry University. 2020, 40(6), 19–26. [Google Scholar]
- Tian, F.; Liu, G.; Wu, J. Exploration on metabolic characteritics of Pengbo semi-wool sheep under the condition of grazing and barn feeding based on non-targeted metabonomics. Chin J Vet Sci Sep. 2020, 40(9), 1854–1863. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).