1. Introduction
World Health Organization (WHO) has estimated that two-thirds of the global burden of disease in the coming decades will be attributable to chronic non-communicable diseases, most of which would be strongly associated with food and associated lifestyle changes [
1]. Many scientific reports have indicated a strong correlation between indirect food contamination and diet-linked diseases [
2,
3]. Indirect contaminations mostly occur at the time of food processing and high temperature-mediated preparation of foods. Generally, deep-frying, grilling, baking, smoking, pan-frying, and more are the most common high-temperature cooking methods in fast food restaurants, domestic kitchens, and processed or packaged household food industries. Due to the general chemical reactions occurring at the time of high temperatures (200–370°C) cooking regular food ingredients may undergo a series of chemical reactions resulting in deteriorative changes (i.e., hydrolytic, oxidative, polymerization, and thermal changes). For instance, polycyclic aromatic hydrocarbons (PAHs), heterocylic amines (HCA), alcohols, ketones, and aldehydes represent a very large group of compounds (more than 10,000 compounds) that are formed and have the potential to cause various toxic effects, including carcinogenic, mutagenic, immunologic and reproductive effects [
4,
5]. While the reaction mechanism consists of multiple steps, both the exact intermediates and the possible reaction steps remain unknown. They are ubiquitous and indirect contaminant materials in food and are well-known causative factors for many chronic diseases (e.g., As the temperature increases., obesity, cancer, diabetes, and more).
Chemically, they consist of fused aromatic rings and heterocylic groups made up of carbon and hydrogen atoms. They are formed during incomplete combustion of all carbon-based materials (i.e., edible oils, flour, meat, and all other food ingredients and additives). Many processed foods, such as baked goods, bakery products, and sweets, contain sucrose in a significant amount, and these foods are often prepared using high-temperature cooking methods. Many research articles have shown that when these foods are cooked at high temperatures, the brown-colored crust or charred areas that form on the surface of the food may contain various heat-induced chemicals, including PAHs, HCAs, and advanced glycation end products (AGEs)[
6]. Typically, AGEs formation occurs through non-enzymatic pathways, involving the condensation of carbonyl groups from reducing sugars with the free amine groups found in nucleic acids, proteins, or lipids. Subsequently, stable compounds are formed after rearrangement of chemical reactions [
7]. As the temperature increases, sucrose starts to caramelize at approximately 180°C (320°F). This means that the sugar molecules will begin to break down and form new compounds, resulting in a characteristic browning and a rich, complex flavor [
8].
For example, high-temperature cooking with food ingredients at approximately 150–270°C produces carbon fumes containing mutagenic compounds such as 1,3-butadiene, benzene, acrolein, and formaldehyde [
9] . Moreover, non-volatile aromatic compounds are generated under pyrolysis. Recent research has shown that glucose and proline pyrolysis increased nitrogen-containing polycyclic aromatic compounds (N-PACs) and polycyclic aromatic hydrocarbons (PAHs) [
10]. Some studies have found that PAHs can also form during the pyrolysis of cellulose [
11]. 5-Hydroxymethylfurfural (HMF) is generated when carbohydrate-containing foods are subjected to high heat, particularly during deep-fat frying. A study revealed that the concentration of glutamic acid, along with the pH level and heating time, had a crucial role in the production of HMF from fructose [
12,
13]. Research has extensively investigated the reaction between reducing sugars and different amino acids under pyrolytic conditions, resulting in the formation of various carbonyl compounds [
14]. Although there are no existing studies on the combination of sucrose and histidine, specifically, this work subject the materials to heat between 150-250ºC and analyzes the resulting organic derivatives under pyrolytic conditions.
Both these carbon-bound hydroperoxides and aldehydes are endogenous reactive chemicals and have inflammatory, mutagenic, and carcinogenic potential. Carbonyl compounds have been found to have toxic and carcinogenic properties, as demonstrated by numerous studies in the field of chemistry and toxicology. Several well-known mutagenic heterocyclic amines are identified on the charred surface of broiled or fried meat [
15]. The chemical analysis of charred food, which contains a vast array of complex derivatives and requires a top-down approach for analysis, presents an extremely challenging task. To overcome this challenge, a bottom-up approach was taken by using a combination of sucrose and histidine. To date, investigations into the uptake of pyrolytic compounds by immune cells have been lacking, leaving a critical knowledge gap in our understanding of their potential effects. It is crucial to explore how these compounds interact with immune cells since certain groups of pyrolytic compounds have been implicated in possible harm to the immune system.
2. Materials and Methods
2.1. Materials
Amino acid and sucrose were purchased from nice chemicals in Mumbai, India. The following materials were obtained from Sigma-Aldrich (St. Louis, MO, USA): DMSO, acridine orange (AO), Ethidium bromide (EB), and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT),. We purchased Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum from Invitrogen (Carlsbad, CA, USA).
2.2. Sample preparation
Sample preparation involved combining 5 grams of sucrose with an equal amount of amino acids in 20 mL of distilled water. The resulting mixture was carefully prepared to ensure thorough mixing and homogeneity. Next, the prepared mixture was subjected to a controlled reaction in a muffle furnace set at a temperature of ≈240°C. This high temperature was chosen to induce the desired charring or pyrolysis process. The reaction duration was carefully monitored and set between 15 to 20 minutes to achieve optimal transformation of the sucrose and amino acids. Following the completion of the reaction, the resulting charred and/or dark brownish materials were retrieved from the muffle furnace. To ensure a uniform dispersion of the obtained materials, they underwent a sonication process. This involved subjecting the materials to ultrasonic vibrations for a period of 10 minutes. The sonication process aimed to break up any agglomerations or clumps and achieve a consistent dispersion throughout the sample. The prepared sample, now uniformly dispersed, was ready for further studies and analyses.
2.3. Identification of pyrolytic compounds through GC-MS
To identify the pyrolytic compounds present in the sample, a gas chromatography system coupled with a mass spectrometer (Agilent GC 7890A/MS5975C) was utilized. The GC-MS analysis involved specific temperature programming to achieve optimal separation and detection of the compounds of interest. The oven temperature was initially set at 60 °C and maintained for 1 minute to ensure the stable baseline. Subsequently, the temperature was increased at a rate of 240°C per 4 minutes. This temperature ramp allowed for the efficient elution and separation of the pyrolytic amino acid and sugar derivatives present in the sample. To identify the compounds, two libraries were utilized: the National Institute of Standards and Technology (NIST) library and the Wiley online library. The detailed GC–MS experimental condition has been followed by Coleman et al. (2002).
2.4. Cell culture
Human monocyte cell line model (THP1) has been procured from the American Type Culture Collection (ATCC, USA) and is already stored in our master bank. For our experiments, the cell line was thawed and cultured in RPMI growth medium with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin in suitable cell culture flasks (T25cm2, 24- or 96-well culture plates) at 37°C in a humidified atmosphere containing 5% CO2. All experiments will be performed using cells from passage 15 or less.
2.5. Cell viability assay
The assessment of cell viability was conducted through the MTT assay. In brief, THP-1 cell line was seeded at a density of 2 x 10^4 cells per well in 200 μL of fresh culture medium. The cells were then exposed to varying concentrations (e.g., 25-400 μg/mL) of Su-His derivatives for 24 and 48 hours. Following the respective incubation periods, 20 μL of MTT solution (5 mg/mL in phosphate-buffered saline, PBS) was added to each well. To protect from light, the plates were covered with aluminum foil and incubated at 37°C for 16 hours. Subsequently, the plates were centrifuged, and the supernatant was carefully discarded. The formazan crystals (displaying a purple color) were dissolved by adding 100 μL of dimethyl sulfoxide (DMSO) to each well. The intensity of the resulting color was measured at 570 nm (measurement) and 630 nm (reference) using a 96-well plate reader (Bio-Rad, CA, USA). The collected data were analyzed utilizing MS-Excel (Microsoft, USA) software for further analysis and interpretation.
2.6. Nuclear and cytoplasmic morphological assessment using fluorescent microscopy
To examine the impact of Su-His derivatives on the cellular nuclear-cytoplasmic morphology, a fluorescent dual staining method called acridine orange/ethidium bromide (AO/EB) was employed following a previously established protocol [
16]. After the completion of the 24-hour and 48-hour incubation periods, the culture medium was cautiously aspirated from the wells. Subsequently, 20μL of the AO/EB staining solution was added to each well. The staining solution was incubated for one minute at 37°C in a CO₂ incubator (Labomiz, USA). Following the incubation period, the staining solution was carefully removed, and images were captured using a camera attached to an epifluorescence microscope Axiovert 40 CFL (Zeiss, USA) equipped with an appropriate fluorescence channel. This imaging technique allowed us to visualize and analyze the nuclear-cytoplasmic morphology in response to the Su-His derivatives.
2.7. Statistical analysis
Statistical analyses were performed using MS Excel, including calculations of % mean, % standard deviation (SD), and T-test analysis. A standard deviation was plotted at the 95% confidence level.
3. Result and discussion
Carbonization and/or pyrolysis, which occur during high-temperature cooking, are integral processes in various cooking methods including baking, frying, grilling, caramelizing, and more. These processes lead to alterations in food chemicals [
13]. Extreme browning or pyrolysis, occurring in the absence of oxygen, mainly generates carbonaceous residues, known as carbonization. The carbonization process occurs as a result of the breakdown of complex organic molecules present in the food substance [
17]. The high temperatures initiate chemical reactions that result in the decomposition of carbohydrates, proteins, and fats, leading to the release of volatile and/or non-volatile compounds, the formation of aromatic compounds, and the production of carbonized residues. For instance, when baking bread or pastries, exposure to high temperatures during the pyrolysis process gives rise to the golden-brown crust and imparts a distinct aroma and taste. Similarly, grilling or barbecuing meats leads to the formation of charred marks and a smoky flavor, enhancing the overall sensory experience. When food substances undergo pyrolysis, the high temperatures initiate complex chemical reactions that result in the generation of various volatile and non-volatile compounds. These compounds include polycyclic aromatic hydrocarbons (PAHs), heterocyclic amines (HCAs), and other potentially harmful substances [
5,
18,
19]. These toxic compounds can be released into the surrounding environment and subsequently come into contact with cells.
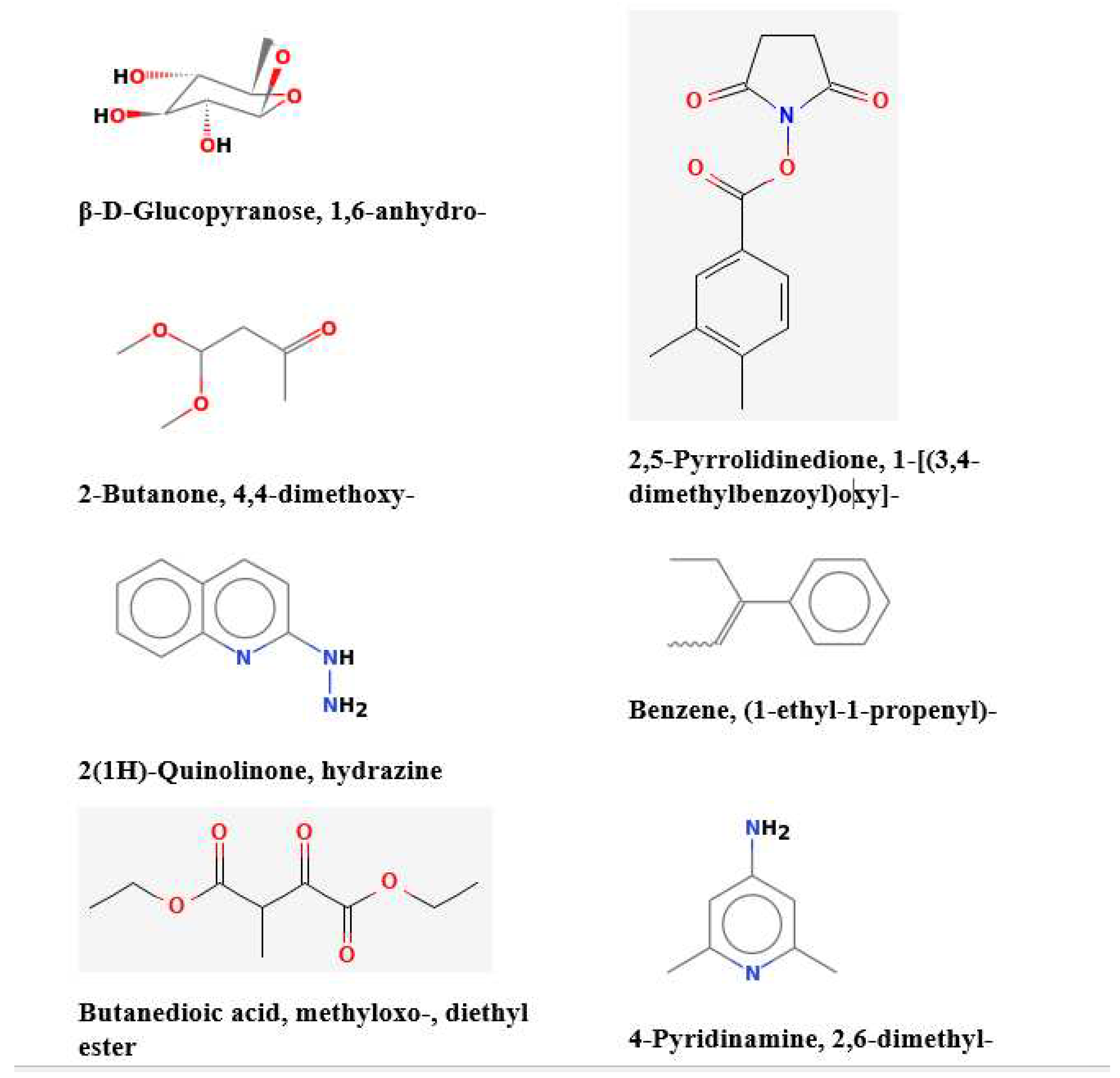
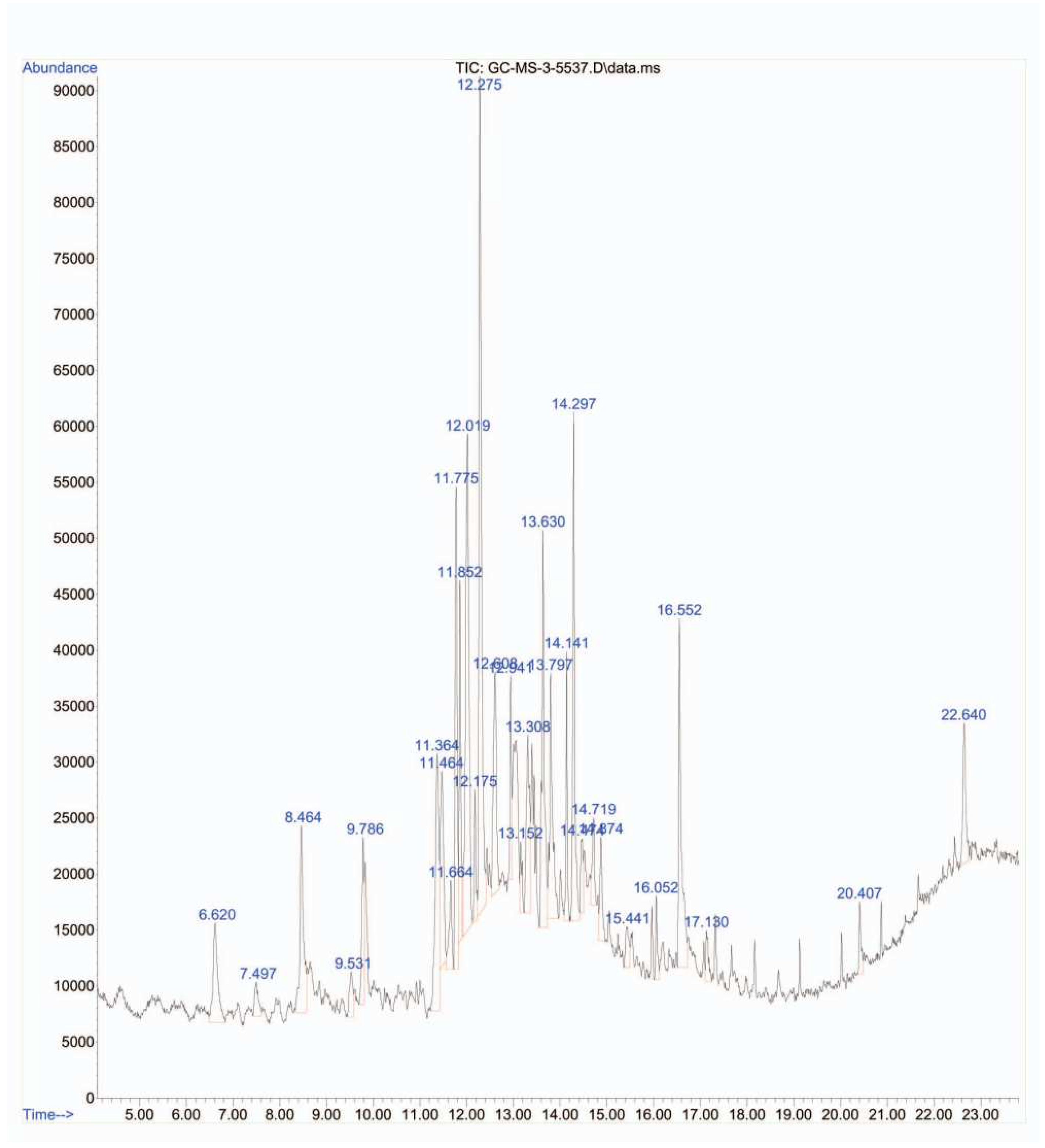
In this study, GC-MS analysis was conducted to determine the levels of pyrolytic molecules in the Su-His derivatives. Each peak in the chromatogram corresponds to a specific compound, and its retention time provides information about its volatility (Graph 1). The mass spectra, obtained from the coupled mass spectrometer, provide further identification and characterization of the pyrolytic molecules based on their unique fragmentation patterns. The data in the table revealed the detection of approximately 25 organic pyrolytic compounds (
Figure 1 and
Table 1). Among them, beta.-D-Glucopyranose, 1,6-anhydro (10.11 %) and 2-Butanone, 4,4-dimethoxy- (12.89 %) were found to be the most abundant. In this study, it was confirmed that 1,6-Anhydro-beta-D-glucopyranose, a type of anhydrohexose produced through the pyrolysis of carbohydrates like starch, was also formed from the pyrolysis of sucrose and histidine [
20]. The degradation of hemicellulose generates 1-hydroxy-2-butanone as one of the products. This compound is commonly found in the pyrolysis liquid generated from hemicelluloses [
21]. The results from this study demonstrated that 2-Butanone, 4,4-dimethoxy- was the major constituent, based on the GC-MS data collected and analyzed. Furthermore, a variety of other aromatic compounds, including 2(1H)-Quinolinone, hydrazine (5.73%), Butanedioic acid, methyloxo-, diethyl ester (6.35%), 2,5-Pyrrolidinedione, 1-[(3,4-dimethylbenzoyl)oxy]- (5.82%), Benzene, (1-ethyl-1-propenyl)- (5.62%), and 4-Pyridinamine, 2,6-dimethyl- (5.52%), were found in significant quantities. Notably, the aforementioned compounds were identified as the major products of pyrolysis during GC-MS analysis of the Su-His derivatives. Interestingly, the data also revealed the identification of various aromatic compounds, which are known to have unique properties and characteristics. Glucose can increase the production of nitrogen-containing polycyclic aromatic compounds (N-PACs) and polycyclic aromatic hydrocarbons (PAHs) during the process of proline pyrolysis [
10]. Some research indicates that the pyrolysis of cellulose can lead to the formation of polycyclic aromatic hydrocarbons (PAHs)[
11]. N-PACs and PAHs are harmful organic compounds with multiple rings of aromatics that can have negative effects on the environment and human health. HCAs exhibit various properties in both microorganisms and eukaryotic organisms, including carcinogenicity, mutagenicity, genotoxicity, metabolic activation and more [
22]. Their precursors are sourced from substances like amino acids, sugars, and amino acids present in high temperature cooked food. Several studies have been investigated their potential to cause cancer, focusing on compounds like 2-aminodipyrido[1,2-a:3′,2′-d]imidazole (Glu-P-2), 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ), 2-amino-6-methyldipyrido[1,2-a:3′-d]imidazole (Glu-P-1), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) [
23]. In a related study, creatinine, together with the sugars and amino acids naturally occurring in meat, plays a pivotal role in the formation of mutagenic derivatives including imidazoquinoxaline and quinoxaline mutagen [
15,
18].
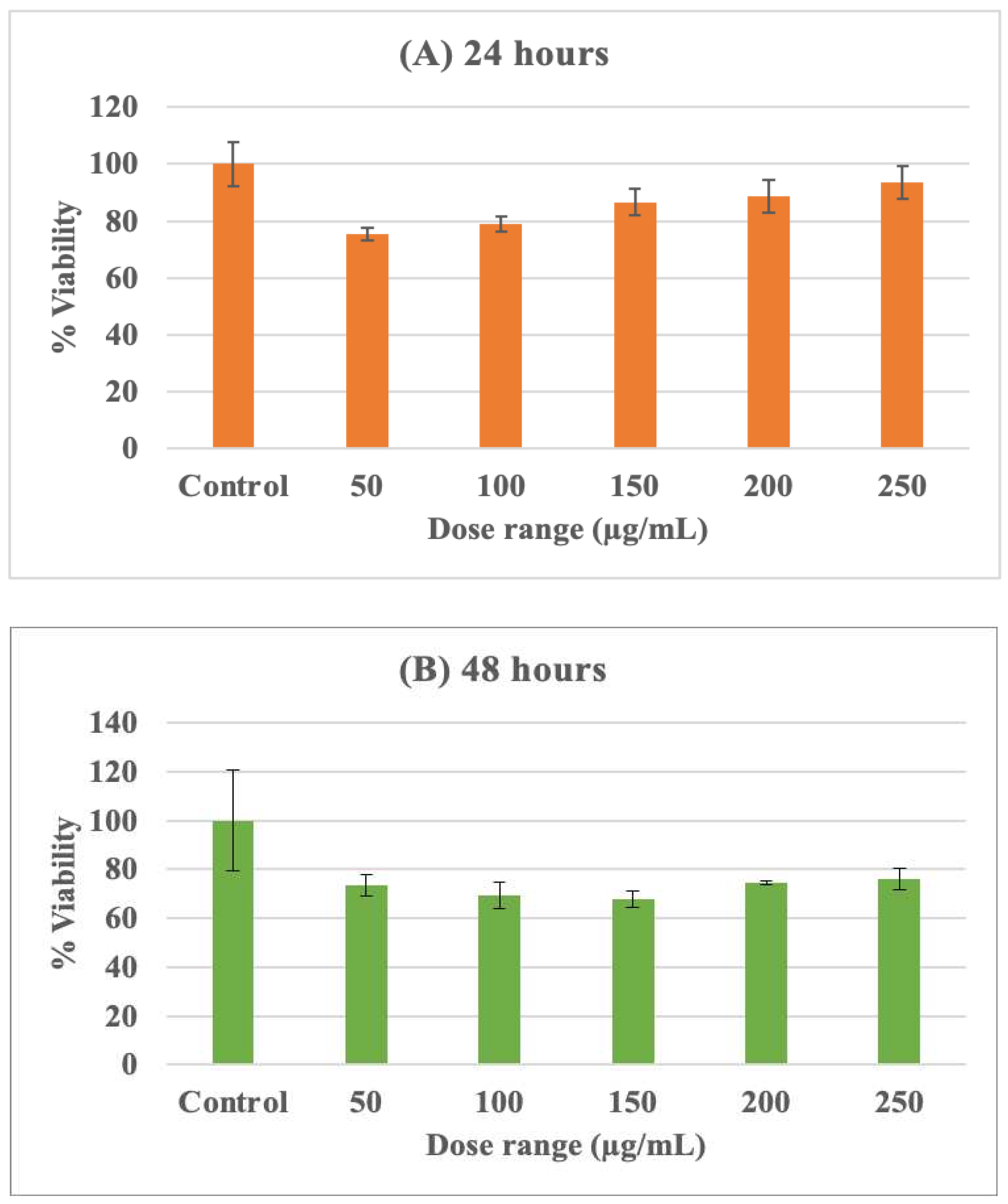
To determine the effects of Su-His derivatives on THP-1 cells, the cells were exposed to varying doses of the compound within the range of 25, 50, 100, 200, and 250 µg/mL for different time periods. The cell viability was then evaluated using the MTT assay, and the results were plotted on a graph. The percentages of viable cells at different concentrations of Su-His derivatives were recorded for both 24 and 48 hours of exposure, and the data are shown in the Graph 1. The results, when compared to a control, indicate that the viability of cells exposed to Su-His derivatives ranges from 80-90% after 24 hours and 70-80% after 48 hours. The figure shows that cell viability decreases with an increase in Su-His derivatives dosage and is dependent on the duration of exposure. This suggests that the aforementioned compounds possess the characteristic of plasma membrane permeability and play a role in the induction of cytotoxicity, which could have significant implications in immune response.
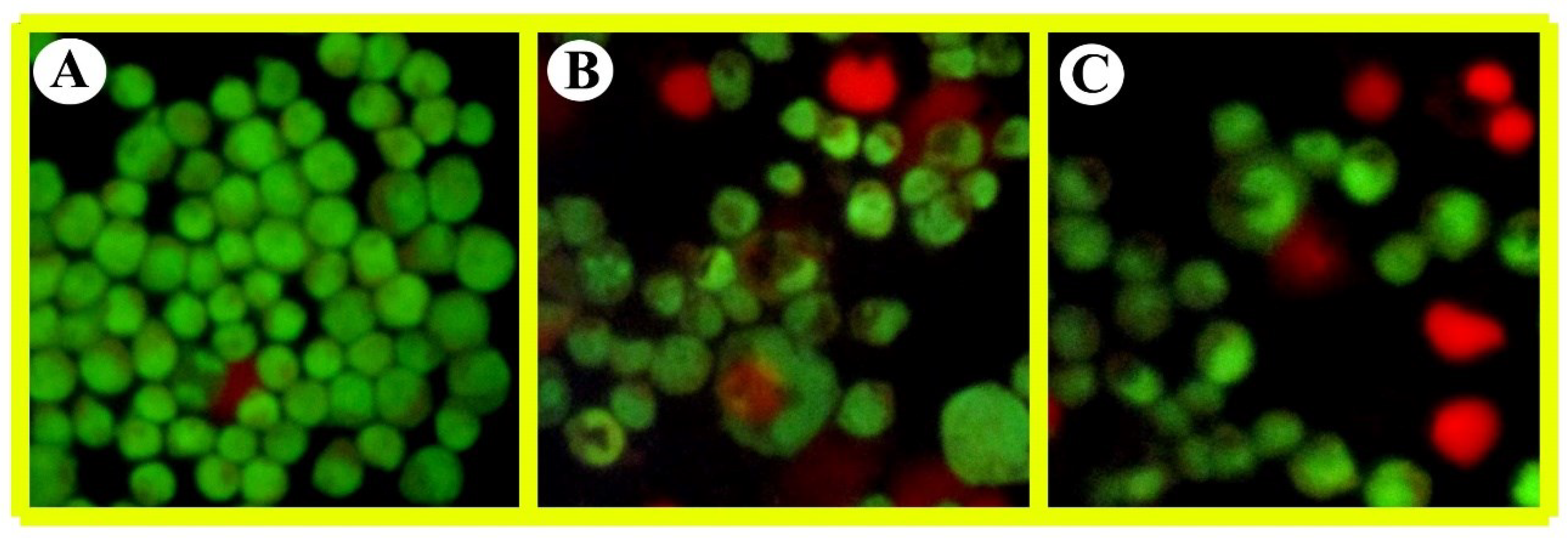
The analysis of THP-1 cells using fluorescence microscopy (AO/EB dual staining) allowed us to observe changes in the structural morphology of both control cells and cells treated with Su-His derivatives. The images of the control and treated cells are presented in
Figure 2. Interestingly, even a low dose of Su-His derivatives induced significant changes in the cells, as highlighted by the observations through fluorescence microscopy. The control cells exhibited a recognizable typical pattern of homogenous nuclear staining, whereas cells exposed to Su-His derivatives presented abnormal nuclear and cytoplasmic morphological features. These observations provide clear evidence of the occurrence of necrosis in the cells treated with Su-His derivatives. Exposure to these compounds may be linked to various adverse outcomes, including cytotoxicity, genotoxicity, oxidative stress, and inflammation in THP-1 cell model. Furthermore, the metabolic activation of certain pyrolytic compounds can lead to the formation of reactive intermediates that can damage cellular components such as DNA, proteins, and lipids.
Figure 2.
Cytotoxicity assay of Su-His derivatives on THP1 cells after (A) 24 hours and (B) 48 hours of exposure.
Figure 2.
Cytotoxicity assay of Su-His derivatives on THP1 cells after (A) 24 hours and (B) 48 hours of exposure.
Figure 3.
Analysis of Acridine Orange/Ethidium Bromide (AO/EB) based morphological features on THP1 cells after (A) untreated, (B) dose 1 treatment, and (C) dose 2 treatment.
Figure 3.
Analysis of Acridine Orange/Ethidium Bromide (AO/EB) based morphological features on THP1 cells after (A) untreated, (B) dose 1 treatment, and (C) dose 2 treatment.
Figure 4.
Major compounds identified from Gas Chromatography-Mass Spectrometry (GC-MS) analysis.
Figure 4.
Major compounds identified from Gas Chromatography-Mass Spectrometry (GC-MS) analysis.
Based on the ADME prediction conducted using the SwissADME predictor, further evidence supports the finding that the majority of the compounds exhibit polar characteristics, possess permeability across the blood-brain barrier (BBB), and have the ability to be absorbed in the gastrointestinal (GI) tract. The breakdown of aromatic compounds in metabolism is a complicated process. These compounds can easily enter cells and interact with important cellular components or go through a redox cycling process that generates oxygen radicals [
24]. Studies have shown that aromatic compounds can be toxic by reducing the amount of antioxidants inside cells. Additionally, exposure to pyrolytic aromatic compounds has been seen to inhibit certain types of cytochrome P450 enzymes[
25]. Altogether, the evidence suggests that a certain group of pyrolytic compounds can be harmful to immune cells. However, despite the existing research on the toxic effects of pyrolytic compounds and carbonized residues, there is still a need for further investigation to gain a comprehensive understanding of the underlying molecular mechanisms responsible for this toxicity. In addition, exploring the impact of pyrolytic compounds on various cell types, tissues, and organs is essential for a thorough understanding of their toxicological effects. Different cell lines, animal models, and in vitro organotypic systems can be employed to investigate the specific cellular and physiological responses to pyrolytic compounds and their metabolites[
26]. This comprehensive approach will enable the identification of tissue-specific effects, potential organ toxicity, and the elucidation of factors influencing the overall toxicity profile.
In conclusion, high-temperature cooking processes such as frying, baking, smoking, or drying can lead to chemical reactions that cause deteriorative changes in food ingredients. The combination of sucrose and histidine (Su-Hi) subjected to charring or pyrolysis at ≈240°C produces carbonyl and aromatic compounds. These compounds, including beta-D-Glucopyranose, 1,6-anhydro, 2-Butanone, 4,4-dimethoxy-, 2(1H)-Quinolinone-hydrazine, Benzenamine, 2,5-Pyrrolidinedione, 1-[(3,4-dimethylbenzoyl)oxy]-, Benzene-(1-ethyl-1-propenyl), and 4-Pyridinamine-2,6-dimethyl, have the ability to permeate cell membranes and contribute to cell death by necrosis. The presence of specific pyrolytic compounds suggests a potential risk to immune cells. Carbonized residues from this process can be formed by incomplete combustion, which can penetrate cells and lead to cellular stress, inflammation, and impaired cellular function. These findings emphasize the importance of considering the potential health risks associated with the consumption of food exposed to high-temperature cooking methods. Further research is needed to fully understand the implications of these chemical reactions on human health and develop appropriate preventive measures.