1. Introduction
Nowadays, non-healing bone fractures or articular cartilage injuries resulting from accidents, surgical resection, age-related pathologies, infections or cancer, possess an elevate epidemiological and clinical incidence [
1,
2]. This kind of injuries consist of non-union fractures in the case of the skeletal bones, or age-related as well as accidental damaged articular joints. In both cases, it is a matter of wounds which are un-reparable through the physiological mechanisms. Since the damaging of these tissues is connected to the loss of the structural function, inflammation and extreme pain, it is fundamental to substitute or repair such injuries and limit the consequent debilitation [
2]. In the clinical practice, graft implantation is the commonly employed technique to fill the gap or substitute defective tissues, in order to recover the native shape and the physiological function of the specific compartment [
3]. Especially, autologous, allogenic, xenogenic or synthetic grafts are used for the scope. Nevertheless, the healing potentiality of these traditional strategies are often limited by the relatively low accessibility, which may occur for autologous grafting, as well as costs, infection potential, risk of rejection and low success rate for allogenic, xenogenic and synthetic grafts [
1].
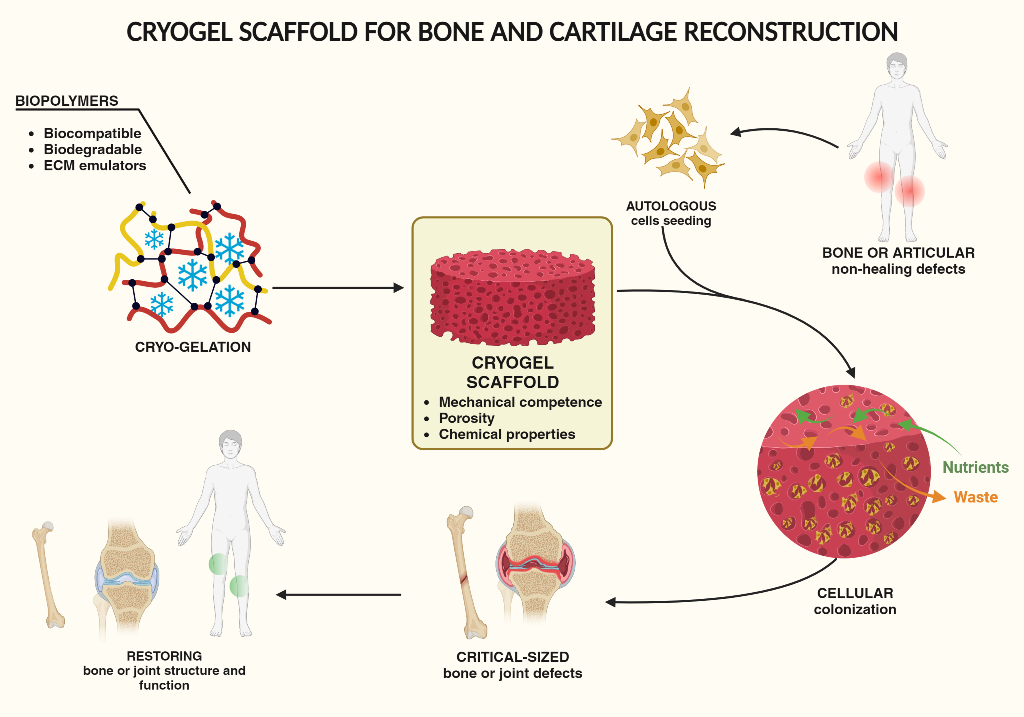
In recent years, one innovative field of the regenerative medicine proposed a valid and promising alternative to traditional autologous or allogenic grafting. The aim of tissue engineering is indeed to reconstitute damaged human tissues by the use of biocompatible materials, in the form of scaffolds, autologous cells and growth-stimulating signals. These three elements are generally referred to as the tissue engineering triad. As schematized in
Figure 1, this technology allows to regenerate tissues both in-vivo or ex-vivo, using cells collected from the patient and subsequently seeded on a scaffold appropriately tailored to match the requirement of the specific tissue. For the successful application of the tissue engineering approach, it is necessary to fabricate a scaffold perfectly compatible and integrable with the host environment, non-immunogenic and potentially able to regenerate the specific tissue, while totally restoring its function [
4,
5]. At the same time, the scaffold should be able to support cellular growing and differentiation. Among the recently reported scaffolds, cryogels represent a suitable and interesting approach for tissue regeneration, including osseous and cartilaginous tissues [
6]. Cryogels represent a technical evolution of hydrogels
, three-dimensional networks of hydrophilic polymers which share the same chemical properties, but differ for the internal structure. Both hydrogels and cryogels possess a porous inner structure, however hydrogels are characterized by small, isolated and not interconnected pores [
7]. Such features of hydrogels, do not allow proper cellular seeding, spreading and proliferation [
1]. On the opposite, cryogels consist of a macro-porous and interconnected network, comparable with a sponge. The internal structure of cryogels offers larger available free space for cell hosting, and generally confers the suitable mechanical properties for tissues reconstruction [
8]. The characteristic interconnected structure of cryogels allows, on the one hand, homogeneous cells infiltration, thus enabling the colonization of the entire scaffold [
1]; on the other hand, it allows unhindered diffusion of nutrients and metabolic wastes during the cell-life cycle [
9,
10].
Several in-vitro and in-vivo studies confirmed the high potential and applicability of cryogels in the field of the orthopaedic non-healing injuries [
11]. One interesting feature of this technology applied to bone and joint regeneration, is the possibility of tailoring physical, mechanical, chemical, and biological properties of the scaffold to closely match those of osteon- and chondro-like tissues and allow their proper reconstruction. Cryogels possess indeed the advantageous to finely tune all these properties by proper selection of the specific scaffold components as well as by accurate control of critical fabrication parameters. Lastly, they can be fabricated according to a specific shape and dimension, which can precisely fit with the defect site [
1,
6,
12].
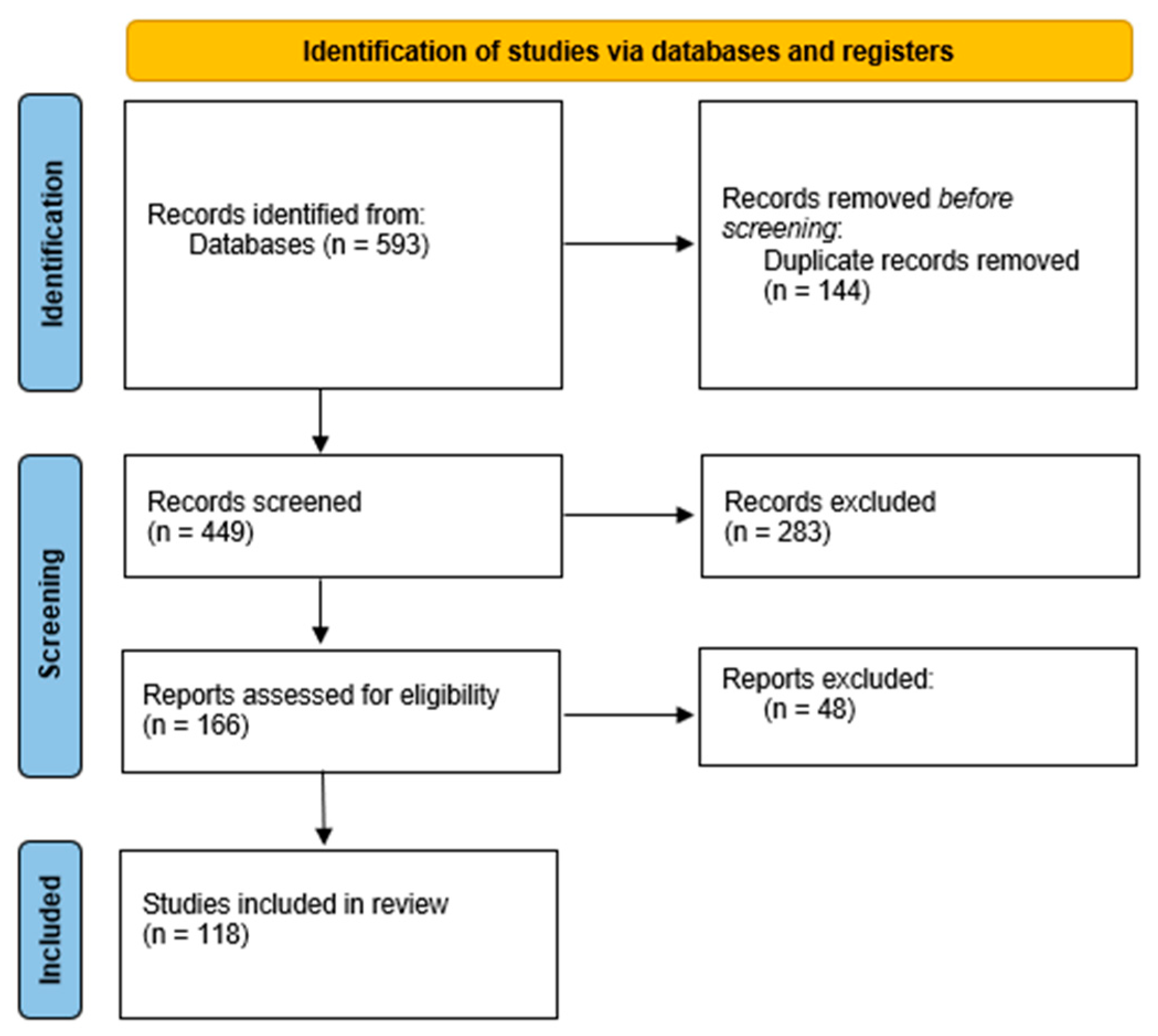
For all these reasons, cryogel scaffolding represent a promising strategy for repairing damaged bones or articular joints. The most recent results achieved in this field will be here reviewed and discussed according to the PRISMA statement for systematic reviews [
13] as reported in the
Figure 2.
2. Cryogel Fabrication
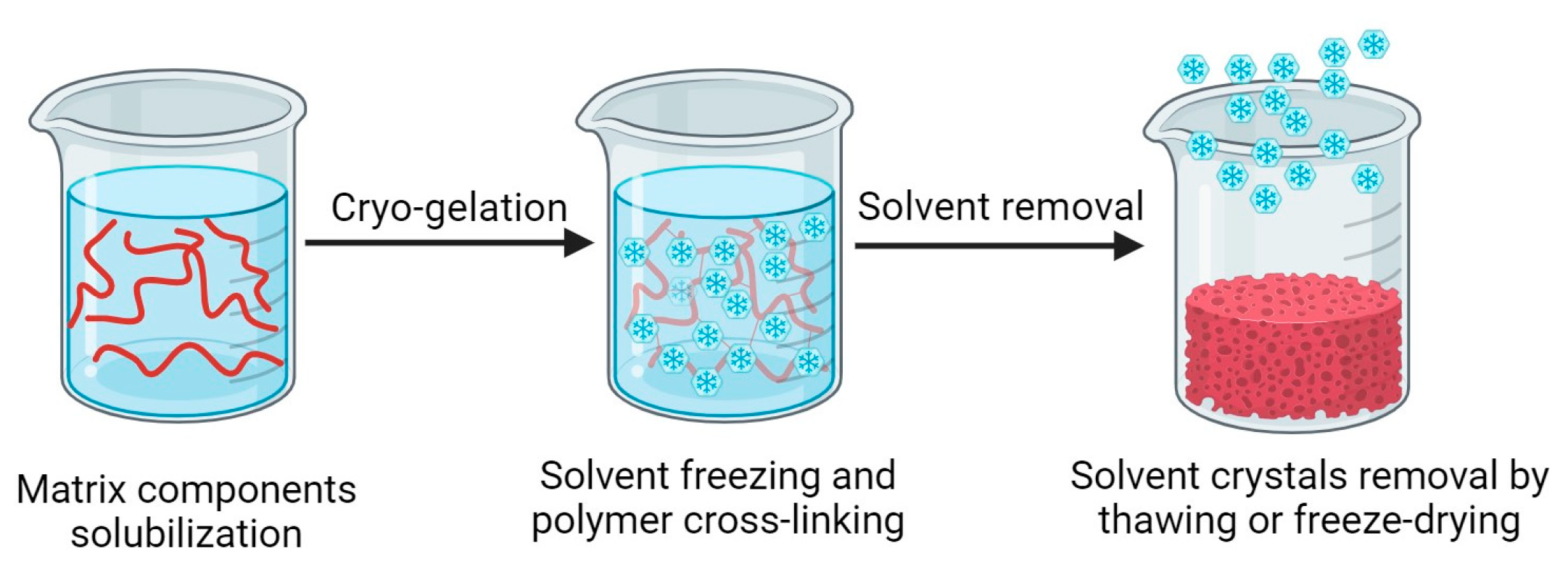
The characteristic inner structure of cryogels is obtained following a pore-templating procedure, which uses solvent crystal as porogen [
14]. All the procedures reported in the literature share a common sequence, which is composed by three different steps, as represented in
Figure 3 [
15,
16,
17]:
solubilization of the matrix components, which can be represented by monomers or macromers, and the cross-linking agents;
cryo-gelation;
solvent crystals removal.
As suggested by the name cryogel, polymer gelation is carried out at sub-zero temperature. In fact, after the solubilization of monomers or macromers, the solution is chilled under the freezing point of the solvent, usually represented by water. Under these conditions, water solidifies and, so formed ice crystals act as a template for the inner open-pore structure of the resulting cryogel [
4]. After the beginning of crystals nucleation, the monomers or macromers polymerization and cross-linking takes act, building the pore walls around the ice crystals. Once the polymer cross-linking is completed, the solvent crystals are removed leaving behind an interconnected continuous pore network as negative replica [
1]. In the following paragraphs, the critical parameters influencing each step of the procedure used for the fabrication of cryogels intended for bone or cartilage reconstruction, will be discussed.
2.1. Solubilization of the Matrix Components
In the first step, an aqueous solution of the matrix components is prepared. Similarly to hydrogels, cryogels are usually obtained by the physical or chemical cross-linking of polymers. Both natural and synthetic polymers can be used in the area of chondrogenic and bone tissues regeneration. The polymers most frequently investigated in this research area will be explored in
Section 3.
2.2. Freezing and Cryo-Gelation
Freezing and cryo-gelation steps are crucial for the architecture and topography of cryogels and, therefore, for their properties, including the mechanical properties. During the cryo-gelation step, the morphology and size of the scaffold pores critically depend on the proper balance between the freezing rate and the gelation kinetics [
1,
4,
9].
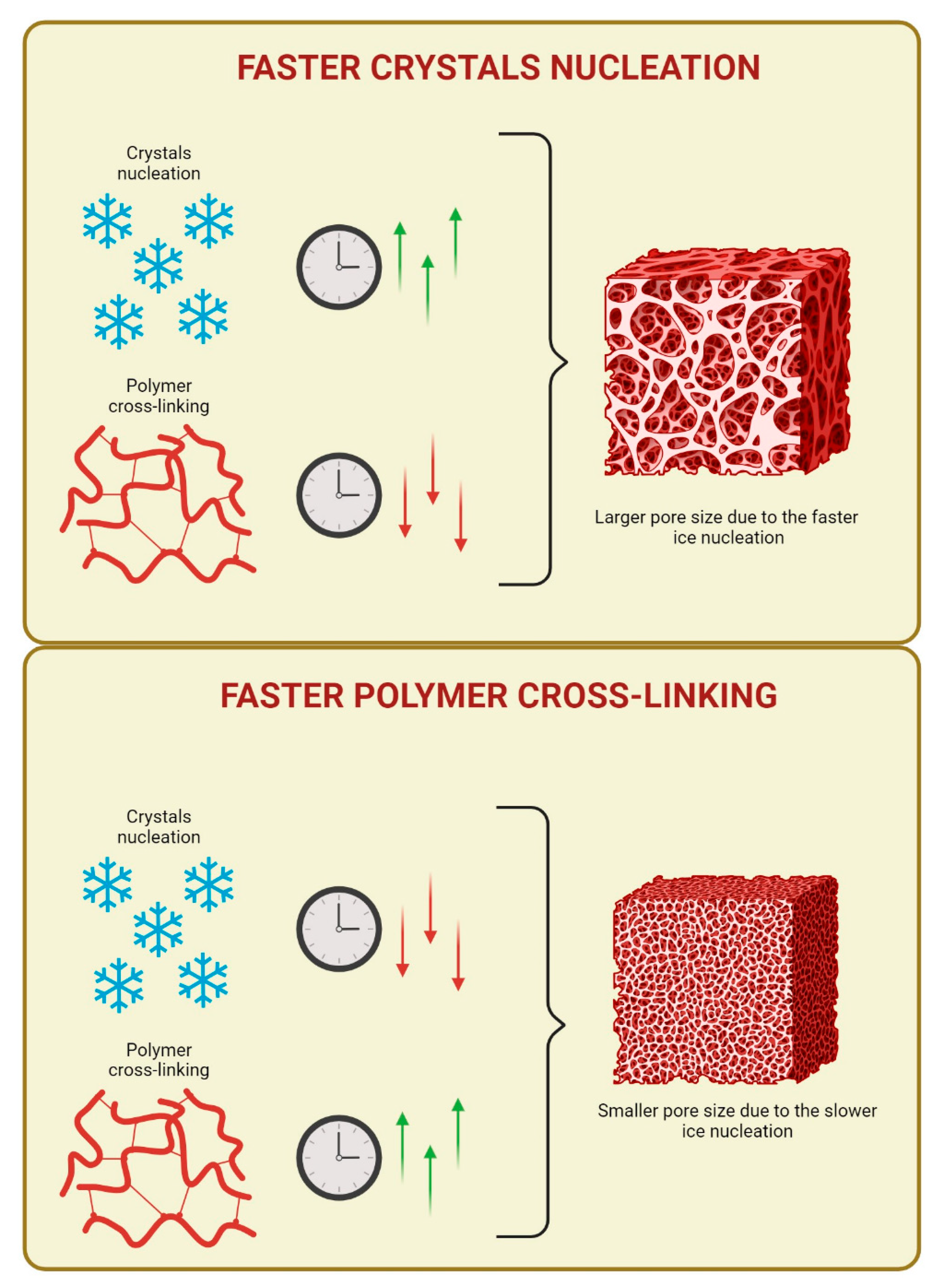
Indeed, the process consists of the combination of two events, which allows for the formation of a porous network. In specific, the cooling of the precursor solution at sub-zero temperature, induces the solvent solidification and, in this way, , two micro-phases are constituted: frozen regions and un-frozen regions [
4]. The frozen fractions are composed by solvent crystals, which will act as porogens, while the un-frozen microphase is where cryo-gelation takes place or rather the polymer chains cross-link around the ice crystals and builds the pore walls [
4,
18]. These two events (solvent crystallization and polymer cross-linking) must occur with a precise hierarchy. Especially, solvent nucleation and crystallization must take place before polymer cross-linking [
4]. When this occurs, all the monomers, macromers and cross-linking reagents of the starting solution undergo a cryo-concentration or freeze-concentration effect [
18,
19], which is one of the major factors responsible for the increase in the rate of the chemical reactions and intensification of intermolecular interactions inside the un-frozen microphases. In other words, these un-frozen microphases act as microreactors [
1,
9,
19]. Indeed, during the freezing process, solvent solidification subtracts water molecules from the un-frozen phase, thus increasing the concentration of all the solutes. Therefore, before the freezing is completed, some chemical reactions can occur and proceed at rather fast rates, despite the low temperature. Alongside this aspect, it is also necessary to consider the cooling rate at which the cryo-gelation process is carried out, as it has direct impact on the ice crystal habits formed, such as size, number and morphology, and this, in turn, impacts the pore size of the polymer network, once the ice crystals are removed. In particular, very high cooling rates lead to the formation of many small ice crystals and the corresponding polymer network will have a hydrogel-like structure. On the opposite, low amounts of ice nucleation sites, which yield to few large ice crystals, are obtained when the freezing process is carried out at slower rates of freezing [
4]. The second event allows the formation of polymer networks characterized by dense and resistant pore walls and properly named cryogels. This result can be achieved when the freezing stage is carried out within the range of -5°C and -20°C [
9,
20,
21,
22,
23,
24,
25,
26].
It is also known that ice crystal morphology is a function of the cooling rate. Specifically, samples frozen at slow freezing rates yield dendritic ice crystals, whereas samples frozen at fast freezing rates produces needle-like structures [
27]. Also this factor, obviously, contributes to determine properties and behaviour of the resulting polymer network. Therefore, all that considered, the cryo-gelation step requires subtle balance between the operating conditions and the composition of the precursor solution, as they have tremendous effect on the structure and properties of the resulting cryogel [
4].
In conclusion, the cryotropic gelation is a delicate interplay between solvent crystallization and polymer cross-linking. The precise sequence and rate of these events, significantly influence the final cryogel structure. Therefore, understanding and careful handling of these parameters is necessary to obtain cryogels with specific properties, which can emulate the complex structure of a physiological tissue.
The cooling process may be carried out by using different techniques. Generally, the immersion of a mold in a freezing-bath (FB) [
28] or unidirectional freezing (UF) system [
29] are used to freeze the precursor solution. In the first case, a temperature-controlled bath is used to freeze the sample at the desired temperature. Since the cooling process takes place with a centripetal direction, crystallisation occurs entropically in the sample [
30]. On the opposite, by the UF it is possible to induce ice crystals formation on a preferential direction. As a consequence, the resulting cryogel will show an internal structure characterized by oriented pores. The freezing direction can be controlled by immersing cooled pins of an inert material, such as cupper, inside the precursor solution. When these pins are cooled-down, solvent crystallisation will occur radially along the pin axis. Due to the proximity of two consecutive pins, crystals elongation is limited. It means that in section, so formed cryogels are structured in macroscopic conic regions made by lamellar pore network. Besides, the upper and lower edges of the cryogels made by UF present a pore network tangential to the cryogel surface. This arcade composition of the inner structure is so constituted by two border regions made by longitudinal fibres and one inner region constituted by a lamellar network, oriented perpendicularly to the edges. UF approach for freezing is a relatively new technique, which has been proposed to meet the peculiar and complex requirements of some specific tissues, such as the physiological structure of cartilage, which is structured in aligned lamellar fibrils. This freezing technique allows tuning the mechanical behaviour of the scaffold, trying to match that of native cartilage. Such feature is particularly important for the regeneration of complex tissues, such as bone and cartilage, because the mechanical behaviour of the scaffold influence morphology and expression pattern of seeded cells [
29]. The correlation between scaffold properties, cellularity and tissue regeneration will be further discussed in
Section 4.
2.3. Cross-Linking Mechanisms
Cryogel formation may involve physical or chemical cross-linking among the polymer chains. However, physically cross-linked cryogels usually present some drawbacks associated to poor mechanical and physical properties, and, for this reason, are barely proposed as scaffolds for tissue engineering. Instead, chemical cryogels based on covalent bonds, usually guarantees a more stable 3D network and resistance to physical or mechanical stresses. Therefore, chemical cryogels appear to be superior scaffolds for tissue regeneration, as reported for cryogels made of chitosan and gelatin [
4]. Indeed, physical cryogels composed by these two polymers resulted brittle and inhomogeneous. On the contrary, when the same polymers were covalently cross-linked, the corresponding cryogel networks presented higher mechanical stability and, in general, resulted more suitable to serve as scaffold for cell seeding and growing. Such stability is also important because it allows scaffold sterilization, even by moist heat. Sterilizability is a fundamental requirement for any biomedical use of cryogels [
31] and steam sterilization represents the easiest, most effective, less expensive, largely employed and FDA-approved method for the sterilization of this kind of structures [
4,
32].
One of the most used cross-linking approach is based on the radical reaction of polymers carrying methacryloyl groups on their chains. The radical cascade is usually triggered with peroxides, such as ammonium peroxydisulfate (APS), a radical initiator, which is usually employed along with N,N,N’,N’-tetrametylethylendiamine (TEMED), used as a catalyst [
17,
33,
34,
35]. The radical cross-linking of methacryloyl or, more in general, vinyl derivatives can also be induced via UV-photocuring. This cross-linking strategy is largely employed for hydrogel fabrication [
36,
37] and involves the use of photoinitiators, such as lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) [
38] or 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (I2959), to initiate the radical cascade [
39]. Some attempts have been made to adopt the UV-induced polymer cross-linking also for the fabrication of cryogels. To do this, the cryo-gelation step is usually divided in two consecutive phases. Firstly, the precursor solution undergoes a partial freezing, which allows the germination of the solvent crystals, but not the full solidification of the sample. The partially frozen samples are then exposed to UV-radiation to initiate the cross-linking process, before the ice crystallization stage is completed [
40].
Other cross-linking protocols, largely used for the fabrication of cryogel scaffolds suitable for bone or cartilage regeneration, are based on coupling reactions with bifunctional agents, such as glutaraldehyde (GA) and divinyl sulfone (DVS) [
4,
41,
42]. GA coupling reaction has been used for the cross-linking of polymers bearing primary amino groups, such as gelatin [
4], collagen [
43] and chitosan [
16]. Instead, polymers having hydroxyl groups such as polysaccharides, have been chemically cross-linked using DVS in alkaline ambient [
42]. The oxygen of two hydroxyl groups can react with the two electronic-deficient double bonds of DVS, leading to the polymer cross-linking.
Another frequently used coupling reaction is known as carbodiimide coupling and is based on the use of (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) used along with N-hydroxysuccinimide (NHS) [
30,
44,
45,
46,
47]. These reagents are used to couple carboxylic groups of one polymer with primary amino groups or hydroxyl groups of the same polymer or a different one. It works through the initial activation of the carboxylic groups, followed by their subsequent reaction with primary amino or hydroxyl groups. The EDC/NHS-mediated coupling reaction has been proposed for the cross-linking of gelatin and collagen [
48].
The above reported chemical cross-linking methods are largely employed for cryogel fabrication, even if some of the employed reactants and their residues raise important safety issues. For this reason, there is a continuous search for alternative and safe cross-linking protocols. Recently, it has been proposed the use of graphene oxide (GO) as cross-linker of gelatin-based scaffolds. The cross-linking reaction involves the primary amino groups of gelatin and the different chemical functions on the surface and the edges of GO sheets [
21].
2.4. Solvent Crystals Removal
After the cryogelation step is completed, the solvent crystals can be removed by thawing at room temperature [
4,
7,
49,
50], or by freeze-drying [
51,
52,
53]. In both cases, a porous structure, which constitutes the negative replica of the solvent crystals, is obtained.
3. Material Used for the Fabrication of Cryogels Suitable for Bone and Cartilage Reconstruction
Scaffolds used for tissue regeneration should be made by polymers which possess precise histological and biological properties to allow proper, cellular adhesion and tissue reconstruction. Moreover, the ideal cryogel components should be biodegradable, since according to the regenerative medicine purpose, it is expected the gradual substitution of the cryogel network by natural histological material [
12]. This means that the non-healing injury is finally substituted by a biological tissue, which will possess the required mechanical properties to support the load-bearing function of the bone tissue and the mechanical articulation of the joint cartilage.
Finally, the scaffold matrix, should reproduce the mechanical and physical qualities of the target tissue, i.e. adequate hardness for the bone and specific tribological behaviour in the case of the articular cartilage [
33,
54,
55]. Nevertheless, the reproduction of the complex structural and functional properties of the biological tissues is very difficult by the employment of a single material [
2,
30]. For this reason, to obtain suitable scaffolds, cryogels are frequently fabricated blending together different materials, including both organic and inorganic biomaterials, as summarized in
Table 1 and
Table 2. When two different biopolymers are combined for the production of a cryogel scaffold, they can be cross-linked each other to form a continuous and interconnected network[
9], or they can be separately cross-linked to form an interpenetrated
network [
56]. The construction of interpenetrated networks is a fine strategy which allows to obtain more complex systems. Besides to the possibility to blend different polymers with different chemical or biological properties, these particular networks possess the advantage of tailoring and customize the porosity and the mechanical behaviour of the final system [
57]. The fabrication of these systems involves the recurrent synthesis of a new cryogel structure inside the macro-porous meshes of a pre-formed cryogel network [
58]. This protocol may be applied both for a second or more polymeric networks [
56]
Cryogel scaffolds for bone and cartilage regeneration have been prepared from both natural and synthetic polymers. Latest advances obtained with the different biomaterials used for cryogel fabrication will be discussed in the following paragraphs.
3.1. Collagen-based Cryogels
Collagen (Coll) is a natural component of the extracellular matrix (ECM) and is largely represented in all mammalian tissues. It contains amino acid sequences, which play a tremendous role in cell adhesion and ECM degradation and remodelling [
30]. These sequences include the tripeptide arginine-glycine-aspartate (RGD), which has been shown to mediate cell adhesion by the interaction with cell integrins, that is a fundamental step for the first-phase of cell colonization of the scaffold [
59]. For these reasons, Coll has been largely used for bone and cartilage regeneration, alone or in combination with other organic or inorganic materials. In this sense, Odabas et al. developed cryogel system combining Coll with carboxymethyl cellulose (CMC) and tricalcium phosphate (TCP). CMC has been proposed as it can mimic aggrecan, an ECM protein which provides gel behaviour to cartilaginous or membranous bone tissue. [
30]. TCP, instead, is usually used to improve the mechanical properties of the scaffold, while providing it with osteoinductive property, that is the ability to induce differentiation into osseous tissue. Particularly, the addition of TCP to Coll/CMC cryogels caused an increase of the compressive resistance from 300 kPa of plain Coll/CMC cryogels to more than 400 kPa of the TCP enriched systems [
30]. Although TCP is able to improve the mechanical properties of cryogels, we are still far from those of native bone and cartilage tissues, which are characterized by compressive moduli in the range 10-20 GPa for bone [
60] and 0.5-1 1 MPa for cartilage [
61]. Therefore, other strategies have been attempted to obtain cryogel scaffolds more suitable from a mechanical point of view. This issue is of fundamental importance for tissue engineering in general, but it is particularly challenging for the reconstruction of load-bearing tissues, such as bone and cartilage. Indeed, there is continuous research aimed at improving the mechanical competence of the scaffolds used for the regeneration of these tissues [
23]. This feature is also important because it has been observed that the stiffness of the scaffold influences its osteoinductive nature, and so its ability to induce mesenchymal cells to differentiate into mature bone-forming cells [
62].
In this context, several works explored the use of hydroxyapatite (HA) (Ca
10(PO
4)
6(OH)
2), which is a major component of the mineral part of the bone [
58,
59,
60,
61,
62,
63,
64,
65]. The mineral fraction of bone trabeculae is indeed constituted by Ca (II) salts, which, in total, represent about 70% of the bone weight. During the early stage of the mineralization process, Ca (II) ions precipitate on the surface of the ECM proteins, firstly as calcium phosphate, which after weeks and months of resorption and replacement become amorphous HA crystals. Therefore, the inclusion of HA within the structure of a cryogel scaffold represent an interesting biomimetic approach, which allows to produce an increase in the storage modulus and mechanical resistance of the scaffold cryogels, dependent on the apatite content [
69,
70]. Moreover, as reported for TCP, HA imparts osteoinductive properties to the scaffold and encourage its mineralization. This feature will be further discussed in
Section 4.3.
3.2. Gelatin-based Cryogels
Coll, as a natural component of the ECM, would represent an ideal candidate for cryogel fabrication, however its actual use is hindered by its low solubility in water. Indeed, harsh acidic conditions are usually needed to allow the preparation of cryogel scaffolds [
69,
71]. For this reason, gelatin (Gel), a derivative produced from the partial hydrolysis of Coll, is most frequently used for the development of scaffolds for tissue regeneration. Compared to native Coll, Gel shows higher solubility in water, while preserving the typical RGD sequences of Coll, and so the innate properties to promote cellular adhesion [
35]. Nevertheless, Gel-based scaffolds possess two major drawbacks: weak mechanical properties and poor thermal stability. To overcome these limits, Gel is frequently chemically cross-linked and combined with other polymers. To improve the thermal stability, Gel has been chemically cross-linked with GA [
72,
73,
74]. However, due to the toxicity of this cross-linking agent, alternative dialdehydes have been proposed to produce stable and mechanical resistant Gel-based networks, such as pullulan dialdehyde [
23] and dextran dialdehyde [
8], obtained from the oxidation of pullulan and dextran respectively. Both these polymers can react with Gel via a Schiff’s base reaction, thus forming stable networks.
Other strategies used to produce Gel-based chemical cryogels involve the modification of the gelatin backbone with specific chemical moieties. One of the most diffuse functionalization of Gel is based on the insertion of methacryloyl groups, with the consequent formation of methacryloyl Gel (GelMA) [
75]. This derivative allows the production of stable three-dimensional networks, with mechanical properties and porosity tuneable by varying the derivatization degree of GelMA [
34,
35,
76], which, however, it is sometimes not appropriately determined [
77] or even non evaluated. Despite this feature, GelMA is rarely used alone for the fabrication of cryogel scaffolds, but it is usually mixed with other biomaterials in order to achieve scaffolds with adequate mechanical resistance for tissue regeneration [
78]. Nevertheless, Di Muzio et al. recently have reported on the possibility of obtaining cryogels made of GelMA only, characterized by high biocompatibility and undisputable mechanical resistance [
9]. These results could be achieved using GelMA derivatives characterized by very low physical gelation point. They supposed, indeed, that if GelMA physical gelation occurs too early during the cryogelation procedure, it may hinder the covalent cross-linking of the polymeric chains and leads to networks with poor mechanical competence, which usually require the addition of mechanical adjuvants. GelMA derivatives with low gelation temperatures can be obtained using Gel obtained from alternative animal sources, such as cold-water fish, as starting material [
79] or adopting a specific synthetic procedure carried out under denaturing-solvent conditions [
77].
When Gel-based scaffolds are used for bone tissues regeneration, they are often decorated by osteoinductive components [
35], such as silica bioglass, which furnishes the inorganic ions necessary to support bone mineralization [
80] and provides an ECM-like environment that enhances osteo regeneration during the bone healing process [
35].
3.3. Silk Fibroin-based Cryogels
Silk fibroin (SF) is a fibrous protein obtained from both silkworms like
Bombyx mori and orb-weaving spiders like
Nephila clavipes [
81]. It is able to form peculiar gels, when solubilized in water. SF chains are characterized by hydrophilic domains and hydrophobic β-sheet-crystallites [
82]. While the hydrophobic domains provide the gel with mechanical strength and resistance, the hydrophilic regions guarantee its wettability and regulate its elasticity and toughness. [
26,
57]. The resulting combination of stiffness and resistance, make SF an interesting material for the fabrication of scaffolds for tissue regeneration, and, more specifically, bone tissue engineering. In this context, stable and mechanically resistant SF-based gels have been developed using ethylene glycol diglycidyl ether as cross-linking agent . This cross-linker is able to trigger the conformational transition of fibroin from random coil to ordered β-sheet structure, which is responsible of fibroin gelation. SF-based cryogels obtained in this way showed unique elasticity feature that allows them to resist complete compression without any crack development. The compressed cryogels immediately swell during unloading to recover their original shape [
82]. Instead, Yetiskin and colleagues studied the design of a silk-fibroin interpenetrated system, which allows the formation of scaffolds with improved mechanical competence [
57].
3.4. Glycosaminoglycans (GAGs)
GAGs are a class of natural polymers frequently employed for the fabrication of scaffolds for bone and cartilage regeneration, as they are components of the ECM proteoglycans. GAGs are linear polysaccharides made up of repeating disaccharide units. Among them, chondroitin sulphate (CS) and hyaluronic acid (Hac) are the GAGs most often explored in the field of tissue regeneration.
Chondroitin sulfate (CS) is a sulphated polysaccharide based on a chain of alternating units of glucuronic acid and N-acetylgalactosamine. It is an essential component of cartilage and plays an important role in the elasticity and functionality of articular cartilage; moreover, it offers stimulus for proteoglycan and type II collagen secretion [
17]. Such features make CS an interesting biomaterial for the fabrication of scaffolds aimed to cartilage regeneration. This GAG exists in various forms related to the nature of the disaccharide unit, sulfation degrees and molecular weight [
83]. Sulfation can occur on either O-4 or O-6 position, giving CS-A and CS-B respectively . Commercially, it is available as variously composed mixtures of CS-A, CS-B and a minor fraction of un-sulfated chondroitin [
84,
85]. Nevertheless, some works have been published reporting the use of pure CS-4-sulfate [
86] or CS-6-sulfate [
87].
HAc is a non-sulphated GAG constituted by disaccharide repeating units made of D-glucuronic acid and N-acetyl-D-glucosamine. It is mostly found in the ECM of connective tissues, where it supports cell migration and proliferation as well as cartilage integration throughout the development of embryonic cartilage [
42,
88,
89].
Rarely, GAGs have been employed alone for cryogel fabrication, whereas they are frequently combined each other or with other biomaterials, to closely mimic natural ECM properties and behaviour. In this sense, Kuo and colleagues prepared macro-porous gelatin/chondoitin-6-sulfate/hyaluronan cryogel scaffolds for cartilage tissue engineering [
87]. Such cryogels showed to be effective to emulate the extra-cellular physiological environment and to exert stimuli for cartilage reconstruction. However, the inclusion of chitosan as a further cryogel component resulted in a general improvement of the biomechanical properties of the scaffold. Chitosan is a cationic (1-4)-2-amino-2-deoxy-β-D-glucan polysaccharide, which is generally considered to resemble the structure of GAG and believed to mimic its function in the modulation of cell behaviour and phenotype, as observed by Kuo and colleagues. These authors evidenced the necessity of blending multiple different biopolymers to mimic as much as possible the complex physiological environment of cartilage, while promoting cell proliferation and differentiation, in order to allow formation of mechanically functional cartilage [
87].
3.5. Other Natural Polymers
Other natural polymers have been employed for the fabrication of cryogels intended for bone or cartilaginous reconstruction. Among them, it has been reported the use of dextran (Dex) and nanocellulose (nCell). Dex is a bacteria-derived polysaccharide, which shows enzymatic degradation and good biocompatibility [
12]. Bölgen and colleagues developed dextran-based cryogels, using Dex as HEMA-L-lactate derivative, obtaining an interconnected network, which demonstrate compatibility with cartilaginous cells. Regarding to the mechanical behaviour, the tested systems showed remarkable mechanical flexibility, softness, and toughness that might simulate tissue softness [
90].
nCell, instead, has been proposed as candidate for tissue engineering due, in particular, to the mechanical properties of its scaffolds [
91]. It exhibits good micro-structuring through the formation of weak interactions (H-bonds, Van der Waals interactions, etc.) among the polymeric chains [
92]. nCell-based cryogels meet the microstructure and mechanical performance requirements of scaffolds for tissue engineering. However, when proposed for bone tissue regeneration, a limiting factor in nCell utilization is the lack of bioactivity to induce proper bone regeneration. Indeed, nCell alone shows poor osteoconductivity, therefore, it must be integrated with bioactive compounds, such as silica bioglass, to promote bone tissue formation and mineralization [
93].
3.6. Synthetic Polymers
Several synthetic polymers have been investigated for the development of scaffolds for bone or cartilage regeneration, including polyethylene glycol (PEG), polyhydroxyethyl- or polyhydroxypropyl-methacrylate (p-HEMA; p-HPMA) and polyvinyl alcohol (PVA). A summary of them is reported in
Table 2.
PEG is a polymer largely used for biomedical application and tissue engineering [
7,
17,
102,
103,
104]. PEG-based scaffolds exhibit good mechanical properties as well as good cyto- and immune-compatibility. Nevertheless, PEG-based cryogels display fast swelling, which can promote homogenous cell spreading within the scaffold [
18]. Despite these positive features, PEG can only be proposed in combination with other biopolymers, because PEG structure do not allow protein adsorption and cell adhesion, which are basic and fundamental events for tissue reconstruction. As an alternative approach, PEG can be modified by the addition of chemical moieties which ensure cellular adhesion (i.e. RGD sequences), as reported by Bruns and colleagues [
106].
p-HEMA and p-HPMA are two other synthetic polymers, which are largely used for medical devices application [
107,
108] and are also proposed for cartilage tissue repairing [
54,
109]. They are synthesized by polymerization of the monomers HEMA and HPMA. The corresponding cryogels are generated by cryogenic radical polymerization of the monomers in the presence of N,N’-methylene-bis-acrylamide [
54].
p-HEMA cryogels, in particular, present marked tribological properties, as they suffer limited erosion, when experience strains comparable to those occurring at the joint surface [
54]. Cryogels produced with two HEMA derivatives, namely N-vinylformammide (HEMA-NVF) and 1-vinyl-2-pyrrolidinone (HEMA-NVP) showed high water absorption and water retention. The improved water intake leads to higher incorporation capacity of the physiological fluids (i.e. blood or serum) like into the joint structure. This property relates to higher lubrification of the scaffold surfaces with consequent resistance to the friction forces, likely to the articular joints.
Another synthetic polymer adopted for cryogel fabrication is PVA. It is a non-toxic and biocompatible material, suitable for cartilage substitution, as it is able to form scaffolds having mechanical characteristics similar to human cartilage [
109,
110]. However, as per PEG, also PVA is a biologically inert polymer and, therefore, it is usually enriched with bioactive and biocompatible components, such as HA [
111,
112]. The inclusion of HA within PVA cryogels gave stable and homogeneous networks and produced a positive effect on the mechanical behaviour of the final construct, as reported for other systems[
113].
4. Cryogels into the Biological Environment: Physical, Chemical and Physiological Properties for Scaffold Cellularity and Body-Implantability
For effective tissue regeneration, the supporting scaffold requires mandatory characteristics for cellular infiltration as well as for the implantation in the physiological environment. To offer a suitable space for cellular colonization, waste-nutrient exchanges, and to guide the differentiation of cells into specialized tissues, specific porosity and stiffness parameters must be respected. Moreover, for the surgical implantation into the mammalian organism, it is necessary to obtain a histo-compatible scaffold, which can be naturally integrated into the host environment without any occurrence of the immune system [
47,
116,
117].
In
Section 4.1,
Section 4.2 and
Section 4.3 the influence of the physical and chemical properties of cryogels on the scaffold cellularity will be discussed. Moreover, in
Section 4.4, the histological integration of cryogel scaffolds
in vivo will be analysed.
4.1. Structural Architecture and Mechanical Properties of Cryogels for Cell Hosting
For the cellular adaptation, it is fundamental that the scaffold possesses a sponge-like architecture and an adequate porosity to offer an adequate surface for cells anchoring and allow generation of organized tissues. Another crucial factor is represented by pore interconnectivity, since the interconnection along a continuous open maze allows the infiltration and ingrowth of cells, even in deeper and remote portions of the scaffold [
4]. Indeed, biomimetic scaffolds usually employed for bone or articular cartilage reconstruction, display a high level of total open porosity, which is comparable to the porosity range of 30% to 95% found in human bone trabecula or cartilaginous joints [
104]. Even the morphology of the porous structure appears to affect cell viability within the scaffold. Generally, a mesh constituted by pores with a uniform size-distribution, allows deeper cellular infiltration and retention among the entire structure. Nevertheless, a nonuniform pores size distribution, appears to increase the scaffold cellularity [
22], perhaps due to the higher retention which non-homogenous meshes can guarantee. Beside this aspect, also pores size appears a relevant parameter to promote cell infiltration and proliferation, even if itis a controversial topic in the literature. It has been reported that adequate cellular infiltration into a cryogel scaffold is reached when the pore size is comprised in the range from 30 to 100 μm [
4]. Under these conditions, osseous and cartilaginous cells are usually able to proliferate and differentiate [
118]. Nevertheless, even if cells are able to colonize scaffolds falling within this pore range, it has been reported that values below 100 μm allow only a partial cellular ingrowth, because they reach a stalemate of the proliferation-rate at a certain time of culturing [
119]. The critical role of pore-size on scaffold cellularity can be related to space limitations, which may hinder cells proliferation and consequently their differentiation [
105]. At the same time, pores size is rationally connected to the ability of the structure to absorb liquids as well as to the diffusion rate through the interconnected channels [
47]. These parameters are crucial to allow both homogenous cell seeding and spreading within the scaffold and easy nutrient-waste exchanges with the culture medium [
38,
120]. Therefore, cryogels with adequate porosity may support a more efficient cellular life-cycle and may consequently lead to a better differentiation profile [
121,
122]. However, the pore diameters subject remains an open issue on which further investigations should be conducted. For instance, the discussion on this topic should include further studies on pores with larger diameters than the above-mentioned ranges, in order to evaluate the effect of larger spans on the scaffold cellularity and on its ability to homogenously spread and retain the seeded cells.
Besides scaffold porosity, also its stiffness appears to tremendously influence cell anchoring and signalling and, consequently, the differentiation process [
5,
62,
98,
121]. The mechanical behaviour of the scaffold should replicate the native ECM of the specific cell lineage, in order to exert a differentiation stimuli on the seeded cells [
121,
123]. For example, Liu and colleagues reported that the ECM rigidity can induce osteogenic differentiation of rMSCs (rat mesenchymal stem cells) through FAK-ERK1/2 signalling pathway, as the focal adhesion kinase (FAK) is an enzyme implied in cellular adhesion, migration and proliferation. [
114]. Same dependency of the cellular differentiation response to the stiffness of the scaffold was reported by Bhat and colleagues that investigated neo-cartilage formation as well as by Häussling and colleagues that studied regeneration of collagenous bone [
5,
119].
As discussed in previous sections, one of the advantages founded in using cryogels for tissue reconstruction, is that the mechanical behaviour of the scaffold can be tailored by the control of the fabrication parameters and the matrix composition. Tailoring these properties, it is possible to drive signalling pathways and gene expression towards cell adhesion, growing and differentiation.
4.2. Chemical Properties of the Scaffolds
Cytocompatibility is of course a mandatory property of cryogel-based scaffolds designed for tissue reconstruction. For this reason, as described in
Section 3, natural polymers characterized by cell tolerability and biocompatibility are usually preferred for cryogel fabrication. Moreover, polymers showing a chemical similarity with the native ECM are able to improve the scaffold cellularity because it determines the gene expression of proteins typically produced in the matrix of native tissues [
87].
A second property of the scaffold that affects cell adhesion and proliferation is the matrix hydrophilicity. Indeed, this attribute allows the homogenous infiltration of biological fluids and cells within the scaffold and promotes the adsorption of ECM proteins, such as albumin, fibrinogen and fibronectin, which are implied in the cellular anchoring, proliferation and differentiation processes, [
104,
124,
125].
The described properties are essential features of cryogels intended for tissue regeneration, however they could be not sufficient for appropriate cell adhesion, which is usually promoted by the presence of specific chemical motifs. Among them, the tripeptide sequence arginine-glycine-aspartate (RGD), largely present in ECM proteins, such fibronectin and collagen, is one of the most important, as it plays a pivotal role in cell adhesion and proliferation. RGD sequences are recognized by integrins, a kind of biding proteins on the cellular membranes, which mediate ECM-proteins binding in the physiological environment. This bridge between the cellular membrane and the ECM proteins, activates biological pathways, which contribute to adhesion, proliferation and morphological adaptation of cells [
59]. In a work published by Koh et al., it was observed an increase in chondrogenic phenotype thanks to the addition of RGD sequences in chondroitin-based cryogels. Indeed, the RGD sequences appeared to up-regulate indirectly the expression of early chondrogenic markers in porcine-derivative chondrocytes, as SOX9, and late chondrogenic markers, such as COL2. Moreover, it was observed the expression of type II collagen and a consequent collagen accumulation into the scaffold network. These results were related to the high cellular viability reached within the scaffold, which was mediated by the adhesion stimulant effect of the RGD sequences [
126].
4.3. Inorganic Decoration as Adjuvant for the Scaffold Cellularity
Cellularity of scaffolds intended especially for bone reconstruction is positively influenced by the addition of some inorganic components, such as calcium phosphate salts (CP) and silica bio-glasses (SB). As already mentioned, this kind of decorations improves the mechanical competence of the scaffold and acts as cellular adjuvant. Considering that mesenchymal differentiation is associated with the precipitation of calcium apatites, the inclusion of calcium sources into the scaffold may encourage the secretion of new matrix, thus establishing a faster and more effective healing process at the wound site [
70]. Moreover, in a work published by Abueva et al., the adjuvant role of CP crystals for scaffold cellularity was related to the adsorption of ECM proteins on the CP crystals surface [
22]. In vitro studies evidenced that the presence of CP had a substantial effect on protein uptake, which influenced the attachment of cells on the material surface and consequently the rate of proliferation of MC3T3-E1 pre-osteoblast cells, which were able to spread evenly within the scaffold reaching the inner portion of the network.
Similar behaviour was reported for scaffolds enriched with other inorganic materials, such as GO [
20] and silica molecules [
62], which showed interesting capacity to induce cell differentiation consequent to the absorption of ECM proteins. Indeed, this scaffold allowed a remarkable up-regulation of the expression of ECM proteins genes related to the osteogenic differentiation, such as the run-related transcription factor 2 (RUNX2) and COL1A1 mRNA [
25]. Also for silicon-nitride (SiN) decorated scaffolds were reported interesting osteoinductive properties, associated with antibacterial activity [
62]. Despite silica biological role in the ossification process remain controversial, in this case it has been supposed that the osteoinductive properties were related to the strong negative charge of SiN, which causes calcium ion binding and, in this way, promotes apatite deposition. Additionally, the acicular polycrystal microstructure of SiN increased the surface area of the scaffold, thus promoting the protein adsorption and, consequently, the bioactivity of the scaffold [
62].
4.4. Histo-Compatibility and Immune-Tolerance
Tissue regeneration can be achieved following an in-vitro or an-situ strategy. The second approach consists in the implantation of tissue-specific biomaterials in combination with cells and/or biomolecules at the tissue defect. Such approach requires the histological integration of the scaffold into the physiological environment. Histo-compatibility of the cryogels is assessed by implantation of the scaffold in animal defect models or by subcutaneous implantation[
127,
128,
129]. Following implantation, the host organism integrates the structure into the native environment. More specifically, the host responds by producing connective tissue and new blood vessels, which are fundamental to activate proliferation and differentiation of mesenchymal cells and, consequently, formation of new bone or cartilaginous structures [
5,
101].
In-vivo implantation of a scaffold also serves to assess the host immune-tolerance [
103]. Indeed, scaffolds which are suitable for tissue reconstruction, must not induce inflammation, degeneration or necrosis at the site of implantation, to allow the integration at the wound site [
74].
5. Conclusions
The pioneering field of tissue engineering recently proposed the use of cryogels to heal critical-sized defects in bone or cartilaginous tissues. Cryogels are innovative polymeric scaffolds, which are formed using a straightforward manufacturing process, which leads to very versatile systems. Thanks to their peculiar porous network, cryogels appear to be able to support cellular growth and differentiation, which, however, can be obtained and aided by opportune selection of the scaffold components. Nevertheless, cryogel versatility and the employment of cutting-edges materials or technologies, open the perspective to obtain systems, which better replicate the complex mechanical and physiological properties of the native tissues.
These encouraging results motivate future research and further investigations to allow effective cryogel use for bone and articular regeneration. As first, these systems might improve their mechanical competence, putting it near to the native tissues. Once reached this goal, the effective clinical use of cryogels need to be assessed in humans, thus moving from preclinical animal models to clinical studies. For this reason, rigorous clinical trials and over extended time studies are now necessary to assess cryogels employability in the clinical practice, as scaffolds for human implants.
Lastly, advancements in technologies like 3D imaging and printing have the potential to implement cryogel technology and enhance the development of personalized cryogel scaffolds. This could increase the chances of successful tissue reconstruction, aligning with the principles of modern personalized medicine.
To sum up, cryogel scaffolds show great potential for rebuilding bone and cartilage tissues. Continuous advancements in scaffold design are expected to bring about breakthroughs that are already revolutionizing the field of regenerative medicine. This offers new hope to patients suffering from articular and skeletal non-healing disorders.
Author Contributions
Conceptualization, V.C.C., L.D.M.; writing—original draft preparation, V.C.C., P.P.; writing—review and editing, P.P, V.C.C.; supervision, P.P., M.A.C., S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A-Abbreviations
| ALP |
Alkaline phosphatase |
| APS |
Ammonium peroxydisulfate |
| Coll |
Collagen |
| CP |
Calcium phosphate |
| CS |
Chondroitin sulfate |
| Dex |
Dextran |
| DexOx |
Oxidized dextran |
| ECM |
Extracellular matrix |
| FB |
Freeze bath |
| GA |
Glutaraldehyde |
| Gel |
Gelatin |
| GelMA |
Methacryloyl gelatin |
| HAc |
Hyaluronic acid |
| HA |
Hydroxyapatite |
| HEMA |
Hydroxyethyl methacrylate |
| HPMA |
Hydroxypropyl methacrylate |
| OC |
Osteocalcin |
| ON |
Osteonectin |
| OP |
Osteopontin |
| PEG |
Polyethylene glycol |
| RUNX2 |
Run-related transcription factor 2 |
| SiN |
Silicon nitride |
| TCP |
Tricalcium phosphate |
| TEMED |
N,N,N’,N’-tetrametylethylendiamine |
| UF |
Unidirectional freezing |
References
- Hixon, K.R.; Eberlin, C.T.; Kadakia, P.U.; McBride-Gagyi, S.H.; Jain, E.; Sell, S.A. A Comparison of Cryogel Scaffolds to Identify an Appropriate Structure for Promoting Bone Regeneration. Biomed. Phys. Eng. Express 2016, 2. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Gelinsky, M.; Williams, D.S.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Marine Collagen-Chitosan-Fucoidan/Chondroitin Sulfate Cryo-Biomaterials Loaded with Primary Human Cells Envisaging Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2023, 241. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Kathuria, N.; Tripathi, A.; Kar, K.K.; Kumar, A. Synthesis and Characterization of Elastic and Macroporous Chitosan-Gelatin Cryogels for Tissue Engineering. Acta Biomater. 2009, 5, 406–418. [Google Scholar] [CrossRef]
- Bhat, S.; Lidgren, L.; Kumar, A. In Vitro Neo-Cartilage Formation on a Three-Dimensional Composite Polymeric Cryogel Matrix. Macromol. Biosci. 2013, 13, 827–837. [Google Scholar] [CrossRef]
- Hixon, K.R.; Melvin, A.M.; Lin, A.Y.; Hall, A.F.; Sell, S.A. Cryogel Scaffolds from Patient-Specific 3D-Printed Molds for Personalized Tissue-Engineered Bone Regeneration in Pediatric Cleft-Craniofacial Defects. J. Biomater. Appl. 2017, 32, 598–611. [Google Scholar] [CrossRef]
- Hwang, Y.; Sangaj, N.; Varghese, S. Interconnected Macroporous Poly(Ethylene Glycol) Cryogels as a Cell Scaffold for Cartilage Tissue Engineering. Tissue Eng.—Part A 2010, 16, 3033–3041. [Google Scholar] [CrossRef]
- Inci, I.; Kirsebom, H.; Galaev, I.Y.; Mattiasson, B.; Piskin, E. Gelatin Cryogels Crosslinked with Oxidized Dextran and Containing Freshly Formed Hydroxyapatite as Potential Bone Tissue-Engineering Scaffolds. J. Tissue Eng. Regen. Med. 2013, 7, 584–588. [Google Scholar] [CrossRef]
- Di Muzio, L.; Sergi, C.; Carriero, V.C.; Tirillò, J.; Adrover, A.; Messina, E.; Gaetani, R.; Petralito, S.; Casadei, M.A.; Paolicelli, P. Gelatin-Based Spongy and Compressive Resistant Cryogels with Shape Recovery Ability as Ideal Scaffolds to Support Cell Adhesion for Tissue Regeneration. React. Funct. Polym. 2023, 105607. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.M.; Saldaña, L.; Benito-Garzón, L.; García-Carrodeguas, R.; de Aza, S.; Vilaboa, N.; Román, J.S. Feasibility of Ceramic-Polymer Composite Cryogels as Scaffolds for Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2012, 6, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Eberlin, C.T.; Pendyala, M.; Alarcon de la Lastra, A.; Sell, S.A. Scaffolds for Use in Craniofacial Bone Regeneration. Methods in Molecular Biology 2022, 2403, 234. [Google Scholar]
- Bölgen, N.; Yang, Y.; Korkusuz, P.; Güzel, E.; El Haj, A.J.; Pişkin, E. 3D Ingrowth of Bovine Articular Chondrocytes in Biodegradable Cryogel Scaffolds for Cartilage Tissue Engineering. J. Tissue Eng. Regen. Med. 2011, 5, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLOS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Takei, T.; Yoshitomi, H.; Fukumoto, K.; Danjo, S.; Yoshinaga, T.; Nishimata, H.; Yoshida, M. Toxic Chemical Cross-Linker-Free Cryosponges Made from Chitosan-Gluconic Acid Conjugate for Chondrocyte Culture. J. Chem. Eng. Jpn. 2017, 50, 142–148. [Google Scholar] [CrossRef]
- Li, P.; Jia, Z.; Wang, Q.; Tang, P.; Wang, M.; Wang, K.; Fang, J.; Zhao, C.; Ren, F.; Ge, X.; et al. A Resilient and Flexible Chitosan/Silk Cryogel Incorporated Ag/Sr Co-Doped Nanoscale Hydroxyapatite for Osteoinductivity and Antibacterial Properties. J. Mater. Chem. B 2018, 6, 7427–7438. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, A.; Kumar, A. Evaluating Potential of Tissue-Engineered Cryogels and Chondrocyte Derived Exosomes in Articular Cartilage Repair. Biotechnol. Bioeng. 2022, 119, 605–625. [Google Scholar] [CrossRef]
- Han, M.-E.; Kim, S.-H.; Kim, H.D.; Yim, H.-G.; Bencherif, S.A.; Kim, T.-I.; Hwang, N.S. Extracellular Matrix-Based Cryogels for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Zhang, C.; Varghese, S. Poly(Ethylene Glycol) Cryogels as Potential Cell Scaffolds: Effect of Polymerization Conditions on Cryogel Microstructure and Properties. J. Mater. Chem. 2010, 20, 345–351. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The Potential of Polymeric Cryogels in Bioseparation.
- Kim, H.D.; Kim, J.; Koh, R.H.; Shim, J.; Lee, J.-C.; Kim, T.-I.; Hwang, N.S. Enhanced Osteogenic Commitment of Human Mesenchymal Stem Cells on Polyethylene Glycol-Based Cryogel with Graphene Oxide Substrate. ACS Biomater. Sci. Eng. 2017, 3, 2470–2479. [Google Scholar] [CrossRef]
- Chopra, V.; Thomas, J.; Sharma, A.; Panwar, V.; Kaushik, S.; Ghosh, D. A Bioinspired, Ice-Templated Multifunctional 3D Cryogel Composite Crosslinked through in Situ Reduction of GO Displayed Improved Mechanical, Osteogenic and Antimicrobial Properties. Mater. Sci. Eng. C 2021, 119. [Google Scholar] [CrossRef]
- Abueva, C.D.G.; Padalhin, A.R.; Min, Y.-K.; Lee, B.-T. Preformed Chitosan Cryogel-Biphasic Calcium Phosphate: A Potential Injectable Biocomposite for Pathologic Fracture. J. Biomater. Appl. 2015, 30, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Nimi, N.; Sivadas, V.P.; Lal, L.P.M.R.; Nair, P.D. Dual Crosslinked Pullulan-Gelatin Cryogel Scaffold for Chondrocyte-Mediated Cartilage Repair: Synthesis, Characterization and in Vitro Evaluation. Biomed. Mater. Bristol 2022, 17. [Google Scholar] [CrossRef]
- Bektas, E.I.; Gurel Pekozer, G.; Kök, F.N.; Torun Kose, G. Evaluation of Natural Gum-Based Cryogels for Soft Tissue Engineering. Carbohydr. Polym. 2021, 271. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.D.; Cengiz, U.; Arslan, Y.E.; Kiran, F.; Ceylan, A. From a Plant Secretion to the Promising Bone Grafts: Cryogels of Silicon-Integrated Quince Seed Mucilage by Microwave-Assisted Sol–Gel Reaction. J. Biosci. Bioeng. 2021, 131, 420–433. [Google Scholar] [CrossRef]
- Ak, F.; Oztoprak, Z.; Karakutuk, I.; Okay, O. Macroporous Silk Fibroin Cryogels. Biomacromolecules 2013, 14, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Assegehegn, G.; Brito-de La Fuente, E.; Franco, J.M.; Gallegos, C. The Importance of Understanding the Freezing Step and Its Impact on Freeze-Drying Process Performance. J. Pharm. Sci. 2019, 108, 1378–1395. [Google Scholar] [CrossRef]
- Singh, D.; Tripathi, A.; Nayak, V.; Kumar, A. Proliferation of Chondrocytes on a 3-d Modelled Macroporous Poly(Hydroxyethyl Methacrylate)-Gelatin Cryogel. J. Biomater. Sci. Polym. Ed. 2011, 22, 1733–1751. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Randriantsilefisoa, R.; Sprecher, C.M.; D’Este, M. Fabrication of Collagen–Hyaluronic Acid Cryogels by Directional Freezing Mimicking Cartilage Arcade-like Structure. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- Odabas, S. Collagen-Carboxymethyl Cellulose-Tricalcium Phosphate Multi-Lamellar Cryogels for Tissue Engineering Applications: Production and Characterization. J. Bioact. Compat. Polym. 2016, 31, 411–422. [Google Scholar] [CrossRef]
- Joshi Navare, K.; Colombani, T.; Rezaeeyazdi, M.; Bassous, N.; Rana, D.; Webster, T.; Memic, A.; Bencherif, S.A. Needle-Injectable Microcomposite Cryogel Scaffolds with Antimicrobial Properties. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Villard, P.; Rezaeeyazdi, M.; Colombani, T.; Joshi-Navare, K.; Rana, D.; Memic, A.; Bencherif, S.A. Autoclavable and Injectable Cryogels for Biomedical Applications. Adv. Healthc. Mater. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Hong, X.; An, Y.-H.; Park, M.J.; Kim, D.-G.; Greene, A.K.; Padwa, B.L.; Hwang, N.S.; Lin, R.-Z.; Melero-Martin, J.M. A Biphasic Osteovascular Biomimetic Scaffold for Rapid and Self-Sustained Endochondral Ossification. Adv. Healthc. Mater. 2021, 10. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Wang, J.; Wang, L.; Gao, C.; Li, B.; Wang, Y.; Wu, J.; Quan, C. Bioactive Gelatin Cryogels with BMP-2 Biomimetic Peptide and VEGF: A Potential Scaffold for Synergistically Induced Osteogenesis. Chin. Chem. Lett. 2022, 33, 1956–1962. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S.S.; Sivashanmugam, A.; Kwon, J.; Kim, S.L.; Noh, M.Y.; Kwon, S.K.; Jayakumar, R.; Hwang, N.S. Bioglass-Incorporated Methacrylated Gelatin Cryogel for Regeneration of Bone Defects. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. 3D Bioprinting of Photocrosslinkable Hydrogel Constructs. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Yang, J.; Wu, F.-H.; Cao, W.-B.; Zhou, T.; Wang, Z.-Y.; Tu, C.-X.; Gou, Z.-R.; Zhang, L.; Gao, C.-Y. A Macroporous Cryogel with Enhanced Mechanical Properties for Osteochondral Regeneration In Vivo. Chin. J. Polym. Sci. Engl. Ed. 2023, 41, 40–50. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, J.; Li, L.; Du, Z.; Cai, Q.; Yang, X. Hydroxyapatite Nanowire Composited Gelatin Cryogel with Improved Mechanical Properties and Cell Migration for Bone Regeneration. Biomed. Mater. Bristol 2019, 14. [Google Scholar] [CrossRef]
- Petrov, P.; Petrova, E.; Tsvetanov, C.B. UV-Assisted Synthesis of Super-Macroporous Polymer Hydrogels. Polymer 2009, 50, 1118–1123. [Google Scholar] [CrossRef]
- Öfkeli, F.; Demir, D.; Bölgen, N. Biomimetic Mineralization of Chitosan/Gelatin Cryogels and in Vivo Biocompatibility Assessments for Bone Tissue Engineering. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Suner, S.S.; Demirci, S.; Yetiskin, B.; Fakhrullin, R.; Naumenko, E.; Okay, O.; Ayyala, R.S.; Sahiner, N. Cryogel Composites Based on Hyaluronic Acid and Halloysite Nanotubes as Scaffold for Tissue Engineering. Int. J. Biol. Macromol. 2019, 130, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Rogulska, O.Y.; Trufanova, N.A.; Petrenko, Y.A.; Repin, N.V.; Grischuk, V.P.; Ashukina, N.O.; Bondarenko, S.Y.; Ivanov, G.V.; Podorozhko, E.A.; Lozinsky, V.I.; et al. Generation of Bone Grafts Using Cryopreserved Mesenchymal Stromal Cells and Macroporous Collagen-Nanohydroxyapatite Cryogels. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2022, 110, 489–499. [Google Scholar] [CrossRef]
- Salgado, C.L.; Teixeira, B.I.B.; Monteiro, F.J.M. Biomimetic Composite Scaffold With Phosphoserine Signaling for Bone Tissue Engineering Application. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.L.; Lee, S.S.; Kim, I.; Kwon, J.; Kwon, S.; Bae, T.; Hur, J.; Lee, H.; Hwang, N.S. Ectopic Transient Overexpression of OCT-4 Facilitates BMP4-Induced Osteogenic Transdifferentiation of Human Umbilical Vein Endothelial Cells. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef]
- Olov, N.; Mirzadeh, H.; Moradi, R.; Rajabi, S.; Bagheri-Khoulenjani, S. Shape Memory Injectable Cryogel Based on Carboxymethyl Chitosan/Gelatin for Minimally Invasive Tissue Engineering: In Vitro and in Vivo Assays. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2022, 110, 2438–2451. [Google Scholar] [CrossRef] [PubMed]
- Tsung, L.H.; Chang, K.-H.; Chen, J.P. OSTEOGENESIS OF ADIPOSE-DERIVED STEM CELLS ON THREE-DIMENSIONAL, MACROPOROUS GELATIN–HYALURONIC ACID CRYOGELS. Biomed. Eng. Appl. Basis Commun. 2011, 23, 127–133. [Google Scholar] [CrossRef]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the Morphological, Mechanical and Degradation Properties of Scaffolds Comprising Collagen, Gelatin and Elastin for Use in Soft Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.C.G.; Shih, Y.-R.V.; Nakasaki, M.; Liu, M.; Varghese, S. Mineralized Biomaterials Mediated Repair of Bone Defects Through Endogenous Cells. Tissue Eng.—Part A 2018, 24, 1148–1156. [Google Scholar] [CrossRef]
- Odabas, S.; Feichtinger, G.A.; Korkusuz, P.; Inci, I.; Bilgic, E.; Yar, A.S.; Cavusoglu, T.; Menevse, S.; Vargel, I.; Piskin, E. Auricular Cartilage Repair Using Cryogel Scaffolds Loaded with BMP-7-Expressing Primary Chondrocytes. J. Tissue Eng. Regen. Med. 2013, 7, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, A. Bacterial Biopolymers and Genetically Engineered Biopolymers for Gel Systems Application. In Handbook of Biopolymer-Based Materials: From Blends and Composites to Gels and Complex Networks; 2013; pp. 87–107.
- Katsen-Globa, A.; Meiser, I.; Petrenko, Y.A.; Ivanov, R.V.; Lozinsky, V.I.; Zimmermann, H.; Petrenko, A.Y. Towards Ready-to-Use 3-D Scaffolds for Regenerative Medicine: Adhesion-Based Cryopreservation of Human Mesenchymal Stem Cells Attached and Spread within Alginate-Gelatin Cryogel Scaffolds. J. Mater. Sci. Mater. Med. 2014, 25, 857–871. [Google Scholar] [CrossRef]
- Mishra, R.; Goel, S.K.; Gupta, K.C.; Kumar, A. Biocomposite Cryogels as Tissue-Engineered Biomaterials for Regeneration of Critical-Sized Cranial Bone Defects. Tissue Eng.—Part A 2014, 20, 751–762. [Google Scholar] [CrossRef]
- Şarkaya, K.; Akıncıoğlu, G.; Akıncıoğlu, S. Investigation of Tribological Properties of HEMA-Based Cryogels as Potential Articular Cartilage Biomaterials. Polym.-Plast. Technol. Mater. 2022, 61, 1174–1190. [Google Scholar] [CrossRef]
- Ayaz, F.; Demir, D.; Bölgen, N. Injectable Chitosan Cryogel Microspheres with Biocompatible Properties on Mammalian Macrophages in Vitro. J. Mater. Sci. 2021, 56, 17268–17277. [Google Scholar] [CrossRef]
- Jain, E.; Srivastava, A.; Kumar, A. Macroporous Interpenetrating Cryogel Network of Poly(Acrylonitrile) and Gelatin for Biomedical Applications. J. Mater. Sci. Mater. Med. 2009, 20, S173–S179. [Google Scholar] [CrossRef]
- Yetiskin, B.; Akinci, C.; Okay, O. Cryogelation within Cryogels: Silk Fibroin Scaffolds with Single-, Double- and Triple-Network Structures. Polymer 2017, 128, 47–56. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, J.; Wu, X.; Lin, Y. Macroporous Double-Network Cryogels: Formation Mechanism, Enhanced Mechanical Strength and Temperature/pH Dual Sensitivity. Soft Matter 2011, 7, 4284. [Google Scholar] [CrossRef]
- Zhang, Y.; Leng, H.; Du, Z.; Huang, Y.; Liu, X.; Zhao, Z.; Zhang, X.; Cai, Q.; Yang, X. Efficient Regeneration of Rat Calvarial Defect with Gelatin-Hydroxyapatite Composite Cryogel. Biomed. Mater. Bristol 2020, 15. [Google Scholar] [CrossRef]
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press, 2002; ISBN 978-0-691-12804-7.
- Pal, S. Design of Artificial Human Joints & Organs; Springer US: Boston, MA, 2014; ISBN 978-1-4614-6254-5. [Google Scholar]
- Lee, S.S.; Laganenka, L.; Du, X.; Hardt, W.-D.; Ferguson, S.J. Silicon Nitride, a Bioceramic for Bone Tissue Engineering: A Reinforced Cryogel System With Antibiofilm and Osteogenic Effects. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Kuo, C.-Y.; Wong, C.-B.; Chien, Y.-M.; Chen, H.-A.; Chen, J.-P. Gelatin/Nanohyroxyapatite Cryogel Embedded Poly(Lactic-Co-Glycolic Acid)/Nanohydroxyapatite Microsphere Hybrid Scaffolds for Simultaneous Bone Regeneration and Load-Bearing. Polymers 2018, 8. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, Y.; Zhang, L.; Huang, Y.; Zuo, D.; Cai, Q.; Yang, X. Comparative Study of Gelatin Cryogels Reinforced with Hydroxyapatites with Different Morphologies and Interfacial Bonding. Biomed. Mater. Bristol 2020, 15. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Tai, W.-C.; Ho, M.-H.; Chang, P.-C. Combination of a Biomolecule-Aided Biphasic Cryogel Scaffold with a Barrier Membrane Adhering PDGF-Encapsulated Nanofibers to Promote Periodontal Regeneration. J. Periodontal Res. 2020, 55, 529–538. [Google Scholar] [CrossRef]
- Kang, H.; Zeng, Y.; Varghese, S. Functionally Graded Multilayer Scaffolds for in Vivo Osteochondral Tissue Engineering. Acta Biomater. 2018, 78, 365–377. [Google Scholar] [CrossRef]
- Liao, H.-T.; Shalumon, K.T.; Chang, K.-H.; Sheu, C.; Chen, J.-P. Investigation of Synergistic Effects of Inductive and Conductive Factors in Gelatin-Based Cryogels for Bone Tissue Engineering. J. Mater. Chem. B 2016, 4, 1827–1841. [Google Scholar] [CrossRef]
- Wu, S.; Ma, S.; Zhang, C.; Cao, G.; Wu, D.; Gao, C.; Lakshmanan, S. Cryogel Biocomposite Containing Chitosan-Gelatin/Cerium–Zinc Doped Hydroxyapatite for Bone Tissue Engineering. Saudi J. Biol. Sci. 2020, 27, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Grenho, L.; Fernandes, M.H.; Colaço, B.J.; Monteiro, F.J. Biodegradation, Biocompatibility, and Osteoconduction Evaluation of Collagen-Nanohydroxyapatite Cryogels for Bone Tissue Regeneration. J. Biomed. Mater. Res.—Part A 2016, 104, 57–70. [Google Scholar] [CrossRef]
- Hixon, K.R.; Eberlin, C.T.; Lu, T.; Neal, S.M.; Case, N.D.; McBride-Gagyi, S.H.; Sell, S.A. The Calcification Potential of Cryogel Scaffolds Incorporated with Various Forms of Hydroxyapatite for Bone Regeneration. Biomed. Mater. Bristol 2017, 12. [Google Scholar] [CrossRef]
- Razavi, M.; Hu, S.; Thakor, A.S. A Collagen Based Cryogel Bioscaffold Coated with Nanostructured Polydopamine as a Platform for Mesenchymal Stem Cell Therapy. J. Biomed. Mater. Res.—Part A 2018, 106, 2213–2228. [Google Scholar] [CrossRef]
- Pandey, G.; Mittapelly, N.; Pant, A.; Sharma, S.; Singh, P.; Banala, V.T.; Trivedi, R.; Shukla, P.K.; Mishra, P.R. Dual Functioning Microspheres Embedded Crosslinked Gelatin Cryogels for Therapeutic Intervention in Osteomyelitis and Associated Bone Loss. Eur. J. Pharm. Sci. 2016, 91, 105–113. [Google Scholar] [CrossRef]
- Gürer, B.; Yılmaz, C.; Yılmaz, Ş.N.; Çabuk, S.; Bölgen, N. A Novel Strategy for Cartilage Tissue Engineering: Collagenase-Loaded Cryogel Scaffolds in a Sheep Model. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 313–321. [Google Scholar] [CrossRef]
- Kemençe, N.; Bölgen, N. Gelatin- and Hydroxyapatite-Based Cryogels for Bone Tissue Engineering: Synthesis, Characterization, in Vitro and in Vivo Biocompatibility. J. Tissue Eng. Regen. Med. 2017, 11, 20–33. [Google Scholar] [CrossRef]
- Park, J.; Kwon, S.; Hwang, N.S.; Kang, B.-J. Clinical Application of Bone Morphogenetic Protein-2 Microcarriers Fabricated by the Cryopolymerization of Gelatin Methacrylate for the Treatment of Radial Fracture in Two Dogs. In Vivo 2018, 32, 575–581. [Google Scholar] [CrossRef]
- Yuan, Z.; Yuan, X.; Zhao, Y.; Cai, Q.; Wang, Y.; Luo, R.; Yu, S.; Wang, Y.; Han, J.; Ge, L.; et al. Injectable GelMA Cryogel Microspheres for Modularized Cell Delivery and Potential Vascularized Bone Regeneration. Small 2021, 17. [Google Scholar] [CrossRef]
- Di Muzio, L.; Cienzo, F.; Paolicelli, P.; Petralito, S.; Garzoli, S.; Brandelli, C.; Trilli, J.; Antonietta Casadei, M. A Convenient Strategy to Synthesize Highly Tunable Gelatin Methacryloyl with Very Low Gelation Temperature. Eur. Polym. J. 2021, 154, 110538. [Google Scholar] [CrossRef]
- Sakr, M.A.; Sakthivel, K.; Hossain, T.; Shin, S.R.; Siddiqua, S.; Kim, J.; Kim, K. Recent Trends in Gelatin Methacryloyl Nanocomposite Hydrogels for Tissue Engineering. J. Biomed. Mater. Res. A 2022, 110, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Young, A.T.; White, O.C.; Daniele, M.A. Rheological Properties of Coordinated Physical Gelation and Chemical Crosslinking in Gelatin Methacryloyl (GelMA) Hydrogels. Macromol. Biosci. 2020, 20, 2000183. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Matsumoto, T.; Sasaki, J.; Egusa, H.; Lee, K.; Nakano, T.; Sohmura, T.; Nakahira, A. Effect of Calcium Ion Concentrations on Osteogenic Differentiation and Hematopoietic Stem Cell Niche-Related Protein Expression in Osteoblasts. Tissue Eng. Part A 2010, 16, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Karakutuk, I.; Ak, F.; Okay, O. Diepoxide-Triggered Conformational Transition of Silk Fibroin: Formation of Hydrogels. Biomacromolecules 2012, 13, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Quality of Different Chondroitin Sulfate Preparations in Relation to Their Therapeutic Activity. J. Pharm. Pharmacol. 2009, 61, 1271–1280. [Google Scholar] [CrossRef]
- Volpi, N. Analytical Aspects of Pharmaceutical Grade Chondroitin Sulfates. J. Pharm. Sci. 2007, 96, 3168–3180. [Google Scholar] [CrossRef]
- Volpi, N. Chondroitin Sulfate Safety and Quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergen, G.; Zhunussova, M.; Mun, E.A.; Ramankulov, Y.; Ogay, V. Macroporous Cell-Laden Gelatin/Hyaluronic Acid/Chondroitin Sulfate Cryogels for Engineered Tissue Constructs. Gels 2022, 8. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Chen, C.-H.; Hsiao, C.-Y.; Chen, J.-P. Incorporation of Chitosan in Biomimetic Gelatin/Chondroitin-6-Sulfate/Hyaluronan Cryogel for Cartilage Tissue Engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, B.; Colombani, T.; Joshi-Navare, K.; Mehta, S.; Kisiday, J.; Bencherif, S.A.; Bajpayee, A.G. Hyaluronic Acid-Based Shape-Memory Cryogel Scaffolds for Focal Cartilage Defect Repair. Tissue Eng.—Part A 2021, 27, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.-H.; Kuo, C.-Y.; Chen, K.-S.; Chen, J.-P. Preparation of Gelatin and Gelatin/Hyaluronic Acid Cryogel Scaffolds for the 3D Culture of Mesothelial Cells and Mesothelium Tissue Regeneration. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Yang, Y.; Korkusuz, P.; Güzel, E.; El Haj, A.J.; Pişkin, E. Three-Dimensional Ingrowth of Bone Cells within Biodegradable Cryogel Scaffolds in Bioreactors at Different Regimes. Tissue Eng.—Part A 2008, 14, 1743–1750. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-Based Foams and Aerogels: Processing, Properties, and Applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Beck, S.; Bouchard, J.; Berry, R. Dispersibility in Water of Dried Nanocrystalline Cellulose. Biomacromolecules 2012, 13, 1486–1494. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Souza, L.P.; Martins, T.M.M.; Lopes, J.H.; Mattos, B.D.; Mariano, M.; Pinheiro, I.F.; Valverde, T.M.; Livi, S.; Camilli, J.A.; et al. Nanocellulose/Bioactive Glass Cryogels as Scaffolds for Bone Regeneration. Nanoscale 2019, 11, 19842–19849. [Google Scholar] [CrossRef]
- Cal, F.; Sezgin Arslan, T.; Derkus, B.; Kiran, F.; Cengiz, U.; Arslan, Y.E. Synthesis of Silica-Based Boron-Incorporated Collagen/Human Hair Keratin Hybrid Cryogels with the Potential Bone Formation Capability. ACS Appl. Bio Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.N.; Lopez-Cebral, R.; Sousa, R.O.; Alves, A.L.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Reis, R.L.; Silva, T.H. Marine Collagen-Chitosan-Fucoidan Cryogels as Cell-Laden Biocomposites Envisaging Tissue Engineering. Biomed. Mater. Bristol 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.Y.; Inci, I.; Egri, S.; Ozturk, A.M.; Yetkin, H.; Goktas, G.; Elmas, C.; Piskin, E.; Erdogan, D. The Treatment of Segmental Bone Defects in Rabbit Tibiae with Vascular Endothelial Growth Factor (VEGF)-Loaded Gelatin/Hydroxyapatite “Cryogel” Scaffold. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 767–774. [Google Scholar] [CrossRef]
- Singh, D.; Singh, D.; Han, S.S. Fabrication of Three Dimensional Macroporous Matrix for Cardiac Regeneration. Int. J. Appl. Eng. Res. 2012, 7, 1226–1228. [Google Scholar]
- Shalumon, K.T.; Liao, H.-T.; Kuo, C.-Y.; Wong, C.-B.; Li, C.-J.; Mini, P.A.; Chen, J.-P. Rational Design of Gelatin/Nanohydroxyapatite Cryogel Scaffolds for Bone Regeneration by Introducing Chemical and Physical Cues to Enhance Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Mater. Sci. Eng. C 2019, 104. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Korkusuz, P.; Vargel, I.; Kiliç, E.; Güzel, E.; Çavuşoǧlu, T.; Uçkan, D.; Pişkin, E. Stem Cell Suspension Injected HEMA-Lactate-Dextran Cryogels for Regeneration of Critical Sized Bone Defects. Artif. Cells Nanomedicine Biotechnol. 2014, 42, 70–77. [Google Scholar] [CrossRef]
- Bölgen, N.; Aguilar, M.R.; Fernández, M.D.M.; Gonzalo-Flores, S.; Villar-Rodil, S.; San Román, J.; Pişkin, E. Thermoresponsive Biodegradable HEMA-Lactate-Dextran-Co-NIPA Cryogels for Controlled Release of Simvastatin. Artif. Cells Nanomedicine Biotechnol. 2015, 43, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Vargel, I.; Korkusuz, P.; Güzel, E.; Plieva, F.; Galaev, I.; Matiasson, B.; Pişkin, E. Tissue Responses to Novel Tissue Engineering Biodegradable Cryogel Scaffolds: An Animal Model. J. Biomed. Mater. Res.—Part A 2009, 91, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Kuo, C.-Y.; Wang, Y.-J.; Chen, J.-P. Dual Function of Glucosamine in Gelatin/Hyaluronic Acid Cryogel to Modulate Scaffold Mechanical Properties and to Maintain Chondrogenic Phenotype for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of Three-Dimensional Chitosan-Agarose-Gelatin Cryogel Scaffold for the Repair of Subchondral Cartilage Defects: An in Vivo Study in a Rabbit Model. Tissue Eng.—Part A 2014, 20, 3101–3111. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Palka, K.; Canal, C.; Kolodynska, D.; Przekora, A. Novel Synthesis Method Combining a Foaming Agent with Freeze-Drying to Obtain Hybrid Highly Macroporous Bone Scaffolds. J. Mater. Sci. Technol. 2020, 43, 52–63. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Quan, C.; Li, X.; Chen, C.; Kang, H.; Hu, W.; Jiang, Q.; Zhang, C. Poly(γ-Glutamic Acid) Induced Homogeneous Mineralization of the Poly(Ethylene Glycol)-Co-2-Hydroxyethyl Methacrylate Cryogel for Potential Application in Bone Tissue Engineering. RSC Adv. 2015, 5, 20227–20233. [Google Scholar] [CrossRef]
- Bruns, J.; McBride-Gagyi, S.; Zustiak, S.P. Injectable and Cell-Adhesive Polyethylene Glycol Cryogel Scaffolds: Independent Control of Cryogel Microstructure and Composition. Macromol. Mater. Eng. 2018, 303. [Google Scholar] [CrossRef]
- Dziubla, T.D.; Torjman, M.C.; Joseph, J.I.; Murphy-Tatum, M.; Lowman, A.M. Evaluation of Porous Networks of Poly(2-Hydroxyethyl Methacrylate) as Interfacial Drug Delivery Devices. Biomaterials 2001, 22, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Casadio, Y.S.; Brown, D.H.; Chirila, T.V.; Kraatz, H.-B.; Baker, M.V. Biodegradation of Poly(2-Hydroxyethyl Methacrylate) (PHEMA) and Poly{(2-Hydroxyethyl Methacrylate)-Co-[Poly(Ethylene Glycol) Methyl Ether Methacrylate]} Hydrogels Containing Peptide-Based Cross-Linking Agents. Biomacromolecules 2010, 11, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jiao, H.; Xu, J.; Liu, Y.; Wei, S. Synthesis of Poly Hydroxypropyl Methacrylate Cryogel Incorporated with Zn/Ce Substituted Hydroxyapatite Nanoparticles for Rejuvenation of Femoral Fracture Treatment in a Rat Model. J. Photochem. Photobiol. B 2019, 201. [Google Scholar] [CrossRef]
- Świȩszkowski, W.; Ku, D.N.; Bersee, H.E.N.; Kurzydlowski, K.J. An Elastic Material for Cartilage Replacement in an Arthritic Shoulder Joint. Biomaterials 2006, 27, 1534–1541. [Google Scholar] [CrossRef]
- Lytkina, D.N.; Fedorishin, D.A.; Kalachikova, P.M.; Plyaskina, A.A.; Babeshin, A.R.; Kurzina, I.A. Cryo-Structured Materials Based on Polyvinyl Alcohol and Hydroxyapatite for Osteogenesis. J. Funct. Biomater. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, R.; Lytkina, D.; Stepanova, K.; Kurzina, I. Synthesis of Biocompatible Composite Material Based on Cryogels of Polyvinyl Alcohol and Calcium Phosphates. Polymers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.S.; Alvarez, V.A. Mechanical Properties of Polyvinylalcohol/Hydroxyapatite Cryogel as Potential Artificial Cartilage. J. Mech. Behav. Biomed. Mater. 2014, 34, 47–56. [Google Scholar] [CrossRef]
- Liu, C.; Lin, C.; Feng, X.; Wu, Z.; Lin, G.; Quan, C.; Chen, B.; Zhang, C. A Biomimicking Polymeric Cryogel Scaffold for Repair of Critical-Sized Cranial Defect in a Rat Model. Tissue Eng.—Part A 2019, 25, 1591–1604. [Google Scholar] [CrossRef]
- Covert, R.J.; Ott, R.D.; Ku, D.N. Friction Characteristics of a Potential Articular Cartilage Biomaterial. Wear 2003, 255, 1064–1068. [Google Scholar] [CrossRef]
- Mao, S.-H.; Chen, C.-H.; Chen, C.-T. Osteogenic Potential of Induced Pluripotent Stem Cells from Human Adipose-Derived Stem Cells. Stem Cell Res. Ther. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Barrias, C.C.; Monteiro, F.J.M. Clarifying the Tooth-Derived Stem Cells Behavior in a 3D Biomimetic Scaffold for Bone Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, Y.; Zou, Y.; Zhu, J.; Liu, L.; Fan, Y. Preparation of Nanochitin Hydrogel with Adjustable Inter-Structure by Sequencial Genipin Crosslinking and Ice-Templating under Acid Condition. Int. J. Biol. Macromol. 2022, 221, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Häussling, V.; Deninger, S.; Vidoni, L.; Rinderknecht, H.; Ruoß, M.; Arnscheidt, C.; Athanasopulu, K.; Kemkemer, R.; Nussler, A.K.; Ehnert, S. Impact of Four Protein Additives in Cryogels on Osteogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering 2019, 6. [Google Scholar] [CrossRef]
- Kumar, A.; Teotia, A.K.; Dienel, K.; Qayoom, I.; Van Bochove, B.; Gupta, S.; Partanen, J.; Seppälä, J. Improved Bone Regeneration in Rabbit Bone Defects Using 3d Printed Composite Scaffolds Functionalized with Osteoinductive Factors. ACS Appl. Mater. Interfaces 2020, 12, 48340–48356. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A. Effect of Plasma Polymerization on Physicochemical Properties of Biocomposite Cryogels Causing a Differential Behavior of Human Osteoblasts. J. Colloid Interface Sci. 2014, 431, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Ling, Y.; Deng, W.; Xue, J.; Sun, P.; Wang, D.-A. A Novel Cell Encapsulatable Cryogel (CECG) with Macro-Porous Structures and High Permeability: A Three-Dimensional Cell Culture Scaffold for Enhanced Cell Adhesion and Proliferation. Biomed. Mater. Bristol 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Offeddu, G.S.; Mela, I.; Jeggle, P.; Henderson, R.M.; Smoukov, S.K.; Oyen, M.L. Cartilage-like Electrostatic Stiffening of Responsive Cryogel Scaffolds. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Fassina, L.; Visai, L.; Magenes, G.; Schelfhout, J.; Bloise, N.; Riva, F.; Omes, C.; Avanzini, M.A.; Cusella De Angelis, M.G.; Benazzo, F.; et al. Ultrasound Stimulus to Enhance the Bone Regeneration Capability of Gelatin Cryogels.; 2013; pp. 846–849.
- Chen, T.-C.; Wong, C.-W.; Hsu, S.-H. Three-Dimensional Printing of Chitosan Cryogel as Injectable and Shape Recoverable Scaffolds. Carbohydr. Polym. 2022, 285. [Google Scholar] [CrossRef]
- Koh, R.H.; Kim, J.; Kim, S.H.L.; Hwang, N.S. RGD-Incorporated Biomimetic Cryogels for Hyaline Cartilage Regeneration. Biomed. Mater. Bristol 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Zhu, S.; Wei, X.; Zhou, N.; Liao, X.; Peng, Y.; Tang, Y.; Zhang, L.; Yang, X.; et al. Cryoprinting of Nanoparticle-Enhanced Injectable Hydrogel with Shape-Memory Properties. Mater. Des. 2022, 223. [Google Scholar] [CrossRef]
- Yang, X.; Meng, H.; Peng, J.; Xu, L.; Wang, Y.; Sun, X.; Zhao, Y.; Quan, Q.; Yu, W.; Chen, M.; et al. Construction of Microunits by Adipose-Derived Mesenchymal Stem Cells Laden with Porous Microcryogels for Repairing an Acute Achilles Tendon Rupture in a Rat Model. Int. J. Nanomedicine 2020, 15, 7155–7171. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, M.S.; Shaikhaliev, A.I.; Korshakov, E.V.; Gasbanov, G.A.; Korgoloev, R.S.; Sinitskaya, E.S.; Sidorskii, E.V.; Yamskova, V.P.; Lozinsky, V.I. Changes in Rat Bone Tissue at the Site of the Defect In Vivo under the Effect of a Cryogenically Structured Albumin Sponge Containing a Bioregulator. Bull. Exp. Biol. Med. 2021, 170, 805–808. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).