1. Introduction
Environmental pollution, and its impact on our lives and those of future generations, is currently arousing significant interest and concern in today's society. One of the largest sources of pollution is also one of the most used materials in our daily lives: plastics.
Plastics are synthetic polymeric materials primarily derived from petroleum. Their properties, such as hydrophobicity, corrosion resistance, lightweight, chemical inertness, and durability, explain their widespread popularity [
1]. Worldwide, the annual plastic production has surged from 2 million tons in the 1950s to 367 million tons in 2020 [
2]. Predictions indicate that the total mass of plastic debris accumulated in the ocean could reach approximately 250 million metric tons (Mt) by 2025, which is an order of magnitude higher than the 2010 figure [
3]. The most used types of plastics include high-density polyethylene (HDPE), low-density polyethylene (LDPE), polyvinyl chloride (PVC), polystyrene (PS), polypropylene (PP), and polyethylene terephthalate (PET), accounting for 90% of the plastics produced worldwide [
4].
Depending on their size, plastics are classified as macroplastics (McP), which have an average size greater than 25 mm, mesoplastics (MsP) with a size between 25 and 5 mm, microplastics (MP), on which this work focuses, whose size may vary according to the literature, but it is established here that these have a size between 5000 and 1 µm and finally, those with a size smaller than 1 µm, nanoplastics (NP). It is precisely these last two groups that are the most difficult to recover due to their small size, and they accumulate in oceans and watery areas (around 8 million tons of PM per year) [
5].
Microplastics (MPs) can be further categorized into primary and secondary MPs. Primary MPs are those specifically manufactured in this size range, which includes, for example, those found in cosmetic skin cleansing products. On the other hand, secondary microplastics result from the degradation of macroplastics (McP) and mesoplastics (MsP). The degradation of these plastics can involve processes such as weathering and aging. Additionally, once they reach the micrometer size range, both primary and secondary MPs can undergo further degradation processes, leading to modifications in some of their properties, such as color or density, which can result in unexpected physical and/or chemical effects on the natural environment.
Since most plastics are reusable materials, one might consider not only their degradation, but also their recovery and subsequent use. However, only about 9% of the world's plastic waste is currently recycled [
6]. As a result, numerous studies have been conducted on microplastic recovery methods, including the utilization of magnetic carbon nanotubes, which have demonstrated a high adsorption capacity for hydrophobic aromatic compounds, such as certain plastics. Another recent method is based on the use of aluminum (III) and iron (III) coagulant salts, which react with water in a basic environment to form metal hydroxide particles that do not dissolve and can be separated once they have settled. These particles are capable of binding with microplastics through complexation, modifying the polymer bonds [
7].
Among the various methodologies and mechanisms listed in
Table 1 for the recovery of microplastics, the use of micromotors has received relatively limited research attention. The process essentially involves the decomposition of hydrogen peroxide by a catalyst to generate oxygen, as described in reaction 1 [
8].
Initially, noble metal catalysts were employed, but their scarcity and cost prompted the development of alternative catalysts. Notably, manganese oxide is one of the catalysts used for this purpose. The production of oxygen bubbles propels the contaminants to the liquid's surface, creating foam that can be collected, within which the microplastics are located. Additionally, the use of these types of catalysts can facilitate the degradation of plastics by generating radicals in the presence of peroxides or persulfate, known as the Fenton process, as indicated in reactions 2 and 3.
In the application of this technology for microplastics recovery, it is sometimes necessary to introduce a surfactant into the medium to aid in the encapsulation of the microplastics within the generated foam. Surfactants alter the surface tension between the microplastics and the medium, enhancing their affinity for water.
In this study, various manganese oxide nanostructures were synthesized and employed for the recovery of synthetic microplastics with a size of approximately 300 nm in the laboratory. Parameters such as the microplastic quantity, structure, morphology, catalyst dosage, hydrogen peroxide concentration, surfactant, and pH were systematically varied. The measurement of total organic carbon (TOC) in the suspension was used as the response variable to determine the optimal conditions for microplastic recovery.
2. Materials and Methods
All reagents used in this work were used without any further purification. KMnO4 (> 99%) and Hydrochloric acid (HCl 37 wt. % in H2O) were obtained from Labkem, Spain. Na2S2O8 (>98%, PanReac AppliChem, Germany). Triton X-100 (Sigma-Aldrich, USA). Hydrogen peroxide solution, (H2O2 30 wt. % in H2O, Honeywell, Germany). Titanium oxysulfate (TiOSO4 27–31 % H2SO4 basis) and Styrene were obtained from Sigma-Aldrich, Germany.
2.1. Synthesis and characterization of MnO2
Different MnO2 structures were synthesized using hydrothermal synthesis, and the specific conditions for each synthesis are provided below.
0.627 g of KMnO
4 were dissolved in 56 mL of deionized water with continuous magnetic stirring. Once the dissolution is homogeneous, 1.4 mL of HCl was added. After 15 min the solution was transferred to a 100 mL autoclave and place it in an oven maintained at 80 °C for 12 h. This sample is referred to as S80 [
18].
The following synthesis was conducted by dissolving 0.363 g of KMnO
4 in 80 mL of deionized water. After achieving homogeneity in the solution, 0.8 mL of HCl was added. Subsequently, the mixture was subjected to continuous magnetic stirring for 1 hour, following which it was transferred to an autoclave and maintained at 140°C for 12 h. This sample was designated as 'S140' [
19].
The synthesis of the sample named S210 was carried out by adding 45 mL of a 0.60 mol/L solution of MnSO
4 dropwise, using a burette, to a beaker containing 28.2 mL of a 0.60 mol/L solution of KMnO
4, while maintaining constant magnetic stirring. The mixture was stirred continuously for 30 min, after which it was transferred to an autoclave and kept in the oven at 210°C for 12 h [
20].
In all cases, the oven was configured with a ramp of 1.5 °C/min until the selected temperature was reached. After 12 h, the temperature was decreased at a ramp of 3°C/min, and then it was allowed to stand for approximately 4 h until it reached room temperature. After this period, each of the synthesized particles was washed with water and ethanol, undergoing centrifugation at 5000 rpm for 3 min, with each process being repeated three times. Once the solid was separated and cleaned, the precipitate was dried in a vacuum oven at 40°C for 24 h.
The structure of the particles was characterized using a Theta/2Theta Bruker D8 diffractometer, which was equipped with a primary monochromator and an ultrafast Lynxeye XE-T multichannel detector with Cu Kα radiation. Diffractograms were recorded in the 2θ range from 5° to 80°, and their profiles were analyzed using the PANalytiical X`Pert High Score program. Micrographs and energy-dispersive X-ray spectroscopy (EDX) analysis were obtained through scanning electron microscopy (SEM) using a Hitachi S-3000N microscope.
The porous structure of the materials, which had been previously outgassed overnight at 150°C to a residual pressure of < 10-3 Torr, was characterized by nitrogen adsorption-desorption using a Micromeritics Tristar 3020 system.
2.2. Synthesis of polystyrene
Different MnO2 structures have been synthesized using a hydrothermal synthesis method. The specific conditions for each synthesis are detailed below.
The synthesis of polystyrene particles was carried out following the methodology previously reported by Lu et al. [
21]. Briefly, 150 mL of distilled water and 10 mL of styrene were placed in a round-bottom flask with a condenser immersed in a thermal bath at 80°C. They were stirred for 30 min under an inert atmosphere of N
2 gas. Subsequently, 5 mL of a 26.4 g/L Na
2S
2O
8 solution was added dropwise with the aid of a syringe. Finally, the reaction was allowed to proceed for 6 h. The resulting suspended particles were thoroughly washed with distilled water and centrifuged at 11,000 rpm repeatedly. Once the microplastics were resuspended, the resulting suspension's concentration was calculated using 0.22 μm Millipore filters. A known volume, typically 1 - 2 mL, was passed through the filter to quantify the amount of microplastics deposited on it.
To analyze the size distribution using an eLINE Plus equipment from Raith GmbH Co. by FESEM, several aliquots were taken and deposited in glass sample holders with the aid of a micropipette. The samples were then allowed to dry at room temperature. Afterward, the glass sample holders were placed on carbon tape affixed to a sample holder and coated with a 10 nm layer of Co to enhance conductivity.
2.2. Experimental setup and conditions for the recovery of microplastics
A 250 mL container was used, into which 100 mL of a solution was added. These experiments were conducted with various parameters being varied, including pH, the amount of MnO2, hydrogen peroxide concentration, microplastics concentration, and surfactant concentration. Initial concentrations of MnO2 were set at 0.1, 0.2, and 0.3 g/L, hydrogen peroxide at 1.6%, 3%, and 6%, and microplastic amounts at 10 ppm, 20 ppm, and 50 ppm. Triton concentrations were 0.001%, 0.005%, and 0.01%, and the pH was adjusted to 3, 7, and 9 using 1 M HCl and NaOH, respectively.
The foam generated was removed continuously for a duration of 20 to 40 min, depending on the test conditions. At this point, the process was considered complete since foam generation had become negligible. From the lower part of the container, samples were taken from the suspension, and the total organic carbon (TOC) content was measured using a colorimeter, specifically the HACH DR900, through a purging method with the aid of a HACH DRB200 instrument and TNT reagents. The TOC measurement in each experiment was compared to the initial total organic carbon content corresponding to the microplastic and Triton surfactant. The remaining peroxide content was quantified using UV-vis titanium complexation with a Perkin Elmer Lambda 365 UV-vis apparatus [
22]. Following each assay, the solution had to be agitated at a temperature of 50°C for approximately 12 h to eliminate excess peroxide, preventing interference with the TOC measurement. In the case of manganese oxide, to prevent interference, all the aliquots were diluted to a 4-fold concentration, and no interference in the measurement was observed.
3. Results and discussion
3.1. Characterization of the MnO2 synthesized
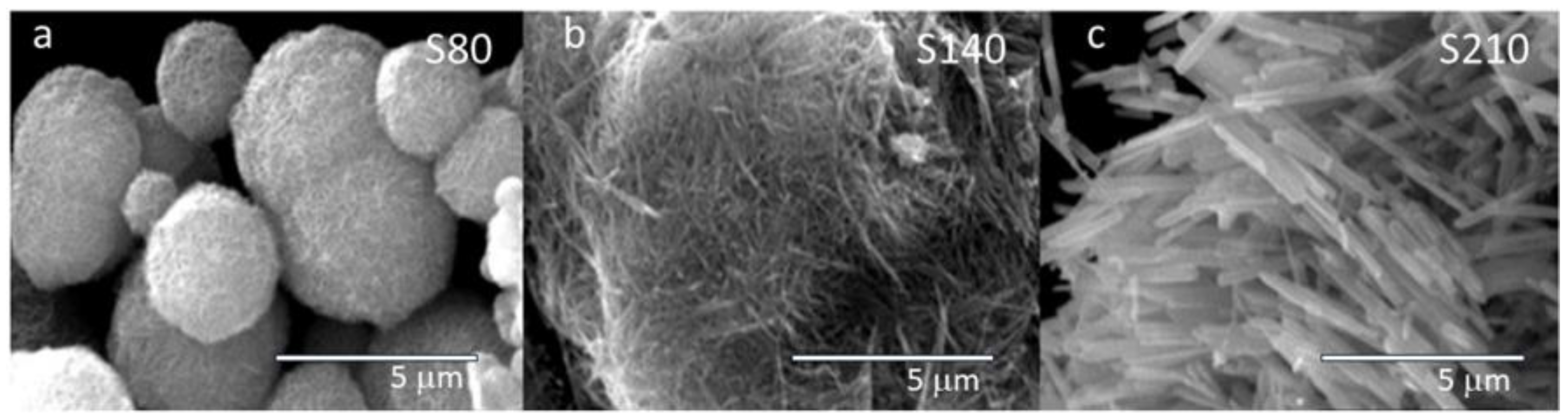
SEM micrographs of the different samples synthesized at various temperatures are depicted in
Figure 1. Their morphologies are distinctly different, but all of them consist of nanowires whose size increases with higher synthesis temperatures. In the case of the sample synthesized at a lower temperature (S80), the nanocrystals agglomerate to form compact spheres of varying sizes (1–3 μm). Sample S210 is composed of well-defined prisms, each about 2–3 μm in length. At an intermediate temperature, sample S140 exhibits agglomerations of ribbon-like nanowires with smaller base and length. In all cases, EDX measurements of the samples confirm the presence of an oxygen-to-manganese ratio very close to 2.
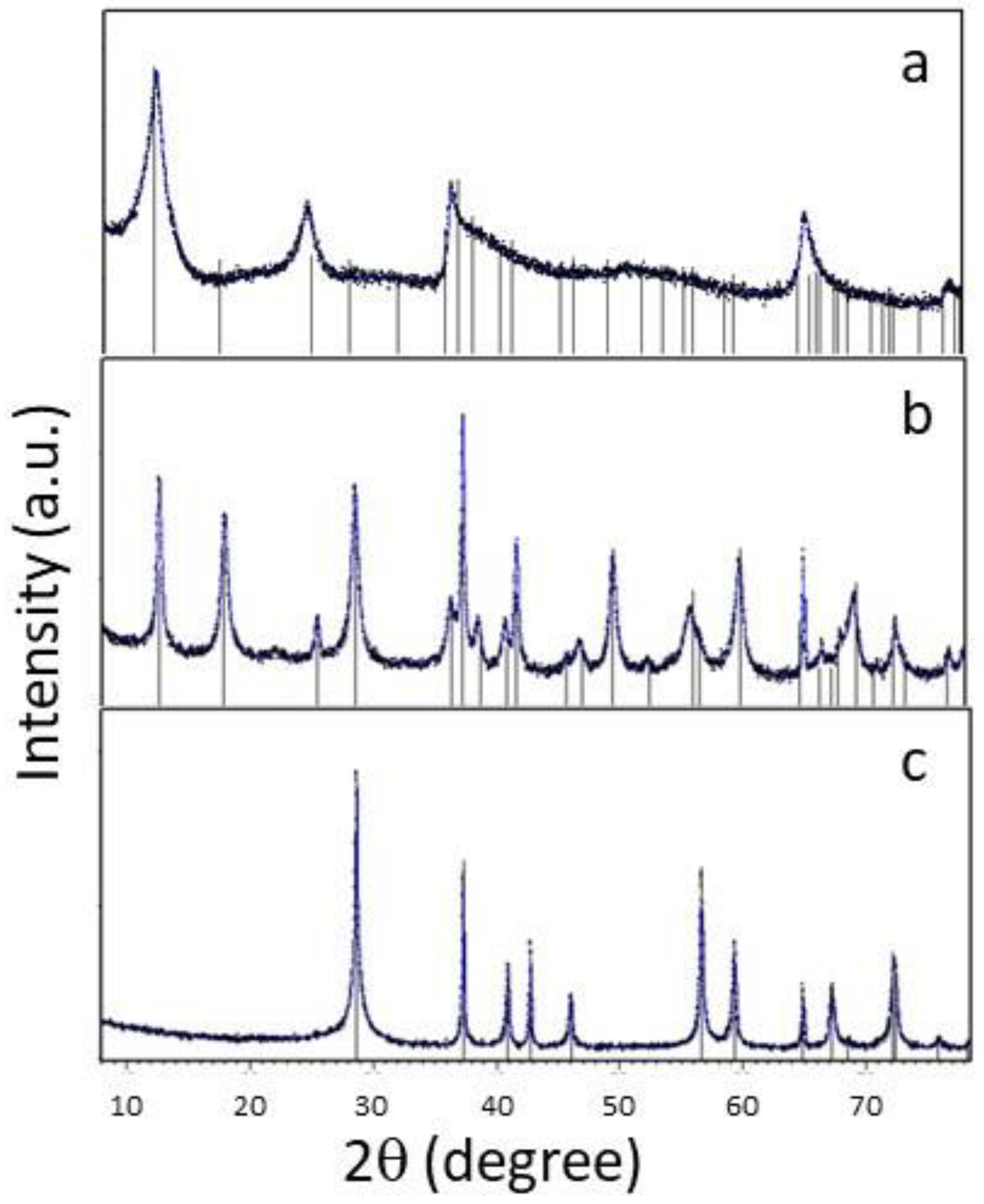
The XRD patterns of the samples resulting from the hydrothermal process under various conditions and temperatures are presented in
Figure 2. Each of these samples corresponds to a pure tetragonal phase of MnO
2. In the diffractogram for the S80 sample, all diffraction peaks can be exclusively indexed as a pure triclinic tetragonal δ-MnO
2 (JCPDS 01-072-1982). The diffractogram for the S150 sample can be indexed as α-MnO
2 corresponding to the tetragonal phase (JCPDS 44-0141), and the sample S210, synthesized at a higher temperature, can be identified as β-MnO
2 (JCPDS 01-081-2261). It's worth noting that both α and β-MnO
2 possess 1D tunnels in their structures, while δ-MnO
2 is a 2D layered compound [
23]. Additionally, the crystallinity of the samples increases with rising temperature, which is consistent with the SEM micrographs.
In addition, N
2 physisorption was conducted to determine the specific surface area (S
BET) value and pore size distribution. The values extracted from the isotherms of the three synthesized samples are presented in
Table 2. In all cases, the S
BET is relatively low, ranging between 57 and 13 m²/g. However, it is observed that the specific surface area decreases as the synthesis temperature in the hydrothermal process increases. At higher temperatures, they exhibit type II isotherms, indicative of non-porous materials with low pore densities. In contrast, samples obtained at lower temperatures exhibit type I isotherms, indicating the presence of micro and mesopores with low volume.
3.2. Recovery of microplastics
Although all three synthesized samples were tested for the recovery of microplastics, the results indicated that the sample with the lowest temperature and the highest surface area yielded the best results. Therefore, the variables influencing the recovery process will be analyzed using the sample labeled S80.
The influence of pH was investigated using conditions with 1.6% H
2O
2, a microplastic concentration of 20 ppm, 0.2 g/L MnO
2, and 0.01% Triton. Under these conditions, foam formation and, consequently, the collection of a certain amount of microplastics only occur at basic pH levels. pH values lower than 9, even after prolonged reaction times, remove a very minimal amount of microplastics. This effect can be explained by the suppression of bubble generation under acidic conditions. Conversely, as pH increases, the catalytically generated microbubbles adsorb suspended microplastics and rise to the surface of the reaction solution, effectively removing them from the environment [
10]. Further increasing the pH beyond 9 does not significantly enhance bubble generation or microplastic recovery, which is why this pH level was selected.
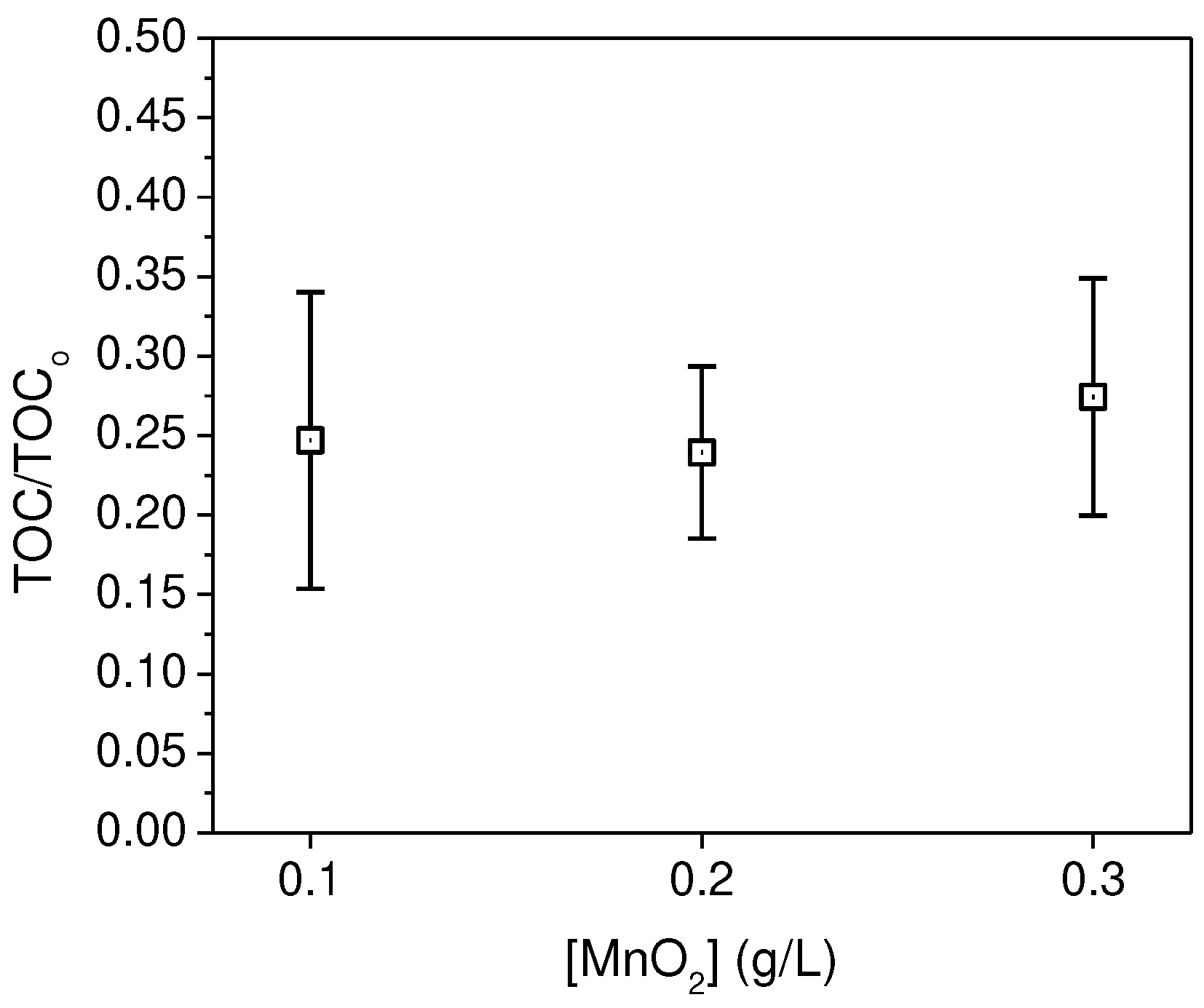
Once the optimal pH was determined, we varied the amount of MnO
2 while keeping other factors constant. In
Figure 3, the ratio between the TOC obtained after the experiment and the initial TOC is depicted for MnO
2 concentrations of 0.1, 0.2, and 0.3 g/L. It is evident that there is no statistically significant difference in the recovery rates when using different MnO
2 concentrations within the studied range, especially in combination with low concentrations of H
2O
2. The determined recovery rate remained at 75%.
Based on the limited impact of MnO
2 concentration on microplastic recovery, we selected a MnO
2 concentration of 0.2 g/L for subsequent experiments. It was also verified that the presence of MnO
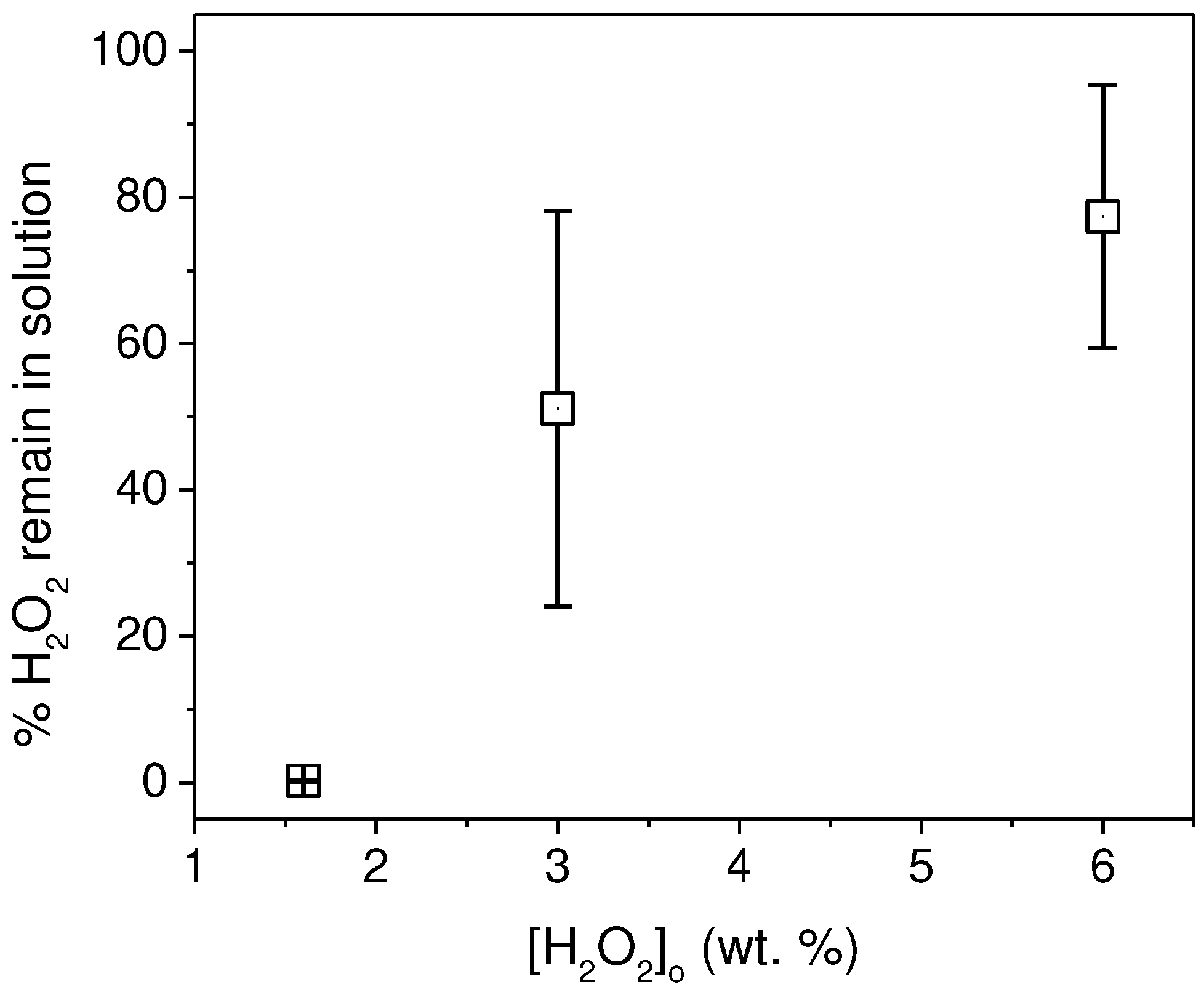
2 particles had a negligible effect on TOC measurements. Although an increase in pH beyond 9 did not result in a significant increase in bubble generation and, consequently, microplastic coating, we opted to maintain a pH of 9 and maintaining the other parameters constant. We observed that as the peroxide concentration in the medium increased, there was a considerable amount of unreacted peroxide left in the medium. This had a significant impact on the TOC measurements. The calibration curves for H
2O
2 are provided in S2, and the quantities measured after performing a calibration curve are presented in
Figure 4.
The remaining amounts of peroxides for the experiments with 3% and 6% concentrations were found to be significantly high, substantially affecting the TOC measurement. To ensure accurate and reproducible measurements, the measurement protocol was modified to ensure that the peroxide concentration prior to TOC measurement was negligible, as detailed in the experimental section.
Figure 5 presents the results obtained for different quantities of microplastics and peroxide.
It can be observed than an increase in peroxide concentration does not result in a higher recovery of microplastics, primarily due to the significant foam formation and the difficulty in its collection. The supplementary material provides information on the foam formation when the peroxide concentration is 6%, occurring within just 5 min. For small amounts of microplastics and low peroxide concentrations, the recovery rate exceeds 80%. This suggests that this procedure is effective at recovering microplastics even at low concentrations, with minimal peroxide usage, as the peroxide is eliminated from the medium and converts into oxygen when 1.6% concentrations are employed. In summary, these MnO2 micromotors act as catalysts in the production of oxygen and can remove over 80% of microplastics through entrainment in just 40 min. The optimal conditions for use should be investigated based on the quantity and type of microplastics to be recovered and the specific environmental conditions.
The results of the current work demonstrate the potential use of these or other micromotors for the removal of emerging pollutants, such as microplastics. The advantage of this methodology is that it utilizes materials that are low-cost, readily available, and can be fabricated on a large scale.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Supplementary Material 1: FSEM images and size distribution of synthesized polystyrene. Calibration curve for the complexation of H
2O
2 with titanium. Analysis of Variance for
Figure 3,
Figure 4, and
Figure 5. Image of foam formed when the peroxide amount is 6%.
Author Contributions
Conceptualization, methodology, writing-original draft preparation, supervision, funding acquisition. P.H. and N.M.; formal analysis, investigation, writing-review and editing, O.C.; investigation, formal analysis, L.S and C.V-M.; writing-review and editing, N. C and P.H All authors have read and agreed to the published version of the manuscript.
Funding
This research has been supported by the Spanish Ministry of Science and Innovation thorough the project PID2021-123431OB-I00.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
O.C. wish to thank CONAHCyT and Fundación TELMEX TELCEL for the scholarships given. C.V-M. wish to thank CONAHCyT for the scholarship given.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Loganathan, Y.; Kizhakedathil, M.P.J. A review on microplastics-an indelible ubiquitous pollutant. Biointerface Res. Appl. Chem. 2023, 13, 126. [Google Scholar]
- Plastics – the Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 1 November 2023).
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; et al. Plastic waste inputs from land into the ocean. Science. 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic Waste in the Marine Environment: A Review of Sources, Occurrence and Effects. Sci. Total Environ. 2016, 566, 333–349. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. The Chemical Behaviors of Microplastics in Marine Environment: A Review. Mar. Pollut. Bull. 2019, 142, 1–14. [Google Scholar] [CrossRef]
- Tiwari, R.; Azad, N.; Dutta, D.; Yadav, B.R.; Kumar, S. A critical review and future perspective of plastic waste recycling. Sci. Total Environ. 2023, 163433. [Google Scholar] [CrossRef] [PubMed]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of Microplastics from the Environment. A Review. Environmental Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Liu, Q.; Duan, X.; Sun, H.; Wang, Y.; et al. Size-tailored porous spheres of manganese oxides for catalytic oxidation via peroxymonosulfate activation. J. Phys. Chem. C. 2016, 120, 16871–16878. [Google Scholar] [CrossRef]
- Gunatilake, U.B.; Morales, R.; Basabe-Desmonts, L.; Benito-Lopez, F. Magneto Twister: Magneto Deformation of the Water–Air Interface by a Superhydrophobic Magnetic Nanoparticle Layer. Langmuir. 2022, 38, 3360–3369. [Google Scholar] [CrossRef]
- Ye, H.; Wang, Y.; Liu, X.; Xu, D.; et al. Magnetically steerable iron oxides-manganese dioxide core–shell micromotors for organic and microplastic removals. J. Colloid Interface Sci. 2021, 588, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Schwarzenberger, K.; Gatzemeier, J.; Lei, Z.; Eckert, K. Magnetically induced aggregation of iron oxide nanoparticles for carrier flotation strategies. ACS Appl. Mater. Interfaces. 2021, 13, 20830–20844. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Liu, Z.; Tian, S.; et al. Coagulation removal of microplastics from wastewater by magnetic magnesium hydroxide and PAM. J. Water Proc. Eng. 2021, 43, 102250. [Google Scholar] [CrossRef]
- Villa, K.; Děkanovský, L.; Plutnar, J.; Kosina, J.; Pumera, M. Swarming of perovskite-like Bi2WO6 microrobots destroy textile fibers under visible light. Adv. Funct. Mater. 2020, 30, 2007073. [Google Scholar] [CrossRef]
- Misra, A. , Zambrzycki, C., Kloker, G., Kotyrba, A., et al. Water purification and microplastics removal using magnetic polyoxometalate-supported ionic liquid phases (magPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chen, W.; Fan, X.; Tian, C.; et al. Cooperative recyclable magnetic microsubmarines for oil and microplastics removal from water. Appl. Mater. Today. 2020, 20, 100682. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; et al. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter. 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 micromotors for removal of microplastics and suspended matter. ACS Appl. Mater. Interfaces. 2019, 11, 32937–32944. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.; Minh, T.D.; Kinnunen, N.; Jänis, J. Manganese Oxide Based Catalytic Micromotors: Effect of Polymorphism on Motion. ACS Appl. Mater. Interfaces. 2016, 8, 32624–32629. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, T.; Phan, N.M.; Kim, K. J.; Huy, N.N.; Dung, N.T. Orange G degradation by heterogeneous peroxymonosulfate activation based on magnetic MnFe2O4/α-MnO2 hybrid. J. Environ. Sci. 2023, 124, 379–396. [Google Scholar]
- Wan, J.; Zhou, L.; Deng, H.; Zhan, F.; Zhang, R. Oxidative degradation of sulfamethoxazole by different MnO2 nanocrystals in aqueous solution. J. Mol. Catal. A Chem. 2015, 407, 67–74. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, K.; Song, W.; Song, G.; et al. Impact of water chemistry on surface charge and aggregation of polystyrene microspheres suspensions Sci. Total Environ. 2018, 630, 951–959. [Google Scholar] [CrossRef]
- Eisenberg, G. Colorimetric determination of hydrogen peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Devaraj, S.; Munichandraiah, N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J. Phys. Chem. C. 2008, 112, 4406–4417. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).