Submitted:
15 November 2023
Posted:
16 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
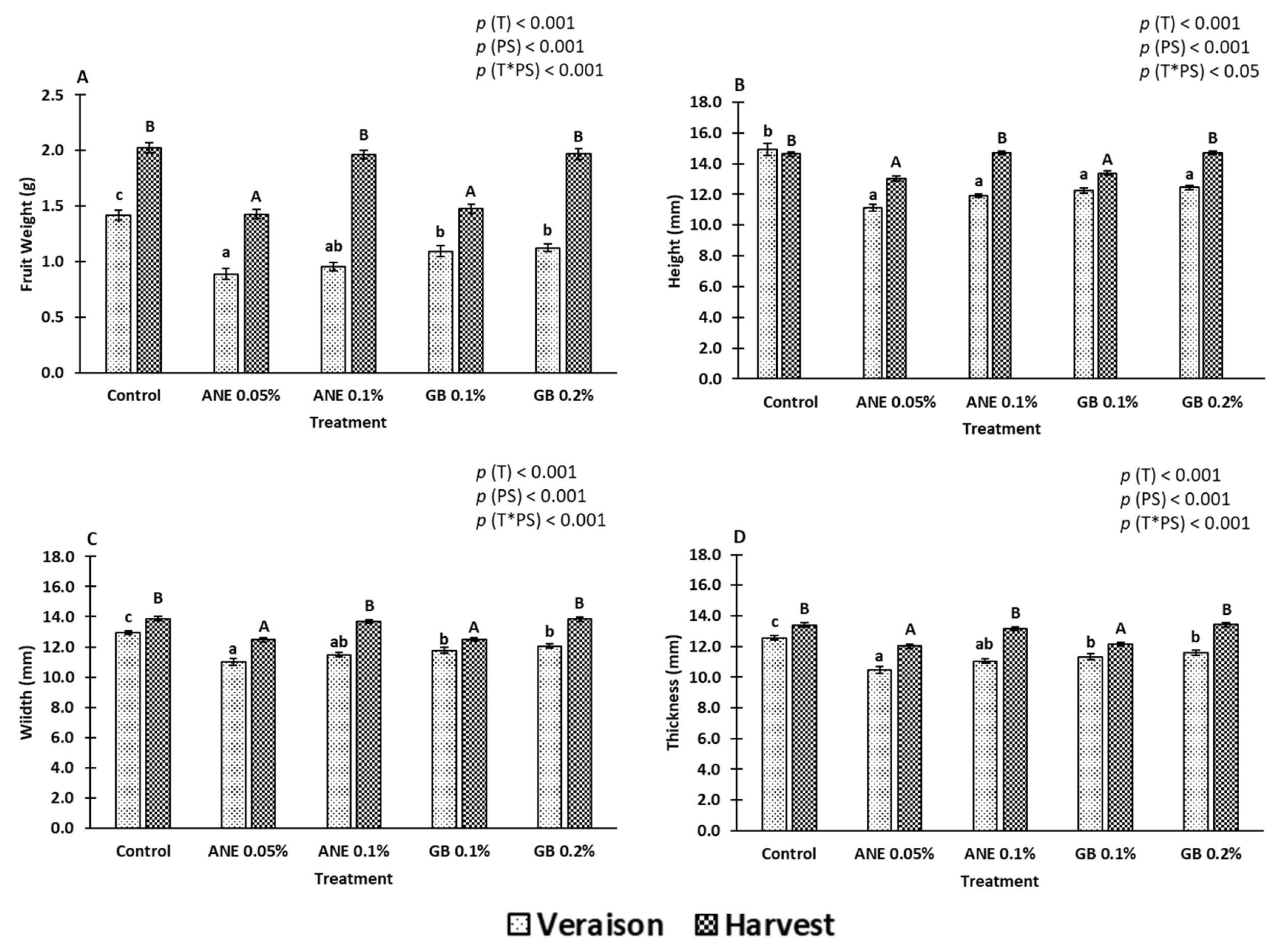
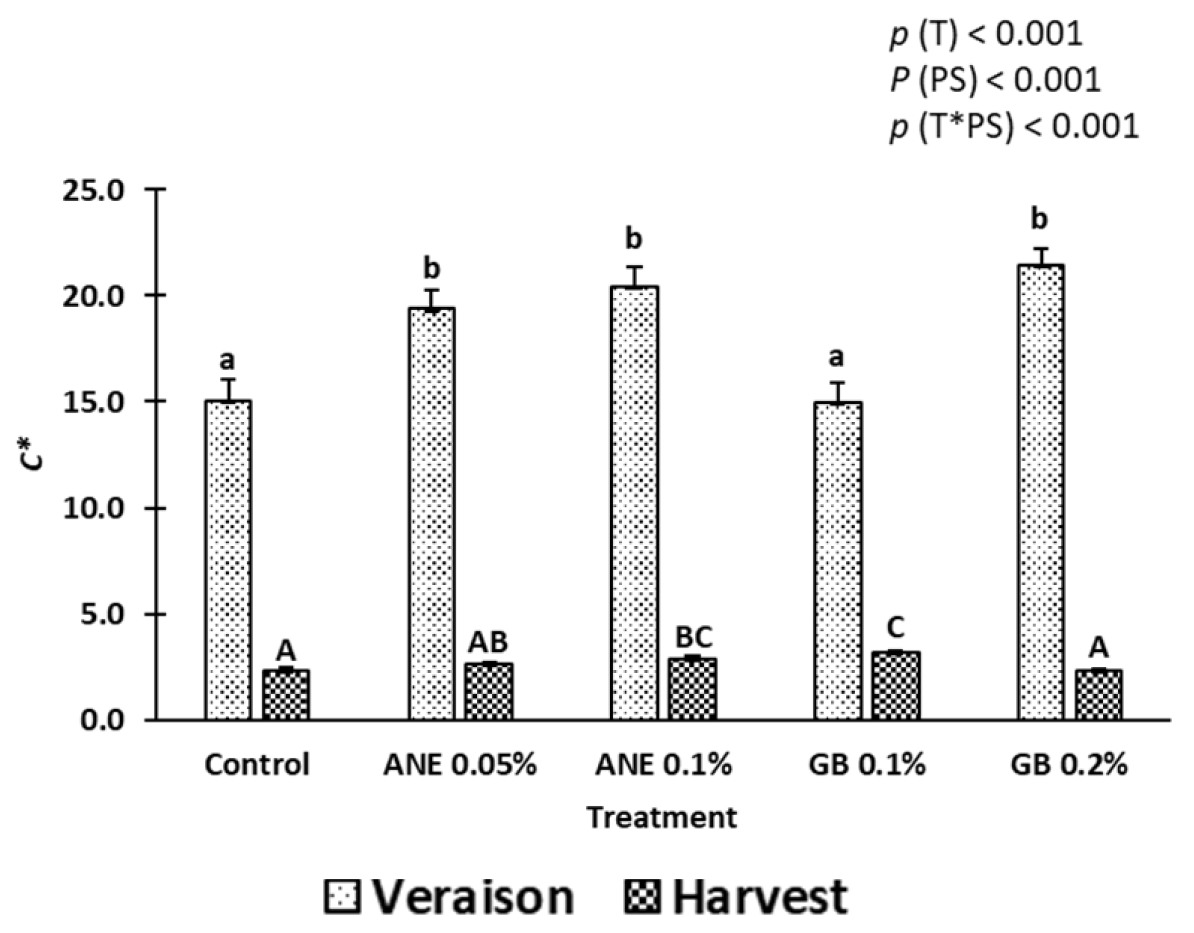
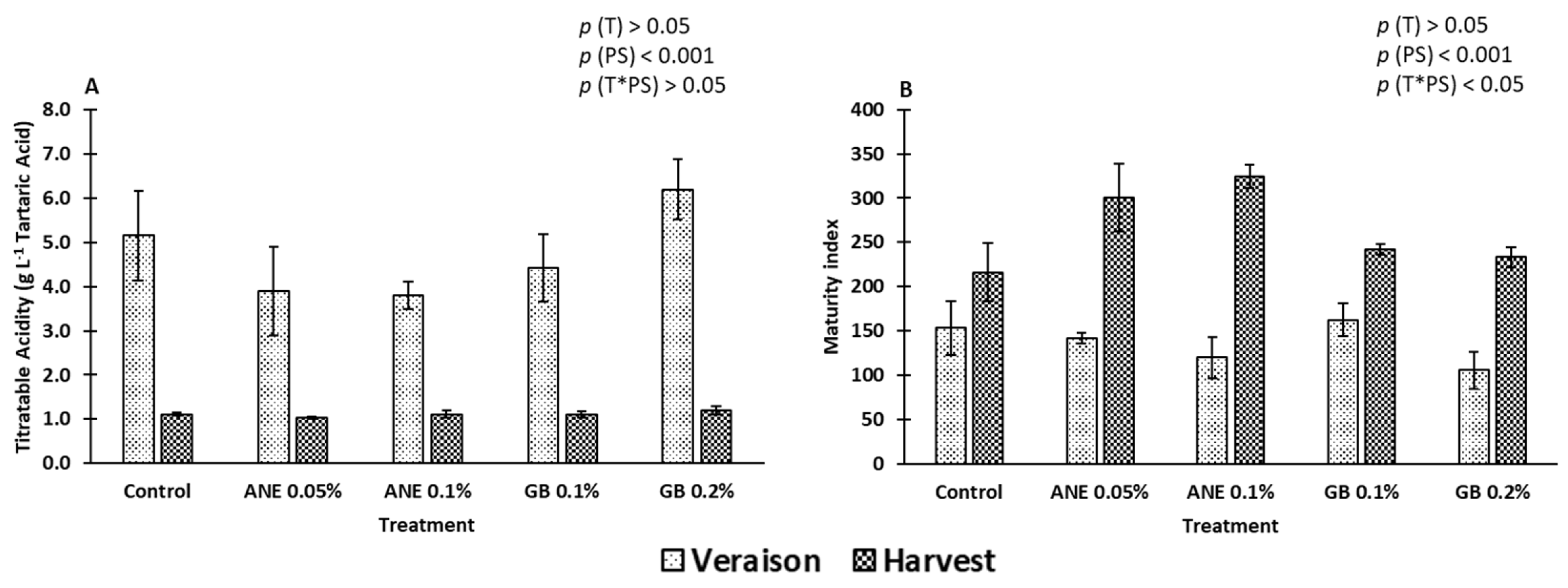
2.1. Berry Quality
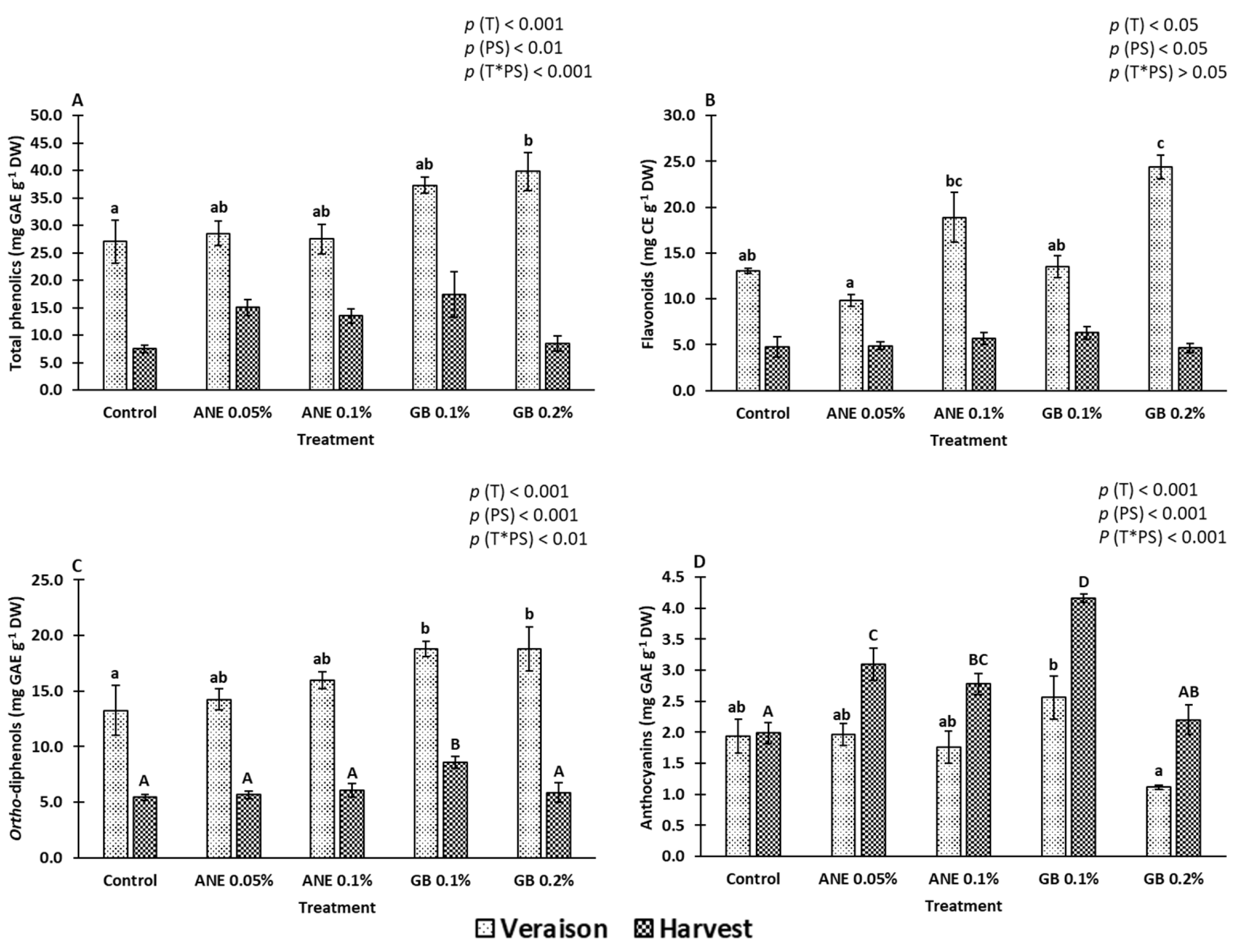
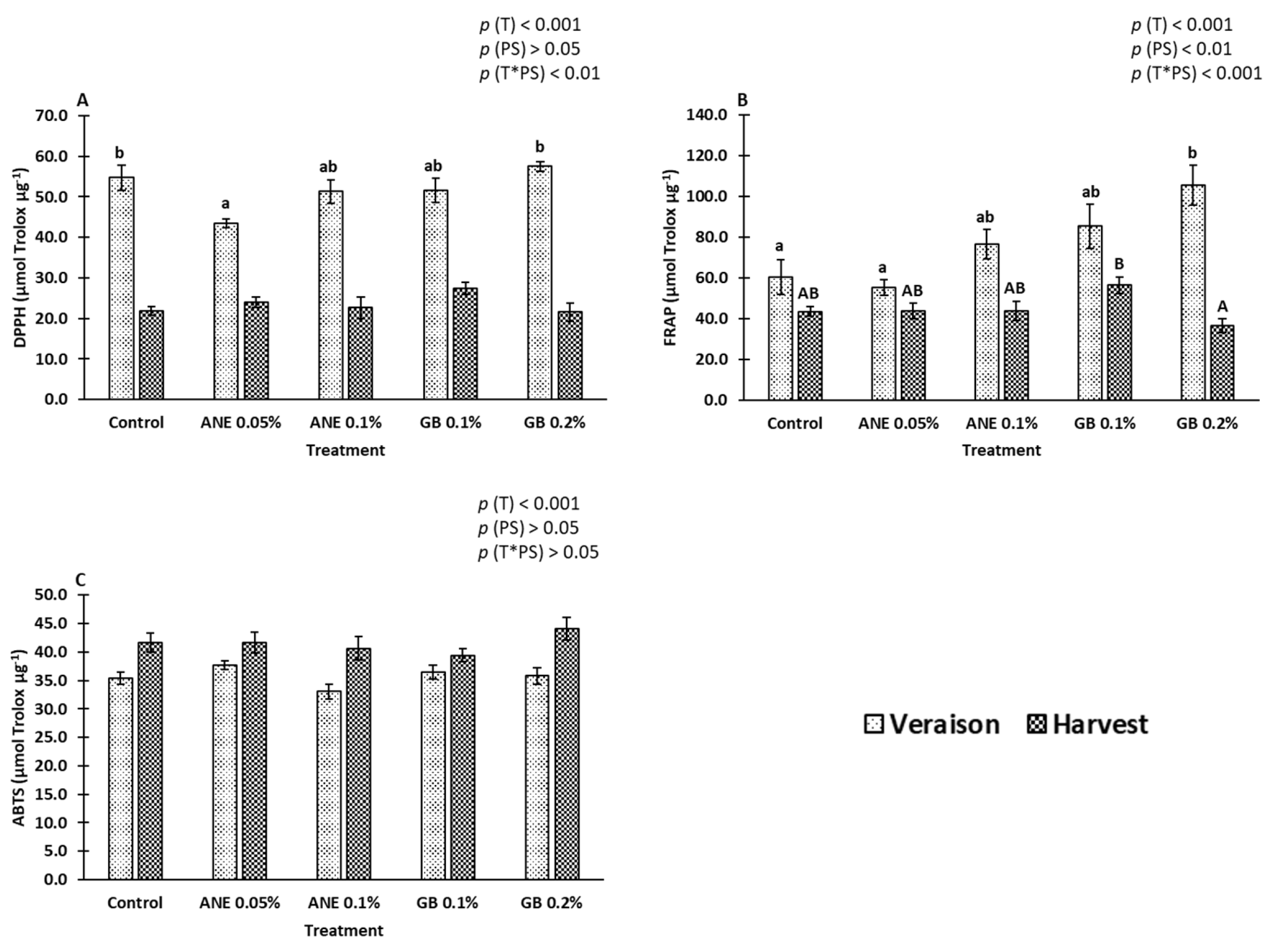
2.2. Bioactive Compounds
2.3. Antioxidant Potential
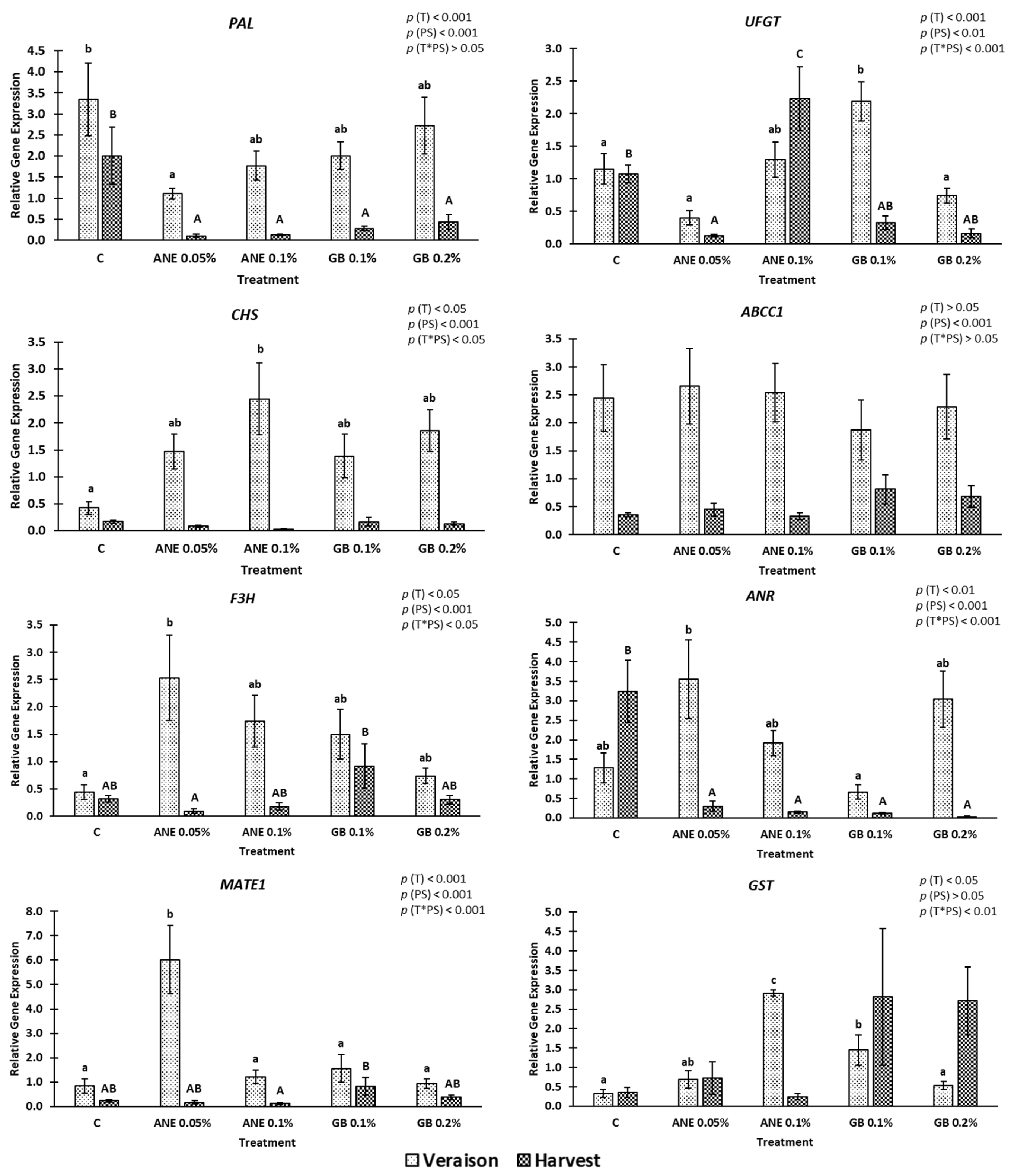
2.4. Gene Expression
3. Discussion
3.1. Impact of Biostimulants on the Quality Attributes of Berries
3.2. Impact of Biostimulants on Bioactive Compounds, Antioxidant Activity, and Gene Expression
4. Materials and Methods
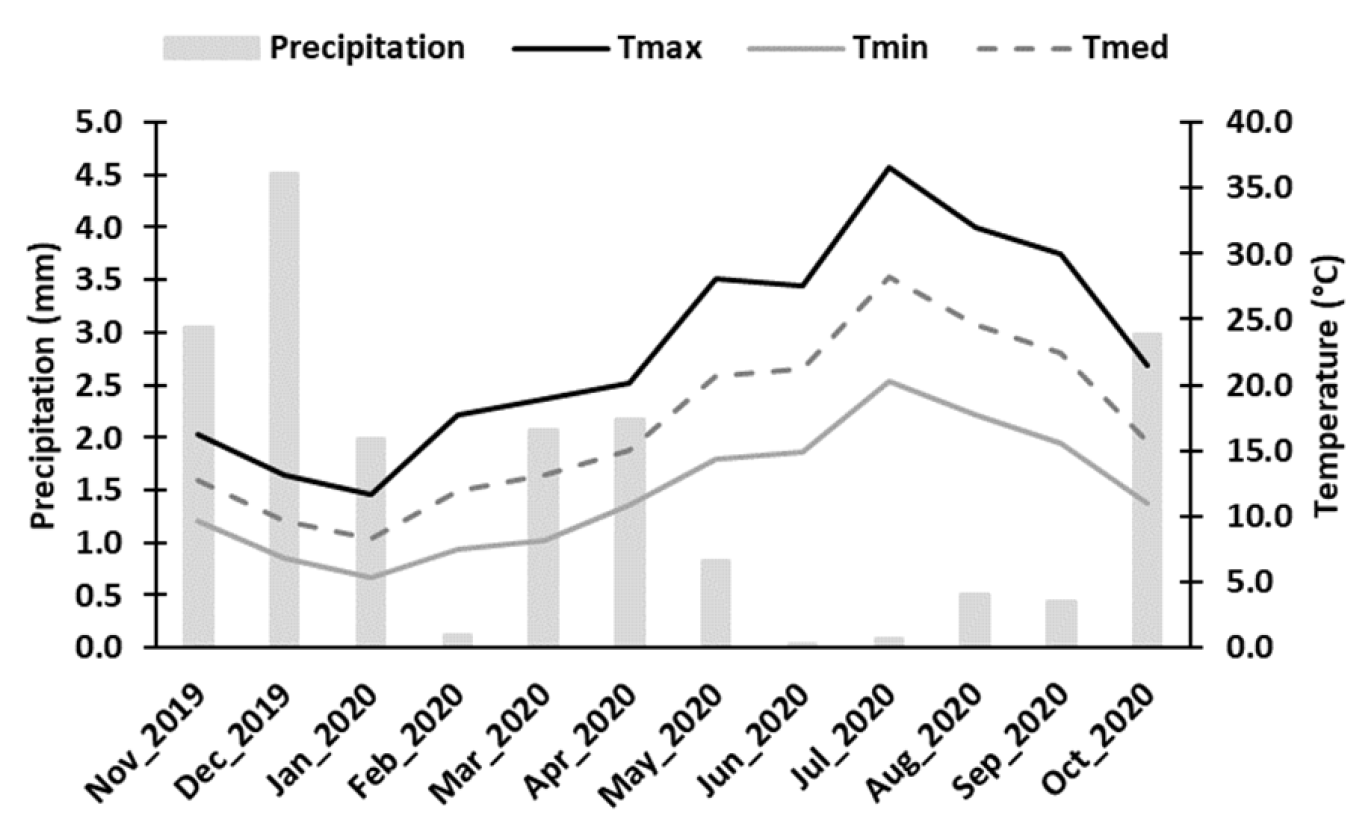
4.1. Plant material and sampling
4.2. Quality Assessment of Fruit
4.3. Determination of bioactive compounds
4.3.1. Total phenolics
4.3.2. Flavonoids
4.3.3. Ortho-diphenols
4.3.4. Total anthocyanins
4.4. Antioxidant activity assays
4.4.1. ABTS•+ radical-scavenging activity
4.4.2. DPPH radical-scavenging activity
4.4.3. FRAP assay
4.5. Total RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR
4.6. Statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV. State of the World Vine and Wine Sector 2022. Organization of Vine and Wine: Dijon, France, 2023.
- IVV. Regiões; Instituto da Vinha e do Vinho, I.P.: Lisboa 2023. Available online: https://www.ivv.gov.pt/np4/regioes/ (accessed on 9 June 2023).
- Fraga H, De Cortázar Atauri IG, Malheiro AC, Moutinho-Pereira J, Santos JA. Viticulture in Portugal: A Review of Recent Trends and Climate Change Projections. OENOOne 2017;51:61–9. [CrossRef]
- Mateus N, Machado JM, De Freitas V. Development changes of anthocyanins in Vitis vinifera grapes grown in the Douro Valley and concentration in respective wines. J Sci Food Agric 2002;82:1689–95. [CrossRef]
- Conde A, Pimentel D, Neves A, Dinis L-T, Bernardo S, Correia CM, et al. Kaolin foliar application has a stimulatory effect on phenylpropanoid and flavonoid pathways in grape berries. Front Plant Sci 2016;7:1–14. [CrossRef]
- Fonseca A, Fraga H, Santos JA. Exposure of Portuguese viticulture to weather extremes under climate change. Clim Serv 2023;30. [CrossRef]
- Frioni T, Sabbatini P, Tombesi S, Norrie J, Poni S, Gatti M, et al. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci Hortic (Amsterdam) 2018;232:97–106. [CrossRef]
- Frioni T, Tombesi S, Quaglia M, Calderini O, Moretti C, Poni S, et al. Metabolic and transcriptional changes associated with the use of Ascophyllum nodosum extracts as tools to improve the quality of wine grapes (Vitis vinifera cv. Sangiovese) and their tolerance to biotic stress. J Sci Food Agric 2019;99:6350–63. [CrossRef]
- Salvi L, Brunetti C, Cataldo E, Niccolai A, Centritto M, Ferrini F, et al. Effects of Ascophyllum nodosum extract on Vitis vinifera: consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol Biochem 2019;139:21–32. [CrossRef]
- Taskos D, Stamatiadis S, Yvin JC, Jamois F. Effects of an Ascophyllum nodosum (L.) Le Jol. extract on grapevine yield and berry composition of a Merlot vineyard. Sci Hortic (Amsterdam) 2019;250:27–32. [CrossRef]
- Baltazar M, Correia S, Guinan KJ, Sujeeth N, Bragança R, Gonçalves B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021;11. [CrossRef]
- Monteiro E, Gonçalves B, Cortez I, Castro I. The role of biostimulants as alleviators of biotic and abiotic stresses in grapevine: A review. Plants 2022;11:396. [CrossRef]
- Hussain Wani S, Brajendra Singh N, Haribhushan A, Iqbal Mir J. Compatible solute engineering in plants for abiotic stress tolerance - Role of glycine betaine. Curr Genomics 2013;14:157–65. [CrossRef]
- Dutta T, Neelapu NRR, Wani SH, Challa S. Compatible solute engineering of crop plants for improved tolerance toward abiotic stresses. In: Biochem. Physiol. Mol. Ave. Combat. Abiotic Stress Toler. Plants, Wani, S.H., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018, p. 221–254. [CrossRef]
- Denaxa N-K, Tsafouros A, Ntanos E, Roussos PA. Role of glycine betaine in the protection of plants against environmental stresses. Plant Stress Mitigators 2023:127–58. [CrossRef]
- Le Mire G, Nguyen ML, Fassotte B, Du Jardin P, Verheggen F, Delaplace P, et al. Implementing plant biostimulants and biocontrol strategies in the agroecological management of cultivated ecosystems. A review. Biotechnol Agron Soc Environ 2016;20:299–313. [CrossRef]
- Gutiérrez-Gamboa G, Romanazzi G, Garde-Cerdán T, Pérez-Álvarez EP. A review of the use of biostimulants in the vineyard for improved grape and wine quality: effects on prevention of grapevine diseases. J Sci Food Agric 2019;99:1001–9. [CrossRef]
- Bulgari R, Cocetta G, Trivellini A, Vernieri P, Ferrante A. Biostimulants and Crop Responses: A review. Biol Agric Hortic 2015;31:1–17. [CrossRef]
- Dinis LT, Bernardo S, Conde A, Pimentel D, Ferreira H, Félix L, et al. Kaolin exogenous application boosts antioxidant capacity and phenolic content in berries and leaves of grapevine under summer stress. J Plant Physiol 2016;191:45–53. [CrossRef]
- Aziz A, Poinssot B, Daire X, Adrian M, Bézier A, Lambert B, et al. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol Plant-Microbe Interact 2003;16:1118–28. [CrossRef]
- Ferrandino A, Lovisolo C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ Exp Bot 2014;103:138–47. [CrossRef]
- Koyama R, Roberto SR, de Souza RT, Borges WFS, Anderson M, Waterhouse AL, et al. Exogenous Abscisic Acid Promotes Anthocyanin Biosynthesis and Increased Expression of Flavonoid Synthesis Genes in Vitis vinifera × Vitis labrusca Table Grapes in a Subtropical Region. Front Plant Sci 2018;9:1–12. [CrossRef]
- Singh RK, Martins V, Soares B, Castro I, Falco V. Chitosan application in vineyards (Vitis vinifera L. cv. Tinto Cão) induces accumulation of anthocyanins and other phenolics in berries, mediated by modifications in the transcription of secondary metabolism genes. Int J Mol Sci 2020;21:306. [CrossRef]
- Robinson SP, Bogs J, McDavid DAJ, Hooper LC, Speirs J, Walker AR. Transgenic grapevines with decreased expression of tannin synthesis genes have altered grape and wine flavonoid composition. Aust J Grape Wine Res 2021;27:106–17. [CrossRef]
- Adak N. Effects of glycine betaine concentrations on the agronomic characteristics of strawberry grown under deficit irrigation conditions. Appl Ecol Environ Res 2019;17:3753–67. [CrossRef]
- Correia S, Queirós F, Ribeiro C, Vilela A, Aires A, Barros AI, et al. Effects of calcium and growth regulators on sweet cherry (Prunus avium L.) quality and sensory attributes at harvest. Sci Hortic (Amsterdam) 2019;248:231–40. [CrossRef]
- Iqbal N, Ashraf MY, Ashraf M. Influence of water stress and exogenous glycine betaine on sunflower achene weight and oil percentage. Int J Environ Sci Technol 2005;2:155–60. [CrossRef]
- Metwaly E-SE, Al-Yasi HM, Ali EF, Farouk HA, Farouk S. Deteriorating harmful effects of drought in cucumber by spraying glycine betaine. Agriculture 2022;12:2166. [CrossRef]
- Taskin S, Ertan E. Exogenous Applications of kaolin and glycine betaine increased the yield and quality of olive fruit and olive oil. Erwerbs-Obstbau 2022. [CrossRef]
- Gonçalves B, Morais M, Sequeira A, Ribeiro C, Guedes F, Silva A, et al. Quality preservation of sweet cherry cv. “Staccato” by using glycine-betaine or Ascophyllum nodosum. Food Chem 2020. [CrossRef]
- Monteiro E, Baltazar M, Pereira S, Correia S, Ferreira H, Alves F, et al. Ascophyllum nodosum Extract and Glycine Betaine Preharvest Application in Grapevine: Enhancement of Berry Quality, Phytochemical Content and Antioxidant Properties. Antioxidants 2023;12:1835. [CrossRef]
- Campbell J, Sarkhosh A, Habibi F, Gajjar P, Ismail A, Tsolova V, et al. Evaluation of biochemical juice attributes and color-related traits in muscadine grape population. Foods 2021;10:1101. [CrossRef]
- Borghezan M. Formation and ripening of grape and effects on the wines: Review. Ciência e Técnica Vitivinícola 2017;32:126–41. [CrossRef]
- Rätsep R, Karp K, Vool E. Yield maturity parameters of hybrid grap Evine (Vitis Sp.) cultivar “Zilga.” Res. Rural Dev., vol. 1, 2014, p. 44–50.
- ADVID. Boletim Ano Vitícola 2020; ADVID: Vila Real, 2020. Available online: www.advid.pt (accessed on 14 June 2023).
- Parađiković N, Teklić T, Zeljković S, Lisjak M, Špoljarević M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur 2019;8:1–17. [CrossRef]
- Bulgari R, Franzoni G, Ferrante A. biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019;9:306. [CrossRef]
- Delaunois B, Farace G, Jeandet P, Clément C, Baillieul F, Dorey S, et al. Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ Sci Pollut Res 2014;21:4837–46. [CrossRef]
- Arakawa O. Photoregulation of anthocyanin synthesis in apple fruit under UV-B and red light. Plant Cell Physiol 1988:1385–1389.
- Mosedale JR, Abernethy KE, Smart RE, Wilson RJ, Maclean I. Climate change impacts and adaptive strategies: lessons from the grapevine. Glob Chang Biol 2016:3814–3828. [CrossRef]
- Lorenz DH, Eichhorn KW, Bleiholder H, Klose R, Meier U, Weber E. Growth stages of the grapevine: phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust J Grape Wine Res 1995;1:100–3. [CrossRef]
- Coombe BG, Dundon RJ, Short AWS. Indices of sugar—acidity as ripeness criteria for winegrapes. J Sci Food Agric 1980;31:495–502. [CrossRef]
- Singleton V, Rossi J. Colorometry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965:144–58.
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 2002:3010–4. [CrossRef]
- Gouvinhas I, De Almeida JM, Carvalho T, Machado N, Barros A. Discrimination and characterisation of extra virgin olive oils from three cultivars in different maturation stages using Fourier transform infrared spectroscopy in tandem with chemometrics. Food Chem 2015;174:226–32. [CrossRef]
- Leal C, Costa CM, Barros A, Gouvinhas I. Assessing the relationship between the phenolic content and elemental composition of grape (Vitis vinifera L.) stems. Waste and Biomass Valorization 2021;12:1313–25. [CrossRef]
- Lee J, Durst RW, Wrolstad RE, Eisele T, Giusti MM, Haché J, et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int 2005;88:1269–78. [CrossRef]
- Meng J-F, Fang Y-L, Qin M-Y, Zhuang X-F, Zhang Z-W. Varietal differences among the phenolic profiles and antioxidant properties of four cultivars of spine grape (Vitis davidii Foex) in Chongyi County (China). Food Chem 2012;134:2049–56. [CrossRef]
- Ali Shehat W, Sohail Akh M, Alam T. Extraction and estimation of anthocyanin content and antioxidant activity of some common fruits. Trends Appl Sci Res 2020;15:179–86. [CrossRef]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231–7. [CrossRef]
- Stratil P, Klejdus B, Kubáň V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J Agric Food Chem 2006;54:607–16. [CrossRef]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol 1995;28:25–30. [CrossRef]
- Sánchez-Moreno C, Larrauri J, Saura-Calixto F. A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 1998:270–6. [CrossRef]
- Siddhraju P, Becker K. Antioxidant properties of various solvents extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam) leaves. J Agric Food Chem 2003:2144–55. [CrossRef]
- Benzie IFF, Strain JJ. The Ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996;239:70–6. [CrossRef]
- Fujita A, Soma N, Goto-Yamamoto N, Mizuno A, Kiso K, Hashizume K. Effect of shading on proanthocyanidin biosynthesis in the grape berry. J Japanese Soc Hortic Sci 2007;76:112–9. [CrossRef]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4):402-8. [CrossRef] [PubMed]
| Gene | Primer sequence | Annealing temperature | Reference | |
|---|---|---|---|---|
| PAL | Phenylalanine ammonia-lyase | F 5’ CCTACTGTTCAGAGCTCCAG 3’ R 5’ GCCACTAGGTATGTGGTAGACA 3’ |
57 °C | [23] |
| CHS | Chalcone synthase | F 5’ CACTCTTCGAACTCGTCTCT 3’ R 5’ CCACCAAGCTCTTCTCTATG 3’ |
57 °C | [23] |
| F3H | Flavanone3-hydroxylase | F 5’ CAGTGCAAGACTGGCGCGAGATCGTA 3’ R 5’ TAGCCTCAGACAACACCTCCAGCAACT 3’ |
57 °C | [23] |
| ANR | Anthocyanidin reductase | F 5’ CTGTCAGGTTCAGTCTCCAT 3’ R 5’ GTTGGGACTTTGTACTGAGG 3’ |
57 °C | [23] |
| UFGT | UDP glucose: flavonoid 3-O-glucosyl transferase | F 5’ TGCAGGGCCTAACTCACTCT 3’ R 5’ GCAGTCGCCTTAGGTAGCAC 3’ |
57 °C | [23] |
| ABCC1 | Anthocyanin transporter | F 5’ CTCCACTGGTCCTCTGCTTC 3’ R 5’ AGCCTGCTTCGAAAGTACCA 3’ |
57 °C | [23] |
| MATE1 | Tonoplast transporter | F 5’ TGCTTTTGTGATTTTGTTAGAGG 3’ R 5’ CCCTTCCCCGATTGAGAGTA 3’ |
57 °C | [23] |
| GST | GlutathioneS-transferase | F 5’ AAGGATCCATGGTGATGAAGGTGTATGGC 3’ R 5’ AACTGCAGAAGCCAACCAACCAACAAAC 3’ |
57 °C | [23] |
| UBI | Ubiquitin | F 5' TCTGAGGCTTCGTGGTGGTA 3’ R 5' AGGCGTGCATAACATTTGCG 3’ |
57 °C | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





