1. Introduction
Nowadays, the need of fresh and safety foods posed Food Technology in the investigation of novel antimicrobial/antioxidant systems to preserve foods. Simultaneously, sustainability and circular economy trends drives the replacement of food chemical additives by natural abundant antimicrobial and antioxidant agents. Antimicrobial agents are necessary for food preservation to prevent the growth of harmful microorganisms that can cause spoilage, reduce the shelf life of food products, and pose health risks to consumers [
1,
2]. Natural compounds like carvacrol, found in essential oils of various herbs, have been shown to have effective antimicrobial activity in food systems, including meat products [
3,
4]. Carvacrol is a natural phenolic monoterpene that is synthesized from the mevalonic acid pathway and commonly found in herbs and spices such as oregano, thyme, and marjoram [
5]. It has been shown to possess potent antimicrobial properties against various microorganisms, including bacteria and fungi, making it a promising natural alternative to synthetic preservatives in the food industry . Carvacrol’s mechanism of action involves disrupting the cell membrane of microorganisms, inhibiting adenosine triphosphate (ATP) synthesis, and leading to cell death [
8,
9]. Additionally, carvacrol has anti-inflammatory, analgesic, and antioxidant effects, which make it a potential candidate for use in various applications in the pharmaceutical and cosmetic industries [
10].
Recent studies have demonstrated the high antimicrobial activity of carvacrol compared to sulfanilamide against various bacteria [
11]. Investigations into the potential of carvacrol for food preservation have included the addition of carvacrol in an osmotic pretreatment to extend the shelf life of a food product by 3 days [
12], the development of carvacrol-loaded zein nanoparticles for active packaging in the meat industry [
13], and a chitosan (CS) coating with essential oils, including carvacrol, to preserve fresh blueberries during postharvest storage [
14]. These findings suggest that carvacrol is a promising natural alternative for food preservation in various forms.
Other studies have shown that controlled-release carvacrol powder can be effective for preserving small fruit, as it has been found to reduce microbial growth, weight loss, and improve sensory quality [
15]. Additionally, microencapsulated thymol and carvacrol in polymer films have been found to be natural antimicrobial solutions for food packaging, which can inhibit the growth of various microorganisms. These findings suggest that carvacrol and thymol have potential as natural alternatives for food preservation in various forms [
16].
Nanotechnology, particularly in the form of nanoemulsions (NEs), offers innovative solutions to challenges in food quality and quantity by employing materials smaller than 100 nm. Designed to deliver bioactive compounds like carvacrol, NEs are composed of oil, water, and emulsifiers that form minuscule droplets, encapsulating the bioactive elements in stabilizers for enhanced structural integrity. Due to their small droplet sizes [
19], NEs not only allow for improved penetration and distribution of active ingredients like carvacrol in food systems, but also enhance their solubility, stability, and bioavailability [
18]. Produced through high-energy methods such as high-pressure homogenization, these nanoemulsions have proven effective in inhibiting microbial growth in various food systems, including meat products [
20,
21]. Their nanoscale dimensions enable the efficient delivery of active substances in reduced dosages, making them versatile for applications across pharmaceutical, medical, and food sectors.
Meat deterioration is a complex process influenced by factors such as lipid oxidation and microbial spoilage. Lipid oxidation is a chemical reaction involving polyunsaturated fatty acids and reactive oxygen species that leads to the formation of volatile and toxic compounds like aldehydes and ketones. These compounds not only degrade the sensory and nutritional quality of the meat but also pose health risks to consumers. Microbial spoilage is exacerbated by factors like pre-slaughter and post-slaughter handling, the bacterial flora of the animal, and processing methods. Stress before slaughter depletes muscle glycogen, leading to the production of lactic acid and changes in meat pH. A higher pH level of 6.4-6.8 results in Dark, Firm, and Dry (DFD) meat, which is more susceptible to bacterial growth [
17]. Therefore, controlling oxidation and microbial contamination are key to preserving the quality and safety of meat products.
Nanoemulsions have been gaining attention for their notable advantages over microemulsions in food applications, particularly in terms of enhanced stability and superior bioactive delivery capabilities. Interestingly, while the efficacy of free essential oils has been extensively documented, a comparative analysis delineating the performance of free essential oil, microemulsions, and nanoemulsions containing carvacrol remains elusive in the literature. Furthermore, the innovative approach of integrating carvacrol into a microemulsion enhanced with CS is a distinctive aspect of the current research, given CS’s renowned biocompatible and antimicrobial properties. For this study, these coatings are applied to fresh pork minced meat. The primary objective is to identify any statistically significant differences in the shelf-life and quality of the meat over a 9-day storage period, aiming to offer insights into the best formulation for meat preservation.
2. Materials and Methods
2.1. Materials
Carvacrol (Cas. No. 98 499-75-2), Ethanol (Cas. No. 493511), TWEEN 80 (CAS No.9005-65-6), Sodium caseinate. (CAS No. 9005-46-3), L-α-Lecithin, Soybean, (CAS 8002-43-5) and glacial acetic acid (CAS No.:62357-86-2) were purchased from Sigma-Aldrich Co., (3050 Spruce Street, St. Louis, MO 63103 USA 314-771-5765). Chitosan with a molecular weight of 100,000–300,000 was purchased from Acros-Organics (Zeel West Zone 2, Janssen Pharmaceuticalaan 3a B2440 Geel, Belgium).
Müeller-Hinton agar plates, Mueller Hinton Broth, Tryptic Soy Agar (TSA) and sterile swabs and forceps were purchased from Sigma-Aldrich Co., (3050 Spruce Street, St. Louis, MO 63103 USA 314-771-5765). Pure Gramm positive bacterial culture of Listeria monocytogenes (DSMZ 27575) (LM) and Staphylococcus aureus (DSMZ 12463) (SA) were obtained from the Institute of Technology of Agricultural Products, ELGO-DEMETER, Lykovryssi, Greece.
2.2. Preparation of carvacrol microemulsions and nanoemulsions
In the
Table 1 the materials and quantities used for the preparation of carvacrol microemulsions and carvacrol nanoemulsion as well as their code names are listed.
The microemulsion containing 2.5% carvacrol (MC) was prepared by the self-emulsification method [
18]. The appropriate amounts (see
Table 1) of distilled water, Tween-80 as surfactant and ethanol as co-surfactant are mixed in the beaker. Then 2.5 ml of carvacrol are added under constant stirring (1000 rpm) and the transparent MC was obtained (see
Table 1).
The microemulsion containing 2.5% carvacrol and diluted chitosan in the aqueous phase (MCC) was prepared similarly to the MC; however, acetic acid was first employed to dissolve CS in a 1:1 ratio before incorporating it into the aqueous phase (see
Table 1).
The nanoemulsion containing 2.5% carvacrol (NC) coating was prepared by mixing the appropriate amounts (see
Table 1) of distilled water, Lecithin, Casein and Carvacrol by using a high-speed ultrasonic homogenizer at 15,000 rpm and at ambient temperature for 20 (see
Figures S1 and S2).
2.3. Characterization of obtained carvacrol based microemulsion and nanoemulsions Dynamic light scattering (DLS) measurements and contact angle measuraments.
The DLS measurements were carried out using a high performance two angle particle and molecular size analyzer (Zetasizer Nano ZS, Malvern). The apparatus operated at a wavelength of 633 nm and was equipped with a helium-neon laser at the standard angle of 173
o. For the experiments a glass cuvette with square aperture was used. The measurements were conducted to evaluate the hydrodynamic diameter and the polydispersity index of the solutions, at 37
o C. The hydrodynamic radius calculation is based on the Einstein-Stokes equation:
where kB is the Boltzmann constant, T is the absolute temperature, η is the solvent viscosity, and D is the diffusion coefficient. The concentration of the solutions was approximately 0.1 wt%.
The study on wetting characteristics of MC, MCC and NC coating samples was carried out using a contact angle instrument (OCA 25, DataPhysics Instruments GmbH, Filderstadt, Germany). Solutions with specific concentrations were prepared and then spin-coated onto silicon wafers under specific conditions (3500 rpm for 30 seconds), resulting in films with a thickness of approximately 40-50 nanometers. The silicon wafers were initially treated with a piranha solution (sulfuric acid/hydrogen peroxide in a 3:1 ratio). During the measurements, 4 μL droplets of deionized water (DI water) were deposited at a consistent rate of 0.5 μL/s. Three separate measurements were conducted on different regions of the same wafer, and the average value was computed and presented in the obtained results. The deviation in all instances did not surpass ±2 degrees, demonstrating the consistency of the results attributable to uniform film deposition.
2.4. Total antioxidant activity of carvacrol based microemulsion and nanoemulsions with DPPH assay.
For the estimation of concentration required to obtain 50% antioxidant activity (EC50) of the obtained MC, MCC, and NC coatings as well as pure carvacrol 20, 40, 60, 80 and 100 μL of each coating (three times each) were added in 2.8 mL of 30 ppm DPPH ethanolic solution, and 0.2 mL of CH
3COONax3H
2O buffer solution. For comparison a blank solution with 2.8 mL of 30 ppm DPPH ethanolic solution, and 0.2 mL of CH
3COONax3H
2O buffer solution was prepared. Next the absorbance at 517 nm at after 1h was measured for all film coatings
as well as for blank coatings
. The %antioxidant activity of all coatings calculated by using the equation:
Next the calculated values of % antioxidant activity of films was plotted as a function of film quantity used and the linear equation from the obtained plot was calculated. From the obtained linear equations for each coating the EC50 value calculated.
2.5. Antibacterial activity test of carvacrol based microemulsion and nanoemulsions.
2.5.1. Inhibition zone tests
The antimicrobial efficacy of various Carvacrol formulations was investigated using the well diffusion method against two critical foodborne pathogenic bacteria, specifically Staphylococcus aureus (DSMZ 12463) and Listeria monocytogenes (DSMZ 27575). These bacterial strains were sourced from the Institute of Technology of Agricultural Products, ELGO DEMETER, situated in Lykovryssi, Greece. To begin the study, the bacterial strains were cultured in Müeller Hinton Broth and incubated at 37 °C for a duration of 24 hours. This enabled bacterial growth and led to a bacterial concentration that ranged between 107 and 108 colony-forming units per milliliter (CFU mL-1). Thereafter, the cultured bacteria were uniformly spread on Müller-Hinton agar plates using a sterile swab. The plates were rotated at intervals of 60 degrees to guarantee an even distribution of bacterial colonies. 6-mm wells were created on the agar surface of each plate using a cork borer that had been sterilized by dipping in alcohol and subsequently flaming. These wells were filled with 100 μl of the Carvacrol suspensions being studied, including the control, MC, MCC, and NC coatings. After the wells had been filled, the agar plates were incubated at 37 °C overnight. Following the incubation period, the diameters of the clear zones around each well were measured using calipers to evaluate the antimicrobial activity of the different Carvacrol formulations. The clear zones represented the area where bacterial growth was effectively inhibited by the respective suspensions. All experimental steps were performed in triplicate to ensure consistency and reliability in the acquired data. By using this methodology, we aimed to offer a comprehensive insight into the antimicrobial capabilities of different Carvacrol formulations against Staphylococcus aureus and Listeria monocytogenes.
2.5.2. Minimum Inhibition Concentration (MIC) tests
The MIC serves as the lowest concentration of the antimicrobial agent at which the visible growth of the microorganism is inhibited, primarily indicating the bacteriostatic effect of the agents without specific data on the microbial population condition. Pure cultures of Listeria monocytogenes (LM), Staphylococcus aureus (SA), and Escherichia coli (EC) were cultivated in Müeller-Hinton broth to concentrations approximating 10-6 CFU/mL. Four types of antimicrobial agents were evaluated: MC, MCC, NC, and FC. These agents were tested at multiple concentrations, including 1000, 500, 250, 125, 62.5, 31.25, 15.625 μg/mL [
19]. Utilizing the Macro dilution method, serial decimal dilutions of the microbial cultures were conducted, and the agents were added at each of these concentrations for evaluation. Following thorough mixing via a vortex mixer, the tubes were incubated at 37 °C for 24 hours. Control tubes, which contained only microbial cultures without any antimicrobial agents, were also maintained and assessed at a concentration of 250 μg/mL. Turbidity in the tubes was observed as an indicator of microbial growth, and additional validation was performed through culture and colony counting techniques. All tests were conducted in triplicate to ensure the reliability and repeatability of the observed results.
2.6. Application of carvacrol based microemulsions and nanoemulsion as active coatings in fresh minced pork meat
Fresh minced pork meat was provided by a local meat processing plant (Aifantis Company-Aifantis Group—Head Quarters, Acheloos Bridge, Agrinio, Greece 30100) and immediately transported to the laboratory. For each of the five treatment categories (Uncoated, FC, MC, MCC, and NC 30 grams of the minced meat was weighed. Each meat coating was then dipped into 25 mL of the appropriate treatment solution to ensure uniform concentration of coated solution across all coated meat samples (see
Figure S3). Following the dipping process, the meat was wrapped in a specialized membrane and stored at a temperature of 4 °C for a period of nine days, physicochemical analyses such pH measuraments, L*a*b Analysis and lipid oxidation analyses, microbiological analyses and sensory analysis were conducted at three-day intervals throughout the storage duration to assess the efficacy of the applied treatments in meat preservation.
2.7. Physicochemical properties of pork minced meat
Chemical parameters including pH, color value (Lab), and thiobarbituric acid reactive substances (TBARs) were analyzed in pork minced meat coatings at specific time intervals: days 0, 3, 6, and 9.
2.7.1. pH analysis
The pH values of the pork minced meat coatings were measured using a portable pH meter fitted with a penetration electrode and a temperature sensor (pH-Star, Matthäus GmbH, Poettmes, Germany). Prior to each set of measurements, the pH meter was calibrated using pH standard solutions of 4.0 and 7.0, and temperature-adjusted to match the meat coating temperature of 4 °C. The entire study was conducted in triplicate, and for each treatment group, ten separate pH readings were taken to ensure accuracy and reliability, as per the methods [
20]. Overall, all coatings displayed an increase in pH over the 9-day analysis period.
2.7.2. L*a*b Analysis
The alterations in the CIELAB color parameters (L*, a*, and b*) of pork minced meat over a period of 9 days under refrigerated storage were assessed using an LS171 colorimeter from Linshang Company (see
Figure S4. Prior to conducting the measurements, the colorimeter was calibrated with a white standard plate in accordance with methods cited in Kang et al. (2019). Color evaluations were conducted directly on the surface of the minced meat coatings, with each treatment group comprising three separate portions. For each of these portions, nine discrete readings were taken to capture a robust assessment of the color. The total color differences (ΔE) were calculated using the equation [
21]:
In this equation, L*0, a*0, b*0 denote the initial color parameters of the pork minced meat at Day 0 post-treatment. L *, a*, b and represent the respective color parameters at different time points during the 9-day refrigerated storage at 4 °C.
2.7.3. Lipid Oxidation TBARS
The level of lipid oxidation in meat coatings was measured using the Thiobarbituric Acid Reactive Substances (TBARS) method, based on Tarladgis et al. (1960) with minor alterations [
22]. A 10-gram coating of meat was combined with 97.5 ml of distilled water and 2.5 ml of 4N HCl in a 500 ml flask. This mixture was steam distillated for roughly 20 minutes to yield 50 ml of distillate by using a steam distillation apparatus (see
Figure S5). From this distillate, a 5 ml coating was mixed with an equal volume of 0.02 M 2-thiobarbituric acid solution in a 15 ml tube. The tube was sealed and heated in a boiling water bath for approximately 35 minutes to form the TBA-Malondialdehyde (TBA-MA) chromogen. After cooling, the absorbance was measured at 532 nm using a DU-8 spectrophotometer. The conversion of TBA-MA absorbance readings to TBA numbers was carried out using a conversion factor of 7.8, as determined by the 1,1,3,3-tetramethoxypropane standard.
2.8. Microbiological analyses of stored fresh pork minced meat.
Fresh pork minced meat coatings were evaluated for mesophilic bacteria, lactic acid bacteria (LAB), and psychrotrophic bacteria on days 0, 3, 6, and 9 of storage at 4 °C. An aseptic transfer of a 10g pork sample was conducted into a stomacher bag, which was subsequently homogenized with 90 ml of peptone water. This was followed by a serial dilution (1:10 ratio). For the quantification of mesophilic bacteria, 0.1 ml from the diluted samples was spread onto PCA agar plates using the spread plate method and then incubated at 37 °C for 48 hours. Psychrotrophic bacteria were enumerated using the pour plate method; 1 ml of the sample was added to the plate, covered twice with the specific agar, and then incubated at 4 °C. For LAB determination, the MRS agar was employed with the pour plate method, and samples were incubated at 30°C for 72 hours [
23]. All results were presented in terms of log CFU/g.
2.9. Sensory evaluation of stored fresh minced pork meat
The sensory attributes of the minced meat samples were evaluated throughout the storage period by a trained panel of 15 members, who have a substantial background in meat and sensory evaluation. The assessment was carried out using a 9-point hedonic scale to rate various attributes such as color, appearance, odor, texture, and overall acceptability, with 9 being “like extremely” and 1 being “dislike extremely.” A score of 5 served as the lower limit for acceptability, as outlined in the methodology proposed by Hasani-Javanmardi, Fallah, & Abbasvali (2021). Each sample was assessed in triplicate for each replicate, resulting in a total of six evaluations per treatment group.
2.10. Statistical Analysis
An entirely random design was employed. The average results of each experiment were recorded, and each was carried out in triplicate. The statistical analyses were conducted using IBM SPSS version 22 (IBM Armonk, NY, USA), and an alpha level of P0.05 was established as a threshold for differentiating the means using Duncan’s multiple range test (after ANOVA) or t-test.
3. Results
3.1. Characterization of carvacrol micro and nanoemulsion coatings
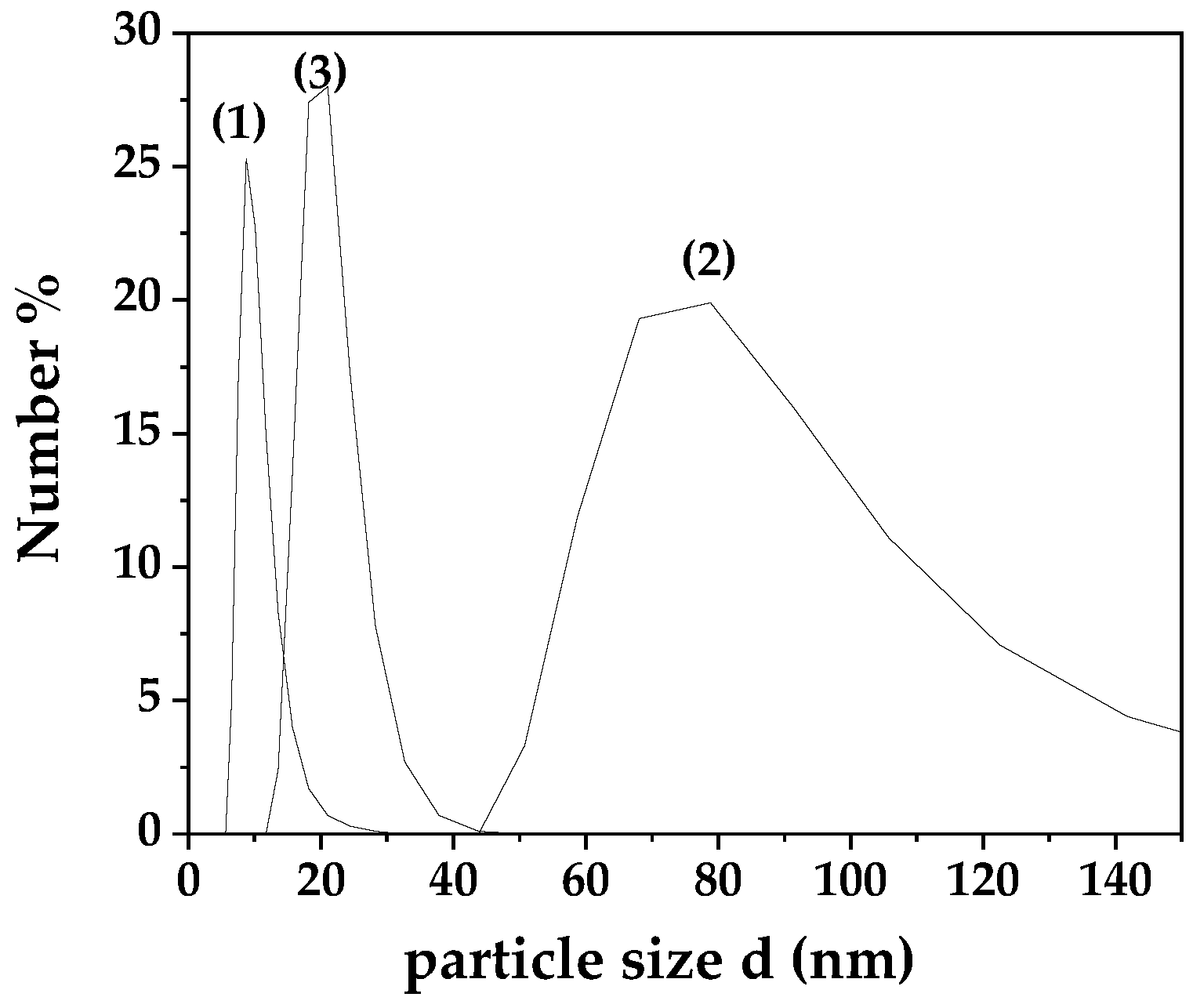
In
Figure 1 are presented the particle size distribution plots obtained from the DLS experiments for MC, MCC and NC samples.
Lines (1), (2) and (3) in
Figure 1 show the particle size distribution of MC, MCC and NC coatings correspondingly. As it is obtained the calculated average particle size hydrodynamic diameter for MC coating is approximately 10 nm, for MCC coating is approximately~ 93 nm, and for NC coating is approximately ~ 21 nm.
The increase in CS NE droplet size has been reported to several studies. Increasing the initial content of essential oils like carvacrol in CS nanoparticles leads to larger droplet sizes, likely due to reduced stability of the nanoparticle dispersion in water and interactions between CS’s amino groups and carvacrol. These interactions result in a less positively charged surface, contributing to droplet enlargement [
24,
25,
26].
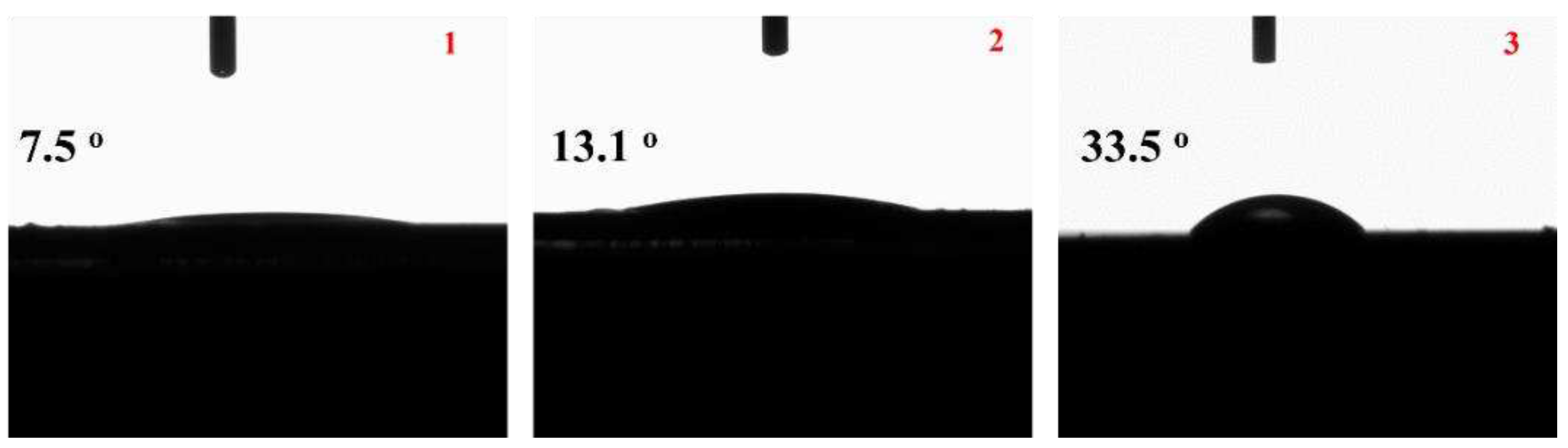
In
Figure 2 the images of water contact angles of the obtained (1) MC, (2) MCC and (3) NC samples it is shown for comparison.
Wettability is an important aspect in the practical applications of such active coatings. To study the wettability of the samples, the surface water contact angles of the films were measured. The contact angle values are shown on
Figure 2. Normally, contact angle < 90° is hydrophilic, while that > 90° is hydrophobic. The results confirm that all the films are hydrophilic. The contact angles were found to be 7.5°, 13.1°, and 33.5° for MC, MCC and NC samples correspondingly. Thus, all obtained coatings are hydrophilic. When CS added in MCC coating the hydrophilicity slightly decreased in comparison to the hydrophilicity of MC coating without CS. Moreover, NC coating sample exhibited the lowest hydrophilicity or the highest hydrophobicity.
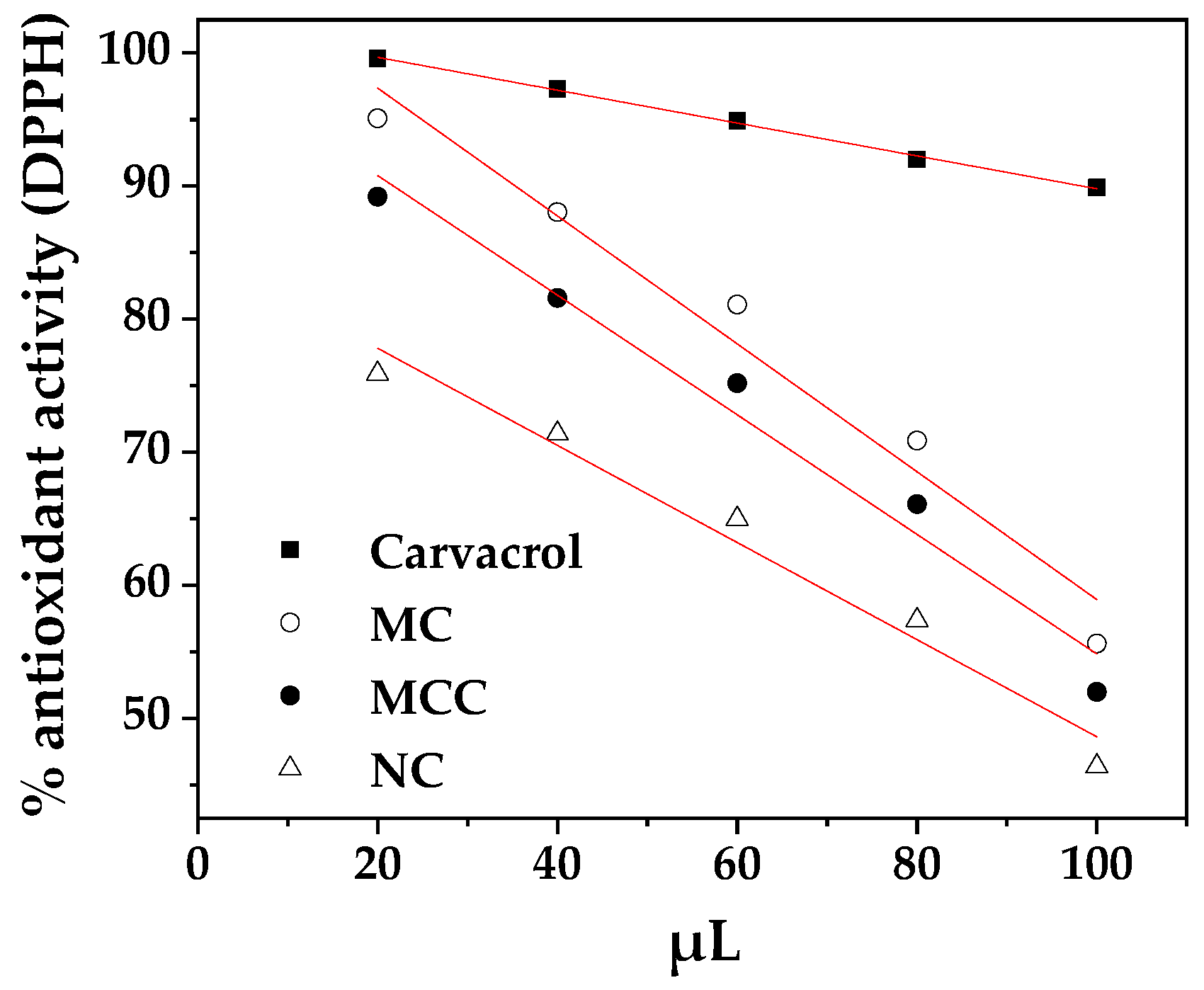
3.2. Antioxidant activity
The variations of obtained antioxidant activity values as a function of MC, MCC and NC quantity used were plotted in the
Figure 2 and the data were fitted to linear equations (see
Table 2).
These equations allowed us to derive the effective concentrations (EC50) required to achieve 50% of the maximum efficacy for each treatment.
With a first glance obtained EC50 values for MC, MCC and NC samples are much lower than the obtained EC50 value of FC. This fact validates both microemulsion and nanoemulsion technologies as technologies which minimize the quantity of active agent used and maximize the obtained bioactivity. Thus, for FC obtained a substantially high EC50 value of 422.27 mg/mL while for MC, MCC and NC samples the obtained EC50 values were 118.60 mg/mL, 110.64 mg/mL and 97.56 mg/mL respectively. The lowest EC50 values is obtained for NC sample implying the superiority of nanoemulsion technology against microemulsion ones. In advance, the obtained lower EC50 value of MCC sample as compared to the obtained EC50 value of MC sample suggest the enhancement of antioxidant activity of microemulsion by the presence of CS chains in the external microemulsion’s aquatic phase. The lower EC50 values for NC and MCC suggest enhanced efficacy compared to FC, echoing findings from previous studies on other types of treatments [
27,
28]. The mechanism underlying the efficacy of these treatments is not completely understood but is believed to involve complex interactions at the molecular level that enhance their effectiveness. These EC50 values serve as critical indicators of the efficiency and potential applications of these treatments in the food industry, particularly in meat preservation. The results of antioxidant effectiveness of nanoemulsions are in line with previous studies [
29,
30], further supporting the potential utility of these formulations.
3.3. Antibacterial activity test of carvacrol based microemulsion and nanoemulsions.
3.3.1. MIC
In the
Table 3 the calculated Minimum Inhibitory Concentration (MIC) values of MC, MCC and NC samples as well as FC sample against two bacterial strains,
Staphylococcus aureus and
Listeria monocytogenes are listed for comparison. Representative images of the obtained diffusion zone of MC, MCC, NC and FC samples are shown in
Figure S6.
The MIC values represent the lowest concentration of carvacrol required to completely inhibit bacterial growth and are expressed in μg/mL. The listed in
Table 3 MIC values reveal that all tested carvacrol microemulsions and nanoemulsions effectively inhibited the growth of both Staphylococcus aureus and Listeria monocytogenes at notably low concentrations, with MIC values ranging from 62.5 to 500 μg/mL. Specifically, the NC sample exhibited the lowest MIC values, at 62.5 μg/mL for both bacterial strains. The inclusion of CS in the carvacrol microemulsion enhanced its antimicrobial activity, with MIC values at 125 μg/mL for both strains (see
Figure S6). These findings agree with earlier studies [
19,
31] and the reason is suggested to be that nanoemulsions are generally more efficacious than microemulsions for delivering antimicrobial agents to bacterial cells due to smaller droplet size and higher surface area [
32]. Overall, the data supports the notion that carvacrol nanoemulsions could serve as a promising antimicrobial agent in food preservation applications, given their potent inhibitory effects on both Staphylococcus aureus and Listeria monocytogenes.
3.3.2. Well Diffusion zone
In the
Table 4 the calculated inhibition zones of MC, MCC, NC samples as well as FC sample
Listeria monocytogenes and
Staphylococcus aureus are listed for comparison. Representative images with the results of MIC of MC, MCC, NC and FC samples are shown in
Figure S7.
As it is obtained from the listed in
Table 4 inhibition zone values, NC sample demonstrated the most potent antibacterial activity, with a minimum inhibition zone measuring 20 mm for Listeria and 21 mm for
Staphylococcus aureus. Following closely, MCC coating achieved inhibition zones of 10.1 mm and 11.2 mm for
Listeria monocytogenes and
Staphylococcus aureus, respectively. The standard MC coating had slightly lower efficacy, with zones measuring 8.3 mm and 9.3 mm for the two bacteria. Notably, the control groups showed no inhibition zones, affirming the antibacterial potential of the Carvacrol microemulsions and nanoemulsion (see
Figure S6). These findings indicate that Carvacrol, particularly in nanoemulsion form, could be a potent candidate for antibacterial applications due to its increased surface area, improved solubility and stability, enhanced penetration into microbial cells, and the ability for targeted delivery of the antimicrobial agent [
19].
3.4. Physicochemical properties of pork minced meat
3.4.1. pH Analysis
In the
Table 5 the obtained pH values for all treatments used and for the 9-day examined period are listed for comparison.
The initial pH levels of the pork samples varied between 5.29 to 5.75, which aligns with previously published data by other researchers [
33]. At the onset of the experiment (Day 0), the pH levels in the treatment categories—FC, MC, MCC, and NC were generally lower compared to the Uncoated control group. The MCC low values can likely be attributed to the acidic nature of the solvents used in some coating processes [
33].
Over the storage period, there was a consistent upward trend in pH across all treatment and control groups. By the 9th day, pH values spanned from 6.05 to 6.74. Of all the treatments, MCC and MC were particularly effective in modulating pH changes over time, as evidenced by their elevated pH levels of 6.55 and 6.74 respectively by the ninth day.
This rise in pH over time is commonly linked to the growth of spoilage microbes that break down proteins and generate alkaline compounds. The slower pace of this increase in the treated coatings suggests that the coatings played a role in mitigating bacterial activity. This could be due to the barrier formed by the coatings, which reduces exposure to air and thus limits microbial proliferation [
25,
33,
34].
3.4.2. Lipid Oxidation
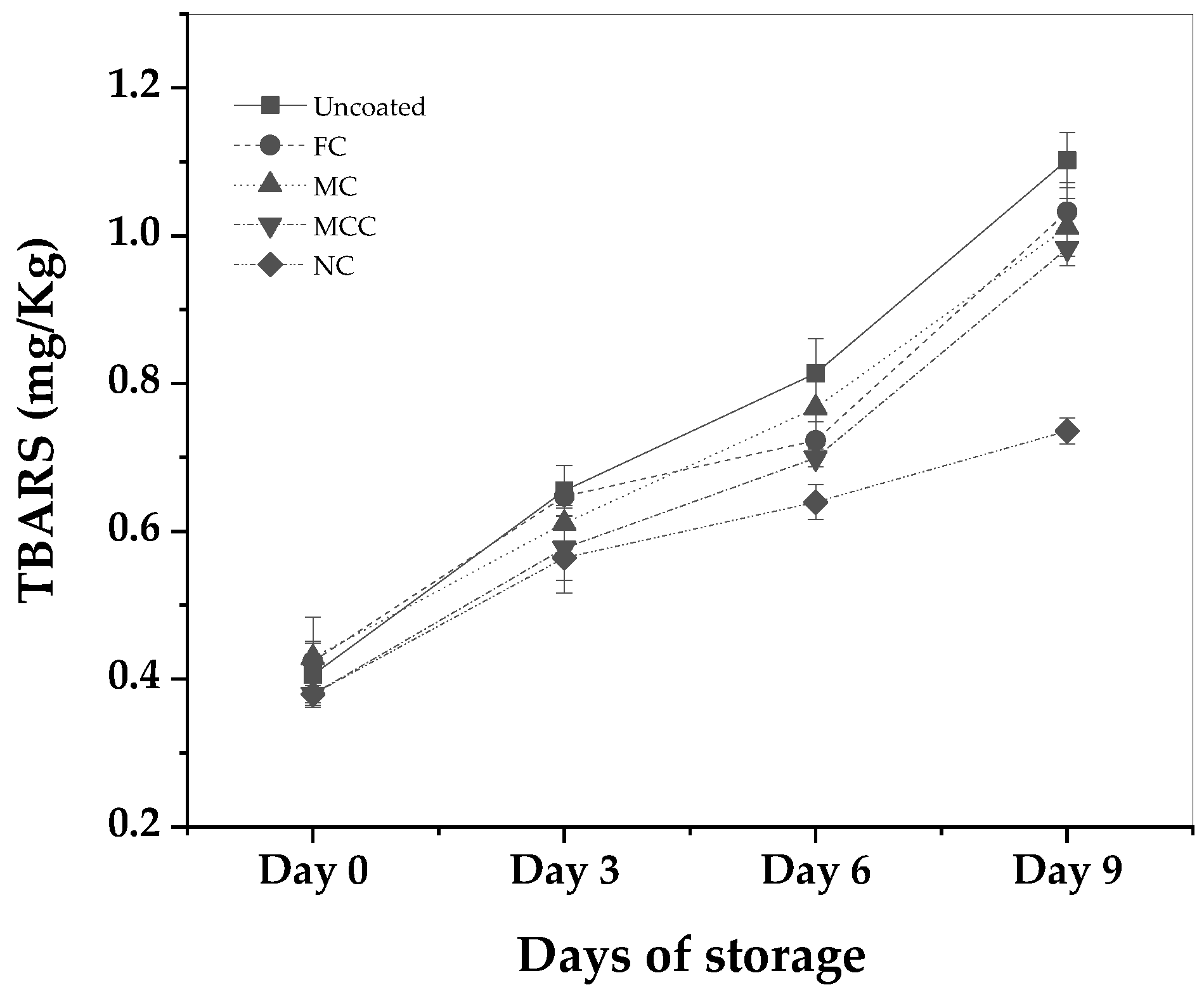
In
Figure 3 there are ploted the calculated TBARS mean values of uncoated pork mink sample as well as of pork minced meat samples coated with FC, MC, MCC, and NC as a function of days of storage.
The obtained TBARS values for day 0 for the pork minced meat were in the range of 0.38 to 0.43 mg MDA/kg, dependent on the coating type. Such values are in line with expectations for freshly processed pork minced meat [
35].
For the uncoated pork minced meat sample, the obtained TBARS values were recorded at 0.655, 0.814, and 1.102 mg MDA/Kg meat on Days 3, 6, and 9 respectively. In comparison, the obtained TBARS values for coated samples with the Free carvacrol (FC), were observed to be 0.647, 0.723, and 1.032 mg MDA/Kg meat over the same intervals.
Regarding the meat samples coated with MC, the obtained TBARS values were 0.611, 0.767, and 1.011 mg MDA/Kg meat on Days 3, 6, and 9 respectively. The MCC coated samples exhibited TBARS values of 0.577, 0.699, and 0.983 mg MDA/Kg meat on Days 3, 6, and 9. The most effective coating in terms of limiting lipid oxidation was NC, where the TBARS values were 0.564, 0.640, and 0.736 mg MDA/Kg meat on Days 3, 6, and 9 respectively. MDA concentrations in meat samples don’t have any regulatory thresholds. However, concentrations exceeding 0.5 mg/kg suggest some degree of oxidation, while levels above 1.0 mg/kg are considered potentially unsatisfactory in various research studies [
36,
37]
It can be noted that the pork minced meat coated with NC displayed the slowest rate of lipid oxidation over the observation period, which suggests the potential antioxidant properties of the nanoemulsion. Specifically, the percentage difference between NC and Uncoated at Day 6 & 9 accounts for 21.4% and 33.3%, respectively. Meanwhile, the difference between NC and Free Carvacrol (control sample) was 11.5% and 28.7%. In comparison, the “Uncoated” and “Free carvacrol” coatings exhibited a more rapid rate of lipid oxidation. Since day 6, the results of NC are statistically significant with uncoated and MCC (See S2 Table). The results are in line with several studies which conclude that encapsulation of essential oil inhibits the lipid oxidation [
33,
37]
3.4.3. L*a*b Analysis
In the
Table 6 the L*a*b values of uncoated pork mink sample as well as of pork minced meat samples coated with FC, MC, MCC, and NC in day 0, day 3, day 6 and day 9 of storage are listed for comparison.
Importantly, the observed discoloration in the pork is attributed to the accumulation of hydrogen peroxide produced by lactic acid bacteria during storage [
25]. Among the samples, uncoated ones were the most susceptible to color degradation, experiencing a 34.6% decrease in the L* values, which underscores their inherent vulnerability to color changes over time (see
Table S3). In stark contrast, samples coated with NC manifested the least decline in L* values, showing a mere 2.4% drop. The initial robustness of these samples can be partly attributed to the casein component in the coating, as they boasted the highest L* value on Day 0. On the other hand, Free Carvacrol-coated samples had a dramatic immediate effect on the coloration, evident from the initial L* value, but saw a 19.8% decline by Day 9. This suggests a less stable color profile in comparison to NC, despite its significant initial color-changing impact. Interestingly, MC coatings exhibited minimal initial impact on color, with an L* value closest to that of the uncoated samples on Day 0. However, by Day 3, the carvacrol in the coating led to a nearly 10-unit increase in L* values, totaling a 9.8% overall increase by Day 9. Conversely, MCC coatings showed only a moderate 3.6% change in L* values but registered the steepest decline among the coated samples. In regards of a* values (see S4 Table), Free Carvacrol was the most volatile, with a startling 114.5% increase, while MCC proved to be the most stable, altering by only 17.9%. For b* values (see S5 Table), uncoated coatings displayed the most change, at a 38% increase, and MCC the least, at a 0.8% change (see
Figure S8). In summary, NC coating emerged as the most promising candidate for preserving color stability, highlighting the importance of effective coatings in mitigating hydrogen peroxide-induced discoloration in food preservation endeavors.
3.5. Results of application of carvacrol based microemulsions and nanoemulsions as active coatings for pork minced meat preservation
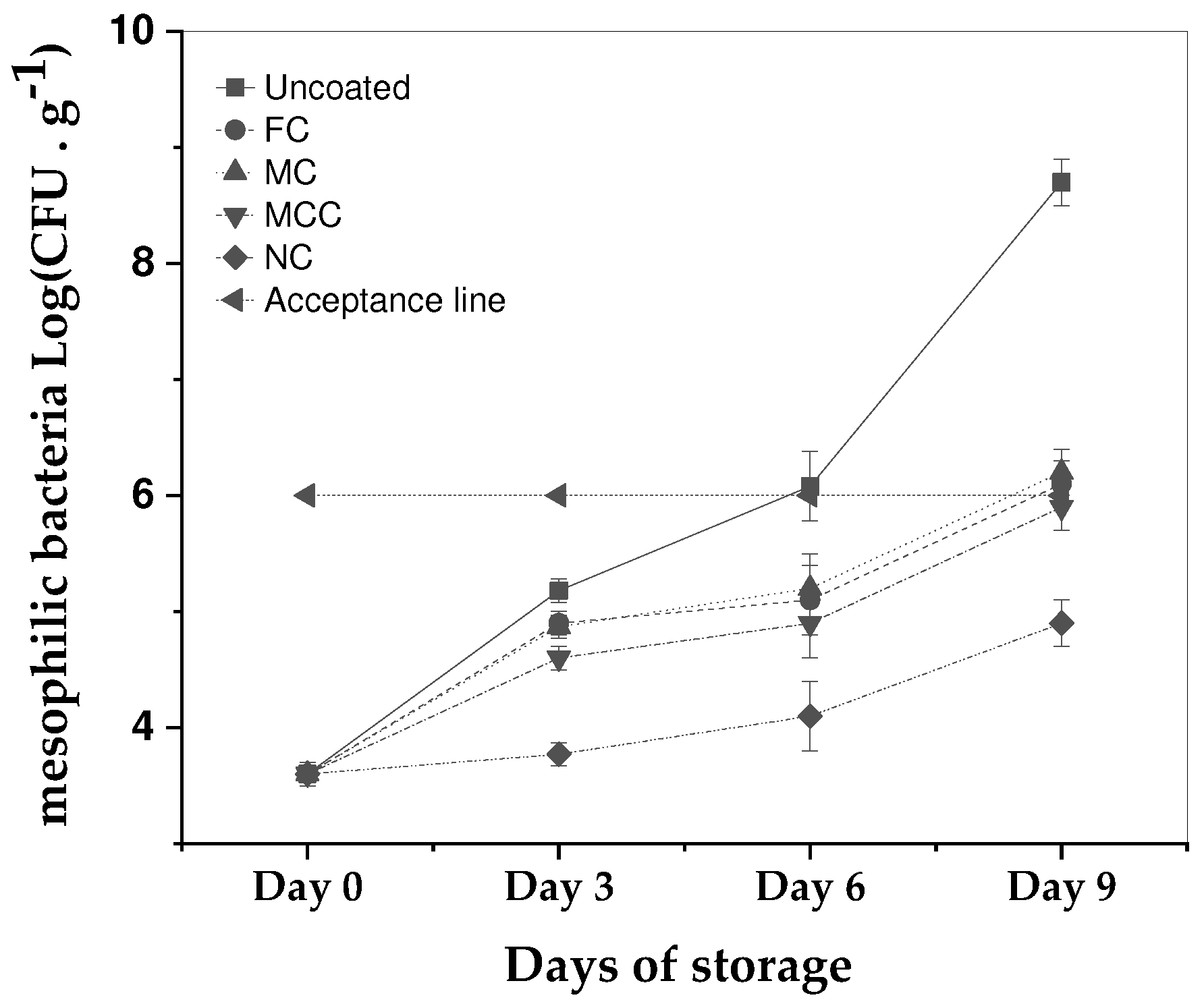
The effectiveness of examined formulations of carvacrol in inhibiting the growth of mesophilic bacteria in minced meat was assessed and presented in
Figure 4 over a storage period of nine days at 4 °C.
The results show that the carvacrol microemulsions and carvacrol nanoemulsion inhibited the rapid growth of the mesophilic bacteria by 3 days. In particular, the sample with no coating experienced a substantial increase in mesophilic bacteria levels, while all carvacrol-based treatments-maintained growth within acceptable limits throughout the nine-day period. Both FC and MC samples crossed the acceptance line of 6 log CFU by Day 9. In contrast, the MCC approached the threshold but remained beneath it. Most strikingly, the NC coating curtailed the microbial proliferation more effectively than Free carvacrol, registering reductions by 1 log and 1.2 logs on Days 6 and 9, respectively. These results for the carvacrol nanoemulsion, are considered as statistically significant at the 5% level on Days 6 and 9.
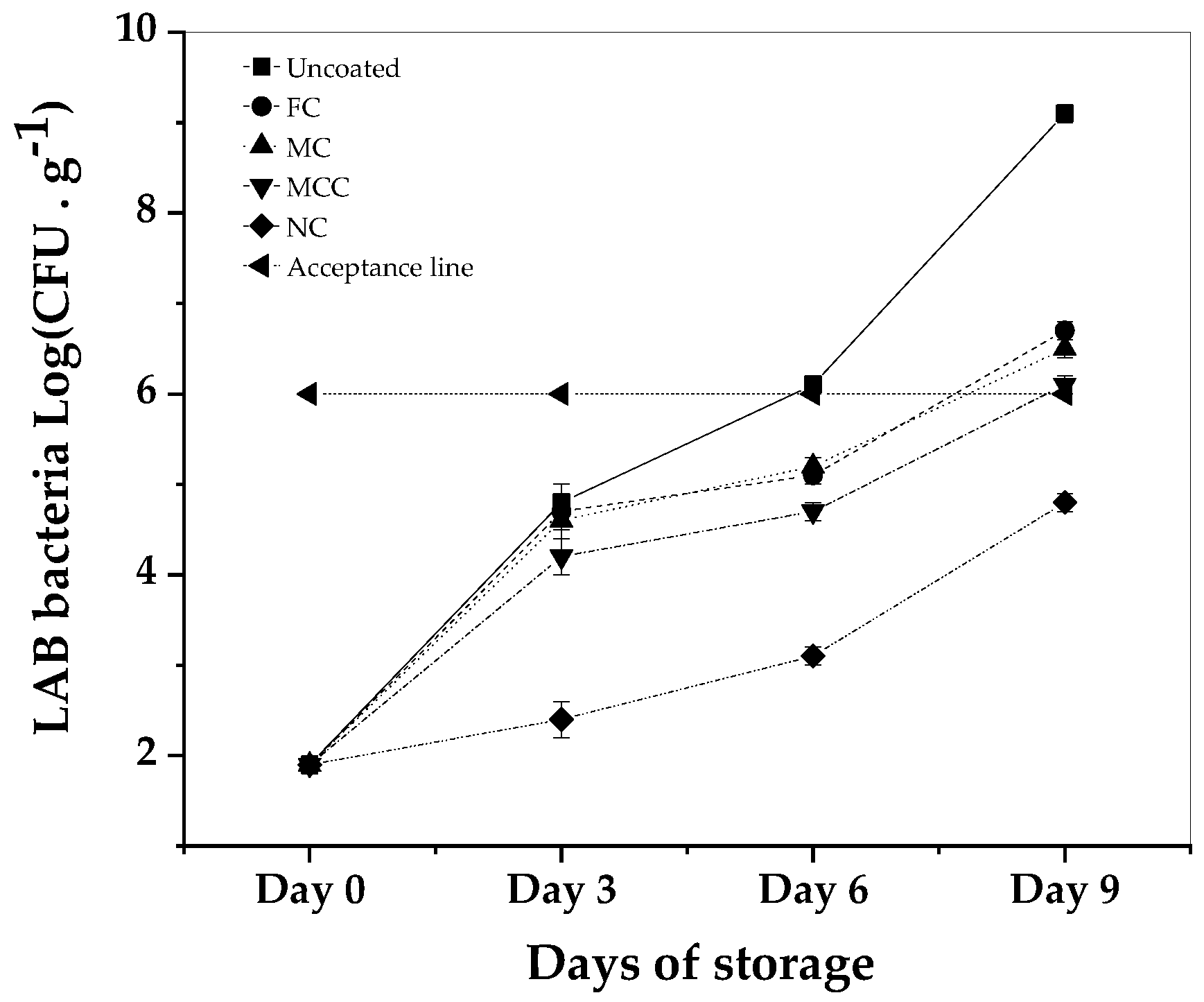
Examining the effectiveness of carvacrol formulated coatings on the growth of LAB bacteria in minced meat over a 9-day storage period at 4°C, the results illustrate varied inhibitory impacts.
All samples began with an identical LAB bacterial count of 1.9 log CFU. Without any coating, the LAB bacterial levels escalated, culminating at 9.1 log CFU by Day 9. In comparison, the sample containing free carvacrol, which served as the control, recorded a rise to 6.7 log CFU over the same duration. The MC exhibited a similar growth trend, settling at 6.5 log CFU on Day 9. Meanwhile, the MCC demonstrated more restraint in bacterial growth, ending at 6.1 log CFU. Most notably, the NC show cased a significant bacterial inhibitory effect, with the LAB bacterial count only reaching 4.8 log CFU by the ninth day, reflecting a log reduction compared to the control. When compared against the set acceptable quality level of 6 log CFU, only the nanoemulsion-coated sample consistently stayed below this threshold. Moreover, the inhibitory effects observed in the NC coated samples are considered statistically significant (P<0.05), especially when compared with the free carvacrol formulations.
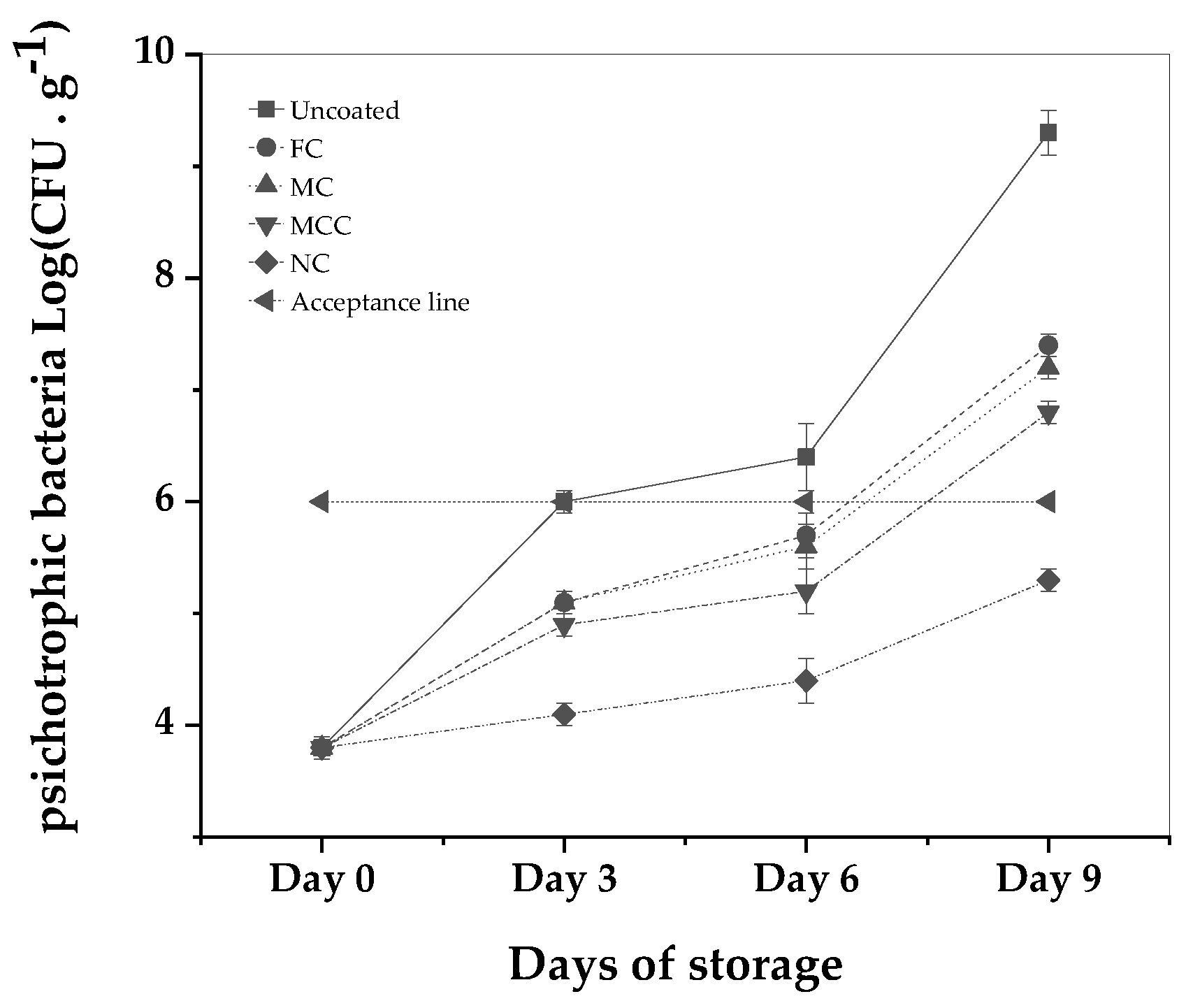
Finally examining the efficacy of carvacrol-formulated samples on psychrotrophic bacteria in minced meat over 9 days at 4°C, distinct patterns emerged.
Figure 6.
Psychrotrophic Bacteria Growth (Log CFU) during 9 Days of storage at 4 °C for incoated minced meat sample and minced meat samples coated with FC, MC, MCC, and NC. See also tatistical analysis results in
Table S9.
Figure 6.
Psychrotrophic Bacteria Growth (Log CFU) during 9 Days of storage at 4 °C for incoated minced meat sample and minced meat samples coated with FC, MC, MCC, and NC. See also tatistical analysis results in
Table S9.
Beginning at 3.8 log CFU, the uncoated sample experienced a significant increase of the psychrotrophic bacteria levels to 9.3 log CFU by Day 9. The free carvacrol, escalated to 7.4 log CFU in the same integral. The MC exhibited a similar trajectory, reaching 7.2 log CFU by Day 9. The presence of CS in the MCC sample resulted in a more moderated increase, peaking at 6.8 log CFU by the end of the observation period.
Most notably, the NC demonstrated a suppressive effect, with bacterial counts modestly rising from the initial 3.8 log CFU to only 5.3 log CFU by Day 9. This translates to a significant 2-log reduction compared to the free carvacrol by Day 9. Furthermore, compared to the set acceptable quality limit of 6 log CFU, the NC remained consistently beneath this standard throughout the storage period, underpinning the superior antibacterial potency of the nanoemulsified carvacrol. This marked reduction by the nanoemulsion was also found to be statistically significant at the P<0.05 level.
Overall, the results are in accordance with other studies which use CS and carvacrol or similar essential oils to preserve meat products and found significant log reduction using nanoemulsions [
38,
39,
40].
3.6. Sensory Analysis
The sensory evaluations of minced pork meat treated with various carvacrol coatings are detailed in
Table 7.
At the beginning of the storage period (Day 0), all the samples across the different coating groups registered high scores in terms of appearance, color, odor, texture, and overall acceptability. As the storage period extended, there was a noticeable trend where the scores for all sensory parameters and overall acceptability witnessed a decline. This decline in sensory scores could likely be attributed to factors such as alterations in meat consistency or the emergence of undesirable flavors or odors over time.
From Day 3, the MCC and NC-coated samples consistently exhibited the highest scores across all sensory attributes, beginning on Day 3 and continuing until the culmination of the observed storage period. Upon assessing longevity of acceptability based on appearance, color, texture, and overall impression, it is evident that the uncoated and FC-coated minced pork meat maintained their acceptability until Day 9. In contrast, the MC, MCC, and NC-coated samples sustained their sensory acceptability through Day 9, with MCC-coated samples displaying marginally superior resilience. With respect to odor scores, the uncoated and FC-coated samples remained within acceptable bounds for 6 days, while the MC, MCC, and NC-coated samples retained their acceptability for a duration of up to 9 days (see
Figure S8).
The data collated suggests that the introduction of specific carvacrol coatings, particularly MCC, can substantially elevate the sensory attributes of minced pork meat throughout the storage phase, potentially extending its desirability and shelf life by a few days. Such findings are in accordance with contemporary research in the meat processing sector [
41]. This study concentrates on the influence of different carvacrol coatings on the sensory attributes of minced pork meat during its storage. A progressive approach would entail an expansive exploration into the sensory properties of carvacrol-coated pork when exposed to various environmental conditions or culinary methodologies.
4. Discussion
As it was shown in the results section both microemulsion and nanoemulsion carvacrol coatings exhibited high performance in preservation of fresh minced pork meat. Overall, NC coating exhibited the highest antioxidant activity according to obtained EC50 values, the highest antibacterial activity against
L. monocytogenes and S. aureus according to obtained MIC values and well diffusion zone results, the lowest pH values increment during the minced pork meat preservation process, the lowest lipid oxidation during the minced pork meat preservation process according to obtained TBRAS values, the highest pork minced pork meat color stability during the preservation process and the lowest mesophilic, LAB and psychrotrophic bacteria growth during the minced pork meat preservation process. The microbiological analysis of fresh minced pork meat stored at 4
oC shown that the uncoated pork meat reaches the upper microbiological limit (6 log cfu/g) for acceptable quality of foods’ according to the ICMSF [
42]. Both MC and MCC coating samples succeed to preserve the stored minced pork meat since the ninth day of storage. NC coating samples succeed to achieve much lower values of mesophilic, LAB and psychrotrophic bacteria growth as compared to the upper microbiological limit of 6 log cfu/g and promises a preservation period of fresh minced pork meat over the nine days of storage. Looking back in the physicochemical characterization of such novel MC, MCC and NC coating it is obtained that the calculated average particle size hydrodynamic diameter for MC coating is approximately 10 nm, for MCC coating is approximately~ 93 nm, and for NC coating is approximately ~ 21 nm. Thus, the obtained particle size of carvacrol nanodroplets inside the MC, MCC and NC nanocoating it is not offering a suitable explanation about the superiority of NC coating in preservation of fresh minced pork meat against both MC and MCC coating samples. A possible explanation could be in the biocapability of NC coating with pork meat against MC and MCC coatings. Casein and lecithin which are used as surfactant and co-surfactant to obtain the NC coating nanostructure are biobased. Casein is known to be a ideal biobased encapsulation agent for such essential oils and their derivatives because its amphiphilic nature [
43,
44,
45,
46]. In accordance with casein’s amphiphilic nature contact angle results of NC coating exhibited the lowest hydrophilicity as compared to MC and MCC coating samples. This lowest hydrophilicity or higher hydrophobicity due to casein could be a logical explanation about the highest capability of such NC nanocoating with minced pork meat which supports the higher diffusion and control release of carvacrol nanodroplets in minced pork meat.
5. Conclusions
The preparation, characterization, and application of carvacrol coatings were elaborated upon in the present study. It was discerned that the nanoemulsion variant of carvacrol, or NC, manifested pronounced in vitro antimicrobial activity against Gram+ bacteria L. monocytogenes and S. aureus. The antimicrobial prowess of the NC was ascertained to be contingent upon its carvacrol essential oil content and the intrinsic characteristics of the microbial strains under examination. In the context of fresh minced pork meat, NC efficaciously curtailed the proliferation of Mesophilic bacteria, LAB, and psychrotrophic bacteria during refrigerated storage. When juxtaposed with FC, the NC also ameliorated the pH, oxidative stability, Lab, and color attributes of the minced pork meat over the 9-day storage period at 4±1 oC. Thus, drawing from the results, it can be posited that NC has the potential to significantly delay spoilage and augment the shelf-life of minced pork meat during chilled storage. Envisioned as an avant-garde natural antimicrobial agent, NC holds promise for the extended preservation of fresh meats. For ensuing studies, a keen focus on the release kinetics of carvacrol essential oil from the nanoemulsion and the overarching stability of NC would be paramount.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Preparation of carvacrol nanoemulsion (NC); Figure S2: Image with Carvacrol microemulsion (MC)(left), carvacrol microemulsion with chitosan (MC)(middle) and carvacrol nanoemulsion (NC)(right) prepared in this study; Figure S3: Minced meat coating process; Figure S4: Representative image with L*a*b measurement in minced pork meat samples; Figure S5: Steam distillation apparatus employed for the calculation of TBARs in meat samples; Figure S6: Representative images of obtained diffusion zone: A) FC, B) MC, C) MCC and D) NC samples; Figure S7: Representative image with the result of MIC in Carvacrol Treatments against Listeria monocytogenes; Figure S8: Minced meat samples after 9 days of storage at 4 oC; Table S1: Tuckey HSD in Inhibition Zones; Table S2: pH statistical analysis; Table S3: Tuckey HSD in Lipid Oxidation Analysis in pork minced meat for 9 days; Table S4: Tuckey HSD L* value during 9 days of meat storage; Table S5: Tuckey HSD a* value during 9 days of meat storage; Table S6: Tuckey HSD b* value during 9 days of meat storage; Table S7: Tuckey HSD in mesophilic analysis of stored fresh pork minced meat for 9 days; Table S8: LAB bacteria microbiological analysis in pork minced meat; Table S9: Psychrotrophic bacteria microbiological analysis in pork minced meat.
Author Contributions
Synthesis experiment design—A.E.G., C.P., and C.E.S.; paper writing—A.E.G., K.Z., A.A., E.R., G.K., and C.E.S.; overall evaluation of this work—A.E.G., C.P., and C.E.S.; experimental data analysis and interpretation—A.E.G., K.Z., A.A., G.K., C.E.S., and C.P.; DLS and contact angle experiments D.M., A. K-M., A.A., N.Z., C.E.S., antioxidant activity experiment K.Z., A.L., MIC and well diffusion zone tests K.Z., E.K., C.P., pH and color measuraments, sensory analysis, K.Z., A.L., A.E.G., Lipid oxidation of stored fresh pork minced meat K.Z., A.L., A.E.G., Microbiological analyses of stored fresh pork minced meat, K.Z., E.K., C.P., A.E.G., All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- El-Saber Batiha, G.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of Natural Antimicrobials in Food Preservation: Recent Views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics (Basel) 2019, 8, 208. [Google Scholar] [CrossRef]
- Jayari, A.; Jouini, A.; Boukhris, H.; Hamrouni, S.; Damergi, C.; Ben Hadj Ahmed, S.; Maaroufi, A. Essential Oils from Thymus Capitatus and Thymus Algeriensis as Antimicrobial Agents to Control Pathogenic and Spoilage Bacteria in Ground Meat. Journal of Food Quality 2021, 2021, e5599374. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and Human Health: A Comprehensive Review. Phytother Res 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Elgayyar, M.; Draughon, F.A.; Golden, D.A.; Mount, J.R. Antimicrobial Activity of Essential Oils from Plants against Selected Pathogenic and Saprophytic Microorganisms. J Food Prot 2001, 64, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems – A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The Mechanisms of Action of Carvacrol and Its Synergism with Nisin against Listeria Monocytogenes on Sliced Bologna Sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Lee, S.F.; Cheng, Z.; Davidson, R.; Rupasinghe, H.P.V. Carvacrol Exhibits Rapid Bactericidal Activity against Streptococcus Pyogenes through Cell Membrane Damage. Sci Rep 2021, 11, 1487. [Google Scholar] [CrossRef]
- Cicalău, G.I.P.; Babes, P.A.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.M.; Ganea, M.; Scrobotă, I. Anti-Inflammatory and Antioxidant Properties of Carvacrol and Magnolol, in Periodontal Disease and Diabetes Mellitus. Molecules 2021, 26, 6899. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Tsironi, T. n.; Taoukis, P. s. Shelf-Life Extension of Gilthead Seabream Fillets by Osmotic Treatment and Antimicrobial Agents. Journal of Applied Microbiology 2012, 112, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, J.; Zhang, Y.; Xv, Q.; Zeng, Q.; Wang, J. Preparation and Characterization of Carvacrol-Loaded Caseinate/Zein-Composite Nanoparticles Using the Anti-Solvent Precipitation Method. Nanomaterials 2022, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of Chitosan-Essential Oil Coatings on Safety and Quality of Fresh Blueberries. Journal of Food Science 2014, 79, M955–M960. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cameron, R.G.; Plotto, A.; Zhong, T.; Ference, C.M.; Bai, J. The Effect of Controlled-Release Carvacrol on Safety and Quality of Blueberries Stored in Perforated Packaging. Foods 2021, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The Antimicrobial Activity of Microencapsulated Thymol and Carvacrol. International Journal of Food Microbiology 2011, 146, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Ghaly, A.E. Meat Spoilage Mechanisms and Preservation Techniques: A Critical Review. American Journal of Agricultural and Biological Sciences 2011, 6, 486–510. [Google Scholar] [CrossRef]
- He, J.; Huang, S.; Sun, X.; Han, L.; Chang, C.; Zhang, W.; Zhong, Q. Carvacrol Loaded Solid Lipid Nanoparticles of Propylene Glycol Monopalmitate and Glyceryl Monostearate: Preparation, Characterization, and Synergistic Antimicrobial Activity. Nanomaterials 2019, 9, 1162. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Mukhamadiev, A.; Feng, J.; Gao, Y.; Zhuansun, X.; Han, R.; Chong, Y.; Jafari, S.M. Formulation Optimization and Characterization of Carvacrol-Loaded Nanoemulsions: In Vitro Antibacterial Activity/Mechanism and Safety Evaluation. Industrial Crops and Products 2022, 181, 114816. [Google Scholar] [CrossRef]
- Janisch, S.; Krischek, C.; Wicke, M. Color Values and Other Meat Quality Characteristics of Breast Muscles Collected from 3 Broiler Genetic Lines Slaughtered at 2 Ages. Poult Sci 2011, 90, 1774–1781. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, R.; Fan, L.; Ma, Y.; Wu, D.; Li, K.; Bai, Y. Microbial Inactivation and Quality of Grapes Treated by Plasma-Activated Water Combined with Mild Heat. LWT 2020, 126, 109336. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan Jr., L. A Distillation Method for the Quantitative Determination of Malonaldehyde in Rancid Foods. Journal of the American Oil Chemists’ Society 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Magiran | Effect of chitosan coating contain Ajwain essential oil on the shelf-life of chicken breast meat during refrigerated condition. Food and Health 3, 20–24.
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Bitter Orange Oil Incorporated into Chitosan Nanoparticles: Preparation, Characterization and Their Potential Application on Antioxidant and Antimicrobial Characteristics of White Button Mushroom. Food Hydrocolloids 2020, 100, 105387. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L. Fabrication and Characterization of Chitosan Nanoemulsions Loading Thymol or Thyme Essential Oil for the Preservation of Refrigerated Pork. Int J Biol Macromol 2020, 162, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, A.; Ashraf, M.; Omer, M.O.; Altaf, I. Carvacrol-Fabricated Chitosan Nanoparticle Synergistic Potential with Topoisomerase Inhibitors on Breast and Cervical Cancer Cells. ACS Omega 2023, 8, 31826–31838. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.D.; do Rosário, D.K.A.; Neto, L.T.; Lelis, C.A.; Conte-Junior, C.A. Antioxidant, Antibacterial and Antibiofilm Activity of Nanoemulsion-Based Natural Compound Delivery Systems Compared with Non-Nanoemulsified Versions. Foods 2023, 12, 1901. [Google Scholar] [CrossRef] [PubMed]
- Preparation and Evaluation of Food-Grade Nanoemulsion of Tarragon (Artemisia Dracunculus L.) Essential Oil: Antioxidant and Antibacterial Properties - ProQuest. Available online: https://www.proquest.com/openview/6d2be0211dbe31dc1c1b63dc288617bb/1?cbl=326253&pq-origsite=gscholar&parentSessionId=pNHqX2y7h%2FQpR8L8Zm6evXNBqYEexLgcyt7CNa7sMpE%3D (accessed on 31 October 2023).
- Zheng, H.; Wang, J.; You, F.; Zhou, M.; Shi, S. Fabrication, Characterization, and Antimicrobial Activity of Carvacrol-Loaded Zein Nanoparticles Using the pH-Driven Method. Int J Mol Sci 2022, 23, 9227. [Google Scholar] [CrossRef]
- Sampaio, C.I.; Bourbon, A.I.; Gonçalves, C.; Pastrana, L.M.; Dias, A.M.; Cerqueira, M.A. Low Energy Nanoemulsions as Carriers of Thyme and Lemon Balm Essential Oils. LWT 2022, 154, 112748. [Google Scholar] [CrossRef]
- Motta Felício, I.; Limongi de Souza, R.; de Oliveira Melo, C.; Gervázio Lima, K. y.; Vasconcelos, U.; Olímpio de Moura, R.; Eleamen Oliveira, E. Development and Characterization of a Carvacrol Nanoemulsion and Evaluation of Its Antimicrobial Activity against Selected Food-Related Pathogens. Letters in Applied Microbiology 2021, 72, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hakemi-Vala, M.; Rafati, H.; Aliahmadi, A.; Ardalan, A. Chapter 13 - Nanoemulsions: A Novel Antimicrobial Delivery System. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier, 2017; pp. 245–266 ISBN 978-0-323-52727-9.
- Wang, L.; Liu, T.; Liu, L.; Liu, Y.; Wu, X. Impacts of Chitosan Nanoemulsions with Thymol or Thyme Essential Oil on Volatile Compounds and Microbial Diversity of Refrigerated Pork Meat. Meat Science 2022, 185, 108706. [Google Scholar] [CrossRef]
- Cao, Y.; Gu, W.; Zhang, J.; Chu, Y.; Ye, X.; Hu, Y.; Chen, J. Effects of Chitosan, Aqueous Extract of Ginger, Onion and Garlic on Quality and Shelf Life of Stewed-Pork during Refrigerated Storage. Food Chemistry 2013, 141, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Muzolf-Panek, M.; Rudzińska, M.; Szablewski, T.; Cegielska-Radziejewska, R. The Effect of Plant Extracts on Pork Quality during Storage. Italian Journal of Food Science 2017, 29, 644–656. [Google Scholar]
- Sheard, P.R.; Enser, M.; Wood, J.D.; Nute, G.R.; Gill, B.P.; Richardson, R.I. Shelf Life and Quality of Pork and Pork Products with Raised N-3 PUFA. Meat Science 2000, 55, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of Oregano Essential Oil and Resveratrol Nanoemulsion Loaded Pectin Edible Coating on the Preservation of Pork Loin in Modified Atmosphere Packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Kang, H.; Peng, X. Antimicrobial and Antioxidant Effects of Edible Nanoemulsion Coating Based on Chitosan and Schizonepeta Tenuifolia Essential Oil in Fresh Pork. Journal of Food Processing and Preservation 2021, 45, e15909. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Xiao, Z.; Bi, W. Effect of Chitosan Nanoparticles Loaded with Cinnamon Essential Oil on the Quality of Chilled Pork. LWT - Food Science and Technology 2015, 63, 519–526. [Google Scholar] [CrossRef]
- Karam, L.; Chehab, R.; Osaili, T.M.; Savvaidis, I.N. Antimicrobial Effect of Thymol and Carvacrol Added to a Vinegar-Based Marinade for Controlling Spoilage of Marinated Beef (Shawarma) Stored in Air or Vacuum Packaging. Int J Food Microbiol 2020, 332, 108769. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, D.; Xiang, Q.; Li, K.; Wang, B.; Bai, Y. Effect of Cinnamon Essential Oil Nanoemulsions on Microbiological Safety and Quality Properties of Chicken Breast Fillets during Refrigerated Storage. LWT 2021, 152, 112376. [Google Scholar] [CrossRef]
- Stewart, G.S.A.B. Micro-Organisms in Food—2. Sampling for Microbiological Analysis: Principles and Specific Applications: ICMSF, Blackwell Scientific Publications, Oxford, 1986. 310 Pp. Price: £19·50 (Cloth). Meat Science 1987, 19, 315. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z. Improved Encapsulation Capacity of Casein Micelles with Modified Structure. Journal of Food Engineering 2022, 333, 111138. [Google Scholar] [CrossRef]
- Soni, M.; Yadav, A.; Maurya, A.; Das, S.; Dubey, N.K.; Dwivedy, A.K. Advances in Designing Essential Oil Nanoformulations: An Integrative Approach to Mathematical Modeling with Potential Application in Food Preservation. Foods 2023, 12, 4017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tang, X.; Wang, Q.; Mao, X.Y. Antibacterial Effect and Hydrophobicity of Yak κ-Casein Hydrolysate and Its Fractions. International Dairy Journal 2013, 31, 111–116. [Google Scholar] [CrossRef]
- Lajnaf, R.; Gharsallah, H.; Attia, H.; Ayadi, M.A. Comparative Study on Antioxidant, Antimicrobial, Emulsifying and Physico-Chemical Properties of Purified Bovine and Camel β-Casein. LWT 2021, 140, 110842. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).