1. Introduction
At present, the development of ways to synthesize multifunctional crystalline nanoparticles (NPs) with specified parameters (composition, morphology, structure) is a topical task. To date, the main methods for obtaining nanocrystals is the production of solid NPs via liquid-solid or solid-liquid-solid phase transitions in various dispersion media. For «green» methods of obtaining NPs with specified parameters the various reactor installations (batch or continuous) that operate under relatively mild conditions (temperature up to several hundred degrees, pressure 1–100 atm) are used. Such methods are called chemical; they allow one to obtain reproducible results and high yields of reaction products with minimal energy consumption, which makes them very attractive for many applications.
For medical applications, it is necessary to obtain colloidal solutions of NPs with the following special properties: NP smaller than 50 nm [
1], narrow size distribution, low degree of toxicity, high photo- and physico-chemical inertness, low degree of crystalline defects, colloidal stability. The ability to form stable colloids is necessary to prevent NPs aggregation and facilitate their further modification. Materials combining such properties can be used to introduce NPs into a living organism to obtain information about biological tissues (bioimaging) or to deliver drugs or other therapeutic effects to disease centers. Thus, dielectric crystalline NPs and stable colloidal solutions based on them have found applications in theranostics including bioimaging and drug delivery carriers.
One of the promising areas in medicine is the study of the effect of ultraviolet radiation in the UV-C spectral range (200–280 nm) on the deactivation of localized microorganisms and biological tissues resistant to antimicrobial drugs or radiation. UV-C photon is not capable of penetrating into biological tissues; therefore, its delivery by some NPs to a closed area of the disease in a living organism is an urgent task. In particular, UV-C emitting NPs are considered as possible radiosensitizers to enhance cancer radiation therapy due to the strong destructive action of UV-C photons on DNA molecules [
2,
3].
As is known, some rare earth ions (REI), namely Ce
3+, Pr
3+, Nd
3+, doped into suitable crystalline matrices, are capable of emitting intense radiation in the UV-C spectral range under the high-energy excitation. The high-intense UV-C radiation can be observed due to the presence of high-energy radiative inter-configurational electronic transitions from the lowest-energy state of excited 4f
n-15d
1 electronic configuration to the ground and some excited states of the 4f
n electronic configuration in the above REI. However, it is necessary to choose the best crystal matrix for effective luminescence due to 4f
n-15d
1 → 4f
n transitions. An important condition for the existence of bright 4f
n-15d
1 → 4f
n UV luminescence of REI under high-energy excitation is an efficient energy transfer from the matrix to the REI optical centers. In particular, phosphate matrices ensure efficient energy transfer to REI [
4,
5,
6,
7]. Lutetium phosphate NPs doped with Pr
3+ ions have been already proposed as efficient UV-C nanoscintillators for biomedical applications [
3,
8].
Some works [
7,
9,
10,
11,
12] show that upon high-energy excitation (X-ray, synchrotron radiation, electron beam) of bulk LaPO
4 crystals with the monazite structure doped with Pr
3+ ions (m-LaPO
4, monoclinic system, space group P2
1/n), it is possible to obtain effective UV-C luminescence (220 – 280 nm) due to the 4f
15d
1 →
3H
4,5,6,
3F
2,3,4 transitions in Pr
3+ ions. A necessary condition for the existence of 4f
15d
1 → 4f
2 radiative transition of Pr
3+ ions in the doped matrix is that the lowest-energy (emitting) 4f15d1 level is below the highest-energy 4f
2 level (
1S
0) [
11] Otherwise, luminescence properties of doping Pr3+ ions will be determined by intra-configurational 4f
2 → 4f
2 transitions from the
1S
0 level (two step photon emission process/photon cascade emission process due to
1S
0 →
1I
6 and
3P
0 →
3H
4 transitions), which occur mainly in the visible spectral range. Thus, one of the ways to deliver the UV-C radiation to biological tissues can be the creation of drugs based on aqueous colloidal solutions of m-LaPO
4: Pr
3+ dielectric crystalline NPs with specified morphological parameters.
According to some studies (see, e.g. [
13]), nanorods can be more effective drug carriers than nanospheres for anti-cancer therapy because nanorods can penetrate tumors more efficiently than nanospheres of the same effective size due to the enhanced transport through porous media of the tumor. Another factor can be an alignment of nanorods with blood flow, which increases the probability of the delivery to the tumor for nanorods. Accordingly, LaPO
4 NPs having a shape of nanorods are of a special interest in our study.
Let us give a small overview of LaPO4: RE3+ NPs synthesis methods. The main method for the synthesis of NPs is their production by various chemical methods using a liquid phase. The use of an intermediate liquid phase makes it possible to reduce the required temperatures for the successful formation of nanocrystals belonging to high-temperature phases without additional annealing of the product. Since the liquid phase separates individual nanocrystals during the synthesis process, the correct choice of synthesis parameters makes it possible to obtain well-faceted crystalline NPs with a low degree of aggregation and stable aqueous colloids based on them.
Recently, Wang et al. [
14] investigated a method for synthesizing HTMW to obtain highly crystalline and highly polarized m-LaPO4:Eu3+ nanorod emitters with an average length of about 100 nm or more. It was shown that LaPO4 NPs can be treated with nitric acid, which leads to the formation of a colloidal solution that remains stable for more than a year. It was also shown that m-LaPO4 NPs in an acidic environment (pH = 2) have a modulus twice as large as the zeta potential than in an alkaline environment (pH = 7 – 12), indicating that the sedimentation stability of NPs is much higher in acidic environment.
Based on the report of Espinoza & Jüstel on UV-emitting LaPO
4 nanocrystals [
15], the X-ray excited optical luminescence (XEOL) spectrum of about 4.5 ± 3 nm m-LaPO
4: Pr
3+ spherical colloidal NPs obtained by a solvothermal method shows a large shift towards UV-B and UV-A spectral ranges. This circumstance does not allow the effective use of such small m-LaPO
4: Pr
3+ NPs as UV-C delivery agents and indicates the need to obtain larger colloidal NPs.
Hilario et al. In [
16], the hydrothermal synthesis of m-LaPO4 doped with Pr3+ or Pr3+ doped with Gd3+ ions was studied. A series of syntheses carried out by the authors at different pH levels, as well as measurements of the zeta potential of the resulting NPs, showed a higher probability of aggregation of the resulting NPs with increasing pH levels.
On the contrary, H. Meyssamy et al. [
17] and H. Song et al. [
18] used the hydrothermal method in alkaline aqueous solutions of ammonium hydroxide (pH>11) to obtain faceted NPs of m-LaPO
4:Eu
3+, Ce
3+ with sizes 10 – 50 nm and low aspect ratio of 1:2. As shown in these works, the treatment of freshly prepared suspensions of LaPO
4 NPs with an HNO
3 solution allows chemical peptization of aggregated NPs with the formation of stable colloidal solutions in acidic conditions.
Riwotzki et al. [
19] used a solvothermal synthesis of m-LaPO
4: Ce
3+, Tb
3+ NPs with mean sizes of 5–6 nm. Similarly, N. Niu et al. [
20] synthesized m-LaPO
4: Ce
3+, Tb
3+ NPs of irregular shape and rounded morphology with sizes about 15 nm using a solvothermal method. As a result of solvothermal synthesis routes, highly luminescent in UV-A region NPs of m-LaPO
4: Ce
3+, Tb
3+ with a hydrophobic surface were obtained.
To control the processes of nucleation and growth of LaPO
4: Ce
3+, Tb
3+ nanowires, X. Zhu et al. [
21] used a combination of an organic dispersion medium, microwave heating and a microfluidic reactor. As a result, non-aggregated hexagonal LaPO
4: Ce
3+, Tb
3+ NPs luminescent in UV-A region with an average length of 60–70 nm and a diameter of about 12 nm were obtained.
The conclusions from this literature review are as follows.
It is possible to obtain a pure monoclinic LaPO4: REI phase of well-crystallized NPs in one step, by using autoclave synthesis methods or synthesis in high-boiling organic solvents.
Depending on the pH values, anion excess ratio, reaction mixture temperature, and the concentration of the initial reagents [
14,
17,
18,
22,
23], the hydrothermal synthesis allows to obtain m-LaPO
4 NPs with characteristic dimensions ranging from 5 to 600 nm with aspect ratio of 2–75. At the same time, the synthesized particles of the LaPO
4 via hydrothermal synthesis are most often obtained with big sizes and without a stabilizing charge on the surface in a neutral or basic aqueous media [
14,
16,
24]. Thus, to prevent aggregation of m-LaPO
4 NPs in the alkaline region, it is necessary to use a surfactant. To modify the surface of lanthanum containing NPs, anionic surfactants are widely used, in particular, compounds of tartaric and citric acids [
25,
26].
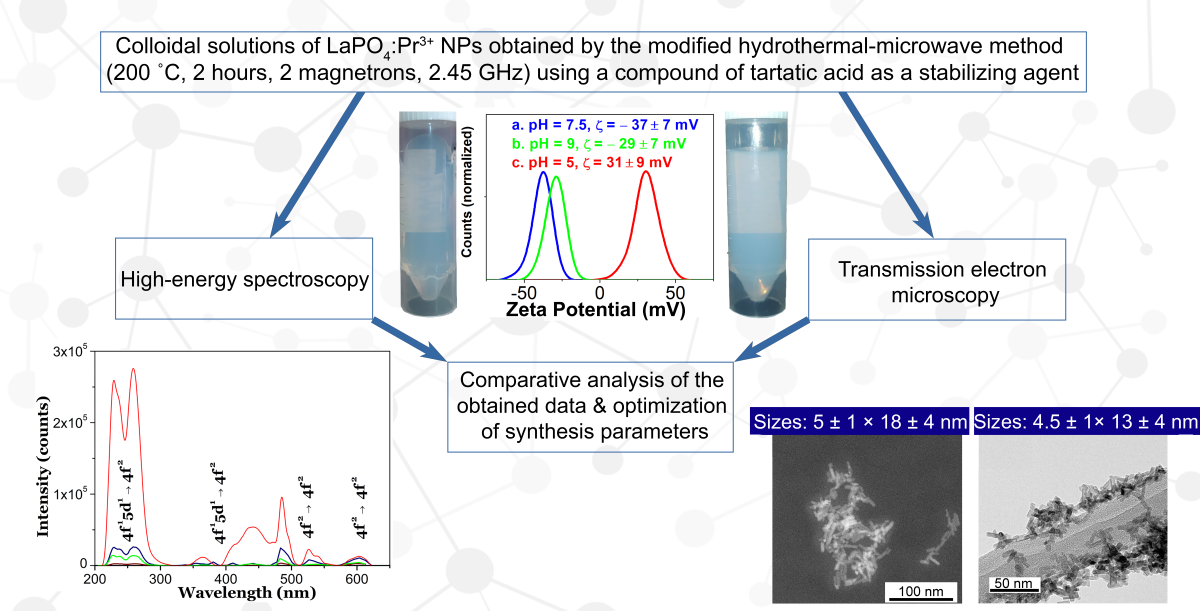
Since it was previously shown [
9,
12] that the monoclinic LaPO
4 phase doped with Pr
3+ ions is an effective UV-C scintillator, we concentrated our efforts on the development of a Hydrothermal Microwave (HTMW) method for the synthesis of m-LaPO
4: Pr
3+ colloidal NPs with specified physicochemical parameters: optimal NP sizes, high colloidal stability, the ability to emit intense luminescence, and the highest intensity in the spectral region of the UV-C radiation spectrum. A surfactant of alkaline solution of ammonium tartrate was used during syntheses in order to provide the colloidal properties to the surface of LaPO
4 NPs for the ability to form the stable aqueous solutions in the neutral or weakly alkaline aqueous media. To study the effect of synthesis parameters on the obtained NPs, several series of syntheses of LaPO
4: Pr
3+ (1 at.%) NPs were carried out by the HTMW method (200 °C, 2 hours, 2 magnetrons, 2.45 GHz).The influence of various parameters on the morphology and intensity of UV-C luminescence of NPs was studied: the use of solutions of tartaric acid (TA) and ammonium hydroxide (NH
4OH) with different amounts of added TA (C
TA = 0.027 or 0.08 M) at pH values of 8 or 9, various ratios of excess anions to cations (excess ratio of K
2HPO
4 = 1 – 10), two concentrations of LaPO
4: Pr
3+ in the reaction mixture (
= 0.005 or 0.01 M).
Aggregation processes of colloidal NPs synthesized by wet-chemistry methods are a well-known problem in their use for medical and other purposes [
27,
28,
29]. They can occur both during the synthesis of NPs and during the storage of their solutions. Monitoring these processes using an electron microscope is a complex and time-consuming procedure and requires drying the sample, which can distort the information obtained as a result of possible changes in the structure of clusters and their additional aggregation during the drying process. This article presents the results of using a simpler and faster method for monitoring aggregation processes, based on highly sensitive optical microscopy visualization of the Brownian motion of single NPs in solutions and subsequent analysis of their trajectories using the Nanoparticle Tracking Analysis (NTA).
4. Discussion
As shown earlier [
14,
16,
24], the LaPO
4: REI NPs have the isoelectric point near pH-neutral region, while stabilization of LaPO
4: REI NPs in aqueous colloids in pH-modified media is explained by the chemical adsorption of H
+ or OH
− ions on the surface nanocrystals. At the same time, the adsorption of H+ ions on the surface of LaPO
4: REI NPs occurs more efficiently than of OH
−. As a result of the disproportion in the adsorption of pH-determining ions, LaPO
4: Pr
3+ NPs without coating agents are more stable in acidic than in alkaline environment [
14,
24]. On the other hand, as has been shown in many works, the use of surfactants with carboxyl groups makes it possible to decrease the aggregation probability of NPs in an alkaline medium [
25,
26,
32].
The reaction of chemical surface peptization of non-modified LaPO
4 NPs as a result of the adsorption of pH-determining ions in an acidic or alkaline environment can be represented by the following expressions:
When TA-modified particles are dispersed in an acidic or alkaline medium, the hydroxyl end of the organic molecule can also undergo reversible chemical transformations:
Thus, for TA-modified NPs placed to an alkaline medium, the total expression of chemical adsorption and neutralization of the hydroxyl groups with OH- ions can be written as
After the adsorption of pH-determining ions, the charged surface of the NP is surrounded by a solvation aqueous shell, which leads to a decrease in the excess energy of the system. For this case, the surface of the LaPO
4 NPs is surrounded by the polar ends of water molecules, opposite in sign to the surface:
The results of these reactions are shown in
Scheme 1.
As can be seen (
Scheme 1), the solvate aqueous shell, as well as the adsorbed tartaric acid molecules, lead to a decrease in the aggregation probability. However, optical microscopy studies have shown, that despite the use of a tartaric acid component as capping agent, the product of modified HTMW synthesis was not individual nanocrystals, but clusters with hydrodynamic radii up to 100 nm and more. It was shown that the synthesized TA-modified NPs have the narrowest hydrodynamic radii distributions in a weakly alkaline aqueous medium, which is a consequence of the adsorption of the tartaric acid compound on the NPs surface. In addition, the resulting particles also have good stability in an acidic environment due to the high efficiency of adsorption of H
+ ions on the surface of LaPO
4: Pr
3+ NPs.
The dense aggregates of TA-modified LaPO
4: Pr
3+ nanocrystals are formed during the synthesis (coprecipitation, HTMW treatment and cooling of the reaction mixture) and not during storage of the colloidal solution. The source of the observed NPs clusters with narrow distributions in modified aqueous media is probably the washing stage (
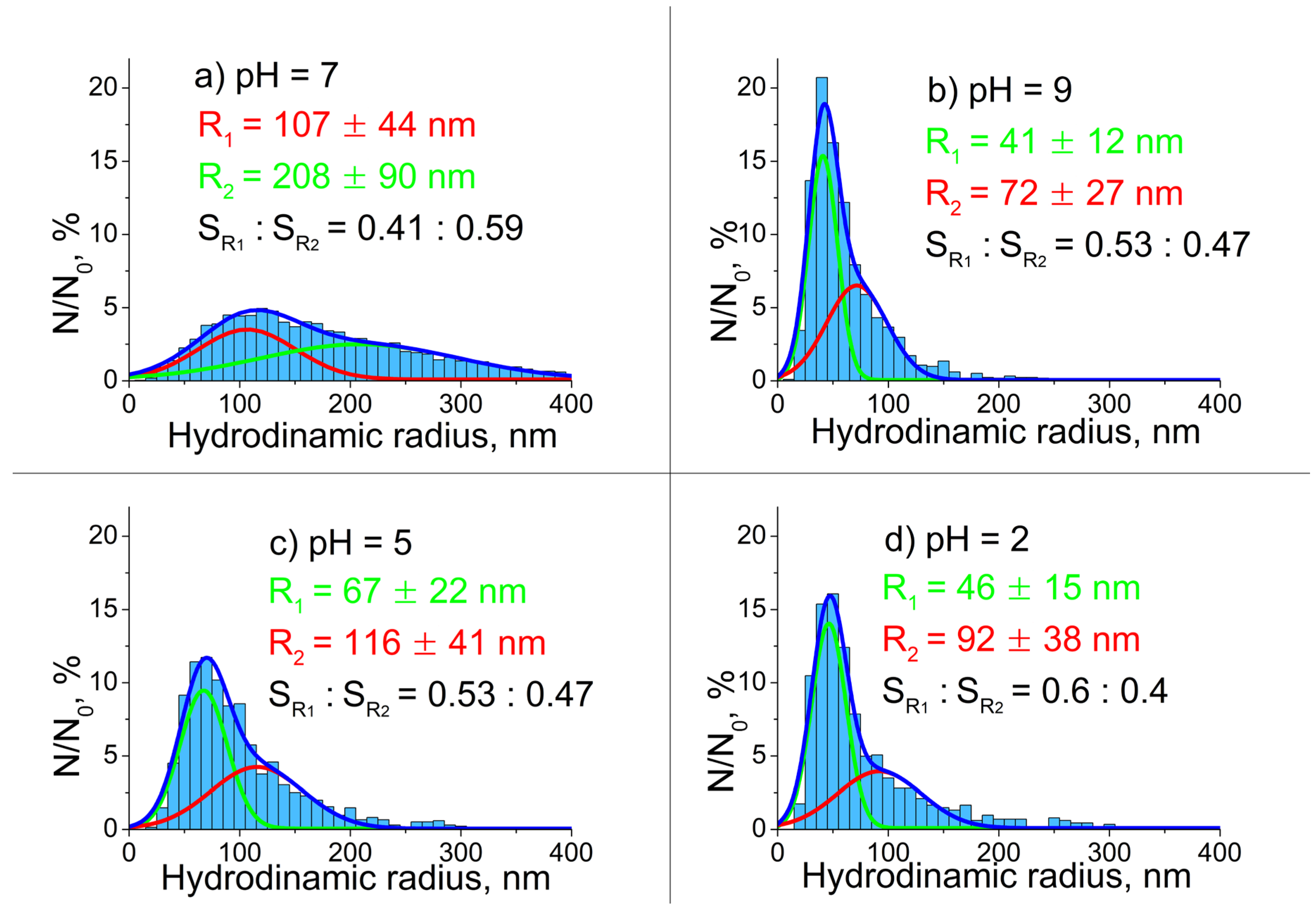
Section 2.1.2). As mentioned earlier, after HTMW synthesis, a rapidly settling precipitate of crystalline particles is formed, while the colloidal properties appear after several washing cycles. As a result of the washing process, loose aggregates break up into smaller aggregates, which leads to transparency and stabilization of the dispersion of NPs. The double distributions of hydrodynamic radii in different pH are likely due to the different properties of hard clusters: size, shape, and density; however, this assumption requires further research.
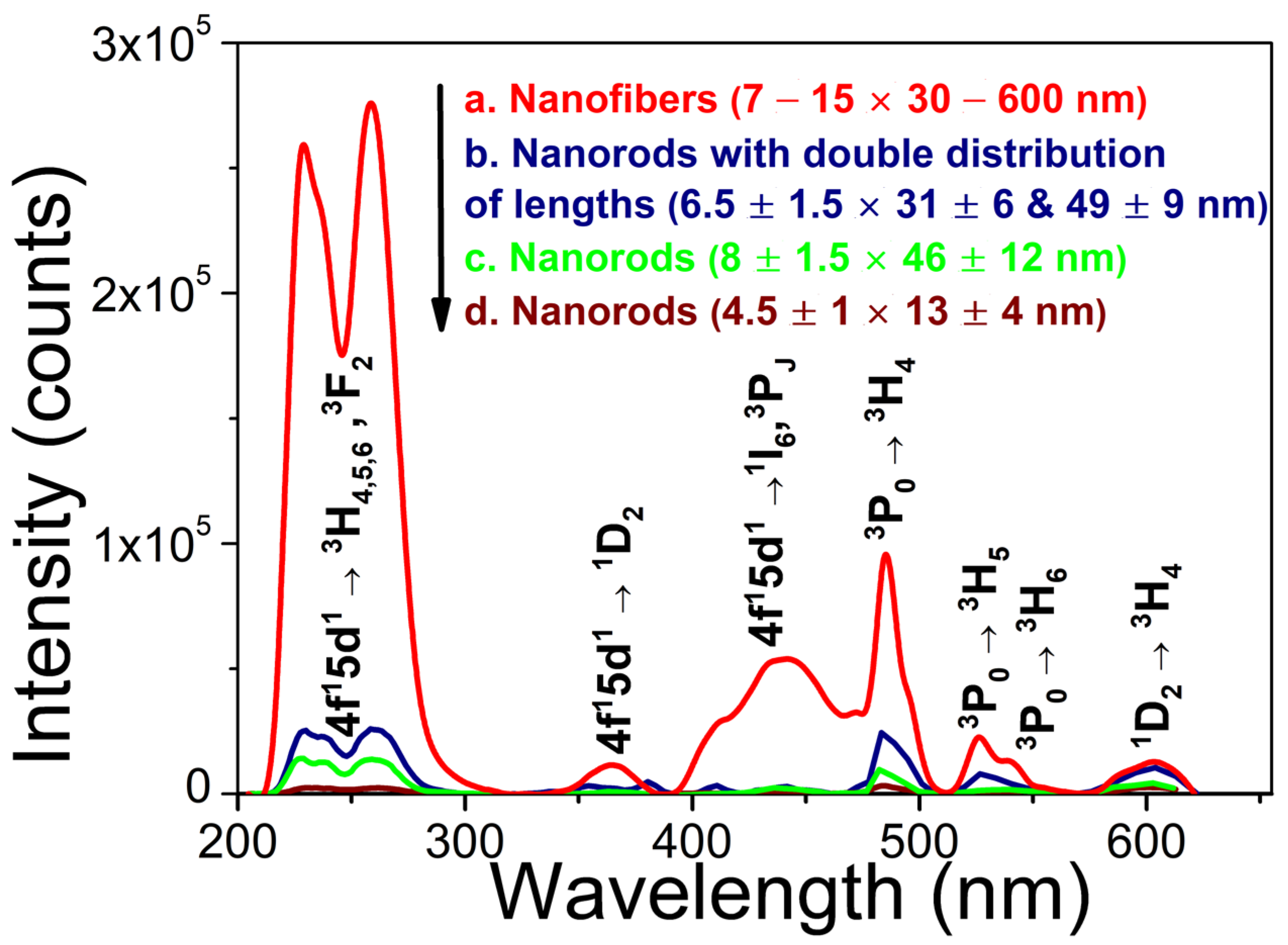
The comparison of obtained data on UV-C luminescence intensities for NPs prepared under various synthesis conditions and having different sizes and aspect ratios between width and length of individual nanocrystals are presented in
Table 1. It should be mentioned that we cannot make reliable quantitative analysis of the dependence of NP luminescence intensities on synthesis conditions (NP morphology) because the procedure of preparing NP powder samples for each measurement of XEOL or CL spectrum can differ from time to time. For example, the temperature and duration of the drying procedure can be slightly different, and the time between synthesis and the measurement also varied in the wide range (from several days to few months) which can induce some aging effects, e.g., NP aggregation. Thus, our qualitative analysis can indicate only some tendencies in changing the NP luminescence intensity depending on synthesis conditions.
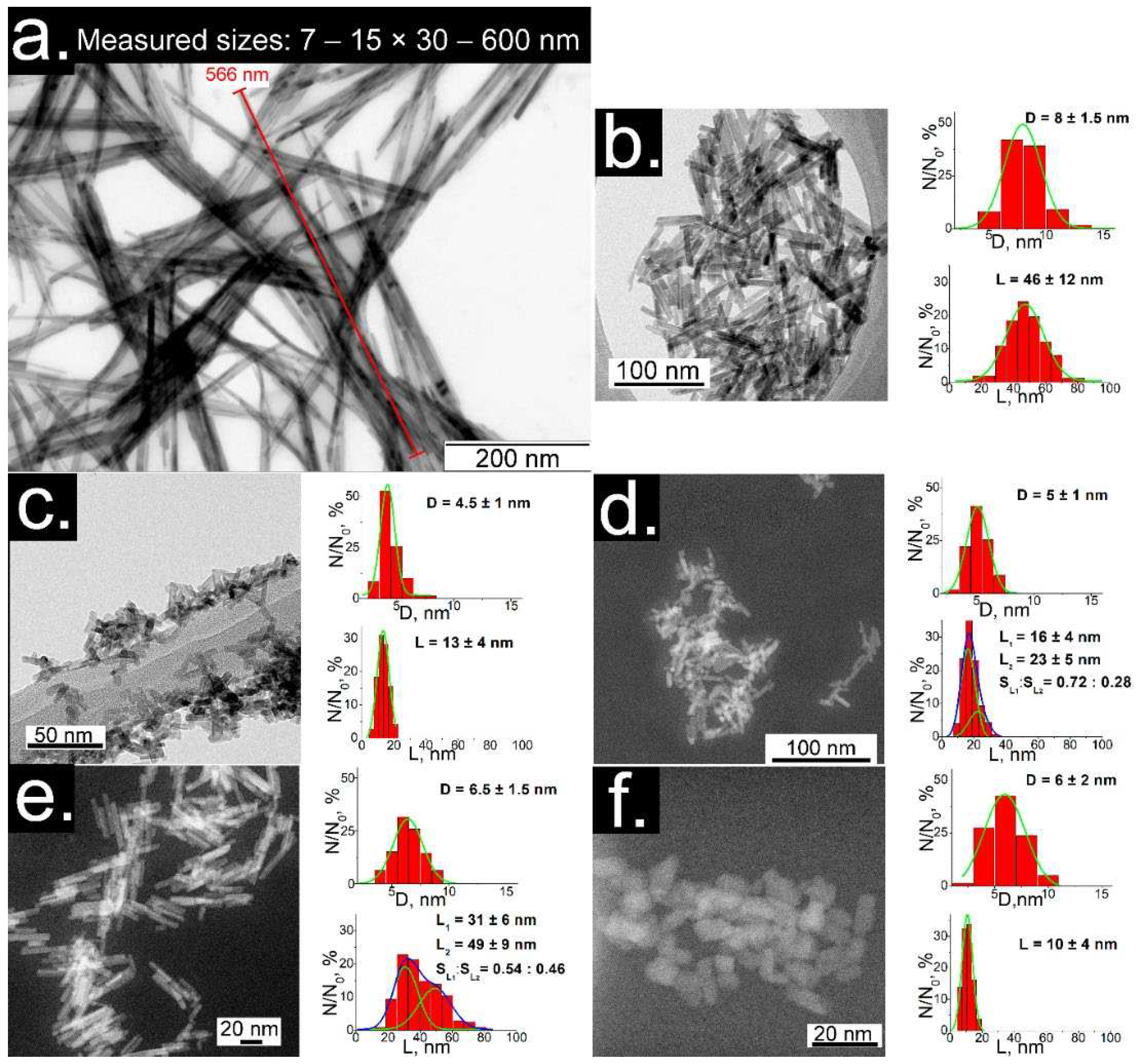
An obvious result, which can be seen in the
Table 1 is that in general the UV-C luminescence intensity is higher for larger size NPs. The highest intensity was obtained for NPs with the size of ~ 7–15 × 30–600 nm synthesized without surfactants (natural pH = 5), while the intensity is much weaker for NPs prepared at basic medium (pH = 8 – 9). As it is commonly accepted the luminescence intensity of NPs depends on the degree of luminescence quenching in the near-surface defect layer as well as on the concentration of defects or impurities in the volume of NPs. Thus, an acidic environment provides a better quality of LaPO
4 NPs due to a more accessible dissolution-growth mechanism during the HTMW treatment, while a basic aqueous medium can inhibit the dissolution of particles and lead to particles with smaller sizes and a larger amount of crystal structural defects (for example, quenching OH
− groups).
Most of performed synthesis experiments resulted in the production of fiber-like morphology of LaPO
4: Pr
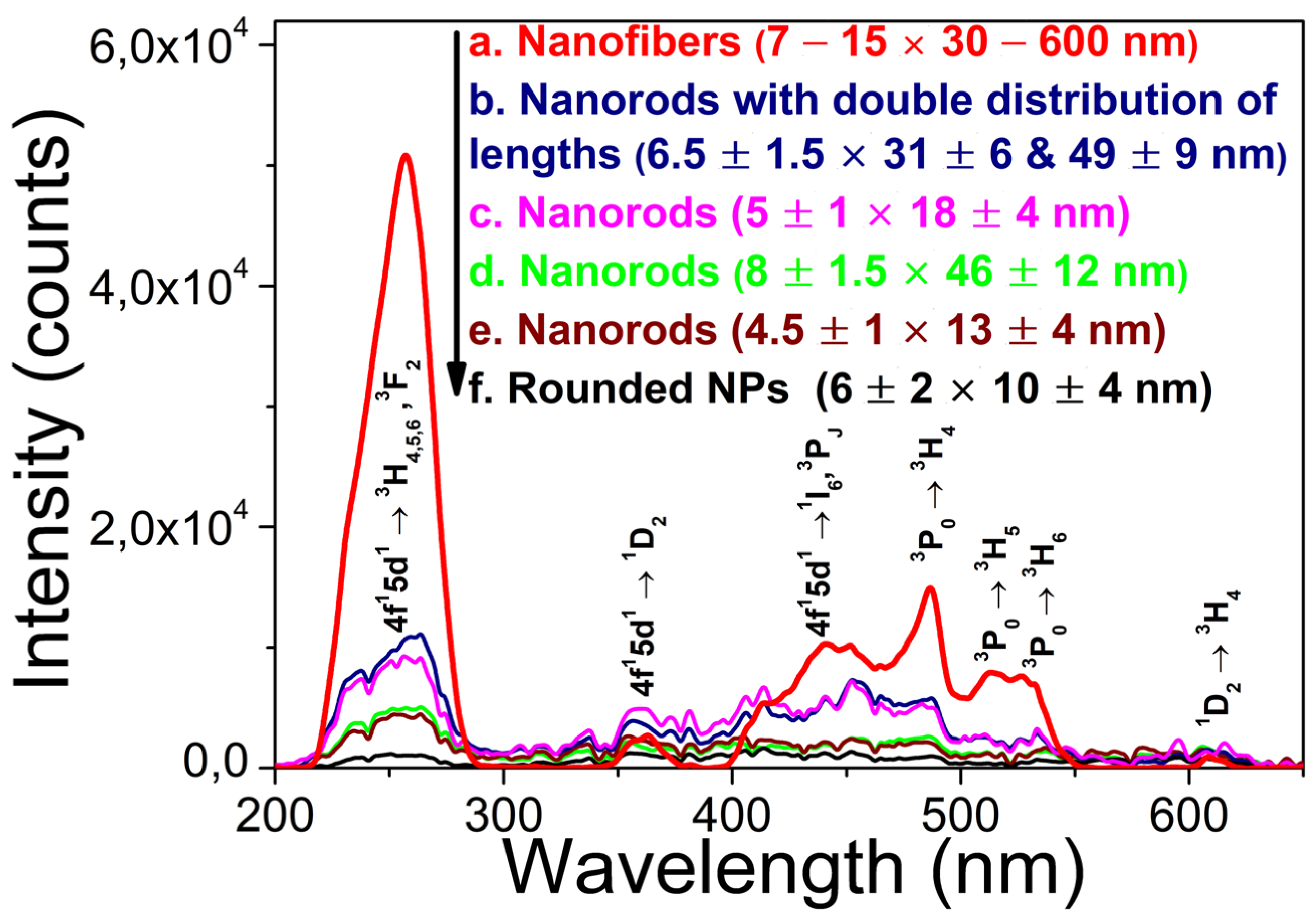
3+ NPs (nanorods, nanowires) having different sizes and aspect ratios between width and length. It is obvious that nanorod have a larger surface-to-volume ratio than rounded NPs with the same volume, i.e., it is expected that near-surface luminescence quenching is stronger for nanofibers than for nanospheres of the same particle volume. However, NPs with a size of 6 × 10 nm, i.e., having a shape close to round, demonstrate a very low intensity of XEOL UV-C luminescence (
Figure 6f), as compared to particles of the similar sizes 4.5 × 13 nm (
Figure 6e). Besides that, the results of CL (
Figure 5b,c) and XEOL (
Figure 6b,d) analysis for samples with comparable lengths but different diameters (
Figure 1b, e) indicate that powders of particles with smaller diameters show better UV-C luminescence intensities. The different intensities of UV-C radiation from samples with similar size distributions can be explained by the lower crystallinity of NPs obtained in a more alkaline medium or with a higher excess of anions.
As a common conclusion from this consideration, it can be assumed that there is no simple direct dependence of Pr3+ UV-C luminescence intensity only on the size of particles but there is some dependence of luminescence intensity on the synthesis conditions, the optimization of which allows to increase the intensity of Pr3+ UV-C luminescence.
Figure 1.
Figure 1.TEM images and size distributions (diameter × length) of m-LaPO
4: Pr
3+ (1 at.%) NPs prepared with HTMW method (200 °C, 2 hours) obtained with various synthesis conditions: a) nanofibers (7 – 15 × 30 – 600 nm),
= 0.01 M, K
2HPO
4 excess ratio = 1.25, no additional components (natural pH = 5); b) nanorods (8 ± 1.5 × 46 ± 12 nm),
= 0.01 M, K
2HPO
4 excess ratio = 1.25, C
TA = 0.08 M, pH=9; c) nanorods (4.5 ± 1 × 13 ± 4 nm),
= 0.01 M, K
2HPO
4 excess ratio = 5, C
TA = 0.08 M, pH=9; d) nanorods (5 ± 1 × 18 ± 4 nm),
= 0.005 M, K
2HPO
4 excess ratio = 5, C
TA = 0.027 M, pH = 8; e) nanorods with double distribution of lengths (6.5 ± 1.5 × 31 ± 6 & 49 ± 9 nm),
= 0.005 M, K
2HPO
4 excess ratio = 2, C
TA = 0.027 M, pH= 8; f) small faceted NPs (6 ± 2 × 10 ± 4 nm),
= 0.005 M, K
2HPO4 excess ratio = 10, C
TA = 0.027 M, pH=8.
Figure 1.
Figure 1.TEM images and size distributions (diameter × length) of m-LaPO
4: Pr
3+ (1 at.%) NPs prepared with HTMW method (200 °C, 2 hours) obtained with various synthesis conditions: a) nanofibers (7 – 15 × 30 – 600 nm),
= 0.01 M, K
2HPO
4 excess ratio = 1.25, no additional components (natural pH = 5); b) nanorods (8 ± 1.5 × 46 ± 12 nm),
= 0.01 M, K
2HPO
4 excess ratio = 1.25, C
TA = 0.08 M, pH=9; c) nanorods (4.5 ± 1 × 13 ± 4 nm),
= 0.01 M, K
2HPO
4 excess ratio = 5, C
TA = 0.08 M, pH=9; d) nanorods (5 ± 1 × 18 ± 4 nm),
= 0.005 M, K
2HPO
4 excess ratio = 5, C
TA = 0.027 M, pH = 8; e) nanorods with double distribution of lengths (6.5 ± 1.5 × 31 ± 6 & 49 ± 9 nm),
= 0.005 M, K
2HPO
4 excess ratio = 2, C
TA = 0.027 M, pH= 8; f) small faceted NPs (6 ± 2 × 10 ± 4 nm),
= 0.005 M, K
2HPO4 excess ratio = 10, C
TA = 0.027 M, pH=8.
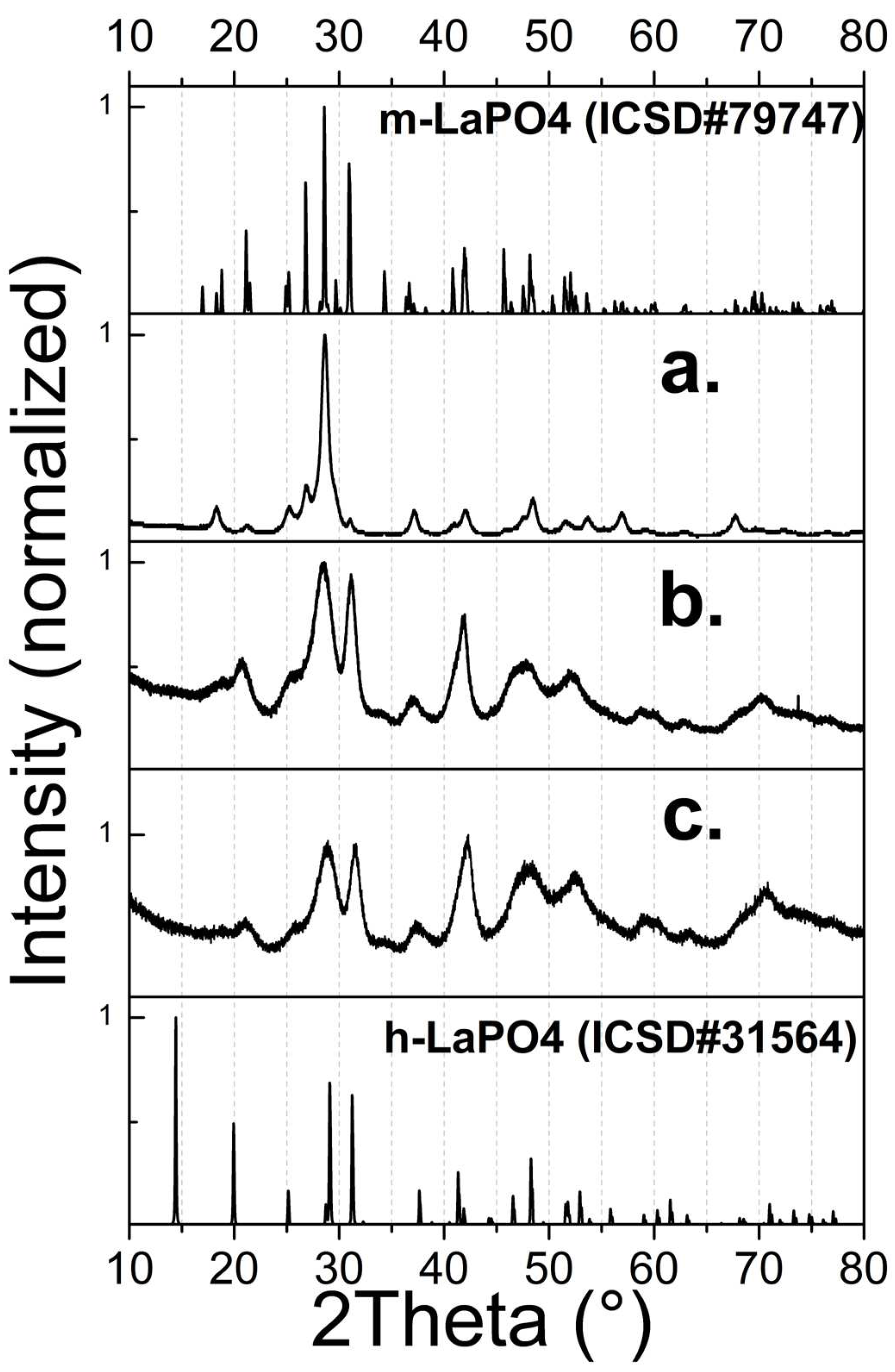
Figure 2.
XRD pattern of LaPO4: Pr3+ (1 at.%) powders prepared with HTMW (200 °C, 2 hours): a) nanofibers obtained with = 0.01 M, K2HPO4 excess ratio = 1.25, no additional components (natural pH = 5); b,c) NPs obtained with = 0.005 M, CTA = 0.027 M, pH = 8, but with K2HPO4 excess ratio equal to five (b) and ten (c).
Figure 2.
XRD pattern of LaPO4: Pr3+ (1 at.%) powders prepared with HTMW (200 °C, 2 hours): a) nanofibers obtained with = 0.01 M, K2HPO4 excess ratio = 1.25, no additional components (natural pH = 5); b,c) NPs obtained with = 0.005 M, CTA = 0.027 M, pH = 8, but with K2HPO4 excess ratio equal to five (b) and ten (c).
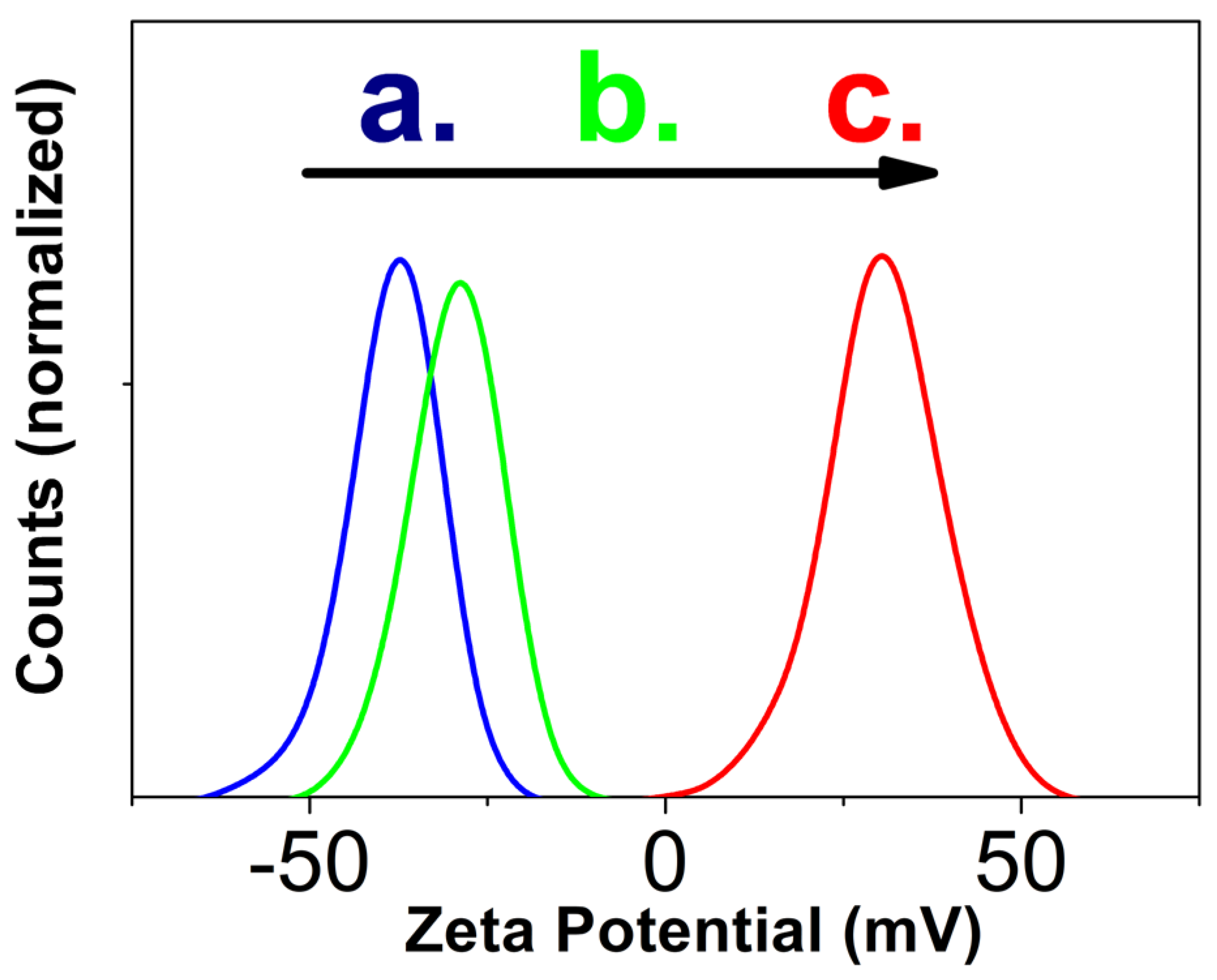
Figure 3.
Zeta potential measurements of the TA-modified LaPO4: Pr3+ (1 at.%) NPs with sizes 5 ± 1 × 18 ± 4 nm (Fig. 1c) obtained by HTMW method (200 , 2 hours) with = 0.005 M, K2HPO4 excess ratio = 5, pH = 8, CTA = 0.027 M, dispersed in different aqueous media by diluting 0.1 mL of colloid with concentration of 10 mg/mL with 0.9 mL of dispersants: a) blue line - DI water, resulting pH = 7.5; b) green line - weak NaOH solution, resulting pH = 9; c) red line - weak HNO3 solution, resulting pH = 5.
Figure 3.
Zeta potential measurements of the TA-modified LaPO4: Pr3+ (1 at.%) NPs with sizes 5 ± 1 × 18 ± 4 nm (Fig. 1c) obtained by HTMW method (200 , 2 hours) with = 0.005 M, K2HPO4 excess ratio = 5, pH = 8, CTA = 0.027 M, dispersed in different aqueous media by diluting 0.1 mL of colloid with concentration of 10 mg/mL with 0.9 mL of dispersants: a) blue line - DI water, resulting pH = 7.5; b) green line - weak NaOH solution, resulting pH = 9; c) red line - weak HNO3 solution, resulting pH = 5.
Figure 4.
The distributions of hydrodynamic radii of the synthesized colloidal LaPO4: Pr3+ (1 at.%) NPs with primary sizes of 5 ± 1 × 18 ± 4 nm (fig. 1c) and the clusters formed by them, measured in various aqueous solutions using the developed high-sensitivity laser ultramicroscope: a) - in a purified deionized water with pH=7; b) - in a weak NaOH solution with pH=9; c,d) in weak HNO3 solutions with pH=5 (c) and pH=2 (d). All distributions are well fitted by the sum of two different Gaussians (R2 > 0.98).
Figure 4.
The distributions of hydrodynamic radii of the synthesized colloidal LaPO4: Pr3+ (1 at.%) NPs with primary sizes of 5 ± 1 × 18 ± 4 nm (fig. 1c) and the clusters formed by them, measured in various aqueous solutions using the developed high-sensitivity laser ultramicroscope: a) - in a purified deionized water with pH=7; b) - in a weak NaOH solution with pH=9; c,d) in weak HNO3 solutions with pH=5 (c) and pH=2 (d). All distributions are well fitted by the sum of two different Gaussians (R2 > 0.98).
Figure 5.
CL spectra of m-LaPO4: Pr3+ (1 at.%) NPs prepared with HTMW method (200 °C, 2 hours) obtained with various synthesis conditions: a) nanofibers (7 – 15 × 30 – 600 nm), = 0.01 M, K2HPO4 excess ratio = 1.25, no additional components (natural pH = 5); b) nanorods with double distribution of lengths (6.5 ± 1.5 × 31 ± 6 & 49 ± 9 nm), = 0.005 M, K2HPO4 excess ratio = 5, CTA = 0.027 M, pH= 8; c) nanorods (8 ± 1.5 × 46 ± 12 nm), = 0.01 M, K2HPO4 excess ratio = 1.25, CTA = 0.027 M, pH= 9; d) nanorods (4.5 ± 1 × 13 ± 4 nm), = 0.01 M, K2HPO4 excess ratio = 5, CTA = 0.08 M, pH = 9.
Figure 5.
CL spectra of m-LaPO4: Pr3+ (1 at.%) NPs prepared with HTMW method (200 °C, 2 hours) obtained with various synthesis conditions: a) nanofibers (7 – 15 × 30 – 600 nm), = 0.01 M, K2HPO4 excess ratio = 1.25, no additional components (natural pH = 5); b) nanorods with double distribution of lengths (6.5 ± 1.5 × 31 ± 6 & 49 ± 9 nm), = 0.005 M, K2HPO4 excess ratio = 5, CTA = 0.027 M, pH= 8; c) nanorods (8 ± 1.5 × 46 ± 12 nm), = 0.01 M, K2HPO4 excess ratio = 1.25, CTA = 0.027 M, pH= 9; d) nanorods (4.5 ± 1 × 13 ± 4 nm), = 0.01 M, K2HPO4 excess ratio = 5, CTA = 0.08 M, pH = 9.
Figure 6.
XEOL spectra of m-LaPO4: Pr3+ (1 at.%) powders prepared with HTMW method (200 °C, 2 hours) obtained with various synthesis conditions: a) nanofibers (7 – 15 × 30 – 600 nm), = 0.01 M, K2HPO4 excess ratio = 1.25, no surfactant (natural pH = 5); b) nanorods with double distribution of lengths (6.5 ± 1.5 × 31 ± 6 & 49 ± 9 nm), = 0.005 M, K2HPO4 excess ratio = 2, CTA = 0.027 M, pH = 8; c) nanorods (5 ± 1 × 18 ± 4 nm), = 0.005 M, K2HPO4 excess ratio = 5, CTA = 0.027 M, pH = 8; d) nanorods (8 ± 1.5 × 46 ± 12 nm), = 0.01 M, K2HPO4 excess ratio = 1.25, CTA = 0.08 M, pH = 9; e) nanorods (4.5 ± 1 × 13 ± 4 nm), = 0.01 M, K2HPO4 excess ratio = 5, CTA = 0.08 M, pH = 9; f) Small faceted nanoparticles (6 ± 2 × 10 ± 4 nm), = 0.005 M, K2HPO4 excess ratio = 10, CTA = 0.027 M, pH = 8.
Figure 6.
XEOL spectra of m-LaPO4: Pr3+ (1 at.%) powders prepared with HTMW method (200 °C, 2 hours) obtained with various synthesis conditions: a) nanofibers (7 – 15 × 30 – 600 nm), = 0.01 M, K2HPO4 excess ratio = 1.25, no surfactant (natural pH = 5); b) nanorods with double distribution of lengths (6.5 ± 1.5 × 31 ± 6 & 49 ± 9 nm), = 0.005 M, K2HPO4 excess ratio = 2, CTA = 0.027 M, pH = 8; c) nanorods (5 ± 1 × 18 ± 4 nm), = 0.005 M, K2HPO4 excess ratio = 5, CTA = 0.027 M, pH = 8; d) nanorods (8 ± 1.5 × 46 ± 12 nm), = 0.01 M, K2HPO4 excess ratio = 1.25, CTA = 0.08 M, pH = 9; e) nanorods (4.5 ± 1 × 13 ± 4 nm), = 0.01 M, K2HPO4 excess ratio = 5, CTA = 0.08 M, pH = 9; f) Small faceted nanoparticles (6 ± 2 × 10 ± 4 nm), = 0.005 M, K2HPO4 excess ratio = 10, CTA = 0.027 M, pH = 8.
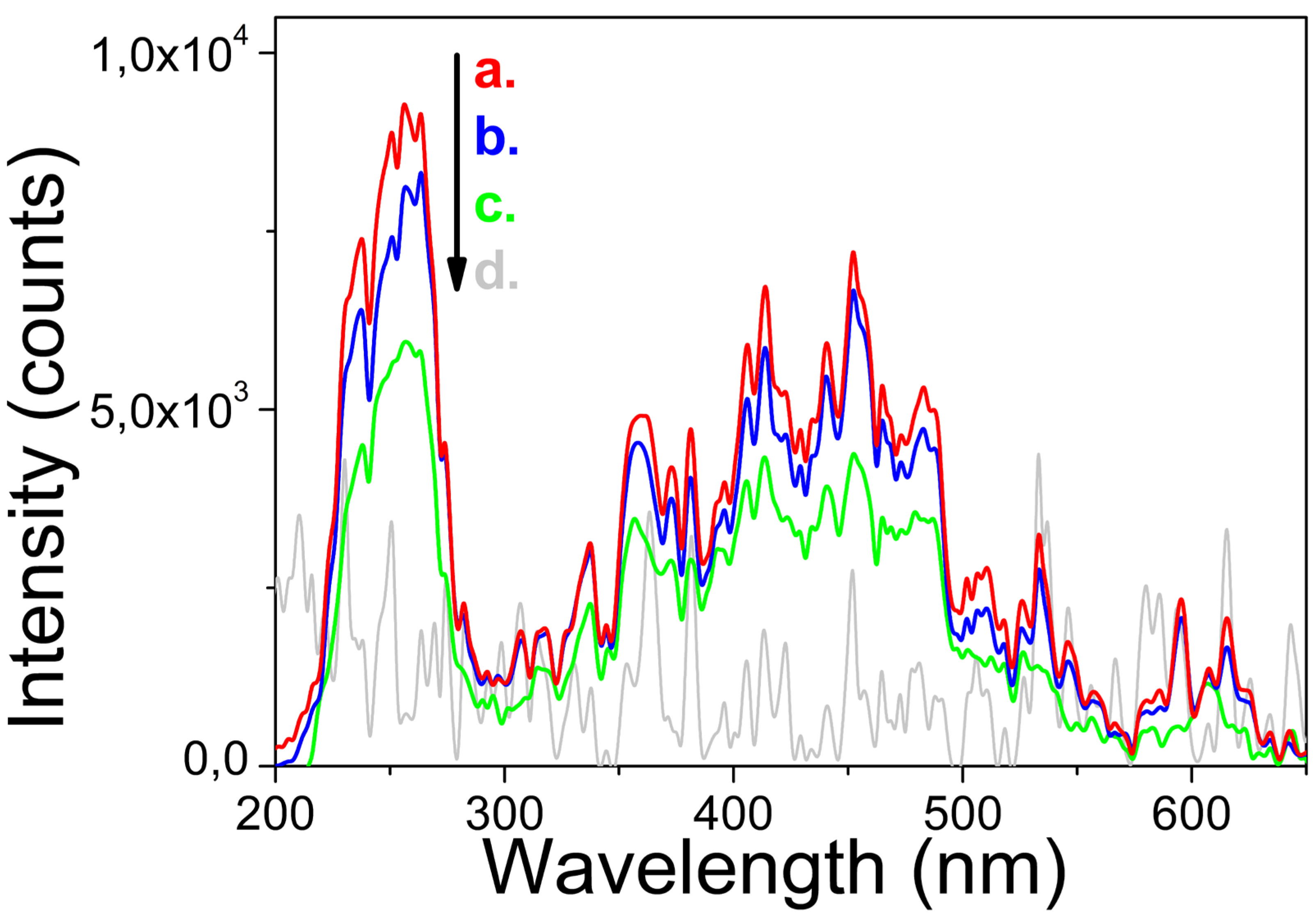
Figure 7.
XEOL spectra of m-LaPO4: Pr3+ (1 at.%) powders prepared by HTMW method (200 °C, 2 hours) obtained with same synthesis conditions = 0.005 M, K2HPO4 excess ratio = 5, CTA = 0.027 M, pH = 8), but with different coprecipitation techniques: a)coprecipitation by adding 0.25 mmol of cations (10 mL solution) to 1.25 mmol anions (15 mL solution) and then diluted with 25 mL of deionized water; b) coprecipitation by adding 1.25 mmol of anions (10 mL solution) to 0.25 mmol cations (15 mL solution) and then diluted with 25 mL of deionized water; c) coprecipitation by adding 0.2 mmol of cations (5 mL solution) to 1.25 mmol cations (45 mL solution);d) background spectrum.
Figure 7.
XEOL spectra of m-LaPO4: Pr3+ (1 at.%) powders prepared by HTMW method (200 °C, 2 hours) obtained with same synthesis conditions = 0.005 M, K2HPO4 excess ratio = 5, CTA = 0.027 M, pH = 8), but with different coprecipitation techniques: a)coprecipitation by adding 0.25 mmol of cations (10 mL solution) to 1.25 mmol anions (15 mL solution) and then diluted with 25 mL of deionized water; b) coprecipitation by adding 1.25 mmol of anions (10 mL solution) to 0.25 mmol cations (15 mL solution) and then diluted with 25 mL of deionized water; c) coprecipitation by adding 0.2 mmol of cations (5 mL solution) to 1.25 mmol cations (45 mL solution);d) background spectrum.
Scheme 1.
Representation of processes on the surface of LaPO4 NP in different aqueous media: (a) pH = 7 is near the isoelectric point of m-LaPO4, no charge in the surface, the near polar H2O molecules (shown by blue circle and red circles) are randomly positioned around NP; (b) pH < 5 is an acidic environment in which H+ ions are adsorbed on the surface of the LaPO4 NP, leading to a positive charge on the surface and polarization of H2O molecules with negative fields around the NP; (c) pH > 9 is a basic environment in which OH− ions are adsorbed on the surface of the LaPO4 NP, which leads to a negative surface charge and polarization of H2O molecules with positive fields around the NP; (d) pH > 9 with TA-modified LaPO4 NP causes the adsorption of OH− ions, as well as deprotonation of the carboxyl groups of tartaric acid, which leads to a higher total negative charge of the modified NPs and to a more stable solvate shell due to stronger interactions between NPs surface and aqueous medium.
Scheme 1.
Representation of processes on the surface of LaPO4 NP in different aqueous media: (a) pH = 7 is near the isoelectric point of m-LaPO4, no charge in the surface, the near polar H2O molecules (shown by blue circle and red circles) are randomly positioned around NP; (b) pH < 5 is an acidic environment in which H+ ions are adsorbed on the surface of the LaPO4 NP, leading to a positive charge on the surface and polarization of H2O molecules with negative fields around the NP; (c) pH > 9 is a basic environment in which OH− ions are adsorbed on the surface of the LaPO4 NP, which leads to a negative surface charge and polarization of H2O molecules with positive fields around the NP; (d) pH > 9 with TA-modified LaPO4 NP causes the adsorption of OH− ions, as well as deprotonation of the carboxyl groups of tartaric acid, which leads to a higher total negative charge of the modified NPs and to a more stable solvate shell due to stronger interactions between NPs surface and aqueous medium.
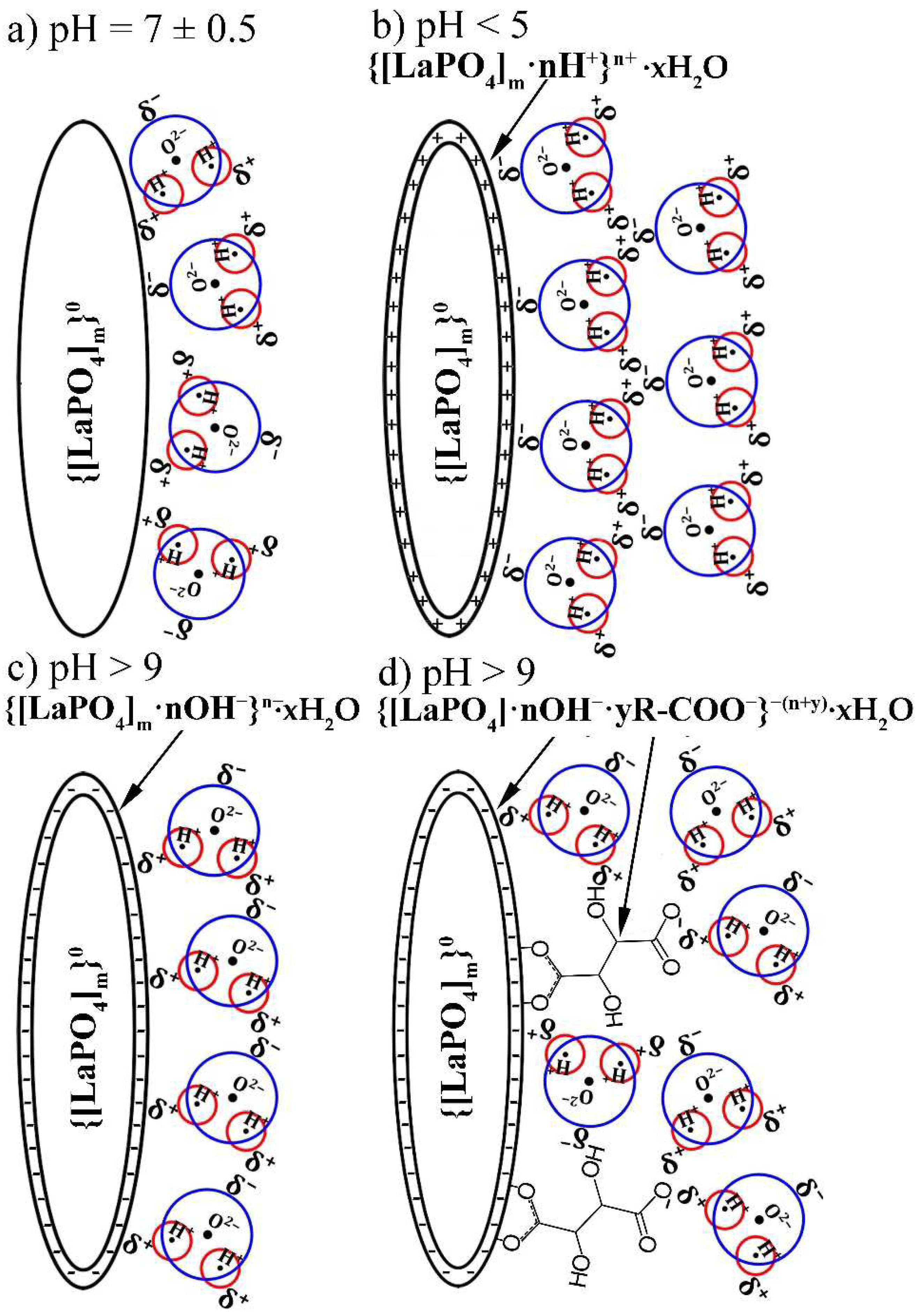
Table 1.
The comparison of morphology parameters with XEOL and CL UV-C intensities for LaPO4: Pr3+ (1 at.%) NPs at room temperature obtained under various synthesis conditions.
Table 1.
The comparison of morphology parameters with XEOL and CL UV-C intensities for LaPO4: Pr3+ (1 at.%) NPs at room temperature obtained under various synthesis conditions.
| Synthesis conditions |
TEM |
CL |
XEOL |
| Cat:An |
, M |
CTA, M |
pH |
Size, nm |
Relative Intensity, arb. units |
Relative Intensity,
arb. units |
| 1:1.25 |
0.01 |
- |
5 |
7 – 15 × 30 – 600 |
1 |
1 |
| 1:2 |
0.005 |
0.027 |
8 |
6.5 ± 1.5 × 31 ± 6 & 49 ± 9 |
0.095 |
0.057 |
| 1:5 |
0.005 |
0.027 |
8 |
5 ± 1 × 18 ± 4 |
- |
0.049 |
| 1:1.25 |
0.01 |
0.08 |
9 |
8 ± 1.5 × 46 ± 12 |
0.052 |
0.028 |
| 1:5 |
0.01 |
0.08 |
9 |
4.5 ± 1× 13 ± 4 |
0.009 |
0.024 |
| 1:10 |
0.005 |
0.027 |
8 |
6 ± 2 × 10 ± 4 |
- |
5.8 10-3
|