1. Introduction
Spinach (
Spinacia oleracea) is commonly consumed as a lutein-rich leafy vegetable in western also, has glycosylates, chlorophylls, vitamins, minerals, and bioactive compounds [
1]. Spinach as a food source of lutein is mostly consumed as a pharmaceutical, health supplement, extract, and functional beverage [
2]. Lutein is the representative macular carotenoid pigment that prevent numerous eye disease due to its different chemical structure compared to carotenes also, known as anti-inflammatory and antioxidant properties [
3]. Generally, carotenoids are composed of 40-carbon skeletons with two 20-carbon precursors conjugated with double bonds and carotenes are subgroups of hydrocarbons that consist of carotenoids [
4]. Lutein is the subgroup that has two hydroxyl groups bound with the ionone rings of the terminal on both sides. This chemical structure also gives polar and hydrophilic properties and improves the activity of reacting with oxygen [
4]. However, chemical changes, instability, low bio accessibility, and bioavailability of lutein is the main limitation of application in the food industry because processing condition which includes high temperature, presence of oxygen, light, and extreme pH (below 4, above 8.0) destroyed integrity of lutein [
5].
For this reason, preserving lutein contents during food processing and increasing bio accessibility using various food processing is required [
6]. Furthermore, to preserve the lutein contents during food processing, extraction is mainly applied rather than other food processing which includes heat but, the pasteurization process should proceed to produce juice product after extraction. Therefore, to improve the extraction yield of lutein, increased quantity of lutein extraction and non-thermal pasteurization technologies are essential to solve these problems.
A previous study has shown that the particle size reduction of raw materials preserves and improves the yield of lutein extraction [
7]. Jet-mill is a prospective superfine grinding technology that produces micro- and nanoparticles (<40 μm) using highly pressurized air streams with preserving hydrophilic and hydrophobic molecular structure characteristics [
8,
9]. Therefore, jet-mill technology has been widely studied and applied in pharmaceutical manufacturing [
10], barley and rye flours [
11], sargassum fusiform [
12], and wheat flour [
13] to improve extraction yield, physicochemical, mechanical, and rheological properties. However, there is no further research on the extraction of lutein from spinach powder using jet-mill without our previous research [
7].
Pulsed electric fields are representative of non-thermal pasteurization technology by using intense electric fields with short duration (milliseconds to microseconds) which preserve the functional compounds compared to thermal pasteurization technology [
14]. Treatment of PEF over 1V initiates the repulsion between the change-carrying molecules and forms the pore on the cell membrane which affects the microbial inactivation depending on the length, duration of field, and number of pulses [
15].However, the excessive treatment of PEF increases the temperature during pasteurization therefore optimization of the condition of PEF is crucial [
16]. Previous studies investigated the effects of PEF on pasteurized orange juice [
17], apple juice [
15], and fruit juice [
18] with no further degradation of bioactive compounds. However, there is no research performed on the pasteurization of spinach juice to preserve the lutein contents.
The aim of the present study is to investigate the application of a Jet mill and PEF to produce spinach juice to increase and preserve the lutein contents. To compare the effects of particle size reduction, coarse, fine, and superfine spinach powder was prepared and investigate the particle size distribution, hydration and oil binding capacity, and lutein contents. After that, PEF1 (20 kV/cm for 110 kJ/L) and PEF2 (20 kV/cm 150 kJ/L) were treated to compare the pasteurize effects on preserving lutein content compared to thermal technologies (Thermal 1 – 95 ℃ for 10 min, Thermal 2 – 121 ℃ for 15 min).
2. Materials and Methods
Fresh whole spinach leaves were purchased from a nonghyup in Anseong, South Korea. Then spinach without stems was dried for 48 s at 50 ℃ in a forced convection drying oven (SFC-203, Shinsaeng, Korea) and ground using a grinder. Raw spinach powder (SP) was passed through two steps (150 µm and 63 µm testing sieve, Nonaka Rikaki, Japan) using a sieve shaker (EML200, Haver & Boecker, Germany). The SP was prepared with sieving using a 150 µm sieve and which cannot pass through the 150 µm was collected as SP-coarse. SP-fine was collected using a 63 µm sieve and which cannot pass through the 63 µm was collected as SP-fine. The SP-superfine was obtained through the fluidized-bed jet mill (CGS 10, NETZSCH, Germany) and the conditions of jet milling were 7 bar of milling pressure and 12000 rpm for the classifier. All SP were stored at -18 ℃ until used for the experiment.
2.1. Determination of particle size distribution
The particle size of the SP used in the experiment was determined in triplicate by a particle size analyzer (Mastersizer 3000, Malvern Instrument Limited, U.K.). The size distribution of each sample was applied using the dry method and a diffraction laser and the operating conditions were set with particle absorption index (0.1), dispersant refractive index (1.00), laser obscurity 1.45 %, and particle refractive index (1.53).
2.2. Physical properties of spinach powder (SP)
To investigate the physical properties of SP, bulk density, tap density, and carr index were measured, and the procedure followed the method with some modifications [
19]. SP samples were poured into the cylinder and the volume was filled to 50 mL then the cylinder weights with 50 mL of powder were measured. The bulk density (g/mL) was calculated as the injection weight of the sample (g) divided by the sample volume (mL). For tap density measurements, SP samples were poured into the cylinder and the volume was filled to 50 mL. Then, the cylinder was tapped strongly 100 times to fill any of the remaining space for homogeneity. The cylinder weights and sample volume of powder were measured. The tap density (g/mL) was calculated as the injection weight of the sample (g) divided by the sample volume (mL). The flowability/compressibility of spinach powder samples was calculated by Shah
, et al. [
20]. The water holding capacity (WHC) and Swelling capacity (SC) were measured and calculated as described in Zhang
, et al. [
21] and the Oil holding capacity (OHC) was determined the procedure followed the method of Choi and Ma [
22] with some modification.
2.3. Scanning electron microscope (SEM)
The microstructures of SP were examined by scanning electron microscope (S-3400N, Hitachi High-Technologies Co., Japan). The sample was freeze-dried and fixed with carbon tape and plate into aluminum specimen stubs. The pretreated samples were coated with platinum-lead (Pt-Pb) and then observed in a vacuum state. Then the microstructure of SP was observed at 40x, 100x, and 200x magnification.
2.4. Spinach juice (SJ) preparation and pasteurization
To prepare the spinach juice (SJ), SP-superfine was extracted using ethanol (SP-superfine: ethanol = 1: 60) and centrifuged at 10,000 rpm for 10 min then the supernatant was collected. The supernatants from extracts were evaporated under vacuum at 40 ℃ then the dried extract was dispersed in distilled water to have a value of 7 brix (%) and used to make juice. The model juice with spinach extract was prepared with added sugar (20 %), citric acid (0.03 %), guar gum (0.01 %), and spinach extract, and each sample was added with 4 mL of spinach extract per 200 mL of distilled water. All samples were put into a glass bottle and homogenized for 3 min at 10,000 rpm using a homogenizer (T18 ultra-turra, IKA, Germany) before the experiment.
The pasteurization treatment condition of SJ was followed; the thermal treatment was performed in two conditions (thermal1- 95 ℃ for 10 min using a water bath (WB-11, Daihan, Korea), thermal2- 121 ℃ for 15 min using an autoclave (BF-AC45, BNF, Korea)) after treatment of pasteurization process the SJ was instantly cooled with ice bath until 4 ℃ reached. Treatment of PEF (Pulsed electric fields, 5 kW, DIL, Germany) with a 5-kW pulse generator (HVP-5; DIL, Germany), a continuous chamber with a 10 mm diameter tubular structure, and pulse of bipolar square type was used for PEF treatment. The input and output temperature were 35 ℃ and the flow rate of the SJ was 35L/h which is controlled using a peristaltic pump. The SJ was treated with 20 kV/cm 110 kJ/L and 20 kV/cm 150 kJ/L. After PEF treatment, SJ was cooled with an ice bath until 4 ℃ reached and stored with different storage temperatures of 15 ℃, 25 ℃, and 35 ℃ for 25 days, and the measurements were performed at 5-day intervals.
2.5. Determination of microbial activity
The reduction of microbial activity of SJ was evaluated by the number of total aerobic bacteria (TAB) which was measured using 3M petrifilm aerobic counts (PAC, 3M). All samples were conducted by 10-fold serial dilution and incubated at 35 ℃ ± 1 for 48 hours and after incubation, the number of total aerobic bacteria was counted.
2.6. Analysis of lutein contents
To extract the lutein in SP, 1 g of the sample was mixed with 50 mL of ethanol using a shaking incubator at 40 ℃ and 300 rpm, for 30 min. The extract was centrifuged at 5,000 rpm for 10 min, then the supernatant was collected as described in Derrien
, et al. [
23]. To prepare the extract of SJ, 5 mL of the sample was mixed with 30 mL of hexane under a shaking incubator at 40 ℃ and 300 rpm for 3 h then centrifuged at 5000 rpm for 10 min. The supernatant was collected, and the residue was centrifuged 2 times more to obtain a decolorized residue. The supernatants from SP and SJ were evaporated under vacuum at 40 ℃. The dry extract was dissolved in mobile phase A and filtered through a 0.2 μm syringe filter before HPLC analysis. The high-performance liquid chromatography (HPLC) system (LC-4000 series, Jasco, Japan) is equipped with a UV detector, set at 450 nm, a C30 YMC column (250 × 4.6 mm id., 5 mm), pump, and autosampler maintained at a constant temperature of 35 ℃, controlled by ChromNAV software. Isocratic conditions were held for 40 min, followed by a 2 % methyl-tertbutyl ether (MTBE) to 60 % of B, held for 25 min, then a subsequent 5 min linear gradient to return to 2 % MTBE, isocratic for 5 min, and total run time was 35 min.
2.7. Determination of antioxidant activities
To evaluate the antioxidant activity of SP and SJ, ABTS and DPPH assay was used., For both ABTS and DPPH assays, the extract of SP and SJ samples were diluted appropriately with ethanol or water and the ascorbic acid was used as a positive control.
For the ABTS assay, the procedure followed the method of Arnao
, et al. [
24] with some modifications. The extract of SP and SJ was diluted to 10 mg/mL and used as a sample solution. The 7.4 mM ABTS solution and 2.6 mM potassium persulfate solution were prepared in equal quantities to react for 24 h at room temperature in the dark. Then diluted by water to obtain an absorbance of 0.7 units using the spectrophotometer at 734 nm. The 180 μL of solution was added to 20 μL of sample solution and allowed to react for 30 min at room temperature in the dark. Then the absorbance was measured at 734 nm using a spectrometer (Spectramax 190 Microplate Reader).
For the DPPH assay, the procedure followed the method of Blois [
25] with some modifications. A stock solution of 0.2 mM DPPH in 99 % ethanol was prepared. The solution was diluted by ethanol to obtain an absorbance of 1.0 units at 517 nm. The 100 μL sample solution was added into the 100 μL solution and allowed to react for 30 min at room temperature in the dark. Then the absorbance was measured at 517 nm using a spectrometer (Spectramax 190 Microplate Reader).
3. Results and Discussion
3.1. Particle properties of Differently sized SP
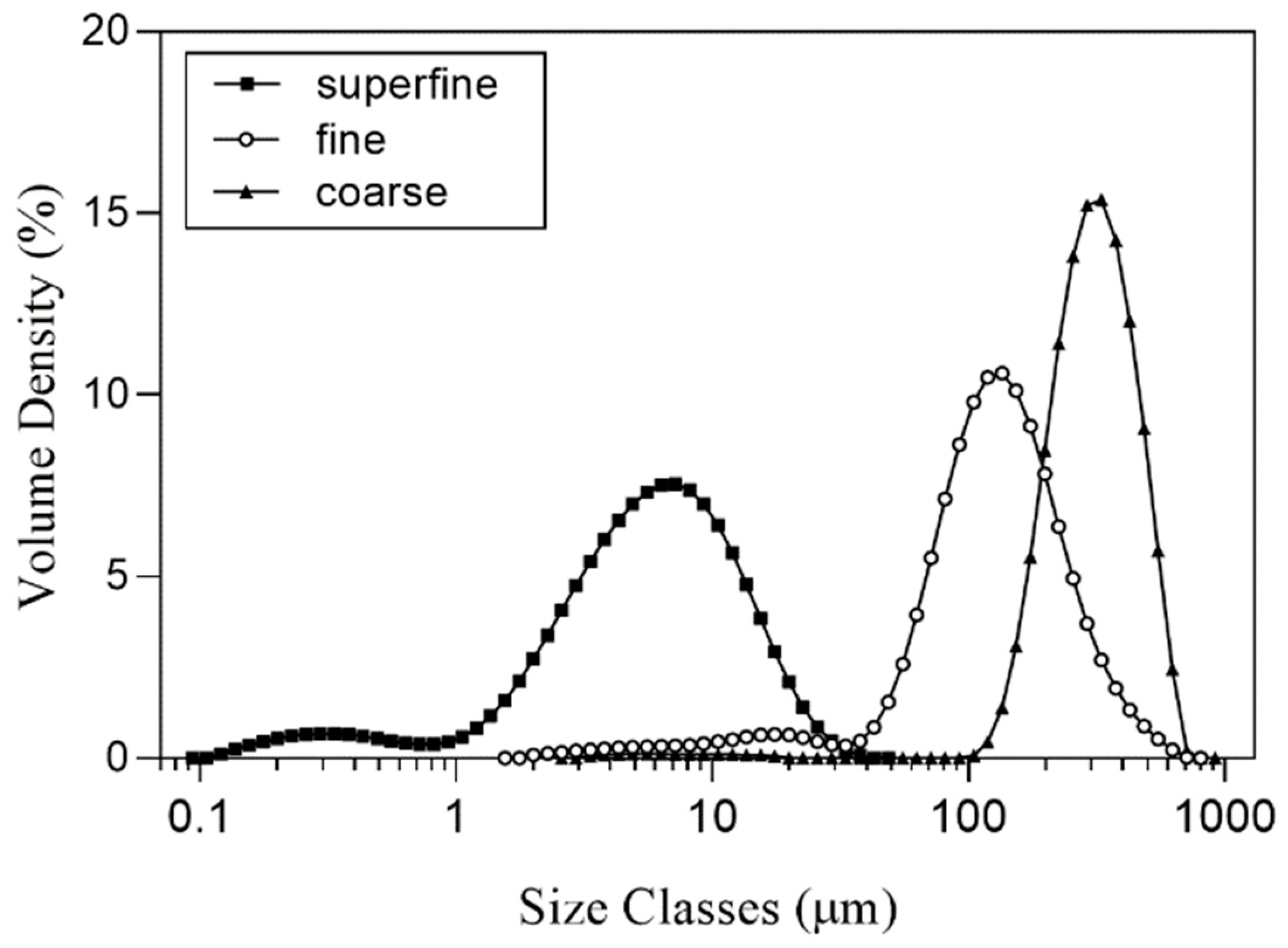
The particle size distribution of SP is presented in
Table 1 and
Figure 1. The Dv50 results of SP-coarse, SP-fine, and SP-superfine were 315.20 ± 3.37 µm, 125.20 ± 1.30 µm and 5.59 ± 0.24 µm, respectively. D[
4,
3] also showed similar values as 329.00 ± 6.44 µm, 143.6 ± 2.07 µm and 6.93 ± 0.24 µm, respectively. The specific surface areas of SP-coarse, SP-fine, and SP-superfine were 97.19 ± 4.11 m²/kg, 334.26 ± 11.33 m²/kg, and 8671.20 ± 414.26 m²/kg, which correlate with the results of particle size reduction. Dv10, Dv50, and Dv90 indicate the equivalent diameters at cumulative volumes of 10 %, 50 %, and 90 %, respectively, and as the decrease in the value of D[
4,
3] indicates the mean of the volume-weighted diameter decreased. An increase in the value of the span indicates the particle has a narrow and homogenous particle size distribution [
19]. Zhang, Song, Peng, Luo, Ming and Zhao [
21] reported that the jet-milled mushroom powder has the highest surface area than the higher particle size group. Also, similar results were found in [
19] that the value of Dv50 and D[
4,
3] which were treated by jet-mill dramatically decreased compared to coarse and fine soybean flour. Regarding the results of particle-size distribution, jet-milling effectively reduces the size of the particle and increases the homogenous particle size distribution which influences the physical properties of powder [
26].
The bulk density of SP-coarse, SP-fine, and SP-superfine were 0.44 ± 0.00 g/mL, 0.44 ± 0.00 g/mL, and 0.22 ± 0.00 g/mL, which increased as the particle size increased. A significant decrease in the bulk density and tap density was observed in the SP-superfine compared to the SP-coarse and SP-fine. The Carr Index of SP-superfine was 43.47 ± 0.49 %, which it means is a fluid powder with poor fluidity. Carr Index of SP-fine and SP-coarse were 24.27 ± 0.59 % and 13.65 ± 0.28 %, respectively, showing better fluidity than SP-superfine. Carr Index value of 0~20 % indicates good fluidity of the powder due to higher moisture content and porosity [
27]. Generally, lower density and cohesive energy correlate with an increase in viscosity than higher density due to the stronger interaction forces [
28]. However, in this study, SP-superfine showed poor flowability and similar research observed that the flowability gradually decreased depending on the particle size reduction due to the agglomeration, and these properties correlated with an increase in surface area [
29]. Moreover, Xu, et al. [
30] reported that due to the decrease in the contents of cellulose induced by the breakdown of the cellular structure during jet-milling could influence the viscosity and flowability of the superfine powder. In this research, jet-milling could significantly reduce the particle size and effectively crush the SP to a superfine scale of less than 10 µm but it suggests that the fluidity of juice could have lower viscosity compared to the coarse and fine particles due to the agglomeration and extremely higher specific surface area.
3.2. Hydration properties and oil holding capacity (OHC) of Differently sized SP
The WHC (water holding capacity) and SC (swelling capacity) of SP-superfine showed the lowest value among the group. Also, the OHC of SP-coarse, SP-fine, and SP-superfine were 2.86 ± 0.01 g/g, 2.51 ± 0.03 g/g, and 2.30 ± 0.04 g/g, which decreased as the particle size increased. In agreement with Gong, et al. [
31], as the particle size decreased, the WHC and OHC were decreased due to the hydrogen bond cleaved by the crushing process thus contents of insoluble cellulose decreased than coarse and fine powder. Ming, et al. [
32] reported that the long cellulose chain may break into the short chain, and disruption of the proteins and polysaccharide structure leads decrease in WHC and OHC. However, rice and barley which were milled by jet mill showed higher WHC due to the damaged starch which observed more water than intact starch [
33]. Also, Zhong, et al. [
34] reported that superfine grape peel powder showed higher solubility due to the larger surface area thus accelerating the dissolution time. Therefore, hydration properties and OHC could be changed by the chemical properties of ingredients. In this research, the reason that the SP-superfine showed lower WHC and OHC is may due to the destroyed cellulose structure.
3.3. Microstructure of Differently sized SP
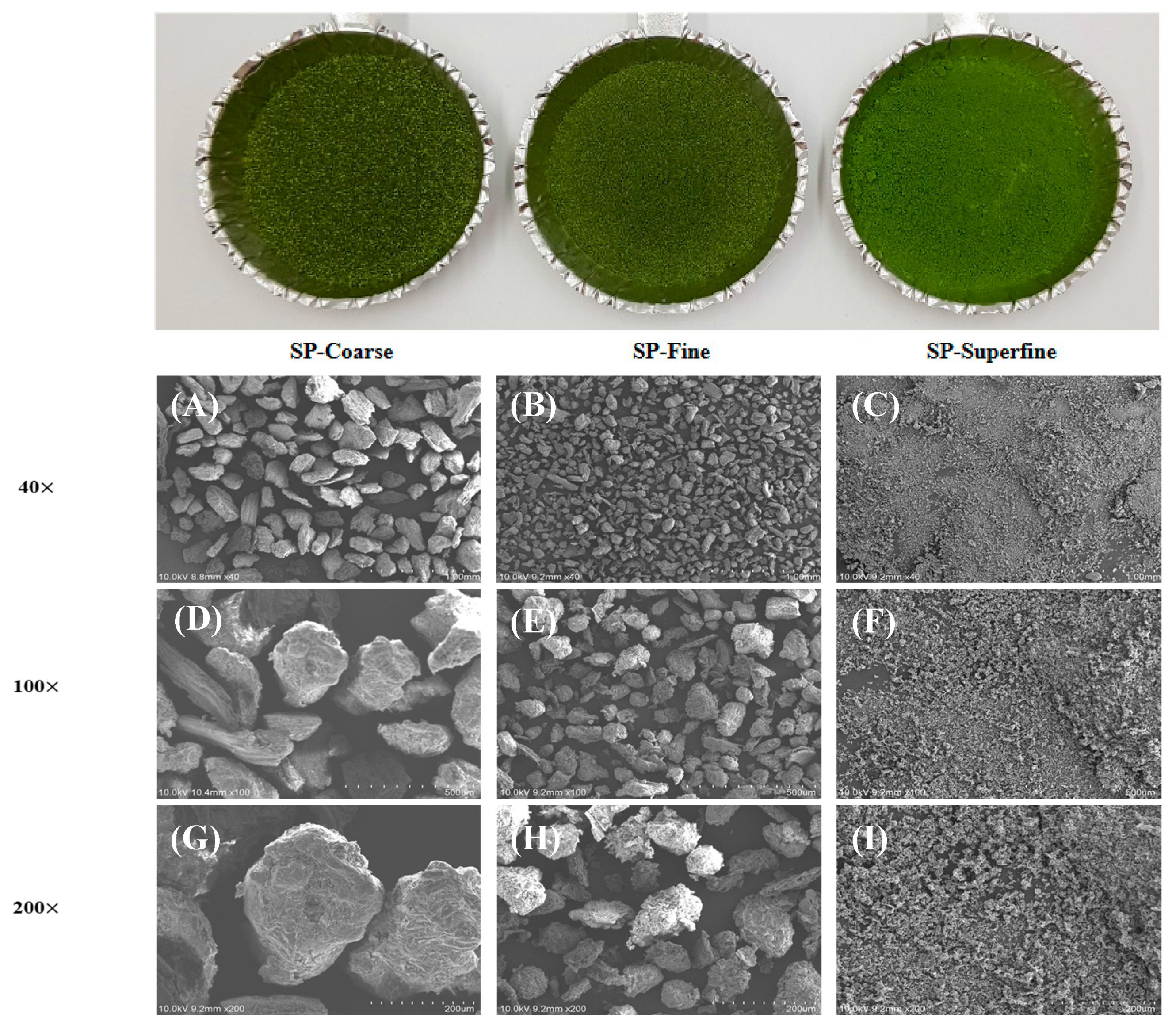
The micrographics are shown in
Figure 2. At the same magnification, the particle size of SP-superfine was significantly smaller than other SP samples. The surface structure of SP-coarse and SP-fine showed a larger and relatively irregular and intact structure than SP-superfine. The changes in microstructure induced by the mechanical force of the jet mill that the significant increase in the surface area gives agglomeration properties of the superfine powder. Similar results were found in Gong, Huang, Guo, Wang, Yue, Yang, Chen, Chang and Chen [
31] that superfine mushroom particles showed smaller, homogenous, and agglomeration powder than coarse (> 60-mesh) and 100-mesh particles. Also, Zhao, et al. [
35] showed, that superfine red grapes powder showed an amorphous domain caused by the breakdown of intermolecular bonds and its morphology properties correlate with the solubility and enzymatic hydrolysis. Muttakin, Kim and Lee [
19] reported that jet-mill induces the collision between the particles due to the high-pressure airflow thus smaller, non-porous, and more sold particles produced.
3.4. Optimization of lutein extraction conditions
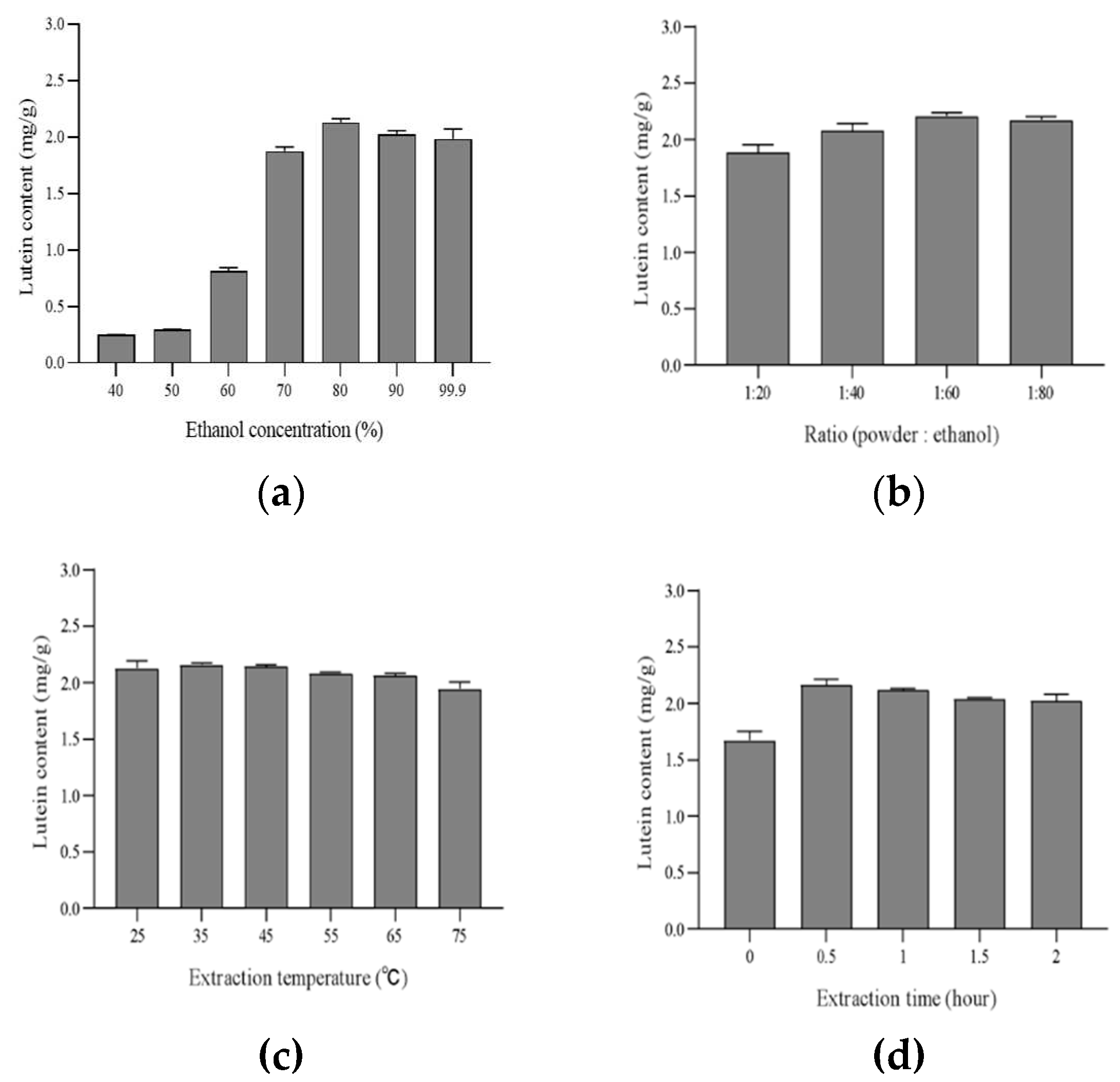
The results of the lutein content extracted by using different concentrations of ethanol are presented in
Figure 3. With the increase of the ethanol concentration from 40 % to 80 % in the extraction system, the lutein content increased from 0.24 to 2.13 mg/g of powder. The content of lutein increased proportionally with the ethanol/water ratio, in line with the lipophilic characteristic of lutein (long aliphatic chain) [
36]. When the ethanol concentration was increased to 80 %, 90 %, and 99 %, there was no significant change in the lutein content. Combined with the above results, the suitable ethanol concentration for the highest content of the lutein was considered 80 %. Solvent to SP ratio was another parameter affecting extraction of lutein however this parameter is a process of finding the minimum volume to avoid the saturation limit of the lutein [
37]. The lutein content increased as the ethanol to SP ratio was increased (20 - 60 mL of ethanol per 1 g of spinach powder) (
Figure 3. B.). Lutein content gives the highest value (2.21 mg/g of powder) when the ratio of SP to ethanol was 1: 60. In addition, the content of lutein increased little when the ratio of SP to ethanol surpassed 1: 60. Combined with the above results, the suitable ratio for highest content of the lutein was considered to be 1: 60. The results of the lutein content extracted at different temperatures (25, 35, 45, 55, 65 and 75 °C) are tabulated in
Figure 3.C. The lutein content extracted from SP at 35 °C showed the highest value (2.16 mg/g of powder) out of the lutein content obtained from extraction temperatures at 25 °C and 45 °C. However, the lutein content was decreased at a temperature above 55 °C, and at temperatures above 60 °C, the yield in lutein starts to decline, due to isomerization and degradation [
36,
38]. Hence, the extraction temperature of 35 °C was used in the subsequent experiments to determine the suitable extraction time. The results of the lutein content extracted at different times (0, 0.5, 1, 1.5, and 2 hours) are illustrated in
Figure 3. D. The increase of the extraction time from 0 to 0.5 hours in the extraction system, the lutein content increased from 1.67 to 2.17 mg/g of powder. When the extraction time continued from 0.5 to 2 h, the lutein content was almost constant, probably because the lutein in SP was already sufficiently extracted [
39]. Therefore, regarding our results, the optimum extraction parameters for lutein in SP were 80 % ethanol, for 0.5 h at 35 °C and with a 1/60 solvent-to-powder ratio.
3.5. Lutein contents and antioxidant activities of SP
Table 2 shows the lutein contents and antioxidant ability of the SP sample. The lutein content of SP-coarse, SP-fine, and SP-superfine were 1.59 ± 0.02 mg/g, 1.70 ± 0.10 mg/g, and 2.09 ± 0.13 mg/g of powder, respectively. The lutein content of SP-superfine was about 30 % higher than that of SP-coarse. These observations indicated that as the particle size decreases, the lutein content increases. The ABTS assay of SP-coarse, SP-fine, and SP-superfine were 56.56 ± 2.01 %, 55.31 ± 0.31 % and 65.01 ± 1.25 %, respectively. The DPPH radical scavenging activity of SP-coarse, SP-fine, and SP-superfine were 39.36 ± 1.94 %, 32.67 ± 1.36 % and 36.82 ± 1.65 %, respectively. ABTS assay showed higher antioxidant ability as the particle size decreased. Similar results were found by Zhong, Zu, Zhao, Li, Ge, Wu, Zhang, Li and Guo [
34] that superfine pomegranate peel powder showed the highest release of polyphenol and flavonoid contents compared to coarse and fine powder at the same time. Moreover, as the particle size of the barley decreased, the total phenolics content also decreased, and the antioxidant capacity due to the higher aggregation of superfine particles [
11]. Also, as the particle size reduced, the polysaccharides that have antioxidant activity extracted more than larger particles [
40]. However, an excessive milling process dramatically increases the surface area which can react with oxygen and the antioxidant compounds oxidation of raw materials increases thus functional compounds decrease which act as an antioxidant [
41]. In this study, the jet mill effectively breaks the cell wall which could accelerate the release of the intracellular compounds due to the high pressure and shear force during the milling process and higher porosity of particle lead accelerating the extraction of lutein.
3.6. Different pasteurization treatment effects on microbial activities of SJ with SP-superfine
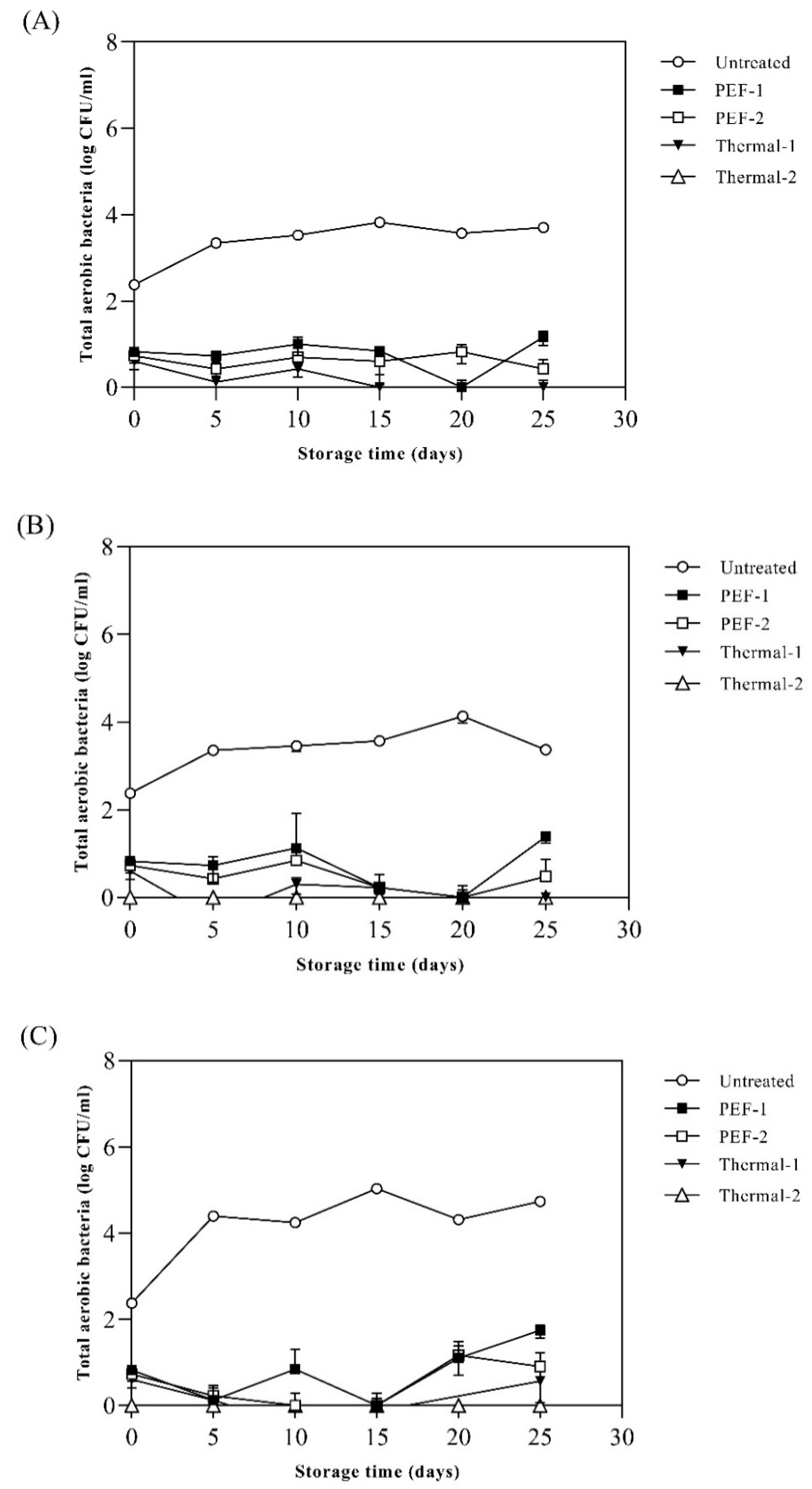
Figure 4 showed total aerobic bacteria (TAB) in SJ samples stored at 15 ℃, 25 ℃, and 35 ℃ for 25 days. The initial number of total aerobic bacteria in the SJ was 2.4 log CFU/mL and when stored at 15 ℃, the number of microorganisms reached 3.8 log CFU/mL in the untreated sample. The PEF and thermal treated SJ showed the number of microorganisms less than 1 log CFU/mL after treatment. The microbial growth stored at 15 ℃ was inhibited for 25 days in PEF-1, PEF-2, thermal-1, and thermal-2 (
Figure 4A). When stored at 25 ℃, the number of microorganisms reached 4.1 log CFU/mL in the untreated sample. The growth of microorganisms stored at 25 °C was inhibited for 25 days under all treatment conditions (
Figure 4B). When stored at 35 °C, the number of microorganisms reached 5.0 log CFU/mL in the untreated samples, and microbial growth was inhibited for 25 days in all pasteurization treatments (
Figure 4C). PEF physically damages the cell membrane which releases the intracellular substances thus when the strengths of PEF exceed the critical value, vegetative cells are inactivated, or irreversible damage is induced. Similar results were found in Jin et al. (1999) that the treatment of PEF (40 kV/cm) on cranberry juice significantly reduced the total aerobic bacteria by approximately 2 log cycles and as the strengths of PEF increased, the TAB decreased (p < 0.05). Moreover, treatment of PEF (over 20 kV/cm) could effectively inhibit the growth of TAB even at room temperature during storage. In agreement with these results, PEF treatment (over 35.5 kV/cm) leads 1-2 log cycle reduction in milk (Odriozola-Serrano et al. 2006). And, in Timmermans, et al. [
42], the TAB of samples treated by PEF showed under detection limit for 2 months at 4 °C without deterioration of initial quality in orange juice. Furthermore, treatment of PEF at 18.8 kV/cm completely inactivated the aerobic bacteria in fermented beverage by approximately 3.7 log cycle reduced also, no growth was observed during 56 days at 4 °C [
43]. However, it is difficult to inactivate microbial activities with a complex matrix because proteins, fats, and other compounds diminish the lethal effect of PEF thus the effects of PEF could be changed by the complexity of the beverage.
3.7. Lutein contents and antioxidant activity of SJ ring storage
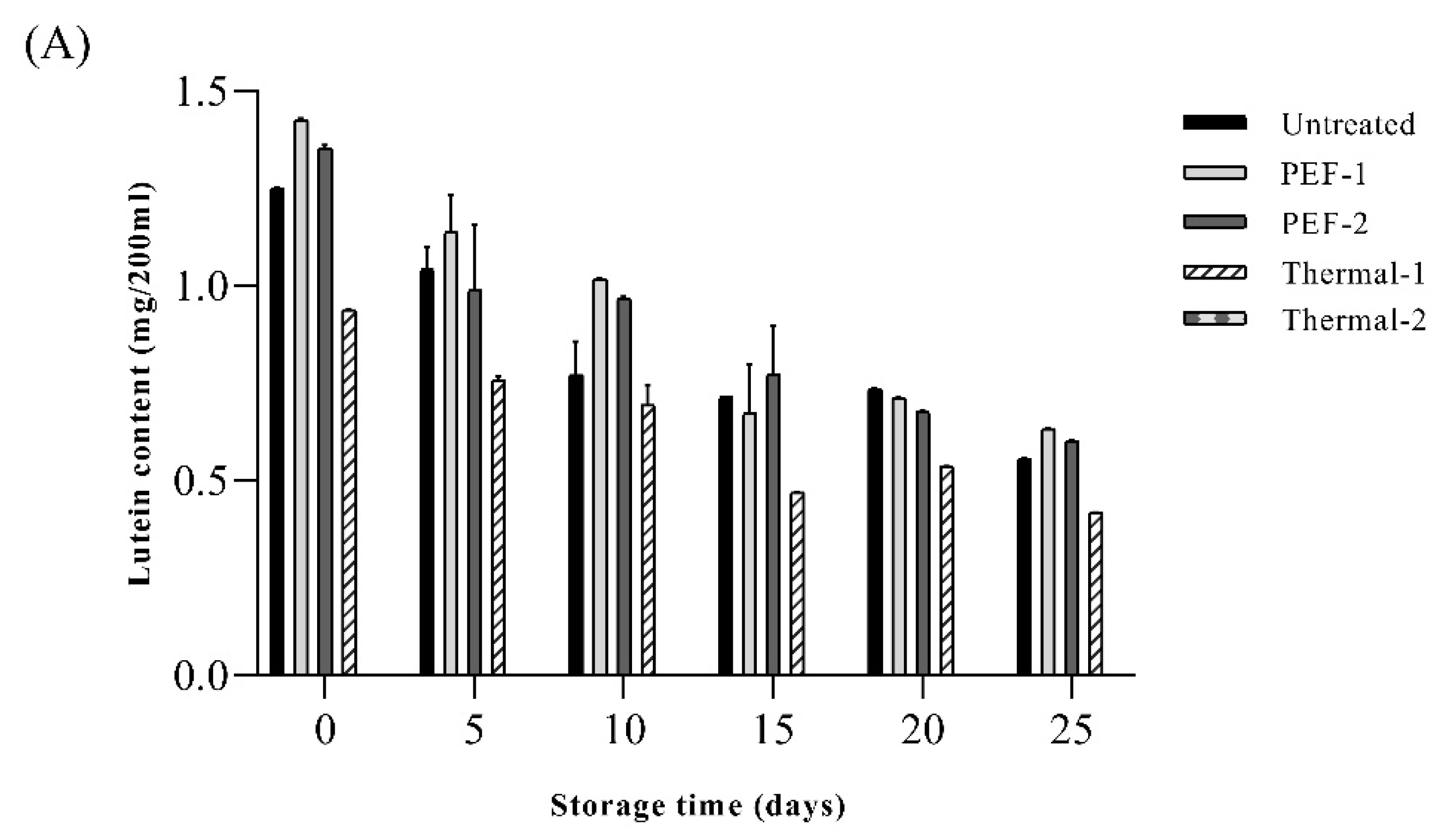
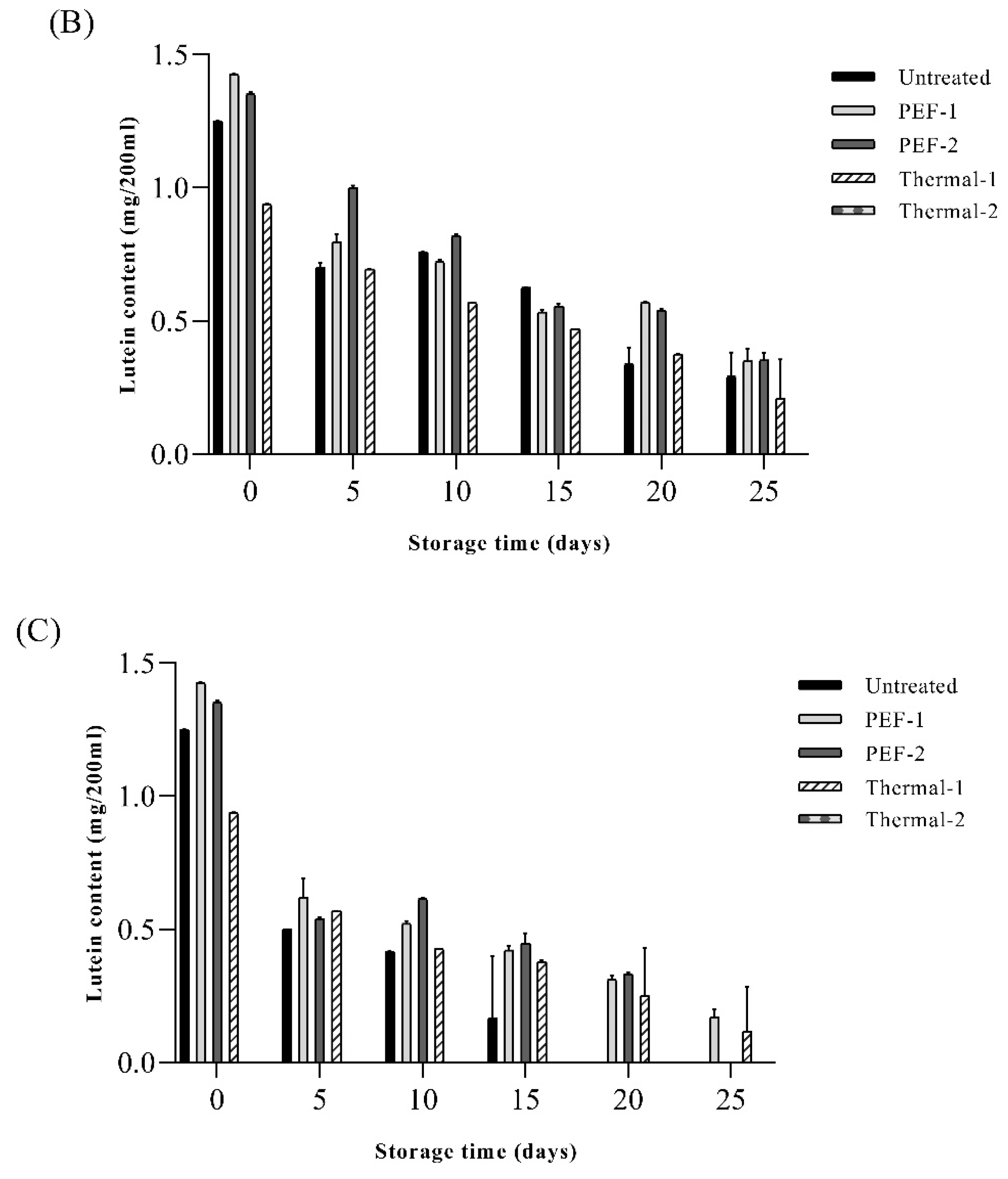
The lutein contents of SJ after pasteurization are shown in
Figure 5. The initial content of lutein in the SJ after PEF treatment (PEF1, PEF2) were 1.42 ± 0.01 mg/200mL, 1.35 ± 0.02 mg/200 mL and the untreated sample was 1.25±0.00 mg/200mL. In the present study, there was no decrease in lutein content by PEF treatment and this result was similar to the previous study, in which PEF treatment inhibited the degradation of pigments in spinach extract [
14]. However, lutein contents of thermal1 were 0.93 ± 0.01 mg/200mL, and thermal2 was not detected. These results were similar to the previous study, which reported that during the pasteurization (85 °C for 25 s) process, the lutein content was slightly decreased and after sterilization (121.5 °C for 15 min), lutein was completely degraded and not detected [
44]. Also, Chung, et al. [
45] reported that regardless of the heating method, heat treatment causes a substantial loss of lutein in spinach. The lutein content of the untreated sample was 1.25 ± 0.00 mg/200 mL, which was higher than that of the heat-treated sample (0.93 ± 0.01 mg/200 mL) and lower than that of the PEF sample (1.42 ± 0.01, 1.35 ± 0.02 mg/200 mL, respectively) on the initial period at 15 ℃ then significantly decreased during storage (p < 0.05). Although reduction of lutein in SJ was found in all samples, lutein content was retained in the PEF1 and PEF2 rather than in the thermal1 and thermal2. When stored at 35 °C, the untreated sample showed a complete decrease in lutein from day 20, and for PEF-2, a complete decrease in lutein occurred on day 25 thus 5 days increased for preserving lutein content in SJ. Similar results were observed in Rios-Corripio et al. (2022) that the sample treated by PEF (18.8 kV/cm) on pomegranate fermented beverage showed constant total phenol and flavonoid content during 56 d storage compared to other pasteurization technologies. Exposure to intense voltage could increase antioxidant capacity due to the release of phenolic compounds that the treatment of PEF (7 kV/cm) on wines increases the bioactive compounds [
46]. Furthermore, treatment of PEF effectively reduces the total aerobic bacteria without alteration in antioxidant compounds such as anthocyanin and total phenolic contents in pomegranate juice [
47]. In summary, it can be established that lutein contents were relatively stable in SJ treated by PEF without excessive degradation of lutein during storage compared to thermal treatment.
4. Conclusions
In this study, a Jet mill could efficiently produce microparticles (SP-superfine powder (Dv50 = 5.59 µm)). Due to the increase in specific surface area, particle size reduction influences the hydration properties and oil holding capacity which could affect the physico-chemical properties and extraction of lutein. The optimum extraction parameters for lutein were 80 % ethanol, for 0.5 h at 35 °C and with 1/60 solvent to spinach powder ratio. As the size of the SP particle decreased, the antioxidant activities and lutein contents significantly increased (p < 0.05). SP-superfine and optimal extraction conditions of lutein, and the 10 mL of the spinach extract have an 11.24 mg lutein content. For the comparison of lutein contents, different pasteurization treatment on SJ was performed using PEF and the results of PEF treatment had little effect on lutein content compared to the untreated sample. However, the lutein content of thermal treatment decreased significantly from 4.46 ± 0.05 mg/200 mL to 3.34 ± 0.01 mg/200 mL (at 95 ℃ for 10 min) and was not detected (at 121 ℃ for 15 min). The lutein content of SJ decreased significantly with increasing storage temperature during storage, but the loss of lutein in the PEF-treated group was relatively smaller than in the thermal-treated group. Regarding all results in this study, we suggest that the complex of Jet-milling and PEF technology could be the promising technology to produce lutein-enhanced SP juice with minimal lutein denaturation and less quality change during storage.
Author Contributions
“Conceptualization, D.-U.L.; methodology, Y.-G.L.; software, J.-H.J.; validation, S.-Y.K.; formal analysis, Y.-G.L.; investigation, S.-Y.K., Y.-G.L., H.-I.J., and J.-H.J.; resources, D.-U.L.; data curation, S.-H.J.; writing—original draft preparation, S.-Y.K. and Y.-G.L.; writing—review and editing, D.-U.L. and S.-H.J.; visualization, J.-H.J.; supervision, D.-U.L.; project administration, D.-U.L.; funding acquisition, D.-U.L.”.
Funding
This work was support by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through High Value-added Food Technology Development Program funded by Ministry of Agriculture, Food, and Rural Affairs (MAFRA)(332018-04).
Data Availability Statement
Data will be made available on request.
Acknowledgments
This research was supported by a Chung-Ang University Graduate Research Scholarship in 2022.
Conflicts of Interest
None
References
- Manzoor, M.F.; Zeng, X.-A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. Journal of Food Science and Technology 2019, 56, 2355–2364. [Google Scholar] [CrossRef]
- Steiner, B.M.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends in Food Science & Technology 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochimica et biophysica acta (BBA)-Molecular and cell biology of lipids 2020, 1865, 158617. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Molecular aspects of medicine 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Gouveia, L.; Empis, J. Relative stabilities of microalgal carotenoids in microalgal extracts, biomass and fish feed: effect of storage conditions. Innovative Food Science & Emerging Technologies 2003, 4, 227–233. [Google Scholar] [CrossRef]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Díaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. Journal of Functional Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Hong, S.-Y.; Choi, H.-S.; Kim, J.-H.; Jeong, S.-H.; Lee, S.-Y.; Kim, S.-H.; Lee, D.-U. Superfine Marigold Powder Improves the Quality of Sponge Cake: Lutein Fortification, Texture, and Sensory Properties. Foods 2023, 12, 508. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Chen, S.-C.; Wang, Q.-L.; Liu, C.-Q.; Xiao, J.-H.; Huang, D.-W. Effects of traditional grinding and superfine grinding technologies on the properties and volatile components of Protaetia brevitarsis larvae powder. LWT 2023, 173, 114307. [Google Scholar] [CrossRef]
- Chamayou, A.; Dodds, J.A. Air jet milling. Handbook of powder technology 2007, 12, 421–435. [Google Scholar] [CrossRef]
- Patel, R.P.; Baria, A.H.; Patel, N.A. An overview of size reduction technologies in the field of pharmaceutical manufacturing. Asian Journal of Pharmaceutics (AJP) 2008, 2. [Google Scholar] [CrossRef]
- Drakos, A.; Kyriakakis, G.; Evageliou, V.; Protonotariou, S.; Mandala, I.; Ritzoulis, C. Influence of jet milling and particle size on the composition, physicochemical and mechanical properties of barley and rye flours. Food Chemistry 2017, 215, 326–332. [Google Scholar] [CrossRef]
- Gan, L.-J.; You, Q.; Luo, Y.; Ye, Y.; Lei, L.; Deng, Z.; Rong, H. Effect of superfine grinding Sargassum fusiforme residue powder on sponge cakes properties. LWT 2022, 165, 113735. [Google Scholar] [CrossRef]
- Angelidis, G.; Protonotariou, S.; Mandala, I.; Rosell, C.M. Jet milling effect on wheat flour characteristics and starch hydrolysis. Journal of food science and technology 2016, 53, 784–791. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Wang, M.-S. Effect of pulsed electric fields (PEFs) on the pigments extracted from spinach (Spinacia oleracea L.). Innovative Food Science & Emerging Technologies 2017, 43, 26–34. [Google Scholar] [CrossRef]
- Noci, F.; Riener, J.; Walkling-Ribeiro, M.; Cronin, D.; Morgan, D.; Lyng, J. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. Journal of food engineering 2008, 85, 141–146. [Google Scholar] [CrossRef]
- Yeom, H.W.; Streaker, C.B.; Zhang, Q.H.; Min, D.B. Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. Journal of Agricultural and Food Chemistry 2000, 48, 4597–4605. [Google Scholar] [CrossRef]
- Rivas, A.; Rodrigo, D.; Martínez, A.; Barbosa-Cánovas, G.; Rodrigo, M. Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice. LWT-Food Science and Technology 2006, 39, 1163–1170. [Google Scholar] [CrossRef]
- Timmermans, R.; Mastwijk, H.; Berendsen, L.; Nederhoff, A.; Matser, A.; Van Boekel, M.; Groot, M.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. International Journal of Food Microbiology 2019, 298, 63–73. [Google Scholar] [CrossRef]
- Muttakin, S.; Kim, M.S.; Lee, D.-U. Tailoring physicochemical and sensorial properties of defatted soybean flour using jet-milling technology. Food Chemistry 2015, 187, 106–111. [Google Scholar] [CrossRef]
- Shah, R.B.; Tawakkul, M.A.; Khan, M.A. Comparative evaluation of flow for pharmaceutical powders and granules. Aaps Pharmscitech 2008, 9, 250–258. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, H.; Peng, Z.; Luo, Q.; Ming, J.; Zhao, G. Characterization of stipe and cap powders of mushroom (Lentinus edodes) prepared by different grinding methods. Journal of Food Engineering 2012, 109, 406–413. [Google Scholar] [CrossRef]
- Choi, S.-M.; Ma, C.-Y. Extraction, purification and characterization of globulin from common buckwheat (Fagopyrum esculentum Moench) seeds. Food Research International 2006, 39, 974–981. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a sustainable purification protocol for lutein and chlorophyll from spinach by-products by a saponification procedure using Box Behnken design and desirability function. Food and Bioproducts Processing 2019, 116, 54–62. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food chemistry 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Nykamp, G.; Carstensen, U.; Müller, B. Jet milling—a new technique for microparticle preparation. International journal of pharmaceutics 2002, 242, 79–86. [Google Scholar] [CrossRef]
- Guerin, E.; Tchoreloff, P.; Leclerc, B.; Tanguy, D.; Deleuil, M.; Couarraze, G. Rheological characterization of pharmaceutical powders using tap testing, shear cell and mercury porosimeter. International journal of pharmaceutics 1999, 189, 91–103. [Google Scholar] [CrossRef]
- Hao, T. Understanding empirical powder flowability criteria scaled by Hausner ratio or Carr index with the analogous viscosity concept. RSC advances 2015, 5, 57212–57215. [Google Scholar] [CrossRef]
- Lv, G.; Zhang, Z.; Pan, H.; Fan, L. Effect of physical modification of mushroom (A. chaxingu) powders on their physical and chemical properties. Food Science and Technology Research 2014, 20, 731–738. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Zhang, N.; Xiong, S.; Zhang, L.; Wang, J. Effect of superfine grinding on the physicochemical properties of straw mushroom (Volvariella volvacea) powders rich in vitamin D2. Journal of Food Processing and Preservation 2022, 46, e17192. [Google Scholar] [CrossRef]
- Gong, P.; Huang, Z.; Guo, Y.; Wang, X.; Yue, S.; Yang, W.; Chen, F.; Chang, X.; Chen, L. The effect of superfine grinding on physicochemical properties of three kinds of mushroom powder. Journal of Food Science 2022, 87, 3528–3541. [Google Scholar] [CrossRef]
- Ming, J.; Chen, L.; Hong, H.; Li, J. Effect of superfine grinding on the physico-chemical, morphological and thermogravimetric properties of Lentinus edodes mushroom powders. Journal of the Science of Food and Agriculture 2015, 95, 2431–2437. [Google Scholar] [CrossRef]
- Phat, C.; Li, H.; Lee, D.-U.; Moon, B.; Yoo, Y.-B.; Lee, C. Characterization of Hericium erinaceum powders prepared by conventional roll milling and jet milling. Journal of Food Engineering 2015, 145, 19–24. [Google Scholar] [CrossRef]
- Zhong, C.; Zu, Y.; Zhao, X.; Li, Y.; Ge, Y.; Wu, W.; Zhang, Y.; Li, Y.; Guo, D. Effect of superfine grinding on physicochemical and antioxidant properties of pomegranate peel. International Journal of Food Science & Technology 2016, 51, 212–221. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Zhang, G.; Tang, W. Effect of superfine grinding on the physicochemical properties and antioxidant activity of red grape pomace powders. Powder Technology 2015, 286, 838–844. [Google Scholar] [CrossRef]
- Latasa, M.; Van Lenning, K.; Garrido, J.; Scharek, R.; Estrada, M.; Rodríguez, F.; Zapata, M. Losses of chlorophylls and carotenoids in aqueous acetone and methanol extracts prepared for RPHPLC analysis of pigments. Chromatographia 2001, 53, 385–391. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). LWT-Food Science and Technology 2017, 79, 170–177. [Google Scholar] [CrossRef]
- Hojnik, M.; Škerget, M.; Knez, Ž. Isolation of chlorophylls from stinging nettle (Urtica dioica L.). Separation and Purification Technology 2007, 57, 37–46. [Google Scholar] [CrossRef]
- XuJie, H.; Na, Z.; SuYing, X.; ShuGang, L.; BaoQiu, Y. Extraction of BaChu mushroom polysaccharides and preparation of a compound beverage. Carbohydrate polymers 2008, 73, 289–294. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Ni, D. Effect of superfine grinding on quality and antioxidant property of fine green tea powders. LWT-Food Science and Technology 2012, 45, 8–12. [Google Scholar] [CrossRef]
- Li, G.; Guo, W.; Gao, X.; Wang, Y.; Sun, S. Effect of superfine grinding on physicochemical and antioxidant properties of soybean residue powder. Food Science & Nutrition 2020, 8, 1208–1214. [Google Scholar] [CrossRef]
- Timmermans, R.; Mastwijk, H.; Knol, J.; Quataert, M.; Vervoort, L.; Van der Plancken, I.; Hendrickx, M.; Matser, A. Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice. Part I: Impact on overall quality attributes. Innovative Food Science & Emerging Technologies 2011, 12, 235–243. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Morales-de la Peña, M.; Welti-Chanes, J.; Guerrero-Beltrán, J.Á. Pulsed electric field processing of a pomegranate (Punica granatum L.) fermented beverage. Innovative Food Science & Emerging Technologies 2022, 79, 103045. [Google Scholar] [CrossRef]
- Jalali-Jivan, M.; Abbasi, S.; Fathi-Achachlouei, B. Lutein extraction by microemulsion technique: Evaluation of stability versus thermal processing and environmental stresses. Lwt 2021, 149, 111839. [Google Scholar] [CrossRef]
- Chung, R.W.; Leanderson, P.; Gustafsson, N.; Jonasson, L. Liberation of lutein from spinach: Effects of heating time, microwave-reheating and liquefaction. Food chemistry 2019, 277, 573–578. [Google Scholar] [CrossRef]
- Vicaş, S.I.; Bandici, L.; Teuşdea, A.C.; Turcin, V.; Popa, D.; Bandici, G.E. The bioactive compounds, antioxidant capacity, and color intensity in must and wines derived from grapes processed by pulsed electric field. CYTA-Journal of Food 2017, 15, 553–562. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Geveke, D.J.; Fan, X.; Sites, J.E.; Wang, L. Evaluation of microbial stability, bioactive compounds, physicochemical properties, and consumer acceptance of pomegranate juice processed in a commercial scale pulsed electric field system. Food and Bioprocess Technology 2014, 7, 2112–2120. [Google Scholar] [CrossRef]
Figure 1.
Particle size distribution of differently sized SP. SP-coarse: coarse spinach powder, SP-fine: fine spinach powder, SP-superfine: superfine spinach powder.
Figure 1.
Particle size distribution of differently sized SP. SP-coarse: coarse spinach powder, SP-fine: fine spinach powder, SP-superfine: superfine spinach powder.
Figure 2.
SEM images of differently sized SP. SP-coarse: coarse spinach powder, SP-fine: fine spinach powder, SP-superfine: superfine spinach powder (A, D, G): SP-coarse, (B, E, H): SP-fine, (C, F, I): SP-superfine. (A)-(C) 40 ×, (D)-(F) 100 ×, (G) -(I) 200 × magnification.
Figure 2.
SEM images of differently sized SP. SP-coarse: coarse spinach powder, SP-fine: fine spinach powder, SP-superfine: superfine spinach powder (A, D, G): SP-coarse, (B, E, H): SP-fine, (C, F, I): SP-superfine. (A)-(C) 40 ×, (D)-(F) 100 ×, (G) -(I) 200 × magnification.
Figure 3.
Lutein content of spinach by extraction conditions (A) ethanol concentration (B) solvent to powder ratio (C) extraction temperature (D) extraction time. Values are expressed as mean ± standard deviation, means with different letters within the same column (a–c) are significantly different (p < 0.05) according to the results of Duncan’s test (n=5).
Figure 3.
Lutein content of spinach by extraction conditions (A) ethanol concentration (B) solvent to powder ratio (C) extraction temperature (D) extraction time. Values are expressed as mean ± standard deviation, means with different letters within the same column (a–c) are significantly different (p < 0.05) according to the results of Duncan’s test (n=5).
Figure 4.
Effects of different pasteurization treatments on the total aerobic bacteria of spinach extract during storage at (A) 15 ℃, (B) 25 ℃, and (c) 35 ℃. Untreated: no pasteurization, PEF-1: treated with 20 kV/cm 110 kJ/L, PEF-2: treated with 20 kV/cm 150 kJ/L, Thermal-1: treated with 95 ℃, 10 min, Thermal-2: treated with 121 ℃, 15 min. Values are expressed as mean ± standard deviation (n = 5).
Figure 4.
Effects of different pasteurization treatments on the total aerobic bacteria of spinach extract during storage at (A) 15 ℃, (B) 25 ℃, and (c) 35 ℃. Untreated: no pasteurization, PEF-1: treated with 20 kV/cm 110 kJ/L, PEF-2: treated with 20 kV/cm 150 kJ/L, Thermal-1: treated with 95 ℃, 10 min, Thermal-2: treated with 121 ℃, 15 min. Values are expressed as mean ± standard deviation (n = 5).
Figure 5.
Effects of pasteurization treatment on lutein content of spinach extract during storage at (A) 15 ℃, (B) 25 ℃, and (c) 35℃. Untreated: no pasteurization, PEF-1: treated with 20 kV/cm 110 kJ/L, PEF-2: treated with 20 kV/cm 150 kJ/L, Thermal-1: treated with 95 ℃, 10 min, Thermal-2: treated with 121 ℃, 15 min. Values are expressed as mean ± standard deviation (n = 5).
Figure 5.
Effects of pasteurization treatment on lutein content of spinach extract during storage at (A) 15 ℃, (B) 25 ℃, and (c) 35℃. Untreated: no pasteurization, PEF-1: treated with 20 kV/cm 110 kJ/L, PEF-2: treated with 20 kV/cm 150 kJ/L, Thermal-1: treated with 95 ℃, 10 min, Thermal-2: treated with 121 ℃, 15 min. Values are expressed as mean ± standard deviation (n = 5).
Table 1.
Particle-size distribution and powder properties with differently-sized SP.
Table 1.
Particle-size distribution and powder properties with differently-sized SP.
| Samples |
Particle-size distribution |
Powder properties flowability |
| Dv10 (μm) |
Dv50 (μm) |
Dv90 (μm) |
D[4,3] (μm) |
Span |
Specific Surface Area (m²/kg) |
Real density (g/mL) |
Bulk density (g/mL) |
Tap density (g/mL) |
Carr index (%) |
| SP-coarse1)
|
191.60±5.41a
|
315.20±3.37a
|
495.00±5.15a
|
329.00±6.44a
|
0.96±0.03c
|
97.19±4.11b
|
1.50±0.00c
|
0.44±0.00a
|
0.51±0.00b
|
13.65±0.28c
|
| SP-fine |
53.22±2.84b
|
125.20±1.30b
|
259.60±4.45b
|
143.6±2.07b
|
1.65±0.04b
|
334.26±11.33b
|
1.55±0.00a
|
0.44±0.00a
|
0.58±0.00a
|
24.27±0.59b
|
| SP-superfine |
1.59±0.08c
|
5.59±0.24c
|
14.16±0.35c
|
6.93±0.24c
|
2.24±0.05a
|
8671.20±414.26a
|
1.52±0.00b
|
0.22±0.00b
|
0.39±0.00c
|
43.47±0.49a
|
Table 2.
Hydration properties, lutein contents, and antioxidant activity with differently-sized SP.
Table 2.
Hydration properties, lutein contents, and antioxidant activity with differently-sized SP.
| Samples |
Hydration properties |
Antioxidant activities |
| WHC (g/g) |
SC (ml/g) |
OHC (g/g) |
Lutein content (mg/g) |
ABTS (%) |
DPPH (%) |
| SP-coarse1)
|
4.55±0.47a
|
1.97±0.06a
|
2.86±0.01c
|
1.59±0.02b
|
56.56±2.01b
|
39.36±1.94a
|
| SP-fine |
3.87±0.08b
|
1.60±0.10b
|
2.51±0.03b
|
1.70±0.10b
|
55.31±0.31b
|
32.67±1.36b
|
| SP-superfine |
3.26±0.23c
|
1.33±0.29b
|
2.30±0.04a
|
2.09±0.13a
|
65.01±1.25a
|
36.82±1.65a
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).